Effects of Aging on Regular and Express Latencies of Vergence
Abstract
Introduction
Methods and Materials
Subjects
Visual display
Fixation and oculomotor tasks: gap and overlap conditions
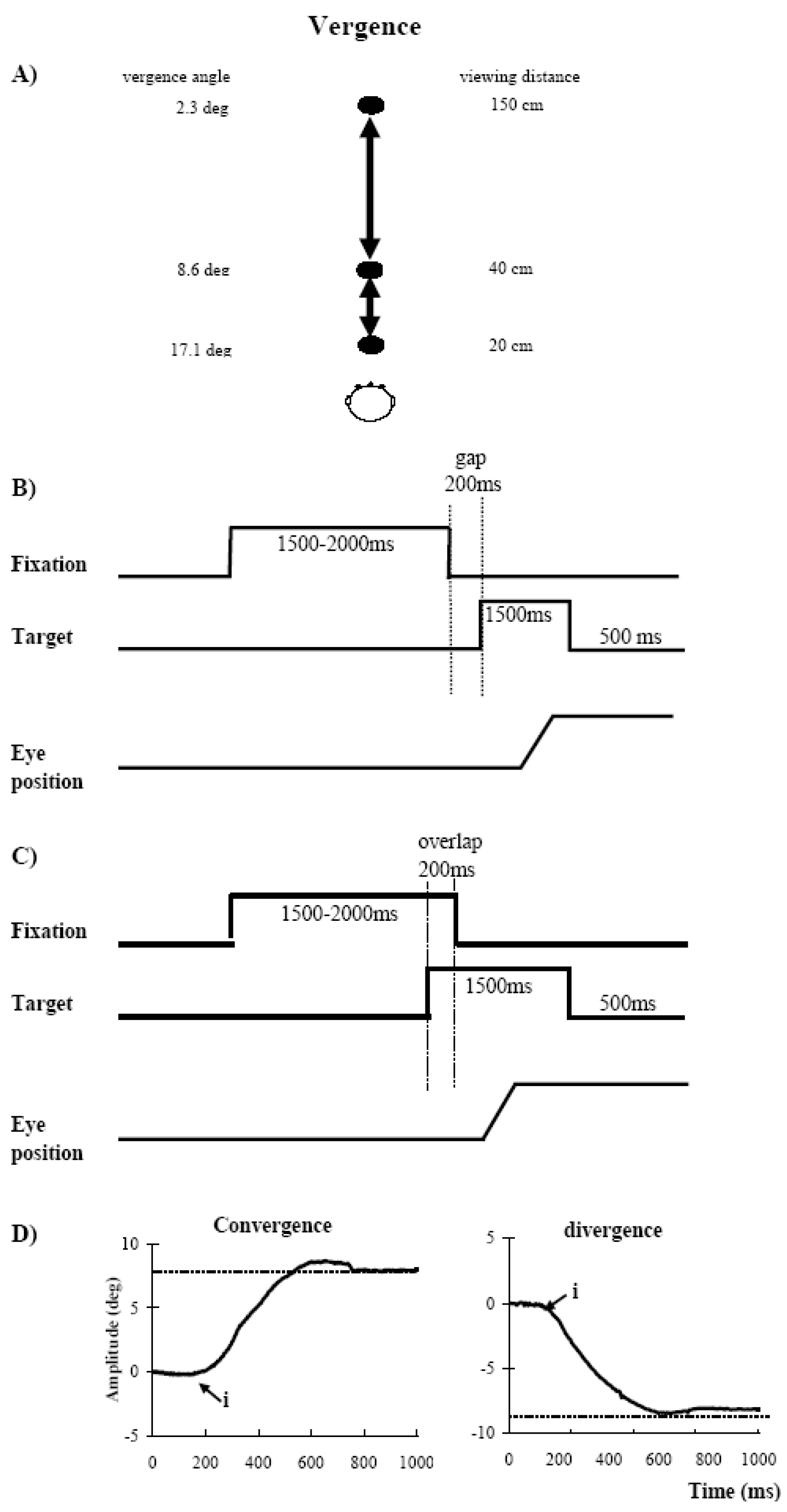
Eye movement recording
Data analysis
Results
Individual and group latencies


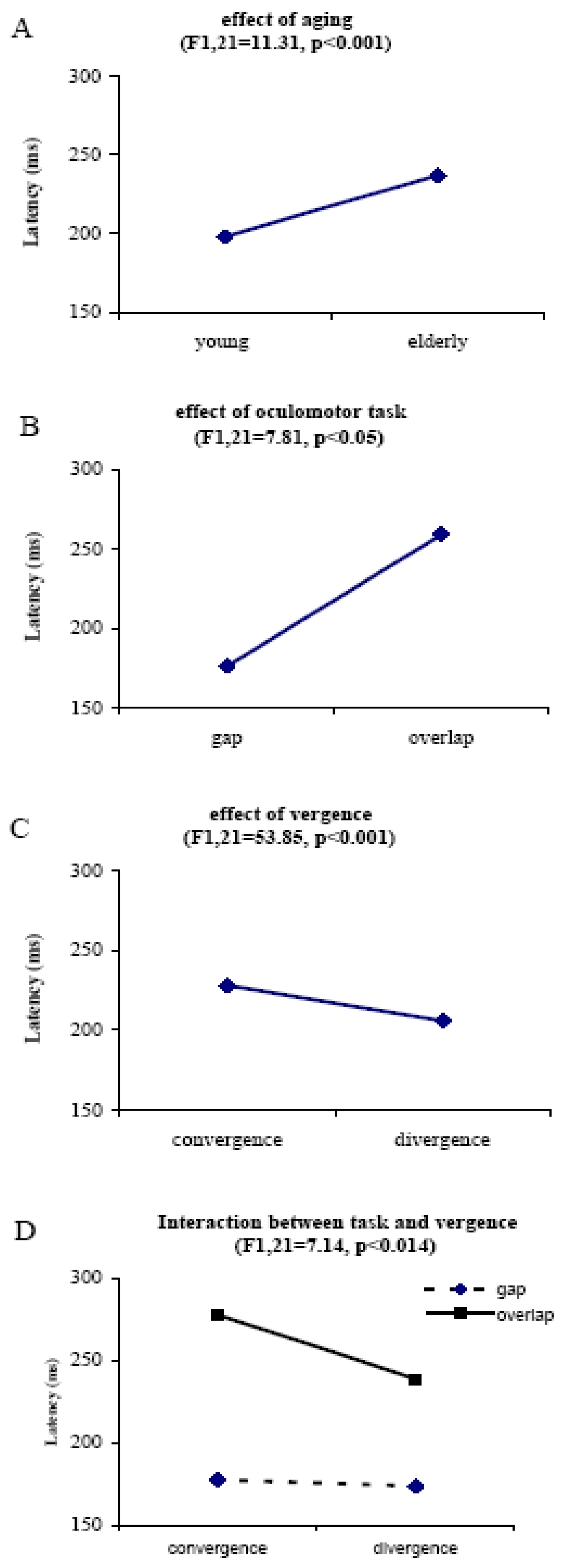
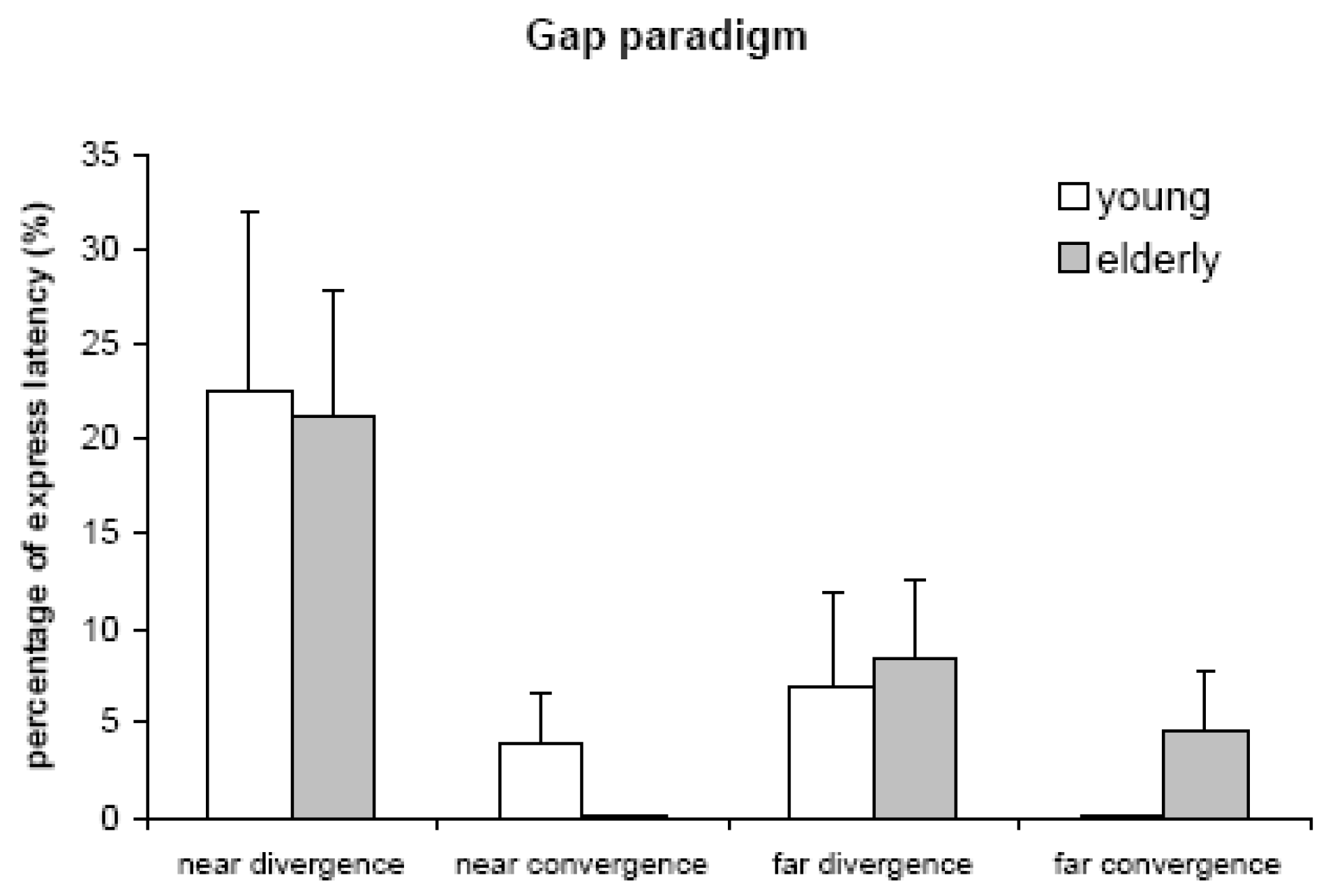
Express latencies
Discussion
Increase of regular vergence latency in elderly
Gap effect
Express divergence
Models of express latency
Convergence vs divergence latency
Acknowledgments
References
- Abel, L. A., B. T. Troost, and L. F. Dell’Osso. 1983. The effects of age on normal saccadic characteristics and their variability. Vision Research 23, 1: 33–37. [Google Scholar] [PubMed]
- Alvarez, T. L., J. L. Semmlow, and C. Pedrono. 2005. Divergence eye movements are dependent on initial stimulus position. Vision Research 45, 14: 1847–1855. [Google Scholar]
- Brown, J. W., D. Bullock, and S. Grossberg. 2004. How laminar frontal cortex and basal ganglia circuits interact to control planned and reactive saccades. Neural Networks 17, 4: 471–510. [Google Scholar]
- Bucci, M. P., N. Pouvreau, Q. Yang, and Z. Kapoula. 2005. Influence of gap and overlap paradigms on saccade latencies and vergence eye movements in seven-year-old children. Experimental Brain Research 164, 1: 48–57. [Google Scholar] [CrossRef]
- Carpenter, R. 1981. Oculomotor procrastination. In Eye movements: cognition and visual perception. Edited by D. Fischer and R. Monty. Erlbaum: Hillsdale, pp. 237–246. [Google Scholar]
- Chaturvedi, V., and J. A. Van Gisbergen. 2000. Stimulation in the rostral pole of monkey superior colliculus: effects on vergence eye movements. Experimental Brain Research 132, 1: 72–78. [Google Scholar] [PubMed]
- Clarke, A. H., J. Ditterich, K. Druen, U. Schonfeld, and C. Steineke. 2002. Using high frame rate CMOS sensors for three-dimensional eye tracking. Behav Res Methods Instrum Comput 34, 4: 549–560. [Google Scholar] [PubMed]
- Coubard, O., G. Daunys, and Z. Kapoula. 2004. Gap effects on saccade and vergence latency. Experimental Brain Research 154, 3: 368–381. [Google Scholar]
- Coubard, O. A., and Z. Kapoula. 2006. Dorsolateral prefrontal cortex prevents short-latency saccade and vergence: a TMS study. Cerebral Cortex 16, 3: 425–436. [Google Scholar]
- Creasey, H., and S. I. Rapoport. 1985. The aging human brain. Annals of Neurology 17, 1: 2–10. [Google Scholar] [CrossRef]
- Csibra, G., M. H. Johnson, and L. A. Tucker. 1997. Attention and oculomotor control: a high-density ERP study of the gap effect. Neuropsychologia 35, 6: 855–865. [Google Scholar]
- de Hemptinne, C., P. Lefevre, and M. Missal. 2006. Influence of cognitive expectation on the initiation of anticipatory and visual pursuit eye movements in the rhesus monkey. Journal of Neurophysiology 95, 6: 3770–3782. [Google Scholar] [PubMed]
- Findlay, J. M., and R. Walker. 1999. A model of saccade generation based on parallel processing and competitive inhibition. Behavioral and Brain Sciences 22, 4: 661–674, discussion 674-721. [Google Scholar] [PubMed]
- Fischer, B., and H. Weber. 1993. Express saccades and visual attention. Behavioral and Brain Sciences 16, 3: 553–610. [Google Scholar]
- Fischer, B., and H. Weber. 1997. Effects of stimulus conditions on the performance of antisaccades in man. Experimental Brain Research 116, 2: 191–200. [Google Scholar]
- Fischer, B., H. Weber, M. Biscaldi, F. Aiple, P. Otto, and V. Stuhr. 1993. Separate populations of visually guided saccades in humans: reaction times and amplitudes. Experimental Brain Research 92, 3: 528–541. [Google Scholar]
- Gamlin, P. D., and K. Yoon. 2000. An area for vergence eye movement in primate frontal cortex. Nature 407, 6807: 1003–1007. [Google Scholar] [PubMed]
- Genovesio, A., and S. Ferraina. 2004. Integration of retinal disparity and fixation-distance related signals toward an egocentric coding of distance in the posterior parietal cortex of primates. Journal of Neurophysiology 91, 6: 2670–2684. [Google Scholar]
- Gezeck, S., and J. Timmer. 1998. Detecting multimodality in saccadic reaction time distributions in gap and overlap tasks. Biological Cybernetics 78, 4: 293–305. [Google Scholar] [CrossRef]
- Goldring, J., and B. Fischer. 1997. Reaction times of vertical prosaccades and antisaccades in gap and overlap tasks. Experimental Brain Research 113, 1: 88–103. [Google Scholar]
- Head, D., R. L. Buckner, J. S. Shimony, L. E. Williams, E. Akbudak, T. E. Conturo, M. McAvoy, J. C. Morris, and A. Z. Snyder. 2004. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cerebral Cortex 14, 4: 410–423. [Google Scholar]
- Henson, C., H. Staunton, and F. M. Brett. 2003. Does ageing have an effect on midbrain premotor nuclei for vertical eye movements? Movement disorder 18, 6: 688–694. [Google Scholar] [CrossRef] [PubMed]
- Huaman, A. G., and J. A. Sharpe. 1993. Vertical saccades in senescence. Investigative Ophthalmology & Visual Science 34, 8: 2588–2595. [Google Scholar]
- Hung, G. K., H. Zhu, and K. J. Ciuffreda. 1997. Convergence and divergence exhibit different response characteristics to symmetric stimuli. Vision Research 37, 9: 1197–1205. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Irving, E. L., M. J. Steinbach, L. Lillakas, R. J. Babu, and N. Hutchings. 2006. Horizontal saccade dynamics across the human life span. Investigative Ophthalmology & Visual Science 47, 6: 2478–2484. [Google Scholar]
- Isa, T., and Y. Kobayashi. 2004. Switching between cortical and subcortical sensorimotor pathways. Progress in Brain Research 143: 299–305. [Google Scholar]
- Kapoula, Z., E. Isotalo, R. M. Muri, M. P. Bucci, and S. Rivaud-Pechoux. 2001. Effects of transcranial magnetic stimulation of the posterior parietal cortex on saccades and vergence. Neuroreport 12, 18: 4041–4046. [Google Scholar] [CrossRef][Green Version]
- Kapoula, Z., Q. Yang, O. Coubard, G. Daunys, and C. Orssaud. 2005a. Contextual influence of TMS on the latency of saccades and vergence. Neuroscience Letters 376, 2: 87–92. [Google Scholar] [CrossRef]
- Kapoula, Z., Q. Yang, O. Coubard, G. Daunys, and C. Orssaud. 2005b. Role of the posterior parietal cortex in the initiation of saccades and vergence: right/left functional asymmetry. Annals of the New York Academy of Sciences 1039: 184–197. [Google Scholar] [CrossRef]
- Katzman, R., and R. Terry. 1983. The neurology of aging. Philadelphia: F.A. Davis Co. [Google Scholar]
- Kingstone, A., and R. M. Klein. 1993. Visual offsets facilitate saccadic latency: does predisengagement of visuospatial attention mediate this gap effect? Journal of Experimental Psychology Human Perception and Performance 19, 6: 1251–1265. [Google Scholar] [CrossRef]
- Knox, P. C., J. H. Davidson, and D. Anderson. 2005. Agerelated changes in smooth pursuit initiation. Experimental Brain Research 165, 1: 1–7. [Google Scholar]
- Krishnan, V. V., F. Farazian, and L. Stark. 1973. An analysis of latencies and prediction in the fusional vergence system. American Journal of Optometry & Archives of American Academy of Optometry 50, 12: 933–939. [Google Scholar]
- Kumar, A. N., Y. Han, S. Garbutt, and R. J. Leigh. 2002. Properties of anticipatory vergence responses. Investigative Ophthalmology & Visual Science 43, 8: 2626–2632. [Google Scholar]
- Leigh, R. J., and D. S. Zee. 2006. The Neurology of Eye Movements. New York: Oxford University Press. [Google Scholar]
- Mays, L. E., J. D. Porter, P. D. Gamlin, and C. A. Tello. 1986. Neural control of vergence eye movements: neurons encoding vergence velocity. Journal of Neurophysiology 56, 4: 1007–1021. [Google Scholar] [PubMed]
- Munoz, D. P., J. R. Broughton, J. E. Goldring, and I. T. Armstrong. 1998. Age-related performance of human subjects on saccadic eye movement tasks. Experimental Brain Research 121, 4: 391–400. [Google Scholar]
- Muri, R. M., A. I. Vermersch, S. Rivaud, B. Gaymard, and C. Pierrot-Deseilligny. 1996. Effects of single-pulse transcranial magnetic stimulation over the prefrontal and posterior parietal cortices during memory-guided saccades in humans. Journal of Neurophysiology 76, 3: 2102–2106. [Google Scholar]
- Ohtsuka, K., S. Maeda, and N. Oguri. 2002. Accommodation and convergence palsy caused by lesions in the bilateral rostral superior colliculus. American Journal of Ophthalmology 133, 3: 425–427. [Google Scholar]
- Pierrot-Deseilligny, C., R. M. Muri, C. J. Ploner, B. Gaymard, and S. Rivaud-Pechoux. 2003. Cortical control of ocular saccades in humans: a model for motricity. Progress in Brain Research 142: 3–17. [Google Scholar]
- Rambold, H., G. Neumann, T. Sander, and C. Helmchen. 2006. Age-related changes of vergence under natural viewing conditions. Neurobiology of Aging 27, 1: 163–172. [Google Scholar]
- Rashbass, C., and G. Westheimer. 1961. Disjunctive eye movements. Journal of Physiology 159: 339–360. [Google Scholar]
- Reuter-Lorenz, P. A., H. C. Hughes, and R. Fendrich. 1991. The reduction of saccadic latency by prior offset of the fixation point: an analysis of the gap effect. Perception & Psychophysics 49, 2: 167–175. [Google Scholar]
- Rosen, R. 1978. Feedforwards and global system failure: a general mechanism for senescence. Journal of Theoretical Biology 74, 4: 579–590. [Google Scholar] [PubMed]
- Ross, L. E., and S. M. Ross. 1980. Saccade latency and warning signals: stimulus onset, offset, and change as warning events. Perception & Psychophysics 27, 3: 251–257. [Google Scholar]
- Ross, S. M., and L. E. Ross. 1981. Saccade latency and warning signals: effects of auditory and visual stimulus onset and offset. Perception & Psychophysics 29, 5: 429–437. [Google Scholar]
- Rossini, P. M., S. Rossi, C. Babiloni, and J. Polich. 2007. Clinical neurophysiology of aging brain: From normal aging to neurodegeneration. Progress in Neurobiology 83, 6: 375–400. [Google Scholar]
- Salat, D. H., J. A. Kaye, and J. S. Janowsky. 2001. Selective preservation and degeneration within the prefrontal cortex in aging and Alzheimer disease. Archives of Neurology 58, 9: 1403–1408. [Google Scholar] [PubMed]
- Saslow, M. G. 1967. Effects of components of displacement-step stimuli upon latency for saccadic eye movement. Journal of the Optical Society of America 57, 8: 1024–1029. [Google Scholar] [PubMed]
- Schiller, P. H., J. H. Sandell, and J. H. Maunsell. 1987. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. Journal of Neurophysiology 57, 4: 1033–1049. [Google Scholar] [PubMed]
- Schiller, P. H., and E. J. Tehovnik. 2005. Neural mechanisms underlying target selection with saccadic eye movements. Progress in Brain Research 149: 157–171. [Google Scholar]
- Semmlow, J., and P. Wetzel. 1979. Dynamic contributions of the components of binocular vergence. Journal of the Optical Society of America 69, 5: 639–645. [Google Scholar]
- Suzuki, S., Y. Suzuki, and K. Ohtsuka. 2004. Convergence eye movements evoked by microstimulation of the rostral superior colliculus in the cat. Neuroscience Research 49, 1: 39–45. [Google Scholar]
- Takagi, M., E. M. Frohman, and D. S. Zee. 1995. Gapoverlap effects on latencies of saccades, vergence and combined vergence-saccades in humans. Vision Research 35, 23-24: 3373–3388. [Google Scholar] [CrossRef]
- Tam, W. J., and H. Ono. 1994. Fixation disengagement and eye-movement latency. Perception & Psychophysics 56, 3: 251–260. [Google Scholar]
- Tzelepi, A., A. Lutz, and Z. Kapoula. 2004. EEG activity related to preparation and suppression of eye movements in three-dimensional space. Experimental Brain Research 155, 4: 439–449. [Google Scholar] [CrossRef] [PubMed]
- Vijayashankar, N., and H. Brody. 1977a. Aging in the human brain stem. A study of the nucleus of the trochlear nerve. Acta Anat (Basel) 99, 2: 169–172. [Google Scholar] [CrossRef] [PubMed]
- Vijayashankar, N., and H. Brody. 1977b. A study of aging in the human abducens nucleus. Journal of Comparative Neurology 173, 3: 433–438. [Google Scholar] [CrossRef] [PubMed]
- Weber, H., and B. Fischer. 1995. Gap duration and location of attention focus modulate the occurrence of left/right asymmetries in the saccadic reaction times of human subjects. Vision Research 35, 7: 987–998. [Google Scholar] [CrossRef]
- Yang, Q., M. P. Bucci, and Z. Kapoula. 2002. The latency of saccades, vergence, and combined eye movements in children and in adults. Investigative Ophthalmology & Visual Science 43, 9: 2939–2949. [Google Scholar]
- Yang, Q., and Z. Kapoula. 2004. TMS over the left posterior parietal cortex prolongs latency of contralateral saccades and convergence. Investigative Ophthalmology & Visual Science 45, 7: 2231–2239. [Google Scholar]
- Yang, Q., and Z. Kapoula. 2006. The control of vertical saccades in aged subjects. Experimental Brain Research 171, 1: 67–77. [Google Scholar] [CrossRef]
- Yang, Q., Z. Kapoula, E. Debay, O. Coubard, C. Orssaud, and M. Samson. 2006. Prolongation of latency of horizontal saccades in elderly is distance and task specific. Vision Research 46, 5: 751–759. [Google Scholar] [CrossRef][Green Version]
© 2009 by the author. 2009 Qing Yang, Thanh-Thuan Lê, Zoi Kapoula
Share and Cite
Yang, Q.; Lê, T.-T.; Kapoula, Z. Effects of Aging on Regular and Express Latencies of Vergence. J. Eye Mov. Res. 2007, 1, 1-12. https://doi.org/10.16910/jemr.1.3.3
Yang Q, Lê T-T, Kapoula Z. Effects of Aging on Regular and Express Latencies of Vergence. Journal of Eye Movement Research. 2007; 1(3):1-12. https://doi.org/10.16910/jemr.1.3.3
Chicago/Turabian StyleYang, Qing, Thanh-Thuan Lê, and Zoi Kapoula. 2007. "Effects of Aging on Regular and Express Latencies of Vergence" Journal of Eye Movement Research 1, no. 3: 1-12. https://doi.org/10.16910/jemr.1.3.3
APA StyleYang, Q., Lê, T.-T., & Kapoula, Z. (2007). Effects of Aging on Regular and Express Latencies of Vergence. Journal of Eye Movement Research, 1(3), 1-12. https://doi.org/10.16910/jemr.1.3.3


