Abstract
Background: Gemcitabine/nab-paclitaxel therapy (GnP) is widely used to treat pancreatic cancer (PC), but chemotherapy-induced peripheral neuropathy (CIPN) is common. The CIPN is also reported to be related by microvascular damage as the basis for toxic neuropathy. However, no sufficient treatment options are available for CIPN. Mirogabalin is a novel drug for treating peripheral neuropathy. We investigated the effects of mirogabalin on CIPN due to GnP. Methods: Patients who had received GnP for PC and had taken mirogabalin for CIPN, were included. Patients completed a questionnaire about their symptoms before and after taking mirogabalin. The outcome was the change in numbness and tingling scores on the patient-reported outcomes version of the Common Terminology Criteria for Adverse Events, the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Chemotherapy-Induced Peripheral Neuropathy (EORTC-QLQ-CIPN20), numerical rating scale, and adverse events (AEs). Results: Increased numbness and tingling severity (1.84 vs. 1.76; p = 0.63) and interference (1.42 vs. 1.44; p = 0.80) were not seen in any of the 25 enrolled patients. The scores on the sensory subscale of the EORTC-QLQ-CIPN improved significantly after treatment (17.5 vs. 15.7; p = 0.02). Adverse events occurred in 22 patients (88%), but there were no serious AEs (≥grade 3). Conclusions: Mirogabalin may control the progression of CIPN caused by GnP and significantly improved sensory neuropathy. However, as the incidence of AEs is high, mirogabalin should be used with caution. (UMIN:R000044039).
1. Introduction
The treatment of pancreatic cancer (PC) is challenging because most cases are detected at an unresectable stage. Chemotherapy plays a central role in the treatment of PC; regimens include FOLFIRINOX (a four-drug combination of 5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) and gemcitabine plus nab-paclitaxel (nab-PTX) (GnP) [1,2,3]. However, FOLFIRINOX is unsuitable for elderly patients due to its severe adverse events (AEs), so GnP therapy is often used as the first-line treatment [4]. Adverse events such as chemotherapy-induced peripheral neuropathy (CIPN) are frequent and serious problems because they not only reduce quality of life, but also force patients to stop or reduce chemotherapy. Various treatments for CIPN are available, but they are rarely effective [5,6]. Mirogabalin monobenzenesulfonate (Daiichi Sankyo, Tokyo, Japan) is a novel, selective oral α2δ ligand [7]. It is useful for peripheral neuropathy [8,9] and may also be effective for CIPN, but studies of its effects on the latter are lacking. Therefore, we evaluated the efficacy and safety of mirogabalin for CIPN due to GnP in PC patients.
2. Method
2.1. Study Design
We conducted a single-center retrospective pilot questionnaire study between January 2019 and August 2020 at Juntendo University Hospital, to evaluate the effects of mirogabalin on CIPN caused by GnP in PC patients. This study was performed in line with the principles of the Declaration of Helsinki. This study was approved by the institutional review board of Juntendo University (H19-0223). All patients provided informed consent.
2.2. Patients and Setting
Patients who had previously or were currently receiving GnP for PC and had taken mirogabalin for at least 2 weeks for numbness, tingling, or pain associated with peripheral neuropathy between January 2019 and June 2020, were included. Participants had to complete a questionnaire to determine the changes in symptoms before and after mirogabalin use. Patients who did not complete the questionnaire due to dementia or refusal were excluded (Figure 1).
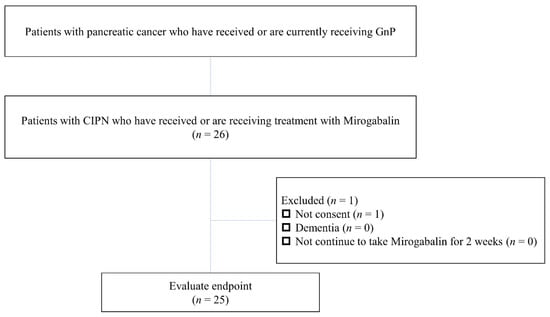
Figure 1.
Study flowchart. GnP, Gemcitabine/nab-paclitaxel therapy; CIPN, chemotherapy-induced peripheral neuropathy.
The primary study endpoint was the change in scores on the patient-reported outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) with respect to numbness and tingling. The frequency and severity of numbness and tingling were determined based on items scored from 0–4, where 0 represents “asymptomatic” and 4 “life-threatening consequences; urgent intervention indicated”. Secondary outcomes were the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire–Chemotherapy-Induced Peripheral Neuropathy (QLQ-CIPN20) score and a numerical rating scale (NRS) pain score. The EORTC-QLQ-CIPN20 comprises nine items assessing sensory neuropathy, eight assessing motor function, and three assessing autonomic neuropathy. However, we excluded items 19 and 20. Item 19 is concerned with the difficulty of using pedals and is applicable only to patients who drive a car. Few of our patients drive because of the efficiency of the Tokyo train network. Item 20 pertains to the difficulty of obtaining or maintaining an erection in males; this question was considered inappropriate because few patients undergoing chemotherapy are sexually active. The EORTC-QLQ-CIPN20 items are also scored from 1–4, with 1 representing “not at all” and 4 “very much”. The NRS pain scores ranged from 0 (asymptomatic) to 10 (extreme pain).
Mirogabalin is available in 2.5, 5, and 10 mg tablets. We gave patients 5–30 mg/day. The final dosage was based on the individual physician’s decision.
2.3. Statistical Analysis
We compared variables before and after taking mirogabalin using paired t-tests and STATA software (ver. 13.0; StataCorp, College Station, TX, USA).
3. Results
3.1. Patient Characteristics
Table 1 summarizes the characteristics of the 25 retrospectively analyzed patients. Their mean age was 65.9 ± 11.2 years. Ten patients (41.7%) were male. Sixteen patients (66.7%) had been diagnosed with diabetes mellitus, of whom ten (41.7%) had received insulin therapy. The mean hemoglobin A1c (HbA1c) was 6.8 ± 0.8%, the mean mirogabalin dose was 20.4 ± 8.2 mg/day and the mean interval between starting mirogabalin and completing the questionnaire was 70 ± 71.5 days. The clinical stage was III in 7 (28%) patients and IV in 16 (64.0%). There were two cases of postsurgical recurrence (8.0%). In terms of chemotherapy status, twenty-four patients received GnP as first-line therapy, and one as second-line therapy after receiving gemcitabine + S-1. Concomitant with CIPN treatment, vitamin B12 was taken by seven patients, non-steroidal anti-inflammatory drugs or acetaminophen by four, the Chinese herbal medicine goshajinkigan by three, and opioids by three, and changing from pregabalin in one patient.

Table 1.
Patient characteristics.
3.2. Efficacy of Mirogabalin
The PRO-CTCAE results showed no significant differences in the numbness or tingling severity after treatment compared to baseline (1.84 ± 0.90 vs. 1.76 ± 0.83; p = 0.63), and in no case did numbness and tingling interfere with daily life (1.42 ± 1.13 vs. 1.46 ± 1.02; p = 0.80). The mean total EORTC-QLQ-CIPN20 score was 32.6 ± 11.1 before taking mirogabalin and 30.6 ± 9.2 thereafter (p = 0.12). The EORTC-QLQ-CIPN sensory subscale score improved significantly after taking mirogabalin (17.5 ± 5.9 vs. 15.7 ± 4.8; p = 0.02), while there were no significant differences in the EORTC-QLQ-CIPN motor (11.7 ± 4.8 vs. 11.3 ± 3.9; p = 0.49) or autonomic (3.4 ± 1.4 vs. 3.6 ± 1.5; p = 0.31) subscale scores. The NRS pain score did not differ significantly before versus after taking mirogabalin (4.4 ± 2.0 vs. 4.1 ± 2.4; p = 0.26) (Table 2).

Table 2.
The efficacy of mirogabalin for CIPN by GnP therapy in PC patients.
Scores for individual items of the sensory and motor neuropathy subscales were also analyzed (Figure 2). The sensory neuropathy-related items showing the largest improvements concerning “tingling in the fingers and hands” and “numbness in the toes and feet”, with a mean change of 0.29. The mean change in “tingling in the toes and feet”, “numbness in the fingers and hands”, and “shooting/burning pain in the toes and feet” was 0.25. The scores for some items pertaining to motor neuropathy improved, while others worsened. The mean change was largest for “cramps in the hands and feet”, at 0.21. The scores for “difficulty holding a pen” and “difficulty climbing stairs” worsened slightly (Figure 3).
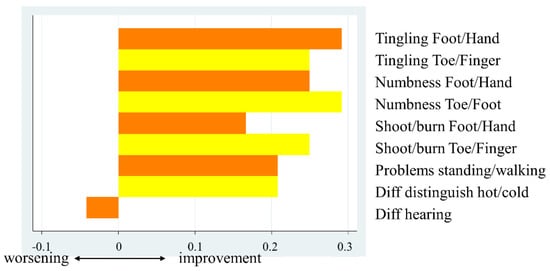
Figure 2.
Mean changes in sensory neuropathy subscale scores before and after taking mirogabalin.
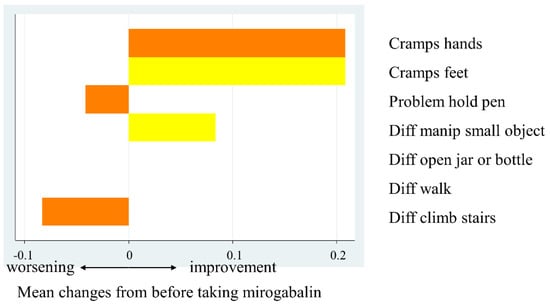
Figure 3.
Mean changes in motor neuropathy subscale scores before and after taking mirogabalin.
3.3. Adverse Events Associated with Mirogabalin
Of the 25 patients, 22 (88.0%) experienced AEs of any grade, including somnolence in 20 patients (grade 1, n = 17; grade 2, n = 3), dizziness in 17 (grade 1, n = 16; grade 2, n = 1), and edema in three (all grade 1). Four patients were unable to continue the medication due to AEs: one each had somnolence and dizziness, somnolence alone, dizziness alone, and difficulty continuing due to edema. The patients who experienced AEs felt that the disadvantages of mirogabalin outweighed the advantages, so discontinued its use (Table 3).

Table 3.
Safety of mirogabalin for CIPN patients.
4. Discussion
This study was to evaluate the efficacy of mirogabalin for CIPN caused by GnP therapy in PC patients. We found that mirogabalin controlled the progression of CIPN, and sensory neuropathy improved significantly. However, the rate of AEs was relatively high.
Despite the high prevalence of CIPN in association with GnP therapy, there are very few established treatments. It is recommended that, in patients who develop CIPN during chemotherapy, the treatment be delayed or discontinued, or the dose decreased. Effective drugs for CIPN are essential; mirogabalin is one such drug. Nab-PTX is a cytotoxic taxane that frequently causes CIPN. Duloxetine is the only drug recommended by the American Society of Clinical Oncology and Japan Pancreas Society [5,10] for treating established, painful CIPN caused by oxaliplatin or paclitaxel. Randomized controlled trials of duloxetine and pregabalin for taxane-induced CIPN found that pregabalin was more effective [11]. Neuropathy caused by nab-PTX usually presents as sensory neuropathy, manifesting as paresthesias, numbness, and tingling in the toes and fingers. These symptoms are like those seen in postherpetic neuralgia and diabetic peripheral neuropathy, and mirogabalin has been shown to be effective for treating them [8,9]. Pregabalin, which has the same mechanism of action as mirogabalin, can improve taxane-induced peripheral neuropathy [9]. We observed no CIPN worsening after taking mirogabalin compared to baseline, suggesting that it controlled CIPN worsening (which occurs due to cumulative toxicity). Mirogabalin significantly improved sensory neuropathy, including symptoms of CIPN such as tingling and numbness, making it a suitable treatment for these patients.
The CTCAE is generally used to evaluate CIPN but has several disadvantages. First, the CTCAE was not developed specifically to assess CIPN; moreover, it is not sensitive to changes in disease status and is associated with significant interrater variability [12]. The CTCAE also has a grade range of only 0–4, which makes it difficult to perform detailed evaluations; the accuracy and precision of the instrument may be inadequate. The PRO-CTCAE was developed to improve the precision of assessment of AEs. The EORTC-QLQ-CIPN has a subscale pertaining specifically to symptoms and functional limitations related to CIPN. These instruments provide a more detailed and reliable assessment of CIPN. In addition, quantification of CIPN using the Pain Vision system was also attempted, and correlations with the questionnaire were found [13].
Adverse effects were more common in this study compared to the literature, where studies have reported somnolence in 8.1–23.9% of patients receiving mirogabalin, dizziness in 4.9–15.5%, and edema in 2.4–8.5% [8,9,14,15]. Most of our patients had somnolence and dizziness. Previous studies on mirogabalin-related AEs focused on diabetic neuropathy and postherpetic neuralgia, in which somnolence and dizziness are less likely compared to CIPN [8,15]. The CIPN patients tend to receive strong chemotherapy; this increases the likelihood of chemotherapy-related AEs, which may be confused with mirogabalin-related ones [4,16]. Studies of AEs associated with mirogabalin reported that the first event tended to occur within 1 month after administration, and that AEs were more frequent in lean women aged over 65 years [8,14,15]. In line with this, we observed somnolence in all our female patients. The frequency of AEs also increases with the mirogabalin dose. In lean elderly women who are prone to AEs, a low dose (5 mg/day) during the first month may reduce the likelihood of AEs. Four of our patients discontinued mirogabalin due to grade 1 AEs. The proportion of patients who discontinued mirogabalin was in line with the literature. Discontinuing or reducing the dosage of mirogabalin is an important option for patients who do not feel that they are deriving any benefits from the medication.
Our study had some limitations. First, it was a single-center retrospective pilot study including a small number of cases. Second, only one questionnaire pertained specifically to nab-PTX, which exhibits cumulative toxicity; prospective studies are therefore necessary. Third, the Japanese version of the EORTC-QLQ-CIPN is still being validated (currently in “stage 3”).
Despite these limitations, this study had several important strengths: it enrolled more patients than the previous study [17] of the effect of mirogabalin on CIPN, and showed that it controlled disease progression. Moreover, using the precise PRO-CTCAE and EORTC-QLQ-CIPN instruments, we demonstrated that mirogabalin is particularly effective for sensory neuropathy.
In conclusion, mirogabalin may slow the progression of CIPN despite cumulative toxicity, and significantly improved sensory neuropathy. However, the rate of AEs was high, so careful dosing and titration are necessary. As this was a pilot study, comparative prospective studies including other drugs are required.
Author Contributions
Data curation, T.F., M.U., S.T., W.Y., A.S., K.I., K.O., K.T., and S.I.; writing—original draft preparation, Y.T.; writing—review and editing, H.I. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no specific funding for this work.
Institutional Review Board Statement
This study was performed in line with the principles of the Declaration of Helsinki. This study was approved by the institutional review board of Juntendo University (H19-0223).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data set is available at https://doi.org/10.6084/m9.figshare.17008838 (accessed on 26 July 2022).
Conflicts of Interest
The authors have no financial or proprietary interests in any material discussed in this article.
References
- Tempero, M.A. NCCN Guidelines Updates: Pancreatic Cancer. J. Natl. Compr. Canc. Netw. 2019, 17, 603–605. [Google Scholar] [PubMed]
- Sohal, D.P.S.; Kennedy, E.B.; Khorana, A.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Krishnamurthi, S.; Moravek, C.; O’Reilly, E.M.; Philip, P.A.; et al. Metastatic Pancreatic Cancer: ASCO Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 2545–2556. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goere, D. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), v56–v68. [Google Scholar] [CrossRef] [PubMed]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loprinzi, C.L.; Lacchetti, C.; Bleeker, J.; Cavaletti, G.; Chauhan, C.; Hertz, D.L.; Kelley, M.R.; Lavino, A.; Lustberg, M.B.; Paice, J.A.; et al. Prevention and Management of Chemotherapy-Induced Peripheral Neuropathy in Survivors of Adult Cancers: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3325–3348. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, S.G.; Kleckner, I.R.; Barton, D.; Mustian, K.; O’Mara, A.; St Germain, D.; Cavaletti, G.; Danhauer, S.C.; Hershman, D.L.; Hohmann, A.G.; et al. The National Cancer Institute Clinical Trials Planning Meeting for Prevention and Treatment of Chemotherapy-Induced Peripheral Neuropathy. J. Natl. Cancer Inst. 2019, 111, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Domon, Y.; Arakawa, N.; Inoue, T.; Matsuda, F.; Takahashi, M.; Yamamura, N.; Kai, K.; Kitano, Y. Binding Characteristics and Analgesic Effects of Mirogabalin, a Novel Ligand for the alpha2delta Subunit of Voltage-Gated Calcium Channels. J. Pharmacol. Exp. Ther. 2018, 365, 573–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, J.; Matsui, N.; Kakehi, Y.; Murayama, E.; Ohwada, S.; Sugihara, M. Mirogabalin for the management of postherpetic neuralgia: A randomized, double-blind, placebo-controlled phase 3 study in Asian patients. Pain 2019, 160, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Matsui, N.; Kuroha, M.; Wasaki, Y.; Ohwada, S. Mirogabalin for the treatment of diabetic peripheral neuropathic pain: A randomized, double-blind, placebo-controlled phase III study in Asian patients. J. Diabetes Investig. 2019, 10, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Okusaka, T.; Nakamura, M.; Yoshida, M.; Kitano, M.; Uesaka, K.; Ito, Y.; Furuse, J.; Hanada, K.; Okazaki, K.; Committee for Revision of Clinical Guidelines for Pancreatic Cancer of the Japan Pancreas Society. Clinical Practice Guidelines for Pancreatic Cancer 2019 From the Japan Pancreas Society: A Synopsis. Pancreas 2020, 49, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Salehifar, E.; Janbabaei, G.; Hendouei, N.; Alipour, A.; Tabrizi, N.; Avan, R. Comparison of the Efficacy and Safety of Pregabalin and Duloxetine in Taxane-Induced Sensory Neuropathy: A Randomized Controlled Trial. Clin. Drug Investig. 2020, 40, 249–257. [Google Scholar] [CrossRef]
- Cavaletti, G.; Frigeni, B.; Lanzani, F.; Mattavelli, L.; Susani, E.; Alberti, P.; Cortinovis, D.; Bidoli, P. Chemotherapy-Induced Peripheral Neurotoxicity assessment: A critical revision of the currently available tools. Eur. J. Cancer 2010, 46, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Satoh, A.; Yamada, T.; Aisu, N.; Matsuoka, T.; Koganemaru, T.; Kajitani, R.; Munechika, T.; Matsumoto, Y.; Nagano, N.; et al. The Relationship Between Evaluation Methods for Chemotherapy-Induced Peripheral Neuropathy. Sci. Rep. 2019, 9, 20361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, J.; Baba, M.; Kuroha, M.; Kakehi, Y.; Murayama, E.; Wasaki, Y.; Ohwada, S. Safety and Efficacy of Mirogabalin for Peripheral Neuropathic Pain: Pooled Analysis of Two Pivotal Phase III Studies. Clin. Ther. 2021, 43, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Inage, K.; Sainoh, T.; Fujiyoshi, T.; Otagiri, T.; Aoki, Y.; Inoue, M.; Eguchi, Y.; Orita, S.; Shiga, Y.; Koda, M.; et al. Frequency of Adverse Drug Reactions and Analgesic Effects of Mirogabalin during Treatment of Peripheral Neuropathic Pain: A Retrospective Clinical Investigation. Spine Surg. Relat. Res. 2020, 4, 354–357. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Ikeda, M.; Ueno, M.; Mizuno, N.; Ioka, T.; Omuro, Y.; Akajima, T.E.; Furuse, J. Phase I/II study of nab-paclitaxel plus gemcitabine for chemotherapy-naive Japanese patients with metastatic pancreatic cancer. Cancer Chemother. Pharmacol. 2016, 77, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Takagi, T.; Suzuki, R.; Konno, N.; Asama, H.; Sato, Y.; Irie, H.; Okubo, Y.; Nakamura, J.; Takasumi, M.; et al. Mirogabalin vs pregabalin for chemotherapy-induced peripheral neuropathy in pancreatic cancer patients. BMC Cancer 2021, 21, 1319. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).