A Retrospective Analysis of Endovascular Stent Insertion for Malignant Superior Vena Cava Obstruction, Focusing on Anticoagulation Practices
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Context
2.2. Sample Collection
2.3. Data Collection
2.4. Statistical Analysis
2.5. Ethical Considerations
3. Results
3.1. Procedural Details
3.2. Procedural Outcomes
3.3. Anticoagulation Practices
3.4. Complications
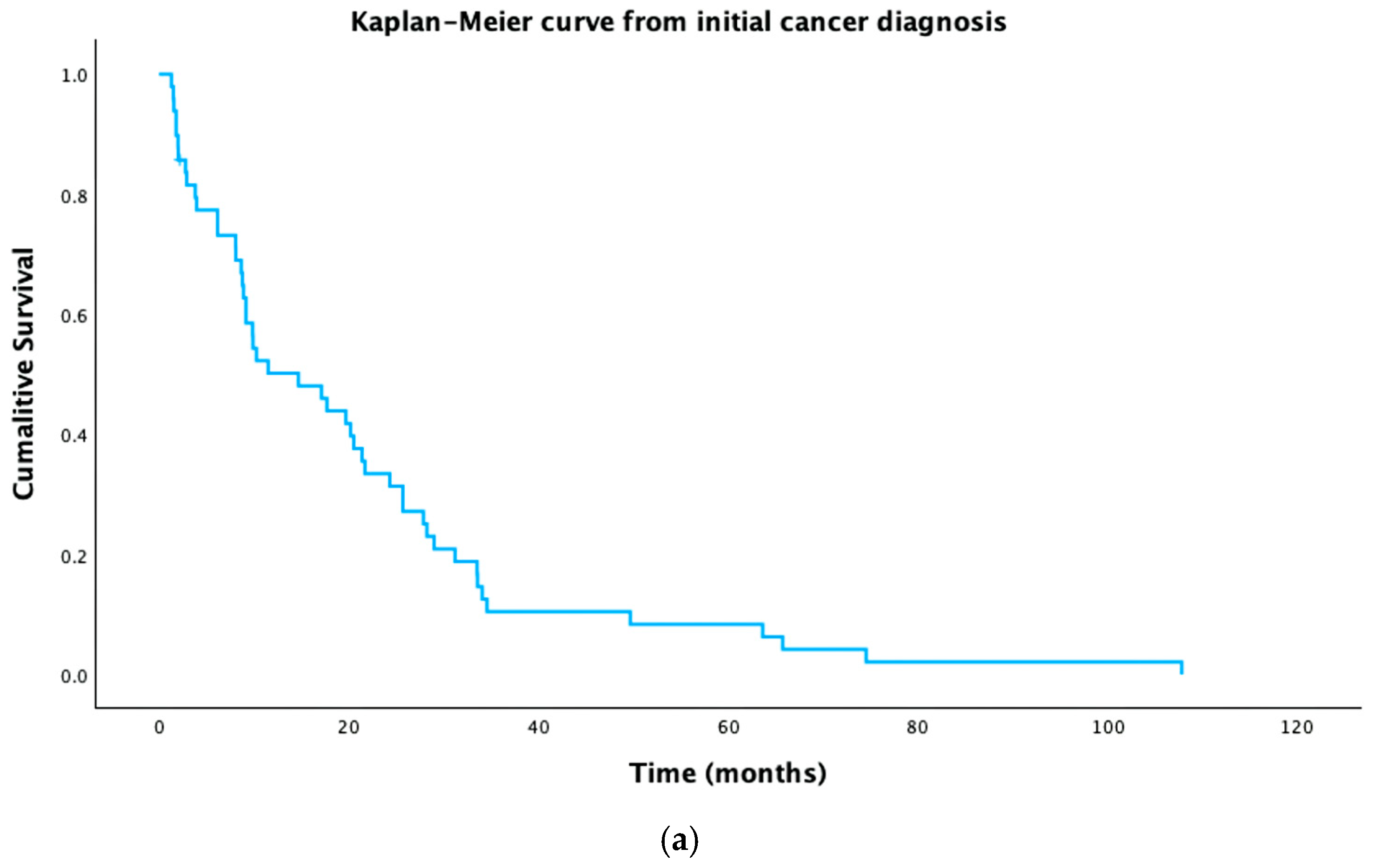
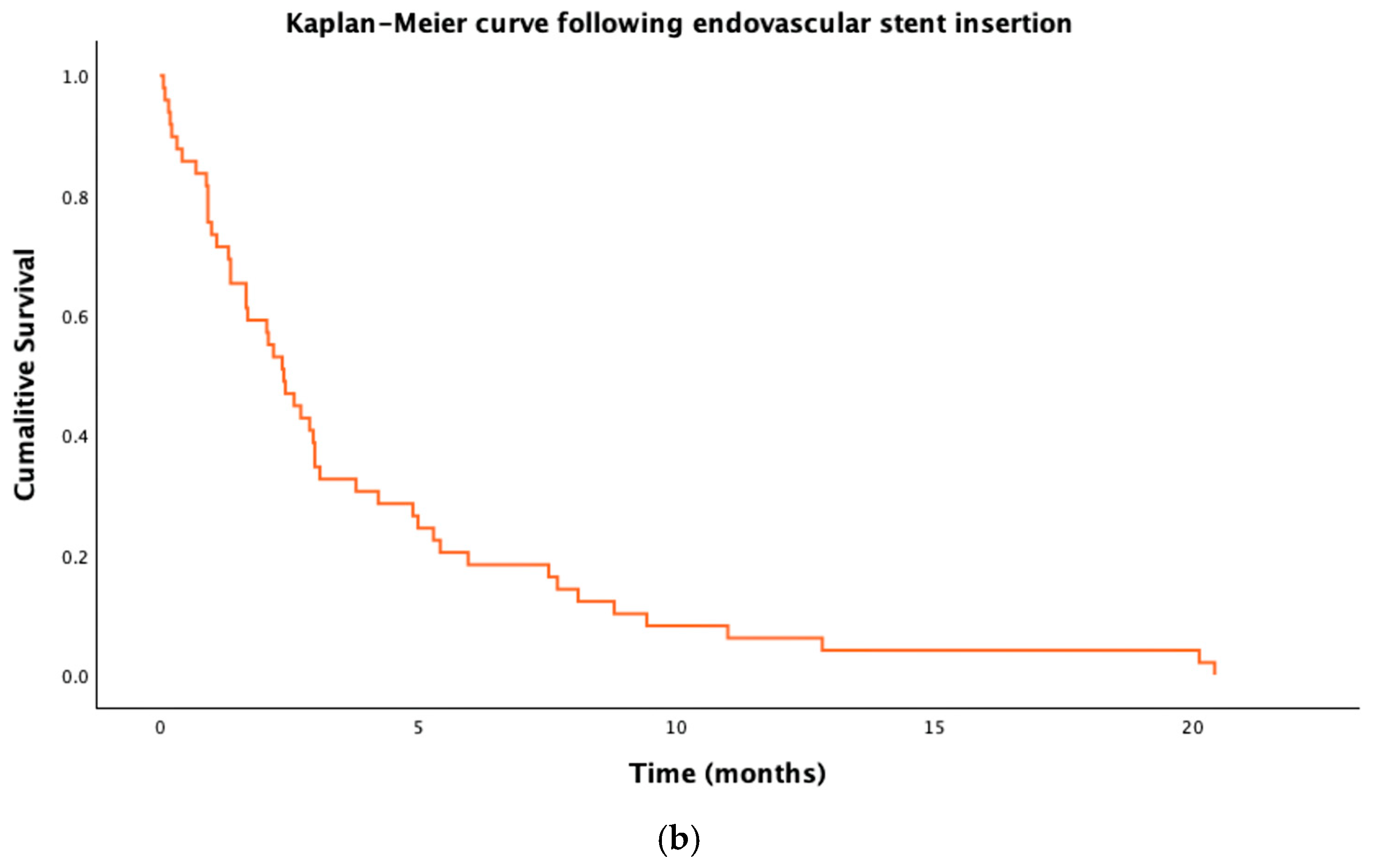
3.5. Survival
4. Discussion
4.1. Summary of Key Findings
4.2. Comparisons with the Literature
4.3. Strengths and Weaknesses
4.4. Implications for Clinicians/Policy Makers/Patients
4.5. Implications for Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SVCO | Superior vena cava obstruction |
| mSVCO | Malignant superior vena cava obstruction |
| CAT | Cancer-associated thrombus |
| LMWH | Low molecular weight heparin |
| Tx LMWH | Treatment-dose low molecular weight heparin |
| Px LMWH | Prophylactically dosed low molecular weight heparin |
| VTE | Venous thromboembolism |
| IQR | Inter quartile range |
| DOAC | Direct oral anticoagulant |
| CIRSE | Cardiovascular and Interventional Society of Europe |
| ESMO | European Society of Medical Oncology |
| IR | Interventional radiology |
| GP | General Practice |
| CT | Computed Tomography |
| IT | Information Technology |
| PS | Performance Status |
References
- Rowell, N.P.; Gleeson, F.V. Steroids, Radiotherapy, Chemotherapy and Stents for Superior Vena Caval Obstruction in Carcinoma of the Bronchus: A Systematic Review. Clin. Oncol. 2002, 14, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Hadi, M.; Walker, H.; Desborough, M.; Basile, A.; Tsetis, D.; Hunt, B.; Müller-Hüllsbeck, S.; Rand, T.; van Delden, O.; Uberoi, R. CIRSE Standards of Practice on Peri-Operative Anticoagulation Management During Interventional Radiology Procedures. Cardiovasc. Interv. Radiol. 2021, 44, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Aung, E.Y.-S.; Khan, M.; Williams, N.; Raja, U.; Hamady, M. Endovascular Stenting in Superior Vena Cava Syndrome: A Systematic Review and Meta-Analysis. Cardiovasc. Interv. Radiol. 2022, 45, 1236–1254. [Google Scholar] [CrossRef] [PubMed]
- Azizi, A.H.; Shafi, I.; Zhao, M.; Chatterjee, S.; Roth, S.C.; Singh, M.; Lakhter, V.; Bashir, R. Endovascular Therapy for Superior Vena Cava Syndrome: A Systematic Review and Meta-Analysis. eClinicalMedicine 2021, 37, 100970. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; McCrae, K.R.; Milentijevic, D.; Fortier, J.; Nelson, W.W.; Laliberté, F.; Crivera, C.; Lefebvre, P.; Yannicelli, D.; Schein, J. Current Practice Patterns and Patient Persistence with Anticoagulant Treatments for Cancer-Associated Thrombosis. Res. Pract. Thromb. Haemost. 2017, 1, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Thony, F.; Fagedet, D.; Michoud, M.; Moro-Sibilot, D.; Ferretti, G.R.; Rodière, M. Anticoagulation Is Not Mandatory after Stenting for Malignant Superior Vena Cava Syndrome. Cardiovasc. Intervent. Radiol. 2014, 37, 1403–1404. [Google Scholar] [CrossRef] [PubMed]
- Uberoi, R. Quality Assurance Guidelines for Superior Vena Cava Stenting in Malignant Disease. Cardiovasc. Intervent. Radiol. 2006, 29, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Antonio, A. ESMO Handbook of Oncological Emergencies; European Society for Medical Oncology: Lugano, Switzerland, 2016; pp. 30–36. [Google Scholar]
- Kordzadeh, A.; Askari, A.; Hanif, M.A.; Gadhvi, V. Superior Vena Cava Syndrome and Wallstent: A Systematic Review. Ann. Vasc. Dis. 2022, 15, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ratzon, R.; Tamir, S.; Friehmann, T.; Livneh, N.; Dudnik, E.; Rozental, A.; Hamburger-Avnery, O.; Pereg, D.; Derazne, E.; Brenner, B.; et al. Thrombosis, Anticoagulation and Outcomes in Malignant Superior Vena Cava Syndrome. J. Thromb. Thrombolysis 2019, 47, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Cella, C.A.; Di Minno, G.; Carlomagno, C.; Arcopinto, M.; Cerbone, A.M.; Matano, E.; Tufano, A.; Lordick, F.; De Simone, B.; Muehlberg, K.S.; et al. Preventing Venous Thromboembolism in Ambulatory Cancer Patients: The ONKOTEV Study. Oncologist 2017, 22, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W. Current Status of Treatment of Cancer-Associated Venous Thromboembolism. Thromb. J. 2021, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Cella, C.A.; Fazio, N.; Lordick, F.; Bagnardi, V.; Frassoni, S.; Gervaso, L.; Valenza, C.; Hozo, I.; Djulbegovic, B. Comparison of Khorana Vs. Onkotev Predictive Score to Individualize Anticoagulant Prophylaxis in Ambulatory Patients with Cancer. Blood 2023, 142, 661. [Google Scholar] [CrossRef]
- Chawla, S.; Zhang, Q.; Gwozdz, A.M.; Wijaya, J.; Tiwana, B.; Tincknell, L.; Turner, B.R.H.; Black, S. Editor’s Choice–A Systematic Review and Meta-Analysis of 24 Month Patency After Endovenous Stenting of Superior Vena Cava, Subclavian, and Brachiocephalic Vein Stenosis. Eur. J. Vasc. Endovasc. Surg. 2025, 69, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Lanciego, C.; Pangua, C.; Chacón, J.I.; Velasco, J.; Boy, R.C.; Viana, A.; Cerezo, S.; García, L.G. Endovascular Stenting as the First Step in the Overall Management of Malignant Superior Vena Cava Syndrome. Am. J. Roentgenol. 2009, 193, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Fagedet, D.; Thony, F.; Timsit, J.-F.; Rodiere, M.; Monnin-Bares, V.; Ferretti, G.R.; Vesin, A.; Moro-Sibilot, D. Endovascular Treatment of Malignant Superior Vena Cava Syndrome: Results and Predictive Factors of Clinical Efficacy. Cardiovasc. Intervent. Radiol. 2013, 36, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Maleux, G.; Gillardin, P.; Fieuws, S.; Heye, S.; Vaninbroukx, J.; Nackaerts, K. Large-Bore Nitinol Stents for Malignant Superior Vena Cava Syndrome: Factors Influencing Outcome. Am. J. Roentgenol. 2013, 201, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Khorana, A.A.; Yannicelli, D.; McCrae, K.R.; Milentijevic, D.; Crivera, C.; Nelson, W.W.; Schein, J.R. Evaluation of US Prescription Patterns: Are Treatment Guidelines for Cancer-Associated Venous Thromboembolism Being Followed? Thromb. Res. 2016, 145, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Kazimi, J.; Bhalla, A.S.; Naranje, P.; Bashir, I. Vascular Thoracic Interventions. In Textbook of Interventional Radiology; Chandrashekhara, S.H., Ed.; Springer Nature: Singapore, 2024; pp. 209–223. ISBN 978-981-97-9601-4. [Google Scholar]
- White, C.; Noble, S.I.R.; Watson, M.; Swan, F.; Allgar, V.L.; Napier, E.; Nelson, A.; McAuley, J.; Doherty, J.; Lee, B.; et al. Prevalence, Symptom Burden, and Natural History of Deep Vein Thrombosis in People with Advanced Cancer in Specialist Palliative Care Units (HIDDen): A Prospective Longitudinal Observational Study. Lancet Haematol. 2019, 6, e79–e88. [Google Scholar] [CrossRef] [PubMed]
- Farge, D.; Frere, C.; Connors, J.M.; Khorana, A.A.; Kakkar, A.; Ay, C.; Muñoz, A.; Brenner, B.; Prata, P.H.; Brilhante, D.; et al. 2022 International Clinical Practice Guidelines for the Treatment and Prophylaxis of Venous Thromboembolism in Patients with Cancer, Including Patients with COVID-19. Lancet Oncol. 2022, 23, e334–e347. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, R.; Gomez, K.; Maraveyas, A.; Noble, S.; Young, A.; Thomas, M.; British Society for Haematology. Cancer-Associated Venous Thrombosis in Adults (Second Edition): A British Society for Haematology Guideline. Br. J. Haematol. 2024, 205, 71–87. [Google Scholar] [CrossRef] [PubMed]
| Descriptor | Number (Percentage/ Range) |
|---|---|
| Total | 49 (100%) |
| Female sex | 27 (55%) |
| Median age at diagnosis (years) | 59 (IQR: 54–72) |
| Malignancy classification | |
| Lung cancer | 40 (82%) |
| Non-small cell | 23 (47%) |
| Adenocarcinoma | 13 (47%) |
| - Squamous | 8 (16%) |
| - Large cell neuro-endocrine | 2 (4%) |
| - Not otherwise specified | 5 (10%) |
| Small cell | 9 (18.5%) |
| Mesothelioma | 3 (6%) |
| Other malignancy | |
| - Breast | 7 (14%) |
| - GI | 1 (2%) |
| - Thyroid | 1 (2%) |
| Performance Status at diagnosis 1 | |
| 0 | 14 (29%) |
| 1 | 19 (39%) |
| 2 | 7 (14%) |
| 3 | 1 (2%) |
| Unknown | 8 (16%) |
| Metastatic disease status at time of stent insertion | |
| No known metastatic disease outside of chest | 13 (27%) |
| One site of metastatic disease outside of chest (nodal, visceral or bone). | 24 (49%) |
| Two or more sites of metastatic disease outside of chest (nodal, visceral or bone). | 12 (24%) |
| Performance status at time of discharge following stent | |
| 0 | 1 (2%) |
| 1 | 9 (18%) |
| 2 | 12 (24%) |
| 3 | 8 (16%) |
| Unknown | 16 (33%) |
| Agent | Number (Percentage/45) |
|---|---|
| Tx LMWH 1 | 28 (62%) |
| Px LMWH 2 | 5 (11%) |
| Nil | 4 (9%) |
| Px LMWH and 75 mg Aspirin | 3 (7%) |
| Tx LMWH and 75 mg Aspirin | 1 (2%) |
| Apixaban 5 mg BD | 1 (2%) |
| Apixaban 2.5 mg BD | 1 (2%) |
| Dual anti-platelet therapy | 1 (2%) |
| Aspirin monotherapy | 1 (2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walker, J.; Ravinthiranathan, A.; Diamantopoulos, A.; Gennatas, S.; Georgiou, A. A Retrospective Analysis of Endovascular Stent Insertion for Malignant Superior Vena Cava Obstruction, Focusing on Anticoagulation Practices. Curr. Oncol. 2025, 32, 601. https://doi.org/10.3390/curroncol32110601
Walker J, Ravinthiranathan A, Diamantopoulos A, Gennatas S, Georgiou A. A Retrospective Analysis of Endovascular Stent Insertion for Malignant Superior Vena Cava Obstruction, Focusing on Anticoagulation Practices. Current Oncology. 2025; 32(11):601. https://doi.org/10.3390/curroncol32110601
Chicago/Turabian StyleWalker, Joshua, Amsajini Ravinthiranathan, Athanasios Diamantopoulos, Spyridon Gennatas, and Alexandros Georgiou. 2025. "A Retrospective Analysis of Endovascular Stent Insertion for Malignant Superior Vena Cava Obstruction, Focusing on Anticoagulation Practices" Current Oncology 32, no. 11: 601. https://doi.org/10.3390/curroncol32110601
APA StyleWalker, J., Ravinthiranathan, A., Diamantopoulos, A., Gennatas, S., & Georgiou, A. (2025). A Retrospective Analysis of Endovascular Stent Insertion for Malignant Superior Vena Cava Obstruction, Focusing on Anticoagulation Practices. Current Oncology, 32(11), 601. https://doi.org/10.3390/curroncol32110601