Characterization of the Population, Treatment Patterns, and Outcomes of Patients with Advanced or Metastatic Non-Small-Cell Lung Cancer (NSCLC) with Epidermal Growth Factor Receptor Mutation (EGFRm): A Retrospective Cohort Study from IPO Porto
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Selection of Patients
2.3. Data Collection
2.4. Outcomes
- Real-world overall survival (rwOS): Time from diagnosis to death from any cause (if the event did not occur, time was censored at data cut-off as death was fully traced);
- Real-world progression-free survival (rwPFS): Time from the start of the first line of therapy (LoT) and first documented disease progression or death from any cause, whichever occurred first (if an event was not identified, time was censored at last contact);
- Real-world time to CNS metastasis: Time between the start of LoT1 and the first detection of a new CNS metastasis (if an event was not identified, time was censored at last contact);
- Real-world time to next treatment (rwTTNT): Time between the start of LoT1 and the initiation of LoT2 (if an event was not identified, time was censored at last contact or death, whichever occurred first);
- Real-world time on treatment (rwToT): Time between the start of LoT1 and the date of the last administered drug of whichever LoT or death, whichever occurred first (if an event was not identified, time was censored at last contact); time during treatment interruptions between LoTs was included in the calculation of rwToT.
2.5. Statistical Analysis
3. Results
3.1. EGFR Mutations
3.2. Demographic and Clinical Characteristics at Diagnosis
3.3. Characteristics at Disease Progression
3.4. Frontline Treatment Intention
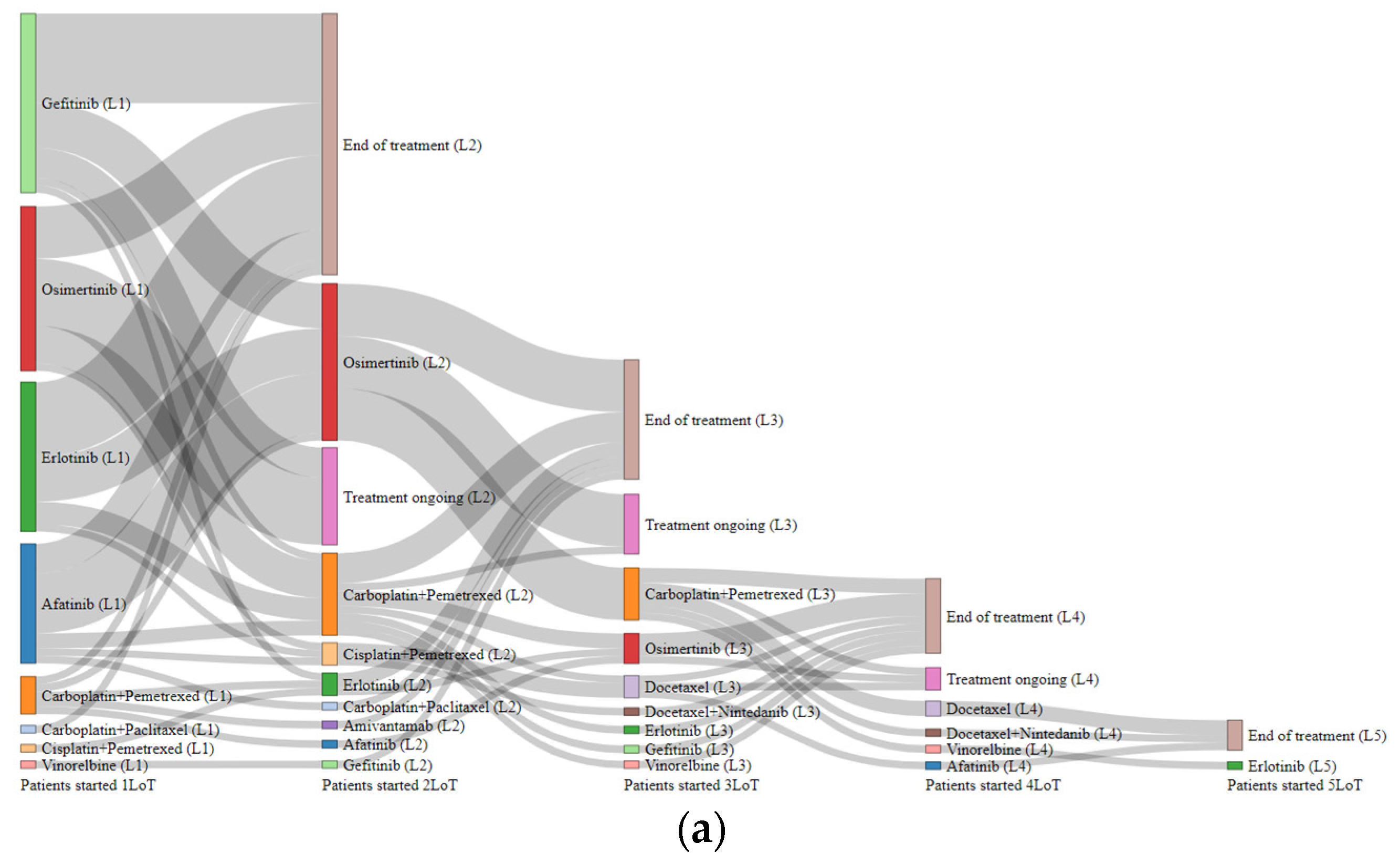
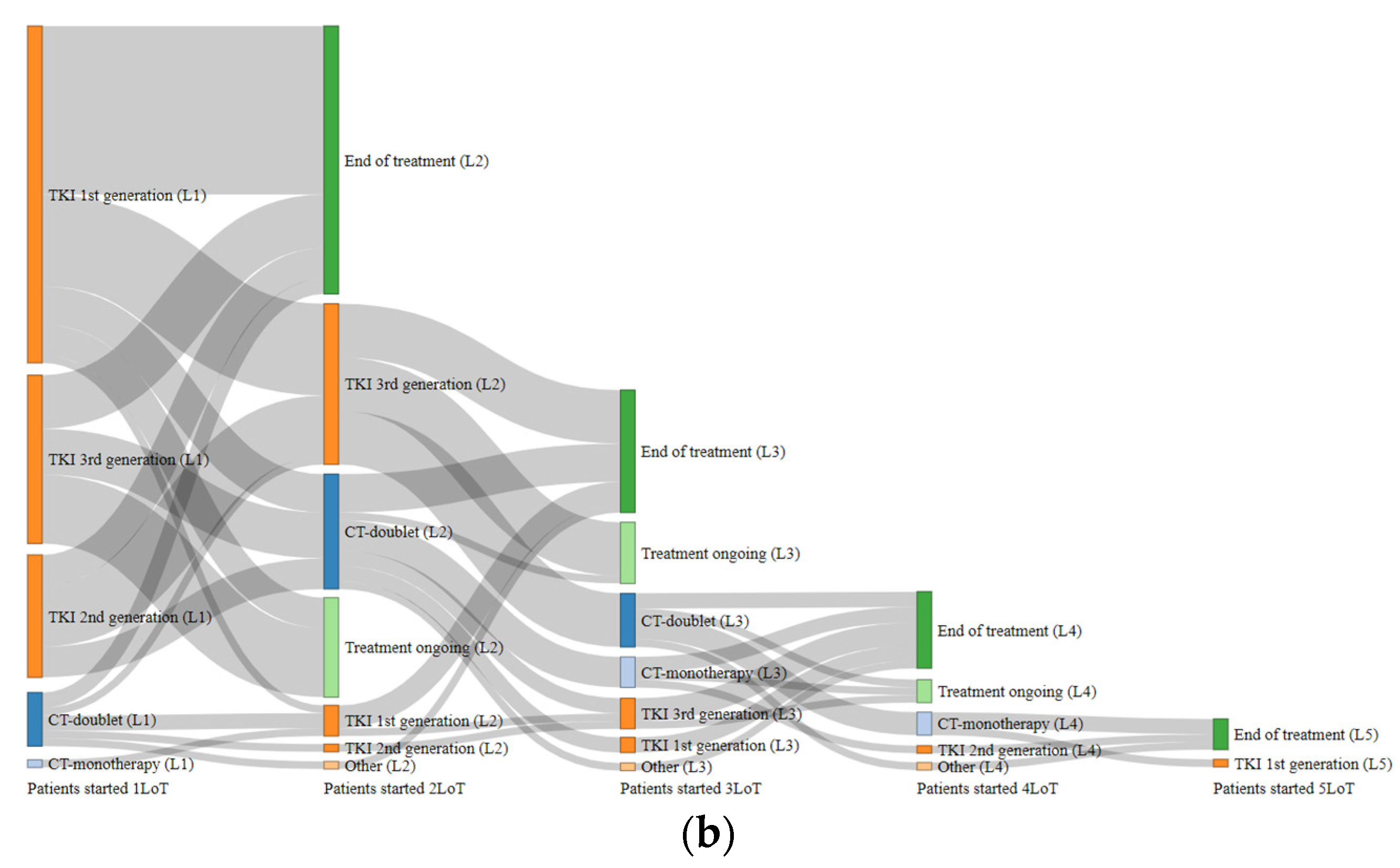
3.5. Treatment Dynamics
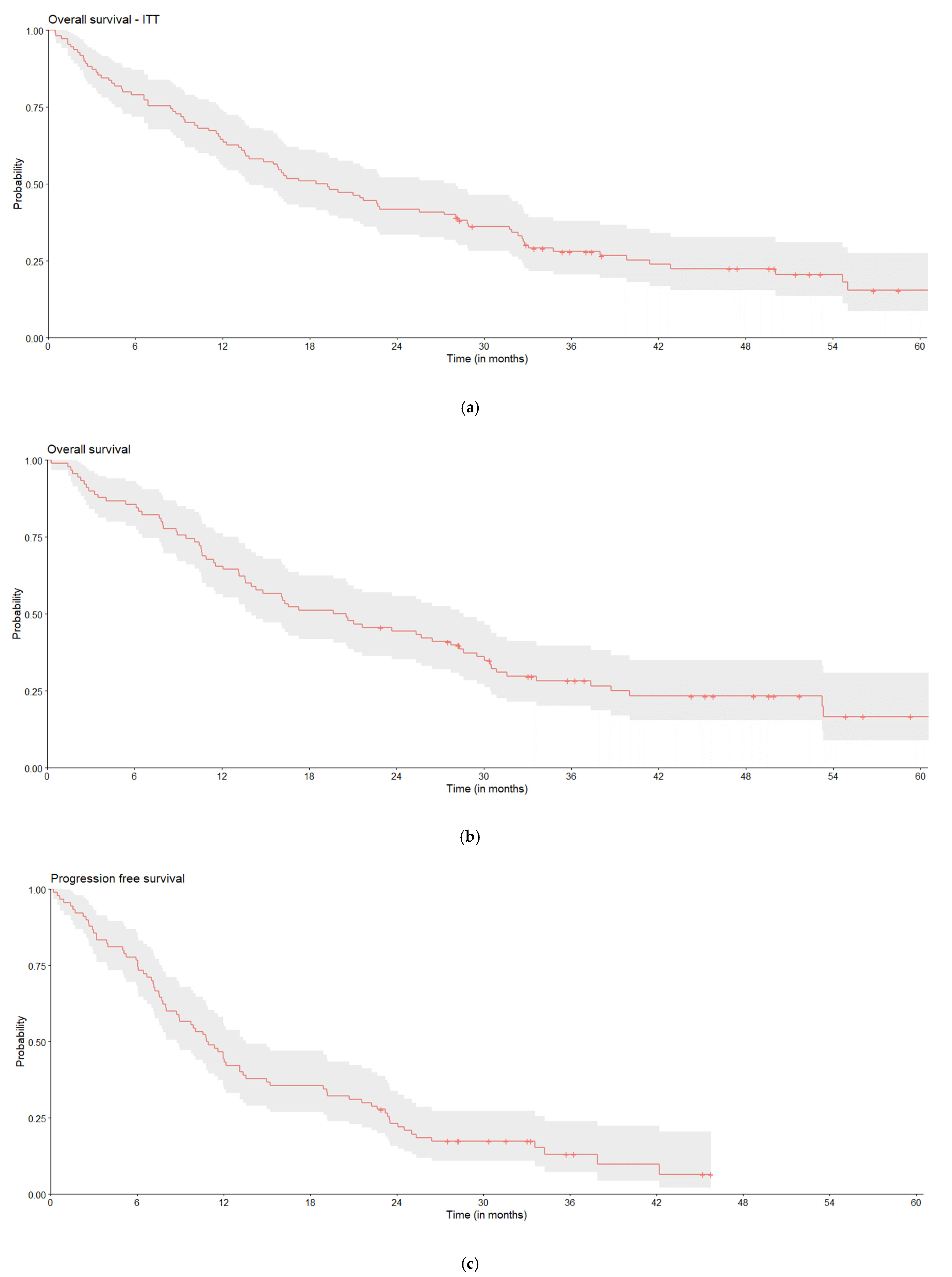
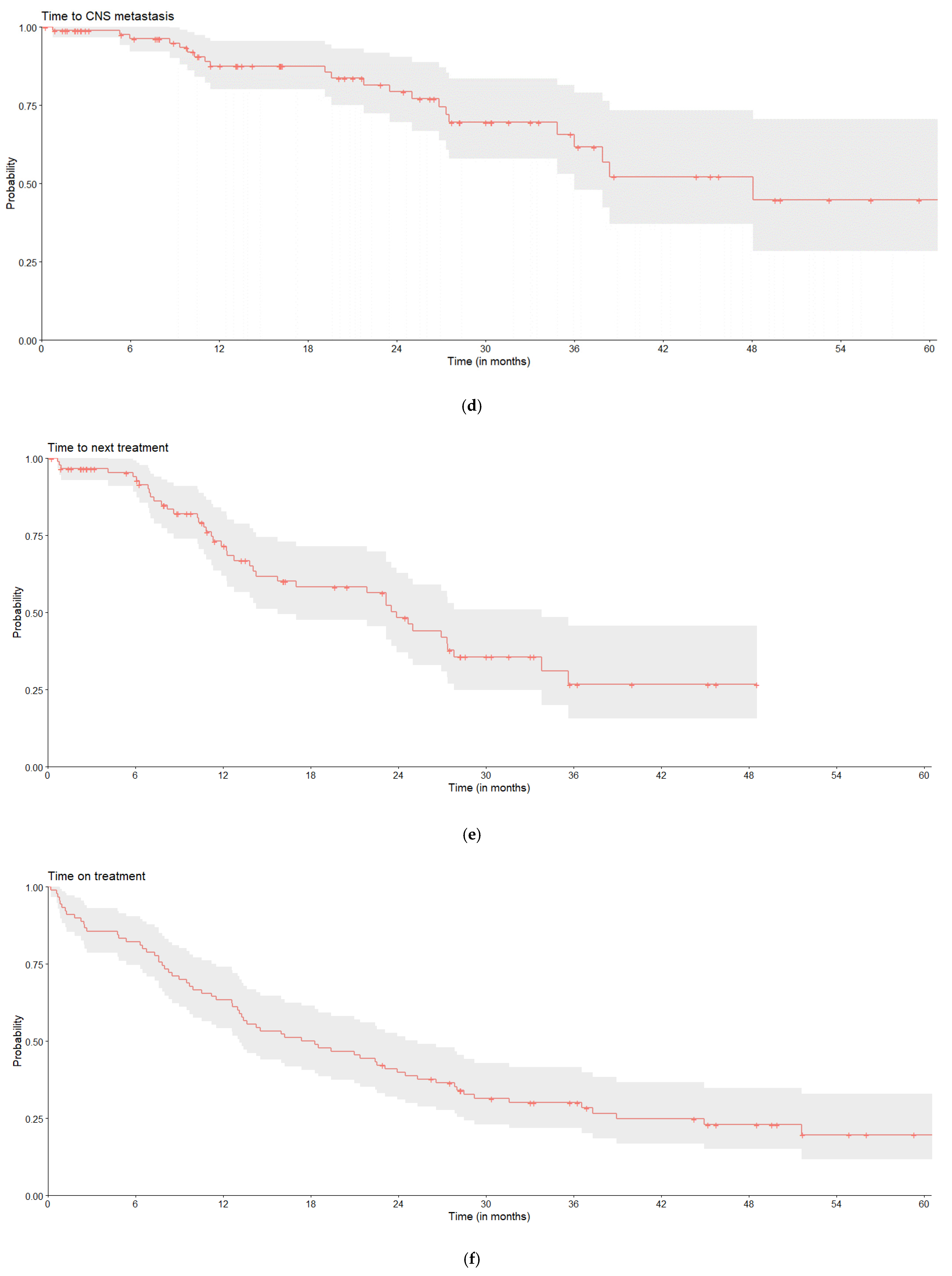
3.6. Real-World Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Cancer Observatory (GLOBOCAN). World Fact Sheet 2022. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/900-world-fact-sheet.pdf (accessed on 5 February 2025).
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Bazhenova, L.; Minchom, A.; Viteri, S.; Bauml, J.M.; Ou, S.I.; Gadgeel, S.M.; Trigo, J.M.; Backenroth, D.; Li, T.; Londhe, A.; et al. Comparative clinical outcomes for patients with advanced NSCLC harboring EGFR exon 20 insertion mutations and common EGFR mutations. Lung Cancer 2021, 162, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Melosky, B.; Kambartel, K.; Häntschel, M.; Bennetts, M.; Nickens, D.J.; Brinkmann, J.; Kayser, A.; Moran, M.; Cappuzzo, F. Worldwide Prevalence of Epidermal Growth Factor Receptor Mutations in Non-Small Cell Lung Cancer: A Meta-Analysis. Mol. Diagn. Ther. 2022, 26, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Yuan, J.Q.; Wang, K.F.; Fu, X.H.; Han, X.R.; Threapleton, D.; Yang, Z.Y.; Mao, C.; Tang, J.L. The prevalence of EGFR mutation in patients with non-small cell lung cancer: A systematic review and meta-analysis. Oncotarget 2016, 7, 78985–78993. [Google Scholar] [CrossRef] [PubMed]
- Van Sanden, S.; Murton, M.; Bobrowska, A.; Rahhali, N.; Sermon, J.; Rodrigues, B.; Goff-Leggett, D.; Chouaid, C.; Sebastian, M.; Greystoke, A. Prevalence of Epidermal Growth Factor Receptor Exon 20 Insertion Mutations in Non-small-Cell Lung Cancer in Europe: A Pragmatic Literature Review and Meta-analysis. Target. Oncol. 2022, 17, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.S.; Vansteenkiste, J.; Planchard, D.; Cho, B.C.; Gray, J.E.; Ohe, Y.; Zhou, C.; Reungwetwattana, T.; Cheng, Y.; Chewaskulyong, B.; et al. FLAURA Investigators. Overall Survival with Osimertinib in Untreated EGFR-Mutated Advanced, NSCLC. N. Engl. J. Med. 2020, 382, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Guo, H.; Xia, Y.; Le, X.; Tan, D.S.W.; Ramalingam, S.S.; Zhou, C. The changing treatment landscape of EGFR-mutant non-small-cell lung cancer. Nat. Rev. Clin. Oncol. 2025, 22, 95–116. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, R. Amivantamab plus Lazertinib in Previously Untreated EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2025, 392, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Planchard, D.; Jänne, P.A.; Cheng, Y.; Yang, J.C.; Yanagitani, N.; Kim, S.W.; Sugawara, S.; Yu, Y.; Fan, Y.; Geater, S.L.; et al. FLAURA2 Investigators. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2023, 389, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Vyse, S.; Huang, P.H. Targeting EGFR exon 20 insertion mutations in non-small cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Wang, F.; Zhao, Y.; Li, S.; Wang, X.; Shou, T.; Luo, Y.; Tang, W. Comparison of clinical outcomes of patients with non-small-cell lung cancer harbouring epidermal growth factor receptor exon 19 or exon 21 mutations after tyrosine kinase inhibitors treatment: A meta-analysis. Eur. J. Clin. Pharmacol. 2016, 72, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Saw, S.P.L.; Le, X.; Hendriks, L.E.L.; Remon, J. New Treatment Options for Patients With Oncogene-Addicted Non-Small Cell Lung Cancer Focusing on EGFR-Mutant Tumors. Am. Soc. Clin. Oncol. Educ. Book 2024, 44, e432516. [Google Scholar] [CrossRef] [PubMed]
- Bauml, J.M.; Viteri, S.; Minchom, A.; Bazhenova, L.; Ou, S.; Schaffer, M.; Le Croy, N.; Riley, R.; Mahadevia, P.; Girard, N. Underdiagnosis of EGFR exon 20 insertion mutation variants: Estimates from NGS-based real-world datasets. J. Thorac. Oncol. 2021, 16, S208–S209. [Google Scholar] [CrossRef]
- Subramanian, J.; Gregg, J.; Berktas, M.; Li, J.; Leighl, N.B. EGFR testing practices, treatment choice and clinical outcomes in advanced NSCLC in a real-world setting: A retrospective analysis of a US-based electronic health records database. Lung Cancer 2025, 201, 108412. [Google Scholar] [CrossRef] [PubMed]
- Hochmair, M.J.; Unk, M.; Spasic, J.; Cerić, T.; Konsoulova, A.; Dediu, M.; Bogos, K.; Hegmane, A.; Oselin, K.; Stojiljkovic, M.; et al. Unmet needs in EGFR exon 20 insertion mutations in Central and Eastern Europe: Reimbursement, diagnostic procedures, and treatment availability. BMC Proc. 2024, 18, 2. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ton, T.G.N.; Pal, N.; Trinh, H.; Mahrus, S.; Bretscher, M.T.; Machado, R.J.M.; Sadetsky, N.; Chaudhary, N.; Lu, M.W.; Riely, G.J. Replication of Overall Survival, Progression-Free Survival, and Overall Response in Chemotherapy Arms of Non-Small Cell Lung Cancer Trials Using Real-World Data. Clin. Cancer Res. 2022, 28, 2844–2853. [Google Scholar] [CrossRef] [PubMed]
- Griffith, S.D.; Miksad, R.A.; Calkins, G.; You, P.; Lipitz, N.G.; Bourla, A.B.; Williams, E.; George, D.J.; Schrag, D.; Khozin, S.; et al. Characterizing the Feasibility and Performance of Real-World Tumor Progression End Points and Their Association With Overall Survival in a Large Advanced Non-Small-Cell Lung Cancer Data Set. JCO Clin. Cancer Inform. 2019, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Burnett, H.; Emich, H.; Carroll, C.; Stapleton, N.; Mahadevia, P.; Li, T. Epidemiological and clinical burden of EGFR Exon 20 insertion in advanced non-small cell lung cancer: A systematic literature review. PLoS ONE 2021, 16, e0247620. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Nyaw, S.F.; Mok, T.S.K.; Curcio, H.; Cortot, A.B.; Kam, T.Y.; Descourt, R.; Chik, Y.K.; Cheema, P.; Gwinnutt, J.M.; et al. Patterns of Treatment and Real-World Outcomes of Patients With Non-small Cell Lung Cancer With EGFR Exon 20 Insertion Mutations Receiving Mobocertinib: The EXTRACT Study. Cancer Med. 2025, 14, e70369. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.I.; Hong, J.L.; Christopoulos, P.; Lin, H.M.; Vincent, S.; Churchill, E.N.; Soeda, J.; Kazdal, D.; Stenzinger, A.; Thomas, M. Distribution and Detectability of EGFR Exon 20 Insertion Variants in NSCLC. J. Thorac. Oncol. 2023, 18, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Nazha, B.; Yang, J.C.; Owonikoko, T.K. Benefits and limitations of real-world evidence: Lessons from EGFR mutation-positive non-small-cell lung cancer. Future Oncol. 2021, 17, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, C.S.; Mustafa, M.A.; Richardson, G.E.; Alam, A.M.; Lee, K.S.; Hughes, D.M.; Escriu, C.; Zakaria, R. Genomic Alterations and the Incidence of Brain Metastases in Advanced and Metastatic NSCLC: A Systematic Review and Meta-Analysis. J. Thorac. Oncol. 2023, 18, 1703–1713. [Google Scholar] [CrossRef] [PubMed]
- Stalker, M.; Grady, C.B.; Watts, A.; Hwang, W.T.; Chandrasekhara, K.; Sun, F.; Liu, G.; Patel, D.; Nieva, J.; Herrmann, A.; et al. Changing Treatment and Metastatic Disease Patterns in Patients with EGFR Mutated NSCLC: An Academic Thoracic Medical Investigator’s Consortium Registry Analysis. JTO Clin. Res. Rep. 2024, 6, 100765. [Google Scholar] [CrossRef] [PubMed]
- Gijtenbeek, R.G.P.; Damhuis, R.A.M.; van der Wekken, A.J.; Hendriks, L.E.L.; Groen, H.J.M.; van Geffen, W.H. Overall survival in advanced epidermal growth factor receptor mutated non-small cell lung cancer using different tyrosine kinase inhibitors in The Netherlands: A retrospective, nationwide registry study. Lancet Reg. Health Eur. 2023, 27, 100592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Characteristics | Overall (n = 110) | Common EGFR Mutation (n = 99) | Uncommon EGFR Mutation (n = 11) |
|---|---|---|---|
| Median age (range), years | 69.0 (37–93) | 70.0 (37–93) | 68.0 (46–81) |
| Female, n (%) | 84 (76.4%) | 75 (75.8%) | 9 (81.8%) |
| Never smoked, n (%) | 89 (83.2%) | 79 (81.4%) | 10 (100.0%) |
| Weight < 80 kg, n (%) | 92 (86.8%) | 84 (88.4%) | 8 (72.7%) |
| ECOG PS, n (%) | |||
| 0 | Not shown | 26 (26.3%) | ≤5 |
| 1 | Not shown | 40 (40.4%) | ≤5 |
| 2 | Not shown | 21 (21.2%) | ≤5 |
| 3–4 | 12 (10.9%) | 12 (12.1%) | 0 (0.0%) |
| Adenocarcinoma, n (%) | 107 (99.1%) | 97 (100.0%) | 10 (90.9%) |
| Unilateral, n (%) | 107 (100.0%) | 96 (100.0%) | 11 (100.0%) |
| TNM stage, n (%) | |||
| IIIB-IIIC | Not shown | 6 (6.1%) | ≤5 |
| IVA | 30 (27.3%) | 24 (24.2%) | 6 (54.5%) |
| IVB | Not shown | 64 (64.6%) | ≤5 |
| IV not specified | 6 (5.5%) | ≤5 | ≤5 |
| No. of metastasis sites, n (%) | |||
| 0–2 | 65 (59.1%) | 58 (58.6%) | 7 (63.6%) |
| 3–4 | Not shown | 32 (32.3%) | ≤5 |
| 5–7 | Not shown | 9 (9.1%) | ≤5 |
| Brain metastasis, n (%) | Not shown | 26 (26.3%) | ≤5 |
| Liver metastasis, n (%) | Not shown | 19 (19.2%) | ≤5 |
| Brain and liver metastasis, n (%) | 6 (5.5%) | 6 (6.1%) | 0 (0.0%) |
| EGFR test type, n (%) | |||
| NGS | Not shown | 70 (70.7%) | ≤5 |
| PCR | Not shown | 29 (29.3%) | ≤5 |
| EGFR mutation, n (%) | |||
| Exon 19 deletion | 64 (58.2%) | 64 (64.6%) | 0 (0.0%) |
| L858R point mutation of Exon 21 | 35 (31.8%) | 35 (35.4%) | 0 (0.0%) |
| Exon 20 insertion | Not shown | 0 (0.0%) | Not shown |
| Other mutation 1 | Not shown | 0 (0.0%) | Not shown |
| LoT1 1 (n = 90) | Median Time (Months) | LoT2 (n = 42) | Median Time (Months) | LoT3 (n = 18) | Median Time (Months) | LoT4 (n ≤ 5) | LoT5 (n ≤ 5) | |
|---|---|---|---|---|---|---|---|---|
| Drug, n (%) | ||||||||
| Afatinib | 16 (17.8%) | 11.5 | ≤5 | 18.0 | 0 (0.0%) | NA | ≤5 | 0 (0.0%) |
| Amivantamab | 0 (0.0%) | NA | ≤5 | 14.8 | 0 (0.0%) | NA | 0 (0.0%) | 0 (0.0%) |
| Carboplatin + paclitaxel | ≤5 | 7.9 | ≤5 | 0.03 | 0 (0.0%) | NA | 0 (0.0%) | 0 (0.0%) |
| Carboplatin + pemetrexed | ≤5 | 4.8 | 11 (26.2%) | 3.7 | 0 (0.0%) | NA | 0 (0.0%) | 0 (0.0%) |
| Cisplatin + pemetrexed | ≤5 | 7.6 | ≤5 | 2.8 | 7 (38.9%) | 2.7 | 0 (0.0%) | 0 (0.0%) |
| Docetaxel | 0 (0.0%) | NA | 0 (0.0%) | NA | ≤5 | 1.7 | ≤5 | 0 (0.0%) |
| Docetaxel + nintedanib | 0 (0.0%) | NA | 0 (0.0%) | NA | ≤5 | 0.03 | ≤5 | 0 (0.0%) |
| Erlotinib | 20 (22.2%) | 10.8 | ≤5 | 5.4 | ≤5 | 1.5 | 0 (0.0%) | ≤5 |
| Gefitinib | 24 (26.7%) | 9.6 | ≤5 | 0.5 | ≤5 | 1.2 | 0 (0.0%) | 0 (0.0%) |
| Osimertinib | 22 (24.4%) | 6.2 | 21 (50.0%) | 7.5 | ≤5 | 1.2 | 0 (0.0%) | 0 (0.0%) |
| Vinorelbine | ≤5 | 0.03 | 0 (0.0%) | NA | ≤5 | 1.0 | ≤5 | 0 (0.0%) |
| Drug class, n (%) | ||||||||
| TKI 1st generation | 44 (48.9%) | 10.3 | ≤5 | 5.4 | ≤5 | 1.4 | 0 (0.0%) | n ≤ 5 |
| TKI 2nd generation | 16 (17.8%) | 11.5 | ≤5 | 18.0 | 0 (0.0%) | NA | ≤5 | 0 (0.0%) |
| TKI 3rd generation | 22 (24.4%) | 6.2 | 21 (50.0%) | 7.5 | ≤5 | 1.2 | 0 (0.0%) | 0 (0.0%) |
| CT-doublet | 7 (7.8%) | 7.6 | 15 (35.7%) | 3.0 | 7 (38.9%) | 2.7 | 0 (0.0%) | 0 (0.0%) |
| CT-mono | ≤5 | 0.03 | 0 (0.0%) | NA | ≤5 | 1.0 | ≤5 | 0 (0.0%) |
| Other | 0 (0.0%) | NA | ≤5 | 14.8 | ≤5 | 0.03 | ≤5 | 0 (0.0%) |
| Real-World Outcomes | Estimates |
|---|---|
| rwOS, overall | |
| No. of pts | 110 |
| No. of events | 85 |
| Median (95% CI), months | 18.9 (13.8–28.1) |
| 1-year rate | 64.5% (56.2–74.1) |
| 2-year rate | 41.8% (33.5–52.1) |
| 3-year rate | 28.0% (20.6–38.0) |
| 5-year rate | 15.4% (8.7–27.4) |
| rwOS, palliative systemic LoT1 | |
| No. of pts | 90 |
| No. of events | 68 |
| Median (95% CI), months | 21.2 (16.1–31.8) |
| 1-year rate | 72.2% (63.5–82.1) |
| 2-year rate | 45.6% (36.3–57.1) |
| 3-year rate | 28.8% (20.7–40.2) |
| 5-year rate | 17.0% (9.2–31.4) |
| rwPFS, palliative systemic LoT1 | |
| No. of pts | 90 |
| No. of events | 78 |
| Median (95% CI), months | 10.9 (8.8–13.6) |
| 1-year rate | 44.4% (35.3–56.0) |
| 2-year rate | 23.1% (15.9–33.8) |
| 3-year rate | 13.0% (7.1–23.9) |
| 5-year rate | NA |
| Time to CNS metastasis, palliative systemic LoT1 | |
| No. of pts | 90 |
| No. of events | 22 |
| Median (95% CI), months | 48.1 (36.0–NA) |
| 1-year rate | 87.6% (80.2–95.6) |
| 2-year rate | 79.3% (69.6–90.4) |
| 3-year rate | 65.7% (53.0–81.5) |
| 5-year rate | 44.7% (28.3–70.5) |
| rwTTNT (LoT1 to LoT2), palliative systemic LoT1 | |
| No. of pts | 90 |
| No. of events | 42 |
| Median (95% CI), months | 23.9 (15.7–33.8) |
| 1-year rate | 71.6% (61.9–82.8) |
| 2-year rate | 48.3% (37.2–62.8) |
| 3-year rate | 26.7% (15.6–45.7) |
| 5-year rate | NA |
| rwToT, palliative systemic LoT1 | |
| No. of pts | 90 |
| No. of events | 67 |
| Median (95% CI), months | 17.8 (13.1–25.3) |
| 1-year rate | 63.3% (54.1–74.1) |
| 2-year rate | 39.9% (31.0–51.5) |
| 3-year rate | 30.1% (21.8–41.5) |
| 5-year rate | 19.7% (11.7–33.0) |
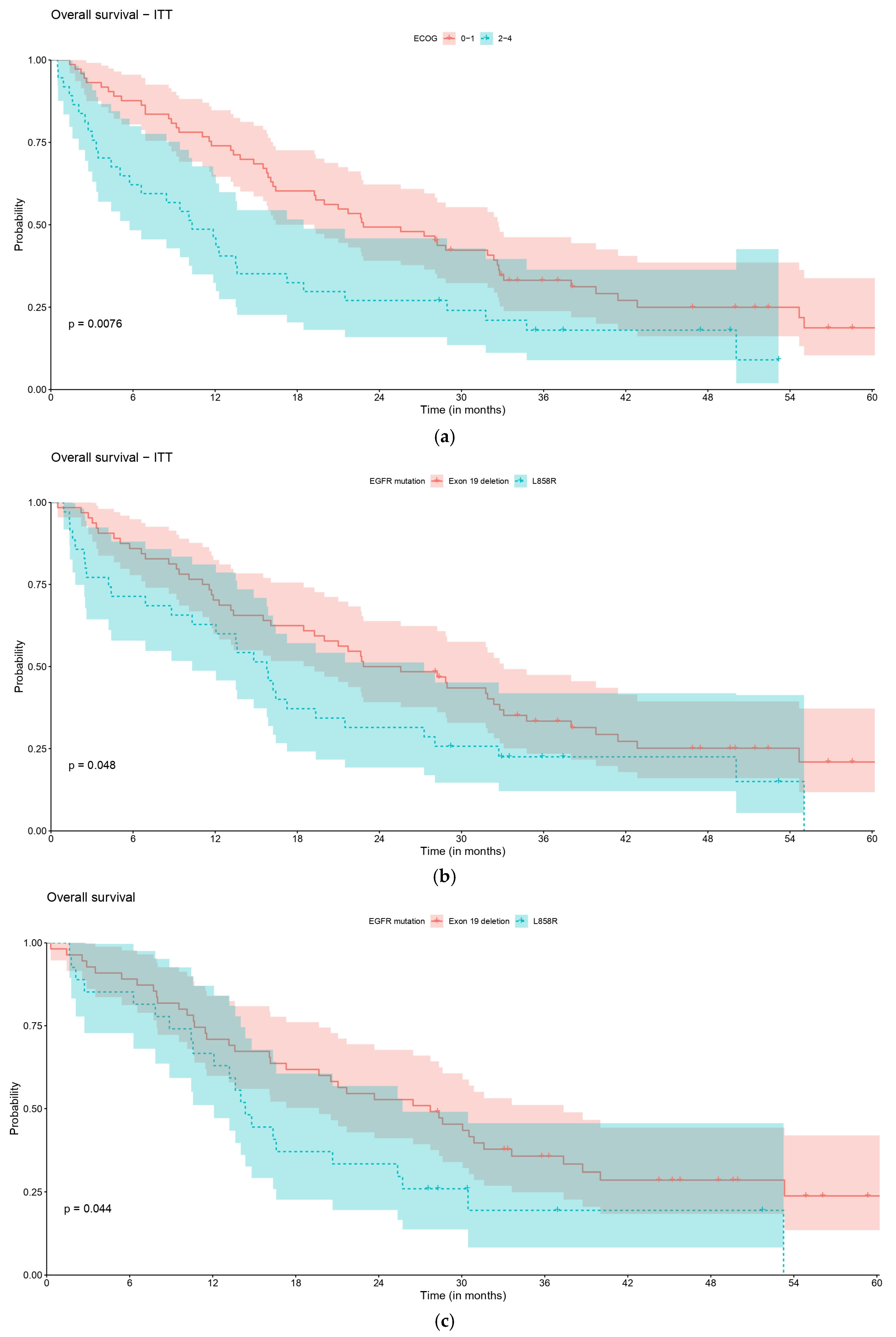
| rwOS, Overall | ECOG 0–1 | ECOG 2–4 | p-Value |
|---|---|---|---|
| No. of pts | 73 | 37 | 0.008 |
| No. of events | 54 | 31 | |
| Median (95% CI), months | 22.8 (19.3–32.7) | 10.3 (5.8–18.5) | |
| 1-year rate | 74.0 (64.6–84.8) | 45.9 (32.4–65.2) | |
| 2-year rate | 49.3 (39.1–62.2) | 27.0 (15.9–45.9) | |
| 3-year rate | 33.2 (23.8–46.2) | 18.0 (8.9–36.4) | |
| 5-year rate | 18.7 (10.4–33.8) | NA | |
| rwOS, overall | E 19 del | L858R | p-value |
| No. of pts | 64 | 35 | 0.048 |
| No. of events | 47 | 29 | |
| Median (95% CI), months | 24.2 (19.3–33.1) | 15.8 (10.3–27.3) | |
| 1-year rate | 70.3 (60.0–82.4) | 62.9 (48.7–81.1) | |
| 2-year rate | 50.0 (39.1–63.9) | 31.4 (19.3–51.3) | |
| 3-year rate | 33.4 (23.5–47.5) | 22.5 (12.1–41.9) | |
| 5-year rate | 20.9 (11.8–37.2) | NA | |
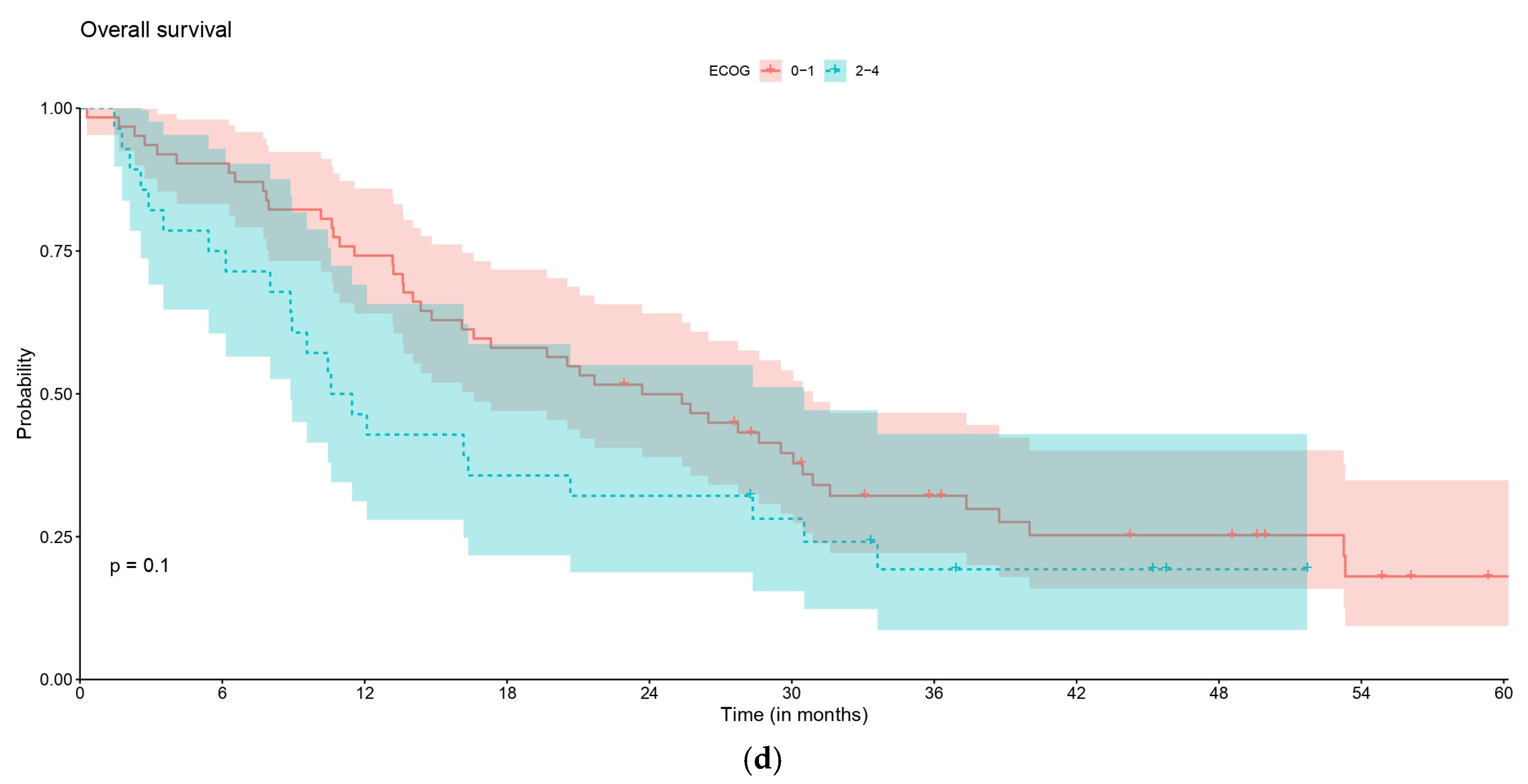
| rwOS, pts who started palliative systemic LoT1 | ECOG 0–1 | ECOG 2–4 | p-value |
| No. of pts | 62 | 28 | 0.086 |
| No. of events | 46 | 22 | |
| Median (95% CI), months | 26.4 (19.4–32.8) | 12.9 (10.1–31.8) | |
| 1-year rate | 79.0 (69.5–89.8) | 57.1 (41.5–78.8) | |
| 2-year rate | 51.6 (40.6–65.7) | 32.1 (18.8–55.1) | |
| 3-year rate | 32.9 (22.9–47.3) | 20.1 (9.4–43.0) | |
| 5-year rate | 18.6 (9.7–35.6) | NA | |
| rwOS, pts who started palliative systemic LoT1 | E 19 del | L858R | p-value |
| No. of pts | 55 | 27 | 0.066 |
| No. of events | 39 | 22 | |
| Median (95% CI), months | 28.8 (21.0–38.0) | 16.2 (13.6–28.1) | |
| 1-year rate | 76.4 (65.9–88.5) | 74.1 (59.3–92.6) | |
| 2-year rate | 54.5 (42.9–69.4) | 33.3 (19.6–56.8) | |
| 3-year rate | 35.7 (25–51.1) | 21.6 (10.4–44.9) | |
| 5-year rate | 23.9 (13.6–42.1) | NA | |
| rwPFS, pts who started palliative systemic LoT1 | ECOG 0–1 | ECOG 2–4 | p-value |
| No. of pts | 62 | 28 | 0.640 |
| No. of events | 54 | 24 | |
| Median (95% CI), months | 12.0 (9.8–19.2) | 8.9 (7.1–20.7) | |
| 1-year rate | 48.4 (37.4–62.6) | 35.7 (21.7–58.7) | |
| 2-year rate | 25.5 (16.6–39.1) | 17.9 (8.1–39.5) | |
| 3-year rate | 13.6 (6.7–27.6) | 11.9 (3.9–36.8) | |
| 5-year rate | NA | NA | |
| rwPFS, pts who started palliative systemic LoT1 | E 19 del | L858R | p-value |
| No. of pts | 55 | 27 | 0.100 |
| No. of events | 47 | 24 | |
| Median (95% CI), months | 12.2 (10.8–22.3) | 9.0 (6.0–13.6) | |
| 1-year rate | 52.7 (41.1–67.7) | 37.0 (22.6–60.6) | |
| 2-year rate | 29.1 (19.3–43.9) | 14.8 (6.0–36.6) | |
| 3-year rate | 17.1 (9.3–31.5) | NA | |
| 5-year rate | NA | NA | |
| Time to CNS metastasis, pts who started palliative systemic LoT1 | E 19 del | L858R | p-value |
| No. of pts | 55 | 27 | 0.350 |
| No. of events | 13 | 6 | |
| Median (95% CI), months | NA | 36.0 (34.9-NA) | |
| 1-year rate | 91.6 (84.1–99.9) | 84.9 (70.5–100) | |
| 2-year rate | 83.0 (72.1–95.6) | 75.5 (56.1–100) | |
| 3-year rate | 73.3 (60.0–89.5) | 50.3 (21.4–100) | |
| 5-year rate | 50.9 (31.4–82.4) | NA | |
| rwTTNT (LoT1 to LoT2), pts who started palliative systemic LoT1 | E 19 del | L858R | p-value |
| No. of pts | 55 | 27 | 0.730 |
| No. of events | 27 | 12 | |
| Median (95% CI), months | 24.7 (14.1-NA) | 23.2 (15.7-NA) | |
| 1-year rate | 70.7 (58.9–85.0) | 74.4 (58.5–94.6) | |
| 2-year rate | 51.0 (38.0–68.4) | 39.0 (20.6–73.7) | |
| 3-year rate | 29.9 (17.8–50.4) | NA | |
| 5-year rate | NA | NA | |
| rwToT (LoT1 to LoT2), pts who started palliative systemic LoT1 | E 19 del | L858R | p-value |
| No. of pts | 55 | 27 | 0.070 |
| No. of events | 38 | 22 | |
| Median (95% CI), months | 22.5 (18.3–37.3) | 13.3 (10.5–25.3) | |
| 1-year rate | 70.9 (59.9–84.0) | 59.3 (43.3–81.0) | |
| 2-year rate | 45.5 (34.0–60.7) | 33.3 (19.6–56.8) | |
| 3-year rate | 37.7 (26.8–53.1) | 20.7 (9.6–45.0) | |
| 5-year rate | 27.4 (17.2–43.7) | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, A.; Pina, M.; Calisto, R.; Leite-Silva, P.; Medeiros, P.; Silva, C.; Silva, A.S.; Redondo, P.; Ramalho-Carvalho, J.; Santos, S.F.; et al. Characterization of the Population, Treatment Patterns, and Outcomes of Patients with Advanced or Metastatic Non-Small-Cell Lung Cancer (NSCLC) with Epidermal Growth Factor Receptor Mutation (EGFRm): A Retrospective Cohort Study from IPO Porto. Curr. Oncol. 2025, 32, 414. https://doi.org/10.3390/curroncol32080414
Rodrigues A, Pina M, Calisto R, Leite-Silva P, Medeiros P, Silva C, Silva AS, Redondo P, Ramalho-Carvalho J, Santos SF, et al. Characterization of the Population, Treatment Patterns, and Outcomes of Patients with Advanced or Metastatic Non-Small-Cell Lung Cancer (NSCLC) with Epidermal Growth Factor Receptor Mutation (EGFRm): A Retrospective Cohort Study from IPO Porto. Current Oncology. 2025; 32(8):414. https://doi.org/10.3390/curroncol32080414
Chicago/Turabian StyleRodrigues, Ana, Marta Pina, Rita Calisto, Pedro Leite-Silva, Pedro Medeiros, Catarina Silva, Ana Sofia Silva, Patrícia Redondo, João Ramalho-Carvalho, Susana Ferreira Santos, and et al. 2025. "Characterization of the Population, Treatment Patterns, and Outcomes of Patients with Advanced or Metastatic Non-Small-Cell Lung Cancer (NSCLC) with Epidermal Growth Factor Receptor Mutation (EGFRm): A Retrospective Cohort Study from IPO Porto" Current Oncology 32, no. 8: 414. https://doi.org/10.3390/curroncol32080414
APA StyleRodrigues, A., Pina, M., Calisto, R., Leite-Silva, P., Medeiros, P., Silva, C., Silva, A. S., Redondo, P., Ramalho-Carvalho, J., Santos, S. F., & Bento, M. J. (2025). Characterization of the Population, Treatment Patterns, and Outcomes of Patients with Advanced or Metastatic Non-Small-Cell Lung Cancer (NSCLC) with Epidermal Growth Factor Receptor Mutation (EGFRm): A Retrospective Cohort Study from IPO Porto. Current Oncology, 32(8), 414. https://doi.org/10.3390/curroncol32080414






