Advances in Therapeutic Vaccines Against HPV: A Review of Human Clinical Trials
Simple Summary
Abstract
1. Introduction
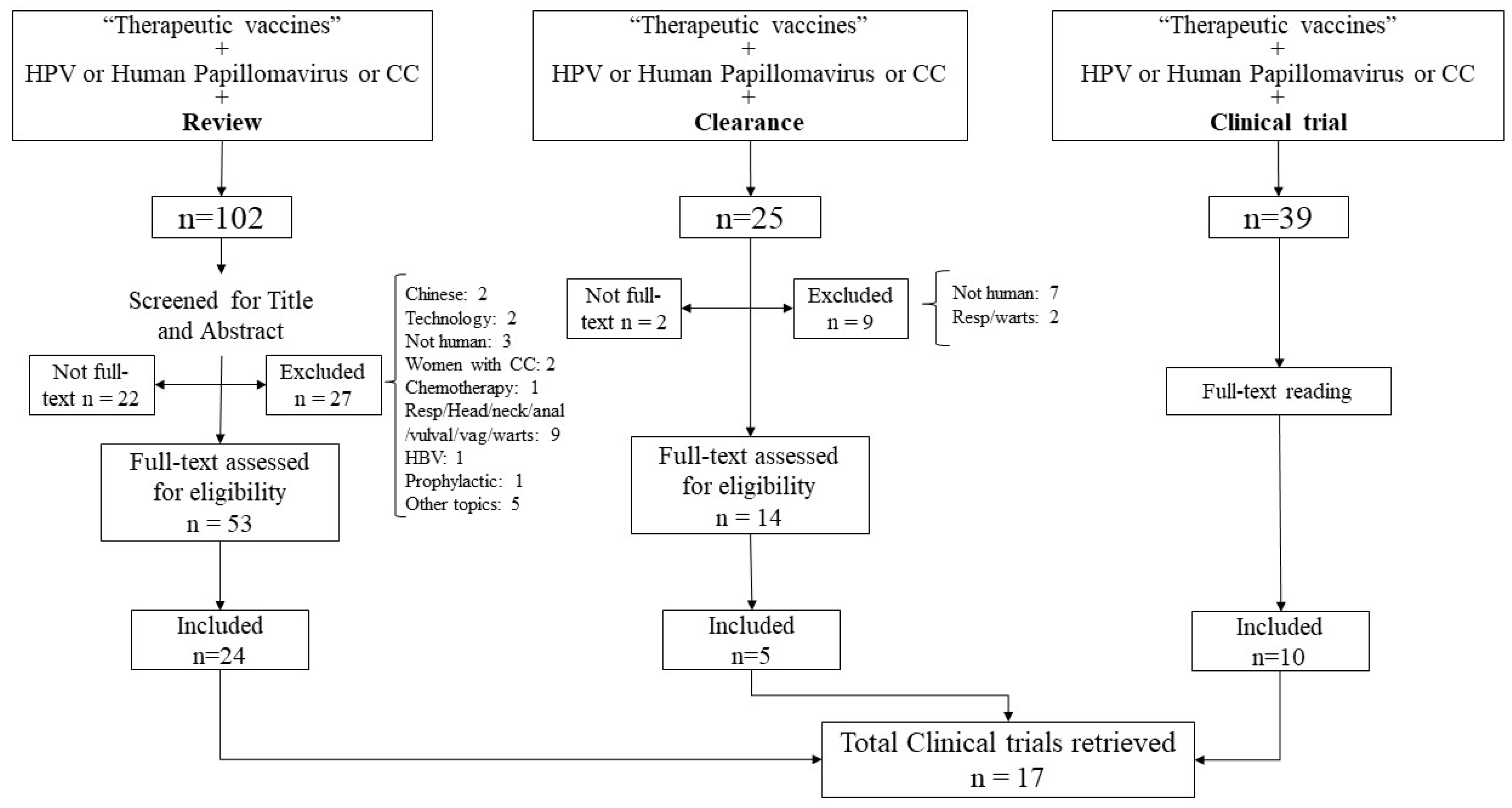
2. Methods
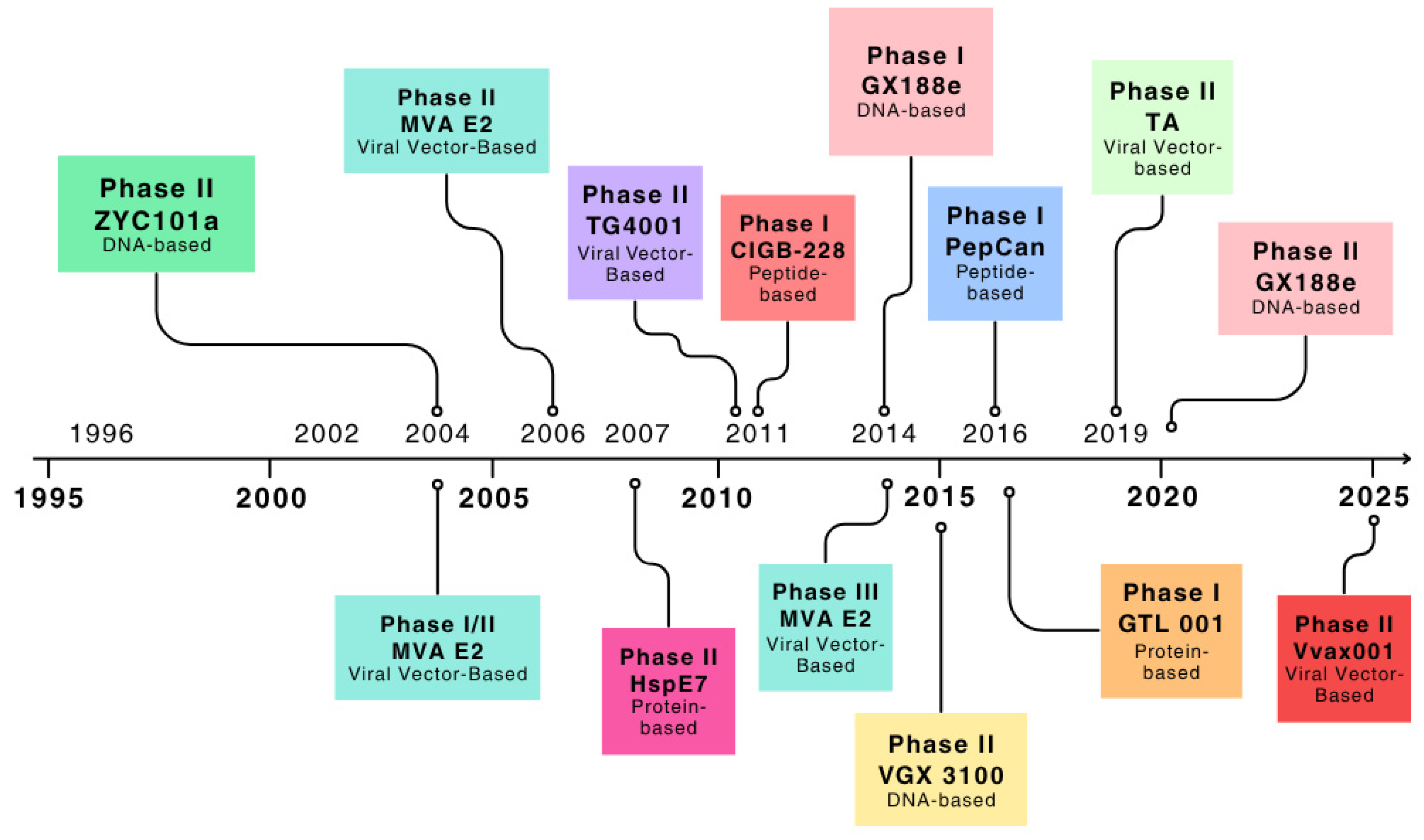
3. Results
3.1. Studies Selection
3.2. HPV Vaccines and Clearance
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cervical Cancer. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/cervical-cancer (accessed on 1 August 2025).
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.-M.; Reis, R.M.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.O.; et al. Cervical cancer in low and middle-income countries. Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef]
- Stelzle, D.; Tanaka, L.F.; Lee, K.K.; Khalil, A.I.; Baussano, I.; Shah, A.S.V.; McAllister, D.A.; Gottlieb, S.L.; Klug, S.J.; Winkler, A.S.; et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob. Health 2021, 9, e161–e169. [Google Scholar] [CrossRef] [PubMed]
- Castellsagué, X.; Muñoz, N. Chapter 3: Cofactors in Human Papillomavirus Carcinogenesis—Role of Parity, Oral Contraceptives, and Tobacco Smoking. JNCI Monogr. 2003, 31, 20–28. [Google Scholar] [CrossRef]
- Hebner, C.M.; Laimins, L.A. Human papillomaviruses: Basic mechanisms of pathogenesis and oncogenicity. Rev. Med. Virol. 2006, 16, 83–97. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Preferred Product Characteristics for Therapeutic Human Papillomavirus Vaccines; WHO Guideline: Geneva, Switzerland, 2024. Available online: https://www.who.int/publications/i/item/9789240092174 (accessed on 1 August 2025).
- Burd, E.M. Human Papillomavirus and Cervical Cancer. Clin. Microbiol. Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef]
- Chen, Y.; Egawa, N.; Zheng, K.; Doorbar, J. How Can HPV E6 Manipulate the Host Cell Differentiation Process to Maintain the Reservoir of Infection? Tumour Virus Res. 2025, 19, 200313. [Google Scholar] [CrossRef]
- Schiller, J.T.; Lowy, D.R. Prospects for Cervical Cancer Prevention by Human Papillomavirus Vaccination. Cancer Res. 2006, 66, 10229–10232. [Google Scholar] [CrossRef] [PubMed]
- Bowden, S.J.; Doulgeraki, T.; Bouras, E.; Markozannes, G.; Athanasiou, A.; Grout-Smith, H.; Kechagias, K.S.; Ellis, L.B.; Zuber, V.; Chadeau-Hyam, M.; et al. Risk factors for human papillomavirus infection, cervical intraepithelial neoplasia and cervical cancer: An umbrella review and follow-up Mendelian randomisation studies. BMC Med. 2023, 21, 274. [Google Scholar] [CrossRef]
- About Genital HPV Infection|STI|CDC. Available online: https://www.cdc.gov/sti/about/about-genital-hpv-infection.html (accessed on 1 August 2025).
- Graham, S.V. The human papillomavirus replication cycle, and its links to cancer progression: A comprehensive review. Clin. Sci. 2017, 131, 2201–2221. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical cancer therapies: Current challenges and future perspectives. Tumour Virus Res. 2022, 13, 200238. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, L.; Zhang, C.; Hong, Z.; Han, Z. Cervical cancer heterogeneity: A constant battle against viruses and drugs. Biomark. Res. 2022, 10, 85. [Google Scholar] [CrossRef]
- World Health Organisation (WHO): Cervical Cancer Elimination Initiative. Available online: https://www.who.int/initiatives/cervical-cancer-elimination-initiative (accessed on 1 August 2025).
- CDC Primary Prevention Methods. Available online: https://www.cdc.gov/std/treatment-guidelines/clinical-primary.htm (accessed on 1 August 2025).
- Sexually Transmitted Infections (STIs). Available online: https://www.who.int/news-room/fact-sheets/detail/sexually-transmitted-infections-(stis) (accessed on 1 August 2025).
- Global Sexually Transmitted Infections Programme. Available online: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/stis/prevention (accessed on 1 August 2025).
- St. Jude Childrens Research Hospital. History of HPV Vaccination. Available online: https://sjr-redesign.stjude.org/content/dam/research-redesign/centers-initiatives/hpv-cancer-prevention-program/hpv-advocacy-campaign/history-hpv-vaccination.pdf (accessed on 1 August 2025).
- Schuind, A.E.; Balaji, K.A.; Du, A.; Yuan, Y.; Dull, P. Human papillomavirus prophylactic vaccines: Update on new vaccine development and implications for single-dose policy. J. Natl. Cancer Inst. Monogr. 2024, 67, 410–416. [Google Scholar] [CrossRef]
- Kutz, J.M.; Rausche, P.; Gheit, T.; Puradiredja, D.I.; Fusco, D. Barriers and facilitators of HPV vaccination in sub-saharan Africa: A systematic review. BMC Public Health 2023, 23, 974. [Google Scholar] [CrossRef]
- Voelker, R.A. Cervical Cancer Screening. JAMA 2023, 330, 2030. [Google Scholar] [CrossRef] [PubMed]
- Marth, C.; Landoni, F.; Mahner, S.; Mccormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef] [PubMed]
- Papilocare Gel Vaginal—Papilocare. Available online: https://papilocare.com/papilocare-gel-vaginal/ (accessed on 1 August 2025).
- Serrano, L.; López, A.C.; González, S.P.; Palacios, S.; Dexeus, D.; Centeno-Mediavilla, C.; Coronado, P.; de la Fuente, J.; López, J.A.; Vanrell, C.; et al. Efficacy of a Coriolus versicolor-Based Vaginal Gel in Women with Human Papillomavirus-Dependent Cervical Lesions: The PALOMA Study. J. Low. Genit. Tract Dis. 2021, 25, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Stern, P.L.; van der Burg, S.H.; Hampson, I.N.; Broker, T.R.; Fiander, A.; Lacey, C.J.; Kitchener, H.C.; Einstein, M.H. Therapy of Human Papillomavirus-Related Disease. Vaccine 2012, 30, F71–F82. [Google Scholar] [CrossRef]
- Cervical Cancer Treatment—NCI. Available online: https://www.cancer.gov/types/cervical/treatment (accessed on 1 August 2025).
- Khairkhah, N.; Bolhassani, A.; Najafipour, R. Current and future direction in treatment of HPV-related cervical disease. J. Mol. Med. 2022, 100, 829–845. [Google Scholar] [CrossRef] [PubMed]
- Skolnik, J.M.; Morrow, M.P. Vaccines for HPV-associated diseases. Mol. Asp. Med. 2023, 94, 101224. [Google Scholar] [CrossRef]
- Beyaert, S.; Machiels, J.P.; Schmitz, S. Vaccine-Based Immunotherapy for Head and Neck Cancers. Cancers 2021, 13, 6041. [Google Scholar] [CrossRef]
- Cheng, M.A.; Farmer, E.; Huang, C.; Lin, J.; Hung, C.F.; Wu, T.C. Therapeutic DNA Vaccines for Human Papillomavirus and Associated Diseases. Hum. Gene Ther. 2018, 29, 971–996. [Google Scholar] [CrossRef]
- Gohar, A.; Ali, A.A.; Elkhatib, W.F.; El-Sayyad, G.S.; Elfadil, D.; Noreddin, A.M. Combination therapy between prophylactic and therapeutic human papillomavirus (HPV) vaccines with special emphasis on implementation of nanotechnology. Microb. Pathog. 2022, 171, 105747. [Google Scholar] [CrossRef]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: A randomised, double-blind, placebo-controlled phase 2b trial. Lancet 2015, 386, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Jin, H.-T.; Hur, S.-Y.; Yang, H.G.; Seon, Y.B.; Hong, S.R.; Lee, C.-W.; Kim, S.; Woo, J.-W.; Park, K.S.; et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat. Commun. 2014, 5, 5317. [Google Scholar] [CrossRef]
- Choi, Y.J.; Hur, S.Y.; Kim, T.-J.; Hong, S.R.; Lee, J.K.; Cho, C.-H.; Park, K.S.; Woo, J.W.; Sung, Y.C.; Suh, Y.S.; et al. A Phase II, Prospective, Randomized, Multicenter, Open-Label Study of GX-188E, an HPV DNA Vaccine, in Patients with Cervical Intraepithelial Neoplasia 3. Clin. Cancer Res. 2020, 26, 1616–1623. [Google Scholar] [CrossRef]
- Gutierrez, C.M.C.; Tinoco, A.; Navarro, T.; Contreras, M.L.; Cortes, R.R.; Calzado, P.; Reyes, L.; Posternak, R.; Morosoli, G.; Verde, M.L.; et al. Therapeutic vaccination with MVA E2 can eliminate precancerous lesions (CIN 1, CIN 2, and CIN 3) associated with infection by oncogenic human papillomavirus. Hum. Gene Ther. 2004, 15, 421–431. [Google Scholar] [CrossRef]
- García-Hernández, E.; González-Sánchez, J.L.; Andrade-Manzano, A.; Contreras, M.L.; Padilla, S.; Guzmán, C.C.; Jiménez, R.; Reyes, L.; Morosoli, G.; Verde, M.L.; et al. Regression of papilloma high-grade lesions (CIN 2 and CIN 3) is stimulated by therapeutic vaccination with MVA E2 recombinant vaccine. Cancer Gene Ther. 2006, 13, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Rosales, R.; López-Contreras, M.; Rosales, C.; Magallanes-Molina, J.-R.; Gonzalez-Vergara, R.; Arroyo-Cazarez, J.M.; Ricardez-Arenas, A.; del Follo-Valencia, A.; Padilla-Arriaga, S.; Guerrero, M.V.; et al. Regression of human papillomavirus intraepithelial lesions is induced by MVA E2 therapeutic vaccine. Hum. Gene Ther. 2014, 25, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Bouillette-Marussig, M.; Hens, A.; De Coster, I.; Depuydt, C.; Goubier, A.; Van Tendeloo, V.; Cools, N.; Goossens, H.; Hercend, T.; et al. GTL001, a therapeutic vaccine for women infected with human papillomavirus 16 or 18 and normal cervical cytology: Results of a phase I clinical trial. Clin. Cancer Res. 2016, 22, 3238–3248. [Google Scholar] [CrossRef]
- Genticel Reports Final Results of GTL001 Phase 2 Trial in HPV16/18-Infected Women. 2016. Available online: https://www.globenewswire.com/news-release/2016/12/13/897347/0/en/Genticel-Reports-Final-Results-of-GTL001-Phase-2-Trial-in-HPV16-18-Infected-Women.html (accessed on 1 August 2025).
- Brun, J.-L.; Dalstein, V.; Leveque, J.; Mathevet, P.; Raulic, P.; Baldauf, J.-J.; Scholl, S.; Huynh, B.; Douvier, S.; Riethmuller, D.; et al. Regression of high-grade cervical intraepithelial neoplasia with TG4001 targeted immunotherapy. Am. J. Obs. Gynecol. 2011, 204, e1–e169.e8. [Google Scholar] [CrossRef]
- Harper, D.M.; Nieminen, P.; Donders, G.; Einstein, M.H.; Garcia, F.; Huh, W.K.; Stoler, M.H.; Glavini, K.; Attley, G.; Limacher, J.-M.; et al. The efficacy and safety of Tipapkinogen Sovacivec therapeutic HPV vaccine in cervical intraepithelial neoplasia grades 2 and 3: Randomized controlled phase II trial with 2.5 years of follow-up. Gynecol. Oncol. 2019, 153, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, W.W.; Stratton, S.L.; Myrick, R.S.; Vaughn, R.; Donnalley, L.M.; Coleman, H.N.; Mercado, M.; Moerman-Herzog, A.M.; Spencer, H.J.; Andrews-Collins, N.R.; et al. A phase I dose-escalation clinical trial of a peptide-based human papillomavirus therapeutic vaccine with Candida skin test reagent as a novel vaccine adjuvant for treating women with biopsy-proven cervical intraepithelial neoplasia 2/3. Oncoimmunology 2015, 4, e1031439. [Google Scholar] [CrossRef]
- Coleman, H.N.; Greenfield, W.W.; Stratton, S.L.; Vaughn, R.; Kieber, A.; Moerman-Herzog, A.M.; Spencer, H.J.; Hitt, W.C.; Quick, C.M.; Hutchins, L.F.; et al. Human papillomavirus type 16 viral load is decreased following a therapeutic vaccination. Cancer Immunol. Immunother. 2016, 65, 563–573. [Google Scholar] [CrossRef]
- Eerkens, A.L.; Esajas, M.D.; Brummel, K.; Vledder, A.; van Rooij, N.; Plat, A.; Haro, S.B.A.; Paijens, S.T.; Slagter-Menkema, L.; Schuuring, E.; et al. Vvax001, a Therapeutic Vaccine, for Patients with HPV16-Positive High-grade Cervical Intraepithelial Neoplasia: A Phase II Trial. Clin. Cancer Res. 2025, 31, 1016–1026. [Google Scholar] [CrossRef]
- Garcia, F.; Petry, K.U.; Muderspach, L.; Gold, M.A.; Braly, P.; Crum, C.P.; Magill, M.; Silverman, M.; Urban, R.G.; Hedley, M.L.; et al. ZYC101a for treatment of high-grade cervical intraepithelial neoplasia: A randomized controlled trial. Obstet. Gynecol. 2004, 103, 317–326. [Google Scholar] [CrossRef]
- Einstein, M.H.; Kadish, A.S.; Burk, R.D.; Kim, M.Y.; Wadler, S.; Streicher, H.; Goldberg, G.L.; Runowicz, C.D. Heat shock fusion protein-based immunotherapy for treatment of cervical intraepithelial neoplasia III. Gynecol. Oncol. 2007, 106, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Roman, L.; Wilczynski, S.; Muderspach, L.; Burnett, A.; O’MEara, A.; Brinkman, J.; Kast, W.; Facio, G.; Felix, J.; Aldana, M.; et al. A phase II study of Hsp-7 (SGN-00101) in women with high-grade cervical intraepithelial neoplasia. Gynecol. Oncol. 2007, 106, 558–566. [Google Scholar] [CrossRef]
- Solares, A.M.; Baladron, I.; Ramos, T.; Valenzuela, C.; Borbon, Z.; Fanjull, S.; Gonzalez, L.; Castillo, D.; Esmir, J.; Granadillo, M.; et al. Safety and Immunogenicity of a Human Papillomavirus Peptide Vaccine (CIGB-228) in Women with High-Grade Cervical Intraepithelial Neoplasia: First-in-Human, Proof-of-Concept Trial. ISRN Obs. Gynecol. 2011, 2011, 292951. [Google Scholar] [CrossRef]
- Kawana, K.; Kobayashi, O.; Ikeda, Y.; Yahata, H.; Iwata, T.; Satoh, T.; Akiyama, A.; Maeda, D.; Hori-Hirose, Y.; Uemura, Y.; et al. Phase I and II randomized clinical trial of an oral therapeutic vaccine targeting human papillomavirus for treatment of cervical intraepithelial neoplasia 2 and 3. JNCI Cancer Spectr. 2023, 7, pkad101. [Google Scholar] [CrossRef]
- Inovio Pharmaceuticals Inc. INOVIO Highlights Key Updates on Phase 3 Program for VGX-3100, Its DNA-Based Immunotherapy for the Treatment of Cervical HSIL Caused by HPV-16 and/or HPV-18. 2021. Available online: https://ir.inovio.com/news-releases/news-releases-details/2021/INOVIO-Highlights-Key-Updates-on-Phase-3-Program-for-VGX-3100-its-DNA-based-Immunotherapy-for-the-Treatment-of-Cervical-HSIL-Caused-by-HPV-16-andor-HPV-18/default.aspx (accessed on 1 August 2025).
- Khalil, A.I.; Zhang, L.; Muwonge, R.; Sauvaget, C.; Basu, P. Efficacy and Safety of Therapeutic HPV Vaccines to Treat CIN2/CIN3 Lesions: A Systematic Review and Meta-Analysis of Phase II/III Clinical Trials. BMJ Open 2023, 13, e069616. [Google Scholar] [CrossRef] [PubMed]
- Stern, P.L.; Roden, R.B. Opportunities to improve immune-based prevention of HPV-associated cancers. Papillomavirus Res. 2019, 7, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Huang, H.; Yu, C.; Li, X.; Wang, Y.; Xie, L. Current Status and Future Directions for the Development of Human Papillomavirus Vaccines. Front. Immunol. 2024, 15, 1362770. [Google Scholar] [CrossRef] [PubMed]
| Vaccine | Type | Reference | Study Design | Period | Country | Population | No. Doses | Follow-Up Duration | Clearance Results |
|---|---|---|---|---|---|---|---|---|---|
| VGX 3100 | 2 synthetic DNA plasmids encoding E6/E7 of HPV16 and HPV18 | [33] | Phase IIb | 2011–2013 | USA Estonia South Africa India Canada Australia Georgia | 169 women CIN2/3 VGX-3100 (n = 125) or placebo (n = 42). | 3 doses (weeks 0, 4, 12) + electroporation | 36 weeks | 49.5% of 107 VGX-3100 recipients and 30.6% of placebo had histopathological regression (p = 0.03). Among those with histopathological regression, viral clearance was:
|
| GX-188E | Plasmid encoding fusion protein of HPV 16/18 E6/E7 linked to Flt3L and tpa | [34] | Phase I | 2012 | Republic of Korea | 9 women CIN3 | 3 doses (weeks 0, 4, 12) + electroporation | 36 weeks | Week 12:
|
| [35] | Phase II | 2014–2016 | Republic of Korea | 87 women CIN3 HPV16/18 | 3 doses (weeks 0, 4, 12) + electroporation | 36 weeks | 52% of patients at 20 weeks and 67% at 36 weeks presented histopathologic regression; 77% of those with histologic regression showed HPV clearance. HPV clearance and histopathologic association: 20 weeks visit (OR = 13.9; 95% CI, 4.1–47.2) 36 weeks visit (OR = 25.3; 95% CI, 4.8–14.9) | ||
| MVA E2 | Modified Vaccinia virus of Ankara (MVA) containing bovine PV E2 protein | [36] | Phase I/II | 2002–2003 | Mexico | 78 CIN1/2/3 MVA E2 (n = 36) Cryosurgery (n = 42) | 6 doses (intralesional weekly) | 24 weeks | 34/36 patients showed complete elimination of precancerous lesions after vaccination. Among patients, 50% completely eliminated HPV, and in remaining 50% of patients, HPV DNA was only 10% of original viral load. |
| [37] | Phase II | 2002–2004 | Mexico | 52 women CIN3 2 women CIN2 (34 vaccine/20 control) | 6 doses (intralesional weekly) | 24 weeks | Histological analysis showed total elimination of high-grade lesions in 20 out of 34 patients, and 11 had a 50% reduction in lesion size. DNA viral load was significantly reduced in MVA E2-treated patients: 12/34 efficiently eliminated all HPV DNA. In some patients, viral load diminished by 95%. In remaining patients, viral load was reduced between 15 and 50%. None of 20 control patients treated by conization eliminated HPV. | ||
| [38] | Phase III | 2007–2012 | Mexico | 1176 women and 180 men with intraepithelial lesions (CIN1/2/3) | Women: 6 doses (intralesional weekly) Men: 5 doses (intraurethral) | 24 weeks | 89.3% of women and 100% of men achieved complete elimination of lesions, and 2.4% of women showed a reduction in CIN1 HPV DNA clearance: 83% | ||
| GTL 001 (ProCervix) | Recombinant E7-CyaA fusion proteins expressed in and purified from Escherichia coli | [39] | Phase I | 2010–2012 | Belgium France | 47 women HPV16/18+ with normal cervical cytology | 2 (intradermal 6 weeks apart) | 24 weeks | In most groups treated with GTL001 600 mg + imiquimod, mean viral loads decreased over time. In randomized portion of trial, sustained clearance occurred in 5 of 6 (83%) patients treated with highest dose of GTL001 (600 mg) versus 1 of 3 (33%) treated with placebo. Trial not designed to detect significant differences in viral clearance. |
| [40] | Phase II | 2013–2015 | 7 countries in Europe | 233 patients HPV16/18 with normal or abnormal (ASCUS/LSIL) cytology | 2 (intradermal 6 weeks apart) | 2 years | No statistical difference in viral clearance between treatment and placebo groups at any point over 2 years | ||
| TG4001 | MVA encoding IL-2 and modified forms of HPV 16 E6 and E7 proteins | [41] | Phase II | 2004–2005 | France | 21 patients with HPV 16-related CIN2/3 | 3 doses (1 week interval) | 12 months | 7/10 clinical responders concomitantly had no HPV 16 mRNA detected (3 other results not available). 8/10 had no HPV 16 DNA present. Median times to disappearance of HSIL: 5 weeks (95% CI, 9–27), Clearance of HPV 16 E6/E7 transcripts: 13.3 weeks (95% CI, 9–18), HR-HPV DNA clearance: 26 weeks (95% CI, 19–33) |
| Tipapkinogen Sovacivec (TA) therapeutic HPV vaccine | Attenuated vaccinia virus (Wyeth strain) encoding IL-2 and modified forms of HPV 16 E6 and E7 proteins | [42] | Phase IIb | 2009–2011 | USA Spain Belgium France Finland | 206 women >=18 years old with confirmed CIN2/3 | 3 doses (1 week interval) | 30 months | Complete resolution significantly higher in vaccine group for CIN 2/3 regardless of 13 HR-HPV types assayed (24% vs. 10%, p < 0.05); as well as for only CIN3, regardless of HR-HPV type (21% vs. 0%, p < 0.01). Irrespective of baseline HPV infection, viral DNA clearance was higher in vaccine group compared to placebo (p < 0.01). Viral DNA clearance of all CIN2/3 and within baseline CIN3, regardless of HR-HPV type, significantly greater in vaccine group (p ≤ 0.01). For specific HR-HPV types, vaccine was superior to placebo for: (1) HPV 16 monoinfected women, (2) any HR-HPV infection except HPV 16, (3) HPV16 co-infected with any other HR-HPV infection. |
| PepCan | 4 peptides HPV16 E6 and Candida skin test reagent as novel adjuvant | [43] | Phase I | 2007–2010 | USA | 24 women CIN2/3 | 4 doses (3 week interval) | 12 weeks | HPV 16 became undetectable in 3/16 subjects after vaccination; viral loads significantly decreased in 9 of 16 HPV16-infected women prior to vaccination. Rate of at least one HPV type becoming undetectable at exit was highest (85%) at 50 μg dose. Systemic T-helper type 1 (Th1) cells were significantly increased after vaccination. |
| [44] | Phase I | 2012–2014 | USA | 37 women CIN2/3 | |||||
| Vvax001 | Replication-deficient recombinant Semliki Forest virus (rSFV) particles encoding E6 and E7 of HPV16 | [45] | Phase II | 2021–2023 | The Netherlands | 18 patients HPV16-positive CIN3 | 3 doses (3 week interval) | 30 months | 10/16 (63%) patients exhibited HPV16 clearance 19 weeks after last vaccination (V8). All patients (100%) with histopathologic regression (8/18) exhibited HPV16 clearance at V8. Among non-responders (8/18), two patients exhibited HPV16 clearance at V8, but two other HPV types (HPV52 and HPV31) were detected in their post-treatment biopsies. |
| ZYC101a | Plasmid-DNA–encoding fragments derived from HPV 16/18 E6/E7 | [46] | Phase II | 2000–2001 | USA Europe | 161 women CIN2/3 | 3 doses (intramuscular) | 6 months | Proportion of subjects who resolved tended to be higher after vaccination compared to placebo (43% vs. 27% p = 0.12). Subjects younger than 25 years with HPV16 or HPV18 at entry, and subjects with other HPV subtypes at entry, experienced higher rates of resolution than placebo subjects (64% vs. 22% and 73% vs. 25%, respectively) |
| HspE7 (SGN-00101) | M. bovis BCG heat shock protein (Hsp65), linked to HPV 16 E7 protein. | [47] | Phase II | 2003–2005 | New York, USA | 58 women CIN3 | 3 doses (monthly subcutaneous vaccinations) | 8 weeks | 22.5% complete pathologic response and 55% partial response. Women who had previous LEEP or ablation for CIN were 2.7 times more likely to have complete response. Decline in HPV dot blot intensity and eventual loss of HPV16 in two patients with complete pathologic response |
| [48] | Phase II | 2001–2004 | California, USA | 21 women CIN2/3 | 4 doses | 12 months | 35% of women had complete regression of their intraepithelial neoplasia, 5% showed regression to CIN1, 55% had stable disease and one progressed. Regression was correlated with immune response. Viral resolution occurred in only one woman participating in this study. | ||
| CIGB-228 | Peptide-based HLA-restricted HPV16 E7 epitope adjuvated with VSSP | [49] | Phase I | 2007–2008 | Cuba | 7 women, HLA-A2 positive, CIN2/3 and HPV16 positive. | 4 doses (weekly) | 12 months | 5/7 women had complete and partial regression. T-cell response was induced in all patients. Concomitant clearance of HPV16 from original lesion sites was observed in three patients who had complete response |
| IGMKK16E7 | HPV16 E7-expressing Lactobacillus casei-based oral vaccine | [50] | Phase I/II | 2019–2021 | Japan | 165 women CIN-2/3 | 4 doses (orally at weeks 1, 2, 4, and 8) | 16–24 weeks | Histopathological regression to normal (complete response) occurred in 32% of high-dose recipients and 12 placebo. Further improvement of IGMKK16E7 is desired to achieve therapeutic effect on viral clearance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, E.; Reina, G.; Carlos, S. Advances in Therapeutic Vaccines Against HPV: A Review of Human Clinical Trials. Curr. Oncol. 2025, 32, 600. https://doi.org/10.3390/curroncol32110600
Martín E, Reina G, Carlos S. Advances in Therapeutic Vaccines Against HPV: A Review of Human Clinical Trials. Current Oncology. 2025; 32(11):600. https://doi.org/10.3390/curroncol32110600
Chicago/Turabian StyleMartín, Elena, Gabriel Reina, and Silvia Carlos. 2025. "Advances in Therapeutic Vaccines Against HPV: A Review of Human Clinical Trials" Current Oncology 32, no. 11: 600. https://doi.org/10.3390/curroncol32110600
APA StyleMartín, E., Reina, G., & Carlos, S. (2025). Advances in Therapeutic Vaccines Against HPV: A Review of Human Clinical Trials. Current Oncology, 32(11), 600. https://doi.org/10.3390/curroncol32110600






