Machine Learning-Based Prognostic Modelling Using MRI Radiomic Data in Cervical Cancer Treated with Definitive Chemoradiotherapy and Brachytherapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Treatment Characteristics
2.3. Image Acquisition
2.4. Tumor Segmentation and Feature Extraction
2.5. Data Cleaning and Preprocessing
2.6. Defining Survival Outcomes
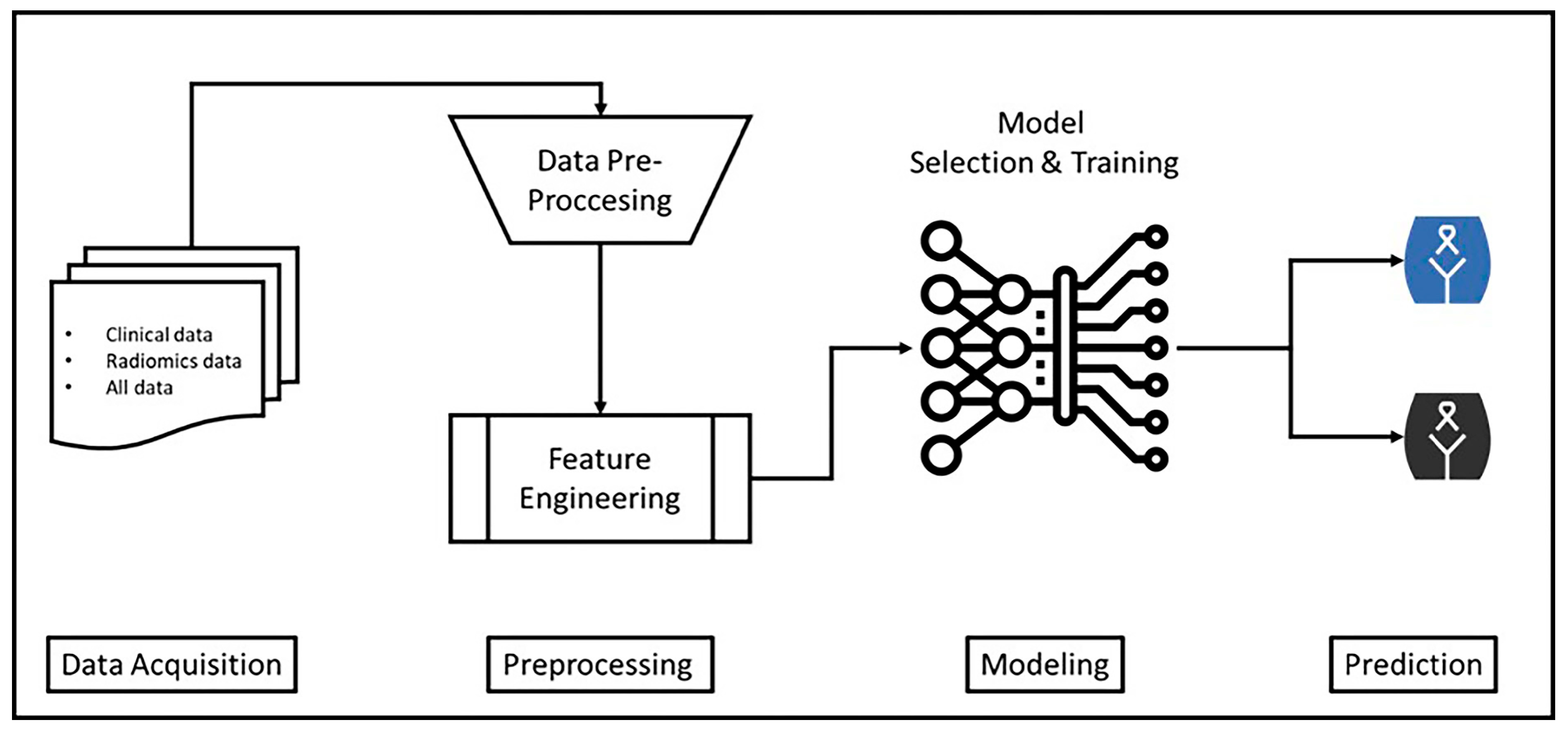
2.7. Machine Learning Modelling Process
2.8. Feature Importances
2.9. Software and Reproducibility
2.10. Statistical Analysis
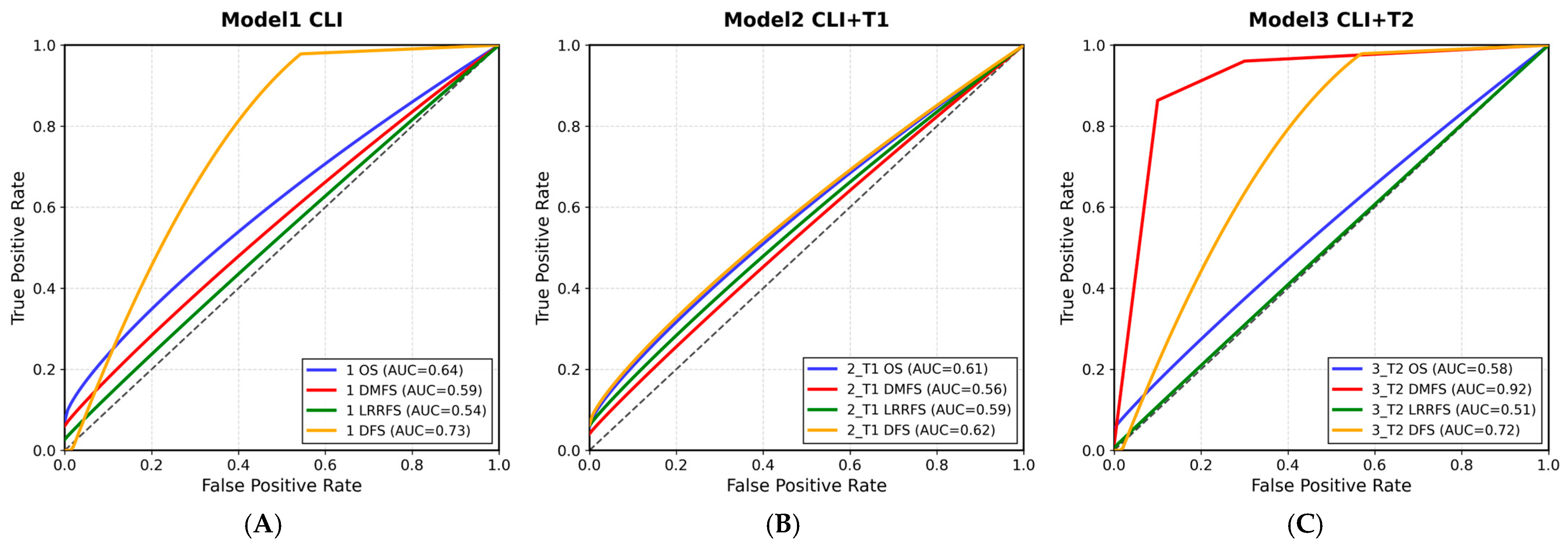
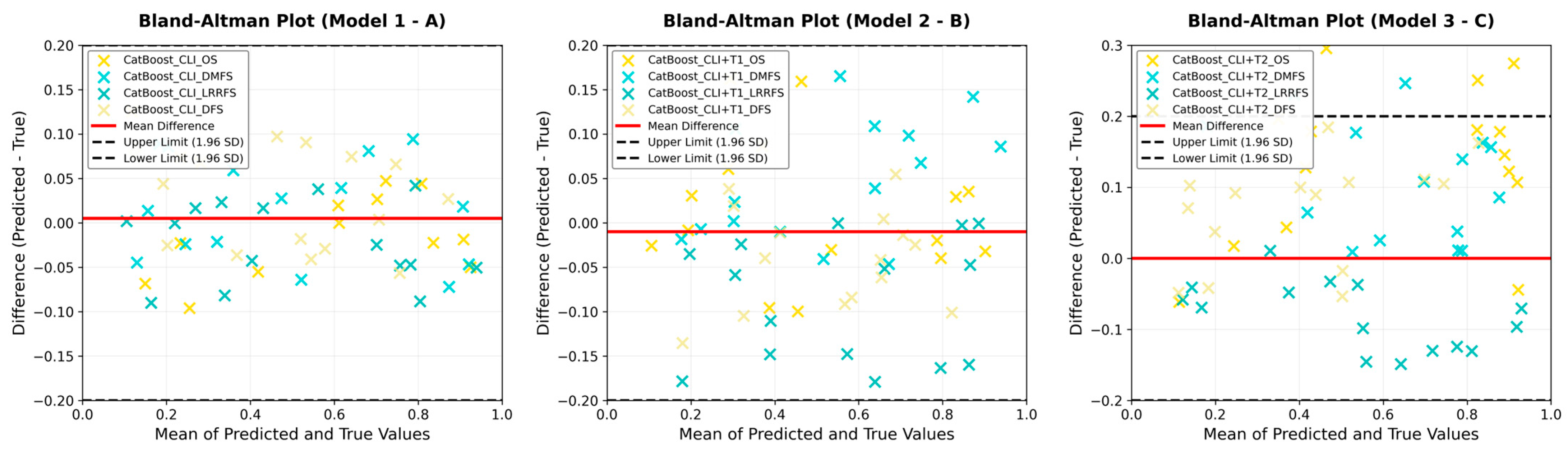
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 18F-FDG-PET/CT | 18F-fluorodeoxyglucose positron emission tomography/computed tomography–maximum standard uptake value |
| 3D-CRT | 3-dimensional conformal radiation therapy |
| ADC | Apparent diffusion coefficient |
| AUC | Area under the curve |
| BT | Brachytherapy |
| ChT | Chemotherapy |
| CLI | Clinic |
| CLI + DWI | Clinic + Diffusion-weighted imaging |
| CLI + T1W | Clinic + contrast-enhanced T1-weighted |
| CLI + T2W | Clinic + T2-weighted |
| CRT | Chemoradiotherapy |
| CT | Computed tomography |
| DFS | Disease-free survival |
| DMFS | Distant metastasis-free survival |
| EBRT | External beam radiation therapy |
| EQD2 | 2 Gy equivalent dose |
| FIGO | The International Federation of Gynaecology and Obstetrics |
| Gd-DTPA | Gadopentetate glucosamine injection |
| Gy | Gray |
| HPV | Human papillomavirus |
| HR-CTV | High-risk clinical target volume |
| IBSI | Image Biomarker Standardization Initiative |
| IGABT | Guided adaptive brachytherapy |
| IMRT | Intensity modulated radiation therapy. |
| KPS | Karnofsky performance status |
| LACC | Locally advanced cervical cancer |
| LRRFS | Local-regional recurrence-free survival |
| MRI | Magnetic resonance imaging |
| OS | Overall survival |
| PACS | Picture Archiving and Communication System |
| ROC | Receiver Operating Characteristic |
| RT | Radiotherapy |
| VMAT | Volumetric modulated arc therapy |
References
- Bary, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Tabibi, T.; Barnes, J.M.; Shah, A.; Osazuwa-Peters, N.; Johnson, K.J.; Brown, D.S. Human papillomavirus vaccination and trends in cervical cancer incidence and mortality in the US. JAMA Pediatr. 2022, 176, 313–316. [Google Scholar] [CrossRef]
- Radin, D.; Burger, E.A.; Atun, R.; Barton, M.; Gospodarowicz, M.; Grover, S.; Hanna, T.P.; Jaffray, D.A.; Knaul, F.M.; Lievens, Y.; et al. Scaling up radiotherapy for cervical cancer in the era of human papillomavirus vaccination in low-income and middle-income countries: A model-based analysis of need and economic impact. Lancet Oncol. 2019, 20, 915–923. [Google Scholar] [CrossRef]
- McCormack, M.; Eminowicz, G.; Gallardo, D.; Diez, P.; Farrelly, L.; Kent, C.; Hudson, E.; Panades, M.; Mathew, T.; Anand, A.; et al. Induction chemotherapy followed by standard chemotherapy versus standard chemotherapy alone in patients with locally advanced cervical cancer (GCIG INTERLACE): An international, multicenter, randomized phase 3 trial. Lancet 2024, 404, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Mileshkin, L.R.; Moore, K.N.; Barnes, E.H.; Gebski, V.; Narayan, K.; King, M.T.; Bradshaw, N.; Lee, Y.C.; Diamante, K.; Fyles, A.W.; et al. Adjuvant chemotherapy following chemoradiotherapy as primary treatment for locally advanced cervical cancer versus chemoradiotherapy alone (OUTBACK): An international, open-label, randomized, phase 3 trial. Lancet Oncol. 2023, 24, 468–482. [Google Scholar] [CrossRef]
- Lorusso, D.; Xiang, Y.; Hasegawa, K.; Scambia, G.; Leiva, M.; Ramos-Elias, P.; Acevedo, A.; Sukhin, V.; Cloven, N.; Pereira de Santana Gomes, A.J.; et al. Pembrolizumab or placebo with chemoradiotherapy followed by pembrolizumab or placebo for newly diagnosed, high-risk, locally advanced cervical cancer (ENGOT-cx11/GGOG-3047/KEYNOTE-A18): A randomized, double-blind, phase 3 clinical trial. Lancet 2024, 403, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Wagar, M.K.; Hsu, H.C.; Hoegl, J.; Rey Valzacchi, G.M.; Fernandes, A.; Cucinella, G.; Sahin Aker, S.; Jayraj, A.S.; Mauro, J.; et al. Cervical cancer: A new era. Int. J. Gynecol. Cancer 2024, 34, 1946–1970. [Google Scholar] [CrossRef] [PubMed]
- Pötter, R.; Tanderup, K.; Schmid, M.P.; Jürgenliemk-Schulz, I.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): A multicenter prospective cohort study. Lancet Oncol. 2021, 22, 538–547. [Google Scholar] [CrossRef]
- Mahantshetty, U.; Krishnatry, R.; Hande, V.; Jamema, S.; Ghadi, Y.; Engineer, R.; Chopra, S.; Gurram, L.; Deshpande, D.; Shrviastava, S. Magnetic resonance image-guided adaptive brachytherapy in locally advanced cervical cancer; An experience from a tertiary cancer center in a low and middle-income countries setting. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 608–617. [Google Scholar] [CrossRef]
- Sturdza, A.; Pötter, R.; Fokdal, L.U.; Haie-Meder, C.; Tan, L.T.; Mazeron, R.; Petric, P.; Segedin, B.; Jurgenliemk-Schulz, I.M.; Nomden, C.; et al. Image-guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother. Oncol. 2016, 120, 428–433. [Google Scholar] [CrossRef]
- Mahantshetty, U.; Poetter, R.; Beriwal, S.; Grover, S.; Lavanya, G.; Rai, B.; Petric, P.; Tanderup, K.; Carvalho, H.; Hegazy, N.; et al. IBS-GEC ESTRO-ABS recommendations for CT-based contouring in image-guided adaptive brachytherapy for cervical cancer. Radiother. Oncol. 2021, 160, 273–284. [Google Scholar] [CrossRef]
- Sturdza, A.E.; Pötter, R.; Kossmeier, M.; Kirchheiner, K.; Mahantshetty, U.; Haie-Meder, C.; Lindegaard, J.C.; Jürgenliemk-Schulz, I.; Tan, L.T.; Hoskin, P.; et al. Nomogram predicting overall survival in patients with locally advanced cervical cancer treated with radiochemotherapy, including image-guided brachytherapy; A Retro-EMBRACE study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 168–177. [Google Scholar] [CrossRef]
- Lindegaard, J.C.; Petric, P.; Schmid, M.P.; Nesvacil, N.; Haie-Meder, C.; Fokdal, L.U.; Sturdza, A.E.; Hoskin, P.; Mahantshetty, U.; Segedin, B.; et al. Prognostic implications of uterine cervical cancer regression during chemoradiation evaluated by the T-score in the multicenter EMBRACE I study. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Bosse, T.; Horeweg, N.; Deodhar, H.; Menon, S.; Rafael, T.; Pai, V.; Rijstenberg, L.; van Kemenade, F.; Kannan, S.; et al. Biomarker expression and clinical outcomes in international study of chemoradiation and magnetic resonance imaging-based image-guided brachytherapy for locally advanced cervical cancer: BIOEMBRACE. Int. J. Radiat. Oncol. Biol. Phys. 2025, 121, 97–106. [Google Scholar] [CrossRef]
- Hatt, M.; Le Rest, C.C.; Tixier, F.; Badic, B.; Schick, U.; Visvikis, D. Radiomics: Data are also images. J. Nucl. Med. 2019, 60 (Suppl. 2), 38S–44S. [Google Scholar] [CrossRef]
- Wang, X.; Su, R.; Li, L.; Qin, Z.; Liu, L.; Zhang, Y. Machine learning-based radiomics for predicting outcomes in cervical cancer patients undergoing concurrent chemoradiotherapy. Comput. Biol. Med. 2024, 177, 108593. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hahm, M.H.; Bae, B.K.; Chong, G.O.; Jeong, S.Y.; Na, S.; Jeong, S.; Kim, J.C. Magnetic resonance imaging features of tumor and lymph node to predict clinical outcome in node positive cervical cancer: A retrospective analysis. Radiat. Oncol. 2020, 15, 86. [Google Scholar] [CrossRef]
- Takada, A.; Yokota, H.; Nemoto, M.W.; Horikoshi, T.; Matsushima, J.; Uno, T. A multi-scanner study of MRI radiomics in uterine cervical cancer: Prediction of in-field tumor control after definitive radiotherapy based on a machine learning method including peritumoral regions. Jpn. J. Radiol. 2020, 38, 265–273. [Google Scholar] [CrossRef]
- Lucia, F.; Visvikis, D.; Desseroit, M.C.; Miranda, O.; Malhaire, J.P.; Robin, P.; Pradier, O.; Hatt, M.; Schick, U. Prediction of outcome using pretreatment 18F-FDG PET/CT and MRI radiomics in locally advanced cervical cancer treated with chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 768–786. [Google Scholar] [CrossRef]
- Lucia, F.; Visvikis, D.; Vallieres, M.; Desseroit, M.C.; Miranda, O.; Robin, P.; Bonaffini, P.A.; Alfieri, J.; Masson, I.; Mervoyer, A.; et al. External validation of a combined PET and MRI radiomics model for prediction of recurrence in cervical cancer patients treated with chemoradiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Chanudom, I.; Tharavichitkul, E.; Laosiritaworn, W. Prediction of cervical cancer patients’ survival period with machine learning techniques. Healthc. Inform. Res. 2024, 30, 60–72. [Google Scholar] [CrossRef]
- Zhang, K.; Sun, K.; Zhang, C.; Ren, K.; Li, C.; Shen, L.; Jing, D. Using deep learning to predict survival outcome in non-surgical cervical cancer patients based on pathological images. J. Cancer Res. Clin. Oncol. 2023, 149, 6074–6083. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, J.; Wang, Y.; Qu, X.; Shi, Z.; Meng, Y.; Qiu, J.; Hua, K. Novel artificial intelligence machine learning approaches to precisely predict survival and site-specific recurrence in cervical cancer: A multi-institutional study. Transl. Oncol. 2021, 14, 101032. [Google Scholar] [CrossRef] [PubMed]
- Mudawi, N.; Alazeb, A. A model for predicting cervical cancer using machine learning algorithms. Sensors 2022, 22, 4132. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Lu, Y.; Shou, H.; Xu, H.; Shi, L.; Geng, X.; Song, T. A 5-year survival status prognosis of nonmetastatic cervical cancer patients through machine learning algorithms. Cancer Med. 2023, 12, 6867–6876. [Google Scholar] [CrossRef]
- Kolasseri, E.A.; B, V. Comparative study of machine learning and statistical survival models for enhancing cervical cancer prognosis and risk factor assessment using SEER data. Sci. Rep. 2024, 14, 22203. [Google Scholar] [CrossRef]
- Ding, D.; Lang, T.; Zou, D.; Tan, J.; Chen, J.; Zhou, L.; Wang, D.; Li, R.; Li, Y.; Liu, J.; et al. Machine learning-based prediction of survival prognosis in cervical cancer. BMC Bioinform. 2021, 22, 331. [Google Scholar] [CrossRef]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuze, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A freeware for radiomic feature calculation in multimodality imaging to accelerate advances in the characterization of tumor heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef]
- Zwanenburg, A.; Vallieres, M.; Abdalah, M.A.; Aerts, H.J.W.L.; Andrearczyk, V.; Apte, A.; Ashrafinia, S.; Bakas, S.; Beukinga, R.J.; Boellaard, R.; et al. The image biomarker standardization initiative: Standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 2020, 295, 328–338. [Google Scholar] [CrossRef]
- Unger, J.M.; Shulman, L.N.; Facktor, M.A.; Nelson, H.; Fleury, M.E. National estimates of the participation of patients with cancer in clinical research studies based on Commission on Cancer accreditation data. J. Clin. Oncol. 2024, 42, 2139–2148. [Google Scholar] [CrossRef]
- Zhang, L.; Janosik, D. Enhanced short-term load forecasting with hybrid machine learning models: CatBoost and XGBoost approaches. Expert Syst. Appl. 2024, 241, 122686. [Google Scholar] [CrossRef]
- Nayyem, M.N.; Sharif, K.S.; Raju, M.A.H.; Al Rakin, A.; Arafin, R.; Khan, M.M. Optimised ensemble learning for chronic kidney disease prognostication: A stratified cross-validation approach. In Proceedings of the 2024 IEEE International Conference on Computing (ICOCO), Kuala Lumpur, Malaysia, 12–14 December 2024; IEEE: Kuala Lumpur, Malaysia, 2024; pp. 553–558. [Google Scholar] [CrossRef]
- Acar, T.O. Comparing measurement reliability estimation techniques: Correlation coefficient vs. Bland–Altman Plot. Meas. Interdiscip. Res. Perspect. 2024, 22, 361–372. [Google Scholar] [CrossRef]
- Kumar, R.; Sherwani, Z.; Lopez, M.; Vergalasova, I.; Zhang, X.; Eckroate, B.; Hollingsworth, J.; Girda, E.; Hathout, L. Dispatities in brachytherapy utilization in cervical cancer in cervical cancer in the United States: A comprehensive literature review. Gynecol. Oncol. 2023, 179, 79–84. [Google Scholar] [CrossRef]
- O’Donnell, B.; Shiao, J.C.; Pezzi, T.A.; Waheed, N.; Sharma, S.; Bonnen, M.D.; Ludwig, M.S. Stereotactic body radiation therapy, intensity modulated radiation therapy, and brachytherapy boost modalities in invasive cervical cancer: A study of National Cancer Data Base. Int. J. Gynecol. Cancer 2018, 28, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Song, C.; Kim, I.A.; Kim, J.S.; Kim, Y.B.; Kim, K.; No, J.H.; Suh, D.H.; Chung, J.B.; Eom, K.Y. Stereotactic ablative body radiotherapy boost for cervical cancer when brachytherapy boost in not feasible. Radiat. Oncol. 2021, 16, 148. [Google Scholar] [CrossRef]
- Gultekin, M.; Yilmaz, M.T.; Sari, S.Y.; Yildiz, D.; Ozyigit, G.; Yildiz, F. Stereotactic body radiotherapy boost in patients with cervical cancer. J. Obstet. Gynaecol. 2022, 42, 3033–3040. [Google Scholar] [CrossRef]
- Turna, M.; Rzazade, R.; Küçükmorkoç, E.; Küçük, N.; Canoğlu, M.D.; Çağlar, H.B. Dose escalation with stereotactic body radiotherapy for cervical cancer treatment. BMC Cancer 2024, 24, 1281. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Nakajima, Y.; Ogawa, H.; Furusawa, A.; Murofushi, K.N.; Kito, S.; Kino, N.; Yasugi, T.; Uno, T.; Karasawa, K. Faz I/II study of stereotactic body radiotherapy boost in patients with cervical cancer ineligible for intracivitary brachytherapy. Jpn. J. Radiol. 2024, 42, 909–917. [Google Scholar] [CrossRef]
- Gazsi, I.; Marcu, L.G. A systematic review of SBRT boost for cervical cancer patients who cannot benefit from brachytherapy. Curr. Oncol. 2025, 32, 170. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.L.; de Miranda, A.V.S.S.; Arruda, G.V.; de Andrade, D.A.P.; Gomes, L.M.; Dos Santos, M.B.; de Medeiros, K.S.; de Melo, A.C. Stereotactic body radiotherapy as an alternative to brachytherapy in cervical cancer (SCORE): A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2025, 213, 104789. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, Y.P.; Xu, X.F.; Shi, Q. Prognostic nomograms for locally advanced cervical cancer based on the SEET database: Integrating Cox regression and competing risk analysis. Medicine 2024, 103, e40408. [Google Scholar] [CrossRef]
- Luo, W.X.; Ding, X.M.; Cheng, J.M.; Liu, X.; Zhou, H.Y. Nomogram based on MRI and clinical features to predict progression-free survival in patients with stage IIIC1r cervical cancer squamous cell carcinoma: A two-center study. Cin. Radiol. 2024, 79, e1031–e1039. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Cao, F.; Zhang, Y.; Dang, Y.; Wang, F. Nomograms for predicting prognostic value of combined neutrophil-to-lymphocyte ratio and Scc-Ag in locally advanced cervical cancer. Transl. Cancer Res. 2024, 13, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Xavier, S.P.; Galvo, N.D.; das Neves, M.A.B.; da Silva, K.M.; Almeida, A.Q.N.; da Silva, A.M.C. Nomogram model for predicting the long-term prognosis of cervical cancer patients: A population-based study in Mato Grosso, Brazil. BMC Cancer 2025, 14, 684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rong, L.; Jiang, H.; Mu, H.; Zhao, H. Construction and validation of nomograms for predicting overall survival and cause-specific survival in cervical cancer patients undergoing radical radiotherapy based on the SEER database. Front. Med. 2025, 12, 1587465. [Google Scholar] [CrossRef]
- Ibis, K.; Ilgin, C.; Suncak, L.; Akbas, C.K.; Bolukbas, D.; Denizli, M.; Azizy, A.; Yilmaz, B.; Ozben, S.G.; Celik, A.I.; et al. Prognostic significance of nomogram and T-score in locally advanced cervical cancer patients treated with curative chemoradiotherapy and image-guided brachytherapy: A single-center retrospective study. Diagnostics 2025, 15, 2142. [Google Scholar] [CrossRef]
- Lindegaatd, J.C.; Petric, P.; Lindegaard, A.M.; Tanderup, K.; Fokdal, L.U. Evaluation of a new prognostic tumor score in locally advanced cervical cancer integrating clinical examination and magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 754–763. [Google Scholar] [CrossRef]
- Lim, K.; Small, W., Jr.; Portelance, L.; Creutzberg, C.; Jurgenliemk-Schulz, I.M.; Mundt, A.; Mell, L.K.; Mayr, N.; Viswanathan, A.; Jhingran, A.; et al. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy for the definitive treatment of cervix cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 348–355. [Google Scholar] [CrossRef]
- Meng, J.; Liu, S.; Zhu, L.; Zhu, L.; Wang, H.; Xie, L.; Guan, Y.; He, J.; Yang, X.; Zhou, Z. Texture analysis as imaging biomarker for recurrence in advanced cervical cancer treated with CCRT. Sci. Rep. 2018, 8, 11399. [Google Scholar] [CrossRef]
- Sittiwong, W.; Dankulchai, P.; Wongsuwan, P.; Prasartseree, W.T.; Thornsri, N.; Tuntapakul, P. Pre-treatment and pre-brachytherapy MRI first-order radiomic features by a commercial software as survival predictors in radiotherapy for cervical cancer objectives. Clin. Transl. Radiat. Oncol. 2025, 53, 100965. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Chen, Y.; Wang, S.; Zhang, J.; An, J.; Xie, L.; Yu, X.; Zhao, X. MRI-based radiomics for pretreatment prediction of response to concurrent chemoradiotherapy in locally advanced cervical squamous cell cancer. Abdom. Radiol. 2023, 48, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, B.; Rojas-García, M.; Rodríguez-Esquivel, J.I.; Marquez-Acosta, J.; Aranda-Flores, C.E.; Cetina-Pérez, L.D.C.; Soto-López, S.; Estevez-García, J.A.; Bahena-Roman, M.; Madrid-Marina, V.; et al. Machine and deep learning for the diagnosis, prognosis, and treatment of cervical cancer: A scoping review. Diagnostics 2025, 15, 1543. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | Treatment Characteristics | ||
|---|---|---|---|
| Characteristics | Numbers (%) Median (Min.–Max.) | Characteristics | Numbers (%) Median (Min.–Max.) |
| Age (years) | n = 161 52 (29–84) | EBRT technique 3D-CRT IMRT/VMAT | n = 161 93 (57.8%) 68 (42.2%) |
| KPS score | n = 161 100 (70–100) | Total EBRT doses | n = 161 50 (45–52.5) |
| Pretreatment haemoglobin (g/dL) | n = 136 12.05 (7.2–15.2) | Total EBRT fractions | n = 161 25 (23–30) |
| Neutrophil/lymphocyte ratio | n = 130 3.06 (1.21–40.20) | Total brachytherapy dose | n = 161 24 (10–35) |
| Menopause status Postmenopause Premenopause Perimenopause | n = 161 89 (55.3%) 64 (39.8%) 8 (5%) | Brachytherapy fractions | n = 161 4 (2–6) |
| Pathology Squamous cell carcinoma Adenocarcinoma Serous papillary carcinoma | n = 161 148 (91.9%) 12 (7.4%) 1 (0.6%) | HR-CTV cc | n = 154 29.95 (4.33–74.76) |
| Tumor diameter | n = 160 4.7 2–12 | HR-CTV group ≤30 cc >30 cc Unknown | n = 161 77 (47.8%) 77 (47.8%) 7 (4.3%) |
| Tumor diameter ≤2 cm 2.1–4 cm >4 cm Unknown | n = 161 2 (1.2%) 48 (29.8%) 110 (68.3%) 1 (0.6%) | HR-CTV D98 EQD2α/β=10Gy | n = 153 73.9 59.50–97.80 |
| 18F-FDG-PET/CT SUVmax | n = 148 16 (6–57) | HR-CTV D90 EQD2α/β=10Gy | n = 161 81.7 (62.5–113.6) |
| Involved lymph node Yes No | n = 161 75 (46.6%) 86 (53.4%) | Right A point D90 EQD2α/β=10Gy | n = 153 66.3 (53.5–89.70) |
| Involved lymph node region Pelvic region Paraaortic region Pelvic + paraaortic region No | n = 161 64 (39.8%) 2 (1.2%) 9 (5.6%) 86 (53.45) | Left A point D90 EQD2α/β=10Gy | n = 153 67 (53.5–89.8) |
| EBRT response Complete response Residue Unknown | n = 161 71 (44%) 86 (53.4%) 4 (2.5%) | Total treatment time (days) | n = 161 82 (52–212) |
| FIGO2018 Staging IB2 IB3 IIA1 IIB IIIA IIIB IIIC1 IIIC2 IVA | n = 161 5 (3.1%) 1 (0.6%) 1 (0.6%) 65 (40.4%) 2 (1.2%) 11 (6.8%) 61 (37.9%) 11 (6.8%) 4 (2.5%) | Total treatment time group ≤80 days >80 days | n = 161 76 (47.2%) 85 (52.8%) |
| Concurrent chemotherapy drugs Cisplatin Carboplatin Low-dose paclitaxel-carboplatin No | n = 161 154 (95.7%) 1 (0.6%) 4 (2.5%) 2 (1.2%) | ||
| FIGO2018 Staging Group Stage I–II Stage III–IV | n = 161 72 (44.7%) 89 (55.3%) | Concurrent chemotherapy cycles | n = 161 4 (0–7) |
| Dataset(s) | Sample Size | Feature Size |
|---|---|---|
| CatBoost_CLI | 161 | 55 |
| CatBoost_CLI + T1W | 116 | 214 |
| CatBoost_CLI + T2W | 161 | 214 |
| CatBoost_CLI + DWI | 68 | 214 |
| (A) Model with Clinical Features (CatBoost_CLI) | ||
| No | Feature(s) | Score |
| 1 | Tumor_diameter_cm | 0.067731 |
| 2 | HR-CTV D90 EQD210Gy | 0.058711 |
| 3 | HR-CTV Volume | 0.043819 |
| 4 | Comorbid condition | 0.043310 |
| 5 | Number of concurrent chemotherapy cycles | 0.042640 |
| 6 | R–A point EQD210Gy | 0.041074 |
| 7 | HR-CTV D98 EQD210Gy | 0.040643 |
| 8 | Age | 0.039858 |
| 9 | L–A_point EQD210Gy | 0.037941 |
| 10 | Pre-treatment 18F-FDG-PET/CT-SUVmax | 0.036313 |
| (B) Clinical + T1W Radiomics Features (CatBoost_CLI + T1) | ||
| No | Feature(s) | Score |
| 1 | MORPHOLOGICAL_RadiusSphereNorm-MaxIntensityCoo | 0.036061 |
| 2 | MORPHOLOGICAL_Maximum3DDiameter(IBSI:L0JK) [mm] | 0.028205 |
| 3 | GLCM_AngularSecondMoment(IBSI:8ZQL) | 0.017962 |
| 4 | INTENSITY-BASED_IntensityVariance(IBSI:ECT3) | 0.017237 |
| 5 | GLRLM_GreyLevelVariance(IBSI:8CE5) | 0.015982 |
| 6 | GLCM_NormalisedInverseDifferenceMoment(IBSI:1QCO) | 0.015428 |
| 7 | GLSZM_SmallZoneHighGreyLevelEmphasis(IBSI:HW1V) | 0.014849 |
| 8 | Number of concurrent chemotherapy cycles | 0.014651 |
| 9 | MORPHOLOGICAL_RadiusSphereNorm-MaxIntensityCoo | 0.013918 |
| 10 | GLSZM_ZoneSizeNonUniformity(IBSI:4JP3) | 0.013632 |
| (C) Clinical + T2W Radiomic Features (CatBoost_CLI + T2) | ||
| No | Feature(s) | Score |
| 1 | INTENSITY-BASED_IntensityBasedQuartileCoeffici | 0.029127 |
| 2 | NGTDM_Contrast(IBSI:65HE) | 0.023912 |
| 3 | MORPHOLOGICAL_RadiusRoiNorm-MaxIntensityCoor | 0.018926 |
| 4 | GLRLM_LongRunsEmphasis(IBSI:W4KF) | 0.017441 |
| 5 | GLCM_Contrast(IBSI:ACUI) | 0.016675 |
| 6 | GLRLM_RunEntropy(IBSI:HJ90) | 0.015700 |
| 7 | INTENSITY-BASED_IntensityBasedEnergy(IBSI:N8CA) | 0.015531 |
| 8 | Age | 0.015137 |
| 9 | INTENSITY-HISTOGRAM_IntensityHistogramSkewness | 0.013715 |
| 10 | INTENSITY-BASED-RIM_RIM-IntensityMean(IBSI:No) | 0.013389 |
| (D) Clinical + DWI Radiomics Features (CatBoost_CLI + DWI) | ||
| No | Feature(s) | Score |
| 1 | Number of concurrent chemotherapy cycles | 0.026246 |
| 2 | MORPHOLOGICAL_Maximum3DDiameter(IBSI:L0JK) [mm] | 0.024132 |
| 3 | GLRLM_LongRunHighGreyLevelEmphasis(IBSI:3KUM) | 0.022260 |
| 4 | GLSZM_GreyLevelVariance(IBSI:BYLV) | 0.021811 |
| 5 | MORPHOLOGICAL_Compacity(IBSI:No) | 0.020744 |
| 6 | GLCM_Autocorrelation(IBSI:QWB0) | 0.019438 |
| 7 | GLSZM_ZoneSizeNonUniformity(IBSI:4JP3) | 0.019039 |
| 8 | INTENSITY-BASED-RIM_RIM-CountingVoxels(IBSI:No) | 0.018038 |
| 9 | MORPHOLOGICAL_MaxIntensityCoor-PerimeterCoor-2 | 0.017279 |
| 10 | INTENSITY-BASED_IntensityKurtosis(IBSI:IPH6) | 0.016723 |
| (A) Model Performance Obtained from Clinical Data (Model 1: CatBoost_CLI) | ||||
| Model(s) | Test_Accuracy (%) | Precision (%) | Recall (%) | F1_Score (%) |
| CatBoost_CLI_DFS | 71.88 | 74.00 | 71.88 | 72.89 |
| CatBoost_CLI_DMFS | 71.88 | 74.54 | 71.88 | 73.18 |
| CatBoost_CLI_LRRFS | 84.38 | 76.21 | 84.38 | 80.08 |
| CatBoost_CLI_OS | 78.12 | 65.52 | 78.12 | 71.27 |
| (B) Clinical + T1W Radiomic Features with Model Performance (Model 2: CatBoost_CLI + T1W) | ||||
| Model(s) | Test_Accuracy (%) | Precision (%) | Recall (%) | F1_Score (%) |
| CatBoost_CLI + T1_DFS | 86.96 | 75.61 | 86.96 | 80.89 |
| CatBoost_CLI + T1_DMFS | 91.30 | 83.36 | 91.30 | 87.15 |
| CatBoost_CLI + T1_LRRFS | 86.96 | 75.61 | 86.96 | 80.89 |
| CatBoost_CLI + T1_OS | 69.57 | 59.63 | 69.57 | 64.21 |
| (C) Clinical + T2W Radiomic Features with Model Performance (Model 3: CatBoost_CLI + T2W) | ||||
| Model(s) | Test_Accuracy (%) | Precision (%) | Recall (%) | F1_Score (%) |
| CatBoost_CLI + T2_DFS | 84.62 | 71.60 | 84.62 | 77.56 |
| CatBoost_CLI + T2_DMFS | 92.31 | 85.21 | 92.30 | 88.62 |
| CatBoost_CLI + T2_LRRFS | 84.62 | 71.60 | 84.62 | 77.56 |
| CatBoost_CLI + T2_OS | 76.92 | 70.51 | 76.92 | 73.58 |
| (D) Model Performance with Clinical + DWI Radiomic Features (Model 4: CatBoost_CLI + DWI) | ||||
| Model(s) | Test_Accuracy (%) | Precision (%) | Recall (%) | F1_Score (%) |
| CatBoost_CLI + DIFF_DFS | 84.38 | 71.19 | 84.38 | 77.22 |
| CatBoost_CLI + DIFF_DMFS | 87.50 | 76.56 | 87.50 | 81.67 |
| CatBoost_CLI + DIFF_LRRFS | 82.41 | 73.12 | 82.25 | 77.41 |
| CatBoost_CLI + DIFF_OS | 75.00 | 60.48 | 75.00 | 66.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibis, K.; Durmaz, M.; Yanik, D.; Bunul, I.; Denizli, M.; Akyuz, E.; Khishigsuren, B.; Celik, A.I.; Kartal, M.G.D.; Kucucuk, N.S.; et al. Machine Learning-Based Prognostic Modelling Using MRI Radiomic Data in Cervical Cancer Treated with Definitive Chemoradiotherapy and Brachytherapy. Curr. Oncol. 2025, 32, 602. https://doi.org/10.3390/curroncol32110602
Ibis K, Durmaz M, Yanik D, Bunul I, Denizli M, Akyuz E, Khishigsuren B, Celik AI, Kartal MGD, Kucucuk NS, et al. Machine Learning-Based Prognostic Modelling Using MRI Radiomic Data in Cervical Cancer Treated with Definitive Chemoradiotherapy and Brachytherapy. Current Oncology. 2025; 32(11):602. https://doi.org/10.3390/curroncol32110602
Chicago/Turabian StyleIbis, Kamuran, Mustafa Durmaz, Deniz Yanik, Irem Bunul, Mustafa Denizli, Erkin Akyuz, Bayarmaa Khishigsuren, Ayca Iribas Celik, Merve Gulbiz Dagoglu Kartal, Nezihe Seden Kucucuk, and et al. 2025. "Machine Learning-Based Prognostic Modelling Using MRI Radiomic Data in Cervical Cancer Treated with Definitive Chemoradiotherapy and Brachytherapy" Current Oncology 32, no. 11: 602. https://doi.org/10.3390/curroncol32110602
APA StyleIbis, K., Durmaz, M., Yanik, D., Bunul, I., Denizli, M., Akyuz, E., Khishigsuren, B., Celik, A. I., Kartal, M. G. D., Kucucuk, N. S., Yirgin, I. K., & Emec, M. (2025). Machine Learning-Based Prognostic Modelling Using MRI Radiomic Data in Cervical Cancer Treated with Definitive Chemoradiotherapy and Brachytherapy. Current Oncology, 32(11), 602. https://doi.org/10.3390/curroncol32110602





