Coral and Coral-Associated Microorganisms: A Prolific Source of Potential Bioactive Natural Products
Abstract
1. Introduction
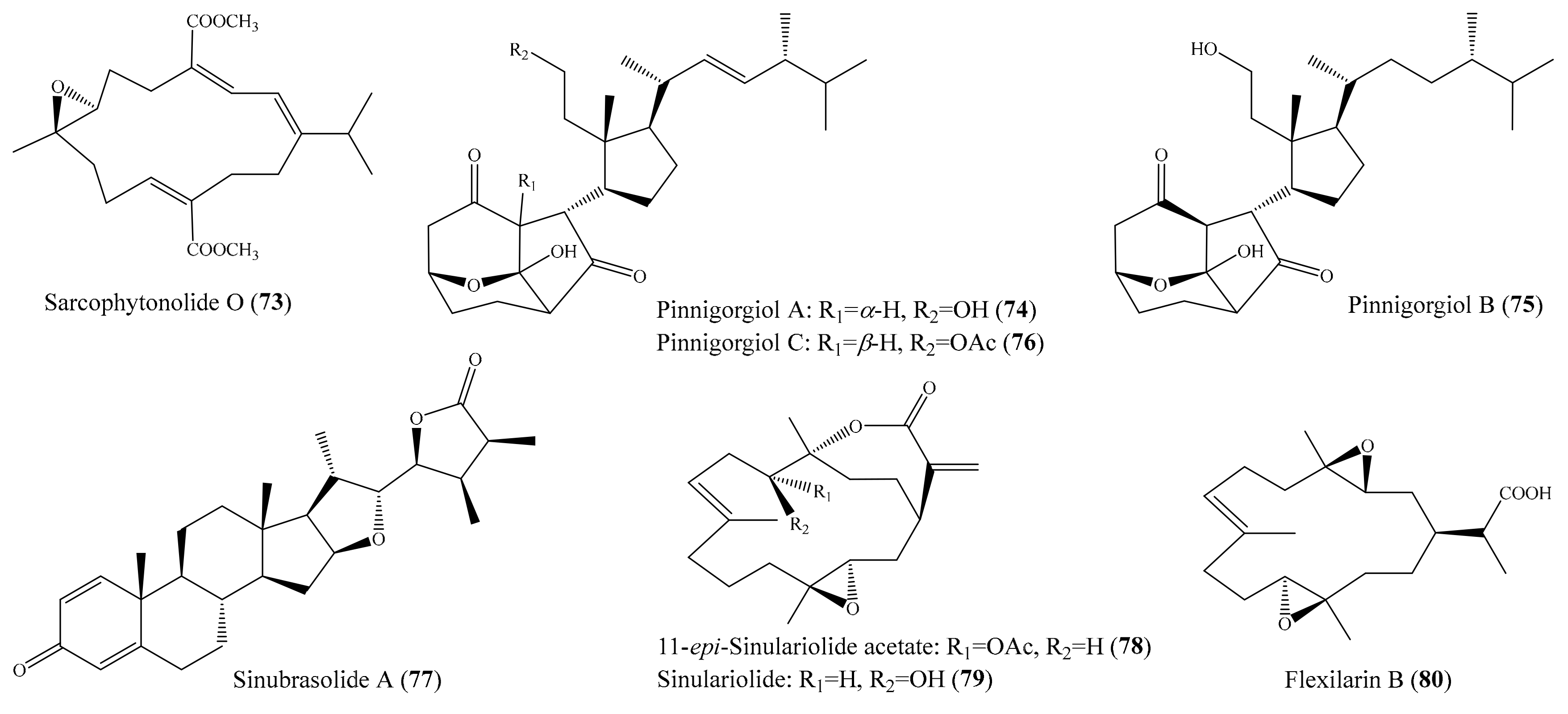
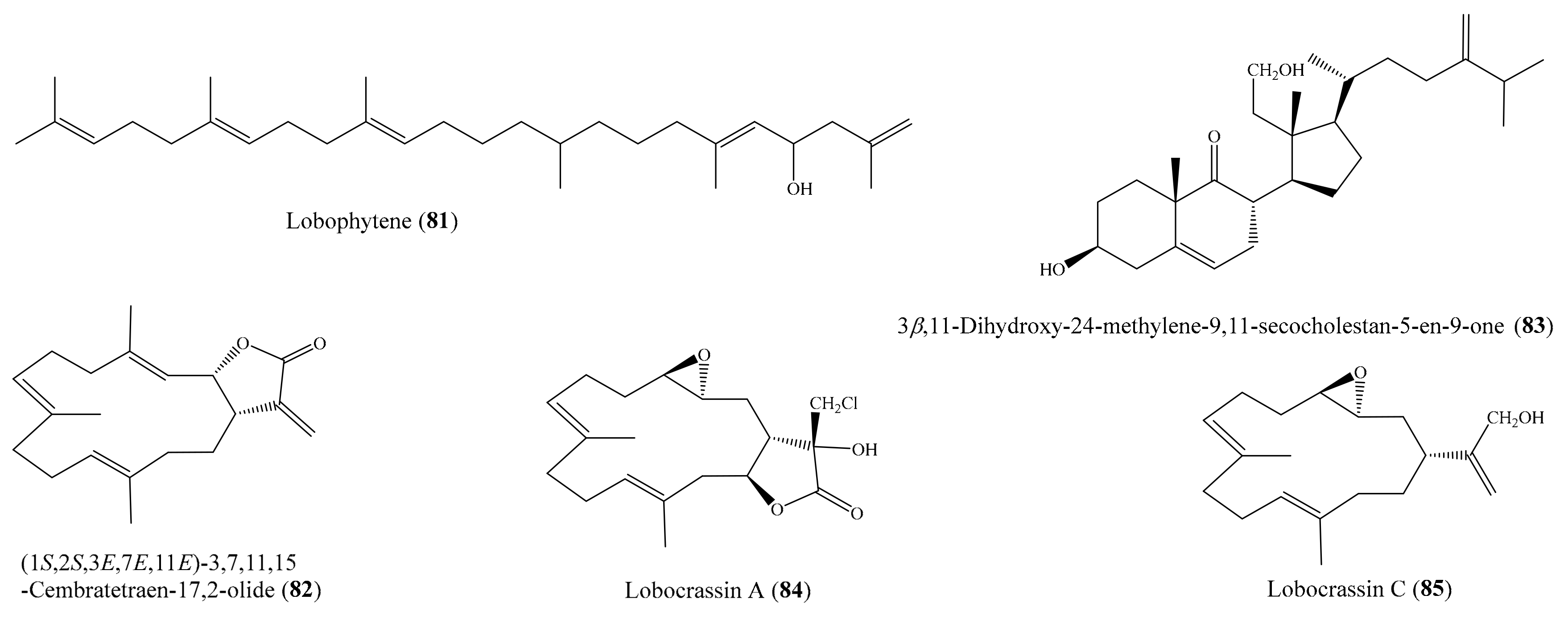
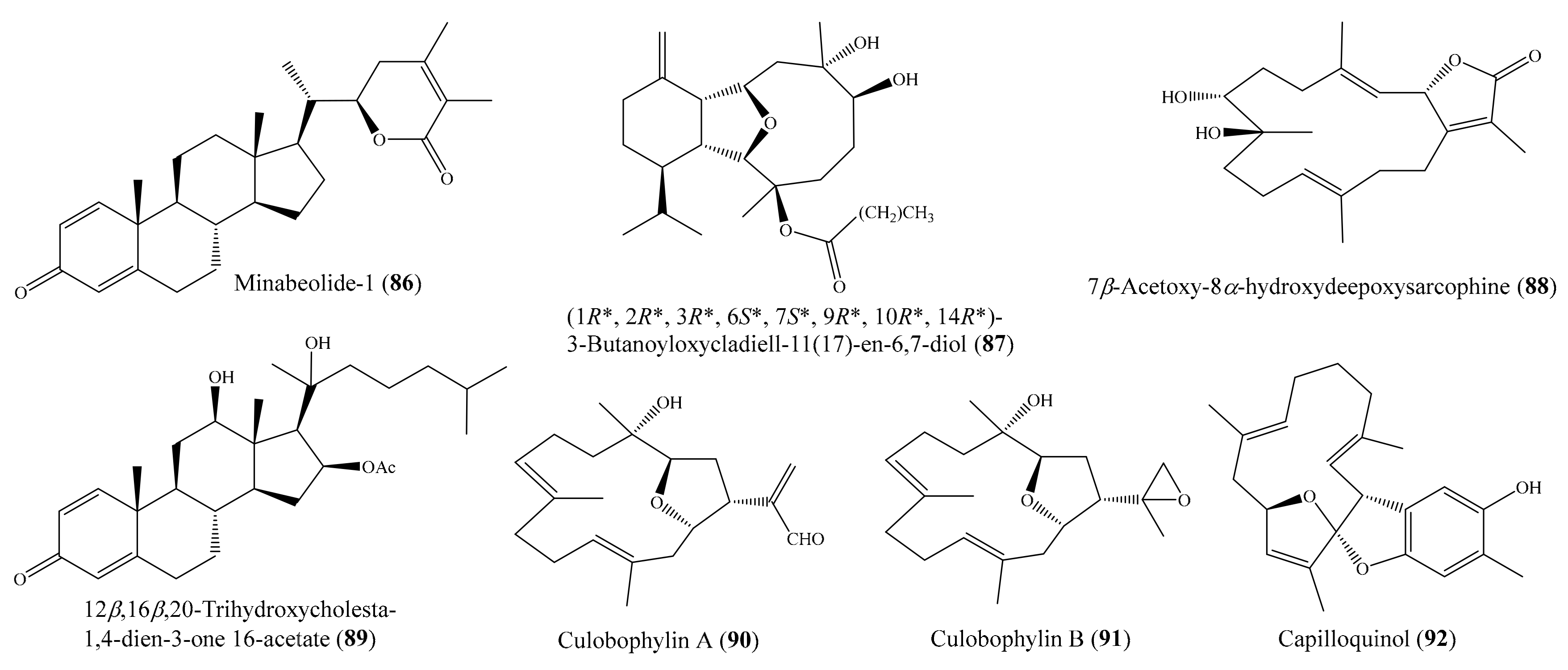
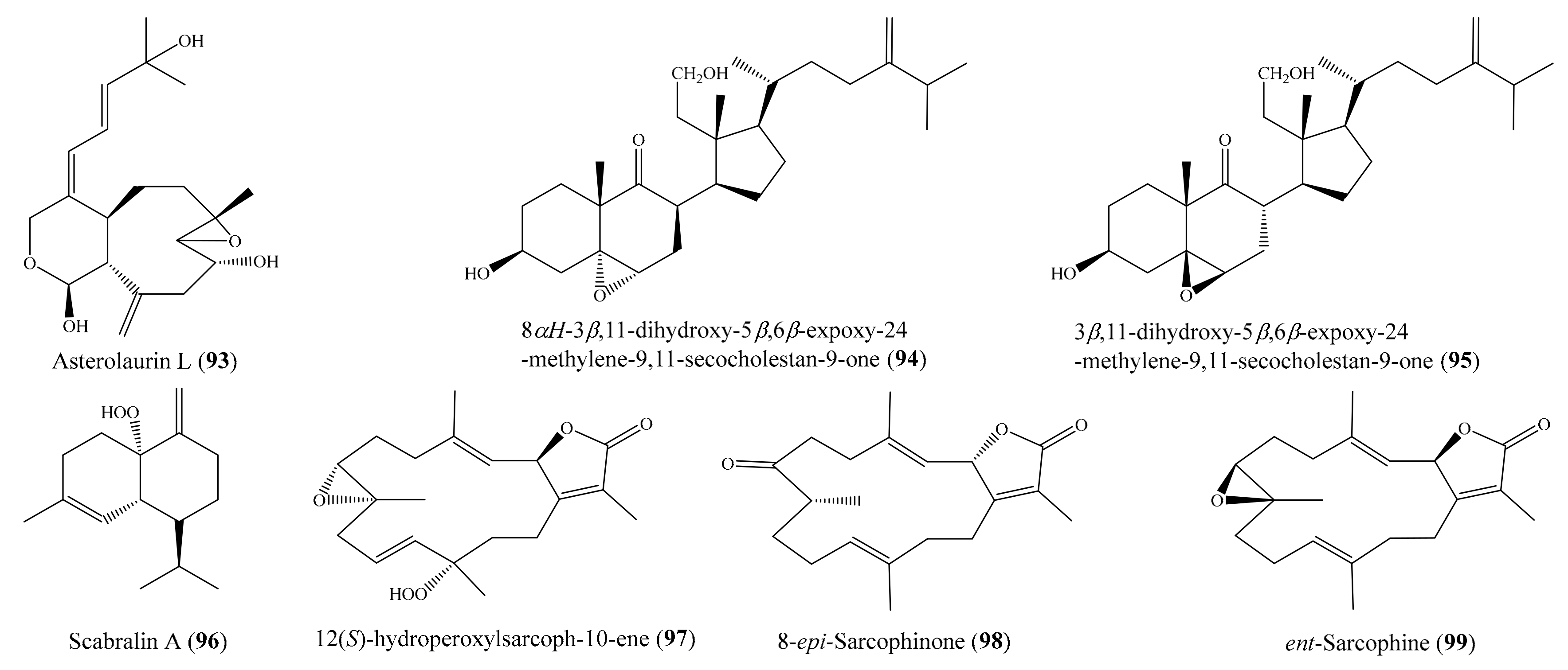
2. Bioactive Compounds from Coral
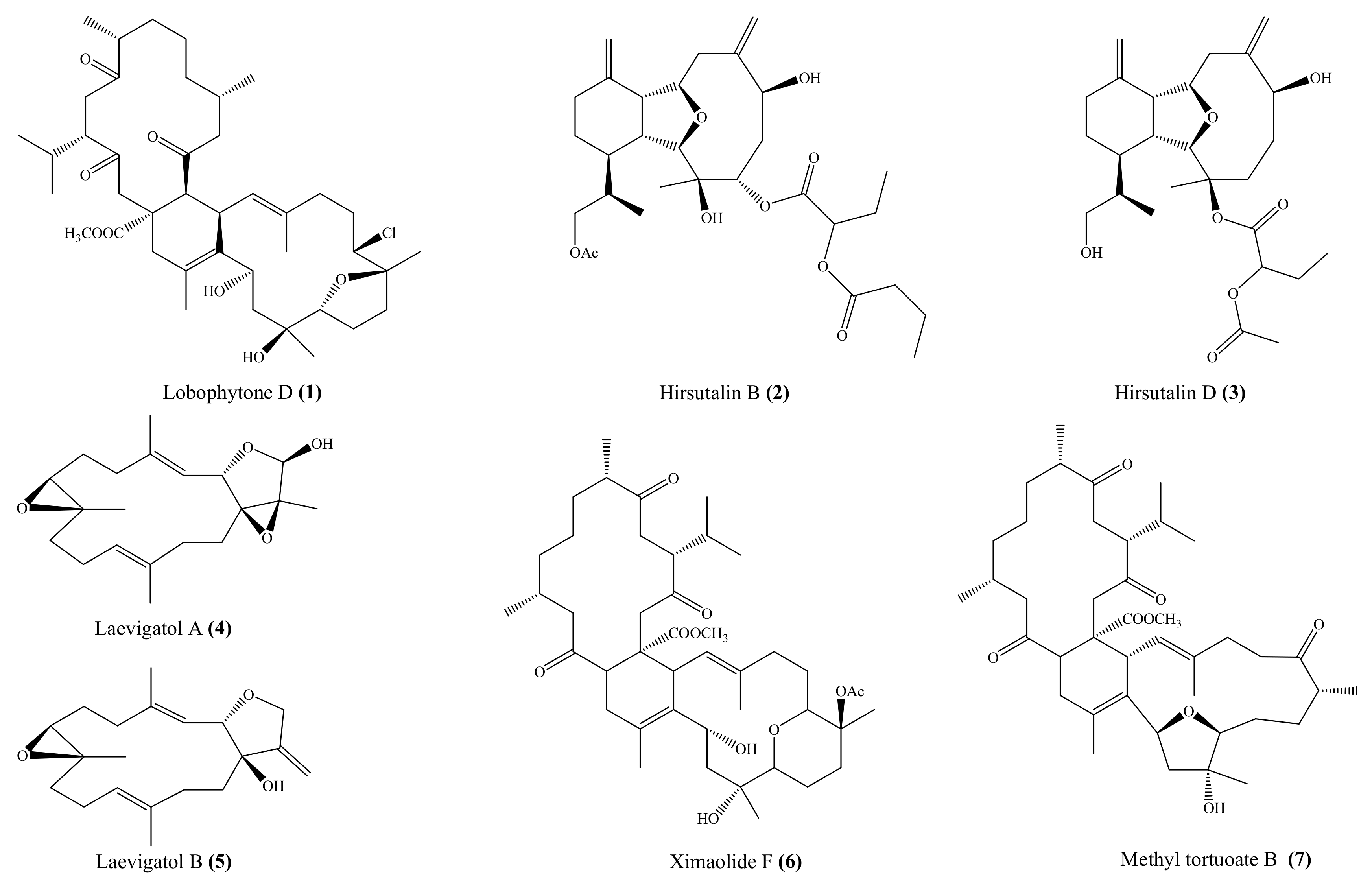
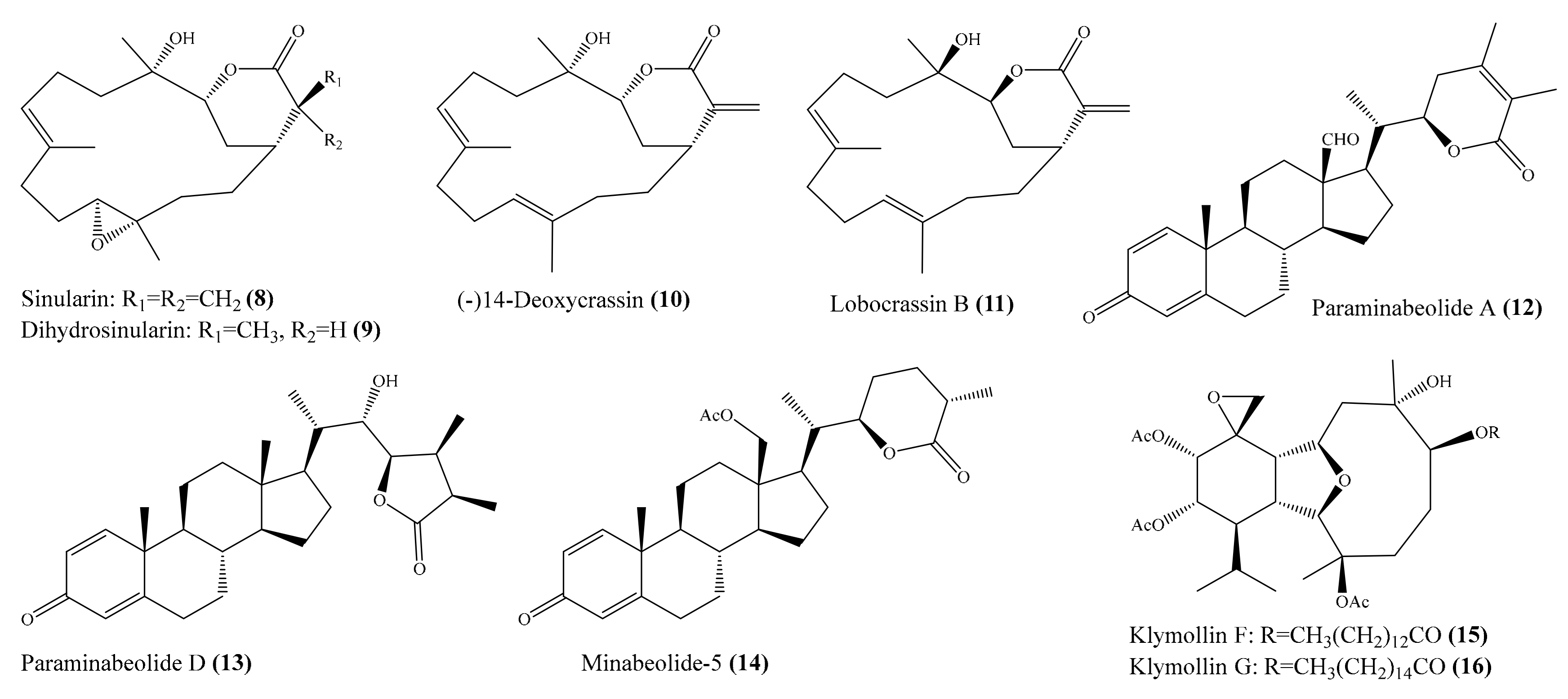
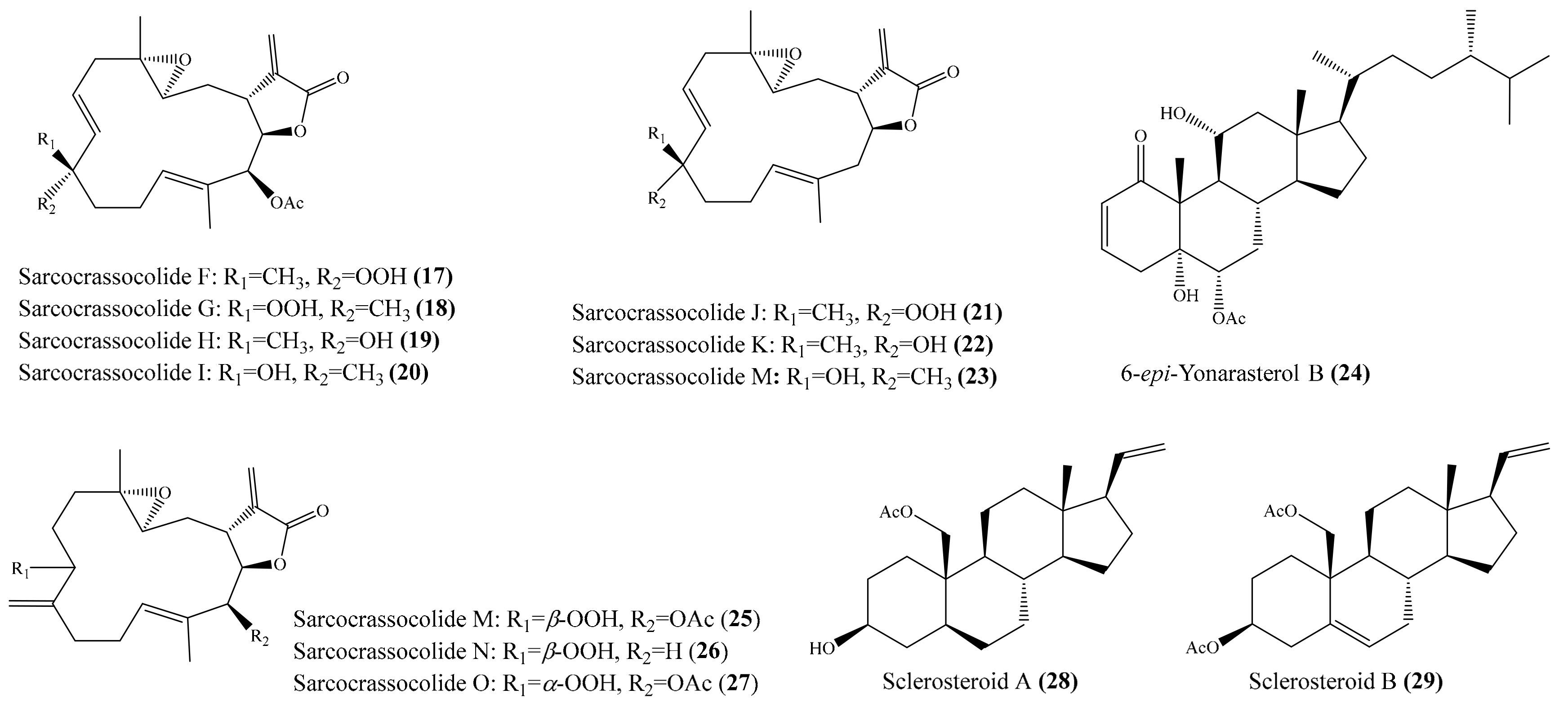
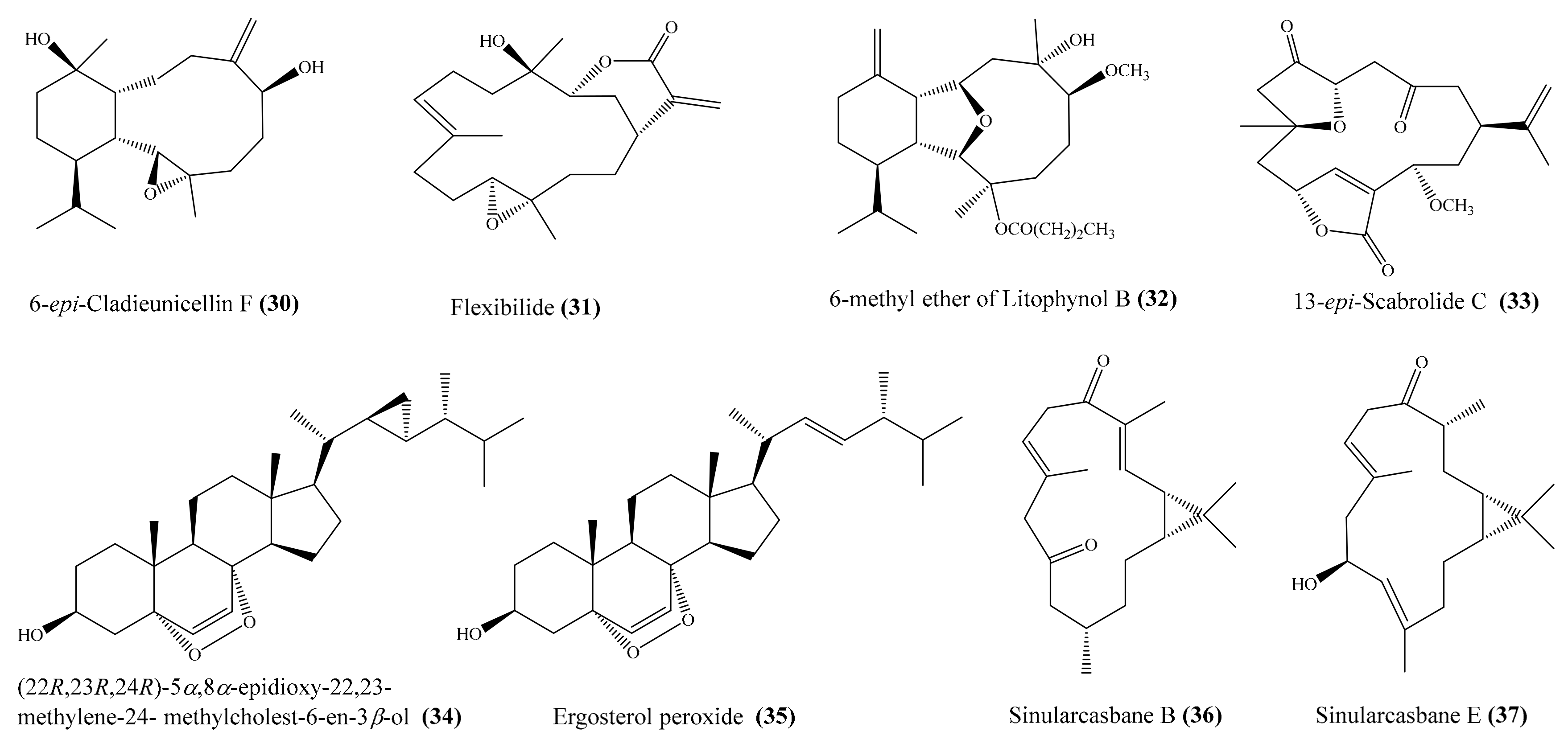
2.1. Anti-Inflammatory Compounds from Coral
2.2. Cytotoxic Compounds from Coral
2.3. Antimicrobial Compounds from Coral
2.4. Antivirus Compounds from Coral
2.5. Antifouling Compounds from Coral
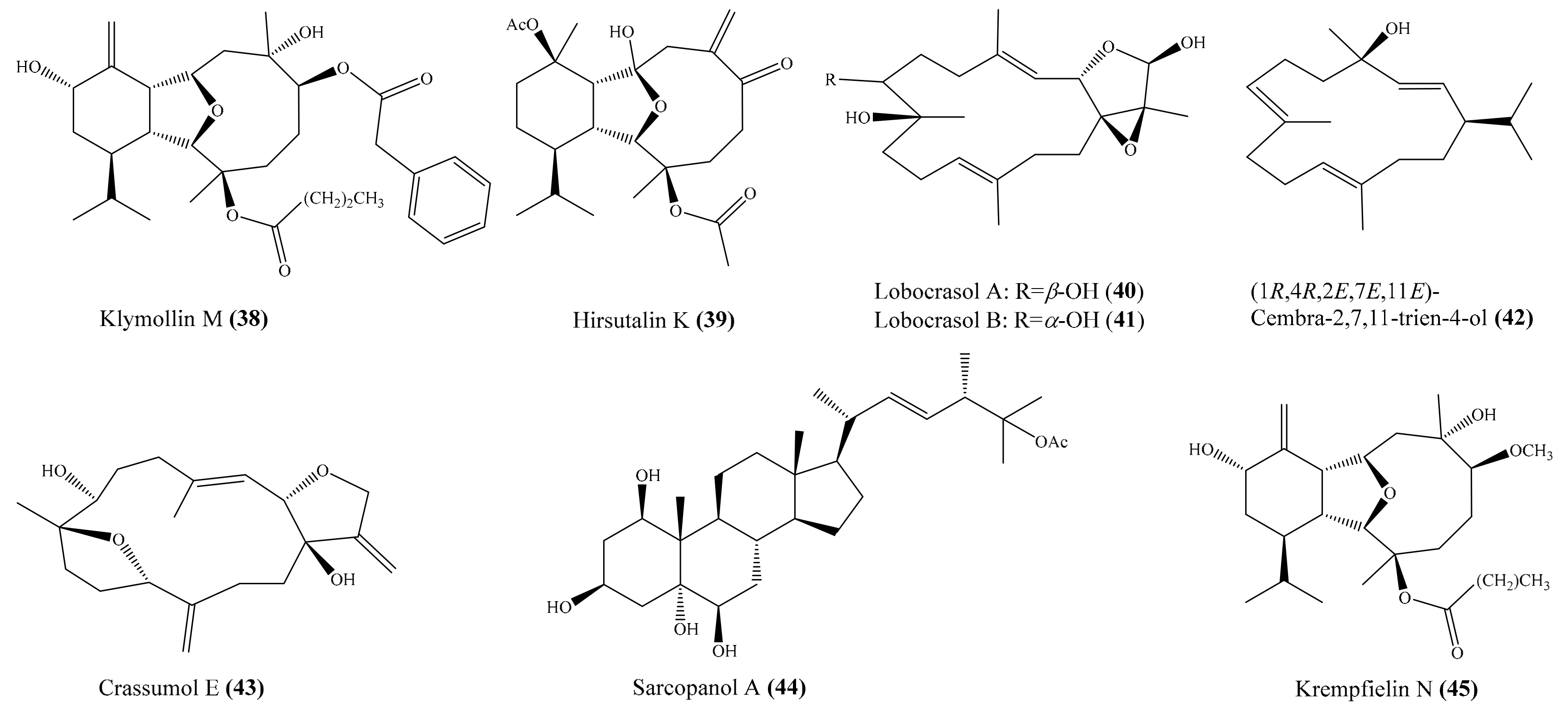
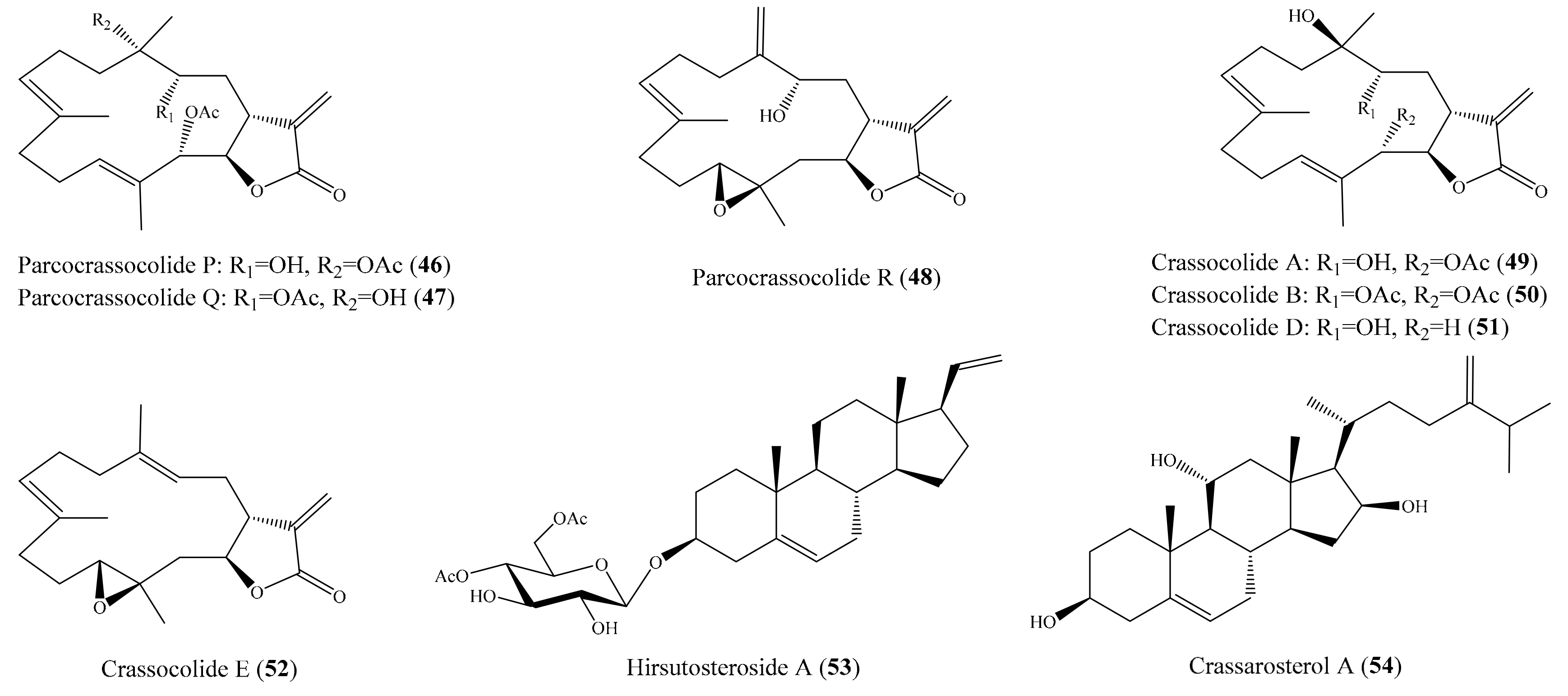
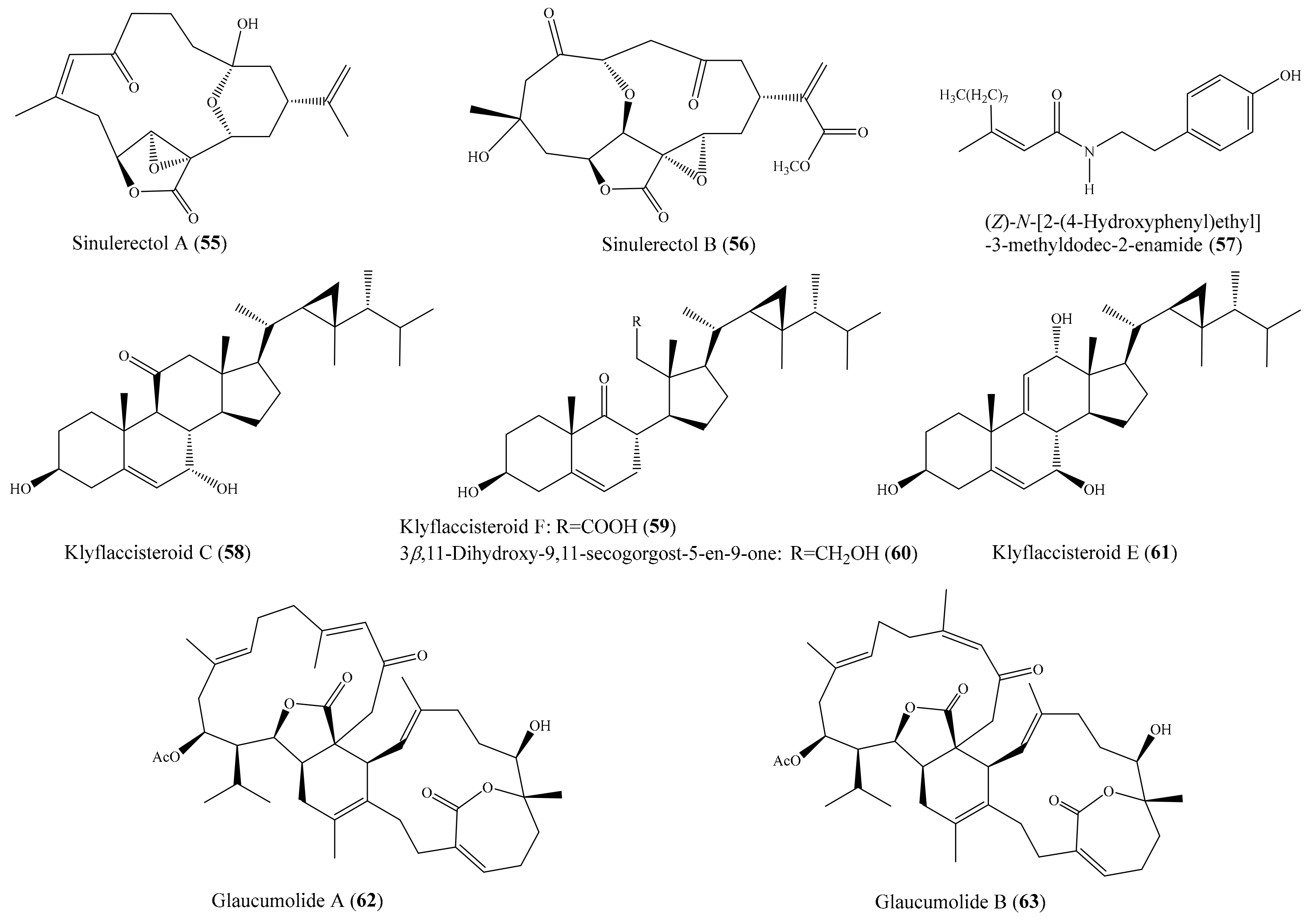
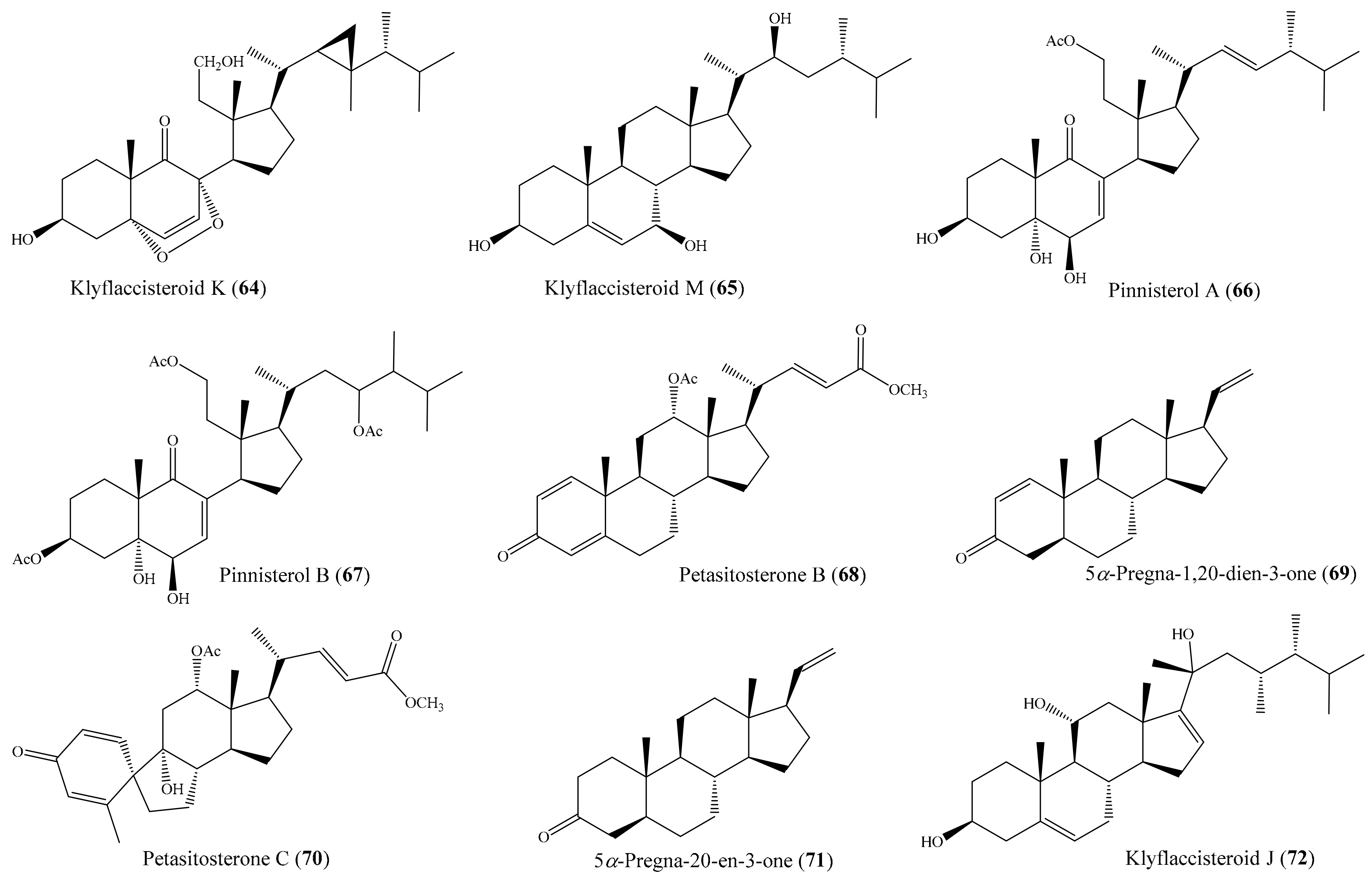
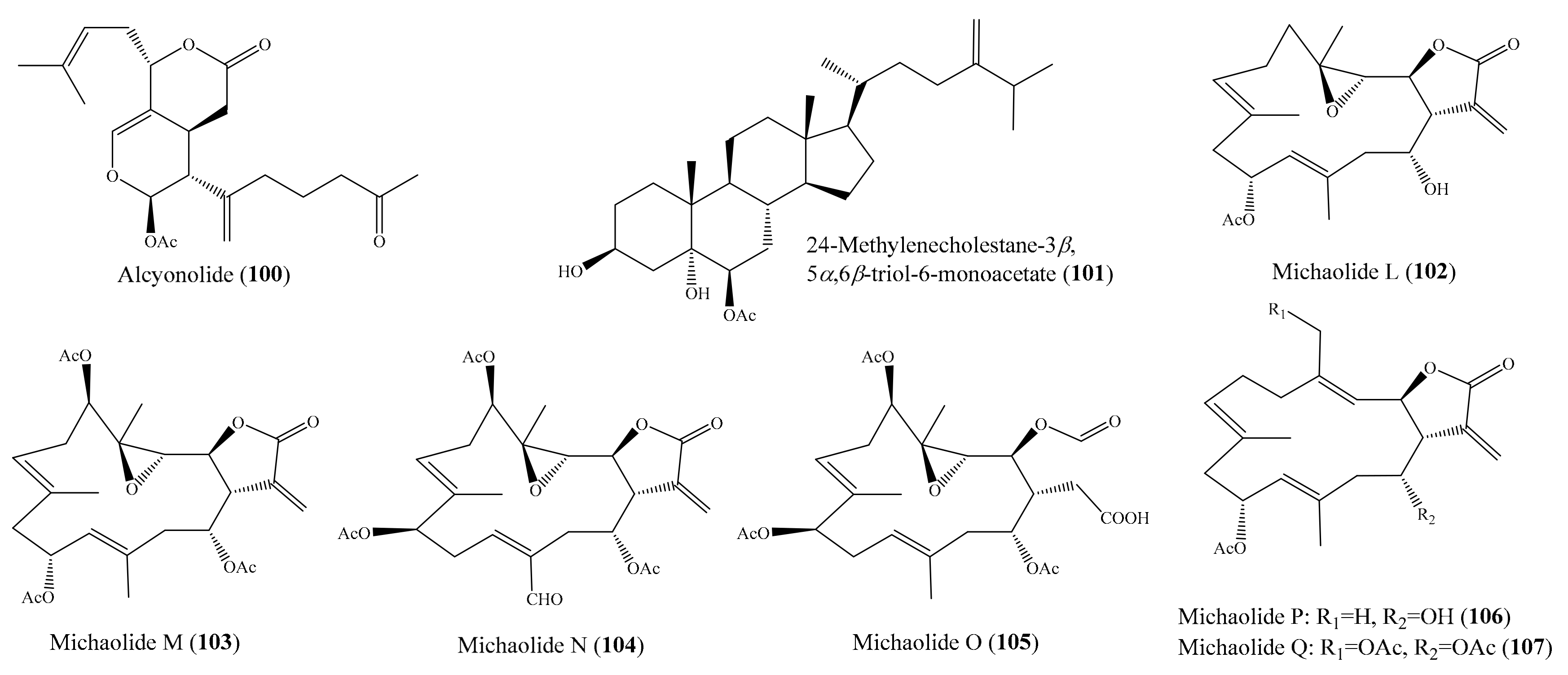
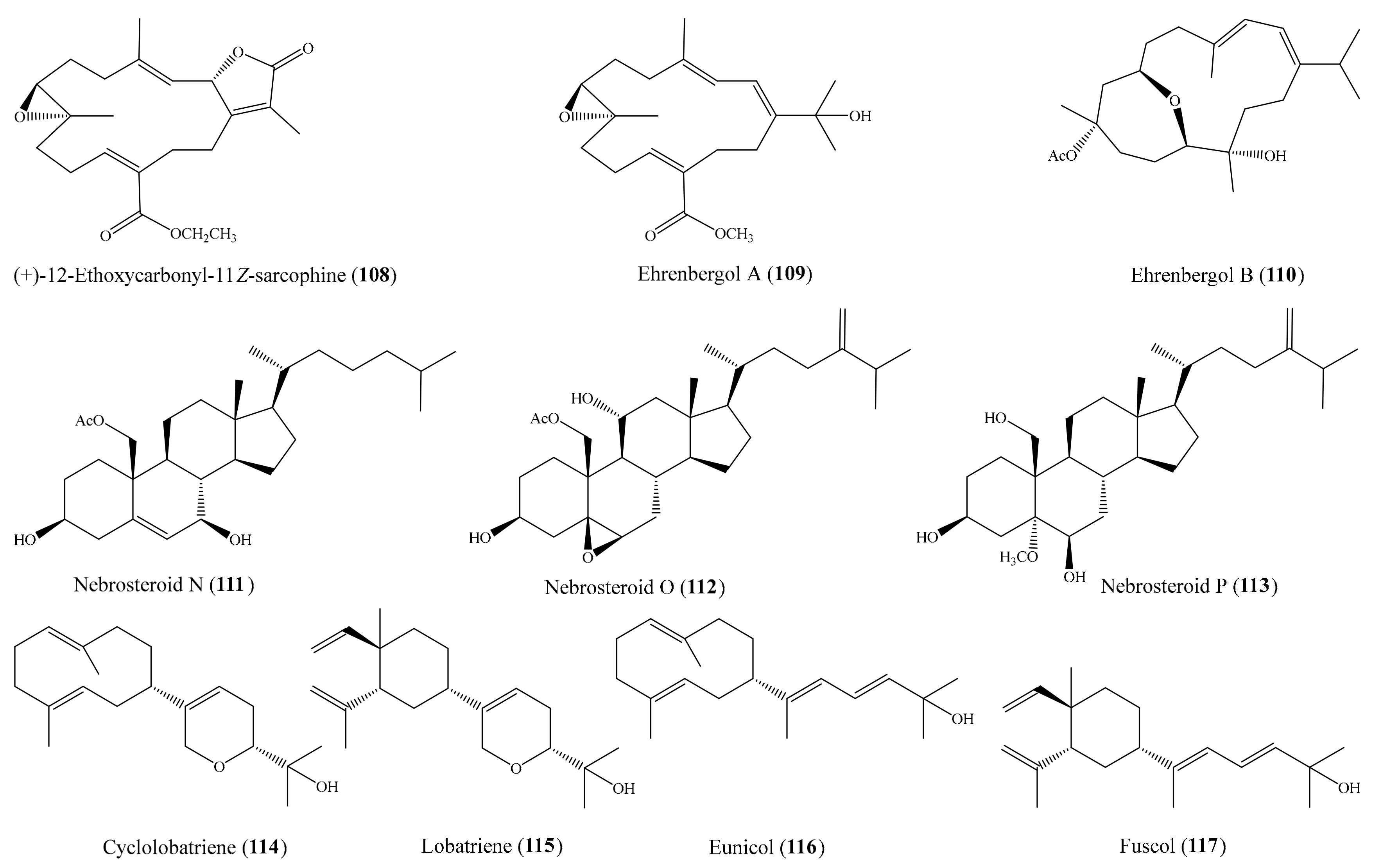
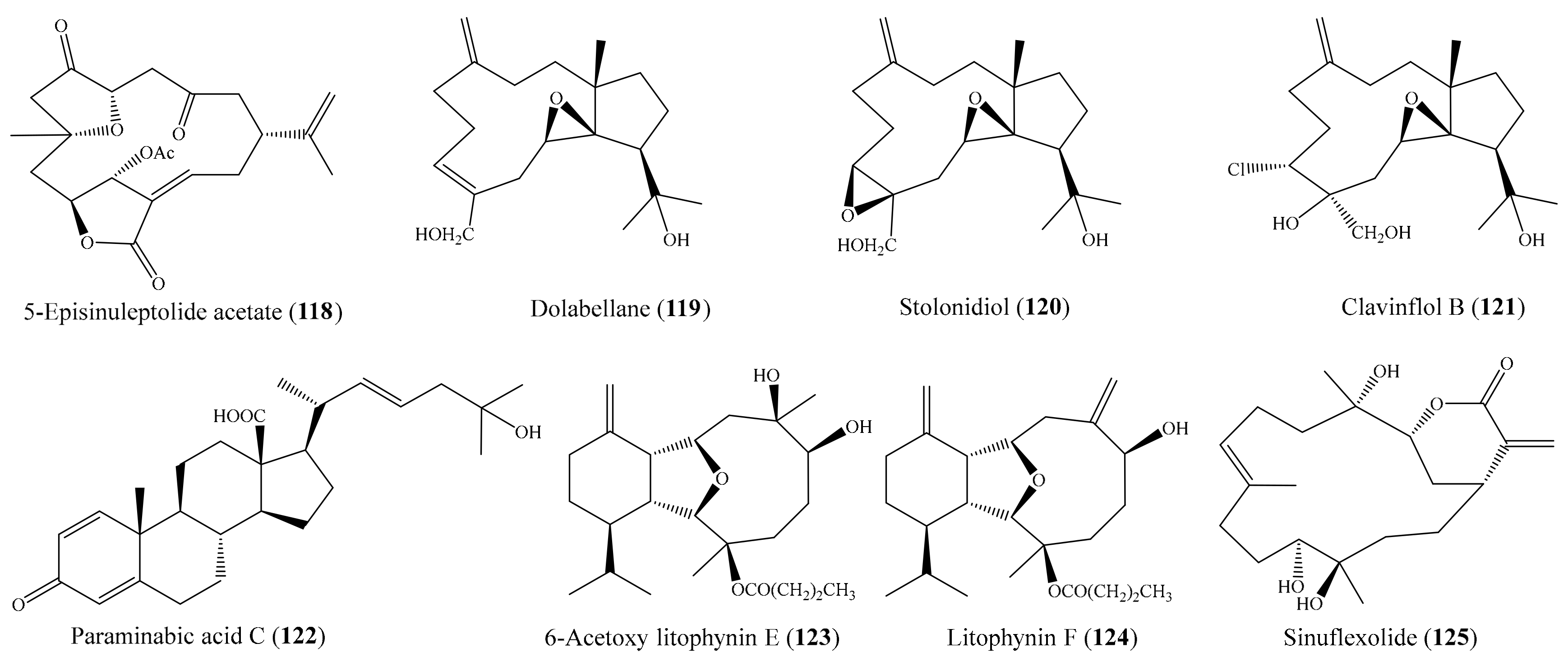
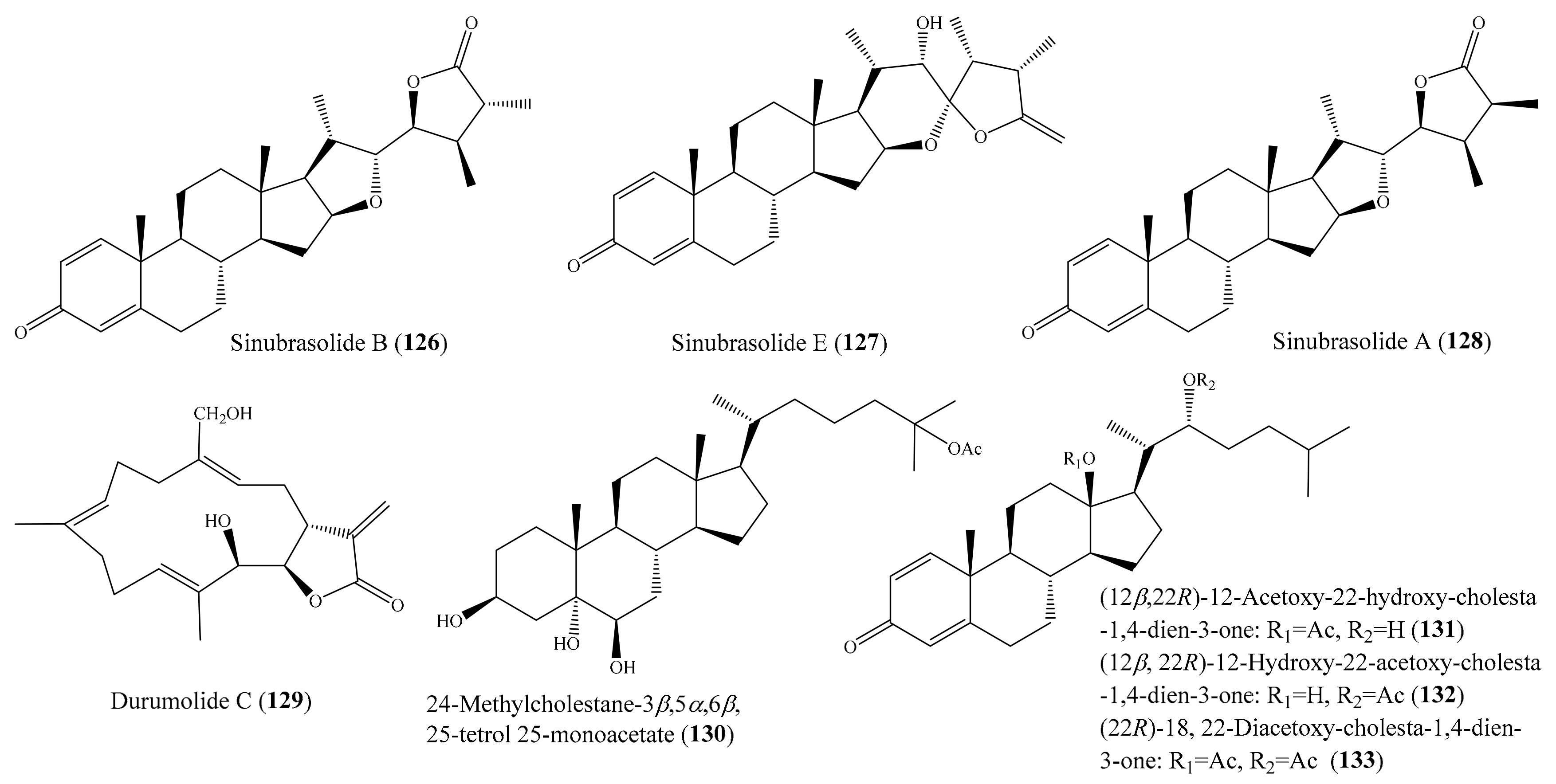
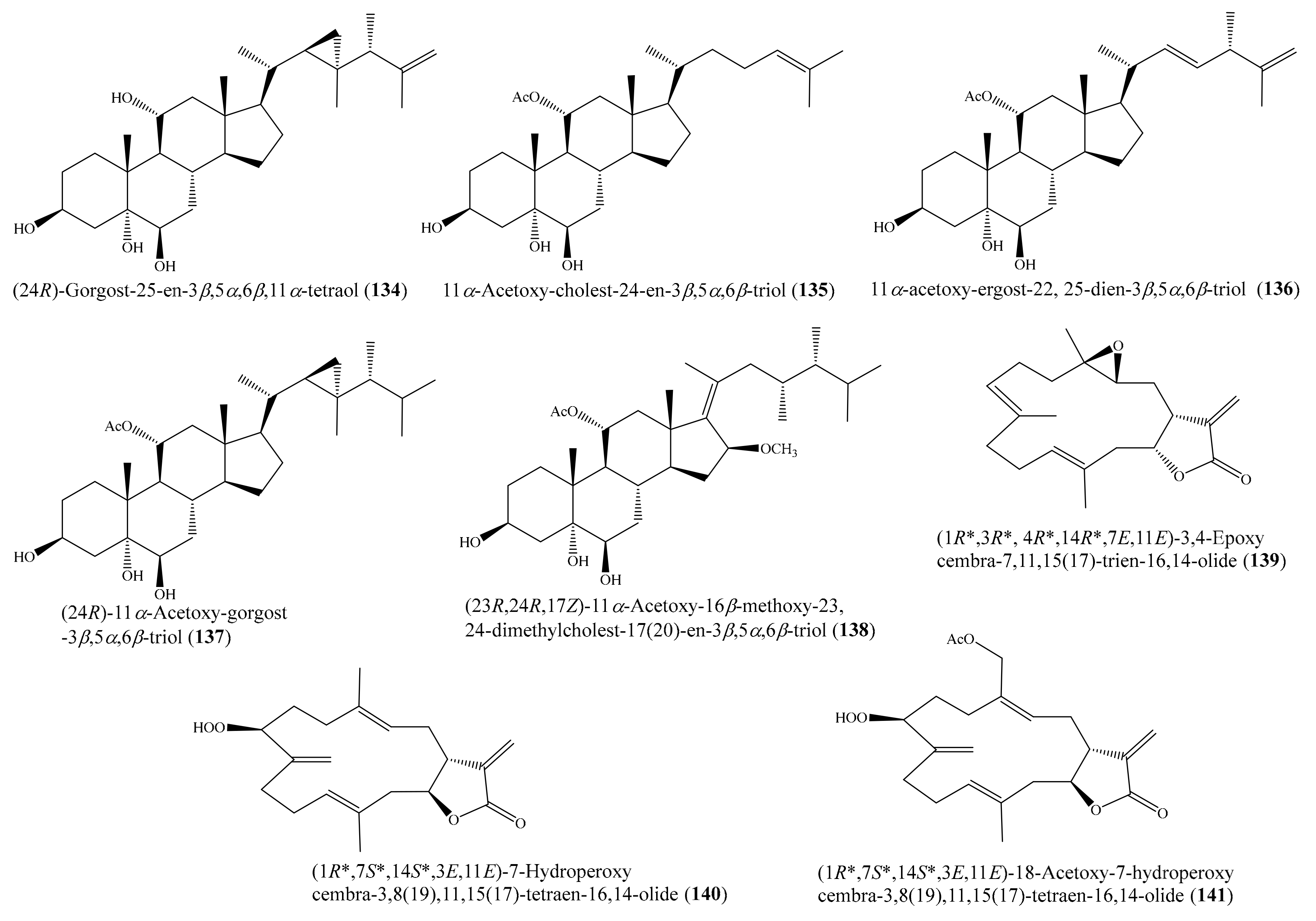
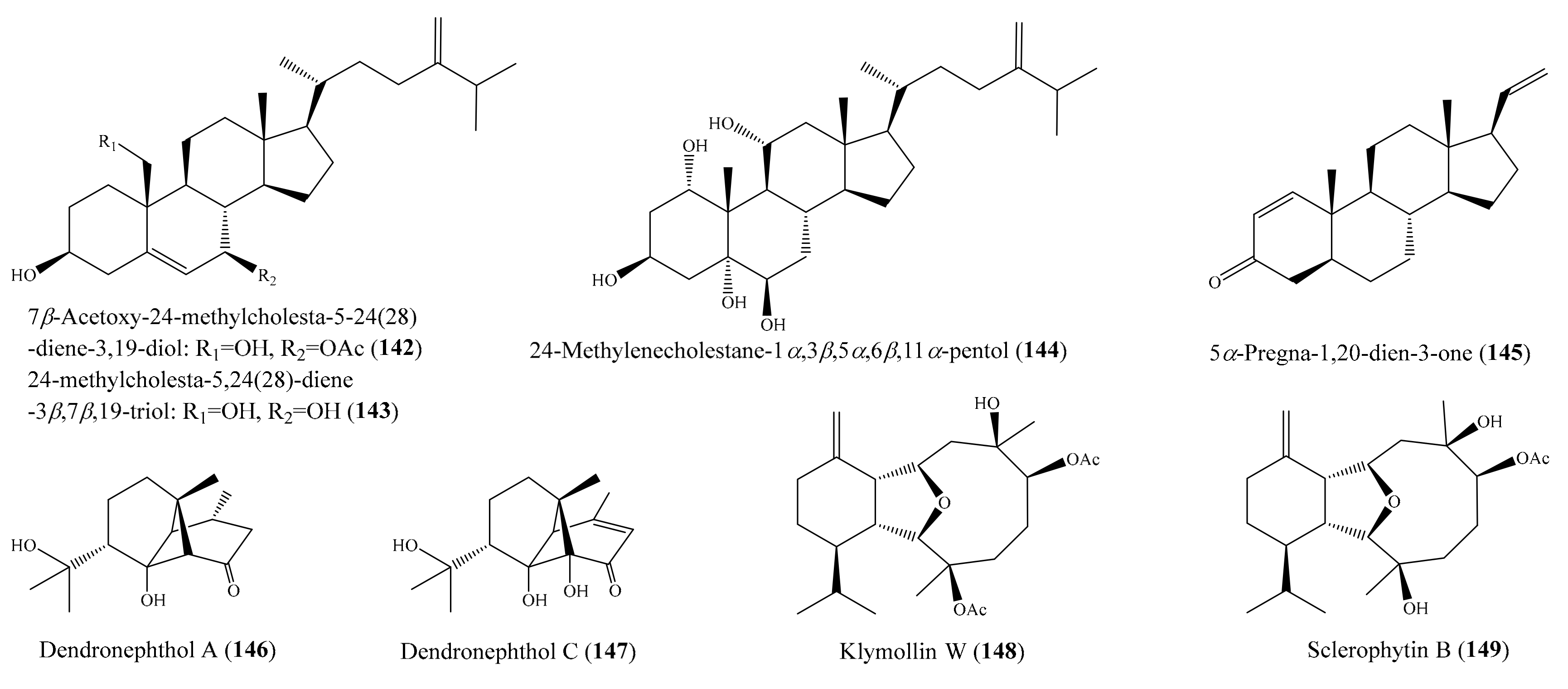
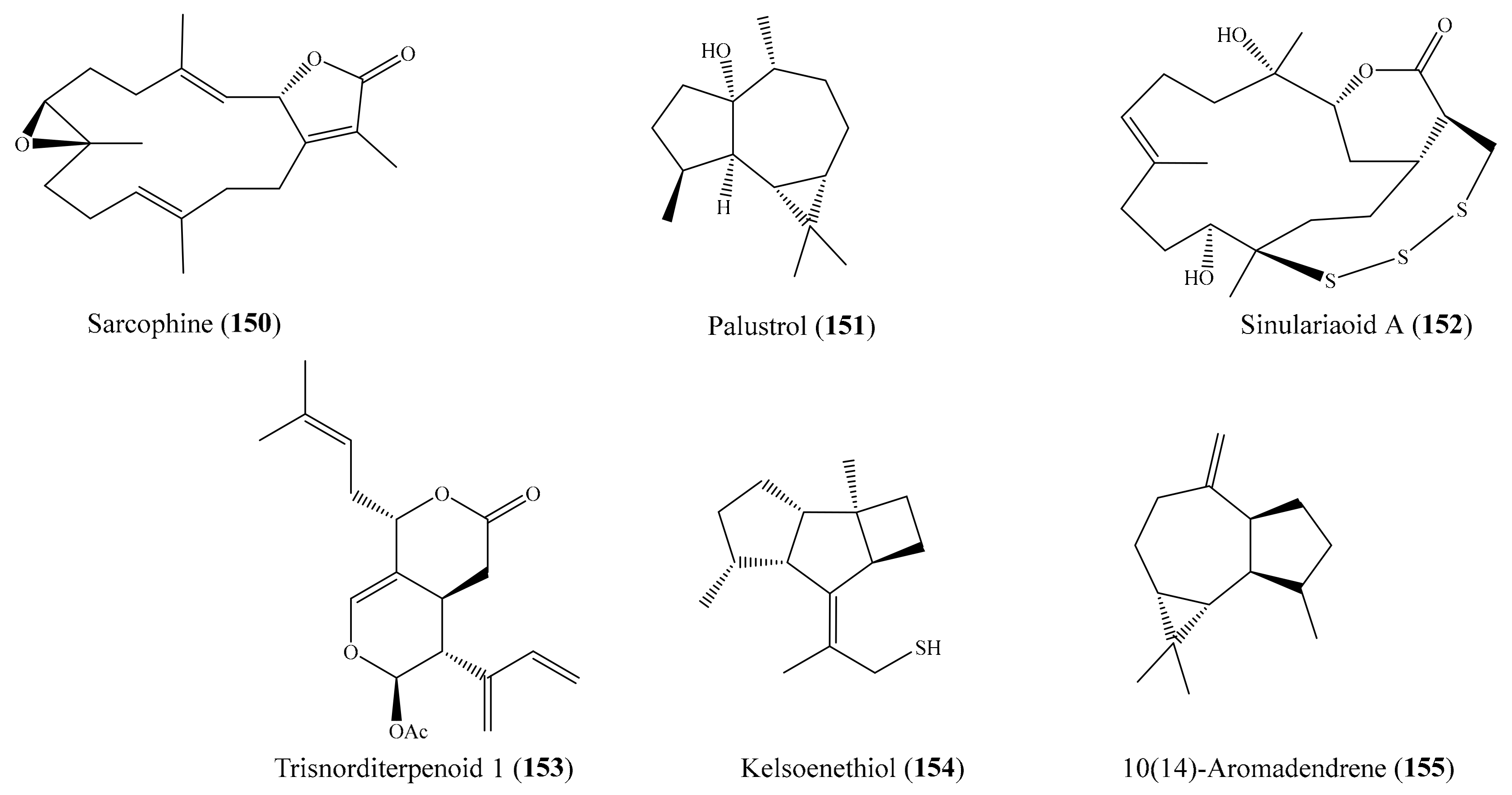
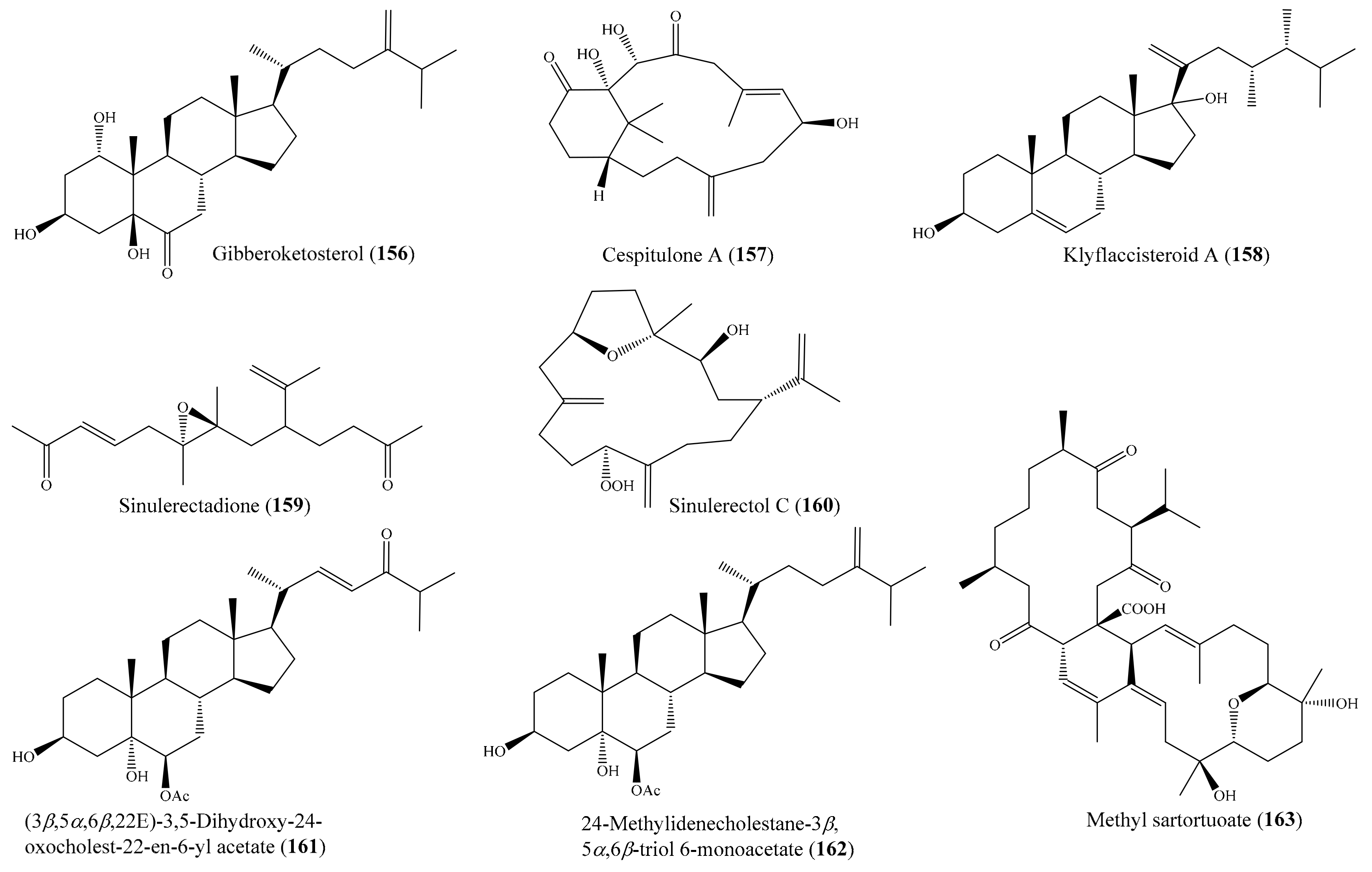
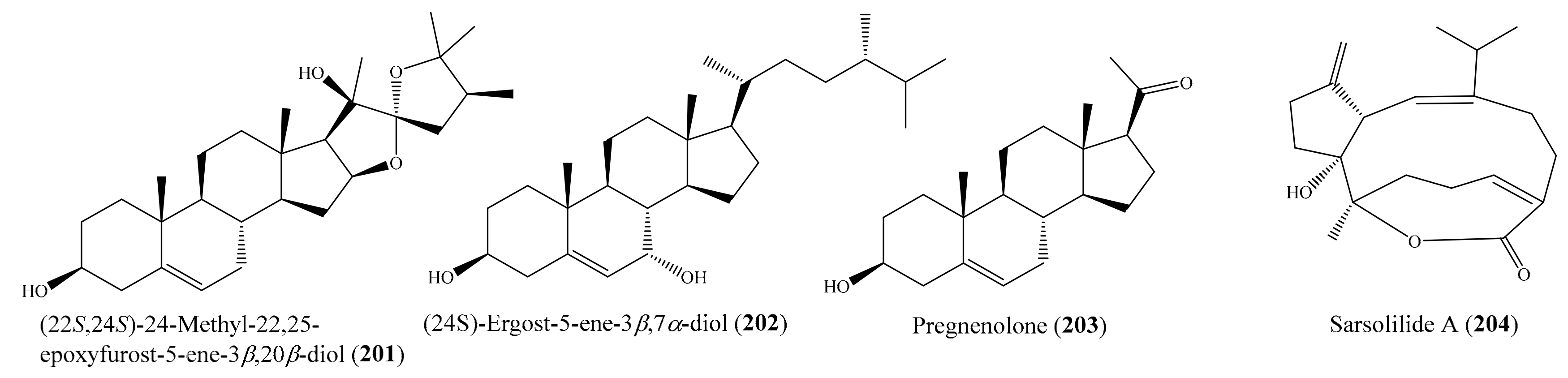
2.6. Other Bioactive Compounds from Coral
3. Bioactive Compounds from Coral-Associated Microorganisms
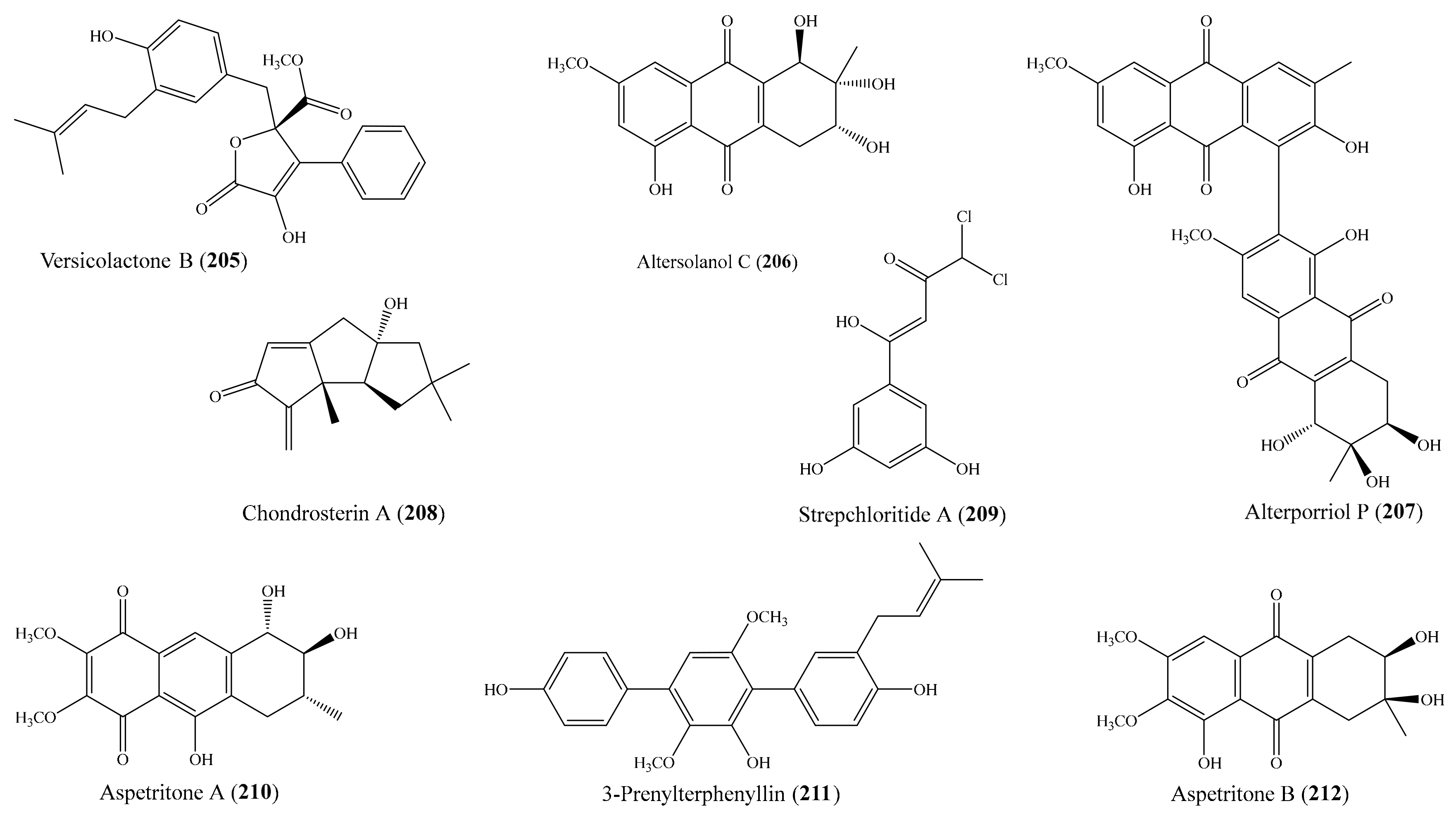
3.1. Anti-Inflammatory and Cytotoxic Compounds from Coral-Associated Microorganisms
3.2. Antimicrobial Compounds from Coral-Associated Microorganisms
3.3. Antivirus Compounds from Coral-Associated Microorganisms
3.4. Antifouling Compounds from Coral-Associated Microorganisms
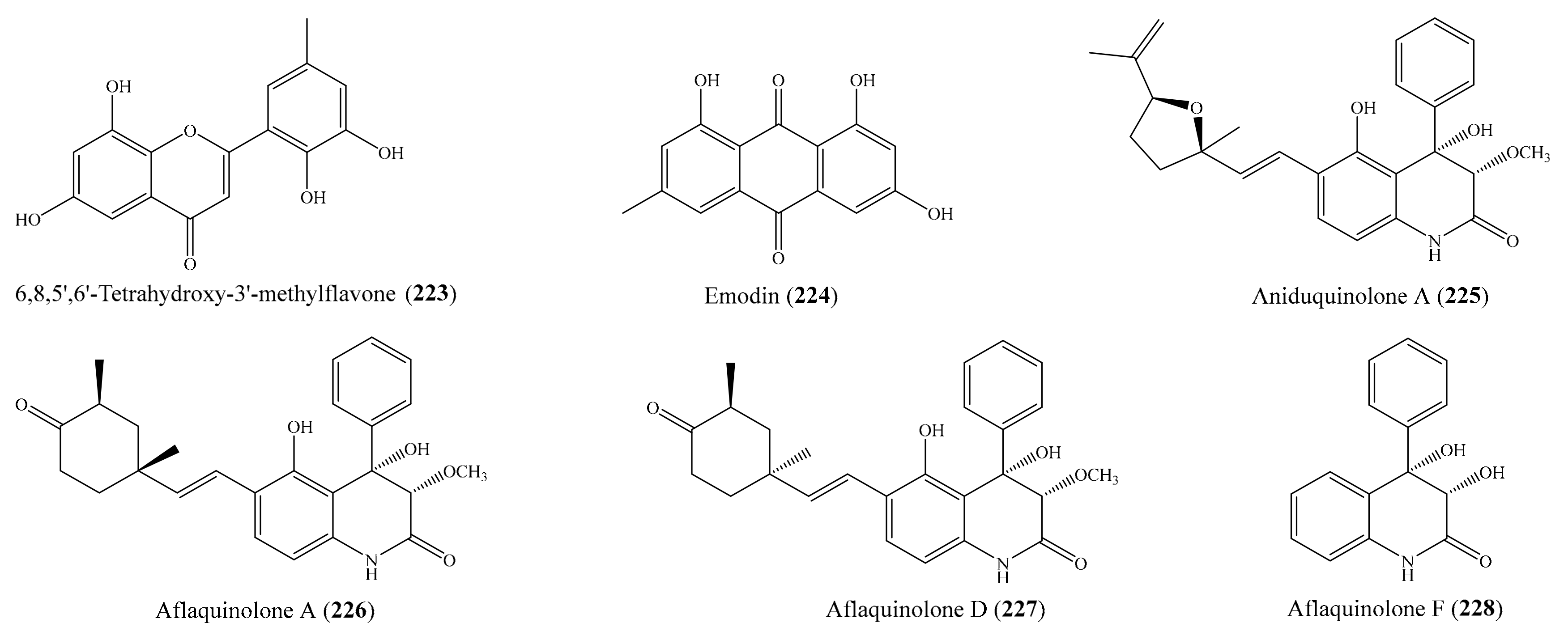
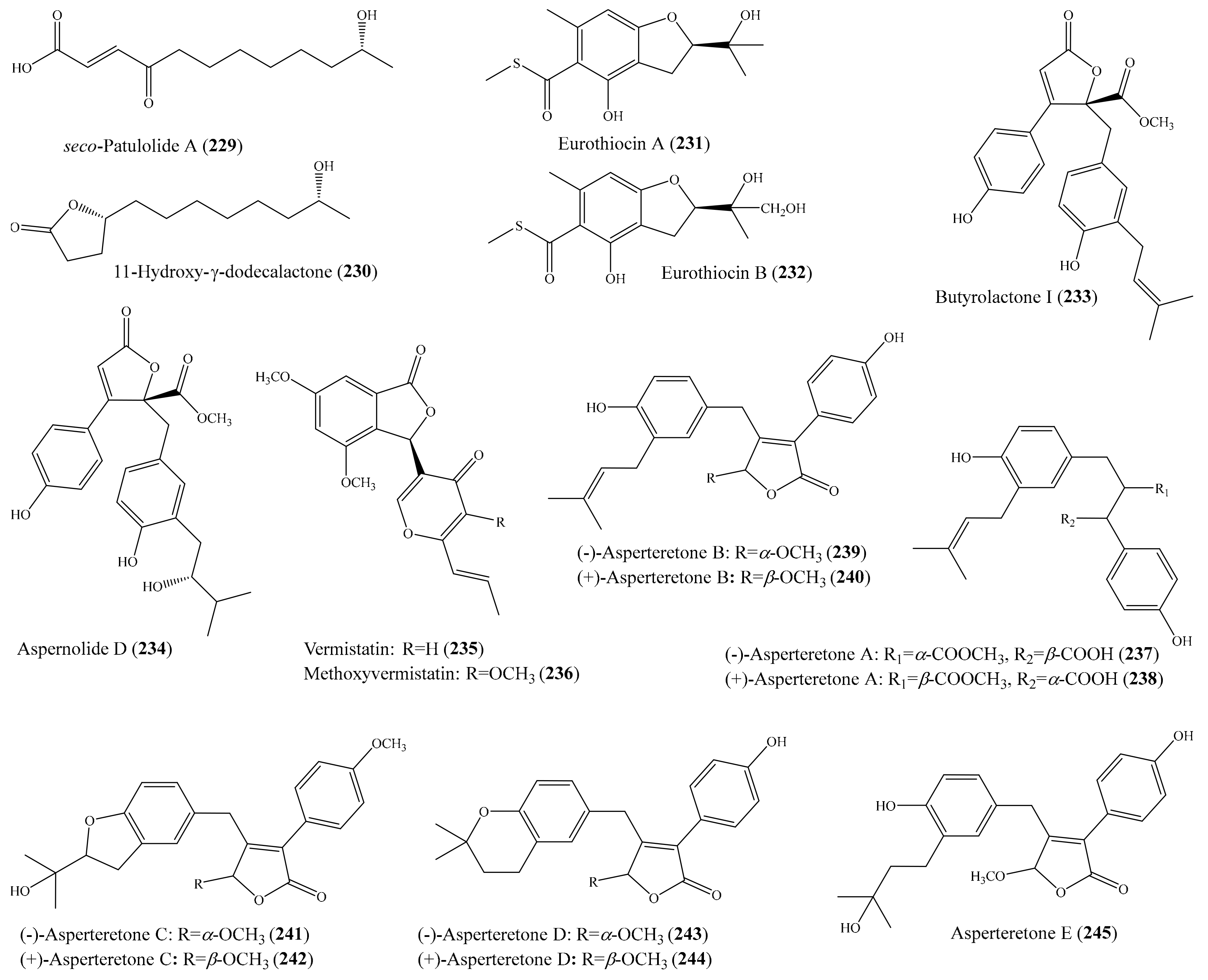
3.5. Other Bioactive Compounds from Coral-Associated Microorganisms
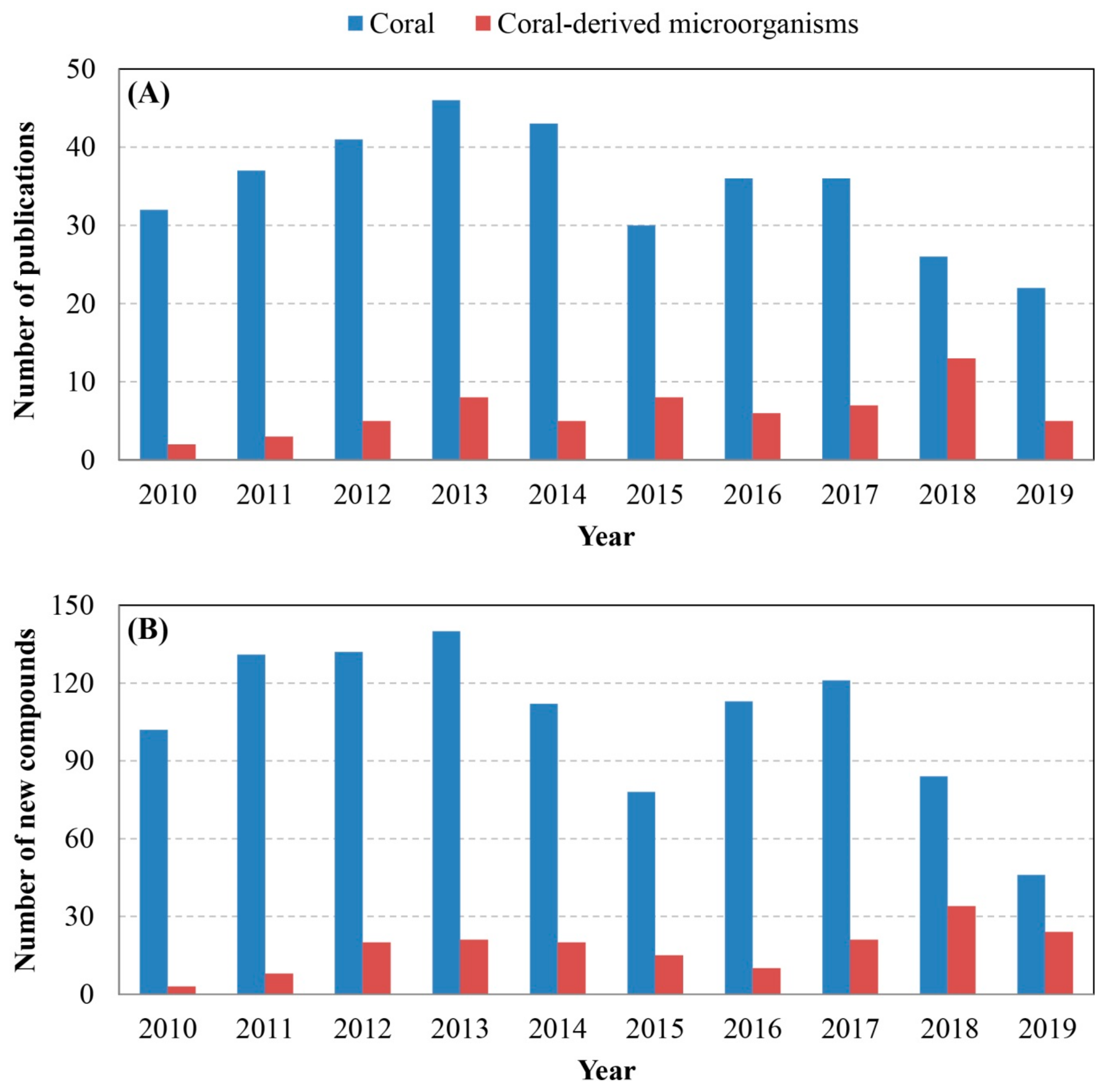
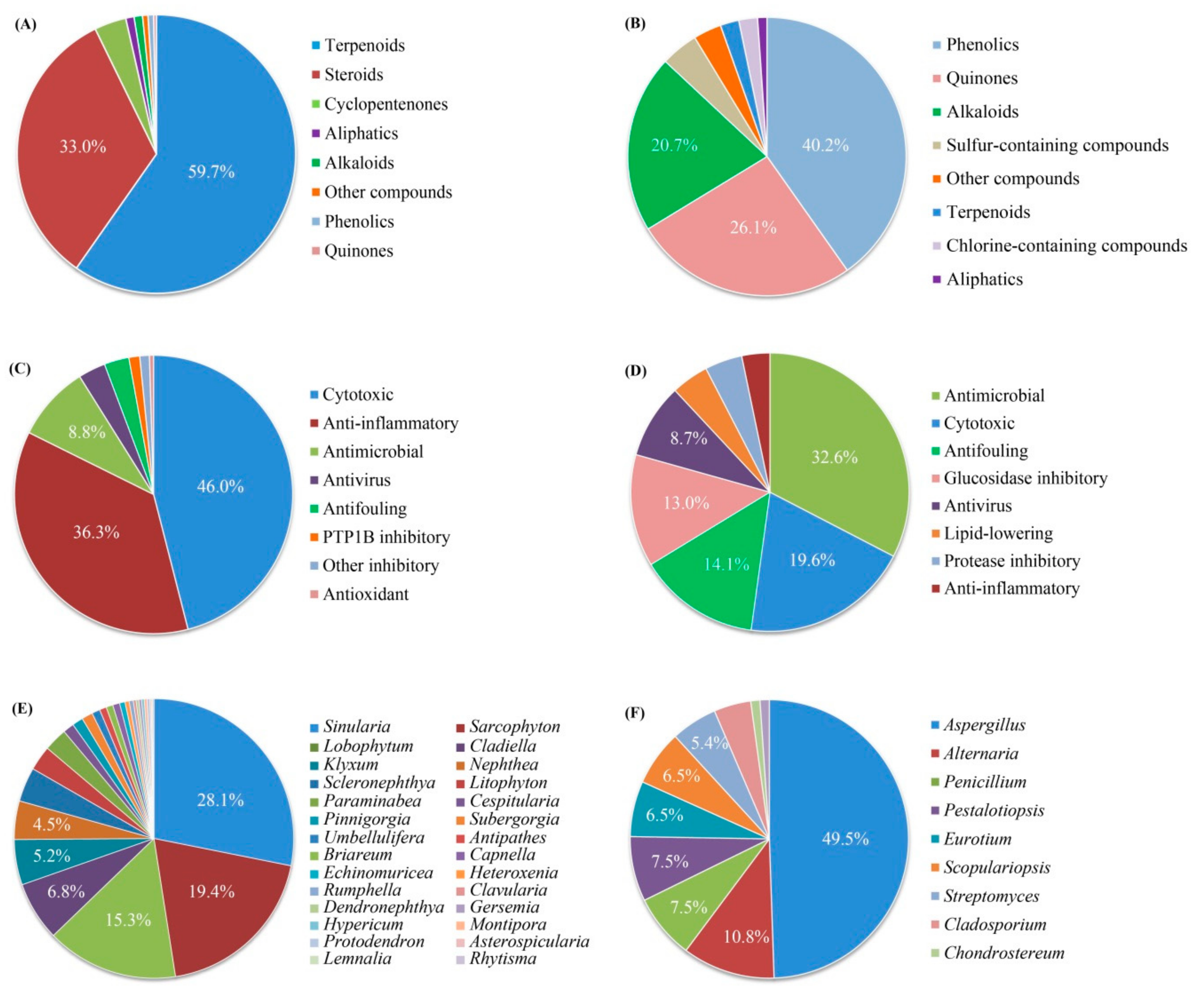
4. Comprehensive Overview and Outlook
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Pietra, F. Biodiversity and Natural Product Diversity, 1st ed.; Pergamon Press: Oxford, UK, 2002; Volume 21. [Google Scholar]
- Hay, M.E.; Fenical, W. Chemical ecology and marine biodiversity: Insights and products from the sea. Oceanography 1996, 9, 10–20. [Google Scholar] [CrossRef]
- Puglisi, M.P.; Sneed, J.M.; Ritson-Williams, R.; Young, R. Marine chemical ecology in benthic environments. Nat. Prod. Rep. 2019, 36, 410–429. [Google Scholar] [CrossRef]
- Rohde, S.; Nietzer, S.; Schupp, P.J. Prevalence and Mechanisms of Dynamic Chemical Defenses in Tropical Sponges. PLoS ONE 2015, 10, e0132236. [Google Scholar] [CrossRef]
- Coll, J.C.; Barre, S.L.; Sammarco, P.W.; Williams, W.T.; Bakus, G.J. Chemical Defences in Soft Corals (Coelenterata: Octocorallia) of the Great Barrier Reef: A Study of Comparative Toxicities. Mar. Ecol. Prog. Ser. 1982, 8, 271–278. [Google Scholar] [CrossRef]
- McClintock, J.B.; Amsler, C.D.; Baker, B.J. Overview of the Chemical Ecology of Benthic Marine Invertebrates along the Western Antarctic Peninsula. Integr. Comp. Biol. 2010, 50, 967–980. [Google Scholar] [CrossRef]
- Lindquist, N. Chemical Defense of Early Life Stages of Benthic Marine Invertebrates. J. Chem. Ecol. 2002, 28, 1987–2000. [Google Scholar] [CrossRef]
- Eskander, R.; Al-Sofyani, A.A.; El-Sherbiny, M.M.O.; Ba-Akdah, M.A.; Satheesh, S. Chemical Defense of Soft Coral Sinularia polydactyla from the Red Sea Against Marine Biofilm-Forming Bacteria. J. Ocean Univ. China 2018, 17, 1451–1457. [Google Scholar] [CrossRef]
- Marris, E. Drugs from the deep. Nature 2006, 443, 904–905. [Google Scholar] [CrossRef]
- Molinski, T.F.; Dalisay, D.S.; Lievens, S.L.; Saludes, J.P. Drug development from marine natural products. Nat. Rev. Drug Discov. 2008, 8, 69–85. [Google Scholar] [CrossRef]
- Lindequist, U. Marine-Derived Pharmaceuticals—Challenges and Opportunities. Biomol. Ther. 2016, 24, 561–571. [Google Scholar] [CrossRef]
- Malve, H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016, 8, 83–91. [Google Scholar] [CrossRef]
- De Zoysa, M. Chapter 9—Medicinal Benefits of Marine Invertebrates: Sources for Discovering Natural Drug Candidates. Adv. Food Nutr. Res. 2012, 65, 153–169. [Google Scholar]
- Hu, G.-P.; Yuan, J.; Sun, L.; She, Z.-G.; Wu, J.-H.; Lan, X.-J.; Zhu, X.; Lin, Y.-C.; Chen, S.-P. Statistical research on marine natural products based on data obtained between 1985 and 2008. Mar. Drugs 2011, 9, 514–525. [Google Scholar] [CrossRef]
- Horta, A.; Alves, C.; Pinteus, S.; Pedrosa, R. The marine origin of drugs. In Phycotoxins, 2nd ed.; John Wiley & Sons: London, UK, 2015. [Google Scholar]
- Simmons, T.L.; Andrianasolo, E.; McPhail, K.; Flatt, P.; Gerwick, W.H. Marine natural products as anticancer drugs. Mol. Cancer Ther. 2005, 4, 333–342. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2013, 30, 237–323. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2012, 29, 144–222. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2011, 28, 196–268. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, J.; Chen, J.; Zhang, H.; Guo, Y.-W.; Wang, H. Terpenoids from Marine Soft Coral of the Genus Lemnalia: Chemistry and Biological Activities. Mar. Drugs 2018, 16, 320. [Google Scholar] [CrossRef]
- Rodrigues, G.I.; Miguel, G.M.; Mnif, W. A Brief Review on New Naturally Occurring Cembranoid Diterpene Derivatives from the Soft Corals of the Genera Sarcophyton, Sinularia, and Lobophytum Since 2016. Molecules 2019, 24, 781. [Google Scholar] [CrossRef]
- Mayer, A.M.S.; Rodríguez, A.D.; Berlinck, R.G.S.; Fusetani, N. Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 191–222. [Google Scholar]
- Kooperman, N.; Ben-Dov, E.; Kramarsky-Winter, E.; Barak, Z.; Kushmaro, A. Coral mucus-associated bacterial communities from natural and aquarium environments. FEMS Microbiol. Lett. 2007, 276, 106–113. [Google Scholar] [CrossRef]
- Rohwer, F.; Breitbart, M.; Jara, J.; Azam, F.; Knowlton, N. Diversity of bacteria associated with the Caribbean coral Montastraea franksi. Coral Reefs 2001, 20, 85–91. [Google Scholar]
- Pollock, F.J.; McMinds, R.; Smith, S.; Bourne, D.G.; Willis, B.L.; Medina, M.; Thurber, R.V.; Zaneveld, J.R. Coral-associated bacteria demonstrate phylosymbiosis and cophylogeny. Nat. Commun. 2018, 9, 4921. [Google Scholar] [CrossRef]
- Huggett, M.J.; Apprill, A. Coral microbiome database: Integration of sequences reveals high diversity and relatedness of coral-associated microbes. Environ. Microbiol. Rep. 2019, 11, 372–385. [Google Scholar] [CrossRef]
- Mahmoud, H.M.; Kalendar, A.A. Coral-associated actinobacteria: Diversity, abundance, and biotechnological potentials. Front. Microbiol. 2016, 7, 204. [Google Scholar] [CrossRef]
- Lawler, S.N.; Kellogg, C.A.; France, S.C.; Clostio, R.W.; Brooke, S.D.; Ross, S.W. Coral-Associated Bacterial Diversity Is Conserved across Two Deep-Sea Anthothela Species. Front. Microbiol. 2016, 7, 458. [Google Scholar] [CrossRef]
- La Rivière, M.; Garrabou, J.; Bally, M. Evidence for host specificity among dominant bacterial symbionts in temperate gorgonian corals. Coral Reefs 2015, 34, 1087–1098. [Google Scholar] [CrossRef]
- van de Water, J.A.J.M.; Melkonian, R.; Voolstra, C.R.; Junca, H.; Beraud, E.; Allemand, D.; Ferrier-Pagès, C. Comparative Assessment of Mediterranean Gorgonian-Associated Microbial Communities Reveals Conserved Core and Locally Variant Bacteria. Microb. Ecol. 2017, 73, 466–478. [Google Scholar] [CrossRef]
- Rosenberg, E.; Koren, O.; Reshef, L.; Efrony, R.; Zilber-Rosenberg, I. The role of microorganisms in coral health, disease and evolution. Nat. Rev. Microbiol. 2007, 5, 355–362. [Google Scholar] [CrossRef]
- Zaneveld, J.R.; Burkepile, D.E.; Shantz, A.A.; Pritchard, C.E.; McMinds, R.; Payet, J.P.; Welsh, R.; Correa, A.M.S.; Lemoine, N.P.; Rosales, S.; et al. Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nat. Commun. 2016, 7, 11833. [Google Scholar] [CrossRef]
- Sunagawa, S.; DeSantis, T.Z.; Piceno, Y.M.; Brodie, E.L.; DeSalvo, M.K.; Voolstra, C.R.; Weil, E.; Andersen, G.L.; Medina, M. Bacterial diversity and White Plague Disease-associated community changes in the Caribbean coral Montastraea faveolata. ISME J. 2009, 3, 512–521. [Google Scholar] [CrossRef]
- Sato, Y.; Willis, B.L.; Bourne, D.G. Successional changes in bacterial communities during the development of black band disease on the reef coral, Montipora hispida. ISME J. 2009, 4, 203–214. [Google Scholar] [CrossRef]
- Morrow, K.M.; Muller, E.; Lesser, M.P. How Does the Coral Microbiome Cause, Respond to, or Modulate the Bleaching Process? In Coral Bleaching: Patterns, Processes, Causes and Consequences; van Oppen, M.J.H., Lough, J.M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 153–188. [Google Scholar]
- Roitman, S.; Joseph Pollock, F.; Medina, M. Coral Microbiomes as Bioindicators of Reef Health. In Population Genomics; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Alagely, A.; Krediet, C.J.; Ritchie, K.B.; Teplitski, M. Signaling-mediated cross-talk modulates swarming and biofilm formation in a coral pathogen Serratia marcescens. ISME J. 2011, 5, 1609–1620. [Google Scholar] [CrossRef]
- Kim, B.R. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar. Ecol. Prog. Ser. 2006, 322, 1–14. [Google Scholar]
- Teplitski, M.; Ritchie, K. How feasible is the biological control of coral diseases? Trends Ecol. Evol. 2009, 24, 378–385. [Google Scholar] [CrossRef]
- Shnit-Orland, M.; Kushmaro, A. Coral mucus-associated bacteria: A possible first line of defense. FEMS Microbiol. Ecol. 2009, 67, 371–380. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Lin, W.H.; Proksch, P. Bioactive Compounds from Marine Bacteria and Fungi. Microb. Biotechnol. 2010, 3, 544–563. [Google Scholar] [CrossRef]
- Lei, H. Diterpenoids of Gorgonian Corals: Chemistry and Bioactivity. Chem. Biodivers. 2016, 13, 345–365. [Google Scholar] [CrossRef]
- Su, Y.-D.; Su, J.-H.; Hwang, T.-L.; Wen, Z.-H.; Sheu, J.-H.; Wu, Y.-C.; Sung, P.-J. Briarane Diterpenoids Isolated from Octocorals between 2014 and 2016. Mar. Drugs 2017, 15, 44. [Google Scholar] [CrossRef]
- Sheu, J.-H.; Chen, Y.-H.; Chen, Y.-H.; Su, Y.-D.; Chang, Y.-C.; Su, J.-H.; Weng, C.-F.; Lee, C.-H.; Fang, L.-S.; Wang, W.-H.; et al. Briarane diterpenoids isolated from gorgonian corals between 2011 and 2013. Mar. Drugs 2014, 12, 2164–2181. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Sheu, J.-H.; Wu, Y.-C.; Sung, P.-J. Terpenoids from Octocorals of the Genus Pachyclavularia. Mar. Drugs 2017, 15, 382. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef]
- Scrivo, R.; Vasile, M.; Bartosiewicz, I.; Valesini, G. Inflammation as “common soil” of the multifactorial diseases. Autoimmun. Rev. 2011, 10, 369–374. [Google Scholar] [CrossRef]
- Sostres, C.; Gargallo, C.J.; Arroyo, M.T.; Lanas, A. Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best Pract. Res. Clin. Gastroenterol. 2010, 24, 121–132. [Google Scholar] [CrossRef]
- Yan, P.; Lv, Y.; van Ofwegen, L.; Proksch, P.; Lin, W. Lobophytones A−G, new isobiscembranoids from the soft coral Lobophytum pauciflorum. Org. Lett. 2010, 12, 2484–2487. [Google Scholar] [CrossRef]
- Chen, B.W.; Chang, S.M.; Huang, C.Y.; Chao, C.H.; Su, J.H.; Wen, Z.H.; Hsu, C.H.; Dai, C.F.; Wu, Y.C.; Sheu, J.H. Hirsutalins A-H, eunicellin-based diterpenoids from the soft coral Cladiella hirsuta. J. Nat. Prod. 2010, 73, 1785–1791. [Google Scholar] [CrossRef]
- Quang, T.H.; Ha, T.T.; Minh, C.V.; Kiem, P.V.; Huong, H.T.; Ngan, N.T.; Nhiem, N.X.; Tung, N.H.; Tai, B.H.; Thuy, D.T.; et al. Cytotoxic and anti-inflammatory cembranoids from the Vietnamese soft coral Lobophytum laevigatum. Bioorg. Med. Chem. 2011, 19, 2625–2632. [Google Scholar] [CrossRef]
- Su, J.H.; Wen, Z.H. Bioactive cembrane-based diterpenoids from the soft coral Sinularia Triangular. Mar. Drugs 2011, 9, 944–951. [Google Scholar] [CrossRef]
- Kao, C.Y.; Su, J.H.; Lu, M.C.; Hwang, T.L.; Wang, W.H.; Chen, J.J.; Sheu, J.H.; Kuo, Y.H.; Weng, C.F.; Fang, L.S.; et al. Lobocrassins A-E: New cembrane-type diterpenoids from the soft coral Lobophytum crassum. Mar. Drugs 2011, 9, 1319–1331. [Google Scholar] [CrossRef]
- Chao, C.H.; Chou, K.J.; Wen, Z.H.; Wang, G.H.; Wu, Y.C.; Dai, C.F.; Sheu, J.H. Paraminabeolides A-F, cytotoxic and anti-inflammatory marine withanolides from the soft coral Paraminabea acronocephala. J. Nat. Prod. 2011, 74, 1132–1141. [Google Scholar] [CrossRef]
- Hsu, F.-J.; Chen, B.-W.; Wen, Z.-H.; Huang, C.-Y.; Dai, C.-F.; Su, J.-H.; Wu, Y.-C.; Sheu, J.-H. Klymollins A–H, bioactive eunicellin-based diterpenoids from the Formosan soft coral Klyxum molle. J. Nat. Prod. 2011, 74, 2467–2471. [Google Scholar] [CrossRef]
- Lin, W.Y.; Lu, Y.; Su, J.H.; Wen, Z.H.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. Bioactive cembranoids from the dongsha atoll soft coral Sarcophyton crassocaule. Mar. Drugs 2011, 9, 994–1006. [Google Scholar] [CrossRef]
- Chung, H.M.; Hong, P.H.; Su, J.H.; Hwang, T.L.; Lu, M.C.; Fang, L.S.; Wu, Y.C.; Li, J.J.; Chen, J.J.; Wang, W.H.; et al. Bioactive compounds from a gorgonian coral Echinomuricea sp. (Plexauridae). Mar. Drugs 2012, 10, 1169–1179. [Google Scholar] [CrossRef]
- Lin, W.Y.; Lu, Y.; Chen, B.W.; Huang, C.Y.; Su, J.H.; Wen, Z.H.; Dai, C.F.; Kuo, Y.H.; Sheu, J.H. Sarcocrassocolides M-O, bioactive cembranoids from the Dongsha Atoll soft coral Sarcophyton crassocaule. Mar. Drugs 2012, 10, 617–626. [Google Scholar] [CrossRef]
- Fang, H.-Y.; Liaw, C.-C.; Chao, C.-H.; Wen, Z.-H.; Wu, Y.-C.; Hsu, C.-H.; Dai, C.-F.; Sheu, J.-H. Bioactive pregnane-type steroids from the soft coral Scleronephthya gracillimum. Tetrahedron 2012, 68, 9694–9700. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Hwang, T.-L.; Su, Y.-D.; Chang, Y.-C.; Chen, Y.-H.; Hong, P.-H.; Hu, L.-C.; Yen, W.-H.; Hsu, H.-Y.; Huang, S.-J. New 6-hydroxyeunicellins from a soft coral Cladiella sp. Chem. Pharm. Bull. 2012, 60, 160–163. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, X.; Huang, H.; Yang, X.-W.; Liu, J.; Lin, X.; Li, X.; Peng, Y.; Liu, Y. New cembrane diterpenoids from a Hainan soft coral Sinularia sp. Mar. Drugs 2012, 10, 2023–2032. [Google Scholar] [CrossRef]
- Tai, C.J.; Su, J.H.; Huang, C.Y.; Huang, M.S.; Wen, Z.H.; Dai, C.F.; Sheu, J.H. Cytotoxic and anti-inflammatory eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs 2013, 11, 788–799. [Google Scholar] [CrossRef]
- Thao, N.P.; Nam, N.H.; Cuong, N.X.; Quang, T.H.; Tung, P.T.; Dat Le, D.; Chae, D.; Kim, S.; Koh, Y.S.; Kiem, P.V.; et al. Anti-inflammatory norditerpenoids from the soft coral Sinularia maxima. Bioorg. Med. Chem. Lett. 2013, 23, 228–231. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Nguyen, H.N.; Nguyen, X.C.; Nguyen, X.N.; Pham, T.T.; Tran, H.Q.; Nguyen, T.T.N.; Phan, V.K.; Chau, V.M.; Kim, Y.H. A new sterol from the soft coral Lobophytum crassum. Bull. Korean Chem. Soc. 2013, 34, 249–251. [Google Scholar] [CrossRef][Green Version]
- Yin, J.; Zhao, M.; Ma, M.; Xu, Y.; Xiang, Z.; Cai, Y.; Dong, J.; Lei, X.; Huang, K.; Yan, P. New casbane diterpenoids from a South China Sea soft coral, Sinularia sp. Mar. Drugs 2013, 11, 455–465. [Google Scholar] [CrossRef]
- Lin, M.C.; Chen, B.W.; Huang, C.Y.; Dai, C.F.; Hwang, T.L.; Sheu, J.H. Eunicellin-based diterpenoids from the Formosan soft coral Klyxum molle with inhibitory activity on superoxide generation and elastase release by neutrophils. J. Nat. Prod. 2013, 76, 1661–1667. [Google Scholar] [CrossRef]
- Chen, B.-W.; Wang, S.-Y.; Huang, C.-Y.; Chen, S.-L.; Wu, Y.-C.; Sheu, J.-H. Hirsutalins I–M, eunicellin-based diterpenoids from the soft coral Cladiella hirsuta. Tetrahedron 2013, 69, 2296–2301. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.; Ngan, N.T.; Song, S.B.; Cuong, N.X.; Nam, N.H.; Kiem, P.V.; Kim, Y.H.; Minh, C.V. New anti-inflammatory cembranoid diterpenoids from the Vietnamese soft coral Lobophytum crassum. Bioorg. Med. Chem. Lett. 2014, 24, 228–232. [Google Scholar] [CrossRef]
- Cuong, N.X.; Thao, N.P.; Luyen, B.T.T.; Ngan, N.T.T.; Thuy, D.T.T.; Song, S.B.; Nam, N.H.; Van Kiem, P.; Kim, Y.H.; Van Minh, C. Cembranoid diterpenes from the soft coral Lobophytum crassum and their anti-inflammatory activities. Chem. Pharm. Bull. 2014, 62, 203–208. [Google Scholar] [CrossRef]
- Thao, N.P.; Luyen, B.T.; Sun, Y.N.; Song, S.B.; Thanh, N.V.; Cuong, N.X.; Nam, N.H.; Kiem, P.V.; Kim, Y.H.; Minh, C.V. NF-kappaB inhibitory activity of polyoxygenated steroids from the Vietnamese soft coral Sarcophyton pauciplicatum. Bioorg. Med. Chem. Lett. 2014, 24, 2834–2838. [Google Scholar] [CrossRef]
- Lee, Y.N.; Tai, C.J.; Hwang, T.L.; Sheu, J.H. Krempfielins N-P, new anti-inflammatory eunicellins from a Taiwanese soft coral Cladiella krempfi. Mar. Drugs 2014, 12, 1148–1156. [Google Scholar] [CrossRef]
- Lin, W.Y.; Chen, B.W.; Huang, C.Y.; Wen, Z.H.; Sung, P.J.; Su, J.H.; Dai, C.F.; Sheu, J.H. Bioactive cembranoids, sarcocrassocolides P-R, from the Dongsha Atoll soft coral Sarcophyton crassocaule. Mar. Drugs 2014, 12, 840–850. [Google Scholar] [CrossRef]
- Chao, C.-H.; Huang, T.-Z.; Wu, C.-Y.; Chen, B.-W.; Huang, C.-Y.; Hwang, T.-L.; Dai, C.-F.; Sheu, J.-H. Steroidal and α-tocopherylhydroquinone glycosides from two soft corals Cladiella hirsuta and Sinularia nanolobata. RSC Adv. 2015, 5, 74256–74262. [Google Scholar] [CrossRef]
- Huang, C.Y.; Tseng, Y.J.; Chokkalingam, U.; Hwang, T.L.; Hsu, C.H.; Dai, C.F.; Sung, P.J.; Sheu, J.H. Bioactive isoprenoid-derived natural products from a Dongsha Atoll soft coral Sinularia erecta. J. Nat. Prod. 2016, 79, 1339–1346. [Google Scholar] [CrossRef]
- Tsai, C.-R.; Huang, C.-Y.; Chen, B.-W.; Tsai, Y.-Y.; Shih, S.-P.; Hwang, T.-L.; Dai, C.-F.; Wang, S.-Y.; Sheu, J.-H. New bioactive steroids from the soft coral Klyxum flaccidum. RSC Adv. 2015, 5, 12546–12554. [Google Scholar] [CrossRef]
- Huang, C.Y.; Sung, P.J.; Uvarani, C.; Su, J.H.; Lu, M.C.; Hwang, T.L.; Dai, C.F.; Wu, S.L.; Sheu, J.H. Glaucumolides A and B, biscembranoids with new structural type from a cultured soft coral Sarcophyton glaucum. Sci. Rep. 2015, 5, 15624. [Google Scholar] [CrossRef]
- Tsai, Y.Y.; Huang, C.Y.; Tseng, W.R.; Chiang, P.L.; Hwang, T.L.; Su, J.H.; Sung, P.J.; Dai, C.F.; Sheu, J.H. Klyflaccisteroids K-M, bioactive steroidal derivatives from a soft coral Klyxum flaccidum. Bioorg. Med. Chem. Lett. 2017, 27, 1220–1224. [Google Scholar] [CrossRef]
- Chang, Y.C.; Kuo, L.M.; Hwang, T.L.; Yeh, J.; Wen, J.H.; Fang, L.S.; Wu, Y.C.; Lin, C.S.; Sheu, J.H.; Sung, P.J. Pinnisterols A–C, new 9,11-secosterols from a Gorgonian Pinnigorgia sp. Mar. Drugs 2016, 14, 12. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chang, C.W.; Tseng, Y.J.; Lee, J.; Sung, P.J.; Su, J.H.; Hwang, T.L.; Dai, C.F.; Wang, H.C.; Sheu, J.H. Bioactive steroids from the Formosan soft coral Umbellulifera petasites. Mar. Drugs 2016, 14, 180. [Google Scholar] [CrossRef]
- Tseng, W.R.; Huang, C.Y.; Tsai, Y.Y.; Lin, Y.S.; Hwang, T.L.; Su, J.H.; Sung, P.J.; Dai, C.F.; Sheu, J.H. New cytotoxic and anti-inflammatory steroids from the soft coral Klyxum flaccidum. Bioorg. Med. Chem. Lett. 2016, 26, 3253–3257. [Google Scholar] [CrossRef]
- Zhao, M.; Cheng, S.; Yuan, W.; Xi, Y.; Li, X.; Dong, J.; Huang, K.; Gustafson, K.R.; Yan, P. Cembranoids from a Chinese collection of the soft coral Lobophytum crassum. Mar. Drugs 2016, 14, 111. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Kuo, L.-M.; Su, J.-H.; Hwang, T.-L.; Kuo, Y.-H.; Lin, C.-S.; Wu, Y.-C.; Sheu, J.-H.; Sung, P.-J. Pinnigorgiols A–C, 9,11-secosterols with a rare ring arrangement from a gorgonian coral Pinnigorgia sp. Tetrahedron 2016, 72, 999–1004. [Google Scholar] [CrossRef]
- Huang, C.Y.; Ahmed, A.F.; Su, J.H.; Sung, P.J.; Hwang, T.L.; Chiang, P.L.; Dai, C.F.; Liaw, C.C.; Sheu, J.H. Bioactive new withanolides from the cultured soft coral Sinularia brassica. Bioorg. Med. Chem. Lett. 2017, 27, 3267–3271. [Google Scholar] [CrossRef]
- Wu, Q.; Li, X.W.; Li, H.; Yao, L.G.; Tang, W.; Miao, Z.H.; Wang, H.; Guo, Y.W. Bioactive polyoxygenated cembranoids from a novel Hainan chemotype of the soft coral Sinularia flexibilis. Bioorg. Med. Chem. Lett. 2019, 29, 185–188. [Google Scholar] [CrossRef]
- Andrea, N.; Angelo, C.; Paola, F.; Pier Mario, B.; Giuseppe, R. Immunotherapy and Hormone-therapy in Metastatic Breast Cancer: A Review and an Update. Curr. Drug Targets 2016, 17, 1127–1139. [Google Scholar]
- Mullard, A. FDA approves first immunotherapy combo. Nat. Rev. Drug Discov. 2015, 14, 739. [Google Scholar] [CrossRef]
- Ali, R.; Mirza, Z.; Ashraf, G.M.D.; Kamal, M.A.; Ansari, S.A.; Damanhouri, G.A.; Abuzenadah, A.M.; Chaudhary, A.G.; Sheikh, I.A. New Anticancer Agents: Recent Developments in Tumor Therapy. Anticancer Res. 2012, 32, 2999–3005. [Google Scholar]
- Khoo, B.L.; Chaudhuri, P.K.; Ramalingam, N.; Tan, D.S.W.; Lim, C.T.; Warkiani, M.E. Single-cell profiling approaches to probing tumor heterogeneity. Int. J. Cancer 2016, 139, 243–255. [Google Scholar] [CrossRef]
- Feinberg, A.P.; Ohlsson, R.; Henikoff, S. The epigenetic progenitor origin of human cancer. Nat. Rev. Genet. 2006, 7, 21–33. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Chau, V.M.; Phan, V.K.; Hoang, T.H.; Nguyen, H.N.; Nguyen, X.C.; Tran, H.Q.; Nguyen, X.N.; Hyun, J.H.; Kang, H.K.; et al. Chemical components from the Vietnamese soft coral Lobophytum sp. Arch. Pharm. Res. 2010, 33, 503–508. [Google Scholar]
- Chau, V.M.; Phan, V.K.; Nguyen, X.; Nguyen, X.C.; Nguyen, P.T.; Nguyen, H.N.; Hoang Le, T.A.; Do, C.T.; Thuy, D.T.; Kang, H.K.; et al. Cytotoxic and antioxidant activities of diterpenes and sterols from the Vietnamese soft coral Lobophytum compactum. Bioorg. Med. Chem. Lett. 2011, 21, 2155–2159. [Google Scholar]
- Tai, C.J.; Su, J.H.; Huang, M.S.; Wen, Z.H.; Dai, C.F.; Sheu, J.H. Bioactive eunicellin-based diterpenoids from the soft coral Cladiella krempfi. Mar. Drugs 2011, 9, 2036–2045. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; El-Beih, A.A.; Moustafa, A.Y.; Hamdy, A.A.; Alhammady, M.A.; Selim, R.M.; Abdel-Rehim, M.; Paré, P.W. Cytotoxic Cembranoids from the Red Sea Soft Coral Sarcophyton glaucum. Nat. Prod. Commun. 2011, 6, 209–222. [Google Scholar] [CrossRef]
- Liao, X.-J.; Tang, L.-D.; Liang, Y.-W.; Geng, H.-W.; Xu, S.-H. Isolation and identification of two new polyhydroxylated sterols from soft coral Sinularia sp. Chem. Res. Chin. Univ. 2011, 27, 217–220. [Google Scholar]
- Lee, N.L.; Su, J.H. Tetrahydrofuran cembranoids from the cultured soft coral Lobophytum crassum. Mar. Drugs 2011, 9, 2526–2536. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Huang, K.J.; Wang, S.K.; Duh, C.Y. Capilloquinol: A novel farnesyl quinol from the Dongsha atoll soft coral Sinularia capillosa. Mar. Drugs 2011, 9, 469–476. [Google Scholar] [CrossRef]
- Lin, Y.S.; Eid Fazary, A.; Chen, C.H.; Kuo, Y.H.; Shen, Y.C. Bioactive xenicane diterpenoids from the Taiwanese soft coral Asterospicularia laurae. Chem. Biodivers. 2011, 8, 1310–1317. [Google Scholar] [CrossRef]
- Huang, C.Y.; Su, J.H.; Duh, C.Y.; Chen, B.W.; Wen, Z.H.; Kuo, Y.H.; Sheu, J.H. A new 9,11-secosterol from the soft coral Sinularia granosa. Bioorg. Med. Chem. Lett. 2012, 22, 4373–4376. [Google Scholar] [CrossRef]
- Su, J.H.; Huang, C.Y.; Li, P.J.; Lu, Y.; Wen, Z.H.; Kao, Y.H.; Sheu, J.H. Bioactive cadinane-type compounds from the soft coral Sinularia scabra. Arch. Pharm. Res. 2012, 35, 779–784. [Google Scholar] [CrossRef]
- Hegazy, M.E.; Gamal Eldeen, A.M.; Shahat, A.A.; Abdel-Latif, F.F.; Mohamed, T.A.; Whittlesey, B.R.; Pare, P.W. Bioactive hydroperoxyl cembranoids from the Red Sea soft coral Sarcophyton glaucum. Mar. Drugs 2012, 10, 209–222. [Google Scholar] [CrossRef]
- Roy, P.K.; Maarisit, W.; Roy, M.C.; Taira, J.; Ueda, K. Five new diterpenoids from an Okinawan soft coral, Cespitularia sp. Mar. Drugs 2012, 10, 2741–2748. [Google Scholar] [CrossRef]
- Li, R.; Shao, C.L.; Qi, X.; Li, X.B.; Li, J.; Sun, L.L.; Wang, C.Y. Polyoxygenated sterols from the South China Sea soft coral Sinularia sp. Mar. Drugs 2012, 10, 1422–1432. [Google Scholar] [CrossRef]
- Wang, S.K.; Duh, C.Y. New cytotoxic cembranolides from the soft coral Lobophytum michaelae. Mar. Drugs 2012, 10, 306–318. [Google Scholar] [CrossRef]
- Wang, S.K.; Hsieh, M.K.; Duh, C.Y. Three new cembranoids from the Taiwanese soft coral Sarcophyton ehrenbergi. Mar. Drugs 2012, 10, 1433–1444. [Google Scholar] [CrossRef]
- Wang, S.K.; Puu, S.Y.; Duh, C.Y. New 19-oxygenated steroids from the soft coral Nephthea chabrolii. Mar. Drugs 2012, 10, 1288–1296. [Google Scholar] [CrossRef]
- Govindam, S.V.; Yoshioka, Y.; Kanamoto, A.; Fujiwara, T.; Okamoto, T.; Ojika, M. Cyclolobatriene, a novel prenylated germacrene diterpene, from the soft coral Lobophytum pauciflorum. Bioorg. Med. Chem. 2012, 20, 687–692. [Google Scholar] [CrossRef]
- Yen, W.H.; Hu, L.C.; Su, J.H.; Lu, M.C.; Twan, W.H.; Yang, S.Y.; Kuo, Y.C.; Weng, C.F.; Lee, C.H.; Kuo, Y.H.; et al. Norcembranoidal diterpenes from a Formosan soft coral Sinularia sp. Molecules 2012, 17, 14058–14066. [Google Scholar] [CrossRef]
- Murni, A.; Hanif, N.; Tanaka, J. A new cytotoxic dolabellane from the Indonesian soft coral Anthelia sp. Indones. J. Chem. 2013, 13, 216–220. [Google Scholar] [CrossRef][Green Version]
- Chao, C.H.; Wu, Y.C.; Wen, Z.H.; Sheu, J.H. Steroidal carboxylic acids from soft coral Paraminabea acronocephala. Mar. Drugs 2013, 11, 136–145. [Google Scholar] [CrossRef]
- Su, C.C.; Wong, B.S.; Chin, C.; Wu, Y.J.; Su, J.H. Oxygenated cembranoids from the soft coral Sinularia flexibilis. Int. J. Mol. Sci. 2013, 14, 4317–4325. [Google Scholar] [CrossRef]
- Huang, C.Y.; Liaw, C.C.; Chen, B.W.; Chen, P.C.; Su, J.H.; Sung, P.J.; Dai, C.F.; Chiang, M.Y.; Sheu, J.H. Withanolide-based steroids from the cultured soft coral Sinularia brassica. J. Nat. Prod. 2013, 76, 1902–1908. [Google Scholar] [CrossRef]
- Aboutabl, S.A.; Azzam, S.M.; Michel, C.G.; Selim, N.M.; Hegazy, M.F.; Ali, A.H.; Hussein, A.A. Bioactive terpenoids from the Red Sea soft coral Sinularia polydactyla. Nat. Prod. Res. 2013, 27, 2224–2226. [Google Scholar] [CrossRef]
- Zhang, J.; Liao, X.J.; Wang, K.L.; Deng, Z.; Xu, S.H. Cytotoxic cholesta-1,4-dien-3-one derivatives from soft coral Nephthea sp. Steroids 2013, 78, 396–400. [Google Scholar] [CrossRef]
- Gong, K.K.; Tang, X.L.; Zhang, G.; Cheng, C.L.; Zhang, X.W.; Li, P.L.; Li, G.Q. Polyhydroxylated steroids from the South China Sea soft coral Sarcophyton sp. and their cytotoxic and antiviral activities. Mar. Drugs 2013, 11, 4788–4798. [Google Scholar] [CrossRef]
- Zhao, M.; Yin, J.; Jiang, W.; Ma, M.; Lei, X.; Xiang, Z.; Dong, J.; Huang, K.; Yan, P. Cytotoxic and antibacterial cembranoids from a South China Sea soft coral, Lobophytum sp. Mar. Drugs 2013, 11, 1162–1172. [Google Scholar] [CrossRef]
- Ellithey, M.S.; Lall, N.; Hussein, A.A.; Meyer, D. Cytotoxic, cytostatic and HIV-1 PR inhibitory activities of the soft coral Litophyton arboreum. Mar. Drugs 2013, 11, 4917–4936. [Google Scholar] [CrossRef]
- Yen, W.H.; Chen, W.F.; Cheng, C.H.; Dai, C.F.; Lu, M.C.; Su, J.H.; Su, Y.D.; Chen, Y.H.; Chang, Y.C.; Chen, Y.H.; et al. A new 5α,8α-epidioxysterol from the soft coral Sinularia gaweli. Molecules 2013, 18, 2895–2903. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Juan, Y.S.; Lu, M.C.; Chiang, M.Y.; Dai, C.F.; Wu, Y.C.; Sung, P.J. Pregnane-type steroids from the Formosan soft coral Scleronephthya flexilis. Int. J. Mol. Sci. 2014, 15, 10136–10149. [Google Scholar] [CrossRef]
- Elkhayat, E.S.; Ibrahim, S.R.M.; Fouad, M.A.; Mohamed, G.A. Dendronephthols A–C, new sesquiterpenoids from the Red Sea soft coral Dendronephthya sp. Tetrahedron 2014, 70, 3822–3825. [Google Scholar] [CrossRef]
- Chang, F.Y.; Hsu, F.J.; Tai, C.J.; Wei, W.C.; Yang, N.S.; Sheu, J.H. Klymollins T-X, bioactive eunicellin-based diterpenoids from the soft coral Klyxum molle. Mar. Drugs 2014, 12, 3060–3071. [Google Scholar] [CrossRef]
- Al-Footy, K.O.; Alarif, W.M.; Asiri, F.; Aly, M.M.; Ayyad, S.-E.N. Rare pyrane-based cembranoids from the Red Sea soft coral Sarcophyton trocheliophorum as potential antimicrobial–antitumor agents. Med. Chem. Res. 2014, 24, 505–512. [Google Scholar] [CrossRef]
- Lei, L.-F.; Chen, M.-F.; Wang, T.; He, X.-X.; Liu, B.-X.; Deng, Y.; Chen, X.-J.; Li, Y.-T.; Guan, S.-Y.; Yao, J.-H.; et al. Novel cytotoxic nine-membered macrocyclic polysulfur cembranoid lactones from the soft coral Sinularia sp. Tetrahedron 2014, 70, 6851–6858. [Google Scholar] [CrossRef]
- Roy, P.K.; Roy, M.C.; Taira, J.; Ueda, K. Structure and bioactivity of a trisnorditerpenoid and a diterpenoid from an Okinawan soft coral, Cespitularia sp. Tetrahedron Lett. 2014, 55, 1421–1423. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Shih, N.L.; Hou, K.Y.; Ger, M.J.; Yang, C.N.; Wang, S.K.; Duh, C.Y. Kelsoenethiol and dikelsoenyl ether, two unique kelsoane-type sesquiterpenes, from the Formosan soft coral Nephthea erecta. Bioorg. Med. Chem. Lett. 2014, 24, 473–475. [Google Scholar] [CrossRef]
- Al-Lihaibi, S.S.; Alarif, W.M.; Abdel-Lateff, A.; Ayyad, S.E.; Abdel-Naim, A.B.; El-Senduny, F.F.; Badria, F.A. Three new cembranoid-type diterpenes from Red Sea soft coral Sarcophyton glaucum: Isolation and antiproliferative activity against HepG2 cells. Eur. J. Med. Chem. 2014, 81, 314–322. [Google Scholar] [CrossRef]
- Tseng, Y.J.; Yang, Y.C.; Wang, S.K.; Duh, C.Y. Numerosol A-D, new cembranoid diterpenes from the soft coral Sinularia numerosa. Mar. Drugs 2014, 12, 3371–3380. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wang, S.S.; Chen, C.H.; Kuo, Y.H.; Shen, Y.C. Cespitulones A and B, cytotoxic diterpenoids of a new structure class from the soft coral Cespitularia taeniata. Mar. Drugs 2014, 12, 3477–3486. [Google Scholar] [CrossRef]
- Zhang, N.X.; Tang, X.L.; van Ofwegen, L.; Xue, L.; Song, W.J.; Li, P.L.; Li, G.Q. Cyclopentenone derivatives and polyhydroxylated steroids from the soft coral Sinularia acuta. Chem. Biodivers. 2015, 12, 273–283. [Google Scholar] [CrossRef]
- Nam, N.H.; Tung, P.T.; Ngoc, N.T.; Hanh, T.T.H.; Thao, N.P.; Thanh, N.V.; Cuong, N.X.; Thao, D.T.; Huong, T.T.; Thung, D.C.; et al. Cytotoxic biscembranoids from the soft coral Sarcophyton pauciplicatum. Chem. Pharm. Bull. 2015, 63, 636–640. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wang, S.K.; Hsieh, M.K.; Duh, C.Y. Polyoxygenated cembrane diterpenoids from the soft coral Sarcophyton Ehrenbergi. Int. J. Mol. Sci. 2015, 16, 6140–6152. [Google Scholar] [CrossRef]
- Hsiao, T.H.; Sung, C.S.; Lan, Y.H.; Wang, Y.C.; Lu, M.C.; Wen, Z.H.; Wu, Y.C.; Sung, P.J. New anti-inflammatory cembranes from the cultured soft coral Nephthea Columnaris. Mar. Drugs 2015, 13, 3443–3453. [Google Scholar] [CrossRef]
- Koncic, M.Z.; Ioannou, E.; Sawadogo, W.R.; Abdel-Razik, A.F.; Vagias, C.; Diederich, M.; Roussis, V. 4alpha-methylated steroids with cytotoxic activity from the soft coral Litophyton mollis. Steroids 2016, 115, 130–135. [Google Scholar] [CrossRef]
- Tsai, T.C.; Huang, Y.T.; Chou, S.K.; Shih, M.C.; Chiang, C.Y.; Su, J.H. Cytotoxic oxygenated steroids from the soft coral Nephthea erecta. Chem. Pharm. Bull. 2016, 64, 1519–1522. [Google Scholar] [CrossRef]
- Urda, C.; Fernandez, R.; Perez, M.; Rodriguez, J.; Jimenez, C.; Cuevas, C. Protoxenicins A and B, cytotoxic long-chain acylated Xenicanes from the soft coral Protodendron repens. J. Nat. Prod. 2017, 80, 713–719. [Google Scholar] [CrossRef]
- Chao, C.H.; Li, W.L.; Huang, C.Y.; Ahmed, A.F.; Dai, C.F.; Wu, Y.C.; Lu, M.C.; Liaw, C.C.; Sheu, J.H. Isoprenoids from the soft coral Sarcophyton glaucum. Mar. Drugs 2017, 15, 202. [Google Scholar] [CrossRef]
- Mohammed, R.; Radwan, M.M.; Ma, G.; Mohamed, T.A.; Seliem, M.A.; Thabet, M.; ElSohly, M.A. Bioactive sterols and sesquiterpenes from the Red Sea soft coral Sinularia terspilli. Med. Chem. Res. 2017, 26, 1647–1652. [Google Scholar] [CrossRef]
- Wu, C.H.; Chao, C.H.; Huang, T.Z.; Huang, C.Y.; Hwang, T.L.; Dai, C.F.; Sheu, J.H. Cembranoid-related metabolites and biological activities from the soft coral Sinularia flexibilis. Mar. Drugs 2018, 16, 278. [Google Scholar] [CrossRef]
- Livermore, D.M. Has the era of untreatable infections arrived? J. Antimicrob. Chemother. 2009, 64 (Suppl. 1), i29–i36. [Google Scholar] [CrossRef]
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heuer, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef]
- Hughes, D.; Andersson, D.I. Environmental and genetic modulation of the phenotypic expression of antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 374–391. [Google Scholar] [CrossRef]
- Genilloud, O. The re-emerging role of microbial natural products in antibiotic discovery. Antonie Van Leeuwenhoek 2014, 106, 173–188. [Google Scholar] [CrossRef]
- Projan, S.J. Why is big Pharma getting out of antibacterial drug discovery? Curr. Opin. Microbiol. 2003, 6, 427–430. [Google Scholar] [CrossRef]
- Bax, R.; Mullan, N.; Verhoef, J. The millennium bugs—The need for and development of new antibacterials. Int. J. Antimicrob. Agents 2000, 16, 51–59. [Google Scholar] [CrossRef]
- Zubair, M.; Alarif, W.; Al-Footy, K.; Ph, M.; Ali, M.; Basaif, S.; Al-Lihaibi, S.; Ayyad, S.-E. New antimicrobial biscembrane hydrocarbon and cembranoid diterpenes from the soft coral Sarcophyton trocheliophorum. Turk. J. Chem. 2016, 40, 385–392. [Google Scholar] [CrossRef]
- Lou, Z.; Sun, Y.; Rao, Z. Current progress in antiviral strategies. Trends Pharmacol. Sci. 2014, 35, 86–102. [Google Scholar] [CrossRef]
- Lurain, N.S.; Chou, S. Antiviral drug resistance of human cytomegalovirus. Clin. Microbiol. Rev. 2010, 23, 689–712. [Google Scholar] [CrossRef]
- Tantillo, C.; Ding, J.; Jacobo-Molina, A.; Nanni, R.G.; Boyer, P.L.; Hughes, S.H.; Pauwels, R.; Andries, K.; Janssen, P.A.J.; Arnold, E. Locations of Anti-AIDS Drug Binding Sites and Resistance Mutations in the Three-dimensional Structure of HIV-1 Reverse Transcriptase: Implications for Mechanisms of Drug Inhibition and Resistance. J. Mol. Biol. 1994, 243, 369–387. [Google Scholar] [CrossRef]
- Morfin, F.; Thouvenot, D. Herpes simplex virus resistance to antiviral drugs. J. Clin. Virol. 2003, 26, 29–37. [Google Scholar] [CrossRef]
- Wang, S.K.; Hsieh, M.K.; Duh, C.Y. New diterpenoids from soft coral Sarcophyton ehrenbergi. Mar. Drugs 2013, 11, 4318–4327. [Google Scholar] [CrossRef]
- Tseng, Y.J.; Wang, S.K.; Duh, C.Y. Secosteroids and norcembranoids from the soft coral Sinularia nanolobata. Mar. Drugs 2013, 11, 3288–3296. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Wang, S.K.; Duh, C.Y. Secocrassumol, a seco-cembranoid from the Dongsha Atoll soft coral Lobophytum crassum. Mar. Drugs 2014, 12, 6028–6037. [Google Scholar] [CrossRef]
- Yuan, W.; Cheng, S.; Fu, W.; Zhao, M.; Li, X.; Cai, Y.; Dong, J.; Huang, K.; Gustafson, K.R.; Yan, P. Structurally diverse metabolites from the soft coral Sinularia verruca collected in the South China sea. J. Nat. Prod. 2016, 79, 1124–1131. [Google Scholar] [CrossRef]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coat. 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Davidson, I.C.; Brown, C.W.; Sytsma, M.D.; Ruiz, G.M. The role of containerships as transfer mechanisms of marine biofouling species. Biofouling 2009, 25, 645–655. [Google Scholar] [CrossRef]
- Schultz, M.P.; Bendick, J.A.; Holm, E.R.; Hertel, W.M. Economic impact of biofouling on a naval surface ship. Biofouling 2011, 27, 87–98. [Google Scholar] [CrossRef]
- Omae, I. Organotin antifouling paints and their alternatives. Appl. Organomet. Chem. 2003, 17, 81–105. [Google Scholar] [CrossRef]
- Qian, P.-Y.; Xu, Y.; Fusetani, N. Natural products as antifouling compounds: Recent progress and future perspectives. Biofouling 2009, 26, 223–234. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. Worldwide occurrence and effects of antifouling paint booster biocides in the aquatic environment: A review. Environ. Int. 2004, 30, 235–248. [Google Scholar] [CrossRef]
- Cresswell, T.; Richards, J.P.; Glegg, G.A.; Readman, J.W. The impact of legislation on the usage and environmental concentrations of Irgarol 1051 in UK coastal waters. Mar. Pollut. Bull. 2006, 52, 1169–1175. [Google Scholar] [CrossRef]
- Muñoz, I.; Martínez Bueno, M.J.; Agüera, A.; Fernández-Alba, A.R. Environmental and human health risk assessment of organic micro-pollutants occurring in a Spanish marine fish farm. Environ. Pollut. 2010, 158, 1809–1816. [Google Scholar] [CrossRef]
- Kwong, T.F.N.; Miao, L.; Li, X.; Qian, P.Y. Novel Antifouling and Antimicrobial Compound from a Marine-Derived Fungus Ampelomyces sp. Mar. Biotechnol. 2006, 8, 634–640. [Google Scholar] [CrossRef]
- Shi, H.; Yu, S.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W. Sinularones A-I, new cyclopentenone and butenolide derivatives from a marine soft coral Sinularia sp. and their antifouling activity. Mar. Drugs 2012, 10, 1331–1344. [Google Scholar] [CrossRef]
- Chen, D.; Chen, W.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W. Asteriscane-type sesquiterpenoids from the soft coral Sinularia capillosa. J. Nat. Prod. 2013, 76, 1753–1763. [Google Scholar] [CrossRef]
- Lai, D.; Geng, Z.; Deng, Z.; van Ofwegen, L.; Proksch, P.; Lin, W. Cembranoids from the soft coral Sinularia rigida with antifouling activities. J. Agric. Food Chem. 2013, 61, 4585–4592. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.-C.; Wang, K.-L.; Liao, X.-J.; Deng, Z.; Xu, S.-H. Pentacyclic hemiacetal sterol with antifouling and cytotoxic activities from the soft coral Nephthea sp. Bioorg. Med. Chem. Lett. 2013, 23, 1079–1082. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Wang, K.L.; Liao, X.J.; Deng, Z.; Xu, S.H. Antifouling steroids from the South China Sea gorgonian coral Subergorgia suberosa. Steroids 2014, 79, 1–6. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wu, H.-X.; Xu, Y.; Shao, C.-L.; Wang, C.-Y.; Qian, P.-Y. Antifouling Activity of Secondary Metabolites Isolated from Chinese Marine Organisms. Mar. Biotechnol. 2013, 15, 552–558. [Google Scholar] [CrossRef]
- Wang, J.; Su, P.; Gu, Q.; Li, W.D.; Guo, J.L.; Qiao, W.; Feng, D.Q.; Tang, S.A. Antifouling activity against bryozoan and barnacle by cembrane diterpenes from the soft coral Sinularia flexibilis. Int. Biodeterior. Biodegrad. 2017, 120, 97–103. [Google Scholar] [CrossRef]
- Quang, T.H.; Ha, T.T.; Minh, C.V.; Kiem, P.V.; Huong, H.T.; Ngan, N.T.; Nhiem, N.X.; Tung, N.H.; Thao, N.P.; Thuy, D.T.; et al. Cytotoxic and PPARs transcriptional activities of sterols from the Vietnamese soft coral Lobophytum laevigatum. Bioorg. Med. Chem. Lett. 2011, 21, 2845–2849. [Google Scholar] [CrossRef]
- Liang, L.-F.; Kurtán, T.; Mándi, A.; Gao, L.-X.; Li, J.; Zhang, W.; Guo, Y.-W. Sarsolenane and capnosane diterpenes from the Hainan soft coral Sarcophyton trocheliophorum Marenzeller as PTP1B Inhibitors. Eur. J. Org. Chem. 2014, 2014, 1841–1847. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, Q.; Wang, J.; Liu, J.; Qi, C.; Lai, Y.; Zhu, H.; Xue, Y.; Hu, Z.; Zhang, Y. Anti-inflammatory butenolide derivatives from the coral-derived fungus Aspergillus terreus and structure revisions of aspernolides D and G, butyrolactone VI and 4′,8′′-diacetoxy butyrolactone VI. RSC Adv. 2018, 8, 13040–13047. [Google Scholar] [CrossRef]
- Zheng, C.J.; Shao, C.L.; Guo, Z.Y.; Chen, J.F.; Deng, D.S.; Yang, K.L.; Chen, Y.Y.; Fu, X.M.; She, Z.G.; Lin, Y.C.; et al. Bioactive hydroanthraquinones and anthraquinone dimers from a soft coral-derived Alternaria sp. fungus. J. Nat. Prod. 2012, 75, 189–197. [Google Scholar] [CrossRef]
- Li, H.J.; Xie, Y.L.; Xie, Z.L.; Chen, Y.; Lam, C.K.; Lan, W.J. Chondrosterins A-E, triquinane-type sesquiterpenoids from soft coral-associated fungus Chondrostereum sp. Mar. Drugs 2012, 10, 627–638. [Google Scholar] [CrossRef]
- Fu, P.; Kong, F.; Wang, Y.; Wang, Y.; Liu, P.; Zuo, G.; Zhu, W. Antibiotic metabolites from the coral-associated Actinomycete Streptomyces sp. OUCMDZ-1703. Chin. J. Chem. 2013, 31, 100–104. [Google Scholar] [CrossRef]
- Wang, W.; Liao, Y.; Tang, C.; Huang, X.; Luo, Z.; Chen, J.; Cai, P. Cytotoxic and antibacterial compounds from the coral-derived fungus Aspergillus tritici SP2-8-1. Mar. Drugs 2017, 15, 348. [Google Scholar] [CrossRef]
- Wei, M.Y.; Li, D.; Shao, C.L.; Deng, D.S.; Wang, C.Y. (±)-Pestalachloride D, an antibacterial racemate of chlorinated benzophenone derivative from a soft coral-derived fungus Pestalotiopsis sp. Mar. Drugs 2013, 11, 1050–1060. [Google Scholar] [CrossRef]
- Zheng, C.J.; Shao, C.L.; Wu, L.Y.; Chen, M.; Wang, K.L.; Zhao, D.L.; Sun, X.P.; Chen, G.Y.; Wang, C.Y. Bioactive phenylalanine derivatives and cytochalasins from the soft coral-derived fungus, Aspergillus elegans. Mar. Drugs 2013, 11, 2054–2068. [Google Scholar] [CrossRef]
- Shao, C.L.; Xu, R.F.; Wang, C.Y.; Qian, P.Y.; Wang, K.L.; Wei, M.Y. Potent antifouling marine dihydroquinolin-2(1H)-one-containing alkaloids from the Gorgonian coral-derived fungus Scopulariopsis sp. Mar. Biotechnol. 2015, 17, 408–415. [Google Scholar] [CrossRef]
- Jia, Y.-L.; Guan, F.-F.; Ma, J.; Wang, C.-Y.; Shao, C.-L. Pestalotiolide A, a new antiviral phthalide derivative from a soft coral-derived fungus Pestalotiopsissp. Nat. Prod. Sci. 2015, 21, 227. [Google Scholar] [CrossRef]
- Bao, J.; Sun, Y.L.; Zhang, X.Y.; Han, Z.; Gao, H.C.; He, F.; Qian, P.Y.; Qi, S.H. Antifouling and antibacterial polyketides from marine gorgonian coral-associated fungus Penicillium sp. SCSGAF 0023. J. Antibiot. 2013, 66, 219–223. [Google Scholar] [CrossRef]
- Zhuang, Y.; Teng, X.; Wang, Y.; Liu, P.; Wang, H.; Li, J.; Li, G.; Zhu, W. Cyclopeptides and polyketides from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Tetrahedron 2011, 67, 7085–7089. [Google Scholar] [CrossRef]
- Hawas, U.W.; El-Desouky, S.; Abou El-Kassem, L.; Elkhateeb, W. Alternariol derivatives from Alternaria alternata, an endophytic fungus residing in red sea soft coral, inhibit HCV NS3/4A protease. Appl. Biochem. Microbiol. 2015, 51, 579–584. [Google Scholar] [CrossRef]
- Zhu, M.; Gao, H.; Wu, C.; Zhu, T.; Che, Q.; Gu, Q.; Guo, P.; Li, D. Lipid-lowering polyketides from a soft coral-derived fungus Cladosporium sp. TZP29. Bioorg. Med. Chem. Lett. 2015, 25, 3606–3609. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, G.; Chen, S.; Liu, Y.; Li, H.; She, Z. Eurothiocin A and B, sulfur-containing benzofurans from a soft coral-derived fungus Eurotium rubrum SH-823. Mar. Drugs 2014, 12, 3669–3680. [Google Scholar] [CrossRef]
- Liu, M.; Qi, C.; Sun, W.; Shen, L.; Wang, J.; Liu, J.; Lai, Y.; Xue, Y.; Hu, Z.; Zhang, Y. α-Glucosidase inhibitors from the coral-associated fungus Aspergillus terreus. Front. Chem. 2018, 6, 422. [Google Scholar] [CrossRef]
- Leal, C.M.; Sheridan, C.; Osinga, R.; Dionísio, G.; Rocha, J.R.; Silva, B.; Rosa, R.; Calado, R. Marine Microorganism-Invertebrate Assemblages: Perspectives to Solve the “Supply Problem” in the Initial Steps of Drug Discovery. Mar. Drugs 2014, 12, 3929–3952. [Google Scholar] [CrossRef]
- Martins, A.; Vieira, H.; Gaspar, H.; Santos, S. Marketed marine natural products in the pharmaceutical and cosmeceutical industries: Tips for success. Mar. Drugs 2014, 12, 1066–1101. [Google Scholar] [CrossRef]
- Carson, M.A.; Clarke, S.A. Bioactive Compounds from Marine Organisms: Potential for Bone Growth and Healing. Mar. Drugs 2018, 16, 340. [Google Scholar] [CrossRef]
- Xue-Mei, H.; Ru-Fang, X.; Yu-Cheng, G.; Chang-Yun, W.; Chang-Lun, S. Biological and Chemical Diversity of Coral-Derived Microorganisms. Curr. Med. Chem. 2015, 22, 3707–3762. [Google Scholar]
- Fehmida, B.; Muhammad, F.; Esam, I.A.; Muhammad, Y.; Sana, A.A.; Mohammad, A.K.; Ikram, U.; Muhammad, I.N. Bacteria from Marine Sponges: A Source of New Drugs. Curr. Drug Metab. 2017, 18, 11–15. [Google Scholar]
- Thomas, T.R.A.; Kavlekar, D.P.; LokaBharathi, P.A. Marine drugs from sponge-microbe association--a review. Mar. Drugs 2010, 8, 1417–1468. [Google Scholar] [CrossRef]
- Raimundo, I.; Silva, S.G.; Costa, R.; Keller-Costa, T. Bioactive Secondary Metabolites from Octocoral-Associated Microbes-New Chances for Blue Growth. Mar. Drugs 2018, 16, 485. [Google Scholar] [CrossRef]
- Keller-Costa, T.; Eriksson, D.; Gonçalves, J.M.S.; Gomes, N.C.M.; Lago-Lestón, A.; Costa, R. The gorgonian coral Eunicella labiata hosts a distinct prokaryotic consortium amenable to cultivation. FEMS Microbiol. Ecol. 2017, 93, fix143. [Google Scholar] [CrossRef]
- Koren, O.; Rosenberg, E. Bacteria associated with mucus and tissues of the coral Oculina patagonica in summer and winter. Appl. Environ. Microbiol. 2006, 72, 5254–5259. [Google Scholar] [CrossRef]
- Nai, C.; Meyer, V. From Axenic to Mixed Cultures: Technological Advances Accelerating a Paradigm Shift in Microbiology. Trends Microbiol. 2018, 26, 538–554. [Google Scholar] [CrossRef]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2012, 11, 21–32. [Google Scholar] [CrossRef]
- Seyedsayamdost, M.R. High-throughput platform for the discovery of elicitors of silent bacterial gene clusters. Proc. Natl. Acad. Sci. USA 2014, 111, 7266–7271. [Google Scholar] [CrossRef]
- Benkendorff, K. 28—Aquaculture and the production of pharmaceuticals and nutraceuticals. In New Technologies in Aquaculture; Burnell, G., Allan, G., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 866–891. [Google Scholar]
- Pomeroy, R.S.; Parks, J.E.; Balboa, C.M. Farming the reef: Is aquaculture a solution for reducing fishing pressure on coral reefs? Mar. Policy 2006, 30, 111–130. [Google Scholar] [CrossRef]
- Leal, M.C.; Calado, R.; Sheridan, C.; Alimonti, A.; Osinga, R. Coral aquaculture to support drug discovery. Trends Biotechnol. 2013, 31, 555–561. [Google Scholar] [CrossRef]
- Mendola, D. Aquaculture of three phyla of marine invertebrates to yield bioactive metabolites: Process developments and economics. Biomol. Eng. 2003, 20, 441–458. [Google Scholar] [CrossRef]
- Loureiro, C.; Medema, M.H.; van der Oost, J.; Sipkema, D. Exploration and exploitation of the environment for novel specialized metabolites. Curr. Opin. Biotechnol. 2018, 50, 206–213. [Google Scholar] [CrossRef]
- Faisal, M.; Saeed, A.; Shahzad, D. Chapter 5–Portrait of the synthesis of some potent anti-inflammatory natural products. In Discovery and Development of Anti-Inflammatory Agents from Natural Products; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 141–183. [Google Scholar]
- Barbosa, L.C.A.; Varejão, J.O.S.; Varejão, E.V.V. Chapter 3—Strategies for Total Synthesis of Furanocembranolides and Related Natural Products from Marine Organisms. In Studies in Natural Products Chemistry; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 52, pp. 115–157. [Google Scholar]
- Nicolaou, K.C.; Xu, J.Y.; Kim, S.; Pfefferkorn, J.; Ohshima, T.; Vourloumis, D.; Hosokawa, S. Total Synthesis of Sarcodictyins A and B. J. Am. Chem. Soc. 1998, 120, 8661–8673. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Xu, J.Y.; Kim, S.; Ohshima, T.; Hosokawa, S.; Pfefferkorn, J. Synthesis of the Tricyclic Core of Eleutherobin and Sarcodictyins and Total Synthesis of Sarcodictyin A. J. Am. Chem. Soc. 1997, 119, 11353–11354. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; van Delft, F.; Ohshima, T.; Vourloumis, D.; Xu, J.; Hosokawa, S.; Pfefferkorn, J.; Kim, S.; Li, T. Total Synthesis of Eleutherobin. Angew. Chem. Int. Ed. Engl. 1997, 36, 2520–2524. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Ohshima, T.; Hosokawa, S.; van Delft, F.L.; Vourloumis, D.; Xu, J.Y.; Pfefferkorn, J.; Kim, S. Total Synthesis of Eleutherobin and Eleuthosides A and B. J. Am. Chem. Soc. 1998, 120, 8674–8680. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Kim, S.; Pfefferkorn, J.; Xu, J.; Ohshima, T.; Hosokawa, S.; Vourloumis, D.; Li, T. Synthesis and Biological Activity of Sarcodictyins. Angew. Chem. Int. Ed. 1998, 37, 1418–1421. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sang, V.T.; Dat, T.T.H.; Vinh, L.B.; Cuong, L.C.V.; Oanh, P.T.T.; Ha, H.; Kim, Y.H.; Anh, H.L.T.; Yang, S.Y. Coral and Coral-Associated Microorganisms: A Prolific Source of Potential Bioactive Natural Products. Mar. Drugs 2019, 17, 468. https://doi.org/10.3390/md17080468
Sang VT, Dat TTH, Vinh LB, Cuong LCV, Oanh PTT, Ha H, Kim YH, Anh HLT, Yang SY. Coral and Coral-Associated Microorganisms: A Prolific Source of Potential Bioactive Natural Products. Marine Drugs. 2019; 17(8):468. https://doi.org/10.3390/md17080468
Chicago/Turabian StyleSang, Vo Thanh, Ton That Huu Dat, Le Ba Vinh, Le Canh Viet Cuong, Phung Thi Thuy Oanh, Hoang Ha, Young Ho Kim, Hoang Le Tuan Anh, and Seo Young Yang. 2019. "Coral and Coral-Associated Microorganisms: A Prolific Source of Potential Bioactive Natural Products" Marine Drugs 17, no. 8: 468. https://doi.org/10.3390/md17080468
APA StyleSang, V. T., Dat, T. T. H., Vinh, L. B., Cuong, L. C. V., Oanh, P. T. T., Ha, H., Kim, Y. H., Anh, H. L. T., & Yang, S. Y. (2019). Coral and Coral-Associated Microorganisms: A Prolific Source of Potential Bioactive Natural Products. Marine Drugs, 17(8), 468. https://doi.org/10.3390/md17080468




