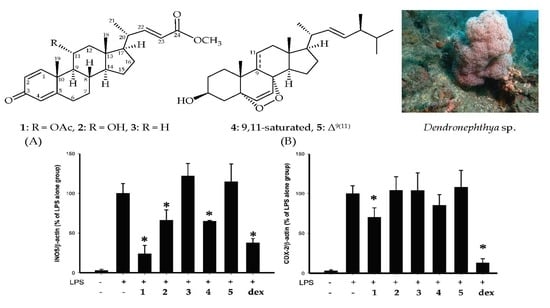
New 1,4-Dienonesteroids from the Octocoral Dendronephthya sp.
Abstract
1. Introduction
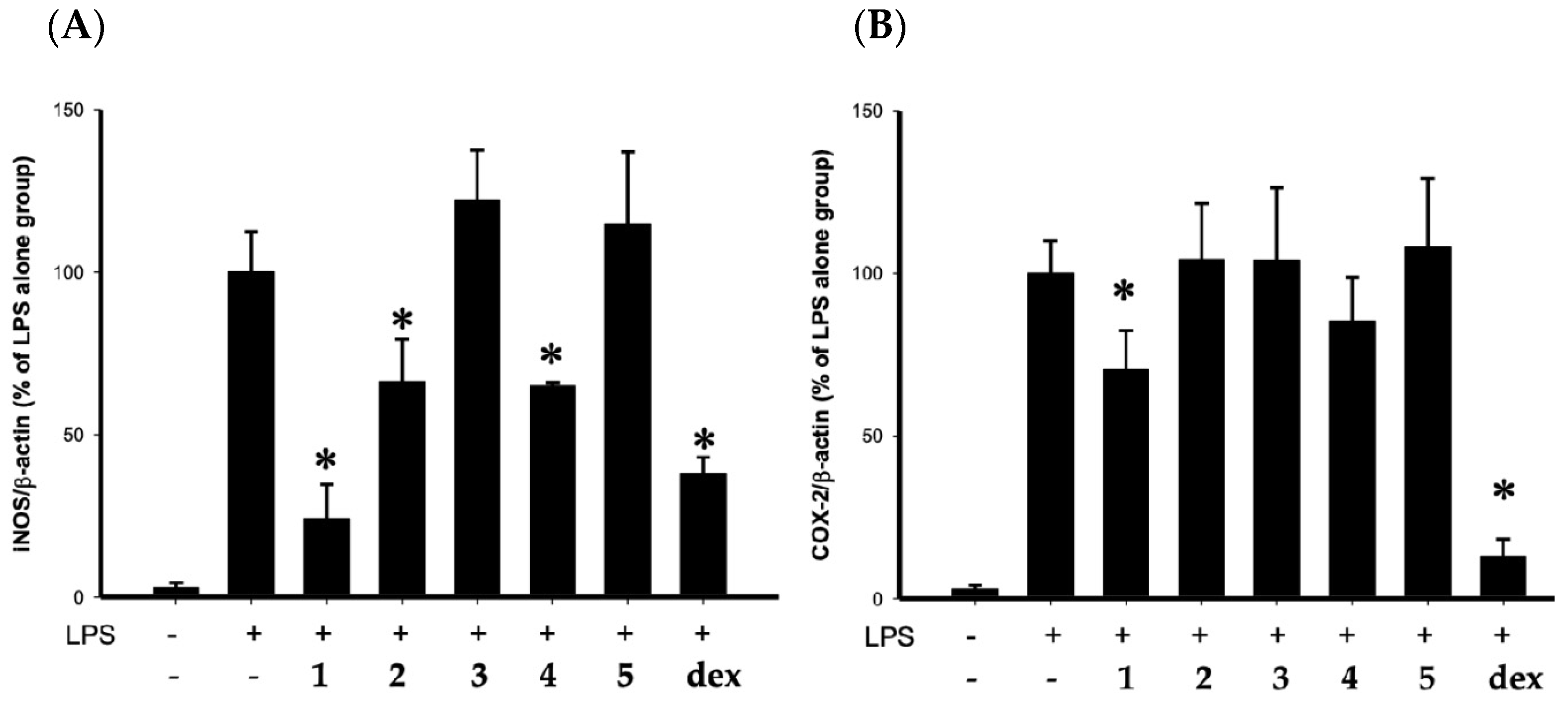
2. Results
3. Discussion
4. Experimental Section
4.1. General Experimental Procedures
4.2. Animal Material
4.3. Extraction and Separation
4.4. In Vitro Anti-Inflammatory Assay
4.5. Cell Viability
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [PubMed]
- Duh, C.Y.; El-Gamal, A.A.H.; Song, P.Y.; Wang, S.K.; Dai, C.F. Steroids and sesquiterpenoids from the soft corals Dendronephthya gigantea and Lemnalia cervicorni. J. Nat. Prod. 2004, 67, 1650–1653. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.H.; Wen, Z.H.; Chen, I.M.; Su, J.H.; Huang, H.C.; Chiang, M.Y.; Sheu, J.H. Anti-inflammatory steroids from the octocoral Dendronephthya griffini. Tetrahedron 2008, 64, 3554–3560. [Google Scholar] [CrossRef]
- Chao, C.H.; Wen, Z.H.; Su, J.H.; Chen, I.M.; Huang, H.C.; Dai, C.F.; Sheu, J.H. Further study on anti- inflammatory oxygenated steroids from the octocoral Dendronephthya griffini. Steroids 2008, 73, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Tomono, Y.; Hirota, H.; Imahara, Y.; Fusetani, N. Four new steroids from two octocorals. J. Nat. Prod. 1999, 62, 1538–1541. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, E.; Abdel-Razik, A.F.; Zervou, M.; Christofidis, D.; Alexi, X.; Vagias, C.; Alexis, M.N.; Roussis, V. 5α,8α-Epidioxysterols from the gorgonian Eunicella cavolini and the ascidian Trididemnum inarmatum: Isolation and evaluation of their antiproliferative activity. Steroids 2009, 74, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Gunatilaka, A.A.L.; Gopichand, Y.; Schmitz, F.J.; Djerassi, C. Minor and trace sterols in marine invertebrates. 26. Isolation and strucutre elucidation of nine new 5α,8α-epidioxy sterols from four marine organisms. J. Org. Chem. 1981, 46, 3860–3866. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chang, C.W.; Tseng, Y.J.; Lee, J.; Sung, P.J.; Su, J.H.; Hwang, T.L.; Dai, C.F.; Wang, H.C.; Sheu, J.H. Bioactive steroids from the Formosan soft coral Umbellulifera petasites. Mar. Drugs 2016, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Liaw, C.C.; Chen, B.W.; Chen, P.C.; Su, J.H.; Sung, P.-J.; Dai, C.F.; Chiang, M.Y.; Sheu, J.H. Withanolide-based steroids from the cultured soft coral Sinularia brassica. J. Nat. Prod. 2013, 76, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.H.; Liu, H.L.; Huang, H.; Li, X.B.; Guo, Y.W. Steroids with aromatic A-rings from the Hainan soft coral Dendronephthya studeri Ridley. J. Nat. Prod. 2011, 74, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Tomono, Y.; Hirota, H.; Fusetani, N. Isogosterones A–D, antifouling 13,17-secosteroids from an octocoral Dendronephthya sp. J. Org. Chem. 1999, 64, 2272–2275. [Google Scholar] [CrossRef]
- Shin, K.; Chin, J.; Hahn, D.; Lee, J.; Hwang, H.; Won, D.H.; Ham, J.; Choi, H.; Kang, E.; Kim, H.; et al. Sterols from a soft coral, Dendronephthya gigantea as farnesoid X-activated receptor antagonists. Steroids 2012, 77, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Sanjeewa, K.K.S.; Kim, H.S.; Kim, S.Y.; Jeon, Y.J. Identification of sterols from the soft coral Dendronephthya gigantea and their anti-inflammatory potential. Environ. Toxicol. Pharm. 2017, 55, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.A.; Ding, Y.; Yang, H.W.; Heo, S.J.; Lee, S.H. Soft coral Dendronephthya puetteri extract ameliorates inflammations by suppressing inflammatory mediators and oxidative stress in LPS-stimulated zebrafish. Int. J. Mol. Sci. 2018, 19, 2695. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, K.; Alderslade, P. Soft Corals and Sea Fans–A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea, 1st ed.; Australian Institute of Marine Science: Townsville, Queensland, Australia, 2001; pp. 50–51; 112–115. [Google Scholar]
- Hung, H.C.; Feng, C.W.; Lin, Y.Y.; Chen, C.H.; Tsui, K.H.; Chen, W.F.; Pan, C.Y.; Sheu, J.H.; Sung, C.S.; Wen, Z.H. Nucleophosmin modulates the alleviation of atopic dermatitis caused by the marine-derived compound dihydroaustrasulfone alcohol. Exp. Mol. Med. 2018, 50, e446. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.H.; Chen, W.F.; Duh, C.Y.; Huang, S.Y.; Hsu, C.H.; Lin, C.S.; Sung, C.S.; Chen, I.M.; Wen, Z.H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 2008, 578, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Y.; Lin, S.C.; Feng, C.W.; Chen, P.C.; Su, Y.D.; Li, C.M.; Yang, S.N.; Jean, Y.H.; Sung, P.J.; Duh, C.Y.; et al. Anti-inflammatory and analgesic effects of the marine-derived compound excavatolide B isolated from the culture-type Formosan gorgonian Briareum excavatum. Mar. Drugs 2015, 13, 2559–2579. [Google Scholar] [CrossRef] [PubMed]
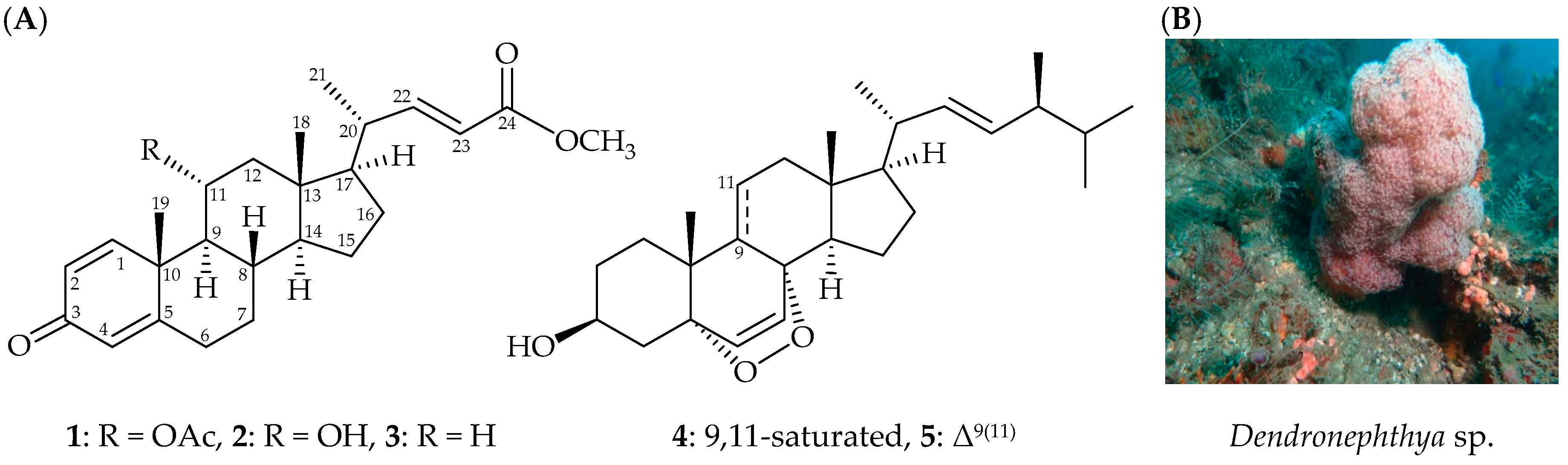
 ) correlations and selective HMBC (
) correlations and selective HMBC ( ) of steroids 1 and 2.
) of steroids 1 and 2.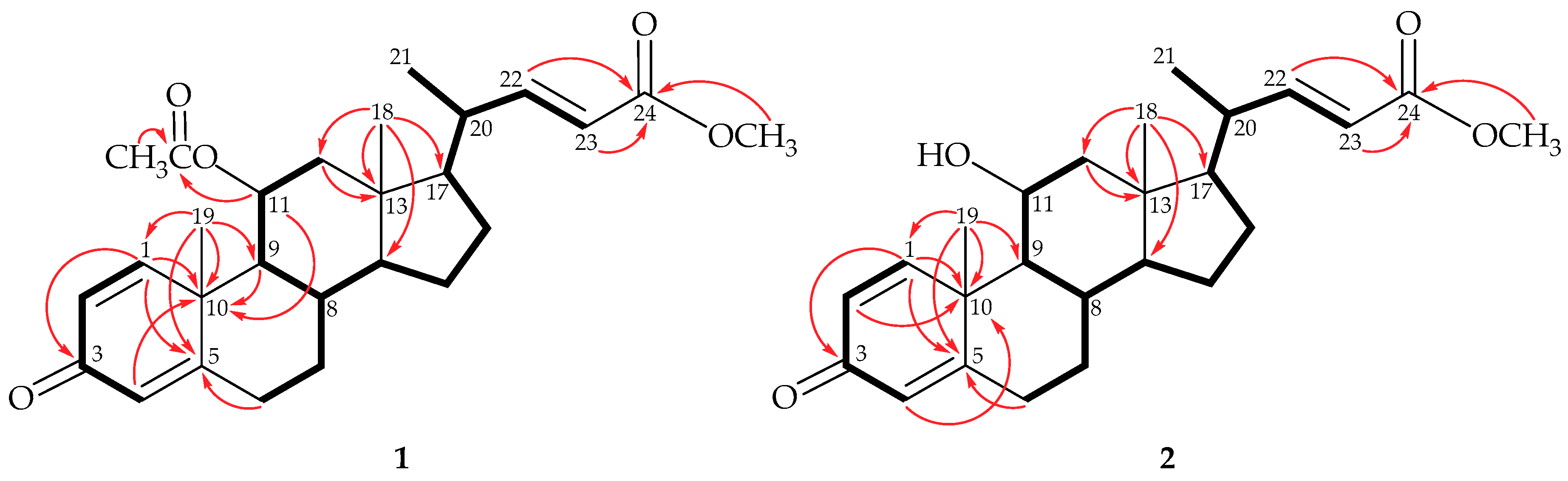
 ) of 1.
) of 1.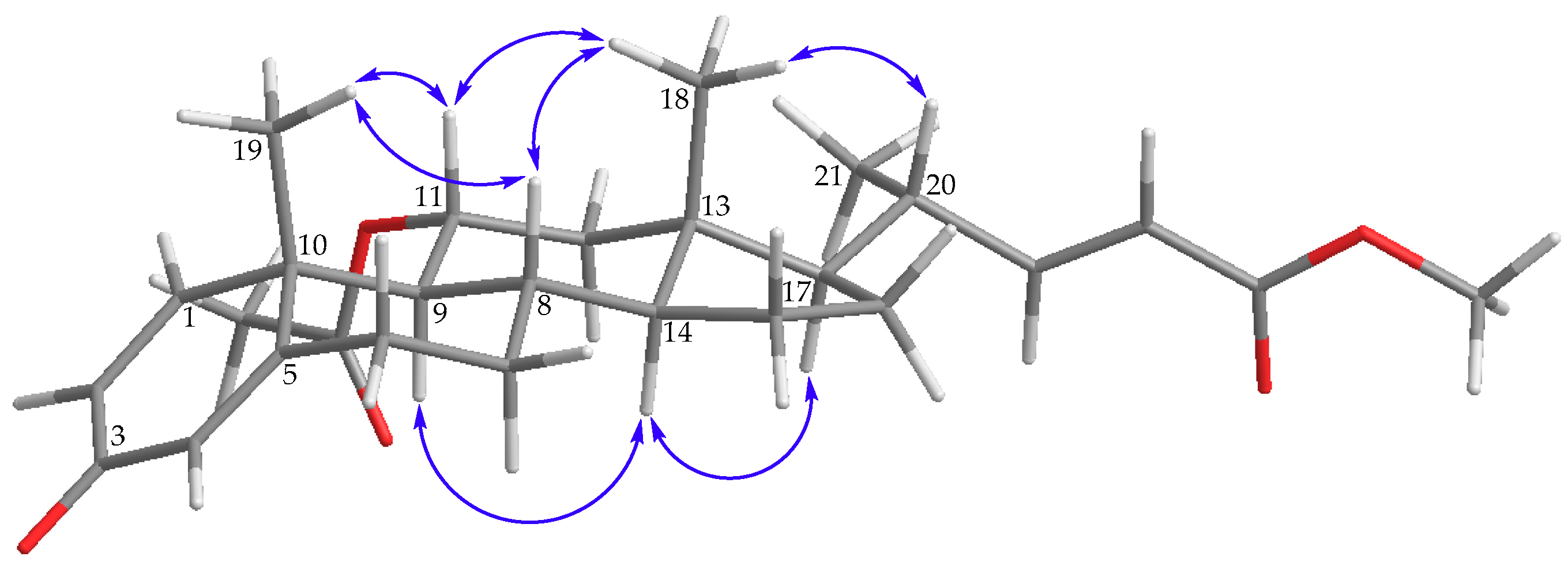
 ) of 2.
) of 2.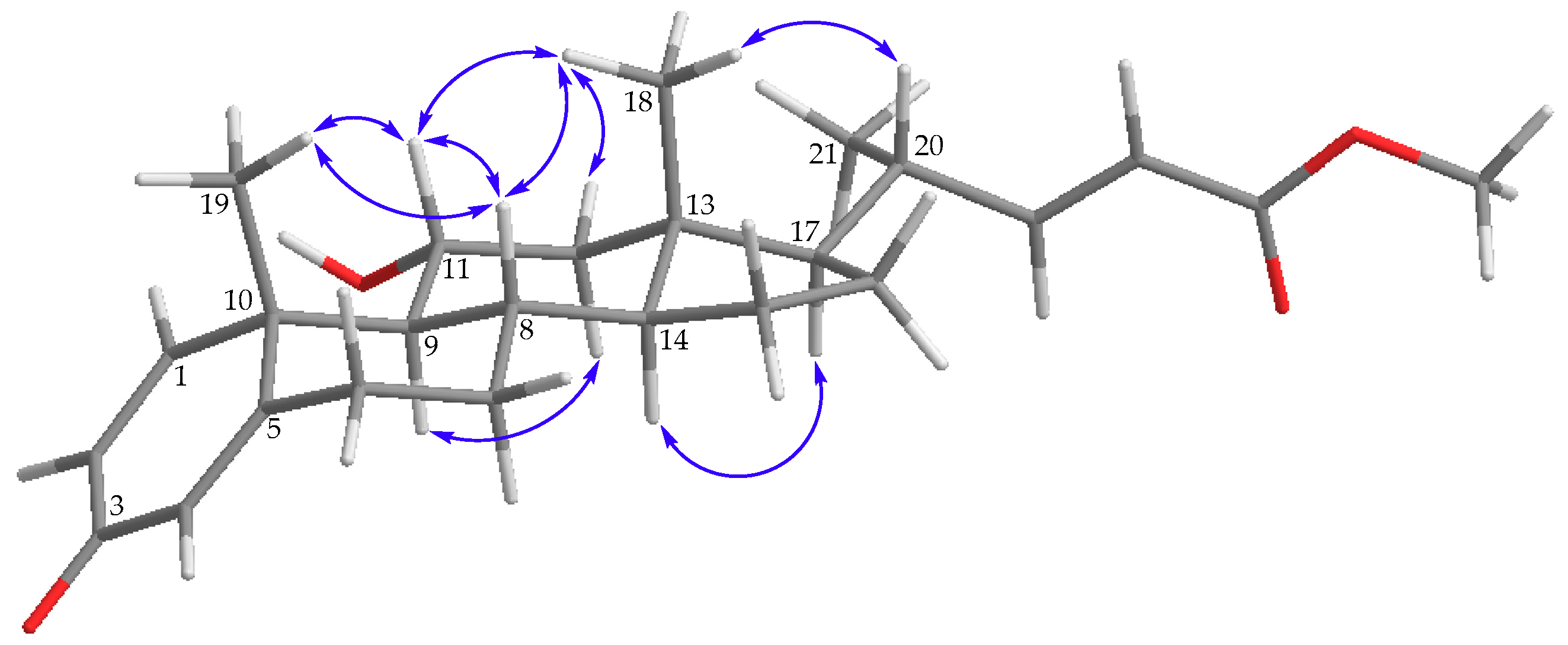
| 1 | 2 | ||||
|---|---|---|---|---|---|
| C/H | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | |
| 1 | 6.78 d (10.8) | 156.2 (CH) | 7.74 d (10.8) | 158.8 (CH) | |
| 2 | 6.13 dd (10.8, 2.0) | 125.7 (CH) | 6.15 dd (10.8, 2.0) | 125.1 (CH) | |
| 3 | 186.2 (C) | 183.8 (C) | |||
| 4 | 6.10 dd (2.0, 1.6) | 124.6 (CH) | 6.09 dd (2.0, 1.2) | 124.6 (CH) | |
| 5 | 167.1 (C) | 167.9 (C) | |||
| 6α β | 2.38 ddd (13.2, 4.4, 2.4) 2.48 ddd (13.2, 13.2, 4.8, 0.8) | 32.8 (CH2) | 2.36 ddd (13.2, 4.4, 2.8) 2.45 ddd (13.2, 13.2, 5.2, 1.6) | 33.2 (CH2) | |
| 7α/β | 1.14 m; 1.97 m | 33.3 (CH2) | 1.09 m; 1.96 m | 33.4 (CH2) | |
| 8 | 1.72 m | 34.4 (CH) | 1.61 m | 34.3 (CH) | |
| 9 | 1.39 dd (10.8, 10.8) | 56.3 (CH) | 1.09 dd (10.4, 10.4) | 60.2 (CH) | |
| 10 | 43.4 (C) | 44.0 (C) | |||
| 11 | 5.17 ddd (10.8, 10.8, 5.6) | 69.8 (CH) | 3.99 m | 67.9 (CH) | |
| 12α/β | 1.00 dd (12.4, 10.8); 2.13 dd (12.4, 5.6) | 44.7 (CH2) | 1.00 m; 2.10 dd (12.0, 4.8) | 50.0 (CH2) | |
| 13 | 42.5 (C) | 42.9 (C) | |||
| 14 | 1.14 m | 53.9 (CH) | 1.09 m | 54.5 (CH) | |
| 15α/β | 1.67 m; 1.16 m | 23.9 (CH2) | 1.63 m; 1.18 m | 24.0 (CH2) | |
| 16α/β | 1.92 m; 1.36 m | 27.4 (CH2) | 1.93 m; 1.38 m | 27.7 (CH2) | |
| 17 | 1.30 dd (9.2, 9.2) | 55.3 (CH) | 1.32 m | 55.3 (CH) | |
| 18 | 0.76 s | 12.9 (CH3) | 0.73 s | 13.3 (CH3) | |
| 19 | 1.26 s | 18.7 (CH3) | 1.25 s | 18.7 (CH3) | |
| 20 | 2.24 m | 39.5 (CH) | 2.25 m | 40.0 (CH) | |
| 21 | 0.97 d (6.4) | 19.8 (CH3) | 0.99 d (6.4) | 20.0 (CH3) | |
| 22 | 6.74 dd (15.6, 10.0) | 154.0 (CH) | 6.84 dd (15.6, 10.4) | 154.8 (CH) | |
| 23 | 5.79 d (15.6) | 119.3 (CH) | 5.81 d (15.6) | 119.2 (CH) | |
| 24 | 166.8 (C) | 167.2 (C) | |||
| OAc-11 | 2.01 s | 169.7 (C) 21.6 (CH3) | |||
| OMe-24 | 3.72 s | 51.3 (CH3) | 3.74 s | 51.5 (CH3) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huynh, T.-H.; Chen, P.-C.; Yang, S.-N.; Lin, F.-Y.; Su, T.-P.; Chen, L.-Y.; Peng, B.-R.; Hu, C.-C.; Chen, Y.-Y.; Wen, Z.-H.; et al. New 1,4-Dienonesteroids from the Octocoral Dendronephthya sp. Mar. Drugs 2019, 17, 530. https://doi.org/10.3390/md17090530
Huynh T-H, Chen P-C, Yang S-N, Lin F-Y, Su T-P, Chen L-Y, Peng B-R, Hu C-C, Chen Y-Y, Wen Z-H, et al. New 1,4-Dienonesteroids from the Octocoral Dendronephthya sp. Marine Drugs. 2019; 17(9):530. https://doi.org/10.3390/md17090530
Chicago/Turabian StyleHuynh, Thanh-Hao, Pei-Chin Chen, San-Nan Yang, Feng-Yu Lin, Tung-Pin Su, Lo-Yun Chen, Bo-Rong Peng, Chiung-Chin Hu, You-Ying Chen, Zhi-Hong Wen, and et al. 2019. "New 1,4-Dienonesteroids from the Octocoral Dendronephthya sp." Marine Drugs 17, no. 9: 530. https://doi.org/10.3390/md17090530
APA StyleHuynh, T.-H., Chen, P.-C., Yang, S.-N., Lin, F.-Y., Su, T.-P., Chen, L.-Y., Peng, B.-R., Hu, C.-C., Chen, Y.-Y., Wen, Z.-H., Wu, T.-Y., & Sung, P.-J. (2019). New 1,4-Dienonesteroids from the Octocoral Dendronephthya sp. Marine Drugs, 17(9), 530. https://doi.org/10.3390/md17090530