Effects of Fucoidans from Five Different Brown Algae on Oxidative Stress and VEGF Interference in Ocular Cells
Abstract
1. Introduction
2. Results
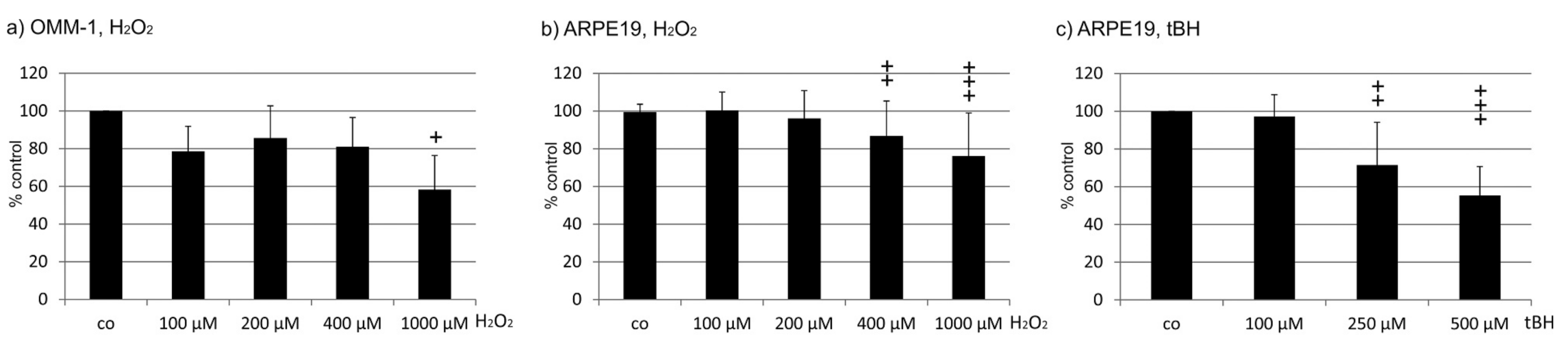
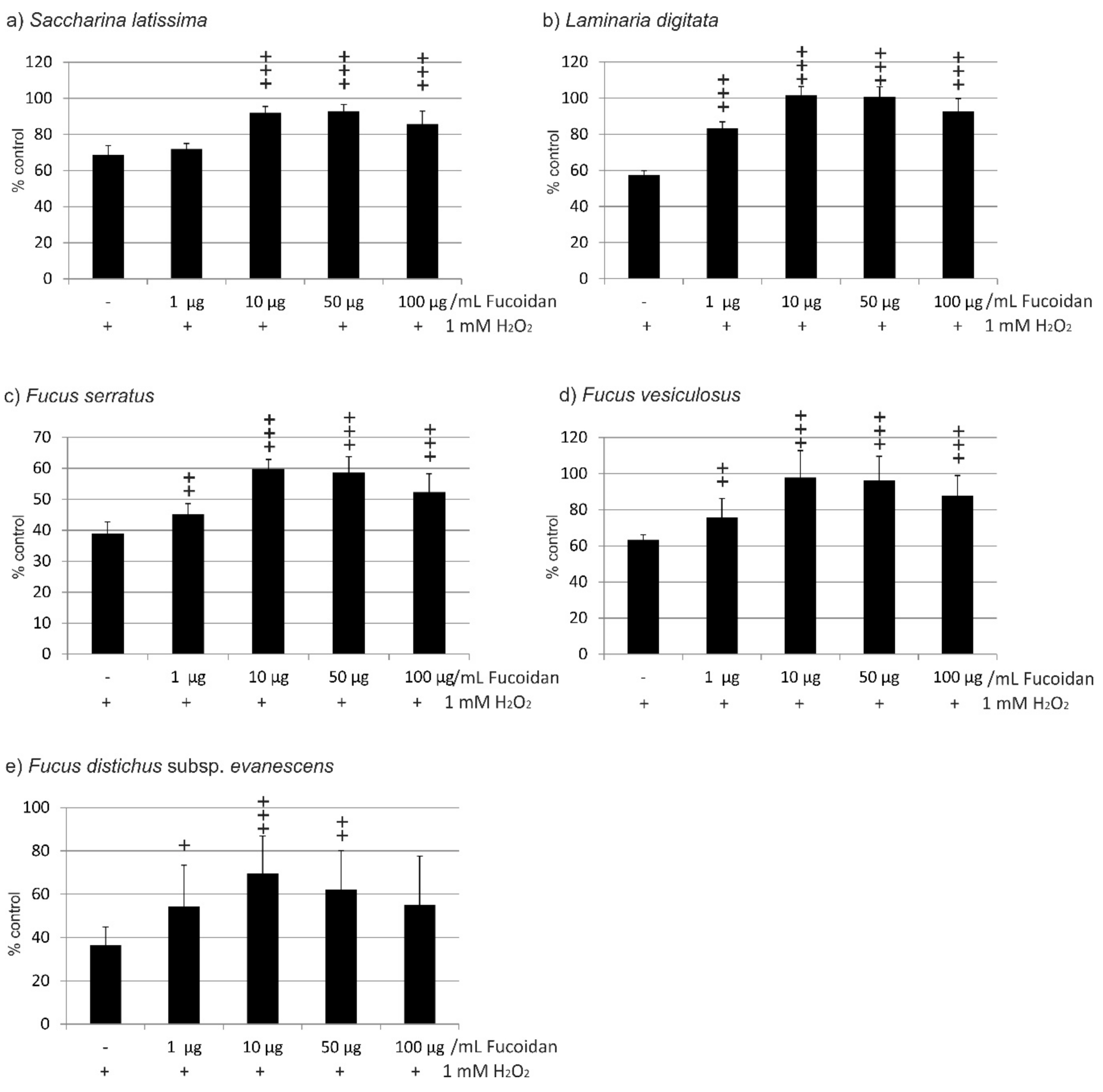
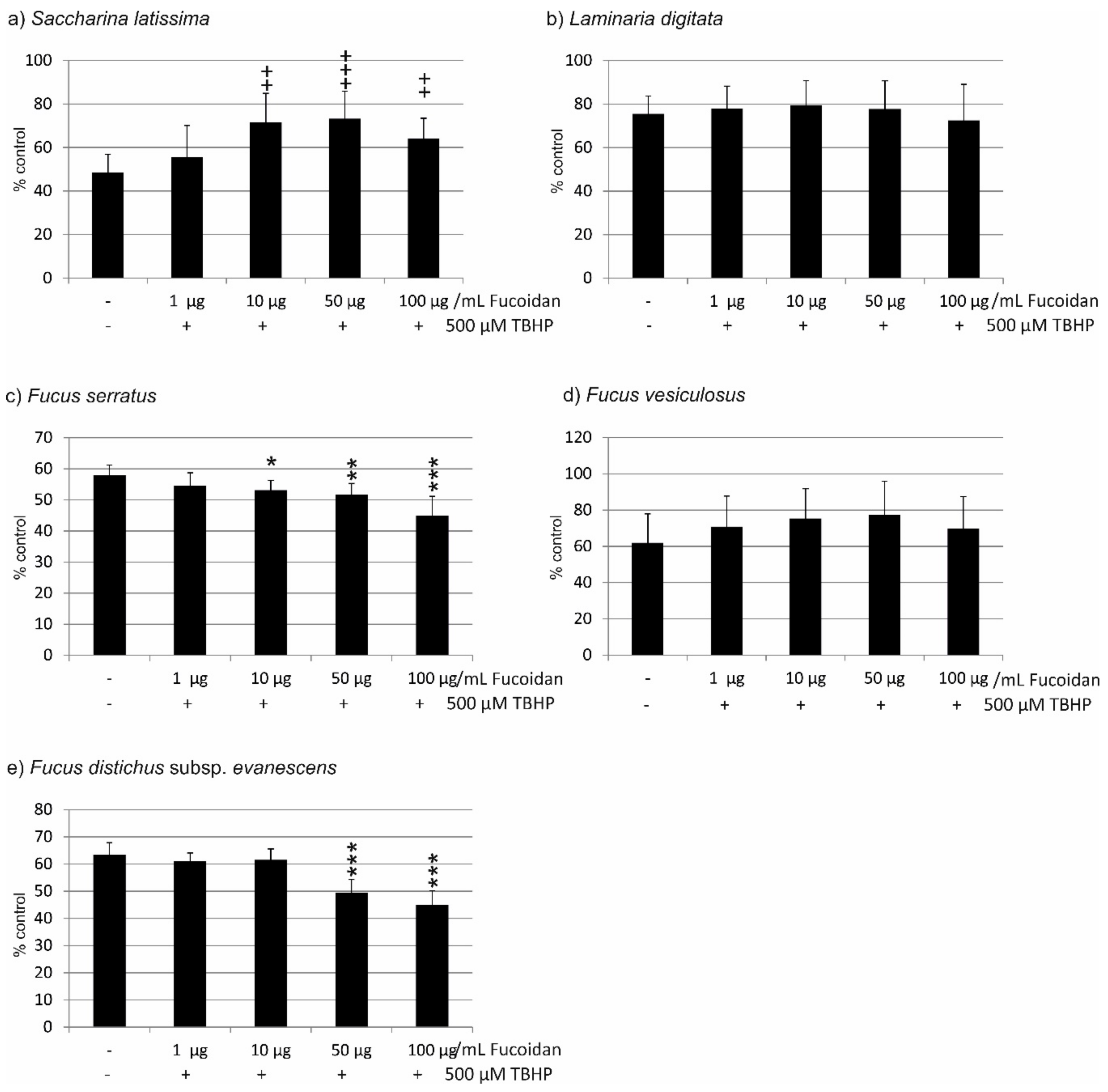
2.1. Oxidative Stress Protection
2.1.1. OMM-1 Cells
2.1.2. ARPE19 Cells
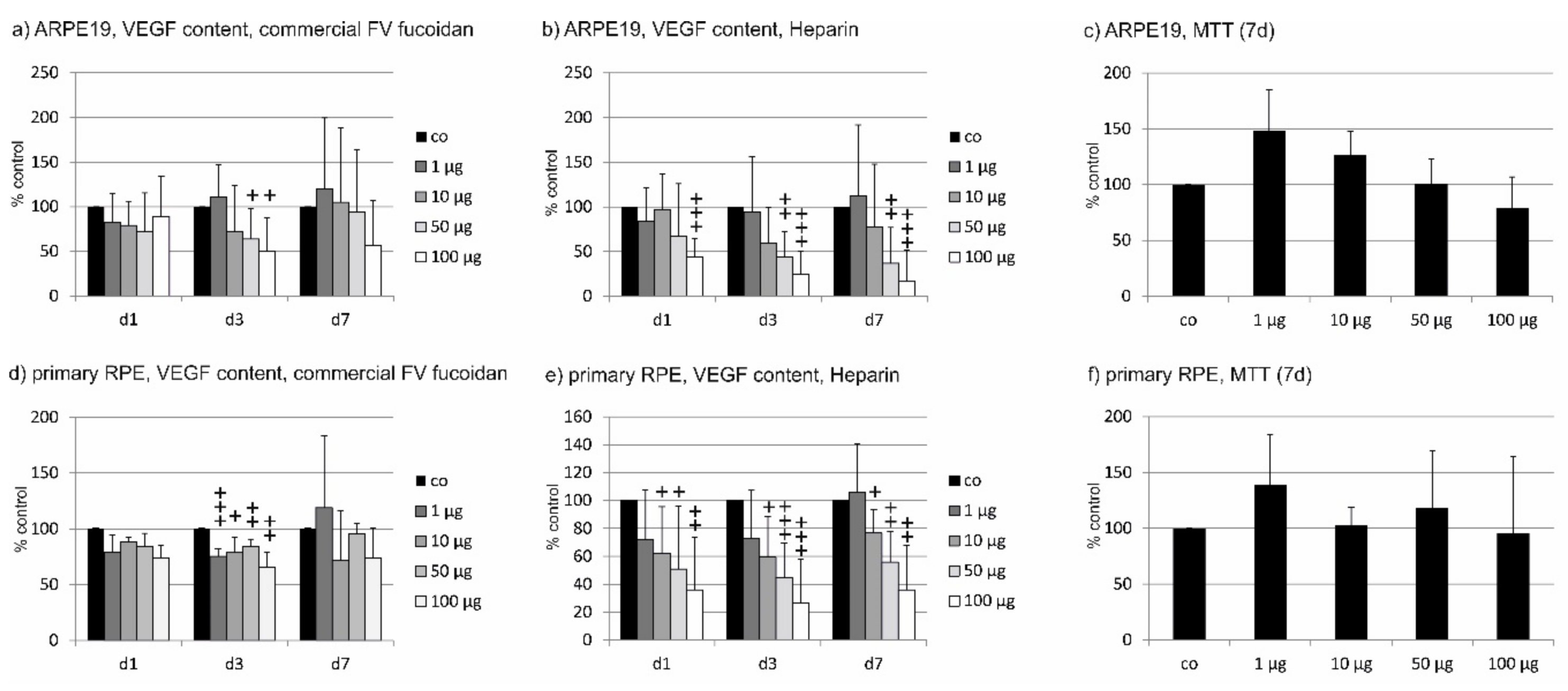
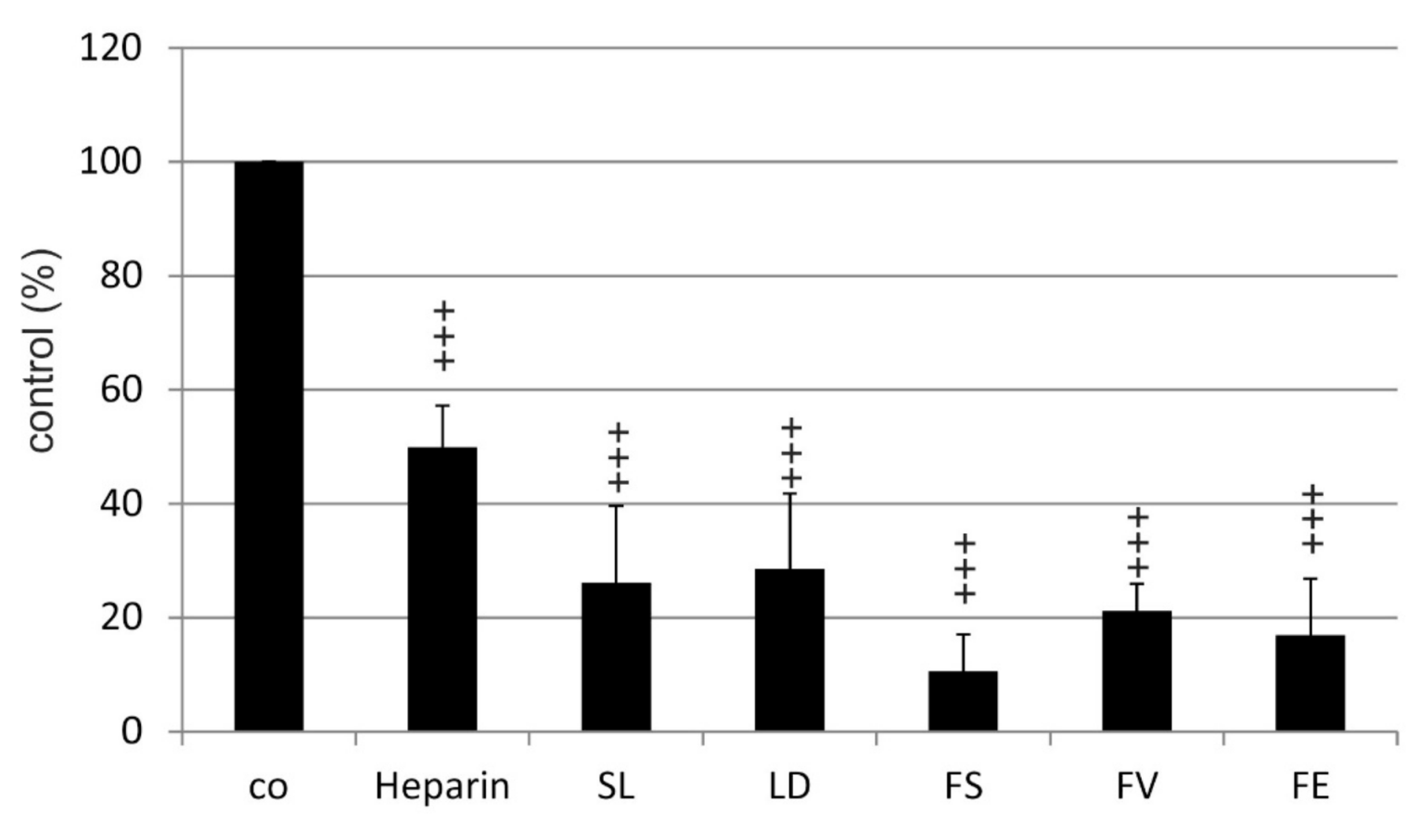
2.2. VEGF Secretion
2.2.1. ARPE19
2.2.2. Primary RPE
2.3. Binding Affinity to VEGF
3. Discussion
4. Material and Methods
4.1. Cell Culture
4.2. Fucoidans
4.3. Oxidative Stress
4.3.1. OMM-1
4.3.2. ARPE19
4.4. Methyl Thiazolyl Tetrazolium (MTT) Assay
4.5. MTS Assay
4.6. VEGF ELISA
4.7. Competitive VEGF Binding Assay
4.8. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed]
- Dithmer, M.; Fuchs, S.; Shi, Y.; Schmidt, H.; Richert, E.; Roider, J.; Klettner, A. Fucoidan reduces secretion and expression of vascular endothelial growth factor in the retinal pigment epithelium and reduces angiogenesis in vitro. PLoS ONE 2014, 9, e89150. [Google Scholar] [CrossRef]
- Klettner, A. Fucoidan as a potential therapeutic for major blinding diseases—A hypothesis. Mar. Drugs 2016, 14, E31. [Google Scholar] [CrossRef]
- Miller, J.W. Age-related macular degeneration revisited--piecing the puzzle: The LXIX Edward Jackson memorial lecture. Am. J. Ophthalmol. 2013, 155, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Klettner, A. Age-related macular degeneration-biology and treatment. Med. Monatsschr. Pharm. 2015, 38, 258–264. [Google Scholar] [PubMed]
- Bellezza, I. Oxidative Stress in Age-Related Macular Degeneration: Nrf2 as Therapeutic Target. Front. Pharmacol. 2018, 9, 1280. [Google Scholar] [CrossRef]
- Copland, D.A.; Theodoropoulou, S.; Liu, J.; Dick, A.D. A Perspective of AMD Through the Eyes of Immunology. Invest. Ophthalmol. Vis. Sci. 2018, 59, AMD83–AMD92. [Google Scholar] [CrossRef]
- Handa, J.T.; Cano, M.; Wang, L.; Datta, S.; Liu, T. Lipids, Oxidized Lipids, Oxidation-specific Epitopes, and Age-related Macular Degeneration. Biochim. Biophys. Acta 2017, 1862, 430–440. [Google Scholar] [CrossRef]
- Toomey, C.B.; Johnson, L.V.; Bowes Rickman, C. Complement factor H in AMD: Bridging genetic associations and pathobiology. Prog. Retin. Eye Res. 2018, 62, 38–57. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Chong, V.; Loewenstein, A.; Larsen, M.; Souied, E.; Schlingemann, R.; Eldem, B.; Monés, J.; Richard, G.; Bandello, F. European Society of Retina Specialists. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br. J. Ophthalmol. 2014, 98, 1144–1167. [Google Scholar] [CrossRef]
- Rofagha, S.; Bhisitkul, R.B.; Boyer, D.S.; Sadda, S.R.; Zhang, K.; SEVEN-UP Study Group. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 2013, 120, 2292–2299. [Google Scholar] [CrossRef]
- Zarubina, A.V.; Gal-Or, O.; Huisingh, C.E.; Owsley, C.; Freund, K.B. Macular Atrophy Development and Subretinal Drusenoid Deposits in Anti-Vascular Endothelial Growth Factor Treated Age-Related Macular Degeneration. Invest. Ophthalmol. Vis. Sci. 2017, 58, 6038–6045. [Google Scholar] [CrossRef]
- Moryak, V.K.; Kim, J.; Kim, E. Algal fucoidan: Structural and size-dependent bioactivities and their perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 71–82. [Google Scholar] [CrossRef]
- Schneider, T.; Ehrig, K.; Liewert, I.; Alban, S. Interference with the CXCL12/CXCR4 axis as potential antitumor strategy: Superiority of a sulfated galactofucan from the brown alga Saccharina latissima and fucoidan over heparins. Glycobiology 2015, 25, 812–824. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef]
- Dithmer, M.; Kirsch, A.M.; Richert, E.; Fuchs, S.; Wang, F.; Schmidt, H.; Coupland, S.E.; Roider, J.; Klettner, A. Fucoidan does not exert anti-tumorigenic effects on uveal melanoma cell lines. Mar. Drugs 2017, 15, 193. [Google Scholar] [CrossRef]
- Klettner, A. Oxidative stress induced cellular signaling in RPE cells. Front. Biosci. (Schol. Ed.) 2012, 4, 392–411. [Google Scholar] [CrossRef]
- Karlsson, M.; Kurz, T. Attenuation of iron-binding proteins in ARPE-19 cells reduces their resistance to oxidative stress. Acta Ophthalmol. 2016, 94, 556–564. [Google Scholar] [CrossRef]
- Folkman, J.; Shing, Y. Control of angiogenesis by heparin and other sulfated polysaccharides. Adv. Exp. Med. Biol. 1992, 313, 355–364. [Google Scholar]
- Blasi, M.A.; Maresca, V.; Roccella, M.; Roccella, F.; Sansolini, T.; Grammatico, P.; Balestrazzi, E.; Picardo, M. Antioxidant pattern in uveal melanocytes and melanoma cell cultures. Invest. Ophthalmol. Vis. Sci. 1999, 40, 3012–3016. [Google Scholar]
- Rupérez, P.; Ahrazem, O.; Leal, J.A. Potential antioxidant capacity of sulfated polysaccharides from the edible marine brown seaweed Fucus vesiculosus. J. Agric. Food Chem. 2002, 50, 840–845. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef]
- Kim, E.A.; Lee, S.H.; Ko, C.I.; Cha, S.H.; Kang, M.C.; Kang, S.M.; Ko, S.C.; Lee, W.W.; Ko, J.Y.; Lee, J.H.; et al. Protective effect of fucoidan against AAPH-induced oxidative stress in zebrafish model. Carbohydr. Polym. 2014, 102, 185–191. [Google Scholar] [CrossRef]
- Abu, R.; Jiang, Z.; Ueno, M.; Okimura, T.; Yamaguchi, K.; Oda, T. In vitro antioxidant activities of sulfated polysaccharide ascophyllan isolated from Ascophyllum nodosum. Int. J Biol. Macromol. 2013, 59, 305–312. [Google Scholar] [CrossRef]
- Lahrsen, E.; Liewert, I.; Alban, S. Gradual degradation of fucoidan from Fucus vesiculosus and its effect on structure, antioxidant and antiproliferative activities. Carbohydr. Polym. 2018, 192, 208–216. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Wei, J.G.; Tu, M.J.; Gu, J.G.; Zhang, W. Fucoidan Alleviates Acetaminophen-Induced Hepatotoxicity via Oxidative Stress Inhibition and Nrf2 Translocation. Int. J. Mol. Sci. 2018, 19, 4050. [Google Scholar] [CrossRef]
- Ryu, M.J.; Chung, H.S. Fucoidan reduces oxidative stress by regulating the gene expression of HO‑1 and SOD‑1 through the Nrf2/ERK signaling pathway in HaCaT cells. Mol. Med. Rep. 2016, 14, 3255–3260. [Google Scholar] [CrossRef]
- Kim, H.; Ahn, J.H.; Song, M.; Kim, D.W.; Lee, T.K.; Lee, J.C.; Kim, Y.M.; Kim, J.D.; Cho, J.H.; Hwang, I.K.; et al. Pretreated fucoidan confers neuroprotection against transient global cerebral ischemic injury in the gerbil hippocampal CA1 area via reducing of glial cell activation and oxidative stress. Biomed. Pharmacother. 2019, 109, 1718–1727. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef]
- Foresti, R.; Bucolo, C.; Platania, C.M.; Drago, F.; Dubois-Randé, J.L.; Motterlini, R. Nrf2 activators modulate oxidative stress responses and bioenergetic profiles of human retinal epithelial cells cultured in normal or high glucose conditions. Pharmacol. Res. 2015, 99, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Pittalà, V.; Fidilio, A.; Lazzara, F.; Platania, C.B.M.; Salerno, L.; Foresti, R.; Drago, F.; Bucolo, C. Effects of Novel Nitric Oxide-Releasing Molecules against Oxidative Stress on Retinal Pigmented Epithelial Cells. Oxid. Med. Cell Longev. 2017, 2017, 1420892. [Google Scholar] [CrossRef] [PubMed]
- Koinzer, S.; Reinecke, K.; Herdegen, T.; Roider, J.; Klettner, A. Oxidative Stress Induces Biphasic ERK1/2 Activation in the RPE with Distinct Effects on Cell Survival at Early and Late Activation. Curr. Eye Res. 2015, 40, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Tode, J.; Richert, E.; Koinzer, S.; Klettner, A.; von der Burchard, C.; Brinkmann, R.; Lucius, R.; Roider, J. Thermal Stimulation of the Retina Reduces Bruch’s Membrane Thickness in Age Related Macular Degeneration Mouse Models. Transl. Vis. Sci. Technol. 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- German, O.L.; Agnolazza, D.L.; Politi, L.E.; Rotstein, N.P. Light, lipids and photoreceptor survival: Live or let die? Photochem. Photobiol. Sci. 2015, 14, 1737–1753. [Google Scholar] [CrossRef]
- Klettner, A.; Roider, J. Treating age-related macular degeneration - interaction of VEGF-antagonists with their target. Mini Rev. Chem. 2009, 9, 1127–1135. [Google Scholar] [CrossRef]
- Platania, C.B.; Di Paola, L.; Leggio, G.M.; Romano, G.L.; Drago, F.; Salomone, S.; Bucolo, C. Molecular features of interaction between VEGFA and anti-angiogenic drugs used in retinal diseases: A computational approach. Front. Pharmacol. 2015, 6, 248. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Cui, N.; Bo, Z.; Xiang, F. Structural Determinant and Its Underlying Molecular Mechanism of STPC2 Related to Anti-Angiogenic Activity. Mar. Drugs 2017, 15, 48. [Google Scholar] [CrossRef]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Chen, H.; Cong, Q.; Du, Z.; Liao, W.; Zhang, L.; Yao, Y.; Ding, K. Sulfated fucoidan FP08S2 inhibits lung cancer cell growth in vivo by disrupting angiogenesis via targeting VEGFR2/VEGF and blocking VEGFR2/Erk/VEGF signaling. Cancer Lett. 2016, 382, 44–52. [Google Scholar] [CrossRef]
- Narazaki, M.; Segarra, M.; Tosato, G. Sulfated polysaccharides identified as inducers of neuropilin-1 internalization and functional inhibition of VEGF165 and semaphorin3A. Blood 2008, 111, 4126. [Google Scholar] [CrossRef]
- Rui, X.; Pan, H.F.; Shao, S.L.; Xu, X.M. Anti-tumor and anti-angiogenic effects of Fucoidan on prostate cancer: Possible JAK-STAT3 pathway. BMC Complement. Altern. Med. 2017, 17, 378. [Google Scholar] [CrossRef]
- Klettner, A.; Westhues, D.; Lassen, J.; Bartsch, S.; Roider, J. Regulation of constitutive vascular endothelial growth factor secretion in retinal pigment epithelium/choroid organ cultures: p38, nuclear factor κB, and the vascular endothelial growth factor receptor-2/phosphatidylinositol 3 kinase pathway. Mol. Vis. 2013, 19, 281–291. [Google Scholar]
- Klettner, A.; Kaya, L.; Flach, J.; Lassen, J.; Treumer, F.; Roider, J. Basal and apical regulation of VEGF-A and placenta growth factor in the RPE/choroid and primary RPE. Mol. Vis. 2015, 21, 736–748. [Google Scholar]
- Cébe Suarez, S.; Pieren, M.; Cariolato, L.; Arn, S.; Hoffmann, U.; Bogucki, A.; Manlius, C.; Wood, J.; Ballmer-Hofer, K. A VEGF-A splice variant defective for heparan sulfate and neuropilin-1 binding shows attenuated signaling through VEGFR-2. Cell. Mol. Life Sci. 2006, 63, 2067–2077. [Google Scholar] [CrossRef]
- Nishiguchi, K.M.; Kataoka, K.; Kachi, S.; Komeima, K.; Terasaki, H. Regulation of pathologic retinal angiogenesis in mice and inhibition of VEGF-VEGFR2 binding by soluble heparan sulfate. PLoS ONE 2010, 5, e13493. [Google Scholar] [CrossRef]
- Croci, D.O.; Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; Piccoli, A.; Totani, L.; Ustyuzhanina, N.E.; Bilan, M.I.; Usov, A.I.; Grachev, A.A.; et al. Fucans, but not fucomannoglucuronans, determine the biological activities of sulfated polysaccharides from Laminaria saccharina brown seaweed. PLoS ONE 2011, 6, e17283. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Maisto, R.; Trotta, M.C.; D’Amico, M.; Rossi, S.; Gesualdo, C.; D’Amico, G.; Balta, C.; Herman, H.; Hermenean, A.; et al. Retinal and circulating miRNA expression patterns in diabetic retinopathy: An in silico and in vivo approach. Br. J. Pharmacol. 2019, in press. [Google Scholar] [CrossRef]
- Platania, C.B.M.; Leggio, G.M.; Drago, F.; Salomone, S.; Bucolo, C. Computational systems biology approach to identify novel pharmacological targets for diabetic retinopathy. Biochem. Pharmacol. 2018, 158, 13–26. [Google Scholar] [CrossRef]
- Luyten, G.P.; Naus, N.C.; Mooy, C.M.; Hagemeijer, A.; Kan-Mitchell, J.; Van Drunen, E.; Vuzevski, V.; De Jong, P.T.; Luider, T.M. Establishment and characterization of primary and metastatic uveal melanoma cell lines. Int. J. Cancer 1996, 66, 380–387. [Google Scholar] [CrossRef]
- Wiencke, A.K.; Kiilgaard, J.F.; Nicolini, J.; Bundgaard, M.; Röpke, C.; La Cour, M. Growth of cultured porcine retinal pigment epithelial cells. Acta Ophthalmol. Scand. 2003, 81, 170–176. [Google Scholar] [CrossRef]
- Klettner, A.; Roider, J. Comparison of bevacizumab, ranibizumab, and pegaptanib in citro: Efficiency and possible additional pathways. Invest. Ophthalmol. Vis. Sci. 2008, 49, 4523–4527. [Google Scholar] [CrossRef]
- Ehrig, K.; Alban, S. Sulfated galactofucan from the brown alga Saccharina latissima--variability of yield, structural composition and bioactivity. Mar. Drugs 2014, 13, 76–101. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual [Internet]; Sittampalam, G.S., Coussens, N.P., Brimacombe, K., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Bejcek, B., Caaveiro, J.M.M., et al., Eds.; (MD), Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2013. [Google Scholar]
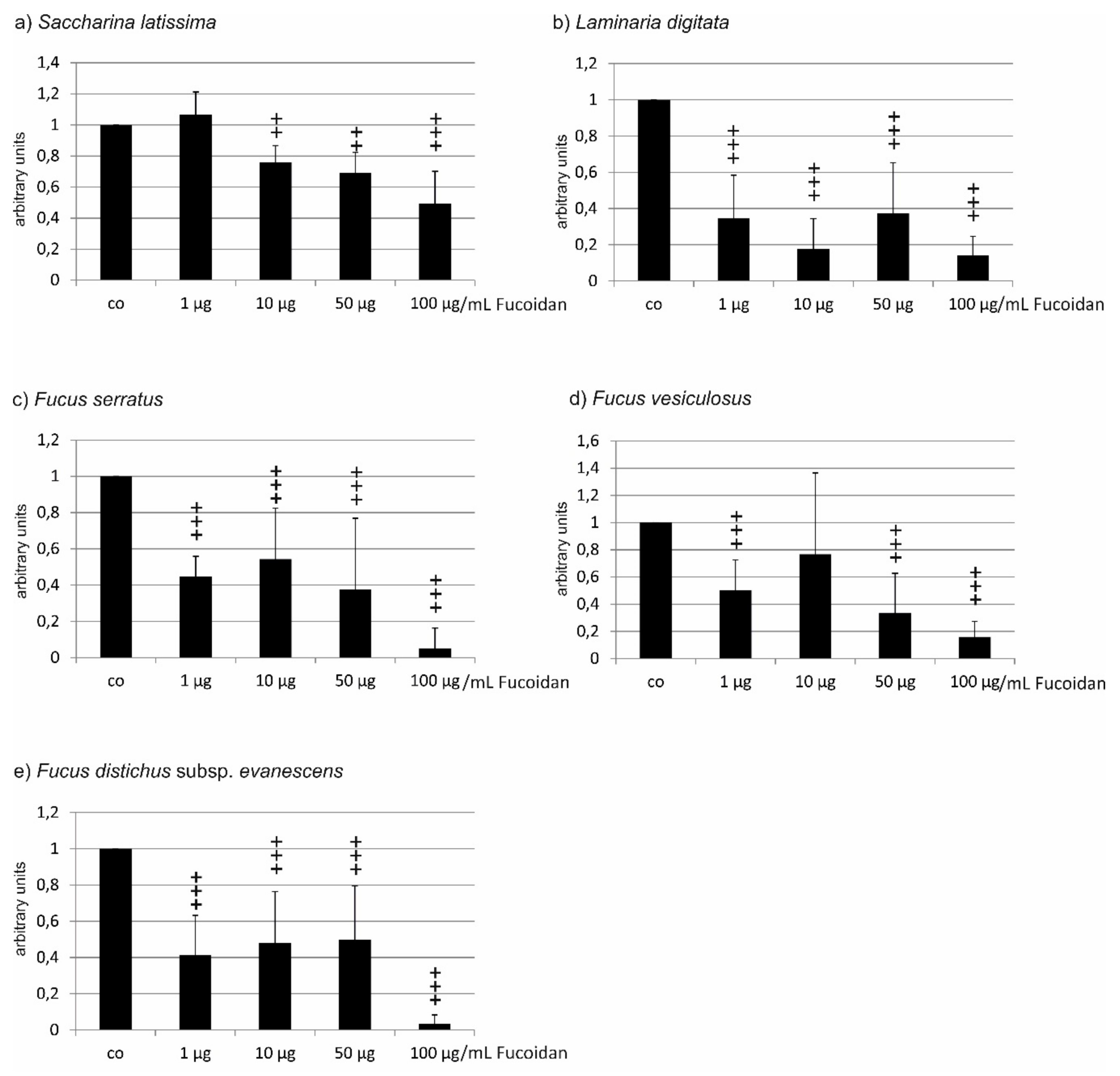
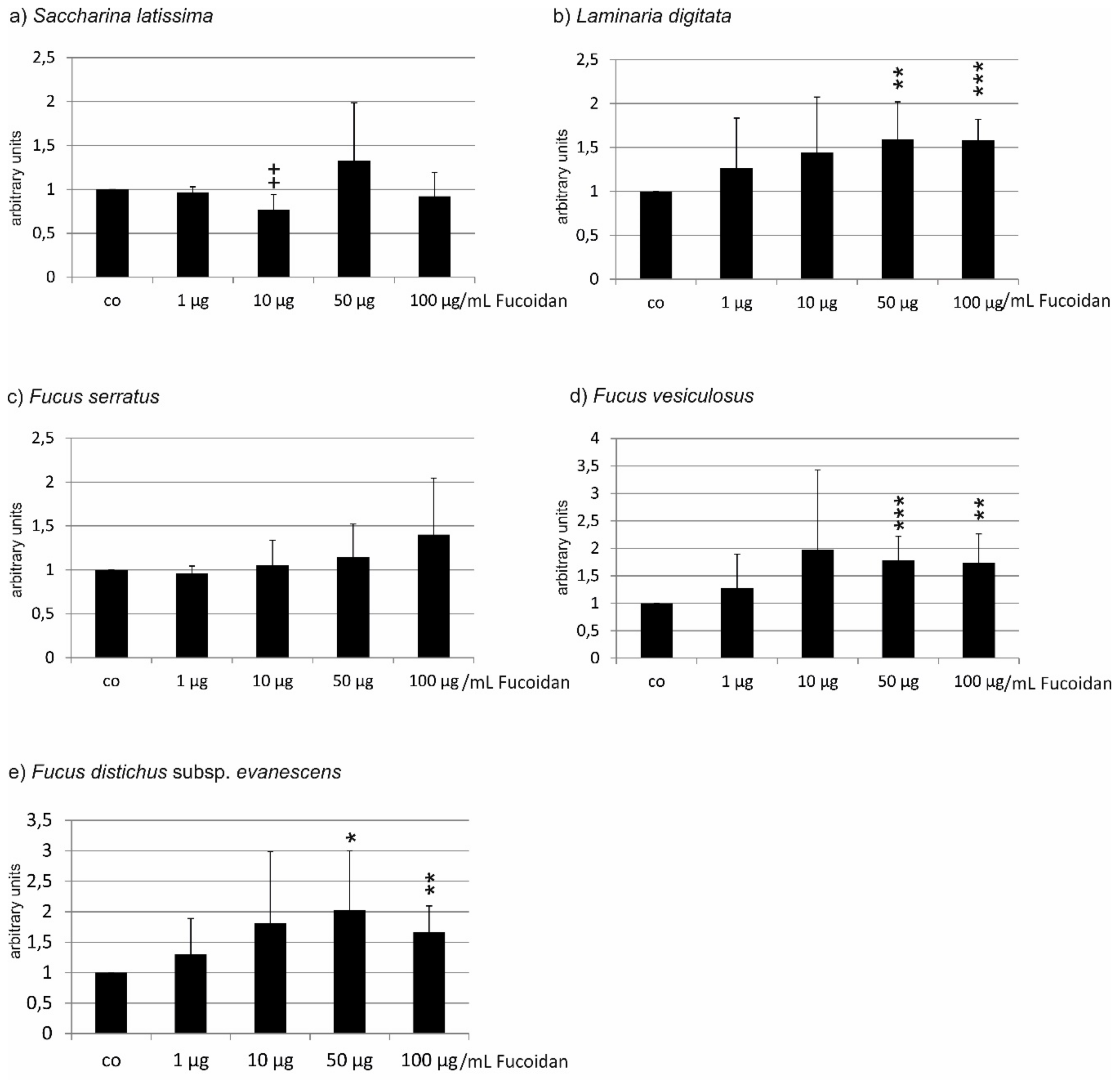
| Compared Fucoidans | 1 µg/mL | 10 µg/mL | 50 µg/mL | 100 µg/mL |
|---|---|---|---|---|
| LD vs. FV | not significant (ns) | ns | ns | ns |
| LD vs. SL | p < 0.001 | p < 0.001 | p < 0.01 | ns |
| LD vs. FE | p < 0.05 | p < 0.001 | p < 0.001 | p < 0.001 |
| LD vs. FS | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| FV vs. SL | ns | ns | ns | ns |
| FV vs. FE | p < 0.05 | p < 0.01 | p < 0.01 | p < 0.01 |
| FV vs. FS | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| SL vs. FE | p < 0.05 | p < 0.01 | p < 0.001 | p < 0.01 |
| SL vs. FS | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
| FE vs. FS | ns | ns | ns | ns |
| Compared Fucoidans | 1 µg/mL | 10 µg/mL | 50 µg/mL | 100 µg/mL |
|---|---|---|---|---|
| LD vs. FV | ns | ns | ns | ns |
| LD vs. SL | p < 0.01 | ns | ns | ns |
| LD vs. FE | p < 0.01 | p < 0.01 | p < 0.001 | p < 0.001 |
| LD vs. FS | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.01 |
| FV vs. SL | ns | ns | ns | ns |
| FV vs. FE | ns | p < 0.05 | p < 0.01 | p < 0.01 |
| FV vs. FS | p < 0.05 | p < 0.01 | p < 0.01 | p < 0.01 |
| SL vs. FE | ns | ns | p < 0.001 | p < 0.001 |
| SL vs. FS | ns | p < 0.01 | p < 0.001 | p < 0.001 |
| FE vs. FS | p < 0.01 | p < 0.001 | ns | ns |
| Compared Fucoidans | 1 µg/mL | 10 µg/mL | 50 µg/mL | 100 µg/mL |
|---|---|---|---|---|
| LD vs. FV | ns | p < 0.05 | ns | ns |
| LD vs. SL | p < 0.001 | p < 0.001 | p < 0.05 | p < 0.01 |
| LD vs. FE | ns | p < 0.05 | ns | p < 0.05 |
| LD vs. FS | ns | p < 0.05 | ns | ns |
| FV vs. SL | p < 0.001 | ns | p < 0.05 | p < 0.01 |
| FV vs. FE | ns | ns | ns | p < 0.05 |
| FV vs. FS | ns | ns | ns | ns |
| SL vs. FE | p < 0.001 | ns | ns | p < 0.001 |
| SL vs. FS | p < 0.001 | ns | ns | p < 0.001 |
| FE vs. FS | ns | ns | ns | ns |
| Compared Fucoidans | 1 µg/mL | 10 µg/mL | 50 µg/mL | 100 µg/mL |
|---|---|---|---|---|
| LD vs. FV | ns | ns | ns | ns |
| LD vs. SL | ns | p < 0.05 | ns | ns |
| LD vs. FE | ns | ns | ns | ns |
| LD vs. FS | ns | ns | ns | ns |
| FV vs. SL | ns | ns | ns | ns |
| FV vs. FE | ns | ns | ns | ns |
| FV vs. FS | ns | ns | p < 0.05 | ns |
| SL vs. FE | ns | ns | ns | ns |
| SL vs. FS | ns | ns | ns | ns |
| FE vs. FS | ns | ns | ns | ns |
| Compared Substances | Significance |
|---|---|
| Heparin vs. FV | p < 0.01 |
| Heparin vs. FS | p < 0.01 |
| Heparin vs. FE | p < 0.01 |
| Heparin vs. LD | ns |
| Heparin vs. SL | ns |
| LD vs. FV | ns |
| LD vs. SL | ns |
| LD vs. FE | ns |
| LD vs. FS | ns |
| FV vs. SL | ns |
| FV vs. FE | ns |
| FV vs. FS | ns |
| SL vs. FE | ns |
| SL vs. FS | ns |
| FE vs. FS | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dörschmann, P.; Bittkau, K.S.; Neupane, S.; Roider, J.; Alban, S.; Klettner, A. Effects of Fucoidans from Five Different Brown Algae on Oxidative Stress and VEGF Interference in Ocular Cells. Mar. Drugs 2019, 17, 258. https://doi.org/10.3390/md17050258
Dörschmann P, Bittkau KS, Neupane S, Roider J, Alban S, Klettner A. Effects of Fucoidans from Five Different Brown Algae on Oxidative Stress and VEGF Interference in Ocular Cells. Marine Drugs. 2019; 17(5):258. https://doi.org/10.3390/md17050258
Chicago/Turabian StyleDörschmann, Philipp, Kaya Saskia Bittkau, Sandesh Neupane, Johann Roider, Susanne Alban, and Alexa Klettner. 2019. "Effects of Fucoidans from Five Different Brown Algae on Oxidative Stress and VEGF Interference in Ocular Cells" Marine Drugs 17, no. 5: 258. https://doi.org/10.3390/md17050258
APA StyleDörschmann, P., Bittkau, K. S., Neupane, S., Roider, J., Alban, S., & Klettner, A. (2019). Effects of Fucoidans from Five Different Brown Algae on Oxidative Stress and VEGF Interference in Ocular Cells. Marine Drugs, 17(5), 258. https://doi.org/10.3390/md17050258






