A New Tyrosinase Inhibitor from the Red Alga Symphyocladia latiuscula (Harvey) Yamada (Rhodomelaceae)
Abstract
:1. Introduction
2. Results
2.1. Effect of Bromophenols on Tyrosinase Activity
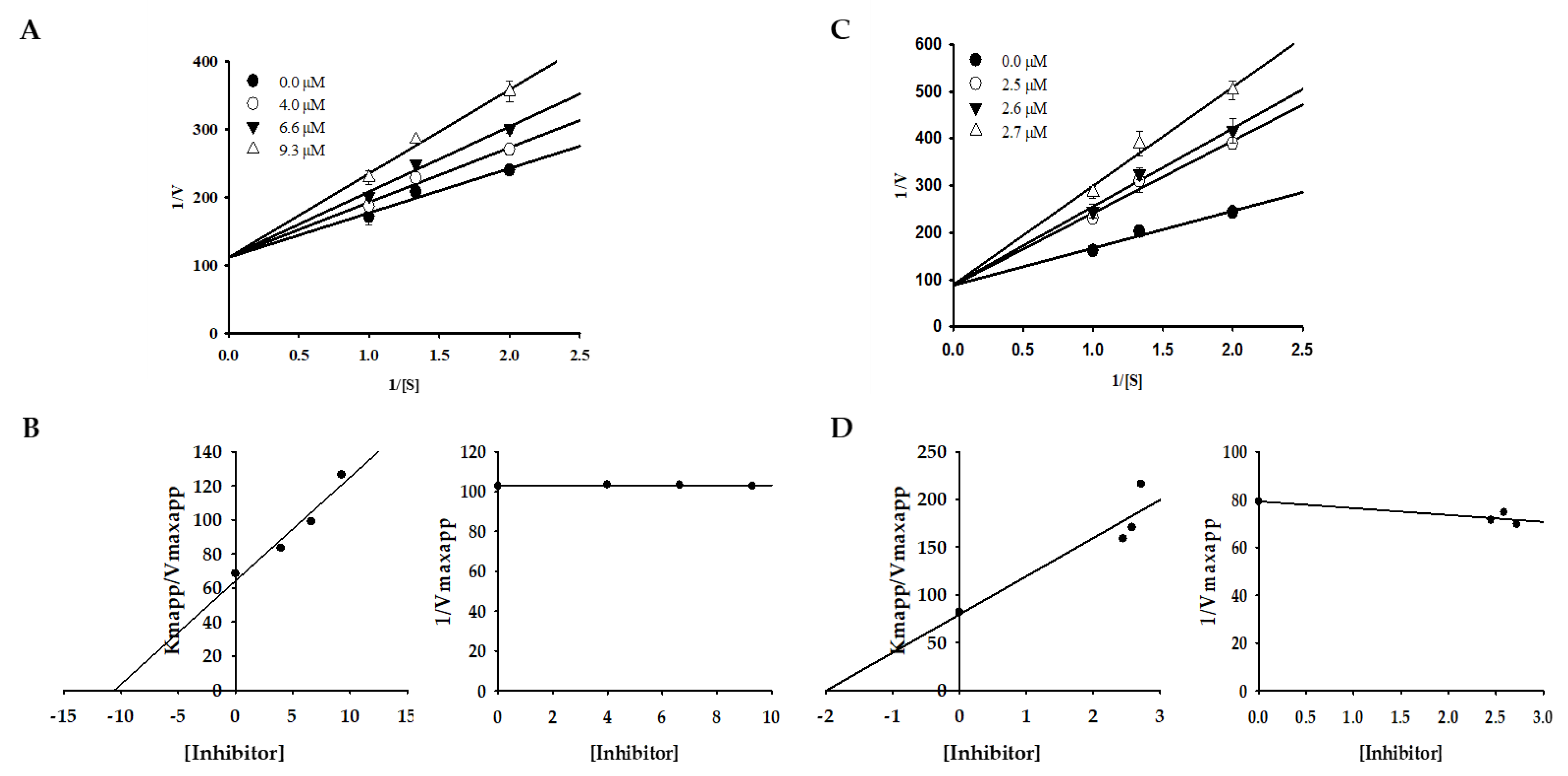
2.2. Effect of Bromophenols on Enzyme Kinetic Inhibition
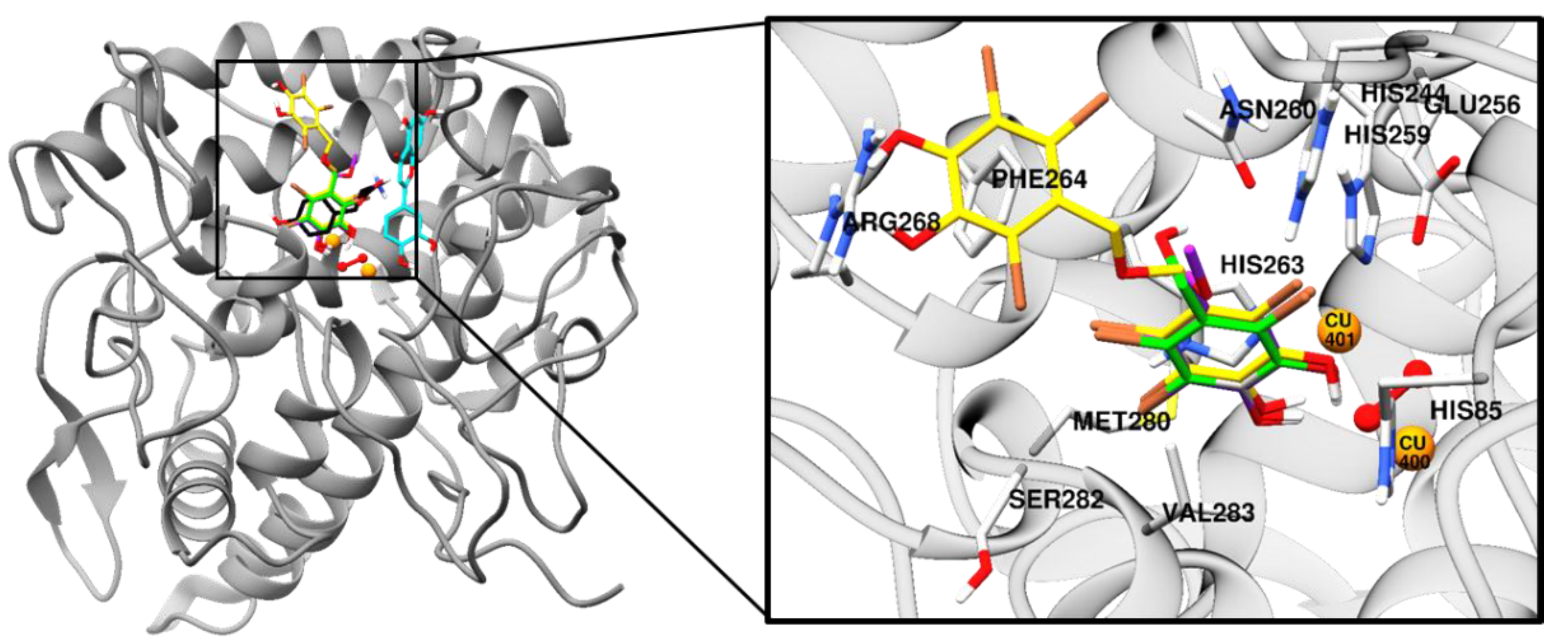
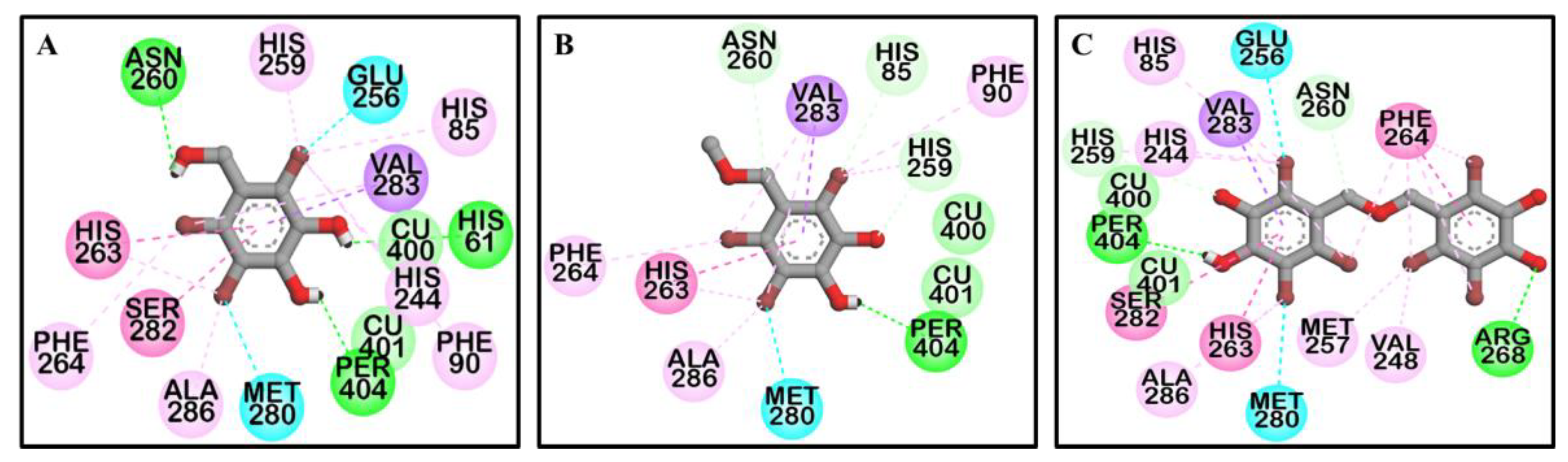
2.3. Molecular Docking Simulation on Tyrosinase Inhibition
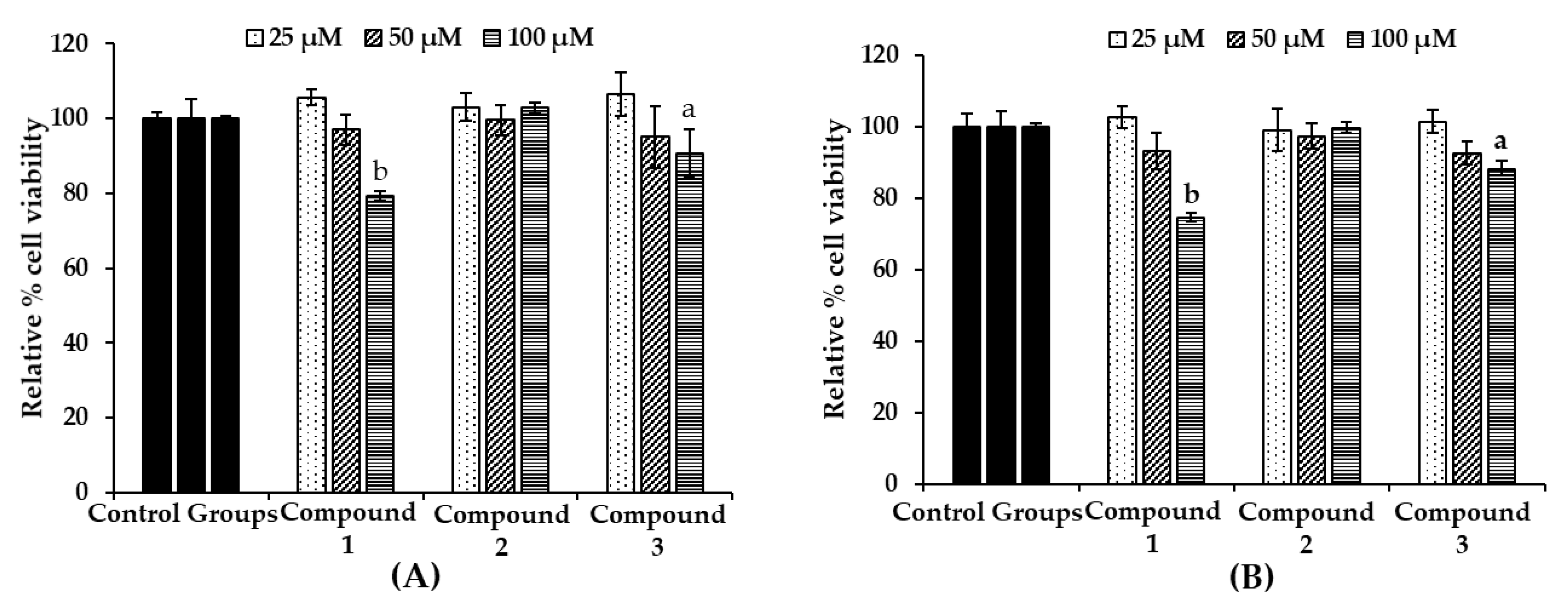
2.4. Effect of Bromophenols on Cell Viability of B16F10 Cells
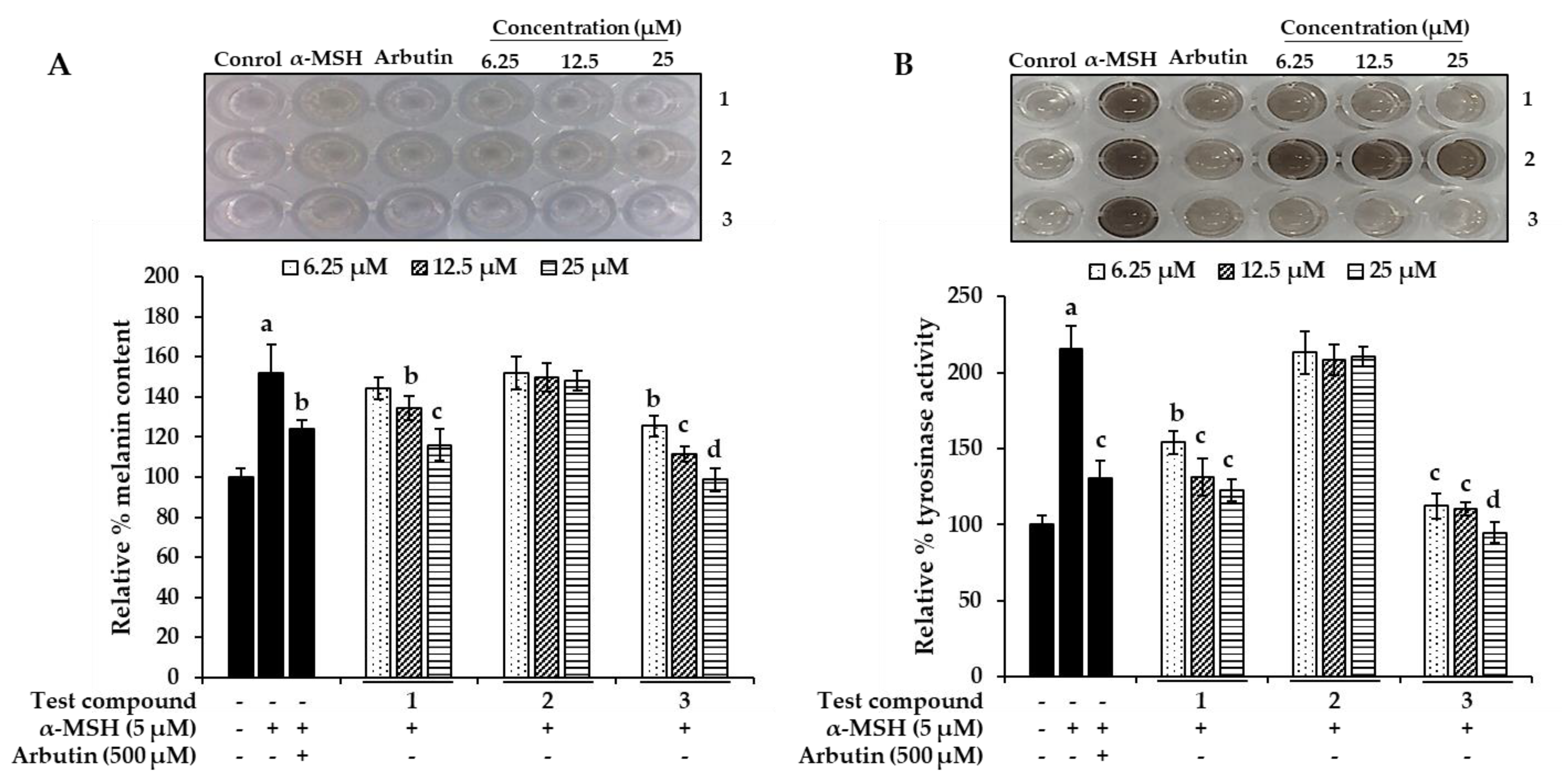
2.5. Effect of Bromophenols on Melanin Content and Intracellular Tyrosinase Activity in B16F10 Cells
2.6. Effect of Bromophenols on Tyrosinase Expression
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Algal Material
4.3. Extraction, Fractionation, and Isolation
4.4. Mushroom Tyrosinase Inhibitory Assay
4.5. Kinetic Study Against Mushroom Tyrosinase
4.6. Molecular Docking Simulation of Mushroom Tyrosinase
4.7. Cell Culture and Viability Assay
4.8. Melanin Content Assay
4.9. Cellular Tyrosinase Assay
4.10. Determination of Tyrosinase Protein Levels via Western Blotting
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rajapakse, N.; Kim, S.K. Nutritional and digestive health benefits of seaweed. In Advances in Food and Nutrition Research; Kim, S.-K., Ed.; Elsevier: San Diego, CA, USA, 2011; Volume 64, pp. 17–28. [Google Scholar]
- Suleria, H.; Osborne, S.; Masci, P.; Gobe, G. Marine-based nutraceuticals: An innovative trend in the food and supplement industries. Mar. Drugs 2015, 13, 6336–6351. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Jeon, Y.J.; Samarakoon, K.W.; Elvitigala, D.A. Marine-derived pharmaceuticals and future prospects. In Springer Handbook of Marine Biotechnology; Springer-Verlag Berlin Heidelberg: Berlin, Germany, 2015; pp. 957–968. [Google Scholar]
- Siahaan, E.A.; Pangestuti, R.; Kim, S.K. Seaweeds: Valuable ingredients for the pharmaceutical industries. In Grand Challenges in Marine Biotechnology; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 49–95. [Google Scholar]
- Thomas, N.; Kim, S.K. Beneficial effects of marine algal compounds in cosmeceuticals. Mar. Drugs 2013, 11, 146–164. [Google Scholar] [CrossRef]
- Lerner, A.B.; Fitzpatrick, T.B. Treatment of melanin hyperpigmentation. JAMA 1953, 152, 577–582. [Google Scholar] [CrossRef]
- Rigopoulos, D.; Gregoriou, S.; Katsambas, A. Hyperpigmentation and melasma. J. Cosmet. Dermatol. 2007, 6, 195–202. [Google Scholar] [CrossRef]
- Brown, D.A. Skin pigmentation enhancers. In Comprehensive Series in Photosciences; Elsevier Science: Amsterdam, The Netherlands, 2001; Volume 3, pp. 637–675. [Google Scholar]
- Costin, G.E.; Hearing, V.J. Human skin pigmentation: Melanocytes modulate skin color in response to stress. FASEB J. 2007, 21, 976–994. [Google Scholar] [CrossRef]
- Cichorek, M.; Wachulska, M.; Stasiewicz, A.; Tymińska, A. Skin melanocytes: Biology and development. Postepy. Dermatol. Alergol. 2013, 30, 30. [Google Scholar] [CrossRef]
- Tsatmali, M.; Ancans, J.; Thody, A.J. Melanocyte function and its control by melanocortin peptides. J. Histochem. Cytochem. 2002, 50, 125–133. [Google Scholar] [CrossRef]
- Kobayashi, T.; Urabe, K.; Winder, A.; Jiménez-Cervantes, C.; Imokawa, G.; Brewington, T.; Solano, F.; García-Borrón, J.; Hearing, V. Tyrosinase related protein 1 (TRP1) functions as a DHICA oxidase in melanin biosynthesis. EMBO J. 1994, 13, 5818–5825. [Google Scholar] [CrossRef]
- Körner, A.M.; Pawelek, J. Dopachrome conversion: A possible control point in melanin biosynthesis. J. Invest. Dermatol. 1980, 75, 192–195. [Google Scholar] [CrossRef]
- Steel, K.; Davidson, D.R.; Jackson, I. TRP-2/DT, a new early melanoblast marker, shows that steel growth factor (c-kit ligand) is a survival factor. Development 1992, 115, 1111–1119. [Google Scholar] [PubMed]
- Valencia, J.C.; Watabe, H.; Chi, A.; Rouzaud, F.; Chen, K.G.; Vieira, W.D.; Takahashi, K.; Yamaguchi, Y.; Berens, W.; Nagashima, K. Sorting of Pmel17 to melanosomes through the plasma membrane by AP1 and AP2: Evidence for the polarized nature of melanocytes. J. Cell Sci. 2006, 119, 1080–1091. [Google Scholar] [CrossRef]
- Kawakami, Y.; Robbins, P.F.; Wang, R.F.; Parkhurst, M.; Kang, X.; Rosenberg, S.A. The use of melanosomal proteins in the immunotherapy of melanoma. J. Immunother. 1998, 21, 237–246. [Google Scholar] [CrossRef]
- Bertolotto, C.; Abbe, P.; Hemesath, T.J.; Bille, K.; Fisher, D.E.; Ortonne, J.P.; Ballotti, R. Microphthalmia gene product as a signal transducer in cAMP-induced differentiation of melanocytes. J. Cell Biol. 1998, 142, 827–835. [Google Scholar] [CrossRef]
- Levy, C.; Khaled, M.; Fisher, D.E. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 2006, 12, 406–414. [Google Scholar] [CrossRef]
- Shibahara, S.; Yasumoto, K.I.; Amae, S.; Udono, T.; Watanabe, K.I.; Saito, H.; Takeda, K. Regulation of pigment cell-specific gene expression by MITF. Pigment Cell Res. 2000, 13, 98–102. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B.; Becker, S.W.; Lerner, A.B.; Montgomery, H. Tyrosinase in human skin: Demonstration of its presence and of its role in human melanin formation. Science 1950, 112, 223–225. [Google Scholar] [CrossRef]
- Ortonne, J.P. Photoprotective properties of skin melanin. Br. J. Dermatol. 2002, 146, 7–10. [Google Scholar] [CrossRef]
- Pomerantz, S.H.; Warner, M.C. 3,4-Dihydroxy-l-phenylalanine as the tyrosinase cofactor occurrence in melanoma and binding constant. J. Biol. Chem. 1967, 242, 5308–5314. [Google Scholar] [PubMed]
- Riley, P. Melanin. Int. J. Biochem. Cell Biol. 1997, 29, 1235–1239. [Google Scholar] [CrossRef]
- Cha, S.H.; Ko, S.C.; Kim, D.; Jeon, Y.J. Screening of marine algae for potential tyrosinase inhibitor: Those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. J. Dermatol. 2011, 38, 354–363. [Google Scholar] [CrossRef]
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch. Pharm. Res. 2004, 27, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.Y.; Eom, T.K.; Kim, M.M.; Kim, S.K. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. In Vitro. 2009, 23, 1123–1130. [Google Scholar] [CrossRef]
- Matos, M.J.; Santana, L.; Uriarte, E.; Delogu, G.; Corda, M.; Fadda, M.B.; Era, B.; Fais, A. New halogenated phenylcoumarins as tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 3342–3345. [Google Scholar] [CrossRef]
- Ismail, T.; Shafi, S.; Srinivas, J.; Sarkar, D.; Qurishi, Y.; Khazir, J.; Alam, M.S.; Kumar, H.M.S. Synthesis and tyrosinase inhibition activity of trans-stilbene derivatives. Bioorg. Chem. 2016, 64, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Onul, N.; Ertik, O.; Mermer, N.; Yanardag, R. Synthesis and biological evaluation of S-substituted perhalo-2-nitrobuta-1,3-dienes as novel xanthine oxidase, tyrosinase, elastase, and neuraminidase inhibitors. J. Chem. 2018, 2018. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Khalil, Z.; Yin, L.; Xiao, X.; Neupane, P.; Bernhardt, P.; Salim, A.; Song, F.; Capon, R. Chemical diversity from a Chinese marine red alga, Symphyocladia latiuscula. Mar. Drugs 2017, 15, 374. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Wang, B.G. Antioxidant capacity and lipophilic content of seaweeds collected from the Qingdao coastline. J. Agric. Food Chem. 2004, 52, 4993–4997. [Google Scholar] [CrossRef]
- Kang, H.S.; Chung, H.Y.; Kim, J.Y.; Son, B.W.; Jung, H.A.; Choi, J.S. Inhibitory phlorotannins from the edible brown alga Ecklonia stolonifera on total reactive oxygen species (ROS) generation. Arch. Pharm. Res. 2004, 27, 194–198. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, H.J.; Jung, H.A.; Chung, H.Y.; Jung, J.H.; Choi, W.C. A cyclohexanonyl bromophenol from the red alga Symphyocladia latiuscula. J. Nat. Prod. 2000, 63, 1705–1706. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.J.; Li, X.M.; Wang, B.G. Highly brominated mono-and bis-phenols from the marine red alga Symphyocladia latiuscula with radical-scavenging activity. J. Nat. Prod. 2007, 70, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yin, L.; Gao, L.; Gao, J.; Chen, J.; Li, J.; Song, F. Two new bromophenols with radical scavenging activity from marine red alga Symphyocladia latiuscula. Mar. Drugs 2013, 11, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Choi, H.R.; Park, H.J.; Choi, J.S.; Choi, W.C. Peroxynitrite scavenging and cytoprotective activity of 2,3,6-tribromo-4,5-dihydroxybenzyl methyl ether from the marine alga Symphyocladia latiuscula. J. Agric. Food Chem. 2001, 49, 3614–3621. [Google Scholar] [CrossRef]
- Choi, J.S.; Bae, H.J.; Kim, S.J.; Choi, I.S. In vitro antibacterial and anti-inflammatory properties of seaweed extracts against acne inducing bacteria, Propionibacterium acnes. J. Environ. Biol. 2011, 32, 313. [Google Scholar]
- Xu, X.; Yin, L.; Wang, Y.; Wang, S.; Song, F. A new bromobenzyl methyl sulphoxide from marine red alga Symphyocladia latiuscula. Nat. Prod. Res. 2013, 27, 723–726. [Google Scholar] [CrossRef]
- Xu, X.; Yin, L.; Gao, J.; Gao, L.; Song, F. Antifungal bromophenols from marine red alga Symphyocladia latiuscula. Chem. Biodivers. 2014, 11, 807–811. [Google Scholar] [CrossRef]
- Park, H.J.; Kurokawa, M.; Shiraki, K.; Nakamura, N.; Choi, J.S.; Hattori, M. Antiviral activity of the marine alga Symphyocladia latiuscula against herpes simplex virus (HSV-1) in vitro and its therapeutic efficacy against HSV-1 infection in mice. Biol. Pharm. Bull. 2005, 28, 2258–2262. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.H.; Park, H.J.; Jung, H.A.; Choi, J.S. Anti-diabetic activity of 2,3,6-tribromo-4,5-dihydroxybenzyl derivatives from Symphyocladia latiuscula through PTP1B downregulation and α-glucosidase inhibition. Mar. Drugs 2019, 17, 166. [Google Scholar] [CrossRef]
- Wang, W.; Okada, Y.; Shi, H.; Wang, Y.; Okuyama, T. Structures and aldose reductase inhibitory effects of bromophenols from the red alga Symphyocladia latiuscula. J. Nat. Prod. 2005, 68, 620–622. [Google Scholar] [CrossRef]
- Jin, H.J.; Oh, M.Y.; Jin, D.H.; Hong, Y.K. Identification of a Taq DNA polymerase inhibitor from the red seaweed Symphyocladia latiuscula. J. Environ. Biol. 2008, 29, 475–478. [Google Scholar]
- Lee, J.H.; Park, S.E.; Hossain, M.A.; Kim, M.Y.; Kim, M.N.; Chung, H.Y.; Choi, J.S.; Yoo, Y.H.; Kim, N.D. 2,3,6-Tribromo-4,5-dihydroxybenzyl methyl ether induces growth inhibition and apoptosis in MCF-7 human breast cancer cells. Arch. Pharm. Res. 2007, 30, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- De Vries, D.J.; Beart, P.M. Fishing for drugs from the sea: Status and strategies. Trends Pharmacol. Sci. 1995, 16, 275–279. [Google Scholar] [CrossRef]
- Guillerme, J.B.; Couteau, C.; Coiffard, L. Applications for marine resources in cosmetics. Cosmetics 2017, 4, 35. [Google Scholar] [CrossRef]
- Kim, S.K. Marine cosmeceuticals. J. Cosmet. Dermatol. 2014, 13, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.W.; Yoo, J.S. Screening for antioxidizing and tyrosinase-inhibitory activities of the extracts of marine algae from Busan coastal area. Ocean Polar Res. 2003, 25, 129–132. [Google Scholar] [CrossRef]
- Wagle, A.; Seong, S.H.; Joung, E.J.; Kim, H.R.; Jung, H.A.; Choi, J.S. Discovery of a highly potent tyrosinase inhibitor, luteolin 5-O-β-d-glucopyranoside, isolated from Cirsium japonicum var. maackii (Maxim.) Matsum., Korean thistle: Kinetics and computational molecular docking simulation. ACS Omega 2018, 3, 17236–17245. [Google Scholar]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Kim, J.H.; Morgan, A.M.; Tai, B.H.; Van, D.T.; Cuong, N.M.; Kim, Y.H. Inhibition of soluble epoxide hydrolase activity by compounds isolated from the aerial parts of Glycosmis stenocarpa. J. Enzyme Inhib. Med. Chem. 2016, 31, 640–644. [Google Scholar] [CrossRef]
- Wagle, A.; Seong, S.H.; Jung, H.A.; Choi, J.S. Identifying an isoflavone from the root of Pueraria lobata as a potent tyrosinase inhibitor. Food Chem. 2019, 276, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Azam, M.S.; Kwon, M.; Choi, J.; Kim, H.R. Sargaquinoic acid ameliorates hyperpigmentation through cAMP and ERK-mediated downregulation of MITF in α-MSH-stimulated B16F10 cells. Biomed. Pharmacother. 2018, 104, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.J.; Ha, Y.M.; Kim, J.A.; Park, J.Y.; Ha, T.K.; Park, D.; Chun, P.; Park, N.H.; Moon, H.R.; Chung, H.Y. A novel synthesized tyrosinase inhibitor: (E)-2-((2,4-dihydroxyphenyl) diazenyl) phenyl 4-methylbenzenesulfonate as an azo-resveratrol analog. Biosci. Biotechnol. Biochem. 2013, 77, 65–72. [Google Scholar] [CrossRef] [PubMed]
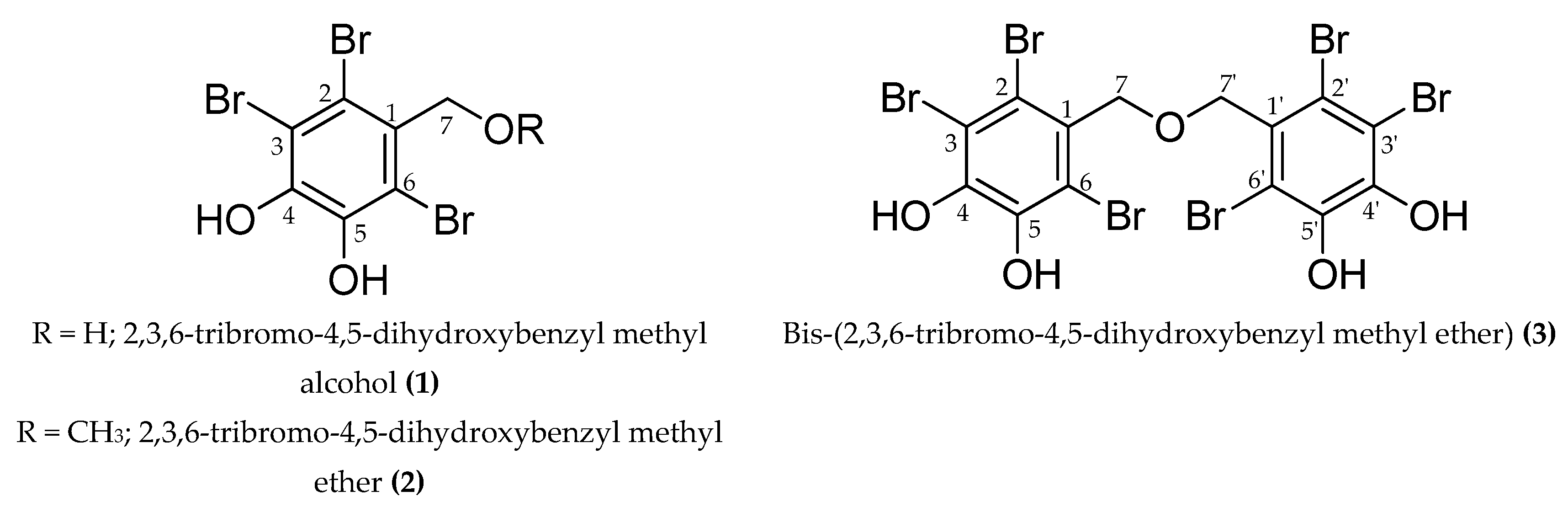

| Compounds | IC50 (µM) a (n = 3) | l-Tyrosine | ||
|---|---|---|---|---|
| l-Tyrosine | l-DOPA | Ki Value (µM) b | Inhibition Type c | |
| 1 | 10.78 ± 0.19 h | 270.53 ± 2.04 f | 10.59 | Competitive |
| 2 | 113.94 ± 0.75 g | >300 | - d | - d |
| 3 | 2.92 ± 0.04 i | 110.91 ± 4.95 g | 1.98 | Competitive |
| Kojic acid e | 3.17 ± 0.07 i | 3.07 ± 0.04 h | - d | - d |
| Arbutin e | 172.82 ± 4.71 f | >300 | - d | - d |
| Compounds | Binding Energy (kcal/mol) | No. of H-Bonds | H-Bond Interactions | Other Interacting Residues |
|---|---|---|---|---|
| 1 | ‒6.19 | 3 | Asn260, His61, and Per404 (O–H bond) | Glu256 and Met280 (O-Br bond), Val283 (Pi-Sigma), His263 (Pi-Pi Stacked), Ser282 (Amide-Pi Stacked), Ala286 and Val283 (Alkyl-Br), His85, Phe90, His244, His259, His263, and Phe264 (Pi-Br), Cu401, and 400 (van der Waals) |
| 2 | ‒6.29 | 1 | Per404 (O–H bond) | Met280 (O-Br bond), Val283 (Pi-Sigma), His263 (Pi-Pi Stacked), Ala286 and Val283 (Alkyl-Br), His85, Phe90, His259, His263, and Phe264 (Pi-Br), Cu401, and 400 (van der Waals) |
| 3 | ‒7.81 | 2 | Arg268 and Per404 (O–H bond) | His259 and Asn260 (C-O bond), Glu256 and Met280 (O-Br bond), Val283 (Pi-Sigma), His263 (Pi-Pi Stacked), Phe264 (Pi-Pi T-shaped), Ser282 (Amide-Pi Stacked), Ala286, Val248, Met257, and Val283 (Alkyl-Br), His85, His244, His259, His263, and Phe264 (Pi-Br), Cu401, and 400 (van der Waals) |
| l-Tyrosine a | ‒6.31 | 5 | His244, Asn260, and Met280 (O–H bond), Glu256 (Salt-bridge) | Ala286 (Pi-Alkyl), Val283(Pi-Sigma), His263 (Pi-Pi Stacked), Cu401, and 400, Per402 (van der Waals) |
| Luteolin a | ‒5.77 | 4 | Cys83, Gly245, Ala246, and Val248 (O–H bond) | Val248 (Pi-Alkyl), His85 (Pi-Sigma), Glu322 (Pi-Anion) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paudel, P.; Wagle, A.; Seong, S.H.; Park, H.J.; Jung, H.A.; Choi, J.S. A New Tyrosinase Inhibitor from the Red Alga Symphyocladia latiuscula (Harvey) Yamada (Rhodomelaceae). Mar. Drugs 2019, 17, 295. https://doi.org/10.3390/md17050295
Paudel P, Wagle A, Seong SH, Park HJ, Jung HA, Choi JS. A New Tyrosinase Inhibitor from the Red Alga Symphyocladia latiuscula (Harvey) Yamada (Rhodomelaceae). Marine Drugs. 2019; 17(5):295. https://doi.org/10.3390/md17050295
Chicago/Turabian StylePaudel, Pradeep, Aditi Wagle, Su Hui Seong, Hye Jin Park, Hyun Ah Jung, and Jae Sue Choi. 2019. "A New Tyrosinase Inhibitor from the Red Alga Symphyocladia latiuscula (Harvey) Yamada (Rhodomelaceae)" Marine Drugs 17, no. 5: 295. https://doi.org/10.3390/md17050295
APA StylePaudel, P., Wagle, A., Seong, S. H., Park, H. J., Jung, H. A., & Choi, J. S. (2019). A New Tyrosinase Inhibitor from the Red Alga Symphyocladia latiuscula (Harvey) Yamada (Rhodomelaceae). Marine Drugs, 17(5), 295. https://doi.org/10.3390/md17050295






