Clinical and Ultrasound Evaluation of Hemiplegic Shoulder Pain in Stroke Patients: A Longitudinal Observational Study Starting in the First Hours After Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Participants
2.3. Study Design
2.4. Clinical Assessment
2.5. Ultrasound Assessment
2.6. Statistical Analysis
3. Results
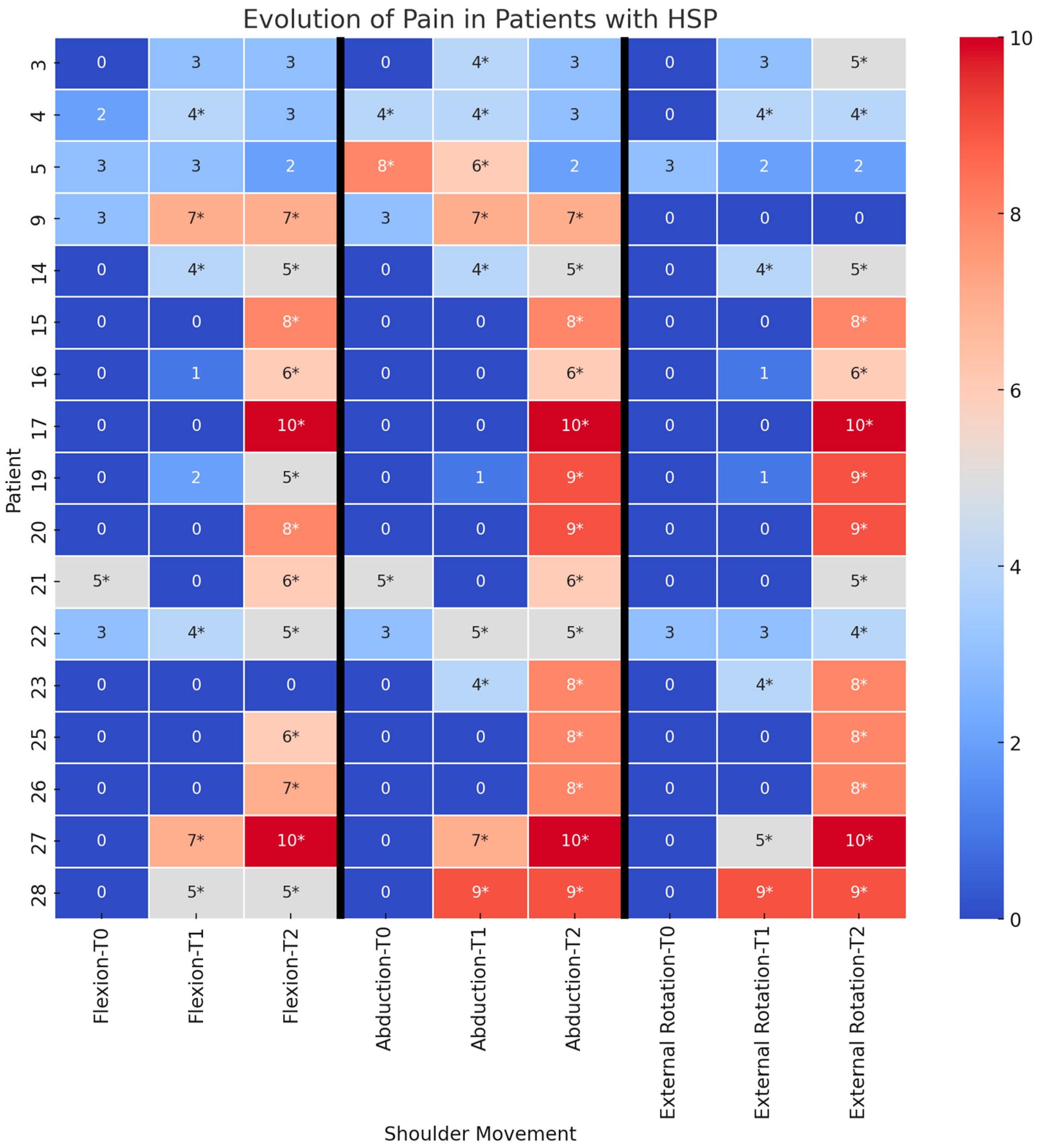
3.1. Time Course of Pain
3.2. Risk Factors for HSP Development
3.3. Time Course of Pain in Patients with and Without Capsular Pathology
3.4. Shoulder Function in Patients with and Without HSP at T2
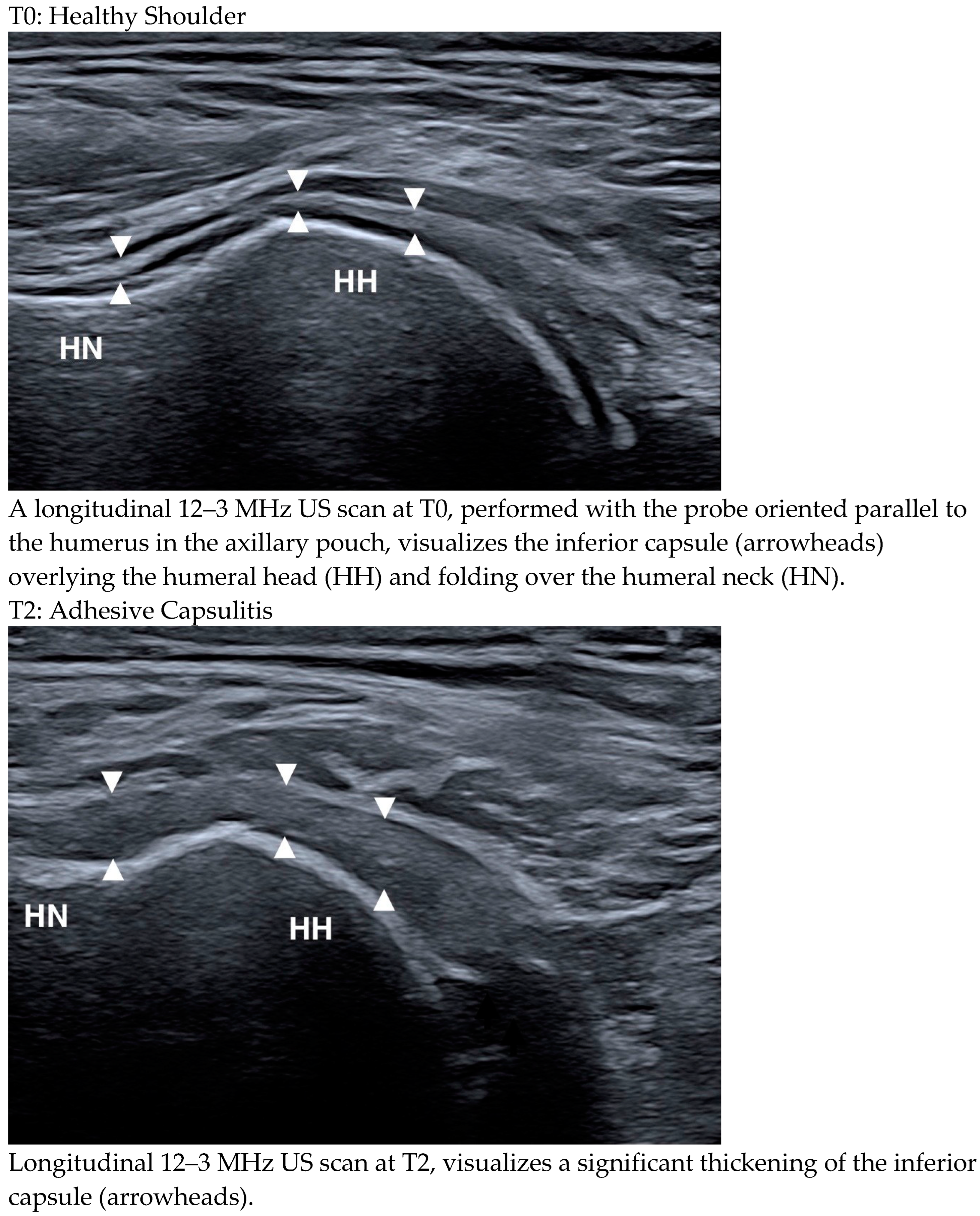
3.5. Ultrasound Features in Patients with and Without HSP at T2
3.6. Ultrasound Changes over Time
4. Discussion
4.1. Ultrasound Changes over Time
4.2. HSP in the Context of the General Clinical Picture
4.3. The Relationship Between HSP and Ultrasound Abnormalities
4.4. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, P.-H. Sonographic Findings of Painful Hemiplegic Shoulder after Stroke. J. Chin. Med. Assoc. 2018, 81, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Murie-Fernández, M.; Carmona Iragui, M.; Gnanakumar, V.; Meyer, M.; Foley, N.; Teasell, R. Painful Hemiplegic Shoulder in Stroke Patients: Causes and Management. Neurología (Engl. Ed.) 2012, 27, 234–244. [Google Scholar] [CrossRef]
- Anwer, S.; Alghadir, A. Incidence, Prevalence, and Risk Factors of Hemiplegic Shoulder Pain: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4962. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, L.; Ratmansky, M. Underlying Pathology and Associated Factors of Hemiplegic Shoulder Pain. Am. J. Phys. Med. Rehabil. 2011, 90, 768–780. [Google Scholar] [CrossRef]
- Adey-Wakeling, Z.; Liu, E.; Crotty, M.; Leyden, J.; Kleinig, T.; Anderson, C.S.; Newbury, J. Hemiplegic Shoulder Pain Reduces Quality of Life After Acute Stroke: A Prospective Population-Based Study. Am. J. Phys. Med. Rehabil. 2016, 95, 758–763. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Jackson, D. Shoulder Pain after Stroke: A Review of the Evidence Base to Inform the Development of an Integrated Care Pathway. Clin. Rehabil. 2002, 16, 276–298. [Google Scholar] [CrossRef]
- Poduri, K.R. Shoulder Pain in Stroke Patients and Its Effects on Rehabilitation. J. Stroke Cerebrovasc. Dis. 1993, 3, 261–266. [Google Scholar] [CrossRef]
- Lindgren, I.; Jönsson, A.-C.; Norrving, B.; Lindgren, A. Shoulder Pain After Stroke: A Prospective Population-Based Study. Stroke 2007, 38, 343–348. [Google Scholar] [CrossRef]
- Paolucci, S.; Iosa, M.; Toni, D.; Barbanti, P.; Bovi, P.; Cavallini, A.; Candeloro, E.; Mancini, A.; Mancuso, M.; Monaco, S.; et al. Prevalence and Time Course of Post-Stroke Pain: A Multicenter Prospective Hospital-Based Study. Pain. Med. 2015, 17, pnv019. [Google Scholar] [CrossRef]
- Nadler, M.; Pauls, M. Shoulder Orthoses for the Prevention and Reduction of Hemiplegic Shoulder Pain and Subluxation: Systematic Review. Clin. Rehabil. 2017, 31, 444–453. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Cui, L.; Bao, Y.; Gu, L.; Pan, H.; Wang, J.; Xie, Q. Prevalence, Risk Factor and Outcome in Middle-Aged and Elderly Population Affected by Hemiplegic Shoulder Pain: An Observational Study. Front. Neurol. 2023, 13, 1041263. [Google Scholar] [CrossRef] [PubMed]
- Ratnasabapathy, Y.; Broad, J.; Baskett, J.; Pledger, M.; Marshall, J.; Bonita, R. Shoulder Pain in People with a Stroke: A Population-Based Study. Clin. Rehabil. 2003, 17, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Gamble, G.E.; Barberan, E.; Laasch, H.; Bowsher, D.; Tyrrell, P.J.; Jones, A.K.P. Poststroke Shoulder Pain: A Prospective Study of the Association and Risk Factors in 152 Patients from a Consecutive Cohort of 205 Patients Presenting with Stroke. Eur. J. Pain 2002, 6, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Aras, M.D.; Gokkaya, N.K.O.; Comert, D.; Kaya, A.; Cakci, A. Shoulder Pain in Hemiplegia: Results from a National Rehabilitation Hospital in Turkey. Am. J. Phys. Med. Rehabil. 2004, 83, 713–719. [Google Scholar] [CrossRef]
- Yelnik, A.P.; Colle, F.M.C.; Bonan, I.V. Treatment of Pain and Limited Movement of the Shoulder in Hemiplegic Patients with Botulinum Toxin A in the Subscapular Muscle. Eur. Neurol. 2003, 50, 91–93. [Google Scholar] [CrossRef]
- De Melo Carvalho Rocha, E.; Riberto, M.; Da Ponte Barbosa, R.; Geronimo, R.M.P.; Menezes-Junior, M. Use of Botulinum Toxin as a Treatment of Hemiplegic Shoulder Pain Syndrome: A Randomized Trial. Toxins 2023, 15, 327. [Google Scholar] [CrossRef]
- Jia, F.; Zhu, X.-R.; Kong, L.-Y.; Fan, J.-C.; Zhu, Z.-J.; Lin, L.-Z.; Zhang, S.-Y.; Yuan, X.-Z. Stiffness Changes in Internal Rotation Muscles of the Shoulder and Its Influence on Hemiplegic Shoulder Pain. Front. Neurol. 2023, 14, 1195915. [Google Scholar] [CrossRef]
- Zheng, P.; Shi, Y.; Qu, H.; Han, M.L.; Wang, Z.Q.; Zeng, Q.; Zheng, M.; Fan, T. Effect of Ultrasound-Guided Injection of Botulinum Toxin Type A into Shoulder Joint Cavity on Shoulder Pain in Poststroke Patients: Study Protocol for a Randomized Controlled Trial. Trials 2024, 25, 418. [Google Scholar] [CrossRef]
- Pompa, A.; Clemenzi, A.; Troisi, E.; Di Mario, M.; Tonini, A.; Pace, L.; Casillo, P.; Cuccaro, A.; Grasso, M.G. Enhanced-MRI and Ultrasound Evaluation of Painful Shoulder in Patients after Stroke: A Pilot Study. Eur. Neurol. 2011, 66, 175–181. [Google Scholar] [CrossRef]
- Doğun, A.; Karabay, İ.; Hatipoğlu, C.; Őzgirgin, N. Ultrasound and Magnetic Resonance Findings and Correlation in Hemiplegic Patients With Shoulder Pain. Top. Stroke Rehabil. 2014, 21, S1–S7. [Google Scholar] [CrossRef]
- Pong, Y.; Wang, L.; Huang, Y.; Leong, C.; Liaw, M.; Chen, H. Sonography and Physical Findings in Stroke Patients with Hemiplegic Shoulders: A Longitudinal Study. J. Rehabil. Med. 2012, 44, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liang, P.; Pong, Y.; Leong, C.; Tseng, C. Physical Findings and Sonography of Hemiplegic Shoulder in Patients after Acute Stroke during Rehabilitation. J. Rehabil. Med. 2010, 42, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Idowu, B.M.; Ayoola, O.O.; Adetiloye, V.A.; Komolafe, M.A. Sonographic Evaluation of Structural Changes in Post-Stroke Hemiplegic Shoulders. Pol. J. Radiol. 2018, 82, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, N.; Yaşar, E.; Demir, Y.; Tezen, Ö.; Gurcay, E. Sonographic Predictors in Patients with Hemiplegic Shoulder Pain: A Cross-Sectional Study. J. Stroke Cerebrovasc. Dis. 2020, 29, 105170. [Google Scholar] [CrossRef]
- Lee, I.S.; Shin, Y.B.; Moon, T.-Y.; Jeong, Y.J.; Song, J.W.; Kim, D.H. Sonography of Patients with Hemiplegic Shoulder Pain After Stroke: Correlation with Motor Recovery Stage. Am. J. Roentgenol. 2009, 192, W40–W44. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Shen, P.-C.; Chang, K.-V.; Wu, W.-T.; Özçakar, L. Shoulder Ultrasound Imaging in the Post-Stroke Population: A Systematic Review and Meta-Analysis. JRM 2023, 55, jrm13432. [Google Scholar] [CrossRef]
- Hsu, C.; Afifi, T.; Isaac, Z. Shoulder Pathology on Advanced Imaging in Asymptomatic Non-Athlete Individuals: A Narrative Review. PMR J. Inj. Funct. Rehabil. 2024, 16, 1264–1275. [Google Scholar] [CrossRef]
- James, M.A. Use of the Medical Research Council Muscle Strength Grading System in the Upper Extremity. J. Hand Surg. 2007, 32, 154–156. [Google Scholar] [CrossRef]
- Meseguer-Henarejos, A.-B.; Sánchez-Meca, J.; López-Pina, J.-A.; Carles-Hernández, R. Inter- and Intra-Rater Reliability of the Modified Ashworth Scale: A Systematic Review and Meta-Analysis. Eur. J. Phys. Rehabil. Med. 2018, 54, 576–590. [Google Scholar] [CrossRef]
- Price, D.D.; Bush, F.M.; Long, S.; Harkins, S.W. A Comparison of Pain Measurement Characteristics of Mechanical Visual Analogue and Simple Numerical Rating Scales. Pain 1994, 56, 217–226. [Google Scholar] [CrossRef]
- Hudak, P.L.; Amadio, P.C.; Bombardier, C.; Beaton, D.; Cole, D.; Davis, A.; Hawker, G.; Katz, J.N.; Makela, M.; Marx, R.G.; et al. Development of an Upper Extremity Outcome Measure: The DASH (Disabilities of the Arm, Shoulder, and Head). Am. J. Ind. Med. 1996, 29, 602–608. [Google Scholar] [CrossRef]
- Kwah, L.K.; Diong, J. National Institutes of Health Stroke Scale (NIHSS). J. Physiother. 2014, 60, 61. [Google Scholar] [CrossRef] [PubMed]
- Grassi, W.; Filippucci, E.; Farina, A.; Cervini, C. Sonographic Imaging of Tendons. Arthritis Rheum. 2000, 43, 969. [Google Scholar] [CrossRef]
- Picasso, R.; Pistoia, F.; Zaottini, F.; Marcenaro, G.; Miguel-Pérez, M.; Tagliafico, A.S.; Martinoli, C. Adhesive Capsulitis of the Shoulder: Current Concepts on the Diagnostic Work-Up and Evidence-Based Protocol for Radiological Evaluation. Diagnostics 2023, 13, 3410. [Google Scholar] [CrossRef] [PubMed]
- Stella, S.M.; Gualtierotti, R.; Ciampi, B.; Trentanni, C.; Sconfienza, L.M.; Del Chiaro, A.; Pacini, P.; Miccoli, M.; Galletti, S. Ultrasound Features of Adhesive Capsulitis. Rheumatol. Ther. 2022, 9, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Cholewinski, J.J.; Kusz, D.J.; Wojciechowski, P.; Cielinski, L.S.; Zoladz, M.P. Ultrasound Measurement of Rotator Cuff Thickness and Acromio-Humeral Distance in the Diagnosis of Subacromial Impingement Syndrome of the Shoulder. Knee Surg. Sports Traumatol. Arthr. 2008, 16, 408–414. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, S.; Yang, E.; Paik, N. Clinical and Sonographic Risk Factors for Hemiplegic Shoulder Pain: A Longitudinal Observational Study. J. Rehabil. Med. 2014, 46, 81–87. [Google Scholar] [CrossRef]
- Dromerick, A.W.; Edwards, D.F.; Kumar, A. Hemiplegic Shoulder Pain Syndrome: Frequency and Characteristics During Inpatient Stroke Rehabilitation. Arch. Phys. Med. Rehabil. 2008, 89, 1589–1593. [Google Scholar] [CrossRef]
- Demirci, A.; Öcek, B.; Köseoğlu, F. Shoulder Pain in Hemiplegic Patients. J. PMR Sci. 2007, 1, 25–30. [Google Scholar]
- Trompetto, C.; Marinelli, L.; Mori, L.; Bragazzi, N.; Maggi, G.; Cotellessa, F.; Puce, L.; Vestito, L.; Molteni, F.; Gasperini, G.; et al. Increasing the Passive Range of Joint Motion in Stroke Patients Using Botulinum Toxin: The Role of Pain Relief. Toxins 2023, 15, 335. [Google Scholar] [CrossRef]
- Trompetto, C.; Marinelli, L.; Mori, L.; Pelosin, E.; Currà, A.; Molfetta, L.; Abbruzzese, G. Pathophysiology of Spasticity: Implications for Neurorehabilitation. BioMed Res. Int. 2014, 2014, 354906. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Fu, Y.; Hai-xin, S.; Yan, D.; Jian-hua, L. The Application of Sonography in Shoulder Pain Evaluation and Injection Treatment after Stroke: A Systematic Review. J. Phys. Ther. Sci. 2015, 27, 3007–3010. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, F.E.; Nye, C.J.S. A Prospective Study of Adhesive Capsulitis of the Shoulder (‘Frozen Shoulder’)in a High Risk Population. QJM: Int. J. Med. 1981, 50, 191–204. [Google Scholar] [CrossRef]
- Hill, J.L. Evidence for Combining Conservative Treatments for Adhesive Capsulitis. TOJ 2024, 24, 47–52. [Google Scholar] [CrossRef]
| Subject | Age | Gender | Type of Stroke | Affected Side | NIHSS |
|---|---|---|---|---|---|
| 1 | 61 | M | haemorragic | R | 11 |
| 2 | 66 | M | ischemic | L | 4 |
| 3 | 66 | F | ischemic | L | 8 |
| 4 | 71 | F | ischemic | L | 16 |
| 5 | 75 | F | ischemic | R | 6 |
| 6 | 69 | M | ischemic | L | 4 |
| 7 | 77 | F | ischemic | R | 3 |
| 8 | 73 | M | ischemic | R | 5 |
| 9 | 53 | M | ischemic | L | 5 |
| 10 | 82 | F | ischemic | R | 15 |
| 11 | 61 | M | haemorragic | R | 6 |
| 12 | 65 | F | haemorragic | R | 3 |
| 13 | 87 | M | haemorragic | L | 10 |
| 14 | 45 | M | haemorragic | L | 23 |
| 15 | 73 | F | ischemic | L | 16 |
| 16 | 46 | M | haemorragic | L | 12 |
| 17 | 73 | F | ischemic | L | 19 |
| 18 | 64 | M | haemorragic | R | 6 |
| 19 | 67 | F | ischemic | R | 11 |
| 20 | 57 | F | ischemic | R | 16 |
| 21 | 57 | M | haemorragic | L | 8 |
| 22 | 77 | M | haemorragic | L | 9 |
| 23 | 61 | M | ischemic | L | 14 |
| 24 | 56 | M | ischemic | R | 19 |
| 25 | 61 | F | haemorragic | L | 10 |
| 26 | 64 | M | haemorragic | L | 17 |
| 27 | 69 | F | ischemic | L | 14 |
| 28 | 64 | M | haemorragic | L | 15 |
| Characteristics | Flexion | Abduction | External Rotation | |||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |
| OR (95% CI) p-Value | Beta (95% CI) p-Value | OR (95% CI) p-Value | Beta (95% CI) p-Value | OR (95% CI) p-Value | Beta (95% CI) p-Value | |
| Age (10-years increase) | 0.42 (0.16; 1.09) 0.076 | 0.46 (0.15; 1.39) 0.170 | 0.38 (0.14; 1.02) 0.056 | 0.38 (0.12; 1.28) 0.120 | 0.56 (0.24; 1.32) 0.187 | --- |
| Gender | 1.29 (0.29; 5.77) 0.743 | --- | 1.00 (0.22; 4.47) 1.000 | --- | 2.57 (0.54; 12.17) 0.234 | --- |
| Type of stroke | 0.58 (0.13; 2.69) 0.490 | --- | 0.74 (0.16; 3.39) 0.699 | --- | 0.94 (0.20; 4.29) 0.934 | --- |
| NIHSS | 1.19 (1.01; 1.41) 0.040 | 1.20 (0.99; 1.46) 0.061 | 1.22 (1.02; 1.46) 0.026 | 1.24 (1.01; 1.53) 0.038 | 1.33 (1.07; 1.64) 0.009 | 1.33 (1.07; 1.64) 0.009 |
| Clinical History (Activities/Diseases) | ||||||
| Overhead Sports/ Occupation | 0.43 (0.09; 1.98) 0.278 | --- | 0.30 (0.06; 1.44) 0.133 | --- | 0.39 (0.08; 1.84) 0.234 | --- |
| Cardiological | 0.11 (0.01; 1.16) 0.066 | 0.12 (0.01; 1.64) 0.113 | 0.15 (0.01; 2.15) 0.093 | 0.15 (0.01; 2.15) 0.164 | 0.17 (0.02; 1.67) 0.128 | --- |
| Endocrine | 0.67 (0.14; 3.19) 0.612 | --- | 0.53 (0.11; 2.56) 0.433 | --- | 0.80 (0.17; 3.77) 0.778 | --- |
| Internal | 2.55 (0.20; 31.86) 0.469 | --- | 2.17 (0.17; 27.08) 0.548 | --- | 1.85 (0.15; 23.07) 0.634 | --- |
| Onco-haematological | 1.18 (0.14; 9.83) 0.877 | --- | 1.00 (0.12; 8.31) 1.000 | --- | 0.85 (0.10; 7.04) 0.877 | --- |
| Neuropsychiatric | 0.50 (0.08; 3.32) 0.473 | --- | 0.42 (0.06; 2.77) 0.365 | --- | 0.35 (0.05; 2.31) 0.273 | --- |
| PNRS Flexion | PNRS Abduction | PNRS External Rotation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No HSP N = 15 (54%) Median (IQR) 0 (0; 0) | HSP N = 13 (46%) Median (IQR) 6 (5; 8) | p Value | No HSP N = 14 (50%) Median (IQR) 0 (0; 0) | HSP N = 14 (50%) Median (IQR) 8 (6; 9) | p Value | No HSP N = 13 (46%) Median (IQR) 0 (0; 0) | HSP N = 15 (54%) Median (IQR) 8 (5; 9) | p Value | |
| TTS > 1 | 5 (33%) | 3 (23%) | 0.686 | 5 (36%) | 3 (21%) | 0.678 | 4 (31%) | 4 (27%) | 1.000 |
| SASD bursitis | 0 (0%) | 3 (23%) | 0.087 | 0 (0%) | 3 (21%) | 0.222 | 0 (0%) | 3 (20%) | 0.226 |
| LHBT tenosynovitis | 0 (0%) | 1 (8%) | 0.464 | 0 (0%) | 1 (7%) | 1.000 | 0 (0%) | 1 (7%) | 1.000 |
| Glenohumeral joint effusion | 0 (0%) | 1 (8%) | 0.464 | 0 (0%) | 1 (7%) | 1.000 | 0 (0%) | 1 (7%) | 1.000 |
| Adhesive capsulitis | 0 (0%) | 6 (46%) | 0.005 | 0 (0%) | 6 (43%) | 0.016 | 0 (0%) | 6 (40%) | 0.018 |
| Shoulder subluxation | 0 (0%) | 6 (46%) | 0.005 | 0 (0%) | 6 (43%) | 0.016 | 1 (8%) | 5 (33%) | 0.173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotellessa, F.; Campanella, W.; Puce, L.; May, M.C.; Ponzano, M.; Picasso, R.; Mordeglia, M.; Subbrero, D.; Cecchella, E.; Mori, L.; et al. Clinical and Ultrasound Evaluation of Hemiplegic Shoulder Pain in Stroke Patients: A Longitudinal Observational Study Starting in the First Hours After Stroke. Medicina 2025, 61, 484. https://doi.org/10.3390/medicina61030484
Cotellessa F, Campanella W, Puce L, May MC, Ponzano M, Picasso R, Mordeglia M, Subbrero D, Cecchella E, Mori L, et al. Clinical and Ultrasound Evaluation of Hemiplegic Shoulder Pain in Stroke Patients: A Longitudinal Observational Study Starting in the First Hours After Stroke. Medicina. 2025; 61(3):484. https://doi.org/10.3390/medicina61030484
Chicago/Turabian StyleCotellessa, Filippo, William Campanella, Luca Puce, Maria Cesarina May, Marta Ponzano, Riccardo Picasso, Matteo Mordeglia, Davide Subbrero, Ester Cecchella, Laura Mori, and et al. 2025. "Clinical and Ultrasound Evaluation of Hemiplegic Shoulder Pain in Stroke Patients: A Longitudinal Observational Study Starting in the First Hours After Stroke" Medicina 61, no. 3: 484. https://doi.org/10.3390/medicina61030484
APA StyleCotellessa, F., Campanella, W., Puce, L., May, M. C., Ponzano, M., Picasso, R., Mordeglia, M., Subbrero, D., Cecchella, E., Mori, L., Sassos, D., Del Sette, M., Formica, M., & Trompetto, C. (2025). Clinical and Ultrasound Evaluation of Hemiplegic Shoulder Pain in Stroke Patients: A Longitudinal Observational Study Starting in the First Hours After Stroke. Medicina, 61(3), 484. https://doi.org/10.3390/medicina61030484






