The Effects of Prednisone/Ketoprofen Administration in Association with Amoxicillin Clavulanate Following Periodontal Surgical Therapy in Patients with Severe Chronic Periodontitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Medical Records
2.3. Oral Examination
2.4. Salivary Cortisol Assessment
2.5. Statistical Analysis
3. Results
3.1. Medical Records and Risk Factors Assessment
3.2. Preintervention Periodontal Assessments
3.3. Pre- and Postintervention Clinical Assessments
3.3.1. BOP Evaluations
3.3.2. Mobility Evaluations
3.4. Salivary Cortisol Assessments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atri, M.; Srivastava, D.; Kharbanda, J.; Bugalia, A.; Yousuf, A.; Anup, N. Occupational stress, salivary cortisol, and periodontal disease: A clinical and laboratory study. J. Int. Oral Health 2015, 7, 65–69. [Google Scholar]
- Alani, A.; Seymour, R. Systemic medication and the inflammatory cascade. Periodontol 2000 2014, 64, 198–210. [Google Scholar] [CrossRef]
- Heasman, P.A.; Hughes, F.J. Drugs, medications and periodontal disease. Nat. Publ. Gr. 2014, 217, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Waite, I.M.; Saxton, C.A.; Young, A.; Wagg, B.J.; Corbett, M. The periodontal status of subjects receiving non-steroidal anti-inflammatory drugs. J. Periodontal Res. 1981, 16, 100–108. [Google Scholar] [CrossRef]
- Kardachi, B.J.R.; Newcomb, G.M. A clinical study of gingival inflammation in renal transplant recipients taking immunosuppressive drugs. J. Periodontol. 1978, 49, 307–309. [Google Scholar] [CrossRef]
- Been, V.; Engel, D. The effects of immunosuppressive drugs on periodontal inflammation in human renal allograft patients. J. Periodontol. 1982, 53, 245–248. [Google Scholar] [CrossRef]
- Pilot, T.; Miyazaki, H. Periodontal conditions in Europe. J. Clin. Periodontol. 1991, 18, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Shiau, H.J.; Reynolds, M.A. Sex differences in destructive periodontal disease: A systematic review. J. Periodontol. 2010, 81, 1379–1389. [Google Scholar] [CrossRef]
- Zee, K.Y. Smoking and periodontal disease. Aust. Dent. J. 2009, 54, S44–S50. [Google Scholar] [CrossRef]
- Hopcraft, M.S.; Morgan, M.V.; Satur, J.G.; Wright, F.A.C.; Darby, I.B. Oral hygiene and periodontal disease in Victorian nursing homes. Gerodontology 2012, 29, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, M.S.; Bissada, N.F.; Borawski, E.A. Obesity and Periodontal Disease in Young, Middle-Aged, and Older Adults. J. Periodontol. 2003, 74, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Ekuni, D.; Yamamoto, T.; Koyama, R.; Tsuneishi, M.; Naito, K.; Tobe, K. Relationship between body mass index and periodontitis in young Japanese adults. J. Periodontal Res. 2008, 43, 417–421. [Google Scholar] [CrossRef]
- Dye, B.A.; Selwitz, R. The relationship between selected measures of periodontal status and demographic and behavioural risk factors. J. Clin. Periodontol. 2005, 32, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Borrell, L.; Crawford, N. Socioeconomic position indicators and periodontitis: Examining the evidence. Periodontol 2000 2012, 58, 69–83. [Google Scholar] [CrossRef]
- Obulareddy, V.T.; Chava, V.K.; Nagarakanti, S. Association of stress, salivary cortisol, and chronic periodontitis: A clinico-biochemical study. Contemp. Clin. Dent. 2018, 9 (Suppl. 2), S299–S304. [Google Scholar]
- Naghsh, N.; Mogharehabed, A.; Karami, E.; Yaghini, J. Comparative evaluation of the cortisol level of unstimulated saliva in patients with and without chronic periodontitis. Dent. Res. J. 2019, 16, 421–427. [Google Scholar]
- Miricescu, D.; Totan, A.; Calenic, B.; Mocanu, B.G.M. Periodontics salivary and serum cortisol in patients with periodontal disease and lichen planus. Stoma. Edu. J. 2015, 1, 47–52. [Google Scholar]
- Dalewski, B.; Kamińska, A.; Szydłowski, M.; Kozak, M.; Sobolewska, E. Comparison of early effectiveness of three different intervention methods in patients with chronic orofacial pain: A randomized, controlled clinical trial. Pain Res. Manag. 2019, 2019, 7954291. [Google Scholar] [CrossRef]
- Anaise, J.Z. Measurement of dental caries experience--modification of the DMFT index. Community Dent. Oral Epidemiol. 1984, 12, 43–46. [Google Scholar] [CrossRef]
- Newman, M. Carranza’s Clinical Periodontology, 12th ed.; Carranza, F., Ed.; Elsevier Saunders: Philadelphia, PA, USA, 2015. [Google Scholar]
- Fleszar, T.J.; Knowles, J.W.; Morrison, E.C.; Burgett, F.G.; Nissle, R.R.; Ramfjord, S.P. Tooth mobility and periodontal therapy. J. Clin. Periodontol. 1980, 7, 495–505. [Google Scholar] [CrossRef]
- Page, R.C.; Eke, P.I. Case definitions for use in population-based surveillance of periodontitis. J. Periodontol. 2007, 78, 1387–1399. [Google Scholar] [CrossRef]
- Navazesh, M. Methods for collecting saliva. Ann. N. Y. Acad. Sci. 1993, 694, 72–77. [Google Scholar] [CrossRef]
- Ishi, E.P.; Bertolo, M.B.; Rossa, C., Jr.; Kirkwood, K.L.; Onofre, M. Periodontal condition in patients with rheumatoid arthritis. Braz. Oral Res. 2008, 22, 72–77. [Google Scholar] [CrossRef]
- Srinivas, M.; Sangeetha Medaiah Girish, S.; Anil, M.; Jagadish Pai, A.W. The effect of ketoprofen in chronic periodontitis: A clinical double-blind study. J. Indian Soc. Periodontol. 2011, 15, 255–259. [Google Scholar] [PubMed]
- Funosas, E.R.; Escovich, L.; Maestri, L. The use of topical subgingival gels of non-steroidal anti-inflammatory drugs (NSAIDs) as an adjunct to non-surgical management of chronic periodontitis. Acta Odontol. Latinoam 2009, 22, 215–219. [Google Scholar]
- Abramson, M.M.; Wolff, L.F.; Offenbacher, S.; Aeppli, D.M.; Hardie, N.D.; Friedman, H.M. Flurbiprofen effect on gingival crevicular fluid prostaglandin and thromboxane levels in humans. J. Periodontal Res. 1992, 27, 539–543. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, T.P.; Roszkowski, M.T.; Wolff, L.F.; Hinrichs, J.E.; Hargreaves, K.M. Effect of a non-steroidal anti-inflammatory drug on tissue levels of immunoreactive prostaglandin E2, immunoreactive leukotriene, and pain after periodontal surgery. J. Periodontol. 1996, 67, 1307–1316. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Lauffart, B.; Brown, P.; Zak, E.; Heasman, P.A. Effects of ketorolac tromethamine mouthrinse (0.1%) on crevicular fluid prostaglandin E2 concentrations in untreated chronic periodontitis. J Periodontol. 1998, 69, 777–783. [Google Scholar] [CrossRef]
- Oduncuoglu, B.F.; Kayar, N.A.; Haliloglu, S.; Serpek, B.; Ataoglu, T.; Alptekin, N.O. Effects of a cyclic NSAID regimen on levels of gingival crevicular fluid prostaglandin E2and Interleukin-1β: A 6-month randomized controlled clinical trial. Niger. J. Clin. Pract. 2018, 21, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Feller, L.; Lemmer, J. Tooth mobility after periodontal surgery. SADJ 2004, 59, 407, 409–411. [Google Scholar] [PubMed]
- Baker, P.J. The role of immune responses in bone loss during periodontal disease. Microbes Infect. 2000, 2, 1181–1192. [Google Scholar] [CrossRef]
- Feldman, R.S.; Szeto, B.; Chauncey, H.H.; Goldhaber, P. Non-steroidal anti-inflammatory drugs in the reduction of human alveolar bone loss. J. Clin. Periodontol. 1983, 10, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Jeffcoat, M.K.; Reddy, M.S.; Haigh, S.; Buchanan, W.; Doyle, M.J.; Meredith, M.P.; Nelson, S.L.; Goodale, M.B.; Wehmeyer, K.R. A comparison of topical ketorolac, systemic flurbiprofen, and placebo for the inhibition of bone loss in adult periodontitis. J. Periodontol. 1995, 66, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Holzhausen, M.; Rossa, C.; Marcantonio, E.; Nassar, P.O.; Spolidório, D.M.P.; Spolidório, L.C. Effect of Selective Cyclooxygenase-2 Inhibition on the Development of Ligature-Induced Periodontitis in Rats. J. Periodontol. 2002, 73, 1030–1036. [Google Scholar] [CrossRef]
- Beeraka, S.S.; Natarajan, K.; Patil, R.; Manne, R.K.; Prathi, V.S.; Kolaparthi, V.S.K. Clinical and radiological assessment of effects of long-term corticosteroid therapy on oral health. Dent. Res. J. 2013, 10, 666–673. [Google Scholar]
- Hilgert, J.B.; Hugo, F.N.; Bandeira, D.R.; Bozzetti, M.C. Stress, cortisol, and periodontitis in a population aged 50 years and over. J. Dent. Res. 2006, 85, 324–328. [Google Scholar] [CrossRef]
- Hobdell, M.H.; Oliveira, E.R.; Bautista, R.; Myburgh, N.G.; Lalloo, R.; Narendran, S.; Johnson, N.W. Oral diseases and socio-economic status (SES). Br. Dent. J. 2003, 194, 91–96. [Google Scholar] [CrossRef]
- Gibson, N.; Ferguson, J.W. Steroid cover for dental patients on long-term steroid medication: Proposed clinical guidelines based upon a critical review of the literature. Br. Dent. J. 2004, 197, 681–685. [Google Scholar] [CrossRef]
- Greabu, M.; Purice, M.; Totan, A.; Spinu, T.; Totan, C. Salivary cortisol-marker of stress response to different dental treatment. Rom. J. Intern. Med. 2006, 44, 49–59. [Google Scholar]
- Miller, C.S.; Dembo, J.B.; Falace, D.A.; Kaplan, A.L. Salivary cortisol response to dental treatment of varying stress. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1995, 79, 436–441. [Google Scholar] [CrossRef]
- Dubar, M.; Clerc-Urmès, I.; Baumann, C.; Clément, C.; Alauzet, C.; Bisson, C. Relations of Psychosocial Factors and Cortisol with Periodontal and Bacterial Parameters: A Prospective Clinical Study in 30 Patients with Periodontitis Before and After Non-Surgical Treatment. Int. J. Environ. Res. Public Health 2020, 17, 7651. [Google Scholar] [CrossRef] [PubMed]
- Debono, M.; Harrison, R.F.; Whitaker, M.J.; Eckland, D.; Arlt, W.; Keevil, D.; Ross, R. Salivary cortisone reflects cortisol exposure under physiological conditions and after hydrocortisone. J. Clin. Endocrinol. Metab. 2016, 101, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
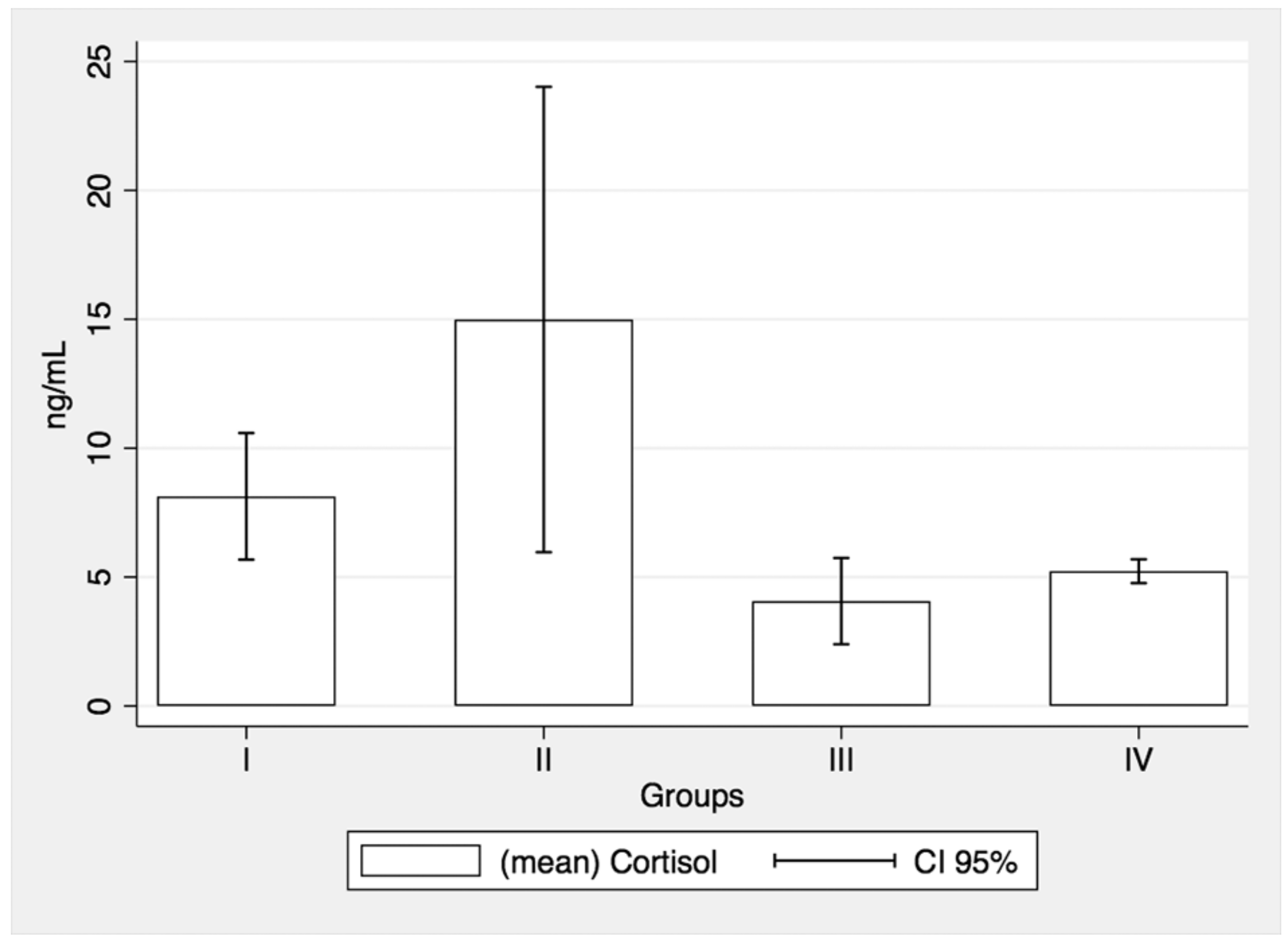
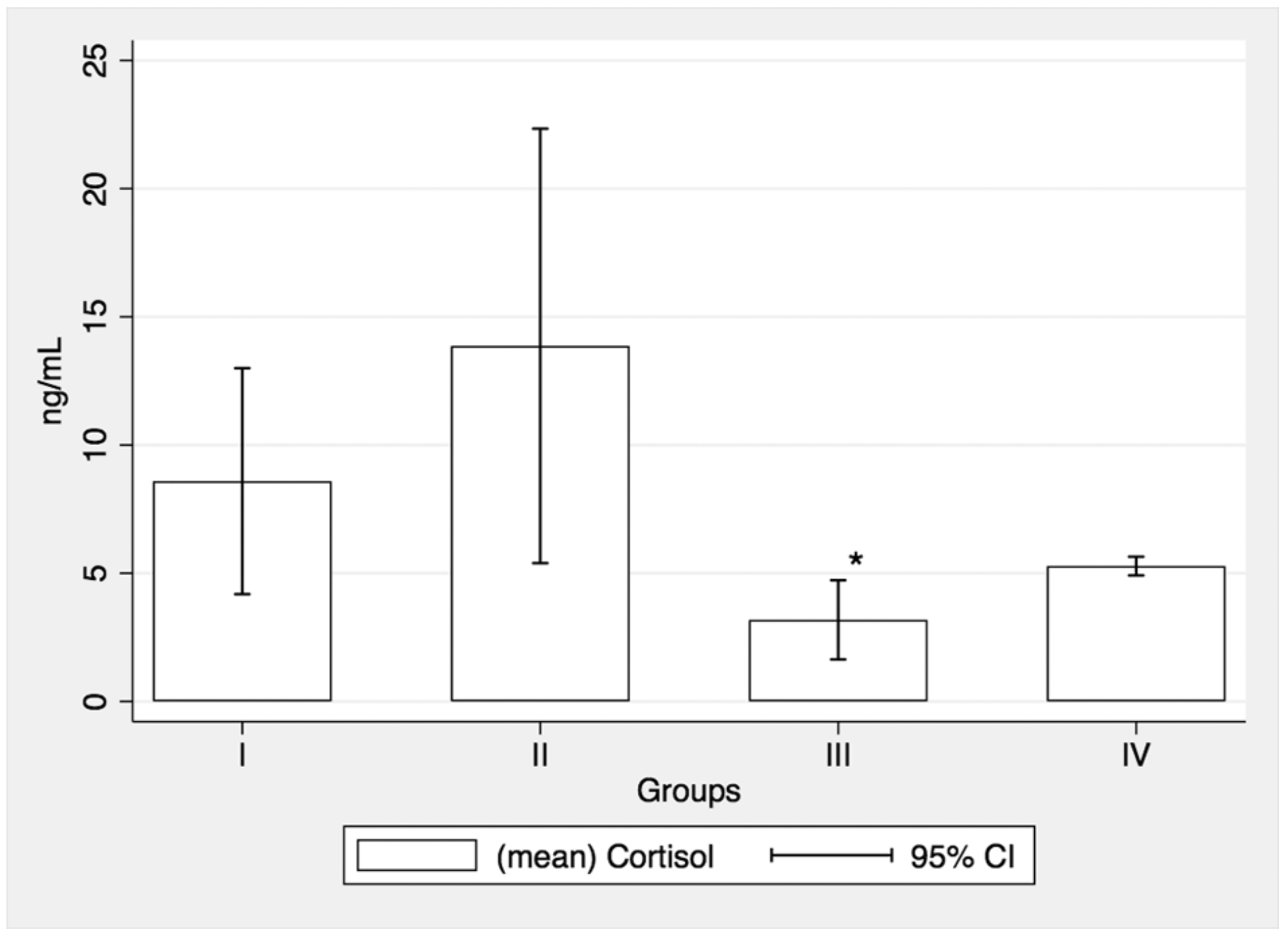
| Groups * | I (n = 8) | II (n = 7) | III (n = 7) | IV (n = 19) | |
|---|---|---|---|---|---|
| Variable | |||||
| Age Mean (SD **; range) | 43.25 (12.8; 24–59) | 45.28 (9.7; 28–54) | 46.28 (16.76; 26–72) | 41.1 (9.84; 28–68) | |
| Male (%) | 62.50 | 42.86 | 57.14 | 57.89 | |
| Smokers (%) | 37.50 | 42.86 | 57.14 | 57.89 | |
| Higher education (%) | 50 | 57.14 | 42.86 | 73.57 | |
| Urban resident (%) | 87.50 | 85.71 | 28.57 | 100 | |
| BMI Mean * (SD **; range) | 28.43 * (9.5; 17.71–47.34) | 24.97 * (4.16; 19.60–30.48) | 23.78 * (3.59; 19.15–27.77) | 26.09 * (5.45; 19.49–41.87) | |
| No scaling per year (%) | 37.5 | 71.43 | 57.14 | 36.84 | |
| Two times/day brushing (%) | 100 | 100 | 71.43 | 89.47 | |
| Using auxiliary methods to brushing (%) | 0 | 14.29 | 0 | 15.79 | |
| Groups * | DMFT | DMFS |
|---|---|---|
| Mean (SD **; Range) | Mean (SD **; Range) | |
| I (n = 8) | 12.5 (6.45; 3–22) | 49 (27.27; 10–86) |
| II (n = 7) | 14 (6.70; 4–21) | 60.14 (37.86; 14–105) |
| III (n = 7) | 11.57 (5.56; 3–18) | 44.14 (26.56; 6–78) |
| IV (n = 19) | 11.65 (4.20; 3–19) | 38.35 (22.84; 7–90) |
| Groups * | CAL (mm) | PPD (mm) | Site |
|---|---|---|---|
| Mean (SD **; Range) | |||
| I (n = 8) | 3.96 (0.62; 3.25–5.35) | 3.49 (0.56; 2.92–4.82) | 155.37 (12.68; 126–168) |
| II (n = 7) | 3.94 (0.23; 3.49–4.25) | 3.69 (0.35; 3.06–4.16) | 145.71 (24.49; 109–180) |
| III (n = 7) | 3.82 (0.99; 2.68–5.44) | 3.17 (0.81; 2.16–4.86) | 159.57 (23.10; 126–191) |
| Groups * | BOP | ||
|---|---|---|---|
| Pre-Treatment | Post-Treatment | Significance | |
| Mean (SD **, Range) | |||
| I (n = 8) | 37.10 (9.91; 24.07–58.33) | 4.63 (3.06; 0–8.33) | p = 0.011 *** |
| II (n = 7) | 49.52 (13.63; 29.16–74.24) | 8.15 (6.37; 0–20.37) | p = 0.018 *** |
| III (n = 7) | 43.05 (16.25; 19.79–71.42) | 27.29 (11.44; 9.52–43.58) | p = 0.022 *** |
| Groups * | Tooth Mobility | ||||
|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | ||
| Mean (SD **; Range) | |||||
| I (n = 8) | Pretreatment | 5.25 (4.29; 0–15) | 10.37 (5.17; 1–14) | 6.37 (2.82; 1–9) | 4.37 (3.70; 0–13) |
| Post-treatment | 12.87 (4.51; 6–12) | 7.12 (1.53; 5–10) | 2.37 (2.78; 0–9) | 0.25 (0.66; 0–2) | |
| II (n = 7) | Pretreatment | 8.71 (4.06; 1–15) | 9.85 (4.58; 3–13) | 3.85 (2.64; 1–8) | 1.57 (0.90; 0–3) |
| Post-treatment | 14.57 (6.18; 6–12) | 5.85 (3.48; 3–16) | 1.14 (8.83; 0–2) | 0 | |
| III (n = 7) | Pretreatment | 16.71 (5.77; 8–26) | 5.71 (2.05; 2–8) | 2.71 (3.14; 0–8) | 1.14 (1.24; 0–3) |
| Post-treatment | 17.28 (6.27; 8–28) | 6.28 (3.75; 0–13) | 1.57 (2.38; 0–7) | 1.14 (1.24; 0–3) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sfetcu, L.; Didilescu, A.C.; Vlădan, C.; Dincă, O.; Miricescu, D.; Băncescu, G.; Bucur, A.; Tribus, L.C. The Effects of Prednisone/Ketoprofen Administration in Association with Amoxicillin Clavulanate Following Periodontal Surgical Therapy in Patients with Severe Chronic Periodontitis. Medicina 2021, 57, 447. https://doi.org/10.3390/medicina57050447
Sfetcu L, Didilescu AC, Vlădan C, Dincă O, Miricescu D, Băncescu G, Bucur A, Tribus LC. The Effects of Prednisone/Ketoprofen Administration in Association with Amoxicillin Clavulanate Following Periodontal Surgical Therapy in Patients with Severe Chronic Periodontitis. Medicina. 2021; 57(5):447. https://doi.org/10.3390/medicina57050447
Chicago/Turabian StyleSfetcu, Lidia, Andreea Cristiana Didilescu, Cristian Vlădan, Octavian Dincă, Daniela Miricescu, Gabriela Băncescu, Alexandru Bucur, and Laura Carina Tribus. 2021. "The Effects of Prednisone/Ketoprofen Administration in Association with Amoxicillin Clavulanate Following Periodontal Surgical Therapy in Patients with Severe Chronic Periodontitis" Medicina 57, no. 5: 447. https://doi.org/10.3390/medicina57050447
APA StyleSfetcu, L., Didilescu, A. C., Vlădan, C., Dincă, O., Miricescu, D., Băncescu, G., Bucur, A., & Tribus, L. C. (2021). The Effects of Prednisone/Ketoprofen Administration in Association with Amoxicillin Clavulanate Following Periodontal Surgical Therapy in Patients with Severe Chronic Periodontitis. Medicina, 57(5), 447. https://doi.org/10.3390/medicina57050447







