Complementarity of Photo-Biomodulation, Surgical Treatment, and Antibiotherapy for Medication-Related Osteonecrosis of the Jaws (MRONJ)
Abstract
1. Introduction
2. Materials and Methods
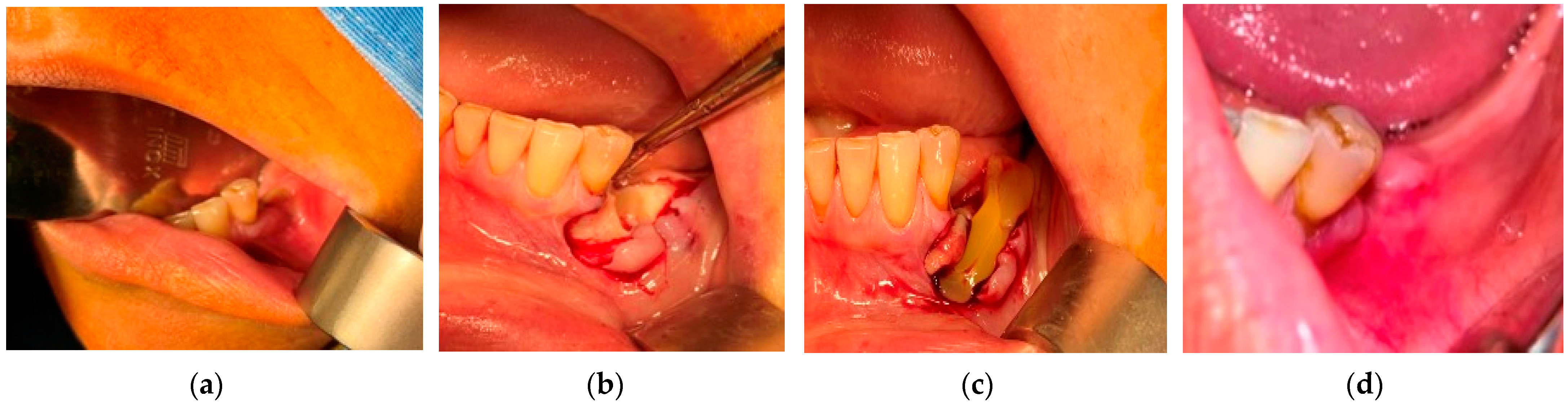
- (a)
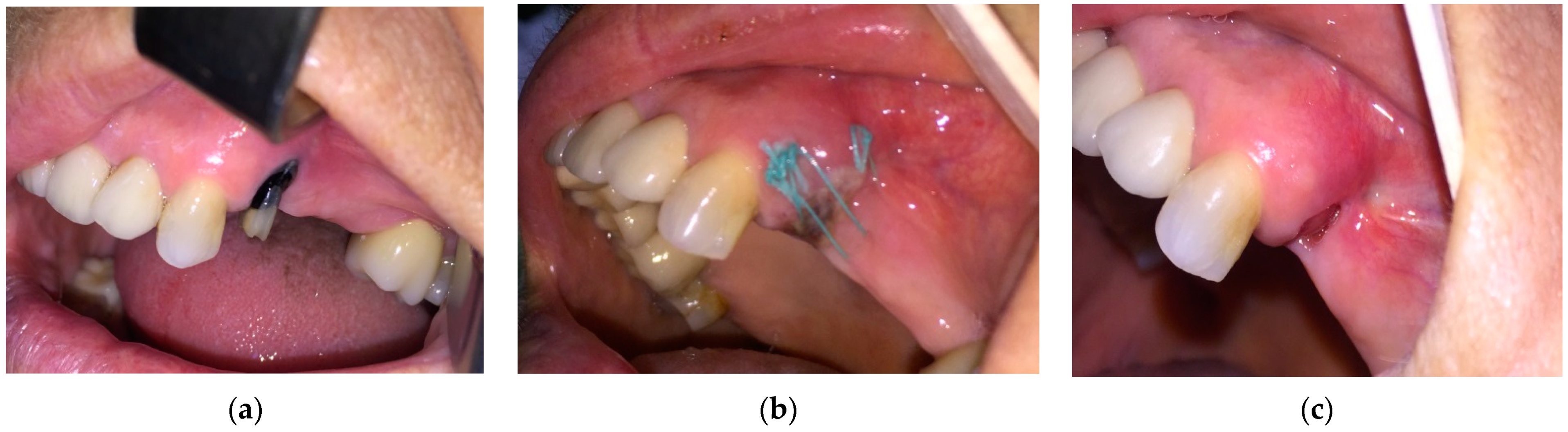
- Patients staged “at-risk” for MRONJ and referred for tooth extraction required oral surgery procedures, but they had no symptoms and oral signs of MRONJ. They received Amoxicillin/Clavulanic acid 1 g/12 h or, in case of drug allergy, 600 mg Clindamycin orally, 3 days before and 7 days after extraction, 2 times a day. Dental extractions were carried out with minimum trauma. After curettage and lavage with saline solution, a crossed horizontal external suture was applied to limit the socket entrance and to cover the bony margins. In the next step, near infrared (NIR) InGaAsP Diode laser (EPIC X™, BIOLASE®, Foothill Ranch, CA, USA), with a center wavelength of 940 nm, was used to photo-biomodulate the socket from the buccal, as well as from the lingual side, perpendicular to the surface. For delivering the laser irradiation, the PBM tissue handpiece by BIOLASE® was used, with a laser beam diameter of 9 mm and with an irradiation area of 0.635 cm2. PBM was performed using the following parameters and settings: power 100 mW, power density 157.4 W/cm2, in continuous mode, irradiation time 40 s on each side, energy 8 J per each session, energy density 3.937 J/cm2 (in non-contact mode, 1 mm from the tissue surface). After tooth extraction, the PBM was performed at 24 h, 48 h, 72 h, day 4, day 5, day 6, and day 7, as well as 3 times/week for the following 2 weeks after surgery. The sutures were removed 10 days after the surgery. Mouth rinsing with 0.12% chlorhexidine digluconate was prescribed for 6 weeks.
- (b)
- The patients in stage 0 of MRONJ received only antibiotic treatment and PBM, with no surgery. The treatment was received for 14 days: Amoxicillin/Clavulanic acid 1 g every 12 h or, in case of drug allergy, 600 mg Clindamycin orally, twice a day. To reduce the local inflammation associated with pain, PBM was performed during the 7 consecutive days, followed by other 6 sessions of laser irradiation distributed in the following 2 weeks.
- (c)
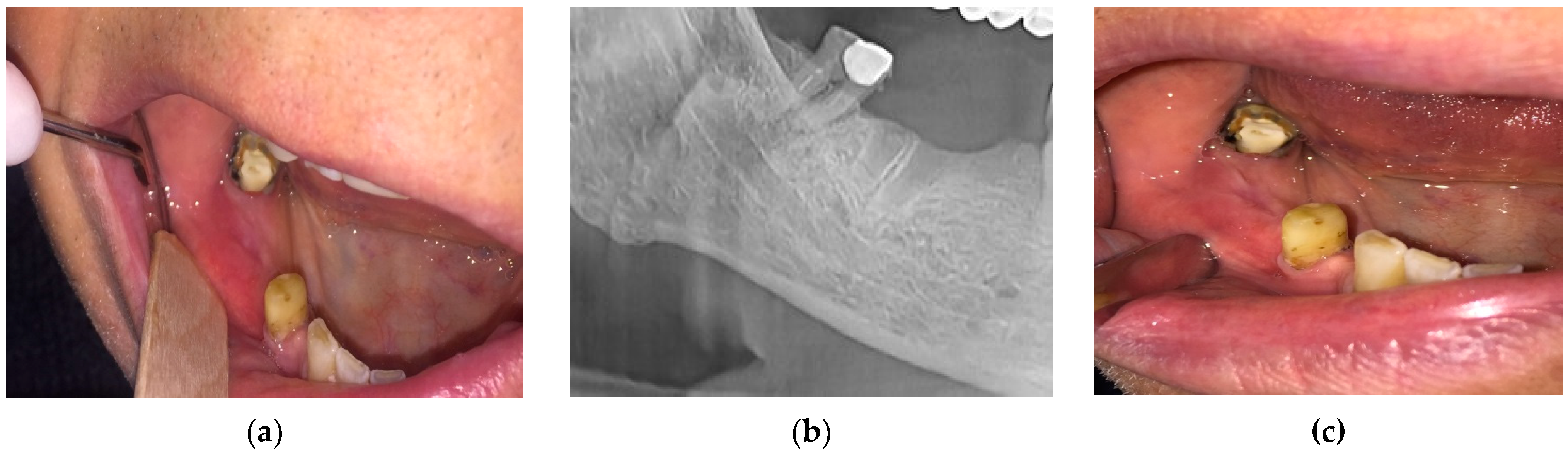
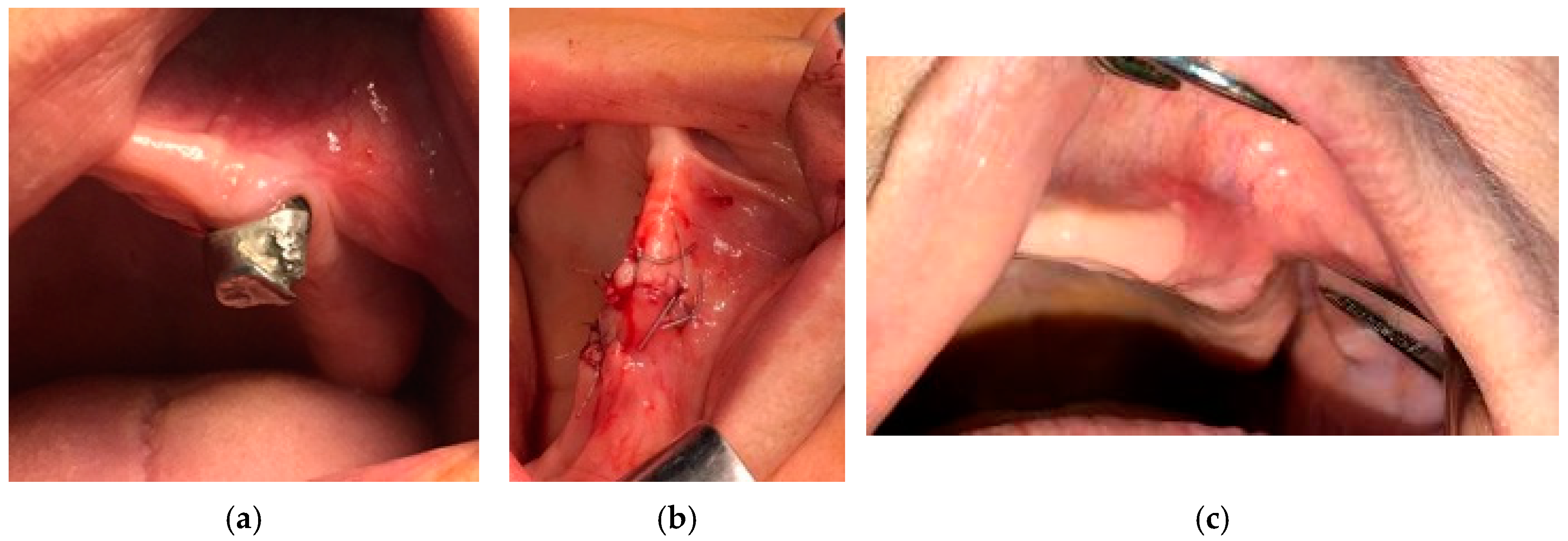
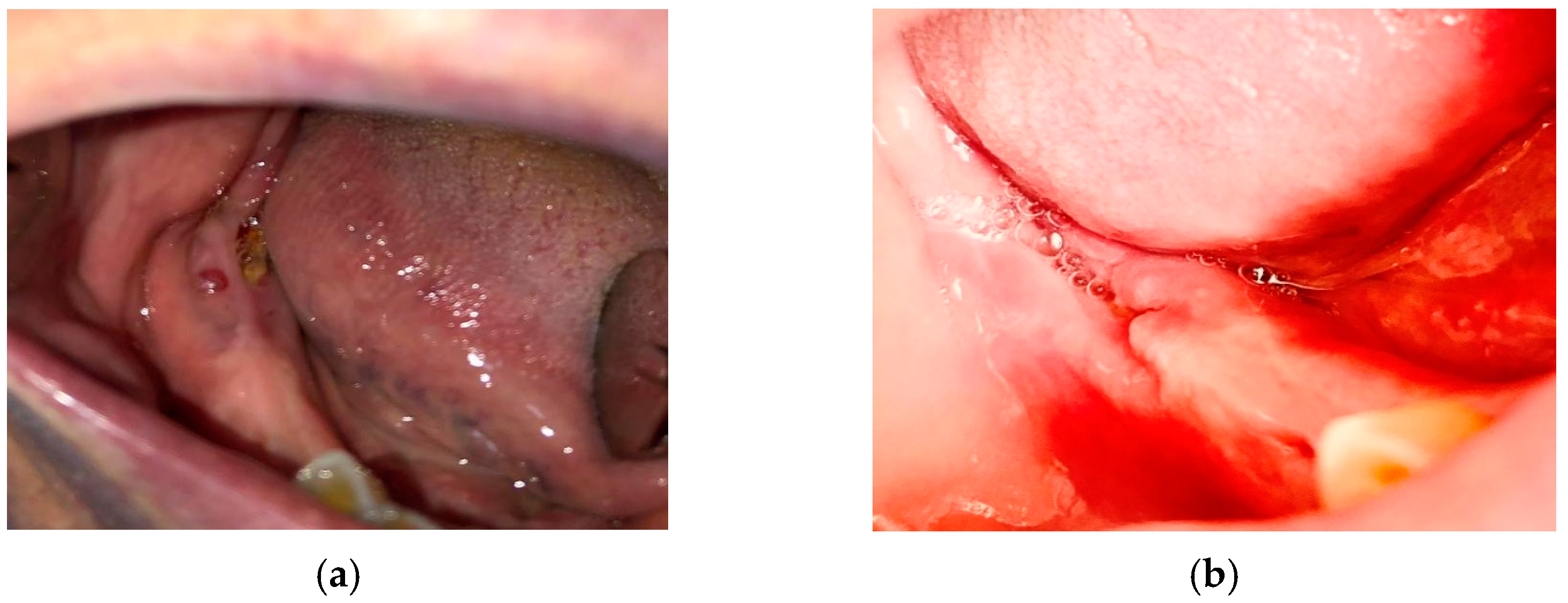
- For all the patients in stages 1, 2, and 3 of MRONJ, the treatment protocol was perioperative antibiotic, preoperative PBM, and surgery. The antibiotic treatment was prescribed 3 days before surgery, as well as 14 days after the surgery. To increase the healing by pre-conditioning the tissues, PBM was applied 3 consecutive days before surgery, with the same parameters mentioned above. The necrotic bone was removed using an ultrasonic device “Piezotome II” (Satelec-ACTEON, France). After the local infiltration of anesthetic solution without vasoconstrictors, a flap was raised to have direct access to the necrotic bone. The procedures required to eliminate the damaged bone (i.e., debridement, sequestrectomy, block resection, and osteoplasty) were performed in accordance with the preoperative radiological findings and with the intraoperative bleeding occurrence within the remaining bone.
3. Results
- (a)
- For the n = 84 patients from the “at risk” group, the extractions were performed under the protocol mentioned above. The healing was complete with a spontaneous bone coverage in all cases. An example of the evolution of such a case is presented in Figure 1.
- (b)
- (c)
- The surgical therapy outcome was analyzed for n = 108 patients with MRONJ in stages 1, 2, and 3, treated with intravenous BP and with oral anti-angiogenic medication. The most common initiating factor was teeth extraction. Most lesions (for n = 82, i.e., for 75.92% of the patients) were placed in the mandible, while a certain number (for n = 26, i.e., for 24.08% of the patients) were placed in the maxilla. The same protocol was applied to all the patients proposed for surgery. A complete disease resolution was obtained in 99 cases (all in stages 1 or 2 of the disease) from the total of 108 cases for which the healing was obtained with the first surgical treatment. In n = 9 cases, n = 2 in stage 2 and n = 7 in stage 3 of MRONJ, a downscaling to stage 1 was obtained, with a significant increase in the quality of life. The specific feature of these two patients in stage 2 who were downscaled is that they were treated for underlying malignant disease with zoledronic acid intravenous associated with an oral treatment with Sunitinib. In a case belonging to stage 3, the recurrence of infection occurred four months after the initial treatment. Thus, for the patients in stage 3 (n = 7), a downscaling to stage 1 was obtained for six cases, corresponding to a healing rate of 85.71%.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruggiero, S.L.; Dodson, T.B.; Assael, L.A.; Landesberg, R.; Marx, R.E.; Mehrotra, B.; American Association of Oral and Maxillofacial Surgeons. Position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J. Oral Maxillofac. Surg. 2009, 67, 2–12. [Google Scholar] [PubMed]
- Bagan, J.V.; Hens-Aumente, E.; Leopoldo-Rodado, M.; Poveda-Roda, R.; Bagan, L. Bisphosphonate-related osteonecrosis of the jaws: Study of the staging system in a series of clinical cases. Oral Oncol. 2012, 48, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Egloff-Juras, C.; Gallois, A.; Salleron, J.; Massard, V.; Dolivet, G.; Guillet, J.; Phulpin, B. Denosumab-related osteonecrosis of the jaw: A retrospective study. J. Oral Pathol. Med. 2018, 47, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Eguia, A.; Bagán-Debón, L.; Cardona, F. Review and update on drugs related to the development of osteonecrosis of the jaw. Med. Oral Patol. Oral Cir. Bucal. 2020, 25, e71–e83. [Google Scholar] [CrossRef]
- Endo, Y.; Kumamoto, H.; Nakamura, M.; Sugawara, S.; Takano-Yamamoto, T.; Sasaki, K.; Takahashi, T. Underlying Mechanisms and Therapeutic Strategies for Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ). Biol. Pharm. Bull. 2017, 40, 739–750. [Google Scholar] [CrossRef]
- de Sales Lima, M.V.; Rizzato, J.; Gracindo Marques, D.V.; Kitakawa, D.; da Silva Peralta, F.; Prado Scherma, A.; Carvalho, L.F.C.S. Denosumab Related Osteonecrosis of Jaw: A Case Report. J. Oral Maxillofac. Res. 2018, 30, e5. [Google Scholar] [CrossRef]
- Di Fede, O.; Panzarella, V.; Mauceri, R.; Fusco, V.; Bedogni, A.; Lo Muzio, L.; Campisi, G. The Dental Management of Patients at Risk of Medication-Related Osteonecrosis of the Jaw: New Paradigm of Primary Prevention. Biomed. Res. Int. 2018, 2684924. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Jung, Y.S.; Park, H.S.; Jung, H.D. Osteonecrosis of the jaw related to everolimus: A case report. Br. J. Oral Maxillofac. Surg. 2013, 51, e302–e304. [Google Scholar] [CrossRef]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1118. [Google Scholar] [CrossRef]
- Aghaloo, T.L.; Felsenfeld, A.L.; Tetradis, S. Osteonecrosis of the jaw in a patient on denosumab. J. Oral Maxillofac. Surg. 2010, 68, 959–963. [Google Scholar] [CrossRef]
- Estilo, C.L.; Fornier, M.; Farook, I.A.; Carlson, D.; Bohle, G.; Huryn, J.M. Osteonecrosis of the jaw related to bevacizumab. J. Clin. Oncol. 2008, 26, 4037–4038. [Google Scholar] [CrossRef] [PubMed]
- Hallmer, F.; Andersson, G.; Götrick, B.; Warfvinge, G.; Anderud, J.; Bjørnland, T. Prevalence, initiating factor, and treatment outcome of medication-related osteonecrosis of the jaw-a 4-year prospective study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Morrison, A.; Kendler, D.L.; Rizzoli, R.; Hanley, D.A.; Felsenberg, D.; McCauley, L.K.; O’Ryan, F.; Reid, I.R.; Ruggiero, S.L.; et al. International Task Force on Osteonecrosis of the Jaw. Case-Based Review of Osteonecrosis of the Jaw (ONJ) and Application of the International Recommendations for Management from the International Task Force on ONJ. J. Clin. Densitom. 2017, 20, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.K.; Solomon, D.H.; Tsacogianis, T.N.; Landon, J.E.; Song, H.J.; Kim, S.C. Comparative Safety and Effectiveness of Denosumab Versus Zoledronic Acid in Patients with Osteoporosis: A Cohort Study. J. Bone Miner. Res. 2017, 32, 611–617. [Google Scholar] [CrossRef]
- AlDhalaan, N.A.; BaQais, A.; Al-Omar, A. Medication-related Osteonecrosis of the Jaw: A Review. Cureus 2020, 12, e6944. [Google Scholar] [CrossRef]
- De Falco, S. Antiangiogenesis therapy: An update after the first decade. Korean J. Intern. Med. 2014, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pimolbutr, K.; Porter, S.; Fedele, S. Osteonecrosis of the Jaw Associated with Antiangiogenics in Antiresorptive-Naïve Patient: A Comprehensive Review of the Literature. BioMed Res. Int. 2018, 8071579. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F.; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Merigo, E.; Manfredi, M.; Meleti, M. Jaw bone necrosis without previous dental extractions associated with the use of bisphosphonates (pamidronate and zoledronate): A four-case report. J. Oral Pathol. Med. 2005, 34, 613–617. [Google Scholar] [CrossRef]
- Migliorati, C.A.; Siegel, M.A.; Elting, L.S. Bisphosphonate associated osteonecrosis: A long-term complication of bisphosphonate treatment. Lancet Oncol. 2006, 7, 508–514. [Google Scholar] [CrossRef]
- Hinchy, N.V.; Jayaprakash, V.; Rossitto, R.A. Osteonecrosis of the jaw—Prevention and treatment strategies for oral health professionals. Oral Oncol. 2013, 49, 878. [Google Scholar] [CrossRef]
- Vandone, A.M.; Donadio, M.; Mozzati, M. Impact of dental care in the prevention of bisphosphonate-associated osteonecrosis of the jaw: A single-center clinical experience. Ann. Oncol. 2012, 23, 193. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Matarese, G.; Cordasco, G.; Rotondo, F.; Crupi, A.; Ramaglia, L. Anticoagulant therapy in patients undergoing dental interventions: A critical review of the literature and current perspectives. Minerva Stomatol. 2015, 64, 21–46. [Google Scholar]
- Isola, G.; Polizzi, A.; Alibrandi, A.; Williams, R.C.; Leonardi, R. Independent impact of periodontitis and cardiovascular disease on elevated soluble urokinase-type plasminogen activator receptor (suPAR) levels. J. Periodontol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Lo Giudice, A.; Polizzi, A.; Alibrandi, A.; Murabito, P.; Indelicato, F. Identification of the different salivary Interleukin-6 profiles in patients with periodontitis: A cross-sectional study. Arch Oral Biol. 2020, 122, 104997. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; Manzano-Moreno, F.J.; Ruiz, C.; Illescas-Montes, R. Salivary Biomarkers and Their Application in the Diagnosis and Monitoring of the Most Common Oral Pathologies. Int. J. Mol. Sci. 2020, 21, 5173. [Google Scholar] [CrossRef] [PubMed]
- Ramaglia, L.; Guida, A.; Iorio-Siciliano, V.; Cuozzo, A.; Blasi, A.; Sculean, A. Stage-specific therapeutic strategies of medication-related osteonecrosis of the jaws: A systematic review and meta-analysis of the drug suspension protocol. Clin. Oral Investig. 2018, 22, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, P.; Burger, M.; von Wilmowsky, C.; Ebker, T.; Lutz, R.; Bauersachs, A.; Nkenke, E.; Neukam, F.W.; Wehrhan, F. The outcome after surgical therapy of bisphosphonate-associated osteonecrosis of the jaw-results of a clinical case series with an average follow-up of 20 months. Clin. Oral Investig. 2014, 18, 1299–1304. [Google Scholar] [CrossRef]
- El-Rabbany, M.; Lam, D.K.; Shah, P.S.; Azarpazhooh, A. Surgical Management of Medication-Related Osteonecrosis of the Jaw Is Associated With Improved Disease Resolution: A Retrospective Cohort Study. J. Oral Maxillofac. Surg. 2019, 77, 1816–1822. [Google Scholar] [CrossRef]
- Hauer, L.; Jambura, J.; Hrusak, D.; Chalupova, M.; Posta, P.; Rusnak, S.; Vyskocil, V. Surgical therapy for medication-related osteonecrosis of the jaw in osteoporotic patients treated with antiresorptive agents. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc 2020, 164, 100–107. [Google Scholar] [CrossRef]
- Steller, D.; Herbst, N.; Pries, R.; Juhl, D.; Hakim, S.G. Positive impact of Platelet-rich plasma and Platelet-rich fibrin on viability, migration and proliferation of osteoblasts and fibroblasts treated with zoledronic acid. Sci. Rep. 2019, 9, 8310. [Google Scholar] [CrossRef]
- Sedghizadeh, P.P.; Kumar, S.K.; Gorur, A.; Schaudinn, C.; Shuler, C.F.; Costerton, J.W. Identification of microbial biofilms in osteonecrosis of the jaws secondary to bisphosphonate therapy. J. Oral Maxillofac. Surg. 2008, 66, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Blus, C.; Szmukler-Moncler, S.; Giannelli, G.; Denotti, G.; Orrù, G. Use of Ultrasonic Bone Surgery (Piezosurgery) to Surgically Treat Bisphosphonate-Related Osteonecrosis of the Jaws (BRONJ). A Case Series Report with at Least 1 Year of Follow-Up. Open Dent. J. 2013, 23, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, L.D.; Bounkeo, J.M.; Brannon, W.M.; Dawes, K.S.; Barham, C.D.; Waddell, D.L.; Enwemeka, C.S. The efficacy of laser therapy in wound repair: A meta-analysis of the literature. Photomed. Laser Surg. 2004, 22, 241–247. [Google Scholar] [CrossRef]
- Posten, W.; Wrone, D.A.; Dover, J.S.; Arndt, K.A.; Silapunt, S.; Alam, M. Low-level laser therapy for wound healing: Mechanism and efficacy. Dermatol. Surg. 2005, 31, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Luca, R.; Todea, C.D.; Duma, V.-F.; Bradu, A.; Podoleanu, A. Quantitative assessment of rat bone regeneration using complex master–slave optical coherence tomography. Quant. Imaging Med. Surg. 2019, 9, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Luca, R.E.; Giuliani, A.; Mănescu, A.; Heredea, R.; Hoinoiu, B.; Constantin, G.D.; Duma, V.-F.; Todea, C.D. Osteogenic Potential of Bovine Bone Graft in Combination with Laser Photobiomodulation: An Ex Vivo Demonstrative Study in Wistar Rats by Cross-Linked Studies Based on Synchrotron Microtomography and Histology. Int. J. Mol. Sci. 2020, 21, 778. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.P.; da Silva, M.A.; Almeida, A.P.; Lombardi, I., Jr.; Matos, A.P. Laser therapy in the tissue repair process: A literature review. Photomed. Laser Surg. 2010, 28, 17–21. [Google Scholar] [CrossRef]
- Fávaro-Pípi, E.; Ribeiro, D.A.; Ribeiro, J.U.; Bossini, P.; Oliveira, P.; Parizotto, N.A.; Tim, C.; de Araújo, H.S.; Renno, A.C. Low-level laser therapy induces differential expression of osteogenic genes during bone repair in rats. Photomed. Laser Surg. 2011, 29, 311–317. [Google Scholar] [CrossRef]
- Obradović, R.R.; Kesić, L.G.; Pesevska, S. Influence of low-level laser therapy on biomaterial osseointegration: A mini-review. Lasers Med. Sci. 2009, 24, 447–451. [Google Scholar] [CrossRef]
- Xu, M.; Deng, T.; Mo, F.; Deng, B.; Lam, W.; Deng, P.; Zhang, X.; Liu, S. Low-intensity pulsed laser irradiation affects RANKL and OPG mRNA expression in rat calvarial cells. Photomed. Laser Surg. 2009, 27, 309–315. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef]
- Manfredi, M.; Merigo, E.; Guidotti, R.; Meleti, M.; Vescovi, P. Bisphosphonate-related osteonecrosis of the jaws: A case series of 25 patients affected by osteoporosis. Int. J. Oral Maxillofac. Surg. 2011, 40, 277–284. [Google Scholar] [CrossRef]
- Martins, M.A.; Martins, M.D.; Lascala, C.A.; Curi, M.M.; Migliorati, C.A.; Tenis, C.A. Association of laser phototherapy with PRP improves healing of bisphosphonate-related osteonecrosis of the jaws in cancer patients: A preliminary study. Oral Oncol. 2012, 48, 79–84. [Google Scholar] [CrossRef]
- Vescovi, P.; Manfredi, M.; Merigo, E.; Guidotti, R.; Meleti, M.; Pedrazzi, G. Early surgical laser-assisted management of bisphosphonate-related osteonecrosis of the jaws (BRONJ): A retrospective analysis of 101 treated sites with long-term follow-up. Photomed. Laser Surg. 2012, 30, 5–13. [Google Scholar] [CrossRef]
- Agrawal, T.; Gupta, G.K.; Rai, V.; Carroll, J.D.; Hamblin, M.R. Pre-conditioning with low-level laser (light) therapy: Light before the storm. Dose Response 2014, 12, 619–649. [Google Scholar] [CrossRef] [PubMed]
- Montebugnoli, L.; Felicetti, L.; Gissi, D.B.; Pizzigallo, A.; Pelliccioni, G.A.; Marchetti, C. Biphosphonate-associated osteonecrosis can be controlled by nonsurgical management. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 473–477. [Google Scholar] [CrossRef]
- Ikeda, T.; Kuraguchi, J.; Kogashiwa, Y.; Yokoi, H.; Satomi, T.; Kohno, N. Successful treatment of bisphosphonate-related osteonecrosis of the jaw (BRONJ) patients with sitafloxacin: New strategies for the treatment of BRONJ. Bone 2015, 73, 217–222. [Google Scholar] [CrossRef]
- Wilde, F.; Heufelder, M.; Winter, K.; Hendricks, J.; Frerich, B.; Schramm, A.; Hemprich, A. The role of surgical therapy in the management of intravenous bisphosphonates-related osteonecrosis of the jaw. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Momesso, G.A.C.; Lemos, C.A.A.; Santiago-Júnior, J.F.; Faverani, L.P.; Pellizzer, E.P. Laser surgery in management of medication-related osteonecrosis of the jaws: A meta-analysis. Oral Maxillofac. Surg. 2020, 24, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Blus, C.; Giannelli, G.; Szmukler-Moncler, S.; Orru, G. Treatment of medication-related osteonecrosis of the jaws (MRONJ) with ultrasonic piezoelectric bone surgery. A case series of 20 treated sites. Oral Maxillofac. Surg. 2017, 21, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Rebaudi, A.; Rebaudi, F.; Barberis, F.; Sammartino, G.; Marenzi, G. Peri-Implant Bone Damage Procured by Piezoelectric and Conventional Implant Site Preparation: An In Vitro Comparison. Appl. Sci. 2020, 10, 8909. [Google Scholar] [CrossRef]
- Statkievicz, C.; Toro, L.F.; de Mello-Neto, J.M.; de Sá, D.P.; Casatti, C.A.; Issa, J.P.M.; Cintra, L.T.A.; de Almeida, J.M.; Nagata, M.J.H.; Garcia, V.G.; et al. Photomodulation multiple sessions as a promising preventive therapy for medication-related osteonecrosis of the jaws after tooth extraction in rats. J. Photochem. Photobiol. B 2018, 184, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Romeo, U.; Galanakis, A.; Marias, C.; Vecchio, A.D.; Tenore, G.; Palaia, G.; Vescovi, P.; Polimeni, A. Observation of pain control in patients with bisphosphonate-induced osteonecrosis using low level laser therapy: Preliminary results. Photomed. Laser Surg. 2011, 29, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Pushalkar, S.; Li, Y.; Glickman, R.; Fleisher, K.; Saxena, D. Antibiotic effects on bacterial profile in osteonecrosis of the jaw. Oral Dis. 2012, 18, 85–95. [Google Scholar] [CrossRef]
- Hoff, A.O.; Toth, B.B.; Altundag, K.; Johnson, M.M.; Warneke, C.L.; Hu, M.; Nooka, A.; Sayegh, G.; Guarneri, V.; Desrouleaux, K.; et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J. Bone Miner. Res. 2008, 23, 826–836. [Google Scholar] [CrossRef]
- Hoefert, S.; Eufinger, H. Relevance of a prolonged preoperative antibiotic regime in the treatment of bisphosphonate-related osteonecrosis of the jaw. J. Oral Maxillofac. Surg. 2011, 69, 362–380. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.R.; Lee, J.H.; Park, J.Y.; Hwang, D.S. Surgical Treatment of Medication-Related Osteonecrosis of the Jaw: A Retrospective Study. Int. J. Environ. Res. Public Health 2020, 17, 8801. [Google Scholar] [CrossRef]
- Sedghizadeh, P.P.; Kumar, S.K.; Gorur, A.; Schaudinn, C.; Shuler, C.F.; Costerton, J.W. Microbial biofilms in osteomyelitis of the jaw and osteonecrosis of the jaw secondary to bisphosphonate therapy. J. Am. Dent. Assoc. 2009, 140, 1259–1265. [Google Scholar] [CrossRef]
- Ganguli, A.; Steward, C.; Butler, S.L. Bacterial adhesion to bisphosphonate coated hydroxyapatite. J. Mater. Sci. Mater. Med. 2005, 16, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Kopel, M.; Degtyar, E.; Banin, E. Surface acoustic waves increase the susceptibility of Pseudomonas aeruginosa biofilms to antibiotic treatment. Biofouling 2011, 27, 701–710. [Google Scholar] [CrossRef]
- Adornato, M.C.; Morcos, I.; Rozanski, J. The treatment of bisphosphonate associated osteonecrosis of the jaws with bone resection and autologous platelet derived growth factors. J. Am. Dent. Assoc. 2007, 138, 971–977. [Google Scholar] [CrossRef]
- Inchingolo, F.; Cantore, S.; Dipalma, G.; Georgakopoulos, I.; Almasri, M.; Gheno, E.; Motta, A.; Marrelli, M.; Farronato, D.; Ballini, A.; et al. Platelet rich fibrin in the management of medication-related osteonecrosis of the jaw: A clinical and histopathological evaluation. J. Biol. Regul. Homeost. Agents 2017, 31, 811–816. [Google Scholar] [PubMed]
- Mozzati, M.; Arata, V.; Gallesio, G. Tooth extraction in patients on zoledronic acid therapy. Oral Oncol. 2012, 48, 817–821. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lin, Y.; Hu, X. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 707–713. [Google Scholar] [CrossRef]
- Asaka, T.; Ohga, N.; Yamazaki, Y.; Sato, J.; Satoh, C.; Kitagawa, Y. Platelet-rich fibrin may reduce the risk of delayed recovery in tooth-extracted patients undergoing oral bisphosphonate therapy: A trial study. Clin. Oral Investig. 2017, 21, 2165–2172. [Google Scholar] [CrossRef] [PubMed]
- Aljohani, S.; Gaudin, R.; Weiser, J.; Tröltzsch, M.; Ehrenfeld, M.; Kaeppler, G.; Smeets, R.; Otto, S. Osteonecrosis of the jaw in patients treated with denosumab: A multicenter case series. J. Craniomaxillofac. Surg. 2018, 46, 1515–1525. [Google Scholar] [CrossRef]
- Choung, H.W.; Lee, S.H.; Ham, A.R.; Lee, N.R.; Kim, B.; Pang, K.M.; Jahng, J.W.; Lee, J.H. Effectiveness of Low-Level Laser Therapy with a 915 nm Wavelength Diode Laser on the Healing of Intraoral Mucosal Wound: An Animal Study and a Double-Blind Randomized Clinical Trial. Medicina 2019, 55, 405. [Google Scholar] [CrossRef]
- Amarillas-Escobar, E.D.; Toranzo-Fernández, J.M.; Martínez-Rider, R.; Noyola-Frías, M.A.; Hidalgo-Hurtado, J.A.; Serna, V.M.; Gordillo-Moscoso, A.; Pozos-Guillén, A.J. Use of therapeutic laser after surgical removal of impacted lower third molars. J. Oral Maxillofac. Surg. 2010, 68, 319–324. [Google Scholar] [CrossRef]
- de Sousa Gomes, P.; Daugela, P.; Poskevicius, L.; Mariano, L.; Fernandes, M.H. Molecular and Cellular Aspects of Socket Healing in the Absence and Presence of Graft Materials and Autologous Platelet Concentrates: A Focused Review. J. Oral Maxillofac. Res. 2019, 10, e2. [Google Scholar]
- Nica, D.F.; Heredea, E.R.; Todea, D. Alveolus soft and bone tissue regeneration after laser biomodulation—A histological study. Rom. J. Morphol. Embryol. 2019, 60, 1269–1273. [Google Scholar] [PubMed]
- Vescovi, P.; Giovannacci, I.; Merigo, E.; Meleti, M.; Manfredi, M.; Fornaini, C.; Nammour, S. Tooth extractions in high-risk patients under bisphosphonate therapy and previously affected with osteonecrosis of the jaws: Surgical protocol supported by low-level laser therapy. J. Craniofacial Surg. 2015, 26, 696–699. [Google Scholar] [CrossRef]
- Tenore, G.; Zimbalatti, A.; Rocchetti, F.; Graniero, F.; Gaglioti, D.; Mohsen, A.; Caputo, M.; Lollobrigida, M.; Lamazza, L.; De Biase, A.; et al. Management of Medication-Related Osteonecrosis of the Jaw (MRONJ) Using Leukocyte- and Platelet-Rich Fibrin (L-PRF) and Photobiomodulation: A Retrospective Study. J. Clin. Med. 2020, 9, 3505. [Google Scholar] [CrossRef] [PubMed]
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Bettini, G.; Biasotto, M.; Colella, G.; Consolo, U.; Di Fede, O.; Favia, G.; Fusco, V.; et al. Medication-Related Osteonecrosis of Jaws (MRONJ) Prevention and Diagnosis: Italian Consensus Update 2020. Int. J. Environ. Res. Public Health 2020, 17, 5998. [Google Scholar] [CrossRef]
- Barca, I.; Novembre, D.; Giofrè, E.; Caruso, D.; Cordaro, R.; Kallaverja, E.; Ferragina, F.; Cristofaro, M.G. Telemedicine in Oral and Maxillo-Facial Surgery: An Effective Alternative in Post COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2020, 17, 7365. [Google Scholar] [CrossRef]
- Vescovi, P. Laser Treatment of Medication-Related Osteonecrosis of the Jaws. In Lasers in Oral and Maxillofacial Surgery; Stübinger, S., Klämpfl, F., Schmidt, M., Zeilhofer, H.F., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2020; pp. 175–193. [Google Scholar]
- Tavares, A.; Carvalho, C.M.; Faustino, M.A.; Neves, M.G.; Tomé, J.P.; Tomé, A.C.; Cavaleiro, J.A.; Cunha, A.; Gomes, N.C.; Alves, E.; et al. Antimicrobial photodynamic therapy: Study of bacterial recovery viability and potential development of resistance after treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, A.; Fedele, S.; Bedogni, G.; Scoletta, M.; Favia, G.; Colella, G.; Agrillo, A.; Bettini, G.; Di Fede, O.; Oteri, G.; et al. Staging of osteonecrosis of the jaw requires computed tomography for accurate definition of the extent of bony disease. Br. J. Oral Maxillofac. Surg. 2014, 52, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Schnödt, E.M.; Haidari, S.; Brunner, T.F.; Aljohani, S.; Mosleh, M.; Ristow, O.; Troeltzsch, M.; Pautke, C.; Ehrenfeld, M.; et al. Autofluorescence-guided surgery for the treatment of medication-related osteonecrosis of the jaw (MRONJ): A retrospective single-center study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, S2212-4403(20)31291-8, Advance online publication. [Google Scholar] [CrossRef]
- Leuci, S.; Coppola, N.; Turkina, A.; Mignogna, M.D. May VelScope Be Deemed an Opportunistic Oral Cancer Screening by General Dentists? A Pilot Study. J. Clin. Med. 2020, 9, 1754. [Google Scholar] [CrossRef]
- Canjau, S.; Todea, D.C.M.; Sinescu, C.; Pricop, M.O.; Duma, V.-F. Fluorescence Influence on Screening Decisions for Oral Malignant Lesions. Rom. J. Morphol. Embryol. 2018, 59, 203–209. [Google Scholar]
- Giudice, A.; Bennardo, F.; Barone, S.; Antonelli, A.; Figliuzzi, M.M.; Fortunato, L. Can Autofluorescence Guide Surgeons in the Treatment of Medication-Related Osteonecrosis of the Jaw? A Prospective Feasibility Study. J. Oral Maxillofac. Surg. 2018, 76, 982–995. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nica, D.F.; Riviș, M.; Roi, C.I.; Todea, C.D.; Duma, V.-F.; Sinescu, C. Complementarity of Photo-Biomodulation, Surgical Treatment, and Antibiotherapy for Medication-Related Osteonecrosis of the Jaws (MRONJ). Medicina 2021, 57, 145. https://doi.org/10.3390/medicina57020145
Nica DF, Riviș M, Roi CI, Todea CD, Duma V-F, Sinescu C. Complementarity of Photo-Biomodulation, Surgical Treatment, and Antibiotherapy for Medication-Related Osteonecrosis of the Jaws (MRONJ). Medicina. 2021; 57(2):145. https://doi.org/10.3390/medicina57020145
Chicago/Turabian StyleNica, Diana Florina, Mircea Riviș, Ciprian Ioan Roi, Carmen Darinca Todea, Virgil-Florin Duma, and Cosmin Sinescu. 2021. "Complementarity of Photo-Biomodulation, Surgical Treatment, and Antibiotherapy for Medication-Related Osteonecrosis of the Jaws (MRONJ)" Medicina 57, no. 2: 145. https://doi.org/10.3390/medicina57020145
APA StyleNica, D. F., Riviș, M., Roi, C. I., Todea, C. D., Duma, V.-F., & Sinescu, C. (2021). Complementarity of Photo-Biomodulation, Surgical Treatment, and Antibiotherapy for Medication-Related Osteonecrosis of the Jaws (MRONJ). Medicina, 57(2), 145. https://doi.org/10.3390/medicina57020145










