Heterologous Overexpression and Functional Analysis of the Isodon suzhouensis IsKS1 Gene in Arabidopsis thaliana
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Analysis of the Metabolome of Isodon Suzhouensis
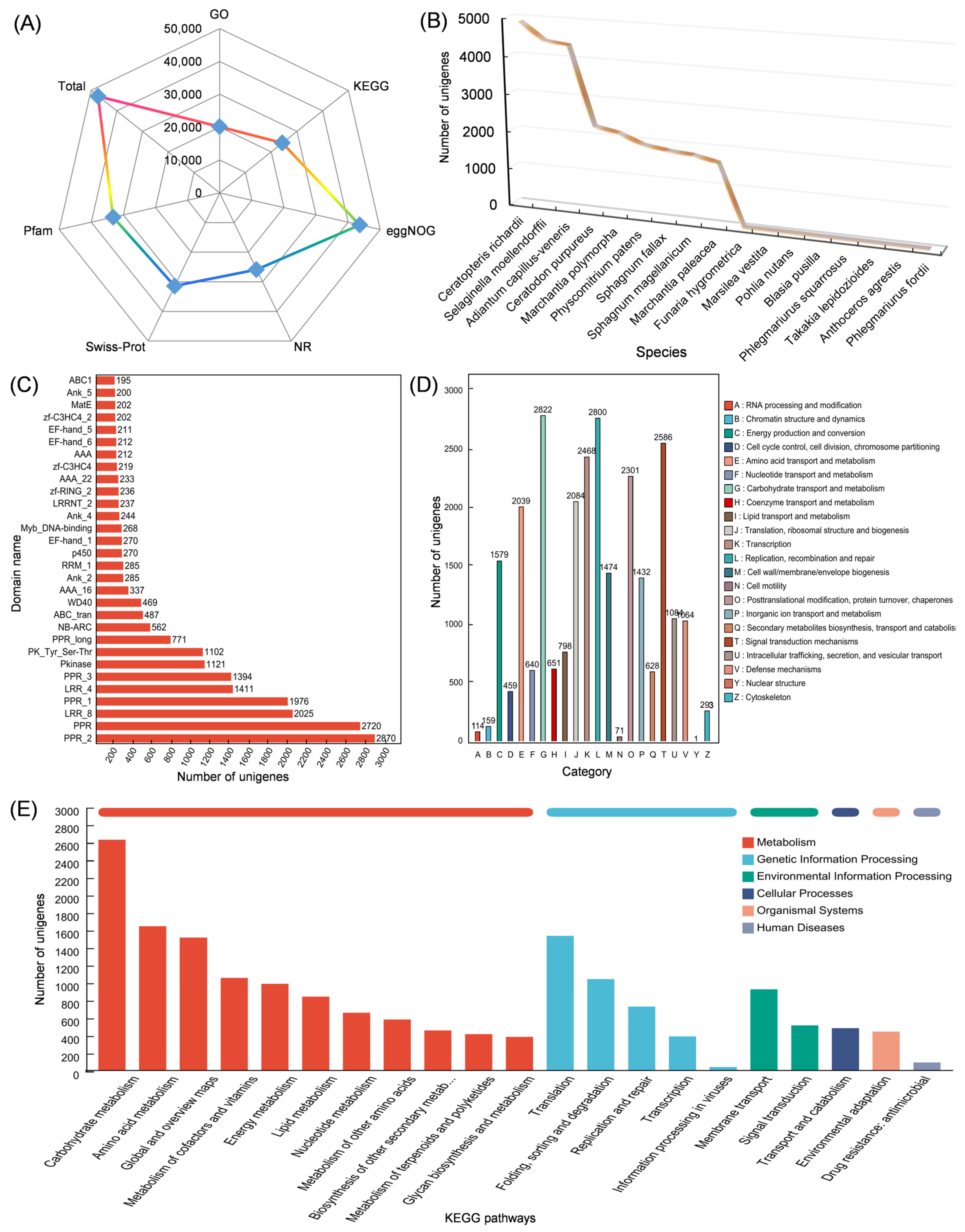
2.3. Transcriptomic Analysis of Isodon Suzhouensis
2.4. Mining, Cloning, and Bioinformatic Analysis of IsKS Gene from Isodon suzhouensis
2.5. Construction of Recombinant Plasmid for IsKS Gene Overexpression and Subcellular Location
2.6. Molecular Docking of the IsKS1 and the Substrates ent-CPP
2.7. Overexpression of the IsKS1 Gene in Arabidopsis and Analysis of Arabidopsis Morphology
2.8. Metabolomic and Transcriptomic Analysis of IsKS1 Gene Overexpression Arabidopsis thaliana
2.9. Statistical Analysis
3. Results
3.1. Differential Metabolites Present in the Leaves and Stems of Isodon suzhouensis
3.2. KEGG Enrichment of DEMs
3.3. Transcriptomic Data from Leaf and Stem of Isodon suzhouensis
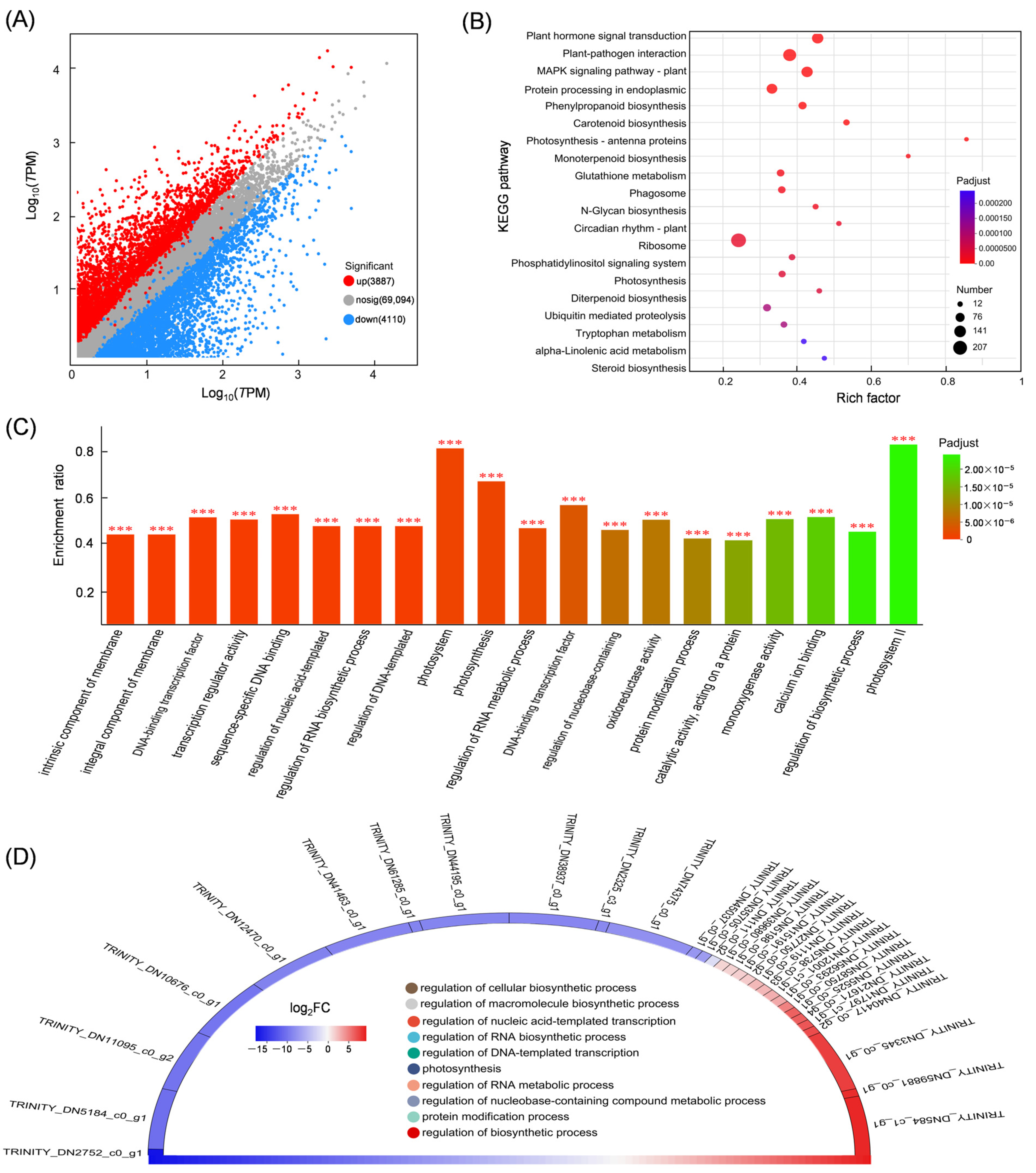
3.4. Differential Expression Gene Analysis
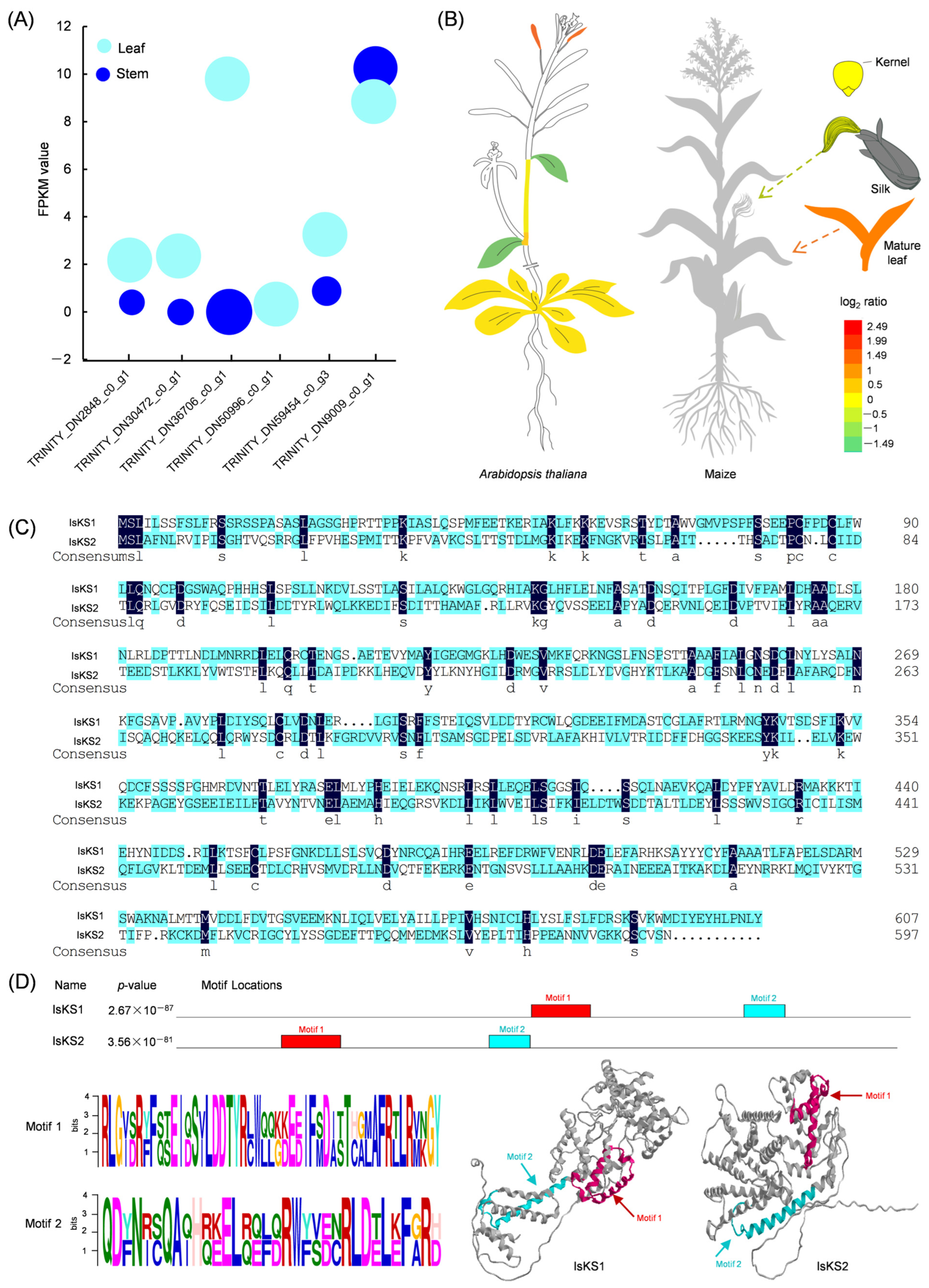
3.5. Mining DEGs Involved in the Diterpenoid Biosynthetic Pathway
3.6. Identification of Two IsKS Genes from Isodon suzhouensis
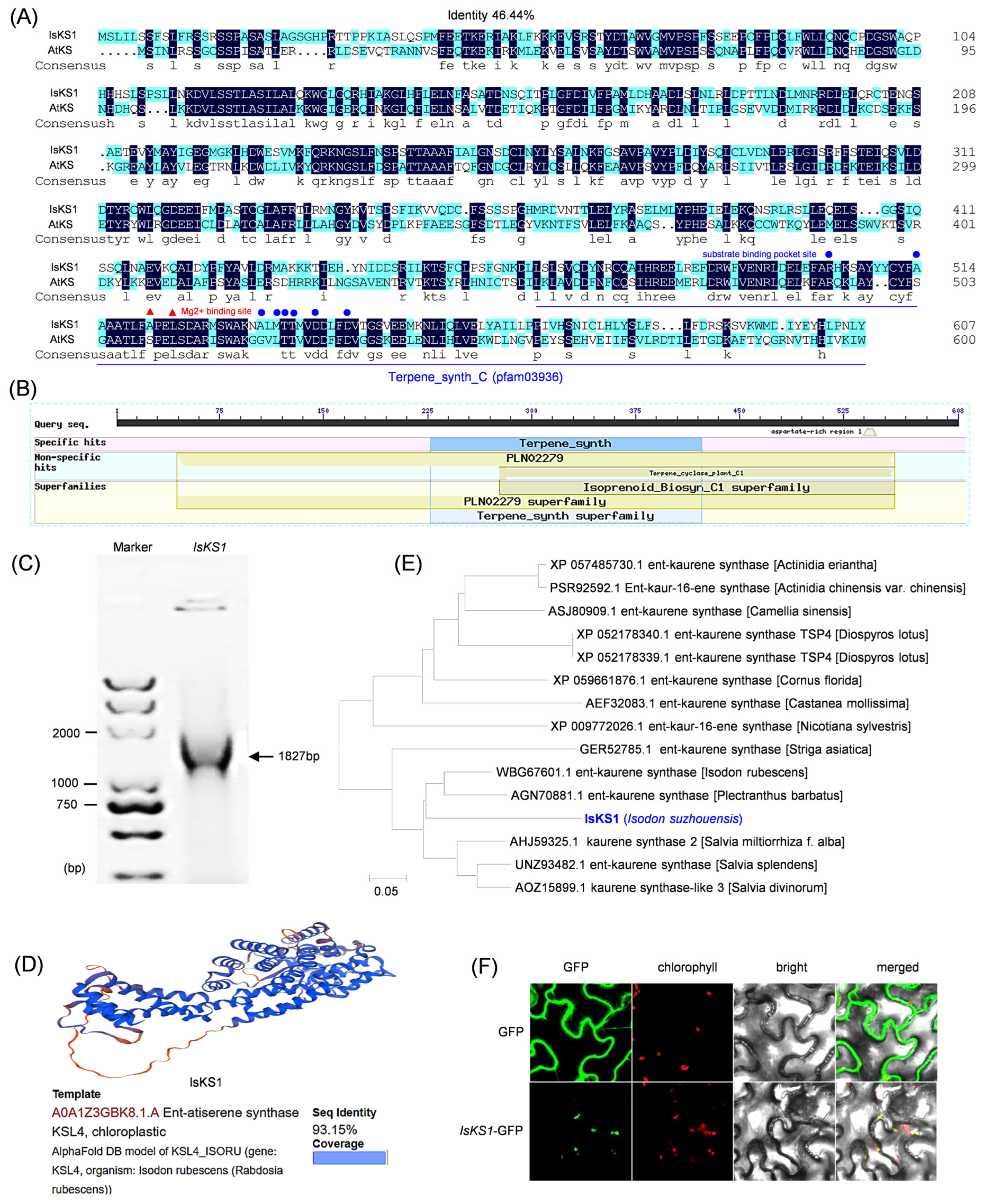
3.7. Cloning, Bioinformatic Analysis, and Subcellular Localization of IsKS1
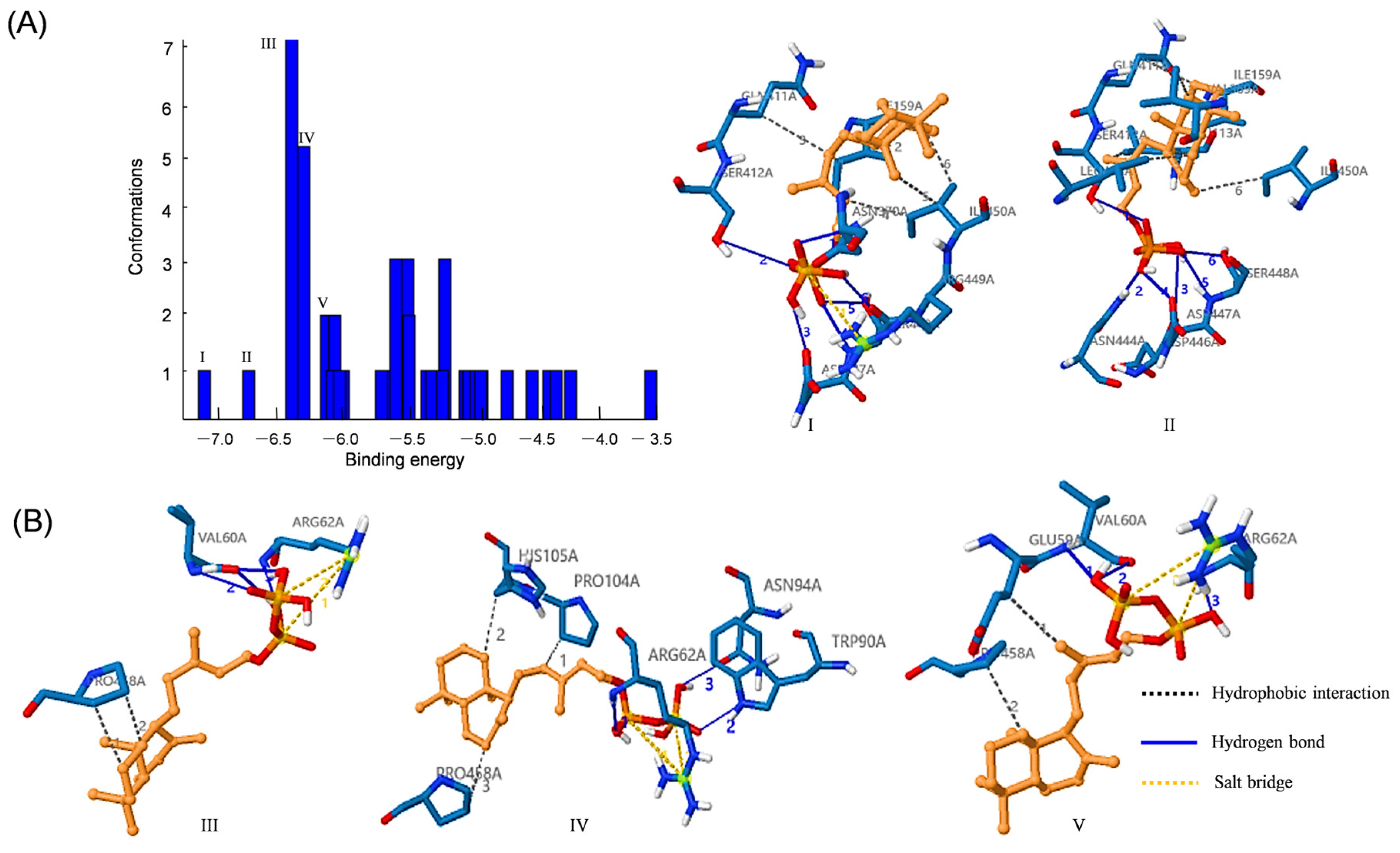
3.8. Docking Analysis of IsKS1 with ent-CPP
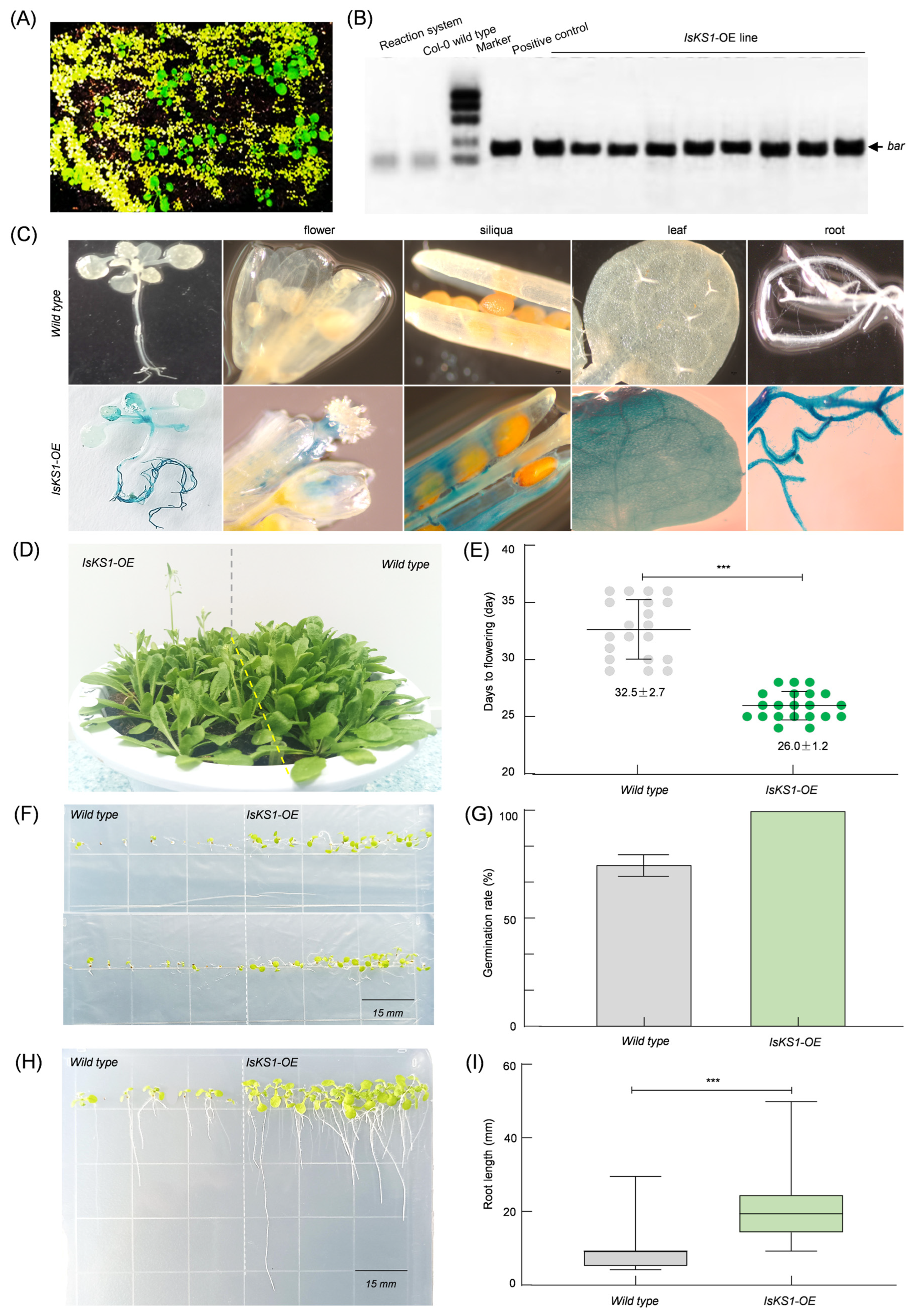
3.9. Overexpression of IsKS1 Arabidopsis thaliana and Phenotypic Analysis
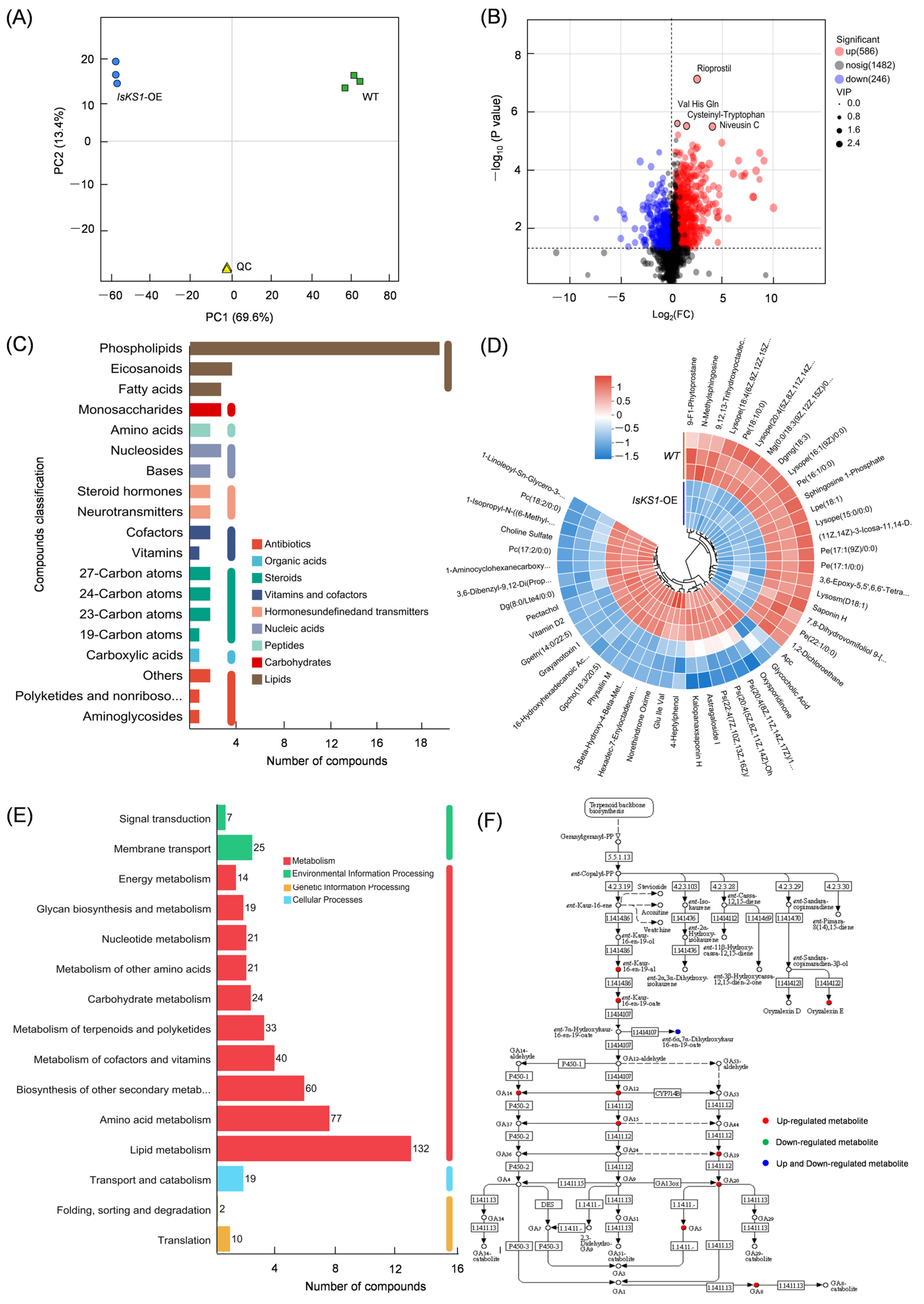
3.10. Detection of GAs and DEGs in the Diterpenoid Biosynthesis Pathway in IsKS1- Overexpressing Arabidopsis thaliana
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, H.; Zhai, K.; Han, Z.; Zhou, S. Isodon suzhouensis (Lamiaceae): A new species from Anhui. J. Suzhou Univ. 2022, 37, 41–45. [Google Scholar] [CrossRef]
- Wei, M.; Chen, L.; Zhang, W.; Sun, M.; Wang, J.; Zhan, J.; Wang, C.; Yan, C. Chemical constituents from Isodon amethystoides and their an-titumor activity. Nat. Prod. Res. Dev. 2025, 37, 262–270. [Google Scholar] [CrossRef]
- Gu, Y.; Wei, L.; Liu, Y.; Tan, T.; Luo, Y. Progress on chemical components and pharmacological effects of Labiatae in recent 10 years. J. Chin. Med. Mater. 2023, 46, 511–524. [Google Scholar] [CrossRef]
- Yang, M.; Mao, J.; Zhu, J.; Zhang, H.; Ding, L. Wangzaozin A, a potent novel microtubule stabilizer, targets both the taxane and laulimalide sites on β-tubulin through molecular dynamics simulations. Life Sci. 2022, 301, 120583. [Google Scholar] [CrossRef] [PubMed]
- Sonia, S.C.; Nancy, M.M.; Jenny, G.S.; Rocío, P.Y.T.; Rebeca, D.M.C. Gibberellin biosynthesis and metabolism: A convergent route for plants, fungi and bacteria. Microbiol. Res. 2018, 208, 85–98. [Google Scholar] [CrossRef]
- Bao, S.J.; Hua, C.M.; Shen, L.S.; Yu, H. New insights into gibberellin signaling in regulating flowering in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 118–131. [Google Scholar] [CrossRef]
- Fleet, C.M.; Yamaguchi, S.; Hanada, A.; Kawaide, H.; David, C.J.; Kamiya, Y.; Sun, T.P. Overexpression of AtCPS and AtKS in Arabidopsis confers increased ent-kaurene production but no increase in bioactive gibberellins. Plant Physiol. 2003, 132, 830–839. [Google Scholar] [CrossRef]
- Sun, Y.; Shao, J.; Liu, H.; Wang, H.; Wang, G.; Li, J.; Mao, Y.; Chen, Z.; Ma, K.; Xu, L.; et al. A chromosome-level genome assembly reveals that tandem-duplicated CYP706V oxidase genes control oridonin biosynthesis in the shoot apex of Isodon rubescens. Mol. Plant. 2023, 16, 517–532. [Google Scholar] [CrossRef]
- Zhai, K.; Wang, W.; Zheng, M.; Khan, G.J.; Wang, Q.; Chang, J.; Dong, Z.; Zhang, X.; Duan, H.; Gong, Z.; et al. Protective effects of Isodon suzhouensis extract and glaucocalyxin A on chronic obstructive pulmonary disease through SOCS3–JAKs/STATs pathway. Food Front. 2023, 4, 511–523. [Google Scholar] [CrossRef]
- Yuan, Q.; Song, W.; Wang, W.; Xuan, L. Scubatines A-F, new cytotoxic neo-clerodane diterpenoids from Scutellaria barbata D. Don. Fitoterapia 2017, 119, 40–44. [Google Scholar] [CrossRef]
- Fan, M.; Zhu, Y.; Zhang, Z.; Du, R.; Zhu, Q.; Wu, X.; Zhao, Q. Salvihispin A and its glycoside, two neo-clerodane diterpenoids with neurotrophic activities from Salvia hispanica L. Tetrahedron Lett. 2018, 59, 143–146. [Google Scholar] [CrossRef]
- Pan, Z.; Cheng, J.; He, J.; Wang, Y.; Peng, L.; Xu, G.; Sun, W.; Zhao, Q. Splendidins A-C, three new clerodane diterpenoids from Salvia splendens. Helv. Chim. Acta 2011, 94, 417–422. [Google Scholar] [CrossRef]
- Angela, B.; Anna, M.S.; Samad, N.E.; Matthias, H.; Giacomo, M.; Gabriella, P.; Giovanni, R.; Fabrizio, D.P.; Nunziatina, D.T. Antibacterial compounds from Salvia adenophora Fernald (Lamiaceae). Phytochemistry 2015, 110, 120–132. [Google Scholar] [CrossRef]
- Zou, J.; Pan, L.; Li, Q.; Pu, J.; Yao, P.; Zhu, M.; Jeffrey, A.B.; Zhang, H.; Sun, H. Rubesanolides C-E: Abietane diterpenoids isolated from Isodon rubescens and evaluation of their anti-biofilm activity. Org. Biomol. Chem. 2012, 10, 5039–5044. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pu, J.; Xiao, W.; Sun, H. ent-Abietane diterpenoids from Isodon xerophilus. Arch. Pharmacal Res. 2011, 34, 875–879. [Google Scholar] [CrossRef]
- Yang, J.; An, Y.; Wu, H.; Liu, M.; Wang, W.; Du, X.; Li, Y.; Pu, J.; Sun, H. Ent-kaurane and ent-abietane diterpenoids from Isodon phyllostachys. Sci. China Chem. 2016, 59, 1211–1215. [Google Scholar] [CrossRef]
- Maria, W.; Smith, S.Y.; Tahar, A.A.; Yuji, K. The first step of gibberellin biosynthesis in pumpkin is catalyzed by at least twocopalyl diphosphate synthases encoded by differentially regulated genes. Plant Physiol. 1998, 118, 1411–1419. [Google Scholar] [CrossRef]
- Aron, L.; Chang, C.; Elzbieta, K.; Sun, T. Developmental regulation of the gibberellin biosynthetic gene GA1 in Arabidopsis thaliana. Plant J. 1997, 12, 9–19. [Google Scholar] [CrossRef]
- Shinjiro, Y.; Sun, T.; Hiroshi, K.; Yuji, K. The GA2 locus of Arabidopsis thaliana encodes ent-kaurene synthase of gibberellin biosynthesis. Plant Physiol. 1998, 116, 1271–1278. [Google Scholar] [CrossRef]
- Guan, S.; Zhang, H.; Jiang, Z.; Jiao, P.; Liu, S.; Ma, Y. Research progress of synergistic effect of photoreceptor and gibberellin on plant flowering. J. Jilin Agric. Univ. 2023, 45, 127–136. [Google Scholar] [CrossRef]
- Norman, B.B.; Brian, P.D. Transcriptional responses to gibberellin in the maize tassel and control by DELLA domain proteins. Plant J. 2022, 112, 493–517. [Google Scholar] [CrossRef]
- Porri, A.; Torti, S.; Romera-Branchat, M.; Coupland, G. Spatially distinct regulatory roles for gibberellins in the promotion of flowering of Arabidopsis under long photoperiods. Development 2012, 139, 2198–2209. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Pan, J.; Li, Y.; Lou, D.; Hu, Y.; Yu, D. The DELLA-CONSTANS transcription factor cascade integrates gibberellic acid and photoperiod signaling to regulate flowering. Plant Physiol. 2016, 172, 479–488. [Google Scholar] [CrossRef] [PubMed]


| Unigene ID | Length | KO_Name | NR_Hit Gene | Swiss-Prot_Description |
|---|---|---|---|---|
| TRINITY_DN48295_c0_g1 | 249 | CYP76AH1 | KAH9573688.1 | Ferruginol synthase |
| TRINITY_DN47553_c0_g4 | 327 | TPS04 | KAI5069968.1 | Santalene and bergamotene synthase |
| TRINITY_DN28685_c0_g1 | 2556 | CPS | UPQ49771.1 | Copalyl diphosphate synthase |
| TRINITY_DN29900_c0_g1 | 1185 | CYP82G1 | KAH9575006.1 | Dimethylnonatriene synthase |
| TRINITY_DN55358_c0_g2 | 434 | GA20ox | KAG6551271.1 | Gibberellin 20 oxidase 1-D |
| TRINITY_DN8466_c0_g2 | 1018 | GA2ox | KAH8968045.1 | Gibberellin 2-beta-dioxygenase |
| TRINITY_DN9483_c0_g2 | 314 | CPS | UPQ49771.1 | Ent-copalyl diphosphate synthase |
| TRINITY_DN40555_c0_g1 | 1351 | GA20ox | KAI5084607.1 | Gibberellin 20 oxidase |
| TRINITY_DN76952_c0_g1 | 1698 | KAO | BAQ20602.1 | Ent-kaurenoic acid oxidase |
| TRINITY_DN30472_c0_g1 | 675 | KSL2 | KAH7437475.1 | Ent-atiserene synthase KSL1 |
| TRINITY_DN56804_c0_g1 | 239 | CYP76AH1 | KAH9555561.1 | Cytochrome P450 76T24 |
| TRINITY_DN50495_c0_g1 | 446 | GA3ox | KAH8945774.1 | Gibberellin 3-beta-dioxygenase |
| TRINITY_DN82414_c0_g1 | 210 | GA20ox | KAH7297002.1 | Gibberellin 20 oxidase |
| TRINITY_DN59454_c0_g3 | 1200 | KSL2 | XP_024380401.1 | Kaurene synthase like |
| TRINITY_DN3391_c0_g1 | 1292 | GA2ox | KAG0616228.1 | Gibberellin 2-beta-dioxygenase |
| TRINITY_DN39640_c0_g1 | 2595 | CPS | UPQ49771.1 | Ent-copalyl diphosphate synthase |
| TRINITY_DN71939_c0_g1 | 1738 | CYP76AH1 | KAI5058385.1 | Ferruginol synthase |
| TRINITY_DN36706_c0_g1 | 254 | KSL2 | APP91796.1 | Ent-atiserene synthase KSL1 |
| TRINITY_DN50996_c0_g1 | 293 | KSL2 | BAQ20600.1 | Ent-atiserene synthase KSL4 |
| TRINITY_DN23337_c0_g1 | 1308 | GA2ox | BAG68569.1 | Gibberellin 2-beta-dioxygenase |
| TRINITY_DN34933_c0_g1 | 2213 | CYP76AH1 | KAI5083804.1 | Ferruginol synthase |
| TRINITY_DN2848_c0_g1 | 3116 | KSL2 | BAQ20600.1 | Ent-atiserene synthase KSL1 |
| TRINITY_DN24082_c0_g1 | 1423 | GA20ox | KAI5084607.1 | Gibberellin 20 oxidase |
| TRINITY_DN19844_c0_g1 | 2557 | GA3 | BAQ20601.1 | Ent-kaurene oxidase |
| TRINITY_DN8466_c0_g1 | 1447 | GA2ox | XP_024399557.1 | Gibberellin 2-beta-dioxygenase |
| TRINITY_DN9884_c0_g4 | 270 | CYP76AH1 | KAG0553548.1 | Ferruginol synthase |
| TRINITY_DN54990_c0_g3 | 442 | CYP76AH1 | KAH8938074.1 | Cytochrome P450 76T24 |
| TRINITY_DN9009_c0_g1 | 2208 | KSL2 | BAQ20600.1 | Miltiradiene synthase KSL2 |
| TRINITY_DN55358_c0_g1 | 938 | GA20ox | KAH7297002.1 | Gibberellin 20 oxidase |
| TRINITY_DN9884_c0_g1 | 1582 | CYP76AH1 | KAH8935636.1 | Ferruginol synthase |
| TRINITY_DN2234_c0_g1 | 1067 | CPS | UPQ49771.1 | Ent-copalyl diphosphate synthase |
| TRINITY_DN9884_c1_g2 | 1645 | CYP76AH1 | KAI5058385.1 | Ferruginol synthase |
| TRINITY_DN9884_c0_g3 | 1109 | CYP76AH1 | KAG0562564.1 | Ferruginol synthase |
| TRINITY_DN58290_c0_g1 | 1127 | GA2ox | KAG0576195.1 | Gibberellin 2-beta-dioxygenase |
| TRINITY_DN56614_c0_g1 | 336 | GA2ox | KAH7297735.1 | Gibberellin 2-beta-dioxygenase |
| TRINITY_DN50495_c0_g2 | 666 | GA3ox | ABX10776.1 | Gibberellin 3-beta-dioxygenase |
| TRINITY_DN2629_c3_g1 | 1183 | GA20ox | KAH7297002.1 | Gibberellin 20 oxidase |
| TRINITY_DN5351_c0_g1 | 1645 | CYP76AH1 | KAH8956371.1 | Carnosic acid synthase |
| TRINITY_DN9483_c0_g1 | 2205 | CPS | UPQ49773.1 | Ent-copalyl diphosphate synthase |
| TRINITY_DN416_c0_g1 | 1925 | GA3 | KAH7284372.1 | Ent-kaurene oxidase |
| TRINITY_DN36338_c0_g1 | 1234 | GA3ox | ABX10776.1 | Gibberellin 3-beta-dioxygenase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Zhai, K.; Xie, D. Heterologous Overexpression and Functional Analysis of the Isodon suzhouensis IsKS1 Gene in Arabidopsis thaliana. Curr. Issues Mol. Biol. 2025, 47, 413. https://doi.org/10.3390/cimb47060413
Liu F, Zhai K, Xie D. Heterologous Overexpression and Functional Analysis of the Isodon suzhouensis IsKS1 Gene in Arabidopsis thaliana. Current Issues in Molecular Biology. 2025; 47(6):413. https://doi.org/10.3390/cimb47060413
Chicago/Turabian StyleLiu, Fawang, Kefeng Zhai, and Dongmei Xie. 2025. "Heterologous Overexpression and Functional Analysis of the Isodon suzhouensis IsKS1 Gene in Arabidopsis thaliana" Current Issues in Molecular Biology 47, no. 6: 413. https://doi.org/10.3390/cimb47060413
APA StyleLiu, F., Zhai, K., & Xie, D. (2025). Heterologous Overexpression and Functional Analysis of the Isodon suzhouensis IsKS1 Gene in Arabidopsis thaliana. Current Issues in Molecular Biology, 47(6), 413. https://doi.org/10.3390/cimb47060413





