Abstract
The interest in regenerative medicine is increasing, and it is a dynamically developing branch of aesthetic surgery. Biocompatible and autologous-derived products such as platelet-rich plasma or adult mesenchymal stem cells are often used for aesthetic purposes. Their application originates from wound healing and orthopaedics. Adipose-derived stem cells are a powerful agent in skin rejuvenation. They secrete growth factors and anti-inflammatory cytokines, stimulate tissue regeneration by promoting the secretion of extracellular proteins and secrete antioxidants that neutralize free radicals. In an office procedure, without cell incubation and counting, the obtained product is stromal vascular fraction, which consists of not only stem cells but also other numerous active cells such as pericytes, preadipocytes, immune cells, and extra-cellular matrix. Adipose-derived stem cells, when injected into dermis, improved skin density and overall skin appearance, and increased skin hydration and number of capillary vessels. The main limitation of mesenchymal stem cell transfers is the survival of the graft. The final outcomes are dependent on many factors, including the age of the patient, technique of fat tissue harvesting, technique of lipoaspirate preparation, and technique of fat graft injection. It is very difficult to compare available studies because of the differences and multitude of techniques used. Fat harvesting is associated with potentially life-threatening complications, such as massive bleeding, embolism, or clots. However, most of the side effects are mild and transient: primarily hematomas, oedema, and mild pain. Mesenchymal stem cells that do not proliferate when injected into dermis promote neoangiogenesis, that is why respectful caution should be taken in the case of oncologic patients. A longer clinical observation on a higher number of participants should be performed to develop reliable indications and guidelines for transferring ADSCs.
1. Introduction
The process of healing has fascinated people for centuries. One of the first descriptions of the human body’s regenerative abilities originates from ancient Greece. In the myth, the titan Prometheus was chained to a rock, and every day an eagle ate a part of his liver, which would regenerate and grow back again. Nowadays, regenerative medicine is a dynamically developing branch of aesthetic surgery.
The term ‘regenerative medicine’ was defined by Daar and Greenwood in 2007 as a multidisciplinary branch of medicine, which stimulates the human body to repair and heal malfunctioning tissues [1]. In aesthetic medicine, the term ‘biostimulation’ is often used to describe the impact of injectables on the function of dermal fibroblasts [2]. Even though more than a decade has passed since a unified description of regenerative medicine has been established, there are still some semantic aspects that need to be clarified. This is especially noticeable with regard to autologous-derived agents and a wide range of different products and nomenclature that varies between authors. However, the aim of this paper was not an attempt to establish nomenclature, but rather to describe the potential use of autologous-derived agents in facial rejuvenation.
2. Harvesting Autologous-Derived Agents
A wide range of autologous products are used for the purpose of facial rejuvenation. The most commonly applied therapies include agents collected from peripheral blood, such as platelet-rich plasma (PRP) or fibrin. These agents are easy to collect and require only cannulation of a peripheral vein. Adipose-derived products are more demanding to harvest and require surgical experience. Fat tissue is a source of micronized fat graft (Microfat) or homogenized gel form (Nanofat) and stromal vascular fraction (SVF). PRP action depends on stress-activated platelets that secrete growth factors and proteins [3] and not on stem cells as is wrongly advertised by some providers. α- granules contained in platelets can secrete over 4000 different proteins. By stimulating fibroblasts, they induce the secretion of collagen, elastin, and the self-synthesis of hyaluronic acid. They also release growth factors for fibroblasts (FGF—fibroblast growth factor), PDGFs (platelet-derived growth factor), TGFβ (transforming growth factor), EGF (epidermal growth factor), and VEGF (vascular endothelial growth factor) [4,5,6,7,8,9]. The main indications for PRP in aesthetics are rejuvenation and fine line correction.
The primary sources of stem cells include bone marrow [10] and subcutaneous fat tissue [11]. Subcutaneous fat tissue contains 500 times more stem cells than bone marrow [11]. Several issues are subject to discussion concerning subcutaneous fat tissue harvesting techniques (Table 1). Subcutaneous fat tissue is feasible for collection in most patients. However, there are some data indicating that certain body areas are richer in stem cells. Padoin et al., as well as Tsekouras, suggested that lipoaspirates from the lower abdomen and the inner thigh had higher concentrations of adipose-derived stem cells (ADSCs) [12,13]. Di Taranto divided subcutaneous white adipose tissue (SWAT) into two parts: superficial and deep. ADSCs harvested from the superficial parts of the SWAT had better survival and presented higher expression of vascular endothelial growth factors (VEGFs) [14]. However, harvesting subcutaneous fat tissue from superficial parts brings the risk of irregularities. The Coleman procedure was first introduced in 1987 [15]. He used local anaesthesia with lidocaine and epinephrine and collected fat with a 2 mm diameter blunt cannula with a suction value amounting to 1 mL on a 10 mL syringe. He believed that too much suction would destroy the cells. There is data suggesting that negative pressure and donor site do not influence ADSC survival for culturing and yield preparation [16], but in terms of clinics and facial lipofilling, there might be a higher resolution of the transfer harvested traumatically [15,17,18,19]. For assisted liposuctions, no differences were observed between power-assisted and ultrasound-assisted liposuction [20], but laser-assisted procedures performed worse than power-assisted [21]. Fontes et al., in a precise review of fat harvesting techniques, described the impact of the cannula—its diameter as well as hole number and diameter—on fat graft survival. They concluded that the size of the cannula should provide minimal shear stress to avoid cell breakage [22].

Table 1.
Clinical studies and reviews considering usage of autologous agents in rejuvenation.
Several techniques of fat tissue processing have been described. The most popular among them are procedures that involve centrifugation. The parameters of spinning differ between the authors and sets [15,23,24,25,26]. Gravity separation and sedimentation requires no specific equipment [32]. There are some sets that enable the washing and infiltration of the fat graft [27]. Collecting ADSC from the fat graft requires micronization of the fat particles [26,28,29,30,31]. In the Arthrex procedure, the lipoaspirate is spun in ACP double syringes for four minutes at 2500 RPM in a Horizon 24-AH swing-out rotor centrifuge (Drucker Diagnostics LLC, Port Matilda, PA, USA). Subsequently, the oil from the destroyed adipocytes on top and the aqueous fraction, containing the anaesthetic and blood cells, are removed. In another step, the aspirate is fractionated by 30 rapid passages through a 1.2 mm female connector and spun for a second time for 4 min at 2500 RPM [26]. The final product that is obtained is the stromal vascular fraction (SVF). Stromal vascular fraction (SVF) contains significantly fewer adipocytes than the fat graft, while the volume of the fat graft comes in 90% from adipocytes [33]. Both SVF and the fat graft consist of numerous active cells such as pericytes, preadipocytes, immune cells, and extra-cellular matrix [33]. That is why most surgeons do not transfer stem cells in an office procedure for aesthetic purposes when the procedure is conducted according to the FDA principles. The proper term for this product is stromal vascular fraction, whereas stem cell transfers require cell incubation and counting [34].
3. The Powerful Action of the ADSCs in Facial Rejuvenation
SVF is a source of stem cells, but its regenerative properties also depend on other active cells (Table 2). ADSCs are multipotent cells, however, in vivo they do not proliferate but act as regulatory cells instead [35]. They are characterized by the expression of CD34+, CD44+, CD31−, and CD45− on the surface [33]. They have a high proliferation ability, and they promote angiogenesis in the dermis [33,36]. They also secrete many growth factors, among others for fibroblasts, the endothelium, as well as anti-inflammatory cytokines. ADSCs stimulate tissue regeneration by promoting the secretion of extracellular proteins, such as collagen and elastin, but also metalloproteinases [37]. The mechanisms triggered by ADSCs are nonspecific paths of immune response [35]. In the paracrine mechanism, they secrete exosome and microvesicles containing proteins, nucleic acids, lipids, and enzymes [38]. Most authors recognize the longevity of the results of the transfer as the actual survival time of ADSCs, but in aesthetic patients the exact in vivo survival time of cells is difficult to determine. Zhang et al., in a mouse model, achieved 28-day survival of cultured stem cells injected intradermally [39]. Another cell population responsible for a strong regenerative attribute of SVF are pericytes. They are also multipotent cells that do not proliferate in vivo into adipocytes or fibroblasts. Their role is limited to neovascularization [40]. Adipocytes are highly active cells with paracrine and autocrine abilities [41]. SVF also contains the extracellular matrix [33] and immune cells that are responsible for the cellular paracrine dialogue [42]. Some authors use sieves to clarify the SVF gel [43], but in our opinion this technique might impoverish the product.

Table 2.
Role of ADSC in skin rejuvenation.
SVFs stimulate collagen I, II, III, and V, oxytalan, and elastin secretion [36,44]. Restoration of ECM fibres prevents skin laxity and atrophy [36]. Activated fibroblasts, via different pathways Wnt/β-catenin, PI3K/Akt, insulin-like growth factor (IGF), IL-1, and TNF-α, secrete extracellular matrix proteins, mainly collagen type I and III [44]. In a micropig model, ADSCs were injected intradermally and skin density was evaluated after a month. Western blot showed an increased expression of dermal collagen [45].
ADSCs improve lesions caused by photoaging. They secrete antioxidants and cytokines that neutralize the effects of the primary indicators of skin damage: UVB and reactive oxygen species (ROS) [38,44]. ROS promote inflammation, damage to cellular membrane, as well as alterations in DNA, RNA, and proteins of the extracellular matrix (ECM). ADSCs secrete growth factors, including the hepatocyte growth factor (HGF) or VEGF, that protect cells from oxidative cells. Interleukin-6 (IL-6) reduces oxidative stress via promotion of the activator of transcription 3 (STAT3), nuclear factor erythroid 2-related factor 2 (Nrf2), and superoxidative dismutase (SOD). Nrf2 downregulates NOX1 and NOX4 responsible for lipid peroxidation. They also secrete glutathione peroxidase (GPx), SOD, and catalase to upregulate antioxidant response, as well as inhibit secretion of myeloperoxidase (MPO) [39,44]. In a rat model, stem cells suppressed the activity of free radicals on the DNA. They inhibit hypoxia and apoptosis by upregulating BCl-2 [38,44]. UVB irradiation is disactivated by the suppression of mitogen-activated protein kinases (MAPKs) and nuclear factor kappa B NF-κB [38].
Photoaging is also manifested by hyperpigmentation. These lesions are acquired pigmentary lesions that mainly affect women. Risk factors include exposure to UV radiation, hormonal changes, and genetic predisposition [50]. These are areas of increased melanin concentration in the skin and epidermis. The accumulation of UV radiation causes inflammation while also stimulating melanogenesis and angiogenesis. Hyperpigmentation is caused not only by the accumulation of melanin, but also occurs due to pathologically dilated vessels [46]. Adipose-derived stem cells secrete TGF-β1, which is a suppressor of tyrosinase, an enzyme necessary for melanin synthesis [44].
Charles-se-Sá et al. transferred isolated stem cells (CD105+/CD90+/CD73+/CD146+/ CD14/CD45−/CD34−) and assessed histopathological specimens of skin from the injection site. Skin biopsies taken before the treatment were diagnosed with elastosis due to the degradation and disorganization of elastic fibres, as well as mild mononuclear dermis infiltration. After the SVFs, the dermis was richer in oxytalan elastic fibres, and a decrease in elastosis was observed in the reticular dermis. The dermis showed new capillary vessels, and the skin was more hydrated [47]. Stem cells inhibit metalloproteinases MMP-1, MMP-2, MMP-3, MMP-9, and MMP-13 [48].
The most recent studies report the presence of ADSCs in subcutaneous tissue, which might suggest their ultimate role in skin repair and wound healing [49]. During injury, expression of the CXCR-4 molecule on ADSCs’ surface increases, and stem cells are recruited to modulate local inflammatory response. ADSCs have the potential to migrate to the site of injury. It has been proven that overexpression of SDF-1 protein is responsible for cell migration. In the initial phases of wound healing, they promote a shift between macrophage population from M1 to M2 and stimulate secretion of anti-inflammatory TNF and IL-10. In later phases, ADSCs stimulate angiogenesis via the secretion of growth factors including VEGF, PDGF, IGF, HGF, b-FGF, SDF-1, TGF-β, and GDF11. During the proliferation phase, ADSCs promote the secretion of ECM proteins [49], as well as secrete fibroblast chemokines [45].
4. Clinical Indications for SVF
The SVF gel can be transferred separately or with the fat transfer (Table 3). CAL (cell-assisted lipotransfer) is a simultaneous transfer of these two adipose-derived agents [51,52]. It was first introduced in 2008 by Yoshimura [53]. The primary reason for using a combination of this kind is the unpredictable survival time of fat grafts. Depending on the author and technique, one-year graft survival varies from 10% to 90% [50]. Adding SVF over the fat graft stimulates the process of neovascularization [54,55], as in the early post-transplant period, fat molecules are nourished by osmosis alone [55]. Due to Yoshimura’s rule, fat particles smaller than 200 µm are the least vulnerable to apoptosis [56]. The effects of the CAL are shown in Figure 1.

Table 3.
ADSC clinical application.
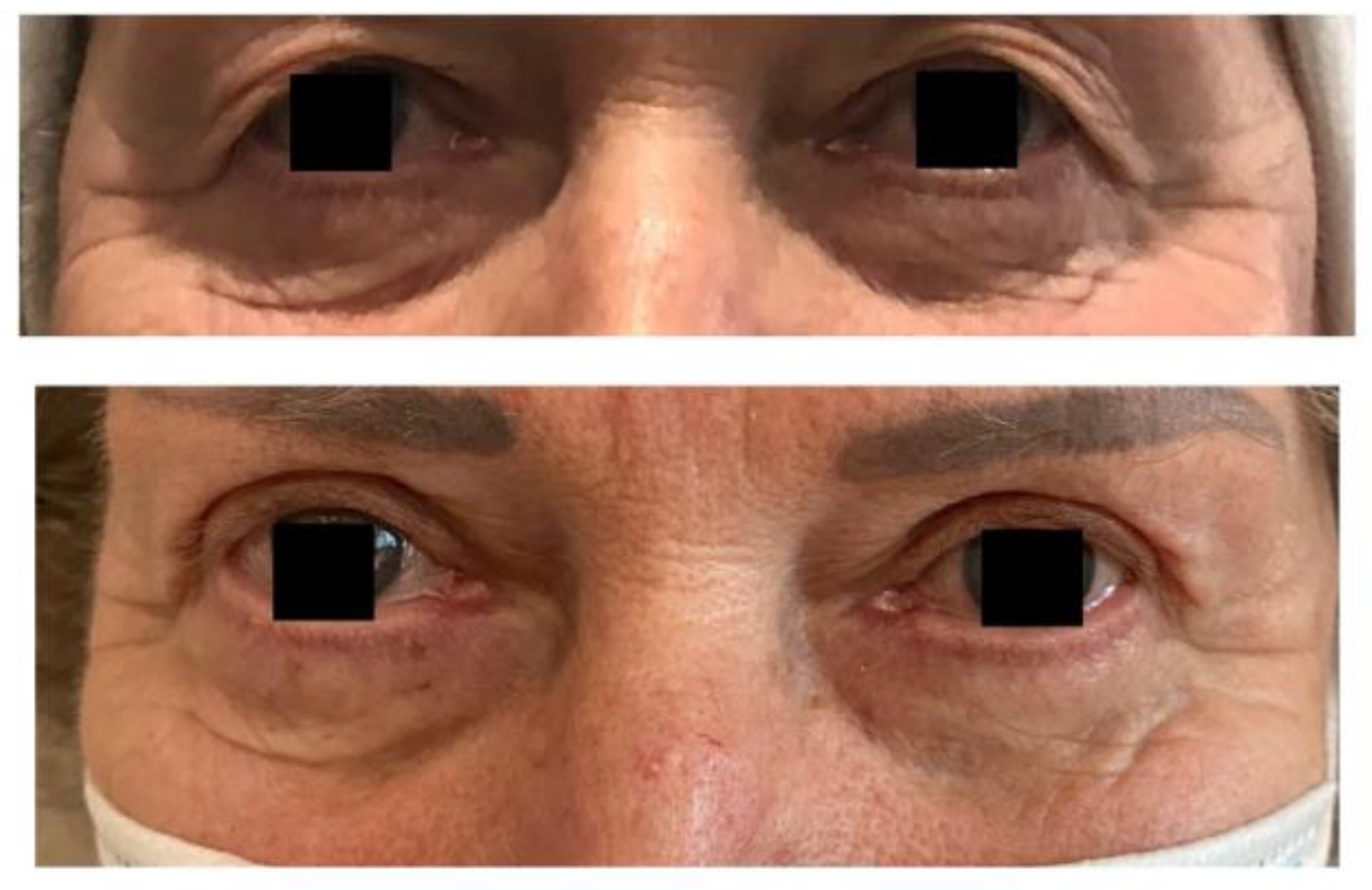
Figure 1.
Results of CAL and upper blepharoplasty.
Adding SVF over the lipotransfer is one of the factors prolonging the result. Schnedel used a 3D computer volumetric analysis to evaluate long-term results. In a 12.6-month observation, 68% of the transferred graft survived [57]. Similar observations were done by Yating Tin et al. The survival time of CAL was higher than in the case of fat transfer alone (77.6% vs. 56.2%) [58]. Adding SVFs to fat grafts may prevent the necessity of secondary procedures and reduce the cost of the treatment [59].
Fat grafts enriched with SVFs have a potential antifibrotic effect. Almadori et al., treated 62 patients with oro-facial fibrosis in systemic sclerosis. Lipotransfer and SVFs reduce fibrosis due to suppression of fibroblast proliferation. The clinical outcomes, including mouth function, were improved in the observation. The levels of transforming growth factor (TGF-β1) and connective tissue growth factor were reduced in the treated population [43].
CAL can be conducted simultaneously with other surgical procedures, such as transconjunctival lower blepharoplasty [60], facelifting [44], threads, or to promote wound healing [61,74].
Jiang et al. described the surgical technique for lower eyelid blepharoplasty and simultaneous SVF gel injection. They used a Coleman procedure for fat harvesting. A transconjunctival fat pad removal was performed using an incision 5 mm below conjunctiva, the opening of the capsulopalpebral fascia was done typically, protruding periorbital fat was resected, and no sutures were left on the conjunctiva. After upright positioning of the patients, SVF gel was injected using one to two entry points with a 0.9mm blunt cannula to position the fat graft between the orbicularis oculi muscle and the infraorbital septum [43].
Berbardini et al. reported successful combined protocols for aesthetic surgeries and simultaneous fat injections. The surgery started from aesthetic surgeries: minimal incision vertical endoscopic lift (n = 51), primary blepharoplasty (n = 35), neck lift (n = 23), and revisional blepharoplasty (n = 12), which were performed in a typical way. In the second step during the same procedure, the harvested and processed fat was injected in superficial layers with a 23G sharp needle. The aim of fat transfer was to restore volume and improve skin quality [62].
Simultaneous fat grafting and PDO barbed threads were reported by Surowiecka [63]. The procedure started with fat injections following the ACA technique [64] and was followed by placing in the subcutis layer a total number of six barbed threads from four different entry points to elevate the midface and to improve the jawline. One entry point was done over the zygomatic ligament, the first thread was directed to the nasolabial folds, through masseteric ligament, placing the end of the threads just behind the nasolabial fold. The second entry point was done over the preauricularis ligament and two threads with a fanning technique were directed to the mental area [63].
Transferring SVFs alone improves skin density and thickness as well as dermis density [47,65]. The results were stable in a 12-month observation [66,67]. The improvement of skin density provides the secondary effect of volumization and tissue elevation [38,57,58]. In a comparison of nanofat, microfat, and SVF, the count of viable cells in SVF increased moderately in comparison with nanofat and microfat [43]. The results are shown in Figure 2.
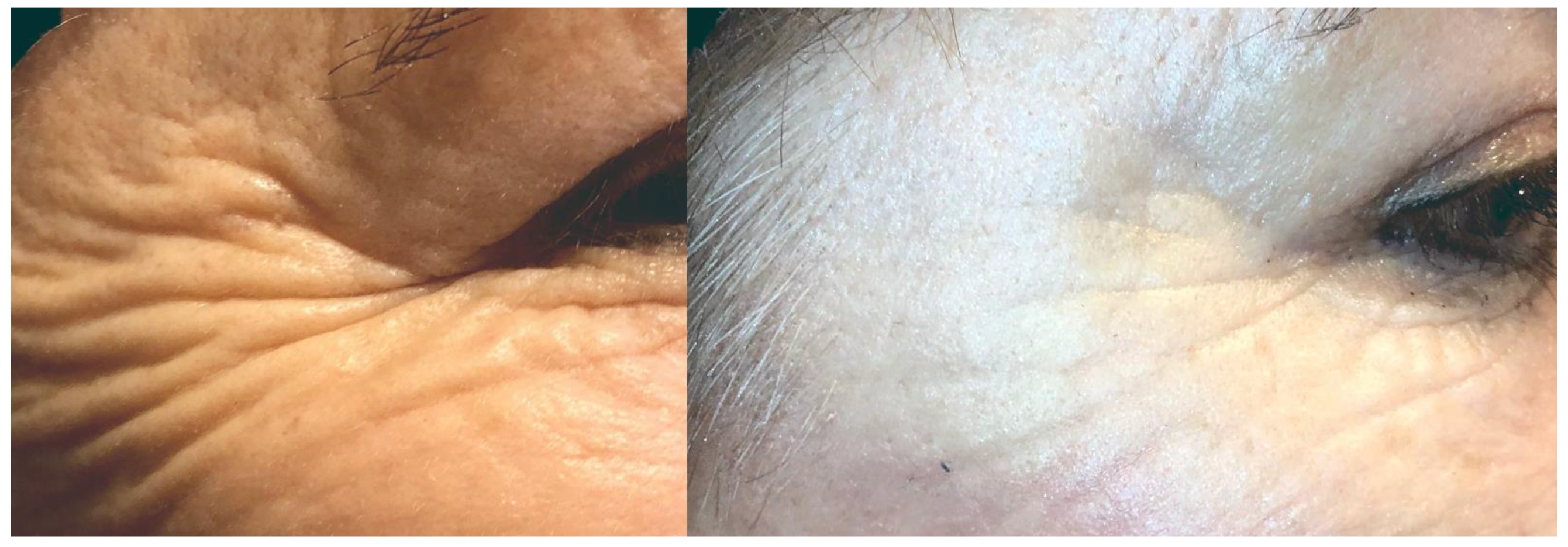
Figure 2.
Results of skin improvements 6 months after SVF injection.
Mesenchymal stem cells undergo senescent changes [68], as does the fat tissue. In in vitro observation, stem cells from elder donors are characterized by an elevated expression of the p53 gene, b-galactosidase, and a decrease in intrinsic antioxidant mechanisms, becoming more vulnerable to apoptosis [68]. Aging cells are found during the G1-phase, and their apoptosis is induced by b-galactosidase and p53 [44,75]. Stem cells may influence senescent lesions in other cells [75]. With age, the number of fat tissue M2 macrophages increases, whereas the number of M1 macrophages decreases. M2 macrophages are known to secrete pro-inflammatory cytokines responsible for atherosclerosis. The macrophages shift decreases the ability to neutralize free fatty acids and promotes weight gain. With time, the ability to proliferate and secrete adiponectin also decreases [76]. Fat tissue aging may impair the final results of lipotransfer in elderly patients even with SVFs.
SVF can be successfully combined with lasers in a combined therapy of scars. Fat grafting has been reported in burn scars in both adults and children. The indications for the lipofilling of post-burn scars include hypotrophic scars without improvement after pressure garments and fibrotic scars around joints including tendon adhesions [69,70]. ADSC were reported to efficiently treat post-ablative laser wounds even in skin type Fitzpatrick III-IV [71]. They significantly reduced erythema after carbon dioxide laser and melanin treatment. The increase of collagen in the skin treated with ADSC was not statistically significant, but there was a short observation time. A topical use of cultured ADSC with niacinamide was reported by Lee. The protocol started from ablative laser CO2 resurfacing. Topical ADSC with niacinamide improved wrinkles and skin texture, as well as skin pigmentation [72]. Verpaele combined liquid nanofat with microneedling and gained satisfactory results [73]. Considering these results, SVFs could also be combined with microneedling during a procedure.
5. Complications
Complications can be related to liposuction and to fat injections. The total rate of reported complications after liposuction is 5% [77] For SVF purposes, a small amount of fat tissue is required; however, the risk associated with the procedure remains. The most common side effects of fat tissue harvesting are mild and transient: primarily hematomas, oedema, and mild pain [78]. A total of 23% cases of reported deaths were caused by pulmonary thromboembolism [77]. Other serious complications include bleeding [79] and fat embolism [77]. Cases of abdominal visceral damage have been described as well [77].
As far as the fat graft is concerned, several cases of graft migration, formation of calcifications and cysts, as well as ischemia, necrosis, and vision loss have been described [80]. Most often these side effects are transient and minor [26]: primarily oedema and hematomas. As a facial soft tissue filler, fat can cause vascular occlusion, which is why a decent anatomy assessment and using blunt cannulas is recommended [26].
ADSCs do not proliferate in vivo and do not undergo neoplastic transformation per se [35], but they promote neoangiogenesis. There is a risk of promoting the neoplastic vascular network, local recurrence, and metastases [80,81]. There are no guidelines for autologous cell therapy in oncologic patients. ADSCs stimulate the process of angiogenesis, therefore the risk of stimulating the neoplastic vascular network and promoting metastasis seems to be very high [82]. One study revealed an improvement in healing and a longer period of remission in head and neck squamous cell carcinoma after the defects of soft tissues had been filled with autologous agents [83]. On the other hand, there are studies showing local progression of ductal adenocarcinoma in the breast and accelerated formation of distant metastases [84]. Klinger et al. in a multicentre study reported that fat transfer for breast reconstruction in breast cancer patients did not worsen the outcome. They used the Coleman technique, without enriching the grafts with adipose-derived stem cells [85]. Calabrese et al. transplanted fat grafts enriched in SVF. It was a prospective study with 41 patients with breast cancer G1 enrolled. Patients diagnosed with breast cancer G2 were treated with lipotransfer alone, and G3 were a control group. Adding SVF to the fat graft in the G1 group did not increase oncological recurrence [86]. In aesthetic indications, it is extremely important to take a proper medical history and examination of the patient before the procedure.
Future Trends
The main limitations of lipotransfers and SVF transfers are the unpredictable outcomes and harvesting procedure. Complications after liposuction can be life-threatening. That is why novel approaches in tissue harvesting are being developed. One of the ideas is to store lipoaspirate in a tissue bank to use it later. Cryopreservation proved to be successful, and the storing process did not influence the number or viability of mesenchymal stem cells [87]. Ohashi et al. divided the lipoaspirate into two parts. The first was injected during the same day of the procedure and the second part was centrifuged with Ringer’s solution, washed, added with cryoprotective solution, then transferred into liquid nitrogen and stored at −196 °C [87].
Fat tissue is highly immunogenic, so allogenic fat transfers are extremely difficult to obtain. There is a case of a successful allogenic fat transfer into post-radiative ulcerations due to cutaneous T-cell lymphoma. The transfer was possible because of the chimeric state between the donor and recipient, who were brothers [88].
Some investigators work on different scaffolds and growth factors that could be safely added to fat grafts to improve its survival [80]. The aim of adding biomaterials is to avoid the necrosis of the stem cells and improve revascularization. There are few scaffolds based on hyaluronic acid reported, as well as beta-glucan or poly(lactide-co-glycoside) [80].
Finally, there are observations that simple techniques, such as botulin, can have a beneficial influence on fat grafting results. By relaxing the moving parts of the face before transferring fat, the mechanical stress of the muscles is diminished, and fat particles are believed to last longer [35].
6. Conclusions
Regenerative medicine is still developing. The mechanism of action of ADSCs and their multidirectional impact on dermis rejuvenation fascinates physicians. ADSCs secrete antioxidative agents, as well as promote wound healing and ECM protein secretion. There are several reports proving that ADSC injections improve skin density and have anti-wrinkle properties. Skin hydration improves and there is a global improvement in appearance. There are some limitations, however. First, it is very difficult to compare studies because of the differences in techniques used. Most fat and stem cell transfers performed due to aesthetic indications are in-office, one-stage procedures without cytometry and cell counting. Stem cell harvesting systems differ and surgeons use different techniques. It seems that the survival and vitality of stem cells is strongly associated with the harvesting technique, and there are no unequivocal guidelines in this area either. Most of the studies provide only a one-year or two-year follow-up on a small number of volunteers. A longer clinical observation on a higher number of participants should be performed to develop reliable indications and guidelines for transferring ADSCs.
7. Key Points
ADSCs are efficient and safe for facial rejuvenation but harvesting ADSC requires some technical skills and adequate equipment.
They improve skin quality in many mechanisms, which makes them superior to other known anti-aging agents. They promote the remodelling of the dermis, which is important in scar treatment and improvement of dermis thickness. ADSCs have an antioxidative effect and can protect fibroblast DNA from free radicals. Oxidative stress is perpetual due to UV radiation, pollution, diet, and smoking. Furthermore, they stimulate secretion of collagen, elastin, and anti-inflammatory cytokines. These features make ADSC an ideal and natural anti-aging solution.
Author Contributions
Conceptualization, A.S. and J.S.; methodology, A.S.; software, A.S.; validation, A.S., J.S.; formal analysis, A.S., investigation, A.S.; resources, J.S.; data curation, A.S., J.S.; writing—original draft preparation, A.S.; writing—review and editing, J.S.; visualization, A.S.; supervision, J.S.; project administration, A.S.; funding acquisition, A.S., J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Daar, A.S.; Greenwood, H.L. A proposed definition of regenerative medicine. J. Tissue Eng. Regen. Med. 2007, 1, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Avantaggiato, A.; Palmieri, A.; Carinci, F.; Pasin, M.; Bertuzzi, G. Biostimulation and biorevitalization: Effects on human skin fibroblasts. Ann. Oral Maxillofac. Surg. 2013, 1, 11. [Google Scholar] [CrossRef][Green Version]
- Gentile, P.; Garcovich, S. Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects. Int. J. Mol. Sci. 2021, 22, 1538. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Ivanovski, S.; Cei, S.; Ducci, F.; Tonetti, M.; Gabriele, M. The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin. Oral Implant. Res. 2006, 17, 212–219. [Google Scholar] [CrossRef]
- Anitua, E.; Sánchez, M.; Zalduendo, M.M.; de la Fuente, M.; Prado, R.; Orive, G.; Andía, I. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 2009, 42, 162–170. [Google Scholar] [CrossRef]
- Maisel-Campbell, A.L.; Ismail, A.; Reynolds, K.A.; Poon, E.; Serrano, L.; Grushchak, S.; Farid, C.; West, D.P.; Alam, M. A systematic review of the safety and effectiveness of platelet-rich plasma (PRP) for skin aging. Arch. Dermatol. Res. 2019, 312, 301–315. [Google Scholar] [CrossRef]
- Alves, R.; Grimalt, R. A Review of Platelet-Rich Plasma: History, Biology, Mechanism of Action, and Classification. Ski. Appendage Disord. 2018, 4, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Stevens, H.P. ACA-Technik: “stromal vascular fraction”, “platelet-rich plasma” und Mikrofett zur körpereigenen Regeneration und Hautverjüngung. J. Ästhetische Chir. 2019, 12, 77–83. [Google Scholar] [CrossRef]
- Elghblawi, E. Platelet-rich plasma, the ultimate secret for youthful skin elixir and hair growth triggering. J. Cosmet. Dermatol. 2017, 17, 423–430. [Google Scholar] [CrossRef]
- Keller, G.; Snodgrass, R. Life span of multipotential hematopoietic stem cells in vivo. J. Exp. Med. 1990, 171, 1407–1418. [Google Scholar] [CrossRef]
- Bacakova, L.; Zarubova, J.; Travnickova, M.; Musilkova, J.; Pajorova, J.; Slepicka, P.; Kasalkova, N.S.; Svorcik, V.; Kolska, Z.; Motarjemi, H.; et al. Stem cells: Their source, potency and use in regenerative therapies with focus on adipose-derived stem cells—A review. Biotechnol. Adv. 2018, 36, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Padoin, A.V.; Braga-Silva, J.; Martins, P.; Rezende, K.; Rezende, A.R.D.R.; Grechi, B.; Gehlen, D.; Machado, D.C. Sources of Processed Lipoaspirate Cells: Influence of Donor Site on Cell Concentration. Plast. Reconstr. Surg. 2008, 122, 614–618. [Google Scholar] [CrossRef]
- Tsekouras, A.; Mantas, D.; Tsilimigras, D.I.; Moris, D.; Kontos, M.; Zografos, G.C. Comparison of the viability and yield of adipose-derived stem cells (ASCs) from different donor areas. In Vivo 2017, 31, 1229–1234. [Google Scholar]
- Di Taranto, G.; Cicione, C.; Visconti, G.; Isgrò, M.A.; Barba, M.; Di Stasio, E.; Stigliano, E.; Bernardini, C.; Michetti, F.; Salgarello, M.; et al. Qualitative and quantitative differences of adipose-derived stromal cells from superficial and deep subcutaneous lipoaspirates: A matter of fat. Cytotherapy 2015, 17, 1076–1089. [Google Scholar] [CrossRef]
- Coleman, S.R. Structural Fat Grafting. Aesthetic Surg. J. 1998, 18, 386–388. [Google Scholar] [CrossRef]
- Travnickova, M.; Pajorova, J.; Zarubova, J.; Krocilova, N.; Molitor, M.; Bacakova, L. The Influence of Negative Pressure and of the Harvesting Site on the Characteristics of Human Adipose Tissue-Derived Stromal Cells from Lipoaspirates. Stem Cells Int. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Centurión, P.; Gamarra, R.; Caballero, G.; Kaufmann, P.; Delgado, P. Optimizing harvesting for facial lipografting with a new photochemical stimulation concept: One STEP technique™. Eur. J. Plast. Surg. 2020, 43, 733–742. [Google Scholar] [CrossRef]
- Mojallal, A.; Auxenfans, C.; Lequeux, C.; Braye, F.; Damour, O. Influence of negative pressure when harvesting adipose tissue on cell yield of the stromal–vascular fraction. Bio-Med. Mater. Eng. 2008, 18, 193–197. [Google Scholar] [CrossRef]
- Cheriyan, T.; Kao, H.K.; Qiao, X.; Guo, L. Low Harvest Pressure Enhances Autologous Fat Graft Viability. Plast. Reconstr. Surg. 2014, 133, 1365–1368. [Google Scholar] [CrossRef]
- Duscher, D.; Atashroo, D.; Maan, Z.; Luan, A.; Brett, E.A.; Barrera, J.; Khong, S.M.; Zielins, E.R.; Whittam, A.J.; Hu, M.S.; et al. Ultrasound-Assisted Liposuction Does Not Compromise the Regenerative Potential of Adipose-Derived Stem Cells. STEM CELLS Transl. Med. 2016, 5, 248–257. [Google Scholar] [CrossRef]
- Chung, M.T.; Zimmermann, A.S.; Paik, K.J.; Morrison, S.D.; Hyun, J.S.; Lo, D.D.; McArdle, A.; Montoro, D.T.; Walmsley, G.G.; Senarath-Yapa, K.; et al. Isolation of Human Adipose-Derived Stromal Cells Using Laser-Assisted Liposuction and Their Therapeutic Potential in Regenerative Medicine. Stem Cells Transl. Med. 2013, 2, 808–817. [Google Scholar] [CrossRef]
- Fontes, T.; Brandão, I.; Negrão, R.; Martins, M.J.; Monteiro, R. Autologous fat grafting: Harvesting techniques. Ann. Med. Surg. 2018, 36, 212–218. [Google Scholar] [CrossRef]
- Rigotti, G.; Marchi, A.; Galie, M.; Baroni, G.; Benati, D.; Krampera, M.; Pasini, A.; Sbarbati, A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plast. Reconstr. Surg. 2007, 119, 1409–1422. [Google Scholar] [CrossRef]
- Ferraro, G.A.; De Francesco, F.; Tirino, V.; Cataldo, C.; Rossano, F.; Nicoletti, G.; D’Andrea, F. Effects of a New Centrifugation Method on Adipose Cell Viability for Autologous Fat Grafting. Aesthetic Plast. Surg. 2010, 35, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Asilian, A.; Siadat, A.H.; Iraji, R. Comparison of fat maintenance in the face with centrifuge versus filtered and washed fat. J. Res. Med Sci. 2014, 19, 556–561. [Google Scholar]
- Surowiecka, A.; Piekarski, M.; Pototschnig, H. Stromal vascular fraction and emulsified fat as regenerative tools in rejuvenation of the lower eyelid area. Dermatol. Ther. 2021, 34, e14937. [Google Scholar] [CrossRef]
- Mestak, O.; Sukop, A.; Hsueh, Y.-S.; Molitor, M.; Mestak, J.; Matejovska, J.; Zarubova, L. Centrifugation versus PureGraft for fatgrafting to the breast after breast-conserving therapy. World J. Surg. Oncol. 2014, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, Z.; Opländer, C.; Almakadi, S.; Fritz, A.; Vogt, M.; Pallua, N. Conventional vs. micro-fat harvesting: How fat harvesting technique affects tissue-engineering approaches using adipose tissue-derived stem/stromal cells. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 1271–1278. [Google Scholar] [CrossRef]
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat grafting: Basic research and clinical applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef]
- Azzam, A.; Kholosy, H.; Abouarab, M. The efficacy of autologous Nanofat Injections in the treatment of infraorbital dark colouration. Egyp. J. Plast. Reconstr. Surg. 2019, 43, 445–452. [Google Scholar]
- Oh, D.S.; Kim, D.H.; Roh, T.S. Correction of Dark Coloration of the Lower Eyelid Skin with Nanofat Grafting. Arch. Aesthetic Plast. Surg. 2014, 20, 92–96. [Google Scholar] [CrossRef]
- Xue, E.Y.; Narvaez, L.; Chu, C.K.; Hanson, S.E. Fat Processing Techniques. Semin. Plast. Surg. 2020, 34, 011–016. [Google Scholar] [CrossRef]
- Shukla, L.; Morrison, W.A.; Shayan, R. Adipose-derived stem cells in radiotheraphy injury: A new frontier. Front. Surg. 2015, 2, 1–12. [Google Scholar] [CrossRef]
- De Ugarte, D.A.; Alfonso, Z.; Zuk, P.A.; Elbarbary, A.; Zhu, M.; Ashjian, P. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol. Lett. 2003, 89, 267–270. [Google Scholar] [CrossRef]
- Strużyna, J.; Pojda, Z. Zastosowania komórek macierzystych z tkanki tłuszczowej w medycynie regeneracyjnej. Chir. Plast. i Oparzenia/Plast. Surg. Burn. 2015, 3, 151–157. [Google Scholar] [CrossRef]
- Suh, A.; Pham, A.; Cress, M.J.; Pincelli, T.; TerKonda, S.P.; Bruce, A.J.; Zubair, A.C.; Wolfram, J.; Shapiro, S.A. Adipose-derived cellular and cell-derived regenerative therapies in dermatology and aesthetic rejuvenation. Ageing Res. Rev. 2019, 54, 100933. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B. Adipose-Derived Stem Cells for Regenerative Medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Yi, C.; Pu, L.L. An Overview of Principles and New Techniques for Facial Fat Grafting. Clin. Plast. Surg. 2020, 47, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dong, Z.; Peng, Z.; Lu, F. Anti-Aging Effect of Adipose-Derived Stem Cells in a Mouse Model of Skin Aging Induced by D-Galactose. PLoS ONE 2014, 9, e97573. [Google Scholar] [CrossRef]
- Esteves, C.L.; Donadeu, F.X. Pericytes and their potential in regenerative medicine across species. Cytom. Part A 2018, 93, 50–59. [Google Scholar] [CrossRef]
- Pond, C.M. Adipose tissue, the anatomists’ Cinderella, goes to the ball at last, and meets some influential partners. Postgrad. Med J. 2000, 76, 671–673. [Google Scholar] [CrossRef][Green Version]
- Kane, H.; Lynch, L. Innate Immune Control of Adipose Tissue Homeostasis. Trends Immunol. 2019, 40, 857–872. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Quan, Y.; Wang, J.; Cai, J.; Lu, F. Fat Grafting for Facial Rejuvenation Using Stromal Vascular Fraction Gel Injection. Clin. Plast. Surg. 2020, 47, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Zhang, L.; Chen, P.; Zhang, C.; Tang, S.; Chen, X. Comparison of the Efficacy and Safety of Cell-Assisted Lipotransfer and Platelet-Rich Plasma Assisted Lipotransfer: What Should We Expect from a Systematic Review with Meta-Analysis? Cell Transplant. 2021, 30, 0963689721989607. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-S.; Jang, K.A.; Sung, J.-H.; Park, J.-S.; Kwon, Y.H.; Kim, K.J.; Kim, W.-S. Adipose-Derived Stem Cells and Their Secretory Factors as a Promising Therapy for Skin Aging. Dermatol. Surg. 2008, 34, 1323–1326. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, Y.C.; Lee, E.-S.; Kang, H.Y. The vascular characteristics of melasma. J. Dermatol. Sci. 2007, 46, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Dobke, M.; Lunyak, V.V. Mesenchymal Stem Cells from Adipose Tissue in Clinical Applications for Dermatological Indications and Skin Aging. Int. J. Mol. Sci. 2017, 18, 208. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, Z.; Xu, J. Application of adipose-derived stem cells in photoaging: Basic science and literature review. Stem Cell Res. Ther. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and Limits of Adipose-Derived Stem Cells (ADSCs) and Mesenchymal Stem Cells (MSCs) in Wound Healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef] [PubMed]
- McKesey, J.; Tovar-Garza, A.; Pandya, A.G. Melasma Treatment: An Evidence-Based Review. Am. J. Clin. Dermatol. 2020, 21, 173–225. [Google Scholar] [CrossRef] [PubMed]
- Menkes, S.; Luca, M.; Soldati, G.; Polla, L. Subcutaneous Injections of Nanofat Adipose-derived Stem Cell Grafting in Facial Rejuvenation. Plast. Reconstr. Surg.-Glob. Open 2020, 8, e2550. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K. Cell-Assisted Lipotransfer and Therapeutic Use of Adipose Stem Cells Thereafter. Aesthetic Plast. Surg. 2020, 44, 1266–1267. [Google Scholar] [CrossRef]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Hirohi, T.; Harii, K. Cell-assisted lipotransfer (CAL) for cosmetic breast augmentation-supportive use of adipose-derived stem/stromal cells. Aesthet. Plast. Surg. 2008, 32, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Modarressi, A. Platlet Rich Plasma (PRP) Improves Fat Grafting Outcomes. World J. Plast. Surg. 2013, 2, 6–13. [Google Scholar]
- Wei, H.; Gu, S.-X.; Liang, Y.-D.; Liang, Z.-J.; Chen, H.; Zhu, M.-G.; Xu, F.-T.; He, N.; Wei, X.-J.; Li, H.-M. Nanofat-derived stem cells with platelet-rich fibrin improve facial contour remodeling and skin rejuvenation after autologous structural fat transplantation. Oncotarget 2017, 8, 68542–68556. [Google Scholar] [CrossRef]
- Eto, H.; Kato, H.; Suga, H.; Aoi, N.; Doi, K.; Kuno, S.; Yoshimura, K. The Fate of Adipocytes after Nonvascularized Fat Grafting. Plast. Reconstr. Surg. 2012, 129, 1081–1092. [Google Scholar] [CrossRef]
- Schendel, S.A. Enriched Autologous Facial Fat Grafts in Aesthetic Surgery: 3D Volumetric Results. Aesthetic Surg. J. 2015, 35, 913–919. [Google Scholar] [CrossRef]
- Yin, Y.; Li, J.; Li, Q.; Zhang, A.; Jin, P. Autologous fat graft assisted by stromal vascular fraction improves facial skin quality: A randomized controlled trial. J. Plast. Reconstr. Aesthetic Surg. 2020, 73, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Almadori, A.; Griffin, M.; Ryan, C.M.; Hunt, D.F.; Hansen, E.; Kumar, R.; Abraham, D.J.; Denton, C.P.; Butler, P.E.M. Stem cell enriched lipotransfer reverses the effects of fibrosis in systemic sclerosis. PLoS ONE 2019, 14, e0218068. [Google Scholar] [CrossRef]
- Sterodimas, A.; Nicaretta, B.; Boriani, F. Composite Face Lifting. Ann. Plast. Surg. 2020, 85, e20–e23. [Google Scholar] [CrossRef]
- Amirkhani, M.A.; Shoae-Hassani, A.; Soleimani, M.; Hejazi, S.; Ghalichi, L.; Nilforoushzadeh, M.A. Rejuvenation of facial skin and improvement in the dermal architecture by transplantation of autologous stromal vascular fraction: A clinical study. BioImpacts 2016, 6, 149–154. [Google Scholar] [CrossRef]
- Bernardini, F.P.; Gennai, A.; Izzo, L.; Zambelli, A.; Repaci, E.; Baldelli, I.; Orcioni, G.F.; Hartstein, M.E.; Santi, P.L.; Quarto, R. Superficial Enhanced Fluid Fat Injection (SEFFI) to Correct Volume Defects and Skin Aging of the Face and Periocular Region. Aesthetic Surg. J. 2015, 35, 504–515. [Google Scholar] [CrossRef]
- Surowiecka, A. The step-up approach in rejuvenation of the midface: A combination of minimally invasive procedures. Funct. Med. Med. Aesthethics 2021. [Google Scholar]
- Stevens, H.P.; Donners, S.; de Bruijn, J. Introducing Platelet-Rich Stroma: Platelet-Rich Plasma (PRP) and Stromal Vascular Fraction (SVF) Combined for the Treatment of Androgenetic Alopecia. Aesthet. Surg. J. 2018, 38, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Cai, J.; Zhang, P.; Liao, Y.; Yuan, Y.; Dong, Z.; Lu, F. Adipose Stromal Vascular Fraction Gel Grafting: A New Method for Tissue Volumization and Rejuvenation. Dermatol. Surg. 2018, 44, 1278–1286. [Google Scholar] [CrossRef]
- Chunlan, L.; Guanchu, L.; Linwang, T.; Jiao, Z. Application of Adipose Stem Cell Glue in Facial and Breast Plastic. Clin. Med. Res. 2020, 9, 132. [Google Scholar] [CrossRef]
- Tonnard, P.; Verpaele, A.; Carvas, M. Fat Grafting for Facial Rejuvenation with Nanofat Grafts. Clin Plast Surg. 2020, 47, 53–62. [Google Scholar] [CrossRef]
- Yang, Y.-H.K.; Ogando, C.R.; See, C.W.; Chang, T.-Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, N.S.; Piccolo, M.S.; Piccolo, M.T.S. Fat Grafting for Treatment of Burns, Burn Scars, and Other Difficult Wounds. Clin. Plast. Surg. 2015, 42, 263–283. [Google Scholar] [CrossRef]
- Condé-Green, A.; Marano, A.A.; Lee, E.S.; Reisler, T.; Price, L.A.; Milner, S.M.; Granick, M. Fat Grafting and Adipose-Derived Regenerative Cells in Burn Wound Healing and Scarring. Plast. Reconstr. Surg. 2016, 137, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.-R.; Xu, Y.; Guo, S.-L.; Wang, Y.; Zhu, F.; Permatasari, F.; Wu, D.; Yin, Z.-Q.; Luo, D. The Effect of Conditioned Media of Adipose-Derived Stem Cells on Wound Healing after Ablative Fractional Carbon Dioxide Laser Resurfacing. BioMed Res. Int. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Lee, Y.I.; Kim, S.; Kim, J.; Kim, J.; Chung, K.B.; Lee, J.H. Randomized controlled study for the anti-aging effect of human adipocyte-derived mesenchymal stem cell media combined with niacinamide after laser therapy. J. Cosmet. Dermatol. 2021, 20, 1774–1781. [Google Scholar] [CrossRef]
- Verpaele, A.; Tonnard, P.; Jeganathan, C.; Ramaut, L. Nanofat Needling. Plast. Reconstr. Surg. 2019, 143, 1062–1065. [Google Scholar] [CrossRef] [PubMed]
- Evans, G.R.D.; Widgerow, A.D. Stem cells and tissue engineering in plastic surgery: An update. Plast. Aesthetic Res. 2020, 2020. [Google Scholar] [CrossRef]
- Fafián-Labora, J.A.; Morente-López, M.; Arufe, M.C. Effect of aging on behaviour of mesenchymal stem cells. World J. Stem Cells 2019, 11, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Yuan, R.; Yang, X.; Qi, Z. Adipose tissue, aging, and metabolism. Curr. Opin. Endocr. Metab. Res. 2019, 5, 11–20. [Google Scholar] [CrossRef]
- Cárdenas-Camarena, L.; Gerardo, L.-P.A.; Durán, H.; Bayter-Marin, J.E. Strategies for Reducing Fatal Complications in Liposuction. Plast. Reconstr. Surg.-Glob. Open 2017, 5, e1539. [Google Scholar] [CrossRef] [PubMed]
- Karina, K.; Rosliana, I.; Rosadi, I.; Schwartz, R.; Sobariah, S.; Afini, I.; Widyastuti, T.; Remelia, M.; Wahyuningsih, K.A.; Pawitan, J.A. Safety of Technique and Procedure of Stromal Vascular Fraction Therapy: From Liposuction to Cell Administration. Sci. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cansancao, A.L.; Condé-Green, A.; David, J.A.; Cansancao, B.; Vidigal, R.A. Use of Tranexamic Acid to Reduce Blood Loss in Liposuction. Plast. Reconstr. Surg. 2018, 141, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.A.; Beeson, W.; Rachel, J.D.; Keller, G.S.; Hanke, C.W.; Waibel, J.; Leavitt, M.; Sacopulos, M. Mesothelial Stem Cells and Stromal Vascular Fraction for Skin Rejuvenation. Facial Plast. Surg. Clin. North Am. 2018, 26, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jin, S.; He, Y.; Zhang, X.; Han, X.; Li, F. Comparison of Microfat, Nanofat and Extracellular Matrix/Stromal Vascular Fraction Gel for Skin Rejuvenation: Basic Research and Clinical Applications. Aesthetic Surg. J. 2021, sjab033. [Google Scholar] [CrossRef]
- Wang, J.V.; Schoenberg, E.; Saedi, N.; Ibrahim, O. Platelet-rich Plasma, Collagen Peptides, and Stem Cells for Cutaneous Rejuvenation. J Clin Aesthet Dermatol 2020, 13, 44–49. [Google Scholar] [PubMed]
- Rubio, D.; Garcia-Castro, J.; Martín, M.C.; De La Fuente, R.; Cigudosa, J.C.; Lloyd, A.C.; Bernad, A. Spontaneous Human Adult Stem Cell Transformation. Cancer Res. 2005, 65, 3035–3039. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Shimono, Y.; Funakoshi, Y.; Imamura, Y.; Toyoda, M.; Kiyota, N.; Minami, H. Adipose-derived stem cells enhance human breast cancer growth and cancer stem cell-like properties through adipsin. Oncogene 2019, 38, 767–779. [Google Scholar] [CrossRef]
- Klinger, M.; Losurdo, A.; Lisa, A.V.E.; Morenghi, E.; Vinci, V.; Corsi, F.; Albasini, S.; Leonardi, M.C.; Jereczek-Fossa, B.A.; Veronesi, P.; et al. Safety of autologous fat grafting in breast cancer: A multicenter Italian study among 17 senonetwork breast units autologous fat grafting safety: A multicenter Italian retrospective study. Breast Cancer Res. Treat. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Kothari, A.; Badylak, S.; Di Taranto, G.; Marcasciano, M.; Sordi, S.; Barellini, L.; Torto, F.L.; Tarallo, M.; Gaggelli, I.; et al. Oncological safety of stromal vascular fraction enriched fat grafting in two-stage breast reconstruction after nipple sparing mastectomy: Long-term results of a prospective study. Eur. Rev. Med Pharmacol. Sci. 2018, 22, 4768–4777. [Google Scholar]
- Ohashi, M. Fat Grafting for Facial Rejuvenation with Cryopreserved Fat Grafts. Clin. Plast. Surg. 2019, 47, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Edelson, R.L.; Sumpio, B.; Kwei, S.; Narayan, D. A Unique Case of Allogeneic Fat Grafting Between Brothers. Plast. Reconstr. Surg.-Glob. Open 2016, 4, e1032. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).