Abstract
Histone deacetylases (HDACs) have become one of the main targets in cancer therapy due to their involvement in various biological processes, including gene regulation, cell proliferation, and differentiation. Microtubules, as key elements of the cell cytoskeleton, also represent important therapeutic targets in anticancer drugs research. These proteins are involved in diverse cellular functions, especially mitosis, cell signaling, and intracellular trafficking. With the emergence of multi-target therapy during the last decades, the combination of HDAC and tubulin inhibitors has been envisioned as a practical approach for optimizing the therapeutic efficacy of antitumor molecules. HDAC/tubulin dual-targeting inhibitors offer the advantages of the synergistic action of both compounds, along with a significant decrease in their respective toxicities and drug resistance. This review will detail the major recent advancements in the development of HDAC/tubulin dual inhibitors over the last decade and their impact on anticancer drugs discovery.
1. Introduction
Cancer is one of the leading causes of death worldwide, with 19.3 million new cases and around 10 million deaths reported in 2020 [1]. This complex disease involves various biological factors, such as epigenetic alterations, which can modulate gene expression. Among these, the significant variation in the reversible acetylation−deacetylation of histones represents a key hallmark of carcinogenesis [2].
Histone deacetylases (HDACs) are a class of epigenetic metalloenzymes capable of removing acetyl groups from N-acetylated lysine residues on histone and non-histone proteins. HDACs mainly target histones to tightly wrap DNA. They can regulate gene expression and protein activity by varying DNA accessibility [3]. In contrast, their dysregulation can lead to the alteration of oncogenes or tumor suppressor gene transcription, inducing carcinogenic events [4]. Hence, HDAC inhibitors (HDACis) have been developed during the last decades as major molecules for cancer treatment [5]. These compounds can inhibit the proliferation and differentiation of cancer cells and promote apoptosis. Their structures are established according to the X-ray crystallographic data of various HDAC isoforms [6,7], and consist of three main features to display effective inhibition: (1) a zinc-binding group (ZBG) chelating the Zn(II) ion in the active site, (2) a linker motif filling the hydrophobic tubular pocket, and (3) a cap unit interacting with the amino acids localized at the edge of the tube-like pocket (Figure 1).
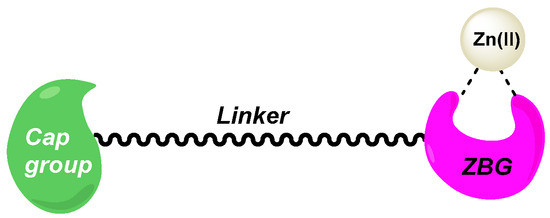
Figure 1.
Designed structure of HDACis.
HDACis are promising antitumor agents, with four US FDA (Food and Drug Administration)-approved HDACis (vorinostat, Panobinostat, Belinostat and Romidepsin) [8]. Clinical trials have confirmed their high efficiency in treating solid and liquid tumors [9]. Recently, Tucinidostat was approved as an HDACi in China for relapsed and refractory peripheral T-cell lymphoma [10] (Figure 2). Nonetheless, the main drawback of these HDACis is their low HDAC isoform specificity, causing significant side effects, e. g. fatigue, nausea, thrombocytopenia, cardiotoxicity, or hematologic toxicity [11]. Furthermore, drug resistance to HDACis could also be noted, probably due to the activation of signal transduction pathways, namely Akt (protein kinase B) or CDK (cyclin-dependent protein kinase) signaling processes [12]. Thus, targeting specific HDAC isoforms remains a challenge in the development of novel anticancer therapies [13]. In fact, the overexpression of various types of HDACs could be correlated to a particular type of solid or hematologic cancer. For instance, the high transcription of HDACs 8 and 10 indicates an advanced state of neuroblastoma [14,15]. High levels of HDACs 2, 5, and 9 are present in medulloblastoma, which is associated with poor prognosis [16,17]. Lung cancer cells exhibit aberrant expression of HDACs 1, 2, 3, 5, and 10 [18,19,20]. The unusual regulation of HDACs 1, 2, 3, 4, and 10 is a hallmark of gastric cancers [21,22,23]. Liver cancer cell lines develop increased levels of HDACs 1, 2, 3, and 5 [24,25,26,27,28], and a downregulation of HDAC 6 [29]. The generation of human pancreatic cancer cells is influenced by the concomitant increase in HDACs 2, 6, and 7 [30,31,32]. HDACs 1, 2, 3, 5, and 7 are highly expressed in colorectal cancer [33,34]. The upregulation of HDACs 1, 2, 3, and 6 has been noted in breast cancer [35,36]. Cervical cancer is characterized by a low concentration of HDAC 10 [37]. HDACs 1, 2, and 3 are overexpressed in ovarian [38], prostate [39], and bladder cancers [40]. Concerning hemotological tumors, some are less sensitive to HDAC isoforms, compared to solid ones. A high rate of HDACs 1–9 has been observed in acute lymphocytic leukemia [41]. Analogously, HDACs 1, 3, 6, 7, 9, and 10 are overexpressed in advanced-stage chronic lymphocytic leukemia [42]. Diffuse large B-cell lymphoma has high HDACs 1, 2, 3, and 6 levels [43,44,45,46]. In contrast, acute myeloid leukemia is more specifically defined by a high quantity of HDAC 6 and a low rate of HDAC 5 [47]. Highly expressed HDAC 2 is a prognosis biomarker for aggressive cutaneous T-cell lymphoma [48]. Likewise, HDACs 1, 2, and 3 are upregulated in Hodgkin’s lymphoma cells [49]. The high expression of HDAC 1 could also be a marker of poor prognosis in myeloma [50].
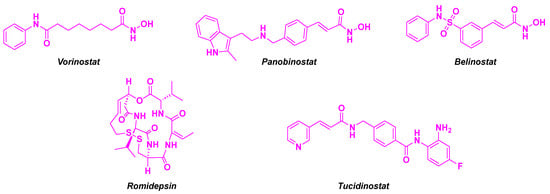
Figure 2.
Approved HDACis.
To circumvent all these issues, research efforts on dual-targeting HDACis have emerged. This strategy involves the association of different pharmacophores in a single drug, which can interact with multiple cancer targets. These hybrid molecules present several advantages over drug combinations, such as more predictable pharmacodynamic and pharmacokinetic properties, lower toxicity, and higher efficiency in advanced-stage diseases owing to their synergistic effects [51,52]. Several promising biological targets have been selected as complementary inhibitors of HDACis [12]. As main examples, dual PTK (Protein Kinase) and HDAC inhibitors have been widely developed, namely EGFR (Epidermal Growth Factor Receptor)-HDAC hybrid inhibitors efficient in liver, pancreatic, breast, and lung human cancer cell lines [53], or hybrid BCR/ABL-HDAC inhibitors displaying promising cytotoxicities against various leukemic cell lines [54]. Given the strong implication of JAK (Janus Kinase) in the STAT (Signal Transducers and Activators of Transcription) proteins pathways in cell functions, JAK-HDAC dual inhibitory agents have also been validated against solid and hematologic cancer cells [55]. CDK (Cyclin-Dependent Kinases) inhibitors can also be coupled with HDACi, affording dual hybrids with nanomolar activities against HDAC1 and CDK2 [56]. Given the major involvement of PI3K (Phosphatidylinositol 3-Kinase) pathway alterations in cancer processes [57], dual inhibitors targeting PI3K and HDAC were developed. These hybrids exhibited significant antiproliferative activities in diverse cancer cell lines and favorable pharmacokinetic parameters in vivo [58]. As topoisomerase inhibitors were widely used in cancer therapy [59], their combination with HDACi paved the way for potent dual hybrids impeding cancer cell proliferation [60]. The key role of NAMPT (NicotinAMide PhosphoribosylTransferase) in the synthesis of NAD+, which regulates the cell redox process, led to the conception of dual NAMPT/HDAC inhibitors. These molecules aimed to hamper cancer cell growth, by diminishing the concentration of NAD+ and favoring apoptosis [61]. Nanomolar values were found for the inhibitory activities toward NAMPT and HDAC 1 for such compounds [61]. The analogous properties of the BET (Bromodomain and Extra Terminal) family and HDACs, such as their action on epigenetic mechanisms, allow access to dual hybrids with subnanomolar cytotoxic and enzymatic activities [62]. The association between PARP (poly (ADP-ribose) polymerase) inhibitors and HDACi was also envisioned, with significant outcomes against cisplatin-resistant SGC7901 cells [63]. Hsp90 (Heat shock protein 90), which participates in the folding of oncoproteins, is a consistent co-target with HDAC in the creation of dual hybrids [64]. In fact, their close interactions in biological pathways, namely between HDAC 6 and Hsp 90 [65], led to the development of dual Hsp90/HDAC inhibitors, with nanomolar inhibitory activities for the corresponding key targets [66].
Among the principal advances made in the field of HDACis with dual-targeting properties, we will particularly focus on dual inhibitors targeting HDACs and tubulin as potential anticancer agents.
Microtubules are also a major therapeutic target in the development of new anticancer drugs. They are composed of dynamic cytoskeletal proteins made of α/β-tubulin heterodimers. These structural cell elements play pivotal roles in the maintenance of cell architecture, migration, and proliferation in eukaryotic cells [67]. Tubulin inhibitors, via their interaction with binding sites (laulimalide, taxane/epothilone, vinca alkaloid, and colchicine sites), would stop the cell cycle and interrupt the mitosis process [68]. Thus, the cell apoptosis pathway could be activated. Through this approach, these tubulin targeting agents demonstrated their ability to kill tumor cells. Among the known microtubule-binding sites, the colchicine site, localized at the interface of α- and β-tubulin subunits [69], is of particular interest with the discovery of a plethora of colchicine-binding site inhibitors (CBSIs) [70]. The CSBIs scaffolds were guided by the colchicine moiety, which was co-crystallized with α/β-tubulin heterodimers [69]. One of the principal elements of these molecules is a trimethoxyphenyl group, which forms H bonds with Cysteine-241 of the β-tubulin binding site [71]. The other main attribute of CSBIs is a tropolone-derived nucleus that interacts with Threonine-179 and Valine-181 of α-tubulin [72] (Figure 3). Moreover, their pharmacochemical properties (complementary angiogenesis inhibition, good pharmacokinetic parameters, and overcoming multidrug resistance) favor their important rise in the development of novel anticancer compounds [73,74,75].

Figure 3.
Designed structure of CSBIs.
In view of the effective properties of HDACis and tubulin inhibitors, combination therapy was envisioned with these compounds. In fact, synergistic effects were observed by associating HDACis with tubulin inhibitors (e.g., paclitaxel, vincristine) [76,77]. Therefore, the construction of molecular hybrid HDAC/tubulin inhibitors is the next logical step in the optimization of new-generation anticancer drugs. With all the benefits of the multi-targeting compounds, numerous research groups have studied the HDAC/tubulin dual inhibitors. These dual-targeted molecules would heighten the synergistic antitumor effect observed in the combination therapy, leading to the suppression of tumor cell proliferation and the induction of the apoptosis process in cancer cells.
Given the wide chemical diversity of these biologically active compounds, this review will mainly classify the main dual molecules developed over the last few decades according to the structural scaffolds of the tubulin inhibitors.
2. Combretastatin-A4 Motif and Related Structures
Combretastatin-A4 (CA-4) is a natural product isolated from Combretum caffrum, an African willow tree, by Pettit et al. [78] (Figure 4). This molecule is identified as a potent anticancer agent, which inhibits tubulin polymerization by interacting with the colchicine site (IC50 value of 2.62 µM for in vitro tubulin polymerization inhibition) [79]. In addition to its selective action on tubulin, CA-4 possesses a nanomolar-scale cytotoxicity against a broad range of human cancer cells, including multidrug-resistant cells [80]. Despite such promising properties, CA-4 shows high instability of the Z-stilbene structure, which could be isomerized during the metabolization process to form the less active E isomer. Thus, the development of CA-4 derivatives and related structural analogs was envisioned over the last decades in various medicinal chemistry research groups [81]. By combining these compounds with HDACis, significant synergistic effects were found for these newly designed antitumor agents.

Figure 4.
Structure of CA-4.
2.1. Chalcone Derivatives
Among the CA-4 related compounds, chalcones constitute one of the main groups. In this manner, Luan et al. described chalcone derivatives as HDAC/tubulin dual-targeting inhibitors. Inspired by the FDA-approved HDACi (see Figure 1) and CA-4 structure, the researchers designed a chalcone platform functionalized with the key methoxy substituents of CA-4, ensuring tubulin inhibition. This part was connected to a hydroxamic acid skeleton, providing HDAC inhibitory activity (Scheme 1). These molecules were constructed through a multi-step synthesis, including a condensation reaction and alkylation step. The HDAC inhibition rates of the prepared hybrid compounds revealed that compounds 1a and 1b exhibited the highest inhibitory activities (87% and 92% inhibition at a concentration of 1 µM, respectively). This result was confirmed in HeLa cell nuclear extracts, with IC50 values ranging from 71.5 to 132.6 nM for the HDACs. A thorough study showed that 1a and 1b have pan-HDAC inhibitory properties, especially with HDAC 1, 6, and 8. After confirming the viability of these molecules as HDACis, their cytotoxicity was evaluated in three cancer cell lines (A549, HeLa, and SGC-7901). Despite its modest HDAC inhibitory activity, molecule 1c was the most active compound with IC50 values of up to 550 nM for A549 cells. Compounds 1a, 1b, and 1c were then subjected to tubulin polymerization inhibition (TPI) assay, with the best inhibition value observed for 1b (IC50 = 5.0 µM). Further exploration of the antiproliferative properties of 1a, 1b, and 1c confirmed their activities against eight human tumor cell lines (LoVo, HCT-116, NCI-H460, NCI-H226, MCF-7, MDA-MB-231, MGC80-3, and BGC-823), especially for 1c (IC50 = 2.40–7.54 µM). More precisely, by analyzing the A549 cells apoptosis, 1c was able to stop the cell cycle at the G2/M phase at a 10 µM dose. Finally, 1c also exhibited an anti-migratory effect on A549 cells at a concentration of 5 or 10 µM, as determined by a wound healing assay [82].
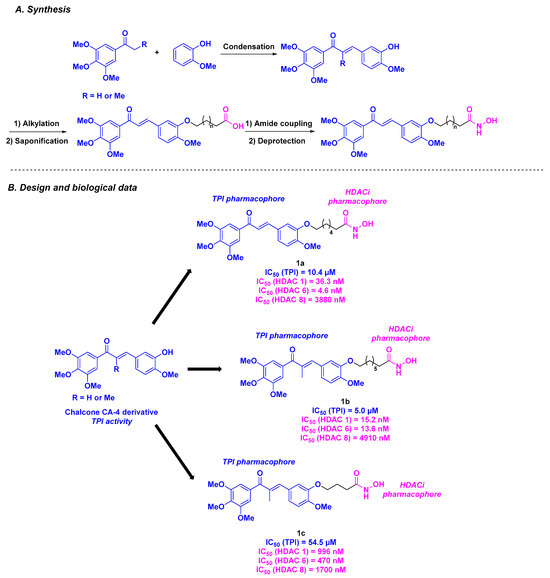
Scheme 1.
HDAC/tubulin inhibitors designed by Luan et al. [82].
Mourad and his colleagues employed analogous scaffolds for their HDAC/tubulin inhibitor hybrids [83]. The group conceived their dual compounds by mixing a chalcone skeleton, which has the same tubulin inhibitory properties as CA-4 [84], with a phthalimide group presenting interesting biological antitumor activities [85]. To ensure HDACi efficiency, a benzamide group was used as an Entinostat analog (Scheme 2). Their chemical synthetic route consists of the condensation of phthalide with potassium phthalimide, followed by addition-elimination and aldolization reactions. In vitro cytotoxicity screening of the prepared molecules revealed that 2a, 2b, and 2c exhibited higher efficacy than CA-4, with IC50 values ranging from 1.62 to 2.21 µM against MCF-7 and Hep G2 cells. The TPI capacities of 2a, 2b, and 2c shed light on compounds 2b and 2c, which offered the best IC50 values of 3.27 and 2.32 µM, respectively. It is worth mentioning that these molecules were selected as hits as their TPI values are comparable to that of CA-4 (IC50 = 2.62 µM). Notably, this outcome was in line with the HDAC inhibitory activity of 2b and 2c, which had a significant specificity for HDAC 1 and 2. Given the probable involvement of 2b and 2c in the cell cycle due to their interaction with tubulin, in vitro DNA flow cytometry was performed. Compounds 2b and 2c appeared to trigger apoptosis by stopping cell growth at the G2/M phase. Calculations of energy binding scores confirmed the strong interaction between 2b and 2c in the colchicine-binding site of tubulin, mainly due to the hydrogen bonds. Molecular docking investigations of the HDAC active site gave the same conclusions as those noted for the tubulin protein.
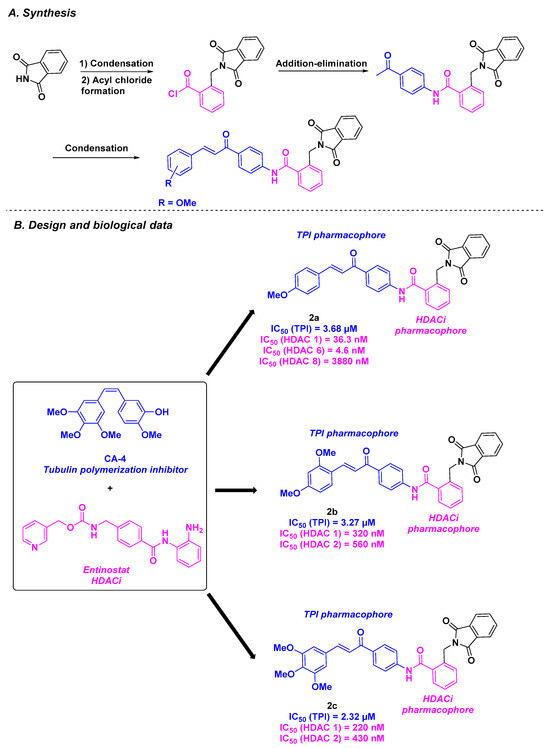
Scheme 2.
HDAC/tubulin inhibitors conceived by Mourad and his colleagues [83].
2.2. Stilbene Derivatives
Inspired by the isoCA-4 structure, a stilbene isomer of CA-4 proposed by the Alami group [86], the Hamze team conceptualized novel dual-targeting inhibitors of tubulin and HDAC by associating the isoCA-4 moiety with the pharmacophore of belinostat as chimeric HDAC/tubulin inhibitors (Scheme 3). Their chemical synthesis involved a palladium-catalyzed Barluenga-Valdés cross-coupling between an aryl halide and the corresponding N-tosylhydrazone. Functionalization with the HDACi pharmacophore (hydroxamic acid) was then realized using different strategies, including alkylation under standard conditions and Sonogashira or Heck couplings. After conducting in vitro antiproliferative assays on HCT-116 cancer cells, the authors validated the importance of the hydroxamic acid group in cytotoxic activity. Compounds with an alkene linker between the stilbene scaffold and hydroxamic acid skeleton exhibited the best GI50 values (1.5 nM for 3a and 8 nM for 3b). The TPI characteristics of the dual compounds were next determined, with promising IC50 values of 1.6 and 2.1 µM for 3a and 3b, respectively. The HDAC inhibitory activity of 3a and 3b was then determined, with a strong selectivity for HDAC 8. The cytotoxicity of these molecules was also verified in nine cancer cell lines (A549, K562, K562R, PC3, U87-MG, MCF7, BXPC3, MiaPaca2, and HT29), with low GI50 values obtained for 3a, ranging from 0.4 to 5.1 nM. Additional studies by incubating 3a at a concentration of 5 or 10 nM with HCT-116, K562, and BL2 cells demonstrated the ability of 3a to stop the cell cycle at the G2/M phase and induce cell death. Finally, the viability of 3a was corroborated by its safety profile on quiescent peripheral blood lymphocytes, with an IC50 value of 7 µM [87].
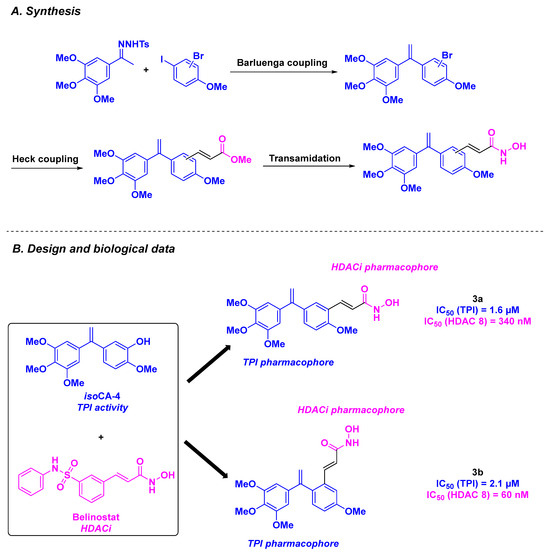
Scheme 3.
HDAC/tubulin dual inhibitors elaborated by the Hamze group [87].
In 2022, the same team proposed a second generation of HDAC/tubulin chimeric inhibitors, by replacing the trimethoxybenzene ring of CA-4 with quinoline or quinazoline cores [88] (Scheme 4). The designed structures were prepared through Buchwald-Hartwig coupling or nucleophilic substitution (SNAr) reaction under acidic conditions, followed by connection to the hydroxamic acid motif via alkylation under basic conditions or through Sonogashira or Heck cross-couplings. The GI50 values in HCT-116 cells for the thirty-one synthesized molecules were determined. Structure activity relationship (SAR) studies confirmed the importance of the hydroxamic acid and the alkene linker for cytotoxicity. Thus, 4a and 4b were recognized as lead compounds, with GI50 values of 0.5 and 0.6 nM, respectively. Encouraged by these outcomes, 4a and 4b were tested against nine additional human cancer cell lines (NCI-N87, K562, K562R, MiaPaca2, SKOV3, A549, MCF7, MDA MB231, and HT-29). Notably, 4a and 4b remained efficient, with average IC50 values of 0.6 and 0.7 nM, respectively. The antiproliferative activities of these second generation of HDAC/tubulin chimeric inhibitors were up to more than 60 times greater than those of the previously described dual molecules, CA-4 and isoCA-4. The HDAC inhibitory profiles of 4a and 4b were next assessed, showing complete inhibition of HDAC 8 and partial effects on HDAC 6 and 11 (60 to 90% inhibition) at 1 µM. The in vitro TPI assay was performed for 4a, with an IC50 value of 20 nM. Given the crucial role of the microtubule network in cell division, additional flow cytometry analyses revealed cell cycle arrest at the G2/M phase at a concentration of 1 nM of 4a and 4b. The authors also demonstrated the involvement of 4a and 4b in mitochondrial dysfunction at 2 nM. The metabolic stabilities of 4a and 4b were also explored in rat and human microsomes. Compound 4a exhibited a longer in vitro half-life, likely due to the presence of its quinoline skeleton and hydroxamic acid scaffold. Moreover, the physicochemical properties of 4a and 4b were evaluated, with a high water solubility of 68.7 and 65 µg/mL, respectively.
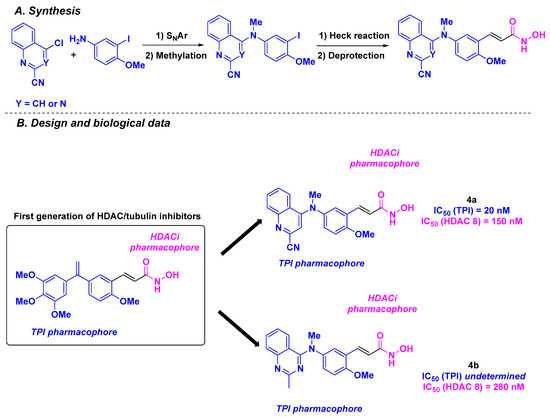
Scheme 4.
Second generation of HDAC/tubulin dual inhibitors elaborated by the Hamze group [88].
The applicability of 4a was further validated using an in vivo allograft mouse model. A significant tumor decrease was observed at 0.25 mg/kg and 0.50 mg/kg doses of 4a injected intratumorally three times per week for over two weeks, without any recidivism after 91 days of treatment. It should be noted that 4a was solubilized in a PEG vehicle for these assays. No toxicity was observed after the intratumoral injection of 4a at the above-mentioned doses.
In 2021, Yao, Yang, Duan and coworkers studied new tubulin/HDAC dual-targeting inhibitors with benzophenones-related and stilbene-derived structures exhibiting antitumor properties [89] (Scheme 5). These compounds were designed through the fusion of CA-4 derivatives (benzophenone or stilbene groups) with HDACi pharmacophores (hydroxamic acid or benzamide moieties). The construction of these dual molecules was based on an alkylation step or palladium-catalyzed Heck cross-coupling, followed by amide bond formation. The generated products were subjected to cytotoxicity assays using six cancer cell lines (MGC-803, MCF-7, U937, A549, HepG2, and HeLa). SAR analysis revealed that stilbene-derived compounds exhibited higher antiproliferative activity than benzophenone analogs. The presence of an alkene linker and benzamide functional group also favored cytotoxicity. Compound 5 was selected as the lead compound, with an IC50 ranging from 16 to 305 nM. The specific activity of 5 toward cancer cell lines was confirmed by testing on non-tumorigenic cells (293T, THP-1-derived macrophages, and L02 cell lines), with no observed toxicity (IC50 values up to 556 nM). The biological analyses of 5 were pursued with the evaluation of its tubulin polymerization and HDAC inhibitory capacity. Compound 5 was found to be a potent tubulin/HDAC inhibitor, with an IC50 value of 1.2 µM for TPI activity, and a pan-HDAC selectivity, especially for HDAC 3, 4, 5, 7, and 9. The TPI phenomenon was also visualized using a confocal microscope by disrupting the cell microtubule network. To explain the high affinity of 5 for its expected targets, molecular docking was performed to detect the binding between 5 and the colchicine site of the tubulin via hydrogen bonds (cf. Figure 3). Similar interactions were observed between 5 and HDAC 7. By deepening the understanding of the mechanism of action of 5, the research group discovered that 5 induced cell cycle arrest at the G2/M phase by upregulating Cyclin B1 and diminishing p21 and p-CDC2 expression. Compound 5 also contributed to cell death by decreasing the concentration Caspase-3, Caspase-7, Caspase-9 and PARP (Poly(ADP-Ribose) Polymerase) and increasing the levels of cleaved corresponding proteins. In addition to this pro-apoptotic pathway, compound 5 altered mitochondria membrane potential and promoted the generation of reactive oxygen species (ROS). A wound healing assay using human umbilical vein endothelial cells (HUVECs) revealed that 5 inhibited cell migration, invasion, and angiogenesis.
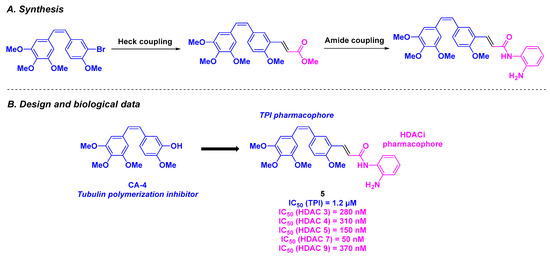
Scheme 5.
HDAC/tubulin dual inhibitor conceived by Yao, Yang, Duan and coworkers [89].
Finally, the effectiveness of 5 was tested in zebrafish embryos, where angiogenesis was impeded at 0.6 µM of 5. Using xenograft zebrafish models, a significant tumor reduction was observed after incubation with 5 at a concentration of 100 nM.
Similarly, the CA-4 derivatives developed by Chen, Xu, and their colleagues featured a stilbene skeleton bearing a quinoline or pyridine motif to enhance tubulin polymerization inhibitory activity. To fulfill the dual properties of these molecules, the research team incorporated a hydroxamic acid structure into the stilbene scaffold as an HDACi [90] (Scheme 6). The construction of the stilbene moiety relied on a Barluenga-Valdés cross-coupling. The hydroxamic acid group was installed via alkylation or a Wittig reaction, followed by amide bond formation. By evaluating the antiproliferative activities of the synthesized dual compounds, compound 6 exhibited the best in vitro IC50 value against K562 cells (3 nM). Further investigations confirmed the superior cytotoxicity of 6 in five cancer cell lines (MCF-7, MDA-MB-231, A549, B16F10, and A2780), with IC50 values ranging from 5 to 36 nM. In contrast, 6 demonstrated low cytotoxicity in normal human lung cells (IC50 (HFL-1) = 230 nM). The HDAC inhibitory character of 6 was validated, with pan-HDAC selectivity, especially for HDAC 1, 2, and 6. The TPI effect of compound 6 was also established in vitro, with a measured IC50 value of 3.84 µM. Competitive inhibition experiments with tritium-labeled colchicine indicated that 6 acts by binding to the colchicine site of tubulin. The dual characteristic of 6 was confirmed in K562 cells, which showed disruption of the microtubule network and accumulation of acetylated α-tubulin. In cellulo assays showed that 6 was able to stop the cell cycle at G2 phase and induce the apoptosis of K562 cells by increasing the concentration of pro-apoptotic proteins Bad and Bax. Compound 6 also exhibited antivascular activity, suppressing HUVECs migration at 8 nM. Finally, an in vivo antitumor assay was performed with 6, using a mouse liver cancer allograft model. Similar to the in vitro results, 6 exhibited excellent efficacy (tumor growth inhibition (TGI) = 73.12% at 20 mg/kg daily for 17 days, by intravenous injection in vehicles containing DMSO (10%), Tween 80 (2%), and saline (88%)), without loss in body weight or significant toxicity in vital organs.
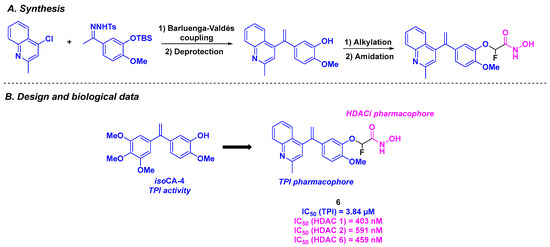
Scheme 6.
HDAC/tubulin dual inhibitor proposed by Chen, Xu and their colleagues [90].
2.3. Amine Derivatives
In 2022, Song, Zhang, and coworkers combined the CA-4-derived skeleton with the key motif of Entinostat, an HDACi, to construct SY-65, a novel aromatic amide scaffold targeting HDAC1 and tubulin (Scheme 7). The synthesis of SY-65 involved a three-step process consisting of two amide couplings and a saponification reaction. This compound exhibited activity against several human cancer cell lines (MGC-803, HGC-27, SGC-7901, HCT-116, and KYSE450), with IC50 values ranging from 39 to 254 nM. With these promising results, SY-65 was then studied as a tubulin polymerization inhibitor in MGC-803 and HGC-27 cells, showing an IC50 of 3.64 µM. Complementary N,N′-ethylene-bis(iodoacetamide) (EBI) competition and cellular thermal shift assays confirmed that SY-65 binds to the colchicine site on β-tubulin. By evaluating the activities of SY-65 against HDACs, the authors reported the inhibition of HDAC1 (IC50 = 525 nM). This effect was also observed in cellulo with MGC-803 and HGC-27 cell lines. In addition, SY-65 exhibited apoptotic properties in MGC-803 and HGC-27 cells, including the reduction of Bcl-xL, Bcl-2, and c-IAP1 levels. This compound also arrested cell proliferation at the G2/M phase in these cell lines, as evidenced by the downregulation of the cell cycle-related proteins Wee1, cdc2, and phosphorylated cdc2. An in vivo nude mouse xenograft model bearing MGC-803 cells confirmed all the biological features of SY-65, especially the inhibition of tumor growth after 14- or 21-day treatment through daily intraperitoneal 30 mg/kg injection [91].
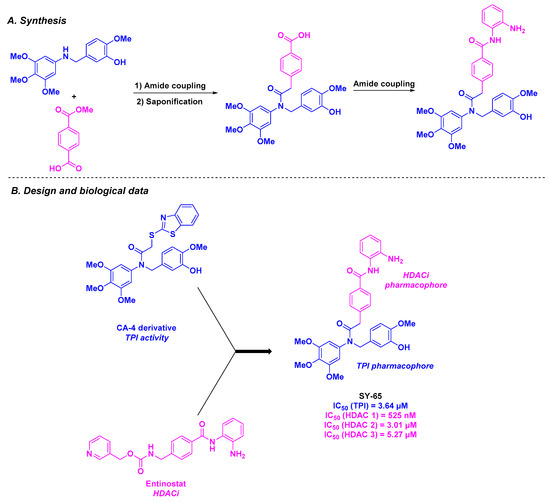
Scheme 7.
HDAC/tubulin inhibitor SY-65 prepared by Song, Zhang and coworkers [91].
2.4. Oxazole Derivatives
In 2019, Höpfner et al. created novel HDAC/tubulin inhibitors by attaching hydroxamic acids to oxazole-bridged CA-4 derivatives (Scheme 8). These bent structures were prepared via a three-step synthesis, with the Von Leusen reaction under basic conditions as the key step. The biological evaluation of these hybrid compounds revealed that bromo derivatives 7a, 7b, and 7c exhibited the highest average antiproliferative activities for six cancer cell lines (518A2, 518A2, HT-29, DLD-1, HCT-116, KB-V1Vbl, MCF-7Topo) with IC50 values ranging from 0.11 to 14.2 µM. It should be noted that 7a–c were also active against Ea.Hy926 cells (endothelial hybrid cells), indicating their potential antivascular capacity as CA-4. In addition, 7a–c showed strong selectivity toward cancer cells, in contrast to their lack of major cytotoxicity in non-malignant HDFa dermal fibroblasts.
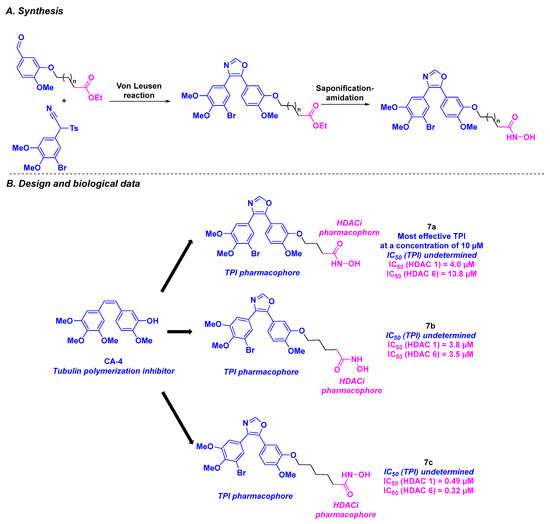
Scheme 8.
HDAC/tubulin inhibitors prepared by Höpfner et al. [92].
Tubulin polymerization assays were then performed with 7a–c, revealing 7a as the most effective tubulin polymerization inhibitor at a concentration of 10 µM. Similar results were observed in 518A2 melanoma cells, with disruption of the microtubule network at 0.5 µM of 7a. The HDAC inhibitory properties of 7a–c were studied next. Although 7a was the most effective compound against cancer cells and microtubules, only moderate HDAC 1 and HDAC 6 inhibition was observed. In contrast, 7c exhibited high specificity for HDAC 1 and HDAC 6, with IC50 values of 0.49 and 0.32 µM, respectively. These data were validated by molecular docking with stronger affinities for HDAC 1 and HDAC 6 for molecules bearing long alkyl linkers (7c vs. 7a). Western blot analyses highlighted that 7c could also impede the acetylation of tubulin, without disturbing the microtubule skeleton, due to its concomitant HDAC 6 inhibitory activity. The FACS (Fluorescence-activated cell sorting) data on 518A2 melanoma cells corroborated the effect of 7a–c on the cell cycle: 7a and 7b led to the interruption of cell growth in the G2/M phase, while 7c caused arrest in the G1 phase. The proof of concept was then verified by determining the toxicity of 7a in an in vivo nude mouse model, with a formulation of 7a containing 10% Tween 80, 10% ethanol, and 80% saline. The treatment was well tolerated at high doses (up to 200 mg/kg orally administered once for two weeks) without causing weight loss [92].
2.5. 1,4-Diarylazetidin-2-one Derivatives
Based on the chiral 1,4-diarylazetidin-2-one structure, a constrained analog of CA-4, capable of inhibiting tubulin by binding the colchicine site [93], and the vorinostat skeleton, Ding, Wang, and coworkers designed an original skeleton as a chimeric inhibitor [94] (Scheme 9). The synthesis of these molecules began with benzylic protection and hydroboration. The alcohol intermediate was subsequently condensed with the corresponding carboxylic acid to afford the desired product. In vitro cytotoxicity studies against four tumor cell lines (BE-(2)-C, A549, U87MG, and HCT-116) revealed that compounds 8a and 8b exhibited the best antiproliferative activities, with IC50 values ranging from 16 to 56 nM. Further exploration of the HDAC isoform inhibitory capacity demonstrated a high selectivity toward HDAC 1 and HDAC 8 for compounds 8a and 8b. It should be noted that 8b exhibited better IC50 values against HDAC 1 and HDAC 8 compared to 8a. The HDAC inhibitory property of 8b was also confirmed in cellulo through western blot analyses, by the detection of non-deacylated substrates of HDAC 1 and HDAC 8. Compound 8b also acted as a proven TPI, with an IC50 value of 5.4 µM for in vitro tubulin polymerization inhibition. Molecular docking supported these results, with a tight fixation of 8b via hydrophobic interactions between the methoxyphenyl groups and the apolar binding site of tubulin (cf. Figure 3). The interaction between 8b and HDAC 8 was ascertained by the chelation of the hydroxamic moiety with Zn2+ localized in the active pocket and the hydrogen bonds with key amino acids (Tyr341, His141, and His142). Additionally, 8b could inhibit the cancer cell colony formation at a concentration of 100 nM. A thorough inspection of 8b’s biological pathway suggested that 8b induced G2/M mitosis arrest and caused the cell apoptosis by activating the cleavage of pro-apoptotic proteins PARP-1 and Bax. The microsomal stability of 8b was also evaluated, revealing a long half-life (t1/2 = 68.8 min) and an intrinsic clearance of 18.1 mL/min/kg in human liver microsomes. A high water solubility was also found for 8b, with a value of 102.2 µg/mL. The antitumor activities of 8b were endorsed in human neuroblastoma xenograft models, with a significant decrease in tumor growth at an intraperitoneal dose of 25 mg/kg every three days, leading to a TGI of 67%. Notably, 8b demonstrated synergistic efficiency, with better TGI values than those observed, with paclitaxel and vorinostat used alone. The in vivo low toxicity of 8b was verified in nude mice, with minor weight loss and adverse effects noted.
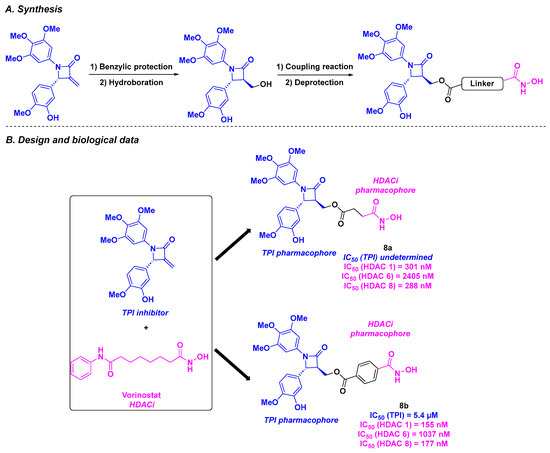
Scheme 9.
HDAC/tubulin inhibitors designed by Ding, Wang and coworkers [94].
2.6. Arylpyridine Derivatives
In 2022, Ding, Wang, and their colleagues proposed a new dual antitumor agent presenting a diarylpyridine scaffold (Scheme 10) [95]. The rational design of these structures relied on the association between pyridine-bridged analogs of CA-4, which exhibited comparable biological properties to CA-4 [96], with hydroxamic acid as the HDACi motif. The synthesis of these frameworks involved the use of commercially available 2,6-dibromopyridine, which underwent two Suzuki-Miyaura cross-couplings to form the corresponding diarylpyridine. The branching of the HDACi pharmacophore was established via an alkylation step or a Heck coupling, followed by amide condensation. The resulting molecules were then subjected to diverse in vitro biological assays. After screening for antiproliferative activities against four cancer cell lines (BE-(2)–C, A549, U87MG, and HCT-116), compound 9 was selected as the hit compound, with IC50 values ranging from 17 to 90 nM. The dual properties of 9 were then examined. On one hand, 9 presented a high specificity for HDAC 8 (IC50 = 117 nM). On the other hand, 9 significantly inhibited tubulin polymerization, with an IC50 value of 2.4 µM. In cellulo experiments showed a disruption of the microtubule network at a concentration of 100 nM of 9. By affecting the microtubule structure, 9 could also arrest cell mitosis at the G2/M phase by upregulating the expression of Cyclin-1 and promoting the hyper-phosphorylation of key mitotic proteins (Bubr-1 and Histone 3). 9 could also participate in the apoptosis process by inducing the formation of cleaved pro-apoptotic markers (PARP-1 and Bax). The evaluation of the metabolic stability in liver microsomes of 9 showed better half-life and clearance values than CA-4 (54.8 min and 22.8 mL/min/kg for 9 vs. 13.6 min and 89.5 mL/min/kg for CA-4). The water solubility of 9 was determined in PBS buffer, with a good value of 138 µg/mL. Finally, compound 9 was tested in vivo using a human neuroblastoma xenograft mouse model. At a unique intraperitoneal dose of 50 mg/kg every three days, 9 had a TGI of 61%, which was higher than those observed for paclitaxel or vorinostat employed alone (N. B.: TGI = 30% at 5 mg/kg of paclitaxel and TGI = 19% at 50 mg/kg of vorinostat). In comparison with the combination of paclitaxel/vorinostat, the TGI of 9 remained higher (61% vs. 44%), demonstrating the synergistic effect of dual compound 9.
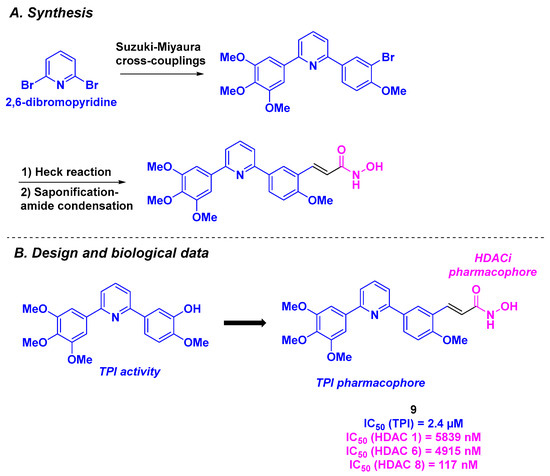
Scheme 10.
HDAC/tubulin inhibitor designed by Ding, Wang and coworkers [95].
More recently, Abdelhafez et al. constructed a library of dual inhibitors by combining the key motifs of CA-4 with the pharmacophore of vorinostat. The linkage between the main functional groups was ensured by a central cyanopyridine core [97] (Scheme 11). The chemical approach for the preparation of these scaffolds consisted of a multicomponent reaction between 4-methoxyacetophenone, 3,4,5-trimethoxybenzaldehyde, and ethyl cyanoacetate. The cyanopyridine derivatives were then subjected to an alkylation reaction and an amide coupling. The antiproliferative assays allowed the identification of the hit compound 10a, which exhibited the best IC50 values for UO-31 (IC50 = 3.52 µM) and T47D cell lines (IC50 = 5.50 µM). The HDAC inhibitory activity of the hybrid compounds was then quantified, highlighting that 10b was more efficient than 10a, especially for HDAC 1 and 6. The TPI assay emphasized that 10a has an IC50 value comparable to that of vinblastine, an anticancer agent with potent TPI capacity (IC50 values of 3.73 and 2.86 µM, respectively). The cytotoxicity of 10a and 10b on WI-38 normal cells was also assessed, showing only minor effects (IC50~30 µM). After obtaining satisfactory results with 10a, its mechanism of action was elucidated through complementary biological studies. Thus, the authors pointed out that 10a favored the cell cycle arrest at the G2/M phase, leading to the apoptosis process. Western blotting showed that the cells were arrested in the G2/M phase by 10a. This outcome demonstrated the clear influence of 10a on cell mitosis arrest via a biological pathway involving HDAC and tubulin.
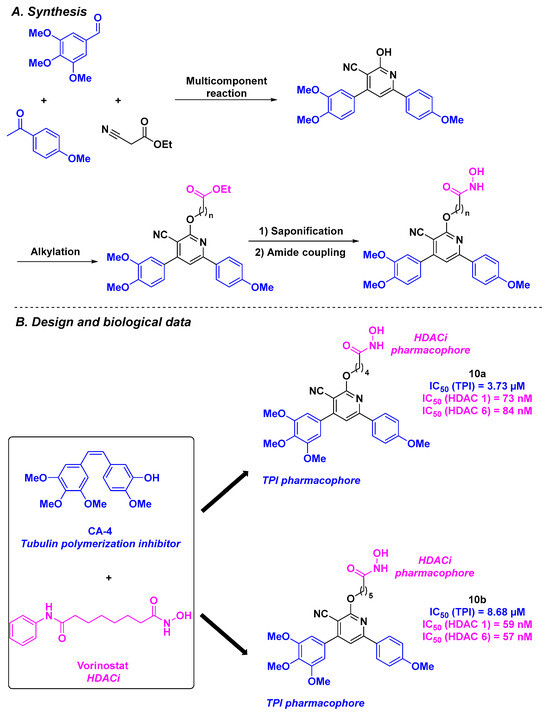
Scheme 11.
HDAC/tubulin inhibitors synthesized by Abdelhafez et al. [97].
3. Colchicine Derivatives
Colchicine, a natural product extracted from the corms of Colchicum autumnale L., is one of the first potent tubulin destabilizers known. Alongside its therapeutic use in familial Mediterranean fever and gout, this compound is also an efficient anticancer agent that inhibits mitosis in diseased cells. Although colchicine efficacy has been well demonstrated in tumor cell lines, its therapeutic index remains narrow. This major drawback results in significant side effects, such as anemia, neutropenia, and bone marrow damage [68].
To overcome these issues, intensive research on colchicine-related molecules has been conducted over the last decade. In this context, tubulin/HDAC dual-targeting inhibitors have been developed.
In 2013, Lu, Chen, and coworkers reported the design and synthesis of tubulin/HDAC inhibitors based on colchicine and hydroxamic acid scaffolds (Scheme 12). The synthetic route for these dual molecules relied on a three-step synthesis, including two amidation reactions mediated by HATU (Hexafluorophosphate Azabenzotriazole Tetramethyl Uronium). The HDAC inhibitory properties of the five elaborated compounds were studied, showing pan-HDAC selectivity for HDAC 1, 3, and 6, especially for compound 11a, with IC50 values ranging from 0.44 to 0.83 µM. By treating BEL-7402 cells with all these tubulin/HDAC inhibitors, the authors noticed that 11b significantly promoted cell cycle arrest at the G2/M phase at a concentration of 1 µM. The viability of the hybrid molecules was validated by exploring their antiproliferative activities against five cancer cell lines (A431, A549, HCT-116, MCF-7, and PC-3). Compound 11b was thus the most active compound, with an IC50 ranging from 0.242 to 4.672 µM [98].
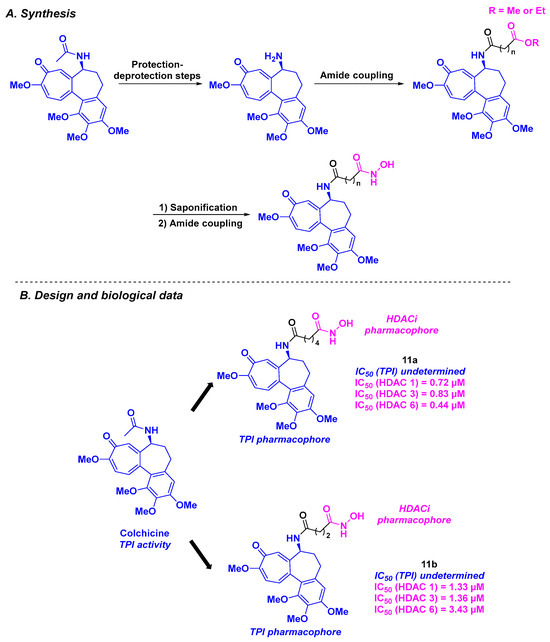
Scheme 12.
HDAC/tubulin inhibitors designed by Lu, Chen, and coworkers [98].
Among the colchicine derivatives employed as HDAC/tubulin inhibitors, the hybrid compounds developed by Fang, Lu, and coworkers merged a colchicine core with TPI activity and a benzamide motif for the HDACi property (Scheme 13). The construction of these hybrid structures was based on amide coupling using HATU as the coupling agent or an alkylation step between the two main chemical entities. The in vitro HDAC inhibitory activity was evaluated for all the synthesized compounds, emphasizing that compound 12a was the most active molecule against HDAC 1, 2, and 3, with IC50 ranging from 0.19 to 1.50 µM. The TPI effect was confirmed for all the prepared hybrid inhibitors, namely 12a and 12b, which possessed activities comparable to free colchicine at a concentration of 10 µM. These outcomes corroborated with the cell cycle analyses, where 12a and 12b were found to block mitosis at the G2/M phase of the cell cycle. The cytotoxicities of 12a and 12b were also explored. Compound 12b exhibited higher antiproliferative activity towards twelve human cancer cell lines (A549, HCT-116, SW620, Hep3B, HeoG2, MHCC97H, SNU-5, SNU-16, MKN-45, PANC-1, and SJSA-1) compared to 12a, with IC50 values ranging from 2 to 106 nM [99].
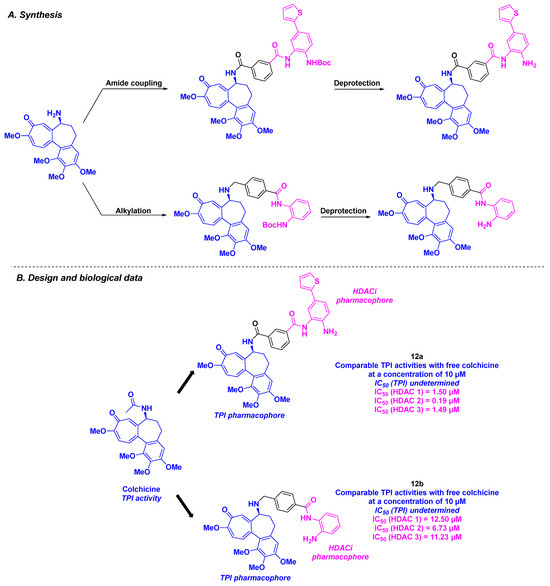
Scheme 13.
HDAC/tubulin inhibitors described by Fang, Lu and coworkers [99].
4. Aminobenzamide Core
Based on their previous outcomes with tubulin inhibitors [100], Xu group reported in 2022 the elaboration of dual molecules displaying an aminobenzamide core capable of inhibiting microtubule polymerization combined with a hydroxamic acid core as an HDACi (Scheme 14). In vitro antiproliferative assays showed that compound 13 exhibited significant IC50 values (26–30 nM) in six cancer cell lines (HepG2, HCT-116, MDA-MB-231, H22, and MCF-7). Satisfactory results were obtained for the in vitro inhibitory potencies against HDACs of compound 13 against HDACs, with an inhibition rate of 93% at 10 µM. Further investigations around the HDAC subtype validated the pan-HDAC-inhibiting property of molecule 13, with a selectivity toward HDAC 1, 2, 3, and 6. Moreover, compound 13 exhibited TPI activity with an IC50 value of 4.06 µM, particularly in the colchicine-binding site. The concomitant role of compound 13 was validated in HepG2 cells, where the HDAC/tubulin inhibitory effects were identified using western blot analysis. Mechanistic insights revealed that molecule 13 could induce cell death through several pathways, such as its ability to stop the cell cycle at the G2/M phase, activate the expression of pro-apoptotic Bad and Bax proteins, induce mitochondrial membrane potential depolarization, or increase ROS levels. Notably, similar to its TPI precursor, compound 13 exhibited significant antivascular activity in HUVECs. Finally, the in vivo antitumor properties of 13 were evaluated using a liver cancer allograft mouse model. A major reduction in tumor weight by 82% (at an intravenous dose of 20 mg/kg per day through a vehicle featuring DMSO (10%), Tween 80 (2%), and saline (88%) for a three-week treatment) was observed in the presence of 13, corroborating all the in vitro efficacies [101].
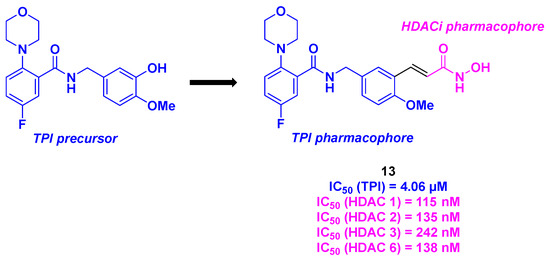
Scheme 14.
HDAC/tubulin hybrid inhibitor bearing an aminobenzamide core developed by the Xu group [101].
5. Amide Derivatives
Zaki and coworkers created a novel series of HDAC/tubulin inhibitors by integrating amide functional groups [102] (Scheme 15). The synthesis of these compounds relied on an oxazole ring-opening with the corresponding aniline or hydrazide. The conceived products were then biologically evaluated, and the best IC50 values against HepG2 cells were observed for 14a and 14b (0.65 and 0.92 µM, respectively). In contrast, low toxicities were reported for 14a and 14b normal liver HL-7022 cell lines (IC50 values of 9.62 and 11.09 µM). Compound 14a exhibited higher HDAC inhibitory activity than 14b, with IC50 values ranging from 47 to 86 nM for HDAC 1 and HDAC 2. A TPI assay was then performed for 14a, yielding an IC50 value of 270 nM. This TPI effect was validated in cellulo, with cell division arrest at the G2/M phase. This phenomenon could lead to HepG2 cell apoptosis after incubation with 14a at a concentration of 0.65 µM for 48 h. This apoptosis pathway was activated through the upregulation of caspases 3 and 7. As caspases 3 and 7 are key markers of apoptosis in mitochondria, the analysis of mitochondrial events was conducted. It was noticed that 14a was able to activate caspases 3 and 7, resulting in mitochondrial apoptosis.
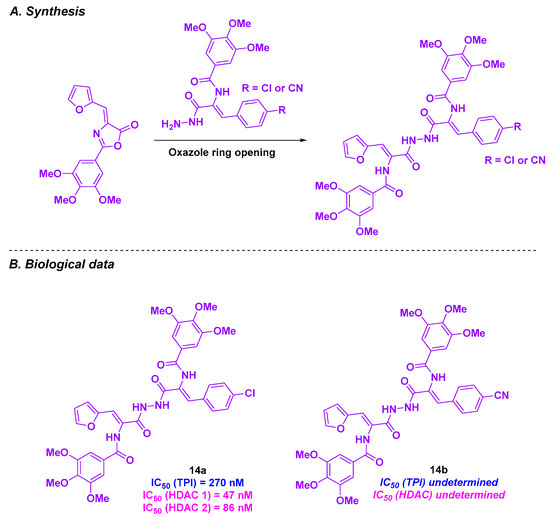
Scheme 15.
HDAC/tubulin hybrid inhibitors created by Zaki and coworkers [102].
6. Quinolone Derivatives
In 2018, Wang et al. conceived a novel series of dual compounds using a quinolone derivative as TPI attached to a hydroxamic acid side chain as HDACi through a triazole linkage (Scheme 16). More precisely, the group designed a TPI pharmacophore based on the interesting properties of levofloxacin derivatives as antitumor agents [103]. The HDAC inhibition activity was first investigated. The prepared dual conjugates presented high affinity for HDAC 1, 2, and 6, especially molecule 15, with IC50 values ranging from 21 to 41 nM. TPI evaluation of the hybrid conjugates showed that all the synthesized compounds were more effective than levofloxacin. As previously reported, compound 15 exhibited the highest efficacy, with an IC50 value of 1.79 µM. After the investigation of the in vitro properties of the dual molecules, the whole cell antiproliferative activity was examined. Again, 15 confirmed its antitumor capacity against five cancer cell lines (A549, HepG2, MCF-7, PC-3, and HeLa), with IC50 values ranging from 0.3 to 4.9 µM. Finally, the viability of HDAC/tubulin inhibitors was established in the healthy epithelial cell line MCF-10A, where negligible toxicity was observed for all these compounds [104].
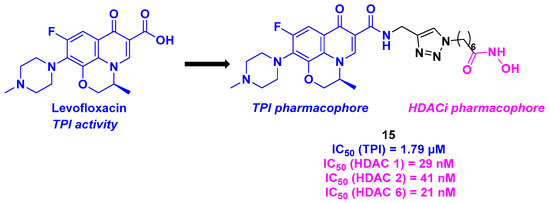
Scheme 16.
Dual HDAC/tubulin inhibitor conceived by Wang et al. [104].
7. Benzofuran Scaffold
Based on their previous work on benzofuran derivatives as inhibitors of tubulin polymerization [105], the Romagnoli group envisaged the development of dual molecules using their lead compound TR187, which binds to the tubulin colchicine site and a hydroxamic acid as an HDACi pharmacophore [106] (Scheme 17). These compounds were synthesized from diversely substituted α-bromo acetophenones, which underwent a cyclization reaction under basic conditions to afford the benzofuran core. The connection with the hydroxamic group was carried out via Heck or Sonogashira coupling. The molecular hybrids thus prepared were subjected to different biological tests. The cytotoxicity studies led to the identification of 16a and 16b as hit molecules, exhibiting promising IC50 values ranging from 0.4 to 23.5 nM against HeLa, MDA-MB-231, A549, HT-29, and MCF-7 cells. Despite these interesting results, the TPI assay demonstrated that 16c and 16d had the best tubulin inhibitory potencies, with IC50 values of 0.56 and 0.42 μM, respectively. Although 16a and 16b were less effective (IC50 values of 0.58 and 0.49 µM, respectively), these compounds remained more potent than CA-4. The ability of these molecules to inhibit HDACs revealed only moderate activity toward HDAC 1, 6, 8, and 10. Compound 16a exhibited pan-HDAC specificity for HDAC 1, 6, and 10, whereas 16b was more selective for HDAC 1, and 16c had a strong affinity for HDAC 6. In contrast, negligible HDAC inhibitory capacity was found for 16d. Based on these outcomes, the authors suggested that the antiproliferative activities of these compounds might mainly result from the TPI effect.
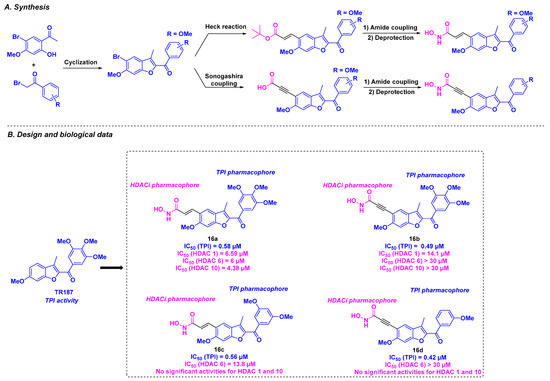
Scheme 17.
Dual HDAC/tubulin inhibitors developed by the Romagnoli group [106].
8. 2-Benzylideneindanone Scaffold
With their long-lasting interest in indanone and benzylideneindanone derivatives as potential anticancer agents [107,108,109], Negi et al. reported a hybrid inhibitor bearing a hydroxamic acid skeleton as an HDACi joined to an indanone core for TPI activity (Scheme 18). The installation of the benzylideneindanone moiety involved a condensation reaction under basic conditions between 3,4,5-trimethoxybenzaldehyde and trimethoxyacetophenone, followed by Nazarov cyclization. The linkage with the hydroxamic acid core was provided by condensation, saponification, amidation, and a final deprotection step. The synthesized benzylideneindanone derivatives were subjected to antiproliferative studies against three human cancer cell lines (MCF-7, MD-AMB-231, and K562). Compounds 17a and 17b were selected for further biological assays based on their satisfactory IC50 values (IC50 = 0.36–49.67 µM). Their low cytotoxicity toward normal Vero cells (IC50 = 100.32 µM and IC50 = 47.23 µM for 17a and 17b, respectively) validated their selectivity for malignant cell lines. Cell cycle analyses in the presence of 17b showed that 17b could promote apoptosis by blocking the G2/M and S phases. Further mechanistic insights indicated a major stabilization effect of tubulin by 17a and 17b at 5 µM. This phenomenon was also observed in confocal microscopy as a disorganization of the cytoskeletal tubulin network. Finally, the authors described the pan-HDAC inhibitory activity of 17a and 17b, with a high specificity of 17b for HDAC 6 at 20 µM, attesting to the dual character of these compounds. The aqueous solubility of 17b was also measured, affording a moderate value of 1.76 µg/mL.
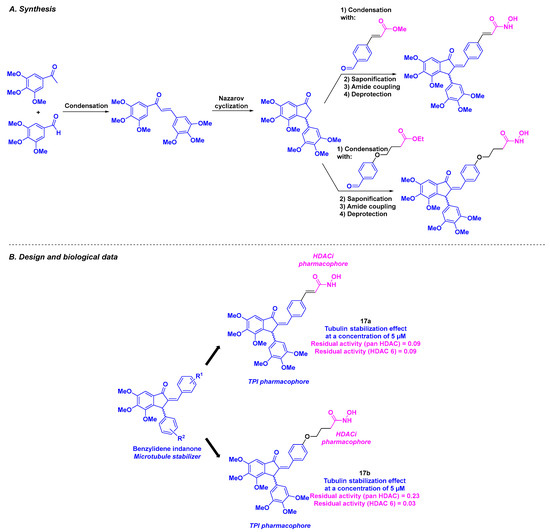
Scheme 18.
Dual HDAC/tubulin inhibitors described by Negi et al. [110].
In addition, 17a and 17b exhibited potential anti-inflammatory properties in macrophage cells, with a significant reduction at 10 µM in the rates of TNF-α and IL-6 (12 to 29%), which are indicators of cancer inflammation. Complementary safety aspects were explored for 17a in mice at various concentrations (5–1000 mg/kg via a single oral administration), identifying 17a as a tolerable and relatively safe agent [110].
9. Aminothiazoles
Building on their recent discoveries on methoxybenzoyl-aryl-thiazole (SMART) derivatives as TPI agents [111], Chen et al. prepared a new class of dual HDAC/tubulin inhibitors in 2021 (Scheme 19). By associating the SMART core with a benzamide scaffold as the HDACi pharmacophore through a multi-step synthesis, a wide range of dual compounds were elaborated and employed in various biological assays. First, the cytotoxic activities of these molecules were evaluated, leading to the identification of compound 18, which was highly potent against HCT-116, B16-F10, Jurkat, and A549 cancer cells (IC50 = 30–140 nM). 18 validated its on-mechanism cytotoxicity in an HDAC inhibitor-resistant cancer cell line (YCC3/7) with an IC50 value of 560 nM. The analysis of the inhibition of HDAC isoforms demonstrated the selectivity of 18 toward HDAC 3 (IC50 = 30 nM). This aspect was reinforced in cellular assays by treating the B16-F10 cancer cell line with 18. The increase in the rate of acetylated histone H3 by enhancing the concentration of 18 proved the HDAC inhibitory activity. The in vitro tubulin polymerization assay was then examined for 18. The TPI property of 18 was thus confirmed with an IC50 value of 12.2 µM. This characteristic was also observed upon incubating 18 in B16-F10 cells, where 18 deeply disrupted the microtubule network. Compound 18 also inhibited cancer cell migration, especially at a concentration of 100 nM. Moreover, 18 interfered with the cell cycle by stopping mitosis at the G2/M phase, thereby inducing apoptosis. In vivo investigations of 18 using a mouse model (10 mg/kg dose of 18 (which was solubilized in a mixture of ethanol/polyoxyethylene castor oil/saline: 1.25/1.25/7.5) via the daily intraperitoneal route for two weeks) revealed a significant reduction in tumor growth (TGI = 70%). Furthermore, no notable changes in body weight were observed during the treatment period. Further safety assessments indicated that 18 did not have significant effects on major organ tissues, including the heart, liver, and kidney [112].
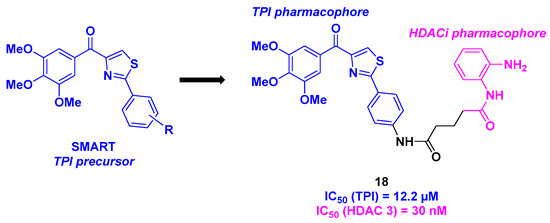
Scheme 19.
Dual HDAC/tubulin inhibitor prepared by Chen et al. [112].
In 2023, the Schiedel group designed an original dual molecule with two specific targets: tubulin deacetylases sirtuin 2 (Sirt2) and HDAC 6. As the disruption of the two enzyme activities could promote cancer processes [113], the team conceived a hybrid structure inspired by the Sirt2 and HDAC6 respective selective inhibitors (Scheme 20). Inspired by the Sirt2 inhibitors previously developed in the laboratory [114], Schiedel and his coworkers installed an aminothiazole core using a diazotation step followed by a Meerwein reaction. This motif was then attached to a hydroxamic acid skeleton, the HDACi pharmacophore, via copper-catalyzed Huisgen cycloaddition. The in vitro biological efficiency of these dual molecules was then analyzed. Among the synthesized compounds, 19 exhibited the highest selectivity and activity toward Sirt2 and HDAC6, with IC50 values of 320 nM and 43 nM, respectively. It should be highlighted that the co-crystallization of 19 and its analogs was achieved with the two respective targets, indicating a strong hydrophobic interaction between Sirt2 and the corresponding pharmacophore, as well as coordination of the hydroxamic acid with the Zn atom. In cellulo assays corroborated the in vitro results, with a high level of acetylated α-tubulin observed by incubating 20 µM of 19 in the PC-3M-cell line. To demonstrate the synergistic effect of hybrid molecule 19, cell viability assays were performed on cancer cells sensitive to Sirt2 and HDAC 6 inhibition. 19 could decrease the viability of HGC27, W1, MCF-7, and PC-3M-luc cells, with EC50 values ranging from 12.9 to 30.1 µM [115].
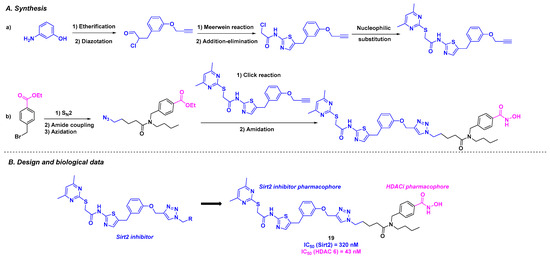
Scheme 20.
Dual Sirt2/HDAC 6 inhibitor developed by the Schiedel group [115].
10. 2-Methoxyestradiol Core
2-Methoxyestradiol, a natural metabolite of estradiol, and its derivatives are known as remarkable tubulin polymerization inhibitors and disruptors of the microtubule skeleton [116]. In this context, Yao et al. described a dual-targeting inhibitor exhibiting a 2-methoxyestradiol scaffold as a TPI pharmacophore linked to a 2-aminobenzamide motif as an HDACi. After a thorough examination of the SAR for forty-seven synthesized hybrid molecules, 20 was identified as the compound exhibiting the best cytotoxicity against six cancer cell lines (MCF-7, MGC-803, HeLa, A549, HepG2, and U937), with IC50 values ranging from 0.371 to 4.840 µM. The inhibitory activities of 20 toward HDAC isoforms showed higher selectivity toward HDAC isoforms 2 and 6, with IC50 values of 60 and 120 nM, respectively (Scheme 21). In vitro immunofluorescence assays showed that compound 20 could disorganize the microtubule network. Moreover, incubating 20 at a concentration of 4 µM with purified porcine brain tubulin significantly inhibited tubulin polymerization. Similar to tubulin polymerization inhibitors, 20 efficiently halted the cell cycle at the G2/M phase, induced apoptosis via a change in mitochondrial membrane potential, increased the ROS rate, and upregulated pro-apoptotic proteins (cleaved forms of caspase 3, 7, 9, and PARP). In addition, 20 could also hamper the proliferation, migration, and invasion of tumor cells. All these antitumor activities were validated in an in vivo zebrafish xenograft tumor model (incubation for 1 h with different concentrations of 20 (1–4 µM) for 3 days) [117].
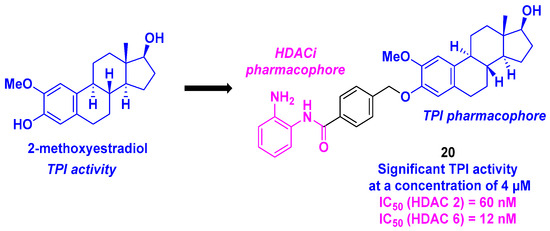
Scheme 21.
HDAC/tubulin dual inhibitor developed by Yao et al. [117].
11. Millepachine Core
More recently, Yin, Kong, and their colleagues developed a new family of dual HDAC/tubulin inhibitors featuring millepachine, a natural chalcone with TPI activity, branched to a hydroxamic acid structure as an HDACi (Scheme 22). After large-molecule-screening against five cancer cell lines (MDA-MB-231, A549, PC-3, U251, and MCF-7), compound 21 was determined to be the best molecule with the highest cytotoxicity, with a notable IC50 value of 16 nM for the human prostate cancer cell line PC-3. The HDAC inhibition activities were then inspected, showing that 21 possessed 67% inhibition of HDACs at a concentration of 1 µM. Furthermore, 21 exhibited pan-HDAC inhibitory activity toward HDAC 1, 2, and 6. The TPI properties of 21 were validated using purified tubulin protein, with an IC50 value of 4.82 µM. This result was confirmed by immunofluorescence assay, where intracellular microtubules were disassembled by incubation with 21. The biological 21 action was then clarified using flow cytometry and Western blot. The authors demonstrated that 21 could interrupt the cell cycle at the G2/M phase by enhancing cleaved PARP and Caspase 3 levels and diminishing Bim and Bcl-2 levels. Compound 21 also inhibited the migration of tumor cells and was involved in the mitochondrial apoptosis process by reducing the mitochondrial membrane potential and increasing ROS levels. In addition, an anti-angiogenesis effect was detected with 21, since a decrease in the HUVECs capillary-like tubular network was observed in the presence of 21. Finally, all the in vitro antitumor aspects of 21 were ascertained in the in vivo PC-3 mice xenografts (intravenous injection every two days, for a three-week treatment), with a TGI of 90% at a 21 dosage of 20 mg/kg (N. B.: For the intravenous dose, 21 was dissolved in a mixture of DMSO/Tween 80/Saline (10/10/80)) [118].
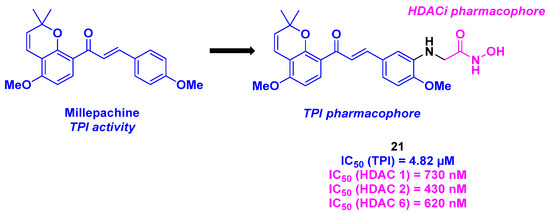
Scheme 22.
Dual HDAC/tubulin inhibitor developed by Yin, Kong and their colleagues [118].
12. Deoxypodophyllotoxin Derivatives
In 2014, Chen, Li, Lu and coworkers proposed a dual HDAC/tubulin inhibitor, integrating a podophyllotoxin (PPT) analog platform. Based on the design of their topoisomerase II/HDAC hybrid inhibitors [119], the team elaborated an analogous approach for the construction of novel molecules. With the well-known TPI properties of deoxypodophyllotoxin (DPT) [120,121] combining with the HDACi activities of benzamides, a novel family of molecular hybrids could be generated (Scheme 23). The DPT core was synthesized from PPT in three steps under standard conditions [122]. The benzamide structure was then linked to the DPT group via amidation using HATU as the coupling agent. The in vitro HDAC inhibition of the established molecules disclosed a pan inhibitory activity for 22a and 22b toward HDAC 1, 2, and 3 (IC50 = 0.75–11.09 µM). By treating HCT-116 cells with dual HDAC/tubulin inhibitors at 40 or 80 nM for 24 h, the authors observed an accumulation of cells in the G2/M phase, indicating cell cycle blocking by the TPI effect. The cytotoxicity of the prepared structures validated the biological activities of 22a and 22b in A549 and HCT-116 cancer cell lines, with IC50 values ranging from 36 to 40 nM [123].
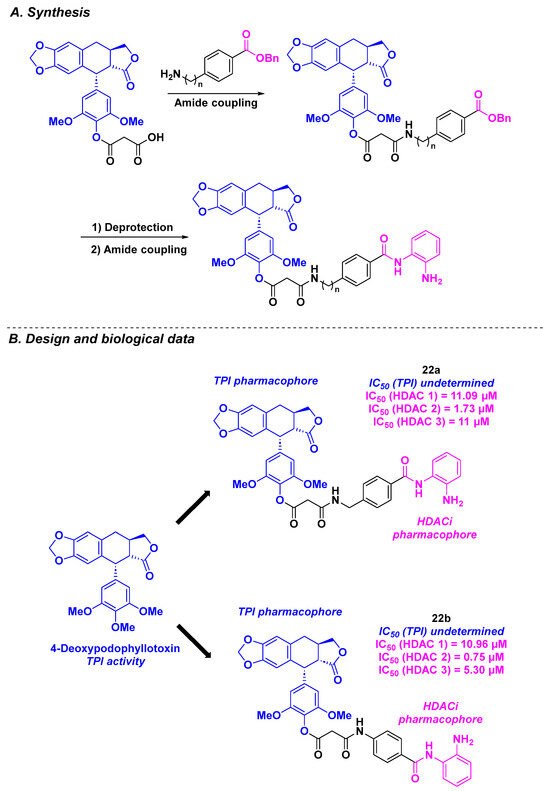
Scheme 23.
Dual HDAC/tubulin inhibitors elaborated by Chen, Li, Lu and coworkers [123].
13. Paclitaxel Scaffold
Given the high efficiency of paclitaxel as an FDA-approved anticancer drug and its activity as a tubulin polymerization inhibitor [124], it would seem obvious to combine this molecule with an FDA-approved HDACi, such as vorinostat. This idea was proposed by Chen and Lu, who conjugated paclitaxel with vorinostat through a glycine or succinic acid linker [125] (Scheme 24). The cytotoxicity of the synthesized dual inhibitors was determined. Surprisingly, compound 23 bearing the glycine motif exhibited higher antiproliferative activity against the drug-resistant cancer cell line MCF-7/ADR compared to paclitaxel alone, with an IC50 value of 1384 nM. This promising result prompted the research teams to explore the synergistic effect of 23 at the cellular level. Compound 23 was able to increase G2/M cell cycle arrest compared to the standard influence of paclitaxel. Western blot analyses indicated that 23 induced hyperacetylation of tubulin in HCT-116, MCF-7, and MCF-7/ADR cells at a concentration of 10 nM.
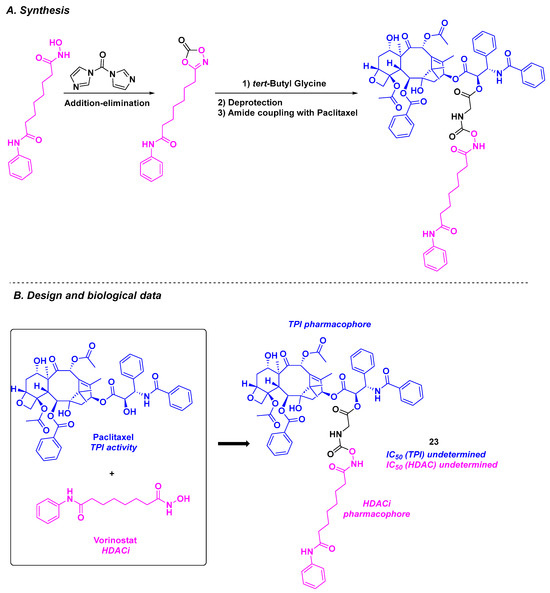
Scheme 24.
Dual HDAC/tubulin inhibitor conceived by the Chen and Lu groups [125].
14. Sulfonamide Scaffold
The sulfonamide skeleton is present in the structure of E-7010, an orally active antitumor compound developed by the Eisai Company. This molecule provided promising biological results, namely, tubulin polymerization inhibition by binding to the colchicine-binding site [126]. Based on these findings, Chen, Liou, and coworkers envisioned the construction of hybrid agents, including a sulfonamide group for TPI activity and a benzamide function as an HDACi (Scheme 25). The connection between the two chemical entities was ensured using a stilbene link. As this motif is not a substrate for membrane-bound P-glycoprotein (P-gp), stilbene can bypass the P-gp-mediated multidrug resistance pathway. Based on this hypothesis, twenty-two dual molecules were prepared and biologically evaluated. Antiproliferative activity revealed that compound 24 was the most cytotoxic agent against KB cell lines, with a GI50 value of 12 nM. It should be highlighted that the same results were noticed in drug-resistant KB cancer cell lines. The HDAC isoform inhibition showed a strong selectivity of 24 for HDAC 1 and 2, with IC50 values of 1.07 and 1.47 µM, respectively. Mechanistic insights revealed that 24 could activate pro-apoptotic markers, such as activated PARP, γH2AX, and caspases 3, 8, and 9, in KB cell lines. In vitro complementary analyses using flow cytometry and fluorescence microscopy, the authors found that 24 blocked the cell cycle at the G2/M phase, with microtubule disassembly [127].
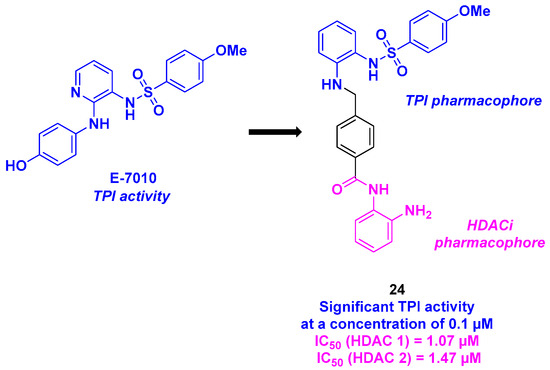
Scheme 25.
Molecular hybrids HDAC/tubulin inhibitor developed by Chen, Liou and coworkers [127].
15. Indoline/Indole-Sulfonamide Scaffold
With the promising biological properties of indoline-sulfonamide derivatives as tubulin inhibitors [128], Liou, Chang, and coworkers designed a hybrid dual molecule incorporating this scaffold and a benzamide group as an efficient HDAC pharmacophore (Scheme 26). The two functional motifs were associated with a benzyl linker installed through reductive amination under standard conditions. Twenty-one dual compounds were synthesized and subjected to biological assays. The antiproliferative activities were determined, with compound 25 being the most cytotoxic against KB, A549, and MKN45 cancer cell lines (IC50 = 49–79 nM). The cell-killing capacity of 25 was confirmed in oral epidermoid carcinoma drug-resistant cell lines (KBVIN10, KB-S15, and KB-7D), with IC50 values ranging from 44 to 65 nM. The TPI abilities of the prepared hybrid molecules were also measured. Compound 25 was identified as the best tubulin inhibitor, with an IC50 value of 1 µM. Further examinations showed that 25 had a better affinity for the colchicine site compared to colchicine at concentrations of 1 or 5 µM. HDAC isoform selectivity was detected for 25, especially with a significant inhibition of HDAC 1, 2, and 6. Moreover, the HDACi efficiency of 25 was verified in A549 cells, with the accumulation of acetylated α-tubulin in the presence of 25 observed. Finally, the dual inhibitory feature of 25 was established in an in vivo A549 xenograft mouse model, with a remarkable reduction in size of the tumor (TGI = 62.9% with a 25 intraperitoneal daily dosage of 50 mg/kg, solubilized in DMSO/Cremophor/Dextrose: 5/5/90). Similar results were also found in the B-cell lymphoma xenograft tumor mouse model (daily intravenous dose of 25 (vehicled in DMSO/Cremophor/Saline: 10/20/70) at 50 mg/kg, 5 times a week) [129].
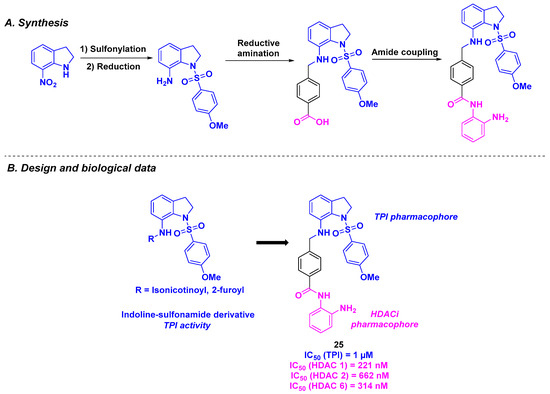
Scheme 26.
Hybrids HDAC/tubulin inhibitors developed by Chen, Liou and their colleagues [129].
The same group envisioned a library of dual-active compounds fusing a potent tubulin assembly inhibitor, currently in phase II clinical trials [130], with an HDACi bearing an indoline-sulfonamide structure [131] (Scheme 27). The construction of the hybrid molecules was based on a three-step synthesis featuring an alkylation step, Heck coupling, and amide condensation [132]. The developed structures demonstrated antitumor activity against A549, HCT-116, and PC-3 cancer cell lines (IC50 values from 179.26 to 484.57 nM) for compound 26a. In contrast, despite its remarkable cytotoxic properties, the tubulin polymerization inhibitory property of 26a remained less important than that of 26b. In addition, 26a and 26b displayed high HDAC 6 specificity, with IC50 values of 275 and 64.5 nM, respectively. In vivo experiments on PC-3 xenograft mice models validated the efficacy of 26b, with the suppression of tumor growth at daily oral doses of 100 and 200 mg/kg (TGI of 24.8% and 68.5%, respectively). Similar outcomes were obtained with multiple myeloma RPMI-8226 xenografts (TGI values of 35.8% and 58.2% for intraperitoneal daily doses of 50 and 100 mg/kg, respectively).
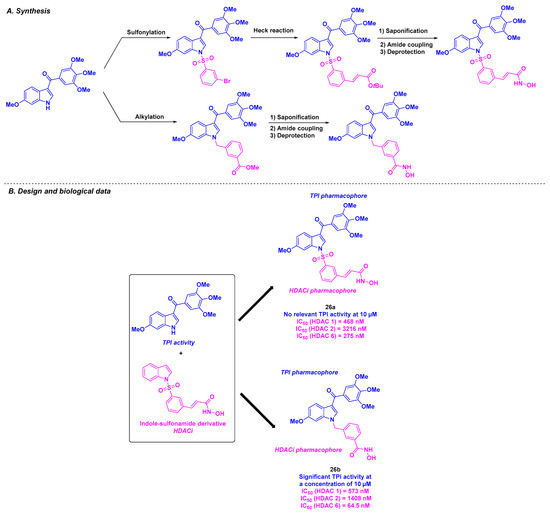
Scheme 27.
Hybrid HDAC/tubulin inhibitors conceived by Yang, Liou and coworkers [132].
Emulating the previous research of the Liou team, Fu et al. deepened the investigation of the properties of chimeric indole-sulfonamide 26a [133] (Scheme 27). Compound 26a also exhibited significant antiproliferative activity against liquid tumor cell lines, such as HL-60, with an IC50 value of 42 nM. Molecular docking validated the dual targeting of 26a, with a partial overlap with colchicine in the tubulin binding site and analogous interactions with vorinostat in HDAC active site. Further biological analyses indicated that this molecule was able to stop the mitosis process at the G2/M phase by significantly varying the G2/M transition protein rates. In addition, 26a could induce cell apoptosis by upregulating pro-apoptotic proteins (cleaved caspases 3, 7, 8, 9, and PARP). Mouse xenograft models confirmed the antitumor effects of 26a, with TGI values of 31.1 and 40.9% in PC-3 and HL-60 grafted mice respectively.
In parallel with the conventional molecular hybrid inhibitors connecting TPI and HDACi pharmacophores, some original dual molecules have recently been conceived based on their HDACi chemical structures.
16. Dual HDAC/Tubulin Inhibitors Inspired by the HDACis
Among the wide diversity of developed dual HDAC/tubulin inhibitors, those the originating from HDACis constitute an important molecule family. In addition to their main function on histones, HDACs could also react with non-histone proteins, such as α-tubulin. For example, HDAC 6 could use tubulin as a substrate, thus regulating the stability of the microtubule network [134,135,136]. Therefore, the development of HDACis with dual HDAC/tubulin activity has emerged. In this frame, medicinal chemists mainly based their compound designs on HDACis developed in the literature. The tubulin inhibitory activity of the synthesized molecules was systematically studied using diverse biological assays.
16.1. Pyrrolo [2,3-d]pyrimidine Skeleton
Emulating the structure of tucidinostat and their recent advances in the development of HDACis [137], Lee, Liou, and their colleagues envisaged at the first glance an ameliorated version of their HDACis (Scheme 28). In this prospect, the team elaborated a rigid and bulky pyrrolo [2,3-d]pyrimidine structure at the cap part of the HDACis, improving the anticancer properties of the molecules by efficiently blocking the active site entrance [137]. As some reported HDACis also target tubulin [138], the group considered their newly designed HDACis as potential dual hybrid HDAC/tubulin inhibitors. The in vitro cytotoxicities of these molecules were then studied. Among the nine synthesized compounds, 27a and 27b exhibited the highest antiproliferative activities against eight solid tumor cell lines (MDA-MB-231, MDA-MB-468, HeLa, DLD-1, HCT-116, H661, H1299, and A549), with IC50 values ranging from 50 to 1150 nM. It should be noteworthy that these cytotoxicity properties were higher than those of tucidinostat. Likewise, 27a and 27b confirmed their antitumor effects in leukemia cell lines (HH, HuT78, HL60, and KG-1), with IC50 values ranging from 80 to 150 nM. Moreover, 27a exhibited significant cell-killing efficacy in the multidrug-resistant cell line MES-SA/Dx5, with an IC50 value of 9.54 µM, three times higher than that of vinorelbine.
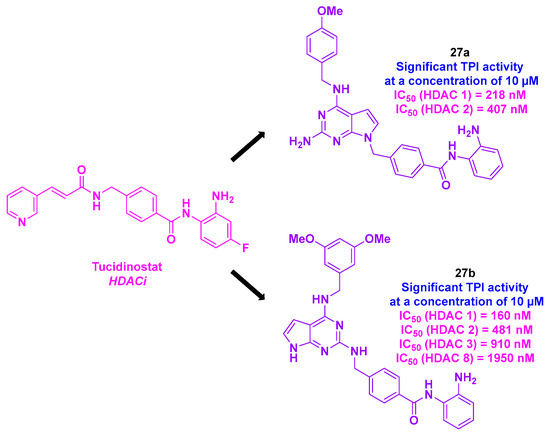
Scheme 28.
Dual HDAC/tubulin inhibitor designed by Lee, Liou and their colleagues [139].
The analysis of HDAC isoform inhibitory activities highlighted that 27a had a strong selectivity for HDAC 1 and 2, whereas 27b showed a wider pan-HDAC inhibitory efficiency for HDAC 1, 2, 3, and 8. Mechanistic studies using flow cytometry suggested that 27a and 27b could promote apoptosis in tumor cells by inducing the production of active forms of caspases-3 and -9 and reducing the formation of anti-apoptotic agents MCL-1 and Bcl-xL. The TPI of 27a and 27b was also scrutinized using an in vitro tubulin polymerization assay. By incubating tubulin proteins with 10 µM of 27a and 27b, the authors noted that these compounds were able to jeopardize the microtubule assembly. It should be noted that no in vitro inhibition was found for HDAC 6 for 27a and 27b, implying an independent TPI pathway for the two dual compounds [139].
16.2. Benzamide Scaffold
In 2021, He, Chen, and coworkers described LT-548-133-1, a tucidinostat analog possessing equivalent HDAC inhibitory activity [140] (Scheme 29). When LT-548-133-1 was incubated with MCF-7 cells for 48 h, an IC50 value of 2.1 mM was obtained. Further investigations indicated that LT-548-133-1 could enhance the rate of acetylated histone H3, probably due to its HDAC inhibitory property. Moreover, LT-548-133-1 promoted cell cycle arrest at the G2/M phase, contrary to tucidinostat, which induced G0/G1 cell mitosis arrest. This result indicated that LT-548-133-1 might follow a different mechanism of action compared to tucidinostat. Complementary western blot analyses indicated that G2/M cell cycle arrest was induced by an increase in CyclinB1 protein expression. In addition, LT-548-133-1 could lead to abnormal cell mitosis and apoptosis in MCF-7 cell lines. Based on these effects on the mitosis process, the authors suggested that LT-548-133-1 could interfere with the microtubule network. Putting LT-548-133-1 with MCF-7 cells revealed by immunofluorescence the destructuration of the microtubules, proving the TPI effect of LT-548-133-1.
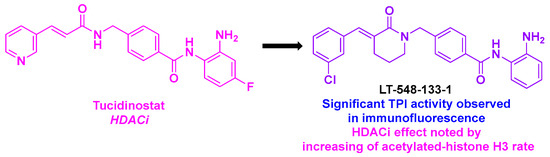
Scheme 29.
Dual HDAC/tubulin inhibitor LT-548-133-1 reported by He, Chen and coworkers [140].
16.3. Quinazoline Scaffold
By scrutinizing the biological pathway of their previously developed SKLB-23bb [141], an HDAC 6 selective inhibitor, the Chen group revealed that this molecule could have a dual-targeting activity [142].
SKLB-23bb contains a 2-methylquinazoline core, a known key bioactive skeleton [53,143], linked to a hydroxamic acid group through an alkyl linker (Scheme 30). SKLB-23bb was obtained through a SNAr reaction involving a highly functionalized aniline, followed by amide condensation [141]. This molecule exhibited high cytotoxic potential, with nanomolar-range IC50 values (36.68 to 116.56 nM) across a wide panel of liquid and solid cancer cell lines. In addition to its HDAC 6 inhibitory property, SKLB-23bb also acts via another biological pathway to hamper cancer cells. The cytotoxic effects of SKLB-23bb were similar in HDAC 6 knockout tumor cell lines compared to wild-type cells. To clarify the exact mechanism of action of this molecule, SKLB-23bb was incubated with fluorescent-stained A2780s cell line. Major morphological modifications in the microtubule network were noted, and microtubule dysfunction was similarly observed upon addition of colchicine to A2780s cells. This result could suggest that this molecule could also bind to the colchicine site of tubulin to block the polymerization process. The validation of this hypothesis was observed in the EBI competition assay. Complementary TPI tests demonstrated that SKLB-23bb could inhibit tubulin polymerization at a concentration of 10 µM. As a result of this biological characteristic, SKLB-23bb could stop the cell cycle at the G2/M phase and trigger apoptosis by promoting the upregulation of pro-apoptotic proteins, such as Bax.
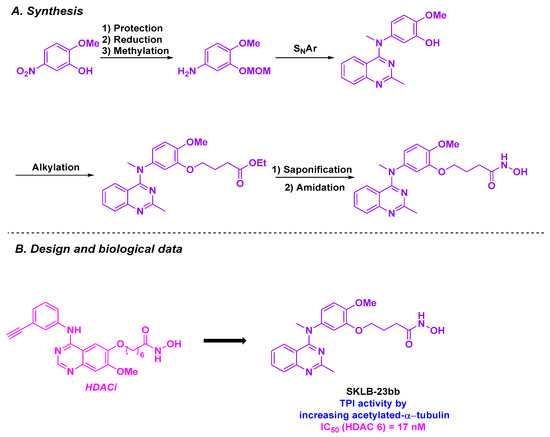
Scheme 30.
Dual HDAC/tubulin inhibitor SKLB-23bb developed by the Chen group [142].
The effectiveness of SKLB-23bb was preserved in vivo in xenograft mice imitating the B-lymphoma model, with a tumor growth inhibition (58.22% tumor-inhibitory rate) at an oral dose of 40 mg/kg three times a week. Analogous results were obtained with solid tumor models (HCT-116 and A2780 xenografts, with an oral dose of 6 or 12.5 mg/kg, thrice a week).
16.4. Imidazolyl Motif
In 2021, Taylor, Tillekeratne et al. developed a novel class of heterocyclic compounds with a dual action on HDACs and tubulin. Inspired by imidazole derivatives and FDA-approved hydroxamic acids as HDACi [144,145,146], the group introduced an imidazolyl skeleton with potential HDAC inhibitory activity. To strengthen the dual property of the newly conceived compounds, a chalcone scaffold was added to the molecule’s structure to impart antimitotic activity (Scheme 31) [147]. The synthesis of these dual inhibitors was achieved via a multi-step synthesis, including a Heck cross-coupling reaction, olefin oxidative cleavage, and aldol condensation as key steps. A preliminary biology study on the HCT-116 cell line was conducted using the prepared compounds, confirming their cytotoxicity in the micromolar range (IC50 = 5.14–6.95 µM). The inhibitory effects of the HDAC isoforms were then evaluated. The in vitro results highlighted that compounds 28a and 28c had pan-HDAC inhibitory character toward HDAC 1, 2, 3, 6, and 8. In contrast, molecule 28b was a selective HDACi for HDAC 8. A similar analysis was carried out by incubating the synthesized compounds with HCT-116 cells. Unexpectedly, no significant variation in histone or tubulin acetylation was observed, implying that these molecules did not mainly target HDACs. Despite these outcomes, complementary assays were performed to evaluate the cytotoxicity of these compounds against a wide range of cancer cell lines. The compounds demonstrated their effectiveness in HeLa and NCI-60 cancer cell lines with micromolar GI50 values. Examination of the antimitotic activity of 28a in HeLa cells revealed that 28a was able to arrest the mitosis process, by destabilizing microtubules. Molecular docking studies have indicated a potential interaction between 28a and tubulin at the colchicine-binding site [148].
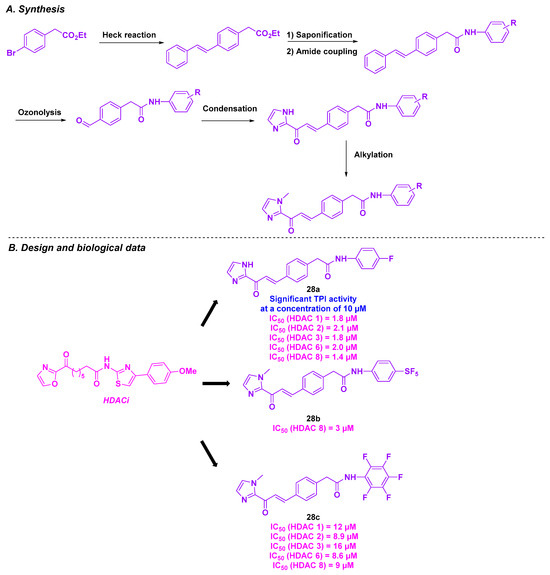
Scheme 31.
Dual HDAC/tubulin inhibitor conceived by Taylor, Tillekeratne et al. [148].
17. Conclusions
The rise of dual-targeting drugs during the last decades represents a promising alternative to conventional chemotherapeutics in cancer treatment. By combining two pharmacophores into a single anticancer agent, medicinal chemists aim to target “two birds in one stone” thereby overcoming the limitations of traditional chemotherapy, such as excessive toxicity, reduction of immunity, and development of drug resistance.
As highlighted in this review, tubulin and HDACs are highly effective and complementary targets for the development of dual-active agents. The remarkable efficacy of the described dual molecules is evident from their outstanding in vitro antiproliferative activities, with nanomolar IC50 values in various human cancer cell lines. These compounds exhibited pan-HDAC inhibitory properties, notably against HDAC 1, 2, and 6 (IC50 in satisfactory nanomolar ranges), and effectively inhibited tubulin polymerization (favorable IC50 micromolar values), which further led to the arrest of the cell cycle at the G2 phase. Some of the HDAC/tubulin inhibitors, such as CA-4 derivatives, also display potent anti-angiogenic properties, contributing to their antitumor characteristics. In addition to their synergistic effects on HDACs and tubulin, these inhibitors showed minimal toxicity at both the cellular and in vivo levels, highlighting their potential for therapeutic application (Table 1).

Table 1.
Summary table of HDAC-tubulin dual-targeting inhibitors.
However, despite their promising preclinical results, no dual HDAC/tubulin inhibitors have entered clinical phase trials yet, as the pharmacokinetics of the most advanced hit molecules are still under investigation. Furthermore, complementary studies should be conducted on dual compounds. For instance, as reported in Table 1, only three agents presented good water solubility (up to 138 µg/mL). Physicochemical data for the other compounds need to be explored. In vivo studies should also be performed for some derivatives after in vitro studies encouraging outcomes.
Hence, the conception of dual-targeting drug candidates remains a challenge in cancer therapy. The elaboration of such molecules would require substantial efforts to synthesize lead compounds, along with thorough pharmacomodulation to meet pharmacokinetics and toxicity criteria.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Fraga, M.F.; Ballestar, E.; Villar-Garea, A.; Boix-Chornet, M.; Espada, J.; Schotta, G.; Bonaldi, T.; Haydon, C.; Ropero, S.; Petrie, K.; et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat. Genet. 2005, 37, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Huang, J.; Zheng, Y.; Sun, Q. Histone acetyltransferase inhibitors: An overview in synthesis, structure-activity relationship and molecular mechanism. Eur. J. Med. Chem. 2019, 178, 259–286. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tian, Y.; Zhu, W.-G. The Roles of Histone Deacetylases and Their Inhibitors in Cancer Therapy. Front. Cell Dev. Biol. 2020, 8, 576946. [Google Scholar] [CrossRef]
- He, X.; Hui, Z.; Xu, L.; Bai, R.; Gao, Y.; Wang, Z.; Xie, T.; Ye, X.-Y. Medicinal chemistry updates of novel HDACs inhibitors (2020 to present). Eur. J. Med. Chem. 2022, 227, 113946. [Google Scholar] [CrossRef]
- Vannini, A.; Volpari, C.; Filocamo, G.; Casavola, E.C.; Brunetti, M.; Renzoni, D.; Chakravarty, P.; Paolini, C.; De Francesco, R.; Gallinari, P.; et al. Crystal Structure of a Eukaryotic Zinc-Dependent Histone Deacetylase, Human HDAC8, Complexed with a Hydroxamic Acid Inhibitor. Proc. Natl. Acad. Sci. USA 2004, 101, 15064–15069. [Google Scholar] [CrossRef]
- Somoza, J.R.; Skene, R.J.; Katz, B.A.; Mol, C.; Ho, J.D.; Jennings, A.J.; Luong, C.; Arvai, A.; Buggy, J.J.; Chi, E.; et al. Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure 2004, 12, 1325–1334. [Google Scholar] [CrossRef]
- Squarzoni, A.; Scuteri, A.; Cavaletti, G. HDACi: The Columbus’ Egg in Improving Cancer Treatment and Reducing Neurotoxicity? Cancers 2022, 14, 5251. [Google Scholar] [CrossRef]
- Yoon, S.; Eom, G.H. HDAC and HDAC Inhibitor: From Cancer to Cardiovascular Diseases. Chonnam Med. J. 2016, 52, 1–11. [Google Scholar] [CrossRef]
- Rai, S.; Kim, W.S.; Ando, K.; Choi, I.; Izutsu, K.; Tsukamoto, N.; Yokoyama, M.; Tsukasaki, K.; Kuroda, J.; Ando, J.; et al. Oral HDAC inhibitor tucidinostat in patients with relapsed or refractory peripheral T-cell lymphoma: Phase IIb results. Haematologica 2023, 108, 811–821. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Qin, M.; Wu, H.-P.; Khamis, M.Y.; Li, Y.-H.; Ma, L.-Y.; Liu, H.-M. A Review of Progress in Histone Deacetylase 6 Inhibitors Research: Structural Specificity and Functional Diversity. J. Med. Chem. 2021, 64, 1362–1391. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Sun, Z.; Kuang, P.; Chen, J. Recent progress on HDAC inhibitors with dual targeting capabilities for cancer treatment. Eur. J. Med. Chem. 2020, 208, 112831. [Google Scholar] [CrossRef]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef]
- Oehme, I.; Deubzer, H.E.; Wegener, D.; Pickert, D.; Linke, J.-P.; Hero, B.; Kopp-Schneider, A.; Westermann, F.; Ulrich, S.M.; von Deimling, A.; et al. Histone Deacetylase 8 in Neuroblastoma Tumorigenesis. Clin. Cancer Res. 2008, 15, 91–99. [Google Scholar] [CrossRef]
- Rettig, I.; Koeneke, E.; Trippel, F.; Mueller, W.C.; Burhenne, J.; Kopp-Schneider, A.; Fabian, J.; Schober, A.; Fernekorn, U.; von Deimling, A. Selective inhibition of HDAC8 decreases neuroblastoma growth in vitro and in vivo and enhances retinoic acid-mediated differentiation. Cell Death Dis. 2015, 6, e1657. [Google Scholar] [CrossRef]
- Ecker, J.; Oehme, I.; Mazitschek, R.; Korshunov, A.; Kool, M.; Hielscher, T.; Kiss, J.; Selt, F.; Konrad, C.; Lodrini, M. Targeting class I histone deacetylase 2 in MYC amplified group 3 medulloblastoma. Acta Neuropathol. Commun. 2015, 3, 22. [Google Scholar] [CrossRef]
- Milde, T.; Oehme, I.; Korshunov, A.; Kopp-Schneider, A.; Remke, M.; Northcott, P.; Deubzer, H.E.; Lodrini, M.; Taylor, M.D.; von Deimling, A.; et al. HDAC5 and HDAC9 in medulloblastoma: Novel markers for risk stratification and role in tumor cell growth. Clin. Cancer Res. 2010, 16, 3240–3252. [Google Scholar] [CrossRef]
- Minamiya, Y.; Ono, T.; Saito, H.; Takahashi, N.; Ito, M.; Mitsui, M.; Motoyama, S.; Ogawa, J. Expression of histone deacetylase 1 correlates with a poor prognosis in patients with adenocarcinoma of the lung. Lung Cancer 2011, 74, 300–304. [Google Scholar] [CrossRef]
- Jung, K.H.; Noh, J.H.; Kim, J.K.; Eun, J.W.; Bae, H.J.; Xie, H.J.; Chang, Y.G.; Kim, M.G.; Park, H.; Lee, J.Y.; et al. HDAC2 overexpression confers oncogenic potential to human lung cancer cells by deregulating expression of apoptosis and cell cycle proteins. J. Cell. Biochem. 2012, 113, 2167–2177. [Google Scholar] [CrossRef]
- Osada, H.; Tatematsu, Y.; Saito, H.; Yatabe, Y.; Mitsudomi, T.; Takahashi, T. Reduced expression of class II histone deacetylase genes is associated with poor prognosis in lung cancer patients. Int. J. Cancer 2004, 112, 26–32. [Google Scholar] [CrossRef]
- Weichert, W.; Röske, A.; Gekeler, V.; Beckers, T.; Ebert, M.P.; Pross, M.; Dietel, M.; Denkert, C.; Röcken, C. Association of patterns of class I histone deacetylase expression with patient prognosis in gastric cancer: A retrospective analysis. Lancet Oncol. 2008, 9, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Sudo, T.; Mimori, K.; Nishida, N.; Kogo, R.; Iwaya, T.; Tanaka, F.; Shibata, K.; Fujita, H.; Shirouzu, K.; Mori, M. Histone deacetylase 1 expression in gastric cancer. Oncol. Rep. 2011, 26, 777–782. [Google Scholar] [PubMed]
- Jin, Z.; Jiang, W.; Jiao, F.; Guo, Z.; Hu, H.; Wang, L.; Wang, L. Decreased expression of histone deacetylase 10 predicts poor prognosis of gastric cancer patients. Int. J. Clin. Exp. Pathol. 2014, 7, 5872–5879. [Google Scholar]
- Xie, H.J.; Noh, J.H.; Kim, J.K.; Jung, K.H.; Eun, J.W.; Bae, H.J.; Kim, M.G.; Chang, Y.G.; Lee, J.Y.; Park, H.; et al. HDAC1 Inactivation Induces Mitotic Defect and Caspase-Independent Autophagic Cell Death in Liver Cancer. PLoS ONE 2012, 7, e34265. [Google Scholar] [CrossRef]
- Buurman, R.; Sandbothe, M.; Schlegelberger, B.; Skawran, B. HDAC inhibition activates the apoptosome via Apaf1 upregulation in hepatocellular carcinoma. Eur. J. Med. Res. 2016, 21, 26. [Google Scholar] [CrossRef]
- Quint, K.; Agaimy, A.; Di Fazio, P.; Montalbano, R.; Steindorf, C.; Jung, R.; Hellerbrand, C.; Hartmann, A.; Sitter, H.; Neureiter, D.; et al. Clinical significance of histone deacetylases 1, 2, 3, and 7: HDAC2 is an independent predictor of survival in HCC. Virchows Arch. 2011, 459, 129–139. [Google Scholar] [CrossRef]
- Fan, J.; Lou, B.; Chen, W.; Zhang, J.; Lin, S.; Lv, F.F.; Chen, Y. Down-regulation of HDAC5 inhibits growth of human hepatocellular carcinoma by induction of apoptosis and cell cycle arrest. Tumour Biol. 2014, 35, 11523–11532. [Google Scholar] [CrossRef]
- Feng, G.; Dong, L.; Shang, W.; Pang, X.; Li, J.; Liu, L.; Wang, Y. HDAC5 promotes cell proliferation in human hepatocellular carcinoma by up-regulating Six1 expression. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 811–816. [Google Scholar]
- Lv, Z.; Weng, X.; Du, C.; Zhang, C.; Xiao, H.; Cai, X.; Ye, S.; Cheng, J.; Ding, C.; Xie, H.; et al. Downregulation of HDAC6 promotes angiogenesis in hepatocellular carcinoma cells and predicts poor prognosis in liver transplantation patients. Mol. Carcinog. 2016, 55, 1024–1033. [Google Scholar] [CrossRef]
- Fritsche, P.; Seidler, B.; Schüler, S.; Schnieke, A.; Göttlicher, M.; Schmid, R.M.; Saur, D.; Schneider, G. HDAC2 mediates therapeutic resistance of pancreatic cancer cells via the BH3-only protein NOXA. Gut 2009, 58, 1399–1409. [Google Scholar] [CrossRef]
- Li, D.; Sun, X.; Zhang, L.; Yan, B.; Xie, S.; Liu, R.; Liu, M.; Zhou, J. Histone deacetylase 6 and cytoplasmic linker protein 170 function together to regulate the motility of pancreatic cancer cells. Protein Cell 2014, 5, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Ouaïssi, M.; Silvy, F.; Loncle, C.; Ferraz da Silva, D.; Martins Abreu, C.; Martinez, E.; Berthézene, P.; Cadra, S.; Le Treut, Y.P.; Hardwigsen, J.; et al. Further characterization of HDAC and SIRT gene expression patterns in pancreatic cancer and their relation to disease outcome. PLoS ONE 2014, 9, e108520. [Google Scholar] [CrossRef] [PubMed]
- Weichert, W.; Röske, A.; Niesporek, S.; Noske, A.; Buckendahl, A.-C.; Dietel, M.; Gekeler, V.; Boehm, M.; Beckers, T.; Denkert, C. Class I Histone Deacetylase Expression Has Independent Prognostic Impact in Human Colorectal Cancer: Specific Role of Class I Histone Deacetylases In vitro and In vivo. Clin. Cancer Res. 2008, 14, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Stypula-Cyrus, Y.; Damania, D.; Kunte, D.P.; Cruz, M.D.; Subramanian, H.; Roy, H.K.; Backman, V. HDAC up-regulation in early colon field carcinogenesis is involved in cell tumorigenicity through regulation of chromatin structure. PLoS ONE 2013, 8, e64600. [Google Scholar] [CrossRef]
- Müller, B.M.; Jana, L.; Kasajima, A.; Lehmann, A.; Prinzler, J.; Budczies, J.; Winzer, K.-J.; Dietel, M.; Weichert, W.; Denkert, C. Differential expression of histone deacetylases HDAC1, 2 and 3 in human breast cancer-overexpression of HDAC2 and HDAC3 is associated with clinicopathological indicators of disease progression. BMC Cancer 2013, 13, 215. [Google Scholar] [CrossRef]
- Hong, Y.R.; Song, B.J.; Jung, S.S.; Kang, B.J.; Kim, S.H.; Chae, B.J.; Seo, J.; Min, S.K.; Park, H.-R.; Kim, D.H. Expression of histone deacetylases HDAC1, HDAC2, HDAC3, and HDAC6 in invasive ductal carcinomas of the breast. J. Breast Cancer 2014, 19, 323–331. [Google Scholar]
- Song, C.; Zhu, S.; Wu, C.; Kang, J. Histone deacetylase (HDAC) 10 suppresses cervical cancer metastasis through inhibition of matrix metalloproteinase (MMP) 2 and 9 expression. J. Biol. Chem. 2013, 288, 28021–28033. [Google Scholar] [CrossRef]
- Hayashi, A.; Horiuchi, A.; Kikuchi, N.; Hayashi, T.; Fuseya, C.; Suzuki, A.; Konishi, I.; Shiozawa, T. Type-specific roles of histone deacetylase (HDAC) overexpression in ovarian carcinoma: HDAC1 enhances cell proliferation and HDAC3 stimulates cell migration with downregulation of E-cadherin. Int. J. Cancer. 2010, 127, 1332–1346. [Google Scholar] [CrossRef]
- Weichert, W.; Röske, A.; Gekeler, V.; Beckers, T.; Stephan, C.; Jung, K.; Fritzsche, F.; Niesporek, S.; Denkert, C.; Dietel, M. Histone deacetylases 1, 2 and 3 are highly expressed in prostate cancer and HDAC2 expression is associated with shorter PSA relapse time after radical prostatectomy. Br. J. Cancer 2008, 98, 604–610. [Google Scholar] [CrossRef]
- Poyet, C.; Jentsch, B.; Hermanns, T.; Schweckendiek, D.; Seifert, H.H.; Schmidtpeter, M.; Sulser, T.; Moch, H.; Wild, P.J.; Kristiansen, G. Expression of histone deacetylases 1, 2 and 3 in urothelial bladder cancer. BMC Clin. Pathol. 2014, 14, 10. [Google Scholar] [CrossRef]
- Moreno, D.A.; Scrideli, C.A.; Cortez, M.A.; de Paula Queiroz, R.; Valera, E.T.; da Silva Silveira, V.; Yunes, J.A.; Brandalise, S.R.; Tone, L.G. Differential expression of HDAC3, HDAC7 and HDAC9 is associated with prognosis and survival in childhood acute lymphoblastic leukaemia. Br. J. Haematol. 2010, 150, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C.; Kafeel, M.I.; Avezbakiyev, B.; Chen, C.; Sun, Y.; Rathnasabapathy, C.; Kalavar, M.; He, Z.; Burton, J.; Lichter, S. Histone Deacetylase in Chronic Lymphocytic Leukemia. Oncology 2012, 81, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Min, S.K.; Koh, Y.H.; Park, Y.; Kim, H.J.; Seo, J.; Park, H.-R.; Cho, S.J.; Kim, I.S. Expression of HAT1 and HDAC1, 2, 3 in diffuse large B-cell lymphomas, peripheral T-cell lymphomas, and NK/T-cell lymphomas. Korean J. Pathol. 2012, 46, 142. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Yoo, C.; Im, S.; Jung, J.H.; Choi, H.J.; Yoo, J. Expression of histone deacetylases in diffuse large B-cell lymphoma and its clinical significance. Int. J. Med. Sci. 2014, 11, 994–1000. [Google Scholar] [CrossRef]
- Marquard, L.; Poulsen, C.B.; Gjerdrum, L.M.; de Nully Brown, P.; Christensen, I.J.; Jensen, P.B.; Sehested, M.; Johansen, P.; Ralfkiaer, E. Histone deacetylase 1, 2, 6 and acetylated histone H4 in B- and T-cell lymphomas. Histopathology 2009, 54, 688–698. [Google Scholar] [CrossRef]
- Gupta, M.; Han, J.J.; Stenson, M.; Wellik, L.; Witzig, T.E. Regulation of STAT3 by histone deacetylase-3 in diffuse large B-cell lymphoma: Implications for therapy. Leukemia 2012, 26, 1356–1364. [Google Scholar] [CrossRef]
- Bradbury, C.A.; Khanim, F.L.; Hayden, R.; Bunce, C.M.; White, D.A.; Drayson, M.T.; Craddock, C.; Turner, B.M. Histone deacetylases in acute myeloid leukaemia show a distinctive pattern of expression that changes selectively in response to deacetylase inhibitors. Leukemia 2005, 19, 1751–1759. [Google Scholar] [CrossRef]
- Marquard, L.; Gjerdrum, L.; Christensen, I.J.; Jensen, P.; Sehested, M.; Ralfkiaer, E. Prognostic significance of the therapeutic targets histone deacetylase 1, 2, 6 and acetylated histone H4 in cutaneous T-cell lymphoma. Histopathology 2008, 53, 267–277. [Google Scholar] [CrossRef]
- Adams, H.; Fritzsche, F.R.; Dirnhofer, S.; Kristiansen, G.; Tzankov, A. Class I histone deacetylases 1, 2 and 3 are highly expressed in classical Hodgkin’s lymphoma. Expert Opin. Ther. Targets 2010, 14, 577–584. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Kalff, A.; Chow, A.; Khong, T.; Spencer, A. Dysregulated Class I histone deacetylases are indicators of poor prognosis in multiple myeloma. Epigenetics 2014, 9, 1511–1520. [Google Scholar] [CrossRef]
- de Lera, A.R.; Ganesan, A. Epigenetic polypharmacology: From combination therapy to multitargeted drugs. Clin. Epigenet. 2016, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Soltan, O.M.; Shoman, M.E.; Abdel-Aziz, S.A.; Narumi, A.; Konno, H.; Abdel-Aziz, M. Molecular hybrids: A five-year survey on structures of multiple targeted hybrids of protein kinase inhibitors for cancer therapy. Eur. J. Med. Chem. 2021, 225, 113768. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhai, H.-X.; Wang, J.; Forrester, J.; Qu, H.; Yin, L.; Lai, C.-J.; Bao, R.; Qian, C. Discovery of 7-(4-(3-Ethynylphenylamino)-7-methoxyquinazolin-6-yloxy)-N-hydroxyheptanamide (CUDC-101) as a Potent Multi-Acting HDAC, EGFR, and HER2 Inhibitor for the Treatment of Cancer. J. Med. Chem. 2010, 53, 2000–2009. [Google Scholar] [CrossRef]
- Mahboobi, S.; Dove, S.; Sellmer, A.; Winkler, M.; Eichhorn, E.; Pongratz, H.; Ciossek, T.; Baer, T.; Maier, T.; Beckers, T. Design of Chimeric Histone Deacetylase- and Tyrosine Kinase-Inhibitors: A Series of Imatinib Hybrides as Potent Inhibitors of Wild-Type and Mutant BCR-ABL, PDGF-Rβ, and Histone Deacetylases. J. Med. Chem. 2009, 52, 2265–2279. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Mustafa, N.; Tan, E.C.; Poulsen, A.; Singh, P.; Duong-Thi, M.-D.; Lee, J.X.T.; Ramanujulu, P.M.; Chng, W.J.; Yen, J.J.Y.; et al. Design and Synthesis of Ligand Efficient Dual Inhibitors of Janus Kinase (JAK) and Histone Deacetylase (HDAC) Based on Ruxolitinib and Vorinostat. J. Med. Chem. 2017, 60, 8336–8357. [Google Scholar] [CrossRef]
- Yu, Y.; Ran, D.; Jiang, J.; Pan, T.; Dan, Y.; Tang, Q.; Li, W.; Zhang, L.; Gan, L.; Gan, Z. Discovery of novel 9H-purin derivatives as dual inhibitors of HDAC1 and CDK2. Bioorg. Med. Chem. Lett. 2019, 29, 2136–2140. [Google Scholar] [CrossRef]
- Millis, S.Z.; Ikeda, S.; Reddy, S.; Gatalica, Z.; Kurzrock, R. Landscape of Phosphatidylinositol-3-Kinase Pathway Alterations Across 19 784 Diverse Solid Tumors. JAMA Oncol. 2016, 2, 1565–1573. [Google Scholar] [CrossRef]
- Thakur, A.; Tawa, G.J.; Henderson, M.J.; Danchik, C.; Liu, S.; Shah, P.; Wang, A.Q.; Dunn, G.; Kabir, M.; Padilha, E.C.; et al. Design, Synthesis, and Biological Evaluation of Quinazolin-4-one-Based Hydroxamic Acids as Dual PI3K/HDAC Inhibitors. J. Med. Chem. 2020, 63, 4256–4292. [Google Scholar] [CrossRef]
- Mastrangelo, S.; Attina, G.; Triarico, S.; Romano, A.; Maurizi, P.; Ruggiero, A. The DNA-topoisomerase inhibitors in cancer therapy. Biomed. Pharmacol. J. 2022, 15, 553–562. [Google Scholar] [CrossRef]
- Guerrant, W.; Patil, V.; Canzoneri, J.C.; Yao, L.-P.; Hood, R.; Oyelere, A.K. Dual-acting histone deacetylase-topoisomerase I inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 3283–3287. [Google Scholar] [CrossRef]
- Chen, W.; Dong, G.; Wu, Y.; Zhang, W.; Miao, C.; Sheng, C. Dual NAMPT/HDAC Inhibitors as a New Strategy for Multitargeting Antitumor Drug Discovery. ACS Med. Chem. Lett. 2018, 9, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Shao, M.; He, L.; Zheng, L.; Huang, L.; Zhou, Y.; Wang, T.; Chen, Y.; Shen, M.; Wang, F.; Yang, Z.; et al. Structure-based design, synthesis and in vitro antiproliferative effects studies of novel dual BRD4/HDAC inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 4051–4055. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Hu, W.; Wang, Z.; Gou, S. Platinum(IV) prodrugs multiply targeting genomic DNA, histone deacetylases and PARP-1. Eur. J. Med. Chem. 2017, 141, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, L.; Lindquist, S.L. HSP90 and the chaperoning of cancer. Nat. Rev. Cancer 2005, 5, 761–772. [Google Scholar] [CrossRef]
- Bali, P.; Pranpat, M.; Bradner, J.; Balasis, M.; Fiskus, W.; Guo, F.; Rocha, K.; Kumaraswamy, S.; Boyapalle, S.; Atadja, P.; et al. Inhibition of Histone Deacetylase 6 Acetylates and Disrupts the Chaperone Function of Heat Shock Protein 90: A Novel Basis for Antileukemia Activity of Histone Deacetylase Inhibitors*. J. Biol. Chem. 2005, 280, 26729–26734. [Google Scholar] [CrossRef]
- Ojha, R.; Huang, H.-L.; HuangFu, W.-C.; Wu, Y.-W.; Nepali, K.; Lai, M.-J.; Su, C.-J.; Sung, T.-Y.; Chen, Y.-L.; Pan, S.-L.; et al. 1-Aroylindoline-hydroxamic acids as anticancer agents, inhibitors of HSP90 and HDAC. Eur. J. Med. Chem. 2018, 150, 667–677. [Google Scholar] [CrossRef]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Xiao, M.; Li, W.; Miller, D.D. An Overview of Tubulin Inhibitors That Interact with the Colchicine Binding Site. Pharm. Res. 2012, 29, 2943–2971. [Google Scholar] [CrossRef]
- Ravelli, R.B.G.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef]
- McLoughlin, E.C.; O’Boyle, N.M. Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review. Pharmaceuticals 2020, 13, 8. [Google Scholar] [CrossRef]
- Li, N.; Guan, Q.; Hong, Y.; Zhang, B.; Li, M.; Li, X.; Li, B.; Wu, L.; Zhang, W. Discovery of 6-aryl-2-(3,4,5-trimethoxyphenyl)thiazole [3,2-b][1,2,4]triazoles as potent tubulin polymerization inhibitors. Eur. J. Med. Chem. 2023, 256, 115402. [Google Scholar] [CrossRef] [PubMed]
- Prota, A.E.; Danel, F.; Bachmann, F.; Bargsten, K.; Buey, R.M.; Pohlmann, J.; Reinelt, S.; Lane, H.; Steinmetz, M.O. The Novel Microtubule-Destabilizing Drug BAL27862 Binds to the Colchicine Site of Tubulin with Distinct Effects on Microtubule Organization. J. Mol. Biol. 2014, 426, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Gao, M.; Zhang, H.; Guan, Q.; Xu, J.; Li, Y.; Qi, H.; Li, Z.; Zuo, D.; Zhang, W.; et al. BZML, a novel colchicine binding site inhibitor, overcomes multidrug resistance in A549/Taxol cells by inhibiting P-gp function and inducing mitotic catastrophe. Cancer Lett. 2017, 402, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.M.; Meegan, M.J.; Zisterer, D.M. Combretastatins: More Than Just Vascular Targeting Agents? J. Pharmacol. Exp. Therapeut. 2015, 355, 212–227. [Google Scholar] [CrossRef]
- Arnst, K.E.; Wang, Y.; Lei, Z.-N.; Hwang, D.-J.; Kumar, G.; Ma, D.; Parke, D.N.; Chen, Q.; Yang, J.; White, S.W.; et al. Colchicine Binding Site Agent DJ95 Overcomes Drug Resistance and Exhibits Antitumor Efficacy. Mol. Pharmacol. 2019, 96, 73–89. [Google Scholar] [CrossRef]
- Zuco, V.; De Cesare, M.; Cincinelli, R.; Nannei, R.; Pisano, C.; Zaffaroni, N.; Zunino, F. Synergistic Antitumor Effects of Novel HDAC Inhibitors and Paclitaxel In Vitro and In Vivo. PLoS ONE 2011, 6, e29085. [Google Scholar] [CrossRef]
- Chao, M.-W.; Lai, M.-J.; Liou, J.-P.; Chang, Y.-L.; Wang, J.-C.; Pan, S.-L.; Teng, C.-M. The synergic effect of vincristine and vorinostat in leukemia in vitro and in vivo. J. Hematol. Oncol. 2015, 8, 82. [Google Scholar] [CrossRef]
- Pettit, G.R.; Singh, S.B.; Hamel, E.; Lin, C.M.; Alberts, D.S.; Garcia-Kendal, D. Isolation and structure of the strong cell growth and tubulin inhibitor combretastatin A-4. Experientia 1989, 45, 209–211. [Google Scholar] [CrossRef]
- Lin, C.M.; Ho, H.H.; Pettit, G.R.; Hamel, E. Antimitotic natural products combretastatin A-4 and combretastatin A-2: Studies on the mechanism of their inhibition of the binding of colchicine to tubulin. Biochemistry 1989, 28, 6984–6991. [Google Scholar] [CrossRef]
- McGown, A.T.; Fox, B.W. Differential cytotoxicity of combretastatins A1 and A4 in two daunorubicin-resistant P388 cell lines. Cancer Chemother. Pharmacol. 1990, 26, 79–81. [Google Scholar] [CrossRef]
- Tseng, C.-H.; Li, C.-Y.; Chiu, C.-C.; Hu, H.-T.; Han, C.-H.; Chen, Y.-L.; Tzeng, C.-C. Combretastatin A-4 derivatives: Synthesis and evaluation of 2,4,5-triaryl-1H-imidazoles as potential agents against H1299 (non-small cell lung cancer cell). Mol. Divers. 2012, 16, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, X.; Gao, J.; Su, L.; Zhang, L.; Xu, H.; Luan, Y. Anti-tumor activity evaluation of novel tubulin and HDAC dual-targeting inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 2638–2645. [Google Scholar] [CrossRef] [PubMed]
- Mourad, A.A.E.; Mourad, M.A.E.; Jones, P.G. Novel HDAC/Tubulin Dual Inhibitor: Design, Synthesis and Docking Studies of α-Phthalimido-Chalcone Hybrids as Potential Anticancer Agents with Apoptosis-Inducing Activity. Drug Des. Devel. Ther. 2020, 14, 3111–3130. [Google Scholar] [CrossRef]
- Sylvie, D. Antimitotic Chalcones and Related Compounds as Inhibitors of Tubulin Assembly. Anticancer Agents Med. Chem. 2009, 9, 336–347. [Google Scholar]
- Belluti, S.; Orteca, G.; Semeghini, V.; Rigillo, G.; Parenti, F.; Ferrari, E.; Imbriano, C. Potent Anti-Cancer Properties of Phthalimide-Based Curcumin Derivatives on Prostate Tumor Cells. Int. J. Mol. Sci. 2019, 20, 28. [Google Scholar] [CrossRef]
- Khelifi, I.; Naret, T.; Renko, D.; Hamze, A.; Bernadat, G.; Bignon, J.; Lenoir, C.; Dubois, J.; Brion, J.-D.; Provot, O.; et al. Design, synthesis and anticancer properties of IsoCombretaQuinolines as potent tubulin assembly inhibitors. Eur. J. Med. Chem. 2017, 127, 1025–1034. [Google Scholar] [CrossRef]
- Lamaa, D.; Lin, H.-P.; Zig, L.; Bauvais, C.; Bollot, G.; Bignon, J.; Levaique, H.; Pamlard, O.; Dubois, J.; Ouaissi, M.; et al. Design and Synthesis of Tubulin and Histone Deacetylase Inhibitor Based on iso-Combretastatin A-4. J. Med. Chem. 2018, 61, 6574–6591. [Google Scholar] [CrossRef]
- Hauguel, C.; Ducellier, S.; Provot, O.; Ibrahim, N.; Lamaa, D.; Balcerowiak, C.; Letribot, B.; Nascimento, M.; Blanchard, V.; Askenatzis, L.; et al. Design, synthesis and biological evaluation of quinoline-2-carbonitrile-based hydroxamic acids as dual tubulin polymerization and histone deacetylases inhibitors. Eur. J. Med. Chem. 2022, 240, 114573. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, M.; Wang, Y.; Qin, J.; Zhang, Y.; Pang, Y.; Yao, Y.; Yang, H.; Duan, Y. Discovery of novel tubulin/HDAC dual-targeting inhibitors with strong antitumor and antiangiogenic potency. Eur. J. Med. Chem. 2021, 225, 113790. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, W.; Liu, Y.; Gao, T.; Zhu, J.; Tan, Y.; Hu, H.; Liang, W.; Zhao, L.; Chen, J.; et al. Synthesis and bioevaluation of novel stilbene-based derivatives as tubulin/HDAC dual-target inhibitors with potent antitumor activities in vitro and in vivo. Eur. J. Med. Chem. 2023, 257, 115529. [Google Scholar] [CrossRef]
- Li, Y.-R.; Liu, F.-F.; Liu, W.-B.; Zhang, Y.-F.; Tian, X.-Y.; Fu, X.-J.; Xu, Y.; Song, J.; Zhang, S.-Y. A novel aromatic amide derivative SY-65 co-targeted tubulin and histone deacetylase 1 with potent anticancer activity in vitro and in vivo. Biochem. Pharmacol. 2022, 201, 115070. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.; Gosch, L.C.; Dittmer, A.; Rothemund, M.; Mueller, T.; Schobert, R.; Biersack, B.; Volkamer, A.; Höpfner, M. Oxazole-Bridged Combretastatin A-4 Derivatives with Tethered Hydroxamic Acids: Structure–Activity Relations of New Inhibitors of HDAC and/or Tubulin Function. Int. J. Mol. Sci. 2019, 20, 383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Liang, Y.; Zhang, H.; Jiang, H.; Feng, K.; Xu, P.; Wang, J.; Wang, X.; Ding, K.; Luo, C.; et al. Design, synthesis, biological evaluation and cocrystal structures with tubulin of chiral β-lactam bridged combretastatin A-4 analogues as potent antitumor agents. Eur. J. Med. Chem. 2018, 144, 817–842. [Google Scholar] [CrossRef]
- Tang, H.; Liang, Y.; Yu, M.; Cai, S.; Ding, K.; Wang, Y. Discovery of chiral 1,4-diarylazetidin-2-one-based hydroxamic acid derivatives as novel tubulin polymerization inhibitors with histone deacetylase inhibitory activity. Bioorg. Med. Chem. 2023, 92, 117437. [Google Scholar] [CrossRef]
- Tang, H.; Liang, Y.; Shen, H.; Cai, S.; Yu, M.; Fan, H.; Ding, K.; Wang, Y. Discovery of a 2,6-diarylpyridine-based hydroxamic acid derivative as novel histone deacetylase 8 and tubulin dual inhibitor for the treatment of neuroblastoma. Bioorg. Chem. 2022, 128, 106112. [Google Scholar] [CrossRef]
- Zheng, S.; Zhong, Q.; Mottamal, M.; Zhang, Q.; Zhang, C.; LeMelle, E.; McFerrin, H.; Wang, G. Design, Synthesis, and Biological Evaluation of Novel Pyridine-Bridged Analogues of Combretastatin-A4 as Anticancer Agents. J. Med. Chem. 2014, 57, 3369–3381. [Google Scholar] [CrossRef]
- El-Zoghbi, M.S.; Bass, A.K.A.; A Abuo-Rahma, G.E.-D.; Mohamed, M.F.A.; Badr, M.; Al-Ghulikah, H.A.; Abdelhafez, E.-S.M.N. Design, Synthesis and Mechanistic Study of New Dual Targeting HDAC/Tubulin Inhibitors. Future Med. Chem. 2024, 16, 601–622. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Tong, L.; Luo, Y.; Su, M.; Zang, Y.; Li, J.; Lu, W.; Chen, Y. The discovery of colchicine-SAHA hybrids as a new class of antitumor agents. Bioorg. Med. Chem. 2013, 21, 3240–3244. [Google Scholar] [CrossRef]
- Zhang, X.; Kong, Y.; Zhang, J.; Su, M.; Zhou, Y.; Zang, Y.; Li, J.; Chen, Y.; Fang, Y.; Zhang, X.; et al. Design, synthesis and biological evaluation of colchicine derivatives as novel tubulin and histone deacetylase dual inhibitors. Eur. J. Med. Chem. 2015, 95, 127–135. [Google Scholar] [CrossRef]
- Zhu, H.; Li, W.; Shuai, W.; Liu, Y.; Yang, L.; Tan, Y.; Zheng, T.; Yao, H.; Xu, J.; Zhu, Z.; et al. Discovery of novel N-benzylbenzamide derivatives as tubulin polymerization inhibitors with potent antitumor activities. Eur. J. Med. Chem. 2021, 216, 113316. [Google Scholar] [CrossRef]
- Zhu, H.; Tan, Y.; He, C.; Liu, Y.; Duan, Y.; Zhu, W.; Zheng, T.; Li, D.; Xu, J.; Yang, D.-H.; et al. Discovery of a Novel Vascular Disrupting Agent Inhibiting Tubulin Polymerization and HDACs with Potent Antitumor Effects. J. Med. Chem. 2022, 65, 11187–11213. [Google Scholar] [CrossRef] [PubMed]
- Al-Warhi, T.; Aldhahrani, A.; Althobaiti, F.; Fayad, E.; Abu Ali, O.A.; Albogami, S.; Abu Almaaty, A.H.; Khedr, A.I.M.; Bukhari, S.N.A.; Zaki, I. Design, Synthesis and Cytotoxic Activity Evaluation of Newly Synthesized Amides-Based TMP Moiety as Potential Anticancer Agents over HepG2 Cells. Molecules 2022, 27, 3960. [Google Scholar] [CrossRef] [PubMed]
- Korolyov, A.; Dorbes, S.; Azéma, J.; Guidetti, B.; Danel, M.; Lamoral-Theys, D.; Gras, T.; Dubois, J.; Kiss, R.; Martino, R.; et al. Novel lipophilic 7H-pyrido [1,2,3-de]-1,4-benzoxazine-6-carboxylic acid derivatives as potential antitumor agents: Improved synthesis and in vitro evaluation. Bioorg. Med. Chem. 2010, 18, 8537–8548. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, X.; Sun, S.; Liu, Y. Synthesis and biological evaluation of novel quinolone derivatives dual targeting histone deacetylase and tubulin polymerization as antiproliferative agents. RSC Adv. 2018, 8, 16494–16502. [Google Scholar] [CrossRef]
- Romagnoli, R.; Baraldi, P.G.; Carrion, M.D.; Cara, C.L.; Cruz-Lopez, O.; Tolomeo, M.; Grimaudo, S.; Cristina, A.D.; Pipitone, M.R.; Balzarini, J.; et al. Design, synthesis and structure–activity relationship of 2-(3′,4′,5′-trimethoxybenzoyl)-benzo[b]furan derivatives as a novel class of inhibitors of tubulin polymerization. Bioorg. Med. Chem. 2009, 17, 6862–6871. [Google Scholar] [CrossRef]
- Mariotto, E.; Canton, M.; Marchioro, C.; Brancale, A.; Hamel, E.; Varani, K.; Vincenzi, F.; De Ventura, T.; Padroni, C.; Viola, G.; et al. Synthesis and Biological Evaluation of Novel 2-Aroyl Benzofuran-Based Hydroxamic Acids as Antimicrotubule Agents. Int. J. Mol. Sci. 2024, 25, 7519. [Google Scholar] [CrossRef]
- Singh, A.; Fatima, K.; Singh, A.; Behl, A.; Mintoo, M.J.; Hasanain, M.; Ashraf, R.; Luqman, S.; Shanker, K.; Mondhe, D.M.; et al. Anticancer activity and toxicity profiles of 2-benzylidene indanone lead molecule. Eur. J. Pharm. Sci. 2015, 76, 57–67. [Google Scholar] [CrossRef]
- Saxena, H.O.; Faridi, U.; Srivastava, S.; Kumar, J.K.; Darokar, M.P.; Luqman, S.; Chanotiya, C.S.; Krishna, V.; Negi, A.S.; Khanuja, S.P.S. Gallic acid-based indanone derivatives as anticancer agents. Bioorg. Med. Chem. Lett. 2008, 18, 3914–3918. [Google Scholar] [CrossRef]
- Negi, A.S.; Prakasham, A.P.; Saxena, A.K.; Luqman, S.; Chanda, D.; Kaur, T.; Gupta, A. Anticancer and Tubulin Polymerisation Activity of Benzylidene Indanones and the Process of Preparing the Same. US Patent US8633242 B2, 21 January 2014. [Google Scholar]
- Kumar, K.; Das, R.; Thapa, B.; Rakhecha, B.; Srivastava, S.; Savita, K.; Israr, M.; Chanda, D.; Banerjee, D.; Shanker, K.; et al. Dual targeted 2-Benzylideneindanone pendant hydroxamic acid group exhibits selective HDAC6 inhibition along with tubulin stabilization effect. Bioorg. Med. Chem. 2023, 86, 117300. [Google Scholar] [CrossRef]
- Li, L.; Quan, D.; Chen, J.; Ding, J.; Zhao, J.; Lv, L.; Chen, J. Design, synthesis, and biological evaluation of 1-substituted -2-aryl imidazoles targeting tubulin polymerization as potential anticancer agents. Eur. J. Med. Chem. 2019, 184, 111732. [Google Scholar] [CrossRef]
- Peng, X.; Chen, J.; Li, L.; Sun, Z.; Liu, J.; Ren, Y.; Huang, J.; Chen, J. Efficient Synthesis and Bioevaluation of Novel Dual Tubulin/Histone Deacetylase 3 Inhibitors as Potential Anticancer Agents. J. Med. Chem. 2021, 64, 8447–8473. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Laurent, G.; Bause, A.S.; Spang, R.; German, N.; Haigis, M.C.; Haigis, K.M. HDAC6 and SIRT2 regulate the acetylation state and oncogenic activity of mutant K-RAS. Mol. Cancer Res. 2013, 11, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Schiedel, M.; Rumpf, T.; Karaman, B.; Lehotzky, A.; Oláh, J.; Gerhardt, S.; Ovádi, J.; Sippl, W.; Einsle, O.; Jung, M. Aminothiazoles as Potent and Selective Sirt2 Inhibitors: A Structure–Activity Relationship Study. J. Med. Chem. 2016, 59, 1599–1612. [Google Scholar] [CrossRef] [PubMed]
- Sinatra, L.; Vogelmann, A.; Friedrich, F.; Tararina, M.A.; Neuwirt, E.; Colcerasa, A.; König, P.; Toy, L.; Yesiloglu, T.Z.; Hilscher, S.; et al. Development of First-in-Class Dual Sirt2/HDAC6 Inhibitors as Molecular Tools for Dual Inhibition of Tubulin Deacetylation. J. Med. Chem. 2023, 66, 14787–14814. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Y.; Qin, J.; Ba, M.; Yao, Y.; Duan, Y.; Liu, H.; Yu, D. Synthesis and biological evaluation of new 2-methoxyestradiol derivatives: Potent inhibitors of angiogenesis and tubulin polymerization. Bioorg. Chem. 2021, 113, 104988. [Google Scholar] [CrossRef]
- Sun, M.; Qin, J.; Kang, Y.; Zhang, Y.; Ba, M.; Yang, H.; Duan, Y.; Yao, Y. 2-Methoxydiol derivatives as new tubulin and HDAC dual-targeting inhibitors, displaying antitumor and antiangiogenic response. Bioorg. Chem. 2022, 120, 105625. [Google Scholar] [CrossRef]
- Xie, S.; Leng, J.; Zhao, S.; Zhu, L.; Zhang, M.; Ning, M.; Zhao, B.; Kong, L.; Yin, Y. Design and biological evaluation of dual tubulin/HDAC inhibitors based on millepachine for treatment of prostate cancer. Eur. J. Med. Chem. 2024, 268, 116301. [Google Scholar] [CrossRef]
- Zhang, X.; Bao, B.; Yu, X.; Tong, L.; Luo, Y.; Huang, Q.; Su, M.; Sheng, L.; Li, J.; Zhu, H.; et al. The discovery and optimization of novel dual inhibitors of topoisomerase II and histone deacetylase. Bioorg. Med. Chem. 2013, 21, 6981–6995. [Google Scholar] [CrossRef]
- Yong, Y.; Shin, S.Y.; Lee, Y.H.; Lim, Y. Antitumor activity of deoxypodophyllotoxin isolated from Anthriscus sylvestris: Induction of G2/M cell cycle arrest and caspase-dependent apoptosis. Bioorg. Med. Chem. Lett. 2009, 19, 4367–4371. [Google Scholar] [CrossRef]
- Shin, S.Y.; Yong, Y.; Kim, C.G.; Lee, Y.H.; Lim, Y. Deoxypodophyllotoxin induces G2/M cell cycle arrest and apoptosis in HeLa cells. Cancer Lett. 2010, 287, 231–239. [Google Scholar] [CrossRef]
- Chen, S.-W.; Gao, Y.-Y.; Zhou, N.-N.; Liu, J.; Huang, W.-T.; Hui, L.; Jin, Y.; Jin, Y.-X. Carbamates of 4′-demethyl-4-deoxypodophyllotoxin: Synthesis, cytotoxicity and cell cycle effects. Bioorg. Med. Chem. Lett. 2011, 21, 7355–7358. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Su, M.; Zhou, Y.; Chen, Y.; Li, J.; Lu, W. Design, synthesis and biological evaluation of 4′-demethyl-4-deoxypodophyllotoxin derivatives as novel tubulin and histone deacetylase dual inhibitors. RSC Adv. 2014, 4, 40444–40448. [Google Scholar] [CrossRef]
- Schiff, P.B.; Fant, J.; Horwitz, S.B. Promotion of microtubule assembly in vitro by taxol. Nature 1979, 277, 665–667. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, K.; Zhu, Q.; Shen, Q.; Zhang, Q.; Yu, J.; Chen, Y.; Lu, W. Synthesis and biological evaluation of paclitaxel and vorinostat co-prodrugs for overcoming drug resistance in cancer therapy in vitro. Bioorg. Med. Chem. 2019, 27, 1405–1413. [Google Scholar] [CrossRef]
- Yoshimatsu, K.; Yamaguchi, A.; Yoshino, H.; Koyanagi, N.; Kitoh, K. Mechanism of Action of E7010, an Orally Active Sulfonamide Antitumor Agent: Inhibition of Mitosis by Binding to the Colchicine Site of Tubulin. Cancer Res. 1997, 57, 3208–3213. [Google Scholar]
- Wu, W.-C.; Liu, Y.-M.; Lin, M.-H.; Liao, Y.-H.; Lai, M.-J.; Chuang, H.-Y.; Hung, T.-Y.; Chen, C.-H.; Liou, J.-P. Design, synthesis, and evaluation of N-phenyl-4-(2-phenylsulfonamido)-benzamides as microtubule-targeting agents in drug-resistant cancer cells, displaying HDAC inhibitory response. Eur. J. Med. Chem. 2020, 192, 112158. [Google Scholar] [CrossRef]
- Chang, J.-Y.; Hsieh, H.-P.; Chang, C.-Y.; Hsu, K.-S.; Chiang, Y.-F.; Chen, C.-M.; Kuo, C.-C.; Liou, J.-P. 7-Aroyl-aminoindoline-1-sulfonamides as a Novel Class of Potent Antitubulin Agents. J. Med. Chem. 2006, 49, 6656–6659. [Google Scholar] [CrossRef]
- Lai, M.-J.; Ojha, R.; Lin, M.-H.; Liu, Y.-M.; Lee, H.-Y.; Lin, T.E.; Hsu, K.-C.; Chang, C.-Y.; Chen, M.-C.; Nepali, K.; et al. 1-Arylsulfonyl indoline-benzamides as a new antitubulin agents, with inhibition of histone deacetylase. Eur. J. Med. Chem. 2019, 162, 612–630. [Google Scholar] [CrossRef]
- Kuo, C.-C.; Hsieh, H.-P.; Pan, W.-Y.; Chen, C.-P.; Liou, J.-P.; Lee, S.-J.; Chang, Y.-L.; Chen, L.-T.; Chen, C.-T.; Chang, J.-Y. BPR0L075, a Novel Synthetic Indole Compound with Antimitotic Activity in Human Cancer Cells, Exerts Effective Antitumoral Activity in Vivo. Cancer Res. 2004, 64, 4621–4628. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Tsai, A.-C.; Chen, M.-C.; Shen, P.-J.; Cheng, Y.-C.; Kuo, C.-C.; Pan, S.-L.; Liu, Y.-M.; Liu, J.-F.; Yeh, T.-K.; et al. Azaindolylsulfonamides, with a More Selective Inhibitory Effect on Histone Deacetylase 6 Activity, Exhibit Antitumor Activity in Colorectal Cancer HCT116 Cells. J. Med. Chem. 2014, 57, 4009–4022. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Lee, J.-F.; Kumar, S.; Wu, Y.-W.; HuangFu, W.-C.; Lai, M.-J.; Li, Y.-H.; Huang, H.-L.; Kuo, F.-C.; Hsiao, C.-J.; et al. 3-Aroylindoles display antitumor activity in vitro and in vivo: Effects of N1-substituents on biological activity. Eur. J. Med. Chem. 2017, 125, 1268–1278. [Google Scholar] [CrossRef]
- Wu, Y.-W.; Hsu, K.-C.; Lee, H.-Y.; Huang, T.-C.; Lin, T.E.; Chen, Y.-L.; Sung, T.-Y.; Liou, J.-P.; Hwang-Verslues, W.W.; Pan, S.-L.; et al. A Novel Dual HDAC6 and Tubulin Inhibitor, MPT0B451, Displays Anti-tumor Ability in Human Cancer Cells in Vitro and in Vivo. Front. Pharmacol. 2018, 9, 205. [Google Scholar] [CrossRef]
- Matsuyama, A.; Shimazu, T.; Sumida, Y.; Saito, A.; Yoshimatsu, Y.; Seigneurin-Berny, D.; Osada, H.; Komatsu, Y.; Nishino, N.; Khochbin, S.; et al. In vivodestabilization of dynamic microtubules by HDAC6-mediated deacetylation. EMBO J. 2002, 21, 6820–6831. [Google Scholar] [CrossRef]
- Hubbert, C.; Guardiola, A.; Shao, R.; Kawaguchi, Y.; Ito, A.; Nixon, A.; Yoshida, M.; Wang, X.-F.; Yao, T.-P. HDAC6 is a microtubule-associated deacetylase. Nature 2002, 417, 455–458. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Caron, C.; Matthias, G.; Hess, D.; Khochbin, S.; Matthias, P. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 2003, 22, 1168–1179. [Google Scholar] [CrossRef]
- Nepali, K.; Chang, T.-Y.; Lai, M.-J.; Hsu, K.-C.; Yen, Y.; Lin, T.E.; Lee, S.-B.; Liou, J.-P. Purine/purine isoster based scaffolds as new derivatives of benzamide class of HDAC inhibitors. Eur. J. Med. Chem. 2020, 196, 112291. [Google Scholar] [CrossRef]
- Schemies, J.; Sippl, W.; Jung, M. Histone deacetylase inhibitors that target tubulin. Cancer Lett. 2009, 280, 222–232. [Google Scholar] [CrossRef]
- Singh, A.; Chang, T.-Y.; Kaur, N.; Hsu, K.-C.; Yen, Y.; Lin, T.E.; Lai, M.-J.; Lee, S.-B.; Liou, J.-P. CAP rigidification of MS-275 and chidamide leads to enhanced antiproliferative effects mediated through HDAC1, 2 and tubulin polymerization inhibition. Eur. J. Med. Chem. 2021, 215, 113169. [Google Scholar] [CrossRef]
- Xue, J.; Wu, G.; Ejaz, U.; Akhtar, F.; Wan, X.; Zhu, Y.; Geng, A.; Chen, Y.; He, S. A novel histone deacetylase inhibitor LT-548-133-1 induces apoptosis by inhibiting HDAC and interfering with microtubule assembly in MCF-7 cells. Investig. New Drugs 2021, 39, 1222–1231. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, T.; Wang, F.; Niu, T.; Liu, Z.; Chen, X.; Long, C.; Tang, M.; Cao, D.; Wang, X.; et al. Discovery of Selective Histone Deacetylase 6 Inhibitors Using the Quinazoline as the Cap for the Treatment of Cancer. J. Med. Chem. 2016, 59, 1455–1470. [Google Scholar] [CrossRef]
- Wang, F.; Zheng, L.; Yi, Y.; Yang, Z.; Qiu, Q.; Wang, X.; Yan, W.; Bai, P.; Yang, J.; Li, D.; et al. SKLB-23bb, A HDAC6-Selective Inhibitor, Exhibits Superior and Broad-Spectrum Antitumor Activity via Additionally Targeting Microtubules. Mol. Cancer Ther. 2018, 17, 763–775. [Google Scholar] [CrossRef]
- Wang, X.-F.; Guan, F.; Ohkoshi, E.; Guo, W.; Wang, L.; Zhu, D.-Q.; Wang, S.-B.; Wang, L.-T.; Hamel, E.; Yang, D.; et al. Optimization of 4-(N-Cycloamino)phenylquinazolines as a Novel Class of Tubulin-Polymerization Inhibitors Targeting the Colchicine Site. J. Med. Chem. 2014, 57, 1390–1402. [Google Scholar] [CrossRef]
- Gryder, B.E.; Sodji, Q.H.; Oyelere, A.K. Targeted Cancer Therapy: Giving Histone Deacetylase Inhibitors All They Need To Succeed. Future Med. Chem. 2012, 4, 505–524. [Google Scholar] [CrossRef]
- Ning, Z.-Q.; Li, Z.-B.; Newman, M.J.; Shan, S.; Wang, X.-H.; Pan, D.-S.; Zhang, J.; Dong, M.; Du, X.; Lu, X.-P. Chidamide (CS055/HBI-8000): A new histone deacetylase inhibitor of the benzamide class with antitumor activity and the ability to enhance immune cell-mediated tumor cell cytotoxicity. Cancer Chemother. Pharmacol. 2012, 69, 901–909. [Google Scholar] [CrossRef]
- Vasudevan, A.; Ji, Z.; Frey, R.R.; Wada, C.K.; Steinman, D.; Heyman, H.R.; Guo, Y.; Curtin, M.L.; Guo, J.; Li, J.; et al. Heterocyclic ketones as inhibitors of histone deacetylase. Bioorg. Med. Chem. Lett. 2003, 13, 3909–3913. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Al-Hamashi, A.A.; Koranne, R.; Dlamini, S.; Alqahtani, A.; Karaj, E.; Rashid, M.S.; Knoff, J.R.; Dunworth, M.; Pflum, M.K.H.; Casero, R.A.; et al. A new class of cytotoxic agents targets tubulin and disrupts microtubule dynamics. Bioorg. Chem. 2021, 116, 105297. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).