Rubus urticifolius Compounds with Antioxidant Activity, and Inhibition Potential against Tyrosinase, Melanin, Hyaluronidase, Elastase, and Collagenase
Abstract
1. Introduction
2. Results
2.1. Isolation and Characterisation of Compounds of R. urticifolius
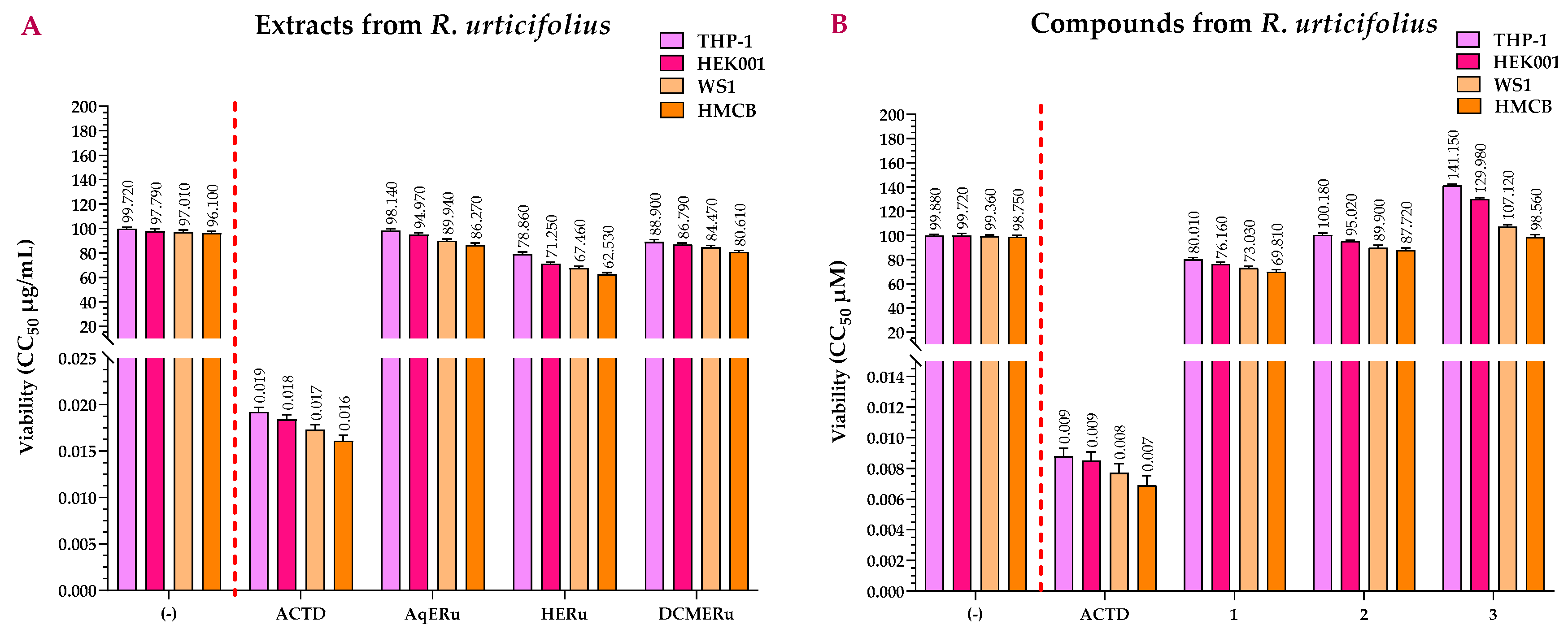
2.2. Viability Assay of the Extracts and Compounds of R. urticifolius
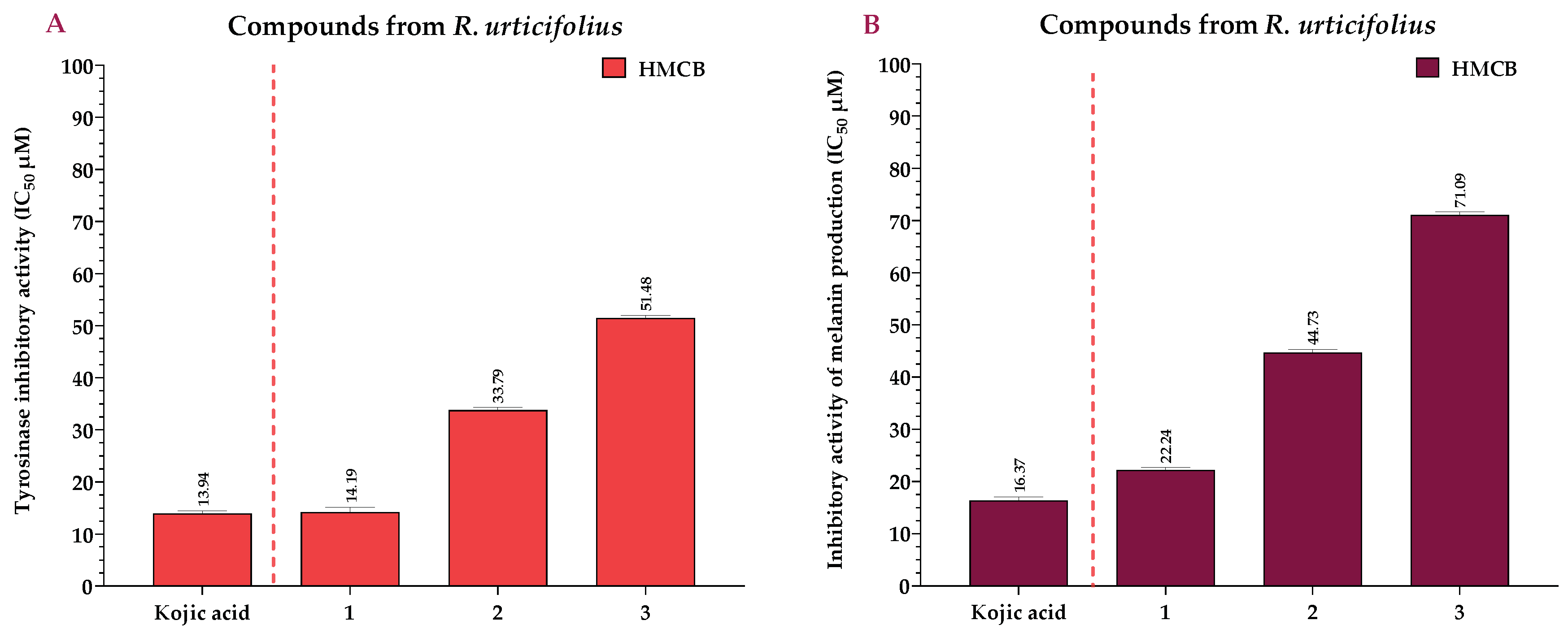
2.3. Effects of the Extracts and Compounds of R. urticifolius on the Inhibition of Tyrosinase and Melanin Production
2.4. Anti-Inflammatory Activity of the Extracts and Compounds of R. urticifolius
2.5. Antioxidant Capacity of the Extracts and Compounds of R. urticifolius
2.6. Anti-Hyaluronidase Activity of the Extracts and Compounds of R. urticifolius
2.7. Anti-Elastase and Anti-Collagenase Activities of the Extracts and Compounds of R. urticifolius
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Extraction and Isolation
4.3. Spectroscopic Data
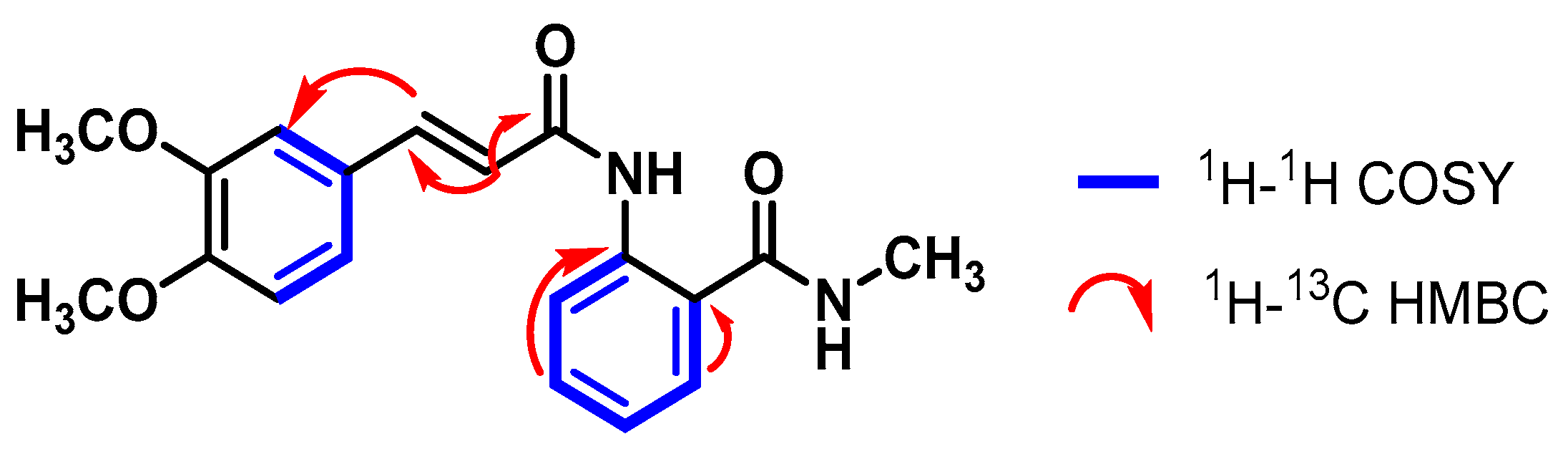
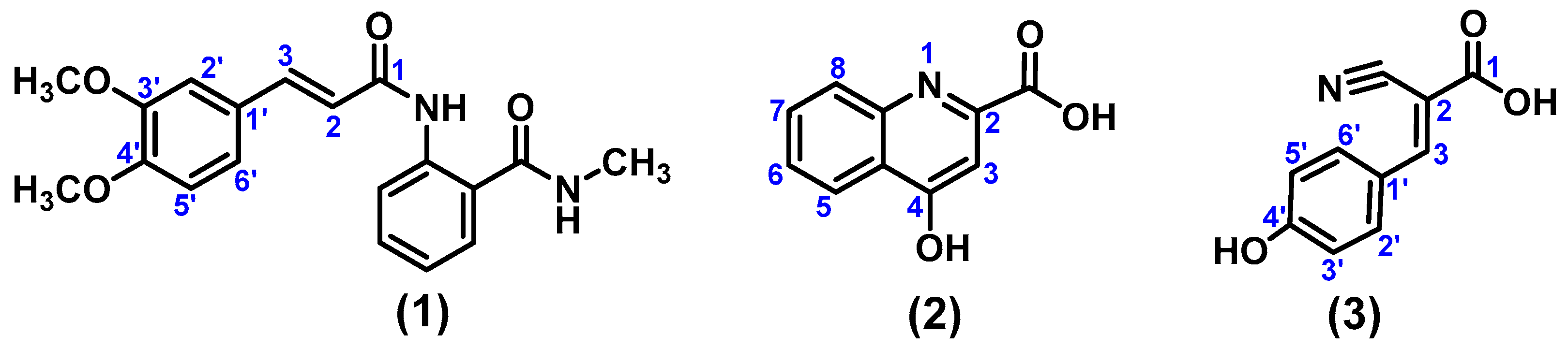
4.3.1. (E)-2-(3-(3,4-Dimethoxyphenyl)acrylamido)-N-methylbenzamide (1)
4.3.2. 4-Hydroxyquinoline-2-carboxylic Acid (2)
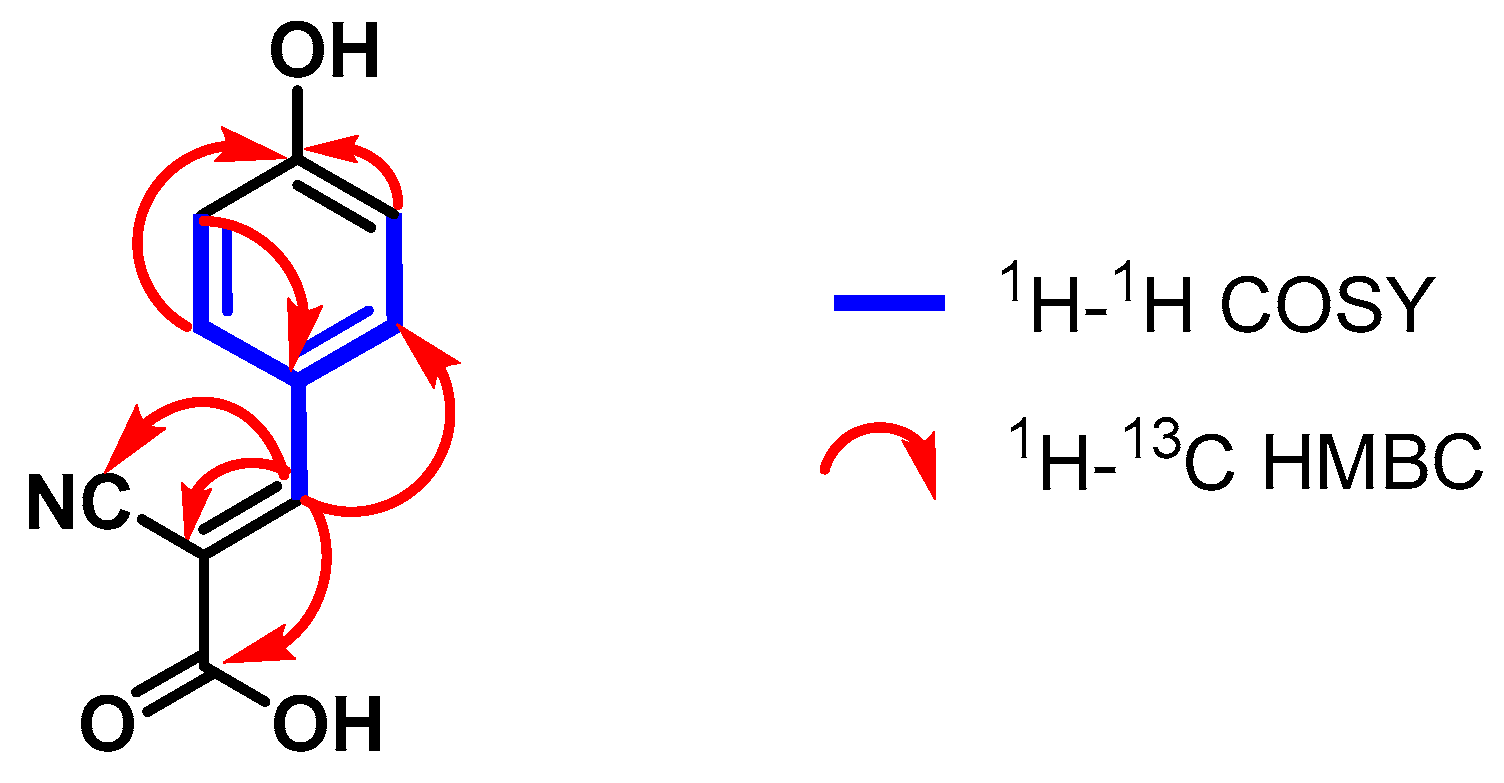
4.3.3. (E)-2-Cyano-3-(4-hydroxyphenyl)acrylic Acid (3)
4.4. Cell Culture
4.5. Statistical Analysis
4.6. In Vitro Viability Assay
4.7. Cellular Tyrosinase Assay
4.8. Melanin Content Assay
4.9. NF-κB Inhibition Assay
4.10. Nrf2 Activity Assay
4.11. Hyaluronidase Inhibition Assay
4.12. Elastase-Inhibitory Effect Assay
4.13. Collagenase Inhibitory Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moolla, S.; Miller-Monthrope, Y. Dermatology: How to Manage Facial Hyperpigmentation in Skin of Colour. Drugs Context 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Beygi, M.; Mohammad, T.F.; Alijanianzadeh, M.; Pillaiyar, T.; Garcia-Molina, P.; Garcia-Canovas, F.; Munoz-Munoz, J.; Saboury, A.A. Targeting Tyrosinase in Hyperpigmentation: Current Status, Limitations and Future Promises. Biochem. Pharmacol. 2023, 212, 115574. [Google Scholar] [CrossRef] [PubMed]
- Lipsker, D.; Lenormand, C. Hyperpigmentations Hyperpigmentation. Ann. Dermatol. Venereol. 2019, 146, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB Signaling in Inflammation. Signal Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Ebanks, J.P.; Wickett, R.R.; Boissy, R.E. Mechanisms Regulating Skin Pigmentation: The Rise and Fall of Complexion Coloration. Int. J. Mol. Sci. 2009, 10, 4066–4087. [Google Scholar] [CrossRef] [PubMed]
- Bang, E.; Kim, D.H.; Chung, H.Y. Protease-Activated Receptor 2 Induces ROS-Mediated Inflammation through Akt-Mediated NF-κB and FoxO6 Modulation during Skin Photoaging. Redox Biol. 2021, 44, 102022. [Google Scholar] [CrossRef] [PubMed]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, J.Y.; Martinez, R.M.; Morocho-Jácome, A.L.; Castillo-Gómez, T.S.; Pereda-Contreras, V.J.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. Skin Impacts from Exposure to Ultraviolet, Visible, Infrared, and Artificial Lights-A Review. J. Cosmet. Laser Ther. 2021, 23, 1–7. [Google Scholar] [CrossRef]
- Lingappan, K. NF-κB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Panich, U. Role of Phytochemicals in Skin Photoprotection via Regulation of Nrf2. Front. Pharmacol. 2022, 13, 823881. [Google Scholar] [CrossRef]
- Galvan-Alvarez, V.; Gallego-Selles, A.; Martinez-Canton, M.; García-Gonzalez, E.; Gelabert-Rebato, M.; Ponce-Gonzalez, J.G.; Larsen, S.; Morales-Alamo, D.; Losa-Reyna, J.; Perez-Suarez, I.; et al. Antioxidant Enzymes and Nrf2/Keap1 in Human Skeletal Muscle: Influence of Age, Sex, Adiposity and Aerobic Fitness. Free Radic. Biol. Med. 2023, 209, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Jiratchayamaethasakul, C.; Ding, Y.; Hwang, O.; Im, S.T.; Jang, Y.; Myung, S.W.; Lee, J.M.; Kim, H.S.; Ko, S.C.; Lee, S.H. In Vitro Screening of Elastase, Collagenase, Hyaluronidase, and Tyrosinase Inhibitory and Antioxidant Activities of 22 Halophyte Plant Extracts for Novel Cosmeceuticals. Fish Aquatic Sci. 2020, 23, 1–9. [Google Scholar] [CrossRef]
- Murthy, R.; Roos, J.C.P.; Goldberg, R.A. Periocular Hyaluronic Acid Fillers: Applications, Implications, Complications. Curr. Opin. Ophthalmol. 2019, 30, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.M.; Avenoso, A.; Campo, S.; D’Ascola, A.; Traina, P.; Calatroni, A. Differential Effect of Molecular Size HA in Mouse Chondrocytes Stimulated with PMA. Biochim. Biophys. Acta 2009, 1790, 1353–1367. [Google Scholar] [CrossRef]
- Jaffray, C.; Yang, J.; Carter, G.; Mendez, C.; Norman, J. Pancreatic Elastase Activates Pulmonary Nuclear Factor Kappa B and Inhibitory Kappa B, Mimicking Pancreatitis-Associated Adult Respiratory Distress Syndrome. Surgery 2000, 128, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Mengshol, J.A.; Vincenti, M.P.; Coon, C.I.; Barchowsky, A.; Brinckerhoff, C.E. Interleukin-1 Induction of Collagenase 3 (Matrix Metalloproteinase 13) Gene Expression in Chondrocytes Requires p38, C-jun N-Terminal Kinase, and Nuclear Factor κB: Differential Regulation of Collagenase 1 and Collagenase 3. Arthritis Rheum. 2000, 43, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Yang, A.; Wang, J.; Huang, D.; Deng, Y.; Zhang, X.; Qu, Q.; Ma, W.; Xiong, R.; Zhu, M.; et al. Potential Application of Natural Bioactive Compounds as Skin-Whitening Agents: A Review. J. Cosmet. Dermatol. 2022, 21, 6669–6687. [Google Scholar] [CrossRef]
- Rathee, P.; Kumar, S.; Kumar, D.; Kumari, B.; Yadav, S.S. Skin Hyperpigmentation and Its Treatment with Herbs: An Alternative Method. Futur. J. Pharm. Sci. 2021, 7, 132. [Google Scholar] [CrossRef]
- Wfoplantlist. Available online: https://wfoplantlist.org/taxon/wfo-4000033581-2023-12?page=1 (accessed on 23 January 2024).
- Rocabado, G.; Bedoya, L.M.; Abad, M.J.F.; Bermejo, P. Rubus-A Review of Its Phytochemical and Pharmacological Profile. Nat. Prod. Commun. 2008, 3, 423–436. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Z.; Wang, X.; Qian, J.; Huang, L.; Li, Z.; Li, W. Studies of Value in Use, Chemical Compositions, Biological and Pharmacological Activities, and Quality Control of Rubus Berries: A Comprehensive Review. J. Food Compos. Anal. 2023, 124, 105707. [Google Scholar] [CrossRef]
- Tian, J.L.; Si, X.; Wang, Y.H.; Gong, E.S.; Xie, X.; Zhang, Y.; Li, B.; Shu, C. Bioactive flavonoids from Rubus corchorifolius inhibit α-glucosidase and α-amylase to improve postprandial hyperglycemia. Food Chem. 2021, 341, 128149. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.T.; Jiang, J.G. Analyses on essential oil components from the unripe fruits of Rubus chingii Hu by different methods and their comparative cytotoxic and anti-complement activities. Food Anal. Methods 2015, 8, 937–944. [Google Scholar] [CrossRef]
- Zhang, T.T.; Wang, M.; Yang, L.; Jiang, J.G.; Zhao, J.W.; Zhu, W. Flavonoid glycosides from Rubus chingii Hu fruits display anti-inflammatory activity through suppressing MAPKs activation in macrophages. J. Funct. Foods 2015, 18, 235–243. [Google Scholar] [CrossRef]
- Sun, Z.L.; Zhang, Y.; Wan, A.H.; Zhang, X.L.; Feng, J. A new active compound against kidney deficiency from the fruits of Rubus corchorifolius. J. Asian Nat. Prod. Res. 2011, 13, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, B.N.; Ligarreto, M.G.A.; Barrero, M.L.S.; Medina, C.C.I. Morphological variability of wild blackberry (Rubus sp.) cultivars in the Andes of Colombia. Rev. Colomb. Cienc. Hortic. 2016, 10, 211–221. [Google Scholar] [CrossRef]
- Moreno, M.; Villarreal, D.; Lagos, T.C.; Ordoñez, H.; Criollo, H. Characterization “In situ” of wild and cultivated genotypes of blackberry Rubus spp. in Pasto municipality. Rev. Cienc. Agríc. 2011, 28, 109–128. [Google Scholar]
- Andrade, J.M.; Lucero Mosquera, H.; Armijos, C. Ethnobotany of Indigenous Saraguros: Medicinal Plants Used by Community Healers “Hampiyachakkuna” in the San Lucas Parish, Southern Ecuador. Biomed. Res. Int. 2017, 2017, 9343724. [Google Scholar] [CrossRef]
- Armijos, C.; Ramírez, J.; Vidari, G. Poorly Investigated Ecuadorian Medicinal Plants. Plants 2022, 11, 1590. [Google Scholar] [CrossRef] [PubMed]
- De Lucca, M.; Zalles, J. Utasan Utjir Qollanaka. Medicinas Junto a Nuestra Casa; Agencia Española de Cooperación Internacional: La Paz, Bolivia, 2006; p. 95. [Google Scholar]
- Jung, H.J.; Lee, H.J.; Cho, H.; Hwang, K.T. Anti-Inflammatory Activities of Rubus Fruit Anthocyanins in Inflamed Human Intestinal Epithelial Cells. J. Food Biochem. 2015, 39, 300–309. [Google Scholar] [CrossRef]
- Ding, H. Extracts and Constituents of Rubus chingii with 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Free Radical Scavenging Activity. Int. J. Mol. Sci. 2011, 12, 3941–3949. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, A.; Kozłowska, W.; Kawiak, A. Chemical composition and biological activity of Rubus idaeus shoots—A traditional herbal remedy of Eastern Europe. BMC Complement. Med. Ther. 2014, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Argoti, J.C.; Salido, S.; Linares-Palomino, P.J.; Ramírez, B.; Insuasty, B.; Altarejos, J. Antioxidant activity and free radical-scavenging capacity of a selection of wild-growing Colombian plants. J. Sci. Food Agric. 2011, 91, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Balloch, L.; Hevia, E.; Kennedy, A.; Mulvey, R.; O’Hara, C.; Robertson, S. Meta-metallation of N,N-dimethylaniline: Contrasting direct sodium-mediated zincation with indirect sodiation-dialkylzinc co-complexation. Beilstein J. Org. Chem. 2011, 7, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Santacruz Cifuentes, L.A. Análisis Químico de Antocianinas en Frutos Silvestres Colombianos. Master’s Thesis, Universidad Nacional de Colombia, Facultad de Ciencias, Departamento de Química, Bogotá, Colombia, 2011. [Google Scholar]
- Wyzgoski, F.J.; Paudel, L.; Rinaldi, P.L.; Reese, R.N.; Ozgen, M.; Tulio, A.Z.; Miller, A.R.; Scheerens, J.C.; Hardy, J.K. Modeling relationships among active components in black raspberry (Rubus occidentalis L.) fruit extracts using high-resolution (1)H nuclear magnetic resonance (NMR) spectroscopy and multivariate statistical analysis. J. Agric. Food Chem. 2010, 58, 3407–3414. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Xue, T.; Su, X.; Liu, Z.; Liu, H.; Tan, Z.; Gan, C.; Xie, Y.; Ye, T. Discovery and evaluation of phenacrylanilide derivatives as novel potential anti-liver fibrosis agents. Eur. J. Med. Chem. 2022, 242, 114685. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, K.; Motlagh, H.R.; Keshavarz, M. Tranilast could has potential therapeutic value in the treatment of psoriasis. Med. Hypotheses 2011, 76, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Tajima, S.; Nishikawa, T. Tranilast inhibits collagen synthesis in normal, scleroderma and keloid fibroblasts at a late passage culture but not at an early passage culture. J. Dermatol. Sci. 1995, 9, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Ciorba, M.A. Kynurenine pathway metabolites: Relevant to vitamin B-6 deficiency and beyond. Am. J. Clin. Nutr. 2013, 98, 863–864. [Google Scholar] [CrossRef] [PubMed]
- Balla, Z.; Kormányos, E.S.; Kui, B.; Bálint, E.R.; Fűr, G.; Orján, E.M.; Iványi, B.; Vécsei, L.; Fülöp, F.; Varga, G.; et al. Kynurenic Acid and Its Analogue SZR-72 Ameliorate the Severity of Experimental Acute Necrotizing Pancreatitis. Front. Immunol. 2021, 12, 702764. [Google Scholar] [CrossRef]
- Bratek-Gerej, E.; Ziembowicz, A.; Godlewski, J.; Salinska, E. The Mechanism of the Neuroprotective Effect of Kynurenic Acid in the Experimental Model of Neonatal Hypoxia-Ischemia: The Link to Oxidative Stress. Antioxidants 2021, 10, 1775. [Google Scholar] [CrossRef]
- Medana, I.M.; Day, N.P.; Salahifar-Sabet, H.; Stocker, R.; Smythe, G.; Bwanaisa, L.; Njobvu, A.; Kayira, K.; Turner, G.D.; Taylor, T.E.; et al. Metabolites of the kynurenine pathway of tryptophan metabolism in the cerebrospinal fluid of Malawian children with malaria. J. Infect. Dis. 2003, 188, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Cihan, M.; Doğan, Ö.; Ceran Serdar, C.; Altunçekiç Yıldırım, A.; Kurt, C.; Serdar, M.A. Kynurenine pathway in Coronavirus disease (COVID-19): Potential role in prognosis. J. Clin. Lab. Anal. 2022, 36, e24257. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Role of the Kynurenine Metabolism Pathway in Inflammation-Induced Depression: Preclinical Approaches. Curr. Top. Behav. Neurosci. 2017, 31, 117–138. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Vigentini, I.; Vitalini, S.; Laganaro, A.; Iriti, M.; Paroni, R.; Foschino, R. Tryptophan Derivatives by Saccharomyces cerevisiae EC1118: Evaluation, Optimization, and Production in a Soybean-Based Medium. Int. J. Mol. Sci. 2021, 22, 472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.F.; Yuan, S.; Zhang, S.; Zheng, H.; Liu, J.; Sun, P.; Gu, Y.; Kurihara, H.; He, R.R.; et al. Bioactivity Focus of α-Cyano-4-hydroxycinnamic acid (CHCA) Leads to Effective Multifunctional Aldose Reductase Inhibitors. Sci. Rep. 2016, 6, 24942. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, Q.H.; Zhuang, J.X.; Zhong, X.; Zhou, J.J.; Guo, Y.J.; Chen, Q.X. Inhibitory effects of α-cyano-4-hydroxycinnamic acid on the activity of mushroom tyrosinase. Food Chem. 2009, 112, 609–613. [Google Scholar] [CrossRef]
- Guan, X.; Morris, M.E. In Vitro and In Vivo Efficacy of AZD3965 and Alpha-Cyano-4-Hydroxycinnamic Acid in the Murine 4T1 Breast Tumor Model. AAPS J. 2020, 22, 84. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.H.; Lee, J.Y.; Park, J.Y.; Jang, G.Y.; Kim, H.D.; Lee, Y.S.; Kim, D.H. Differences in anti-inflammatory effect of immature and mature of Rubus coreanus fruits on LPS-induced RAW 264.7 macrophages via NF-κB signal pathways. BMC Complement. Altern. Med. 2019, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Tolentino, F.; Araújo, P.A.; de Marques, E.S.; Petreanu, M.; Andrade, S.F.; Niero, R.; Perazzo, F.F.; Rosa, P.C.; Maistro, E.L. In vivo evaluation of the genetic toxicity of Rubus niveus Thunb. (Rosaceae) extract and initial screening of its potential chemoprevention against doxorubicin-induced DNA damage. J. Ethnopharmacol. 2015, 164, 89–95. [Google Scholar] [CrossRef]
- Holden, L.; Burke, C.S.; Cullinane, D.; Keyes, T.E. Strategies to promote permeation and vectorization, and reduce cytotoxicity of metal complex luminophores for bioimaging and intracellular sensing. RSC Chem. Biol. 2021, 2, 1021–1049. [Google Scholar] [CrossRef]
- Sucu, B.O.; Koc, E.B.; İpek, Ö.Ş.; Mirat, A.; Almas, F.; Guzel, M.A.; Doğan, B.; Uludağ, D.; Karakaş, N.; Durdağı, S.; et al. Design and synthesis of novel caffeic acid phenethyl ester (CAPE) derivatives and their biological activity studies in glioblastoma multiforme (GBM) cancer cell lines. J. Mol. Graph. Model. 2022, 113, 108160. [Google Scholar] [CrossRef] [PubMed]
- Basson, C.; Serem, J.C.; Hlophe, Y.; Bipath, P. An in vitro investigation of l-kynurenine, quinolinic acid, and kynurenic acid on B16 F10 melanoma cell cytotoxicity and morphology. Cell Biochem. Funct. 2023, 41, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Turski, W.A.; Małaczewska, J.; Marciniak, S.; Bednarski, J.; Turski, M.P.; Jabłoński, M.; Siwicki, A.K. On the toxicity of kynurenic acid in vivo and in vitro. Pharmacol. Rep. 2014, 66, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Niero, E.L.; Machado-Santelli, G.M. Cinnamic acid induces apoptotic cell death and cytoskeleton disruption in human melanoma cells. J. Exp. Clin. Cancer Res. 2013, 32, 1–14. [Google Scholar] [CrossRef]
- Desmiaty, Y.; Saputri, F.C.; Hanafi, M.; Prastiwi, R.; Elya, B. Anti-Elastase, Anti-Tyrosinase and Anti-Oxidant of Rubus fraxinifolius Stem Methanolic Extract. Pharmacogn. J. 2020, 12, 271–275. [Google Scholar] [CrossRef]
- García-Borrón, J.C.; Solano, F. Molecular anatomy of tyrosinase and its related proteins: Beyond the histidine-bound metal catalytic center. Pigment Cell Res. 2002, 15, 162–173. [Google Scholar] [CrossRef]
- Varghese, P.K.; Abu-Asab, M.; Dimitriadis, E.K.; Dolinska, M.B.; Morcos, G.P.; Sergeev, Y.V. Tyrosinase Nanoparticles: Understanding the Melanogenesis Pathway by Isolating the Products of Tyrosinase Enzymatic Reaction. Int. J. Mol. Sci. 2021, 22, 734. [Google Scholar] [CrossRef] [PubMed]
- Batoryna, O.; Kamila, Ż.; Banyś, A.; Morawiec, E. The Pluripotent Activities of Caffeic Acid Phenethyl Ester. Molecules 2021, 26, 1335. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Branquinho, M.S.; Silva, M.B.B.; Castilho, G.A.; Cavalcante, J.; Barros, S.B.M.; Clara, R.O.; Maria-Engler, S.S.; Campa, A. Kynurenine inhibits melanogenesis in human melanocyte-keratinocyte co-cultures and in a reconstructed 3D skin model. Exp. Dermatol. 2022, 31, 427–432. [Google Scholar] [CrossRef]
- Hubková, B.; Valko-Rokytovská, M.; Čižmárová, B.; Zábavníková, M.; Mareková, M.; Birková, A. Tryptophan: Its Metabolism along the Kynurenine, Serotonin, and Indole Pathway in Malignant Melanoma. Int. J. Mol. Sci. 2022, 23, 9160. [Google Scholar] [CrossRef]
- Li, J.; Min, X.; Zheng, X.; Wang, S.; Xu, X.; Peng, J. Synthesis, Anti-Tyrosinase Activity, and Spectroscopic Inhibition Mechanism of Cinnamic Acid-Eugenol Esters. Molecules 2023, 28, 5969. [Google Scholar] [CrossRef] [PubMed]
- Mermer, A.; Demirci, S. Recent advances in triazoles as tyrosinase inhibitors. Eur. J. Med. Chem. 2023, 259, 115655. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Oh, S.M.; Lim, S.S.; Shin, H.K.; Oh, Y.S.; Kim, J.K. Antiinflammatory activities of Rubus coreanus depend on the degree of fruit ripening. Phytother. Res. 2008, 22, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Maslov, O.; Komisarenko, M.; Kolisnyk, S.; Derymedvid, L. Evaluation of Anti-Inflammatory, Antioxidant Activities and Molecular Docking Analysis of Rubus idaeus Leaf Extract. Jordan, J. Pharm. Sci. 2024, 17, 105–122. [Google Scholar] [CrossRef]
- Lee, J.E.; Cho, S.M.; Park, E.; Lee, S.M.; Kim, Y.; Auh, J.H.; Choi, H.K.; Lim, S.; Lee, S.C.; Kim, J.H. Anti-inflammatory effects of Rubus coreanus Miquel through inhibition of NF-κB and MAP Kinase. Nutr. Res. Pract. 2014, 8, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; González-Sánchez, F.A.; Sánchez-López, A.L.; Garcia-Amezquita, L.E.; Garcia-Varela, R. Antioxidant, Anti-Inflammatory and Cytotoxic Activity of Phenolic Compound Family Extracted from Raspberries (Rubus idaeus): A General Review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef] [PubMed]
- Armutcu, F.; Akyol, S.; Ustunsoy, S.; Turan, F.F. Therapeutic potential of caffeic acid phenethyl ester and its anti-inflammatory and immunomodulatory effects (Review). Exp. Ther. Med. 2015, 9, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chu, K.H.; Liang, Y.C.; Lin, Y.; Chiang, B. Caffeic acid phenethyl ester inhibits nuclear factor-κB and protein kinase B signalling pathways and induces caspase-3 expression in primary human CD4+ T cells. Clin. Exp. Immunol. 2010, 160, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ji, L.; Zhang, D.; Guo, H.; Wang, Y.; Li, W. Synthesis and Anti-Inflammatory Activity of 1-Methylhydantoin Cinnamoyl Imides. Molecules 2022, 27, 8481. [Google Scholar] [CrossRef]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Günther, J. Kynurenic acid: The Janus-faced role of an immunomodulatory tryptophan metabolite and its link to pathological conditions. Front. Immunol. 2018, 8, 1957. [Google Scholar] [CrossRef]
- Giridharan, S.; Srinivasan, M. Mechanisms of NF-κB p65 and strategies for therapeutic manipulation. J. Inflamm. Res. 2018, 11, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Al-Shabib, N.A.; Khan, J.M.; Malik, A.; Rehman, M.T.; Husain, F.M.; AlAjmi, M.F.; Hamdan Ali Alghamdi, O.; Khan, A. Quinoline yellow dye stimulates whey protein fibrillation via electrostatic and hydrophobic interaction: A biophysical study. J. Dairy Sci. 2021, 104, 5141–5151. [Google Scholar] [CrossRef] [PubMed]
- Munch, G.; Holthoff, H.P.; Ungerer, M.; Kramer, B.; Dormeyer, M. Activation Specific Inhibitors of NF-ΚB And Method of Treating Inflammatory Processes in Cardio-Vascular Diseases. Patent US20060194819A1, 31 August 2006. [Google Scholar]
- Chen, P.; Ruan, A.; Zhou, J.; Huang, L.; Zhang, X.; Ma, Y.; Wang, Q. Cinnamic Aldehyde Inhibits Lipopolysaccharide-Induced Chondrocyte Inflammation and Reduces Cartilage Degeneration by Blocking the Nuclear Factor-Kappa B Signaling Pathway. Front. Pharmacol. 2020, 11, 949. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Reyes-Carmona, J.; Yousef, G.G.; Martínez-Peniche, R.A.; Lila, M.A. Antioxidant Capacity of Fruit Extracts of Blackberry (Rubus sp.) Produced in Different Climatic Regions. J. Food Sci. 2005, 70, s497–s503. [Google Scholar] [CrossRef]
- Ligarda-Samanez, C.A.; Choque-Quispe, D.; Ramos-Pacheco, B.S.; Yanahuillca-Vargas, A.; Huamán-Carrión, M.L.; Moscoso-Moscoso, E.; Palomino-Rincón, H. Taxonomic, physicochemical, phenolic and antioxidant comparison in species of high Andean wild fruits: Rubus and Hesperomeles. Acta Agron. 2023, 72, 30–37. [Google Scholar] [CrossRef]
- Zhang, L.; Gavin, T.; Barber, D.S.; LoPachin, R.M. Role of the Nrf2-ARE pathway in acrylamide neurotoxicity. Toxicol. Lett. 2011, 205, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.S.; Biasibetti-Brendler, H.; Pierozan, P.; Schmitz, F.; Bertó, C.G.; Prezzi, C.A.; Manfredini, V.; Wyse, A.T.S. Kynurenic Acid Restores Nrf2 Levels and Prevents Quinolinic Acid-Induced Toxicity in Rat Striatal Slices. Mol. Neurobiol. 2018, 55, 8538–8549. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, X.; Zhou, Y.; Du, J.; Lu, C.; Zhang, L.; Sun, S.; Wang, S.; Li, Y. Kynurenic acid inhibits macrophage pyroptosis by suppressing ROS production via activation of the NRF2 pathway. Mol. Med. Rep. 2023, 28, 211. [Google Scholar] [CrossRef]
- Malakoutikhah, Z.; Mohajeri, Z.; Dana, N.; Haghjooy Javanmard, S. The dual role of Nrf2 in melanoma: A systematic review. BMC. Mol. Cell. Biol. 2023, 24, 5. [Google Scholar] [CrossRef] [PubMed]
- Hering, A.; Stefanowicz-Hajduk, J.; Hałasa, R.; Olech, M.; Nowak, R.; Kosiński, P.; Ochocka, J.R. Polyphenolic Characterization, Antioxidant, Antihyaluronidase and Antimicrobial Activity of Young Leaves and Stem Extracts from Rubus caesius L. Molecules 2022, 27, 6181. [Google Scholar] [CrossRef] [PubMed]
- Marquina, M.A.; Corao, G.M.; Araujo, L.; Buitrago, D.; Sosa, M. Hyaluronidase inhibitory activity from the polyphenols in the fruit of blackberry (Rubus fruticosus B.). Fitoterapia 2002, 73, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Küpeli Akkol, E.; Süntar, I.; Ilhan, M.; Aras, E. In vitro enzyme inhibitory effects of Rubus sanctus Schreber and its active metabolite as a function of wound healing activity. J. Herb Med. 2015, 5, 207–210. [Google Scholar] [CrossRef]
- Araujo, L.; Buitrago, D.; Marquina, M.; Morales, N.; Méndez, G.C.; Pernia, T.; Sosa, M. Comparation of the polyphenols anti-inflammatory activity present in the fruits: Blackberry (Rubus fruticosus B) strawberry (Fragaria vesca L) and grapefruit (Citrus paradasi M). Rev. Fac. Farm. 2002, 44, 64–69. [Google Scholar]
- Nakahara, K.; Miyagawa, K.; Kodama, T.; Fujii, W. Hyaluronidase Inhibitor Containing God-Type Ellagitannin as Active Ingredient. Patent US5843911A, 21 September 1999. [Google Scholar]
- Osés, S.M.; Marcos, P.; Azofra, P.; de Pablo, A.; Fernández-Muíño, M.Á.; Sancho, M.T. Phenolic Profile, Antioxidant Capacities and Enzymatic Inhibitory Activities of Propolis from Different Geographical Areas: Needs for Analytical Harmonization. Antioxidants 2020, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Gębalski, J.; Graczyk, F.; Załuski, D. Paving the way towards effective plant-based inhibitors of hyaluronidase and tyrosinase: A critical review on a structure-activity relationship. J. Enzyme Inhib. Med. Chem. 2022, 37, 1120–1195. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Watahiki, M.; Tanaka, Y.; Miyase, T.; Yoshizaki, F. Hyaluronidase inhibitors from Takuran, Lycopus lucidus. Chem. Pharm. Bull. 2010, 58, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Kakegawa, H.; Matsumoto, H.; Satoh, T. Activation of hyaluronidase by metallic salts and compound 48/80, and inhibitory effect of anti-allergic agents on hyaluronidase. Chem. Pharm. Bull. 1985, 33, 642–646. [Google Scholar] [CrossRef]
- Kaessler, A.; Nourrisson, M.R.; Duflos, M.; Jose, J. Indole carboxamides inhibit bovine testes hyaluronidase at pH 7.0 and indole acetamides activate the enzyme at pH 3.5 by different mechanisms. J. Enzyme Inhib. Med. Chem. 2008, 23, 719–727. [Google Scholar] [CrossRef][Green Version]
- Osorio, E.; Bravo, K.; Cardona, W.; Yepes, A.; Osorio, E.H.; Co, J.C. Antiaging activity, molecular docking, and prediction of percutaneous absorption parameters of quinoline-hydrazone hybrids. Med. Chem. Res. 2019, 28, 1959–1973. [Google Scholar] [CrossRef]
- Do Prado, F.G.; Pagnoncelli, M.G.B.; Prado, M.R.M.; Corazza, M.L.; Soccol, V.T.; de Melo Pereira, G.V.; Soccol, C.R. Enhancing the Recovery of Bioactive Compounds of Soybean Fermented with Rhizopus oligosporus Using Supercritical CO2: Antioxidant, Anti-Inflammatory, and Oxidative Proprieties of the Resulting Extract. J. Fungi 2022, 8, 1065. [Google Scholar] [CrossRef] [PubMed]
- Thippeswamy, N.B.; Rajeshwara, N.A. Inhibitory effect of phenolic extract of carum carvi on inflammatory enzymes, hyaluronidase and trypsin. World J. Pharm. Pharm. Sci. 2014, 2, 350–356. [Google Scholar]
- Desmiaty, Y.; Hanafi, M.; Saputri, F.C.; Elya, B.; Rifai, E.A.; Syahdi, R.R. Two triterpenoids from Rubus fraxinifolius leaves and their tyrosinase and elastase inhibitory activities. Sci. Rep. 2021, 11, 20452. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Shin, S.Y.; Kim, S.; Lee, K.H.; Shin, J.; Park, K.M. Comparison of antiaging, anti-melanogenesis effects, and active components of Raspberry (Rubus occidentalis L.) extracts according to maturity. J. Food Biochem. 2020, 44, e13464. [Google Scholar] [CrossRef] [PubMed]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Assessment of cytotoxicity and antioxidant properties of berry leaves as by-products with potential application in cosmetic and pharmaceutical products. Sci. Rep. 2021, 11, 3240. [Google Scholar] [CrossRef] [PubMed]
- Deniz, F.S.; Salmas, R.E.; Emerce, E.; Cankaya, I.I.; Yusufoglu, H.S.; Orhan, I.E. Evaluation of collagenase, elastase and tyrosinase inhibitory activities of Cotinus coggygria Scop. through in vitro and in silico approaches. S. Afr. J. Bot. 2020, 132, 277–288. [Google Scholar] [CrossRef]
- Chung, S.W.; Park, I.H.; Hong, S.M.; Cho, J.S.; Moon, J.H.; Kim, T.H.; Lee, H.M. Role of caffeic Acid on collagen production in nasal polyp-derived fibroblasts. Clin. Exp. Otorhinolaryngol. 2014, 7, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Vanjare, B.D.; Seok Eom, Y.; Raza, H.; Hassan, M.; Hwan Lee, K.; Ja Kim, S. Elastase inhibitory activity of quinoline Analogues: Synthesis, kinetic mechanism, cytotoxicity, chemoinformatics and molecular docking studies. Bioorg. Med. Chem. 2022, 63, 116745. [Google Scholar] [CrossRef]
- Min, J.H.; Kim, M.G.; Kim, S.M.; Park, J.W.; Chun, W.; Lee, H.J.; Oh, S.R.; Ahn, K.S.; Lee, J.W. 3,4,5-Trihydroxycinnamic acid exerts a protective effect on pulmonary inflammation in an experimental animal model of COPD. Int. Immunopharmacol 2020, 85, 106656. [Google Scholar] [CrossRef]
- Morikawa, T.; Inoue, N.; Nakanishi, Y.; Manse, Y.; Matsuura, H.; Okino, K.; Hamasaki, S.; Yoshikawa, M.; Muraoka, O.; Ninomiya, K. Collagen synthesis-promoting and collagenase inhibitory activities of constituents isolated from the rhizomes of Picrorhiza kurroa Royle ex Benth. Fitoterapia 2020, 143, 104584. [Google Scholar] [CrossRef] [PubMed]
- Truchado, P.; Martos, I.; Bortolotti, L.; Sabatini, A.G.; Ferreres, F.; Tomas-Barberan, F.A. Use of quinoline alkaloids as markers of the floral origin of chestnut honey. J. Agric. Food Chem. 2009, 57, 5680–5686. [Google Scholar] [CrossRef] [PubMed]
- Mahy, R.; Oulmidi, A.; Challioui, A. Synthesis of Cinnamic Derivatives using Pyridine and Piperidine via Simple Knoevenagel Condensation Reaction. Chem. Res. J. 2018, 3, 28–32. [Google Scholar] [CrossRef]
- Gomez Perez, M.; Fourcade, L.; Mateescu, M.A.; Paquin, J. Neutral Red versus MTT assay of cell viability in the presence of copper compounds. Anal. Biochem. 2017, 535, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Apaza Ticona, L.; Thiebaut Estrada, C.; Rumbero Sánchez, Á. Inhibition of melanin production and tyrosinase activity by flavonoids isolated from Loranthus acutifolius. Nat. Prod. Res. 2021, 35, 4690–4693. [Google Scholar] [CrossRef] [PubMed]
- Apaza Ticona, L.; Rumbero Sánchez, Á.; Sánchez Sánchez-Corral, J.; Iglesias Moreno, P.; Ortega Domenech, M. Anti-inflammatory, pro-proliferative and antimicrobial potential of the compounds isolated from Daemonorops draco (Willd.) Blume. J. Ethnopharmacol. 2021, 268, 113668. [Google Scholar] [CrossRef] [PubMed]
- Sahasrabudhe, A.; Deodhar, M. Anti-hyaluronidase, anti-elastase activity of Garcinia indica. Int. J. Bot. 2010, 6, 299–303. [Google Scholar] [CrossRef]
- Tu, P.T.; Tawata, S. Anti-oxidant, anti-aging, and anti-melanogenic properties of the essential oils from two varieties of Alpinia zerumbet. Molecules 2015, 20, 16723–16740. [Google Scholar] [CrossRef]
- Wang, L.; Lee, W.; Oh, J.Y.; Cui, Y.R.; Ryu, B.; Jeon, Y.J. Protective effect of sulphated polysaccharides from Celluclast-assisted extract of Hizikia fusiforme against ultraviolet B-induced skin damage by regulating NF-κB, AP-1, and MAPKs signaling pathways in vitro in human dermal fibroblasts. Mar. Drugs 2018, 16, 239. [Google Scholar] [CrossRef]
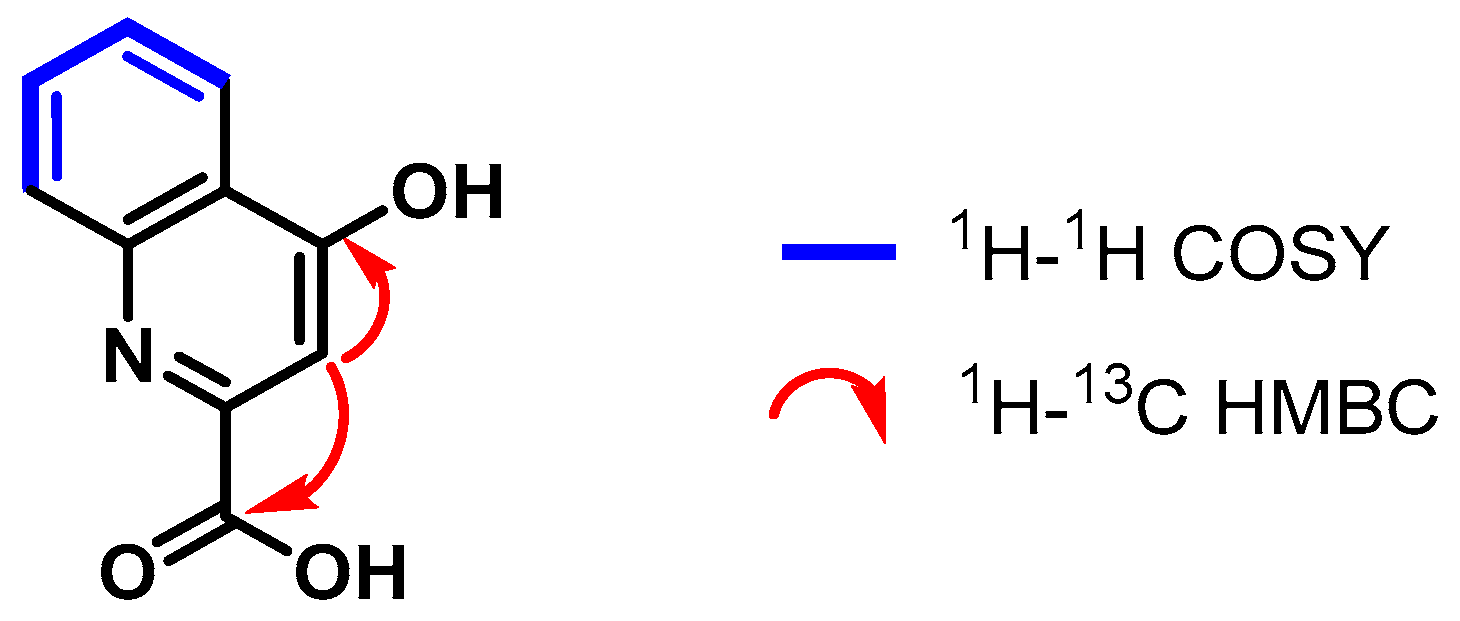


| Samples No. | NF-κB Inhibition at 72 h IC50 ± SEM (μM) a | |||
|---|---|---|---|---|
| THP-1 | HEK001 | WS1 | HMCB | |
| AqERu (*) | 37.39 ± 0.50 | 33.53 ± 0.95 | 31.73 ± 0.32 | 31.21 ± 0.83 |
| HERu (*) | 55.47 ± 0.55 | 50.54 ± 0.47 | 46.75 ± 0.91 | 39.14 ± 0.61 |
| DCMERu (*) | 29.10 ± 0.35 | 28.06 ± 0.31 | 23.03 ± 0.59 | 19.86 ± 0.75 |
| Celastrol (*) | 3.35 ± 0.09 | 3.33 ± 0.03 | 3.29 ± 0.05 | 3.27 ± 0.03 |
| Compound 1 | 12.72 ± 0.34 | 11.24 ± 0.23 | 11.02 ± 0.67 | 9.82 ± 0.65 |
| Compound 2 | 37.21 ± 0.72 | 35.42 ± 0.12 | 32.66 ± 0.51 | 30.66 ± 0.98 |
| Compound 3 | 58.77 ± 0.86 | 55.97 ± 0.32 | 53.21 ± 0.23 | 51.42 ± 0.13 |
| Celastrol | 7.62 ± 0.43 | 7.57 ± 0.23 | 7.41 ± 0.75 | 7.36 ± 0.53 |
| Samples No. | Anti-Hyaluronidase Activity | |
|---|---|---|
| IC50 (μM) | Hyaluronidase Inhibition (%) | |
| AqERu (*) | 111.20 ± 2.97 | 57.43 ± 1.92 |
| HERu (*) | 165.30 ± 2.66 | 36.71 ± 1.75 |
| DCMERu (*) | 71.72 ± 1.65 | 72.54 ± 1.68 |
| Quercetin (*) | 250.03 ± 3.10 | 95.72 ± 1.88 |
| Compound 1 | 79.71 ± 1.59 | 61.12 ± 1.78 |
| Compound 2 | 142.59 ± 2.63 | 30.45 ± 1.92 |
| Compound 3 | 171.97 ± 2.97 | 16.12 ± 1.34 |
| Quercetin | 820.01 ± 6.58 | 99.99 ± 1.19 |
| Samples No. | Anti-Elastase | Anti-Collagenase | ||
|---|---|---|---|---|
| IC50 (μM) | Elastase Inhibition (%) | IC50 (μM) | Collagenase Inhibition (%) | |
| AqERu (*) | 57.09 ± 1.92 | 52.86 ± 1.94 | 56.42 ± 1.32 | 52.86 ± 1.22 |
| HERu (*) | 102.35 ± 1.85 | 15.49 ± 1.76 | 75.96 ± 1.96 | 36.53 ± 1.68 |
| DCMERu (*) | 30.42 ± 1.49 | 74.88 ± 1.51 | 11.60 ± 1.69 | 90.31 ± 1.77 |
| EGCG (*) | 114.38± 3.07 | 94.44 ± 1.30 | 114.04± 3.07 | 95.29 ± 2.09 |
| Compound 1 | 80.13 ± 1.92 | 60.76 ± 1.44 | 76.59 ± 1.31 | 62.24 ± 1.42 |
| Compound 2 | 168.11 ± 3.09 | 17.68 ± 1.46 | 152.40 ± 3.73 | 24.87 ± 1.34 |
| Compound 3 | 191.58 ± 3.78 | 6.19 ± 1.44 | 187.17 ± 2.61 | 7.73 ± 1.21 |
| EGCG | 250.37 ± 3.25 | 98.08 ± 1.74 | 250.54 ± 3.65 | 98.81 ± 1.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apaza Ticona, L.; Sánchez Sánchez-Corral, J.; Díaz-Guerra Martín, C.; Calderón Jiménez, S.; López González, A.; Thiebaut Estrada, C. Rubus urticifolius Compounds with Antioxidant Activity, and Inhibition Potential against Tyrosinase, Melanin, Hyaluronidase, Elastase, and Collagenase. Pharmaceuticals 2024, 17, 937. https://doi.org/10.3390/ph17070937
Apaza Ticona L, Sánchez Sánchez-Corral J, Díaz-Guerra Martín C, Calderón Jiménez S, López González A, Thiebaut Estrada C. Rubus urticifolius Compounds with Antioxidant Activity, and Inhibition Potential against Tyrosinase, Melanin, Hyaluronidase, Elastase, and Collagenase. Pharmaceuticals. 2024; 17(7):937. https://doi.org/10.3390/ph17070937
Chicago/Turabian StyleApaza Ticona, Luis, Javier Sánchez Sánchez-Corral, Carolina Díaz-Guerra Martín, Sara Calderón Jiménez, Alejandra López González, and Cristina Thiebaut Estrada. 2024. "Rubus urticifolius Compounds with Antioxidant Activity, and Inhibition Potential against Tyrosinase, Melanin, Hyaluronidase, Elastase, and Collagenase" Pharmaceuticals 17, no. 7: 937. https://doi.org/10.3390/ph17070937
APA StyleApaza Ticona, L., Sánchez Sánchez-Corral, J., Díaz-Guerra Martín, C., Calderón Jiménez, S., López González, A., & Thiebaut Estrada, C. (2024). Rubus urticifolius Compounds with Antioxidant Activity, and Inhibition Potential against Tyrosinase, Melanin, Hyaluronidase, Elastase, and Collagenase. Pharmaceuticals, 17(7), 937. https://doi.org/10.3390/ph17070937








