Abstract
Cadmium (Cd) is a potentially toxic element able to interfere with cellular functions and lead to disease or even death. Cd accumulation has been demonstrated in cartilage, where it can induce damage in joints. The aim of this study was to evaluate the effect of CdCl2 on primary cultures of human chondrocytes and the possible protective effect of seleno-methionine (Se-Met). Human primary articular chondrocytes were cultured and treated as follows: control groups, cells challenged with 7.5 μM and 10 μM CdCl2 alone, and cells pretreated with 10 and 20 μM Se-Met and then challenged with 7.5 μM and 10 μM CdCl2. Twenty-four hours after incubation, cell viability, histological evaluation with hematoxylin–eosin stain, and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay were performed. Furthermore, reverse transcription-PCR was carried out to evaluate mRNA levels of BAX, BAK1, CASP-3, and CASP-9. After CdCl2 challenge at both doses, a reduced cell viability and an overexpression of BAX, BAK1, CASP-3, and CASP-9 genes, as well as a high number of TUNEL-positive cells, were demonstrated, all parameters becoming higher as the dose of CdCl2 was increased. The pretreatment with Se-Met lowered the expression of all considered genes, improved cell viability and morphological changes, and reduced the number of TUNEL-positive cells. It was concluded that Se-Met plays a protective role against CdCl2-induced structural and functional changes in chondrocytes in vitro, as it improved cell viability and showed a positive role in the context of the apoptotic pathways. It is therefore suggested that a translational, multifaceted approach, with plant-based diets, bioactive functional foods, nutraceuticals, micronutrients, and drugs, is possibly advisable in situations of environmental pollution caused by potentially toxic elements.
1. Introduction
Cadmium (Cd) is a potentially toxic element (PTE) [1] present in the environment because of natural and anthropogenic processes. It is therefore able to occur in the contexts of water, air, and soil pollution, resulting in its entrance and accumulation in the food chain [2]. As a result, Cd can pass into the human body through inhalation from the environment or following cigarette smoking, or by eating or drinking contaminated food or water. Moreover, Cd can penetrate into the body following occupational exposure, owing to its use in industries associated with anticorrosive materials, plastic stabilizers, electronic devices, batteries, and paint pigments [3]. Once absorbed, it can reach different organs through blood circulation, where about 90% of Cd is bound to α2-macroglobulin and albumin in serum [4], compromising human health [5].
Cd is potentially toxic even under low-dose exposure conditions [6,7]. It is not required in any mammalian physiological process, but it can mimic essential metal ions by competing with their transporters, or replacing them in intracellular macromolecules, thus interfering with cellular functions and leading to disease or even death [8]. As its half-life is 16–30 years [9], the International Agency for Research on Cancer and the USA National Toxicology Program have indicated that Cd is a type I carcinogen, being related to higher risks of lung, breast, liver, colon, and genitourinary cancers [10].
Several in vitro studies have assessed Cd toxicity in different cellular lines, such as normal mouse renal MM55.K cells [11], duck renal tubular epithelial cells [12], human renal proximal-tubule epithelial cells (RPTEC/TERT1) [13], human liver carcinoma HepG2 and human normal liver THLE-3 cells [14], alpha mouse liver 12 (AML12) cells [15], SN56 cholinergic neurons [16], PC12 cells and primary rat cerebral cortical neurons [17,18], and cerebellar cells [19]. Cd toxicity has been linked as a cause to oxidative stress, endoplasmic reticulum stress, and apoptosis. In more detail, these studies have reported an inhibitory effect of Cd on antioxidant enzymes, one of which causes reactive oxygen species (ROS) production [14,19]; endoplasmic reticulum stress [11]; oxidative stress-mediated apoptosis with increased Bax, Bak-1, and Caspase-3 expression [12]; activation of the NLRP3 inflammasome [13]; and inhibition of the autophagic flux [15]. Cd also has caused cholinergic neuron death by blocking muscarinic acetylcholine receptor (M1) [16], initiated Fas/FasL-mediated mitochondrial apoptotic pathways in neuronal cells [17], and caused mitochondrial dysfunction via ROS-mediated Sirtuin 1 (SIRT1) suppression [18].
In the body, Cd is deposited mainly in the kidney, testis, brain, liver, and bones [20,21]. Furthermore, Cd accumulation has also been demonstrated in cartilage, enabling it to induce damage in joints [22]. In fact, in vivo, Cd is able to induce in mice a significant reduction, in comparison to control animals, in cartilage volume and surface area, as well as number of chondrocytes [23], and a positive correlation between its concentration in the body and arthritis has been shown [24].
In this context, the possibility of relieving the negative effects of Cd on chondrocytes could be of particular interest. A protective role of selenium (Se) against Cd toxicity has been demonstrated in vivo [25,26,27,28]. Among other trace elements, Se can be found both in inorganic species, such as SeO32− and SeO42−, and in organic forms, such as amino acids like selenocysteine and seleno-methionine (Se-Met), or proteins like selenoalbumin and antioxidase, thus playing a significant biological role in living organisms [29,30,31,32]. In fact, Se is involved in the balance of redox reactions, in strengthening the immune system, and in detoxification processes [33].
However, whether Se reduces the toxic effect of Cd on chondrocytes in vitro remains uncertain; therefore, the role of a pretreatment of Se-Met before cadmium chloride (CdCl2) addition in primary cultures of human chondrocytes, with particular regard to cell viability and apoptosis, was evaluated. This line of research hopes to add a novel aspect to the translational multifaceted approaches (i.e., healthy dietary habits, nutraceuticals, medical foods, micronutrients, pharmaceuticals, and drugs) employed against environmental pollution caused by PTEs such as Cd.
2. Results
2.1. Effects of Se-Met on CdCl2-Induced Cell Injury
CdCl2 significantly reduced chondrocyte viability in a dose-dependent manner, mostly at concentrations of 7.5 and 10 μM (Figure 1; *** p < 0.001 vs. CTRL). The pretreatment with Se-Met before CdCl2 addition partially restores cell viability compared to cells incubated with CdCl2 alone, at each of the studied concentrations (5, 10, and 20 μM), in a dose-dependent manner (Figure 1; ° p < 0.05 vs. 5 μM CdCl2; ^^ p < 0.01 and ^^^ p < 0.001 vs. 7.5 μM CdCl2; §§ p < 0.01 and §§§ p < 0.001 vs. 10 μM CdCl2).
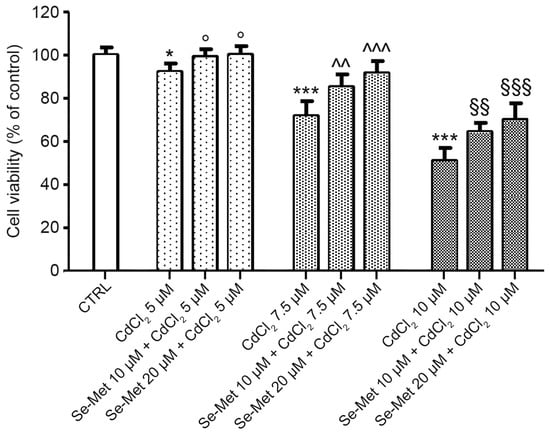
Figure 1.
Evaluation of cell viability in control chondrocytes and in chondrocytes pre-treated with Se-Met before CdCl2 addition. Control chondrocytes, from culture medium alone, or from culture medium supplemented with only 10 μM Se-Met, and 20 μM Se-Met. Control groups showed normal viability, and control data were incorporated and presented as a single value. Values are the mean ± SD of no less than five experiments and are expressed as the % increase versus controls (CTRL). * p < 0.05 and *** p < 0.001 vs. CTRL; ° p < 0.05 vs. 5 μM CdCl2; ^^ p < 0.01 and ^^^ p < 0.001 vs. 7.5 μM CdCl2; §§ p < 0.01 and §§§ p < 0.001 vs. 10 μM CdCl2.
2.2. Se-Met Preserves Chondrocytes from the Damage Induced by CdCl2
The control chondrocytes, which received no CdCl2, were those from (i) culture medium alone, or from culture medium supplemented with (ii) 10 μM Se-Met, and (iii) 20 μM Se-Met alone. All control groups showed cells with normal morphology. For this reason, for sake of simplification, data from (i), (ii), and (iii) were combined, and they are presented in all figures as a single image. In all controls, cells showed uniform size, regular distribution, many processes, and euchromatic nuclei (Figure 2A). In chondrocytes treated with 7.5 μM CdCl2 (Figure 2B), a statistically significant reduction in their number was observed (Figure 2H; * p < 0.05 versus CTRL) and normal or necrotic cells together with cells with heterochromatic nuclei were present. When these chondrocytes were pre-treated with 10 μM Se-Met, a mild increase in cells/UA was observed (Figure 2H; * p < 0.05 versus CTRL; § p < 0.05 versus CdCl2 7.5 μM alone), bur their morphology was not improved (Figure 2C). If instead chondrocytes pre-treated with 20 μM Se-Met and then challenged with 7.5 μM CdCl2 were considered, a significant increase in cells/UA was observed (Figure 2H; * p < 0.05 versus CTRL; § p < 0.05 versus CdCl2 7.5 μM alone) and the presence of elongated cells with thin processes was observed; however, some cells with heterochromatic nuclei were still present (Figure 2D). In chondrocytes treated with 10 μM CdCl2 alone, a sharp reduction in cells/UA was demonstrated (Figure 2H; * p < 0.05 versus CTRL). Only a few normal cells were present, while the others were small, with heterochromatic nuclei and thin, elongated processes (Figure 2E). The pretreatment with 10 μM Se-Met induced a significant increase in cells/UA (Figure 2H; * p < 0.05 versus CTRL, † p < 0.05 versus CdCl2 10 μM alone) and the maintenance of a close-to-normal morphology of a large part of the cells (Figure 2F). If instead chondrocytes were pre-treated with 20 μM Se-Met, the absolute number of cells/UA was increased (Figure 2H; * p < 0.05 versus CTRL, † p < 0.05 versus CdCl2 10 μM alone) and both elongated cells with thin processes and small cells with heterochromatic nuclei were observed (Figure 2G).
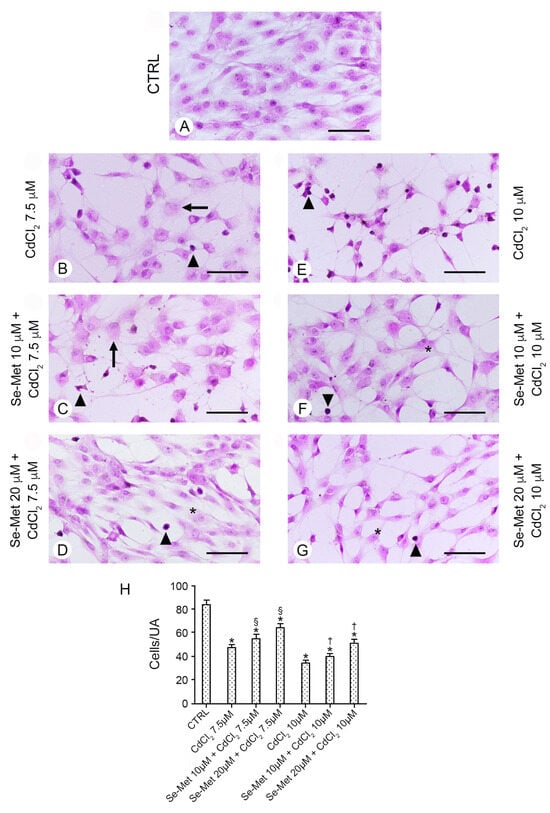
Figure 2.
Histological organization of the different groups of chondrocytes evaluated with hematoxylin–eosin stain. (Scale bar: 100 μm). (A) Control chondrocytes, from culture medium alone, or from culture medium supplemented with only 10 μM Se-Met, and 20 μM Se-Met. All control groups showed normal morphology, and control data were incorporated and presented as a single image. In all controls, cells show uniform size, regular distribution, many processes, and euchromatic nuclei. (B) Chondrocytes treated with 7.5 μM CdCl2 alone. A lower number of cells is present; normal, necrotic cells (arrow), and cells with heterochromatic nuclei (arrowhead) are evident. (C) Chondrocytes pre-treated with 10 μM Se-Met plus 7.5 μM CdCl2. A mild increase in cells/UA is observed, but normal, necrotic cells (arrow), and cells with heterochromatic nuclei (arrowhead) are still present. (D) Chondrocytes pre-treated with 20 μM Se-Met plus 7.5 μM CdCl2. A significant increase in cells/UA is observed; many of them are elongated (*) and show thin processes, even if some have heterochromatic nuclei (arrowhead). (E) Chondrocytes treated with 10 μM CdCl2 alone. Only a few normal cells are present; the other are small, with heterochromatic nuclei and thin, elongated processes (arrowhead). (F) Chondrocytes pre-treated with 10 μM Se-Met plus 10 μM CdCl2. The largest number of the cells show a close-to-normal morphology (*), but some cells with heterochromatic nuclei (arrowhead) are evident. (G) Chondrocytes pre-treated with 20 μM Se-Met plus 10 μM CdCl2. Normal cells with thin processes (*) and occasional cells with heterochromatic nuclei (arrowhead) are observed. (H) Histogram of the cells/UA in the different chondrocyte groups (mean ± SD). * p < 0.05 versus CTRL; § p < 0.05 versus CdCl2 7.5 μM alone; † p < 0.05 versus CdCl2 10 μM alone.
2.3. Se-Met Effects on Transcription of Apoptotic Genes
The expression of pro-apoptotic genes BAX, BAK1, CASP-3 and CASP-9 in cultured cells was evaluated to assess the effect of Se-Met on CdCl2-induced apoptosis. CdCl2 was able to increase mRNA levels of all genes, in a dose-dependent manner (Figure 3; *** p < 0.001 vs. CTRL). The addition of Se-Met decreased the upregulation promoted by CdCl2, showing a protective effect at both doses (Figure 3; ° p < 0.05, °° p < 0.01 and °°° p < 0.001 vs. 7.5 μM CdCl2 alone; ^^ p < 0.01 and ^^^ p < 0.001 vs. 10 μM CdCl2 alone).
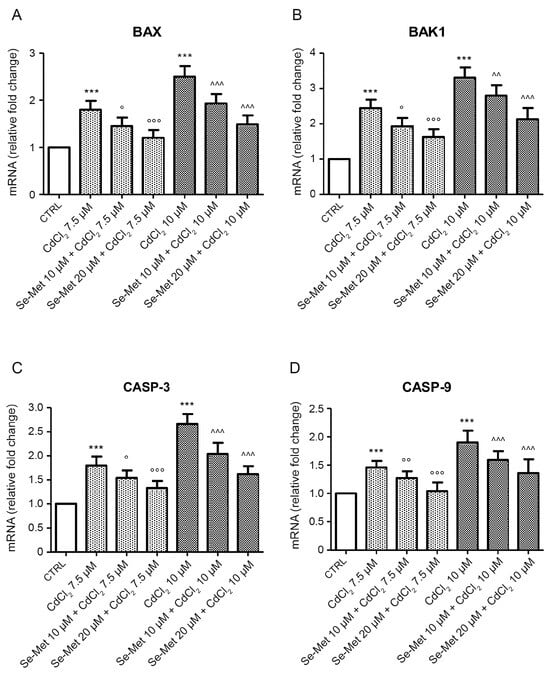
Figure 3.
Evaluation of mRNA levels of BAX (A), BAK1 (B), CASP-3 (C), and CASP-9 (D) in control chondrocytes and in chondrocytes pre-treated with Se-Met before CdCl2 addition. Control chondrocytes, from culture medium alone, or from culture medium supplemented with only 10 μM Se-Met, and 20 μM Se-Met showed similar mRNA levels of apoptotic genes and data were incorporated and presented as a single value. Values are the mean ± S.D. of no less than five experiments and are expressed as fold change with respect to controls. *** p < 0.001 vs. CTRL; ° p < 0.05, °° p < 0.01 and °°° p < 0.001 vs. 7.5 μM CdCl2 alone; ^^ p < 0.01 and ^^^ p < 0.001 vs. 10 μM CdCl2 alone.
2.4. Se-Met Effects on Apoptosis with TUNEL Assay
No positive cells were observed in chondrocytes from all control groups with TUNEL assay (Figure 4A). In chondrocytes challenged with CdCl2 alone at the concentration of 7.5 μM, a significantly high number of positive cells was observed (Figure 4B,H; * p < 0.05 versus CTRL). When chondrocytes challenged with CdCl2 at the concentration of 7.5 μM were pretreated with Se-Met at a dose of 10 μM, a mild, but statistically unsignificant reduction in TUNEL-positive chondrocytes versus CdCl2 alone was demonstrated (Figure 4C,H; * p < 0.05 versus CTRL). The pretreatment with Se-Met at a dose of 20 μM to chondrocytes challenged with CdCl2 at the same concentration of 7.5 μM induced an evident and significant reduction in TUNEL-positive cells (Figure 4D,H; * p < 0.05 versus CTRL; § p < 0.05 versus 7.5 μM CdCl2 alone). In chondrocytes challenged with CdCl2 alone at the concentration of 10 μM, the number of TUNEL-positive cells was high and statistically significant versus controls (Figure 4E,H; * p < 0.05 versus CTRL). When chondrocytes challenged with CdCl2 at the concentration of 10 μM were pretreated with Se-Met at a dose of 10 μM, an evident and significant reduction in the number of TUNEL-positive cells was observed (Figure 4F,H; * p < 0.05 versus CTRL; † p < 0.05 versus 10 μM CdCl2 alone). The pretreatment with Se-Met at a dose of 20 μM of chondrocytes challenged with 10 μM CdCl2 was able to induce an evident and statistically significant reduction in TUNEL-positive cells, even if lower when compared to chondrocytes of Se-Met 20 μM plus CdCl2 7.5 μM group (Figure 4G,H; * p < 0.05 versus CTRL; † p < 0.05 versus 10 μM CdCl2 alone).
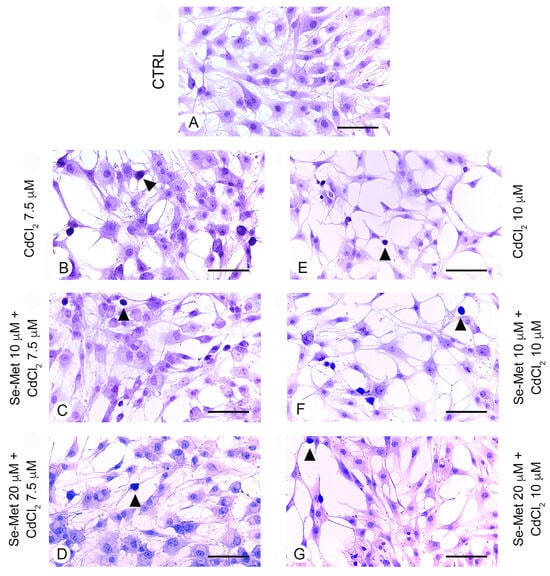
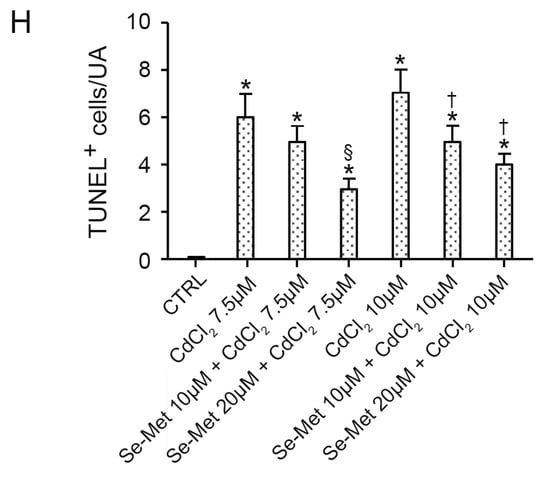
Figure 4.
Assessment of apoptosis in the different groups of cells with TUNEL technique (scale bar: 100 μm). (A) Control chondrocytes, from culture medium alone, or from culture medium supplemented with only 10 μM Se-Met, and 20 μM Se-Met. All groups showed similar morphology, and control data were combined and presented as a single image. No TUNEL-positive chondrocytes are observed. (B) In chondrocytes challenged with 7.5 μM CdCl2 alone, the number of positive cells (arrowhead) is significantly high versus controls. (C) After a pretreatment with 10 μM Se-Met, TUNEL-positive chondrocytes (arrowhead) challenged with 7.5 μM CdCl2 show a mild, but statistically unsignificant reduction versus CdCl2 alone. (D) Chondrocytes pretreated with 20 μM Se-Met and challenged with 7.5 μM CdCl2. An evident and statistically significant reduction in TUNEL-positive cells (arrowhead) is present. (E) Chondrocytes challenged with 10 μM CdCl2 alone. TUNEL-positive cells (arrowhead) are significantly more numerous versus controls. (F) In chondrocytes pretreated with 10 μM Se-Met and challenged with 10 μM CdCl2, a significant reduction in TUNEL-positive cells (arrowhead) is observed. (G) In chondrocytes pretreated with 20 μM Se-Met and challenged with 10 μM CdCl2, a significant reduction in TUNEL-positive cells (arrowhead) is demonstrated. (H) Histogram of TUNEL-positive cells/UA in the different groups of chondrocytes (mean ± SD). * p < 0.05 versus CTRL; § p < 0.05 versus 7.5 μM CdCl2 alone; † p < 0.05 versus 10 μM CdCl2 alone.
3. Discussion
Cd is a PTE [1], able to induce damage in most tissues and organs, including cartilage [24], where it induces oxidative stress with changes in composition and function [34]. On this basis, the possible role of the pretreatment with Se-Met, a well-known micronutrient with multiple positive effects on cell health [35], has been tested in the present in vitro model to suggest novel translational multifaceted approaches against environmental pollution caused by PTEs, such as Cd.
Cd toxicity derives mainly from its inhibitory effects on antioxidant enzymes, such as glutathione reductase [36] and superoxide dismutase [37]. In fact, it binds with high-affinity -SH and disulfide -S-S- groups, negatively interfering with the functions of the biological structures containing these chemical groups. As a result, Cd causes oxidative stress and ROS production, inducing oxidation and degradation of proteins, DNA, lipids, and phospholipids of the cellular membrane and triggering cell apoptosis and necrosis. Furthermore, Cd affects oxidative phosphorylation and ATP synthesis, inhibiting the mitochondrial electron transport chain [8].
Few studies are, however, available on the effect of Cd on chondrocytes in in vitro studies. Fernández-Torres et al. [38] showed that Cd can negatively influence the viability of chondral cells. Zamudio-Cuevas et al. [39], in micromass cultures of human chondrocytes exposed to Cd, showed loss of collagen II, aggrecan, proteoglycans, and glycosaminoglycans in the extracellular matrix, increased expression of metalloproteinases (MMP), and activation of Interleukin (IL)-1β and IL-6. Recently, in chondrocytes obtained from chicken embryos, Gu et al. [40] demonstrated toxic effects of Cd, such as an increased expression of proapoptotic Bax and of MMP-9, and a reduced expression of collagen IIα1 and acid mucopolysaccharides.
It was also observed that functional changes of cartilage induced by Cd can be a risk factor, in addition to genetics, obesity, sex, and age, for the evolution of joint diseases, such as osteoarthritis (OA), as it interferes with the uptake of essential elements necessary for cartilage balance between matrix synthesis and degradation [41,42].
In the present experimental model, a dose–response analysis of different concentrations of CdCl2 (2.5, 5, 7.5, 10, 12.5, 15 μM), some based on a previous paper [39], was performed, demonstrating that the intermediate doses of 7.5 and 10 μM induced changes in chondrocytes.
Among the mechanisms involved in Cd toxicity, apoptosis plays an important role. Chondrocytes are present only in cartilage, and, owing to their low or absent reproductive capacity, their response to Cd-induced apoptosis is critical, as it causes irreversible damages in the extracellular matrix [43]. As an apoptotic effect on chondrocytes induced by Cd has not been yet demonstrated, this topic was evaluated in human chondrocyte cultures. The results showed a peculiar involvement of the pathways of apoptosis after CdCl2 challenge. In particular, an overexpression of BAX, BAK1, CASP-3, CASP-9 genes and an elevated number of TUNEL-positive cells were demonstrated, which was higher as the dose of CdCl2 increased. In agreement with similar findings reported by other authors [44,45,46], these results confirmed the induction of apoptosis by Cd, indicating the activation of the intrinsic pathway.
In order to counteract the apoptosis induced by Cd, many pharmacological approaches were performed in many tissues [47,48,49,50,51,52,53]. In particular, the protection of chondrocytes against the toxic action of Cd can be oriented towards the use of natural substances able to prevent the apoptosis of cultured cells.
Among them, Se, an essential trace element, showed protective action against Cd toxicity both in vivo [54] and in vitro [55] in all its forms (selenite, nanoSe, Se-Met) [56]. In particular, Se showed a significant antagonistic effect on apoptosis in chicken ovary [57] and positively regulated anti-apoptosis pathways in TM3 cells [58]. Se forms complexes with Cd, thus preventing its accumulation in tissues [56], increases antioxidant selenoprotein levels, such as glutathione peroxidase, catalyzing the reaction between glutathione and hydrogen peroxide, and reduces malondialdehyde levels [56].
In cartilage, Se influences the metabolism of extracellular matrix [59] and, when deficient, it can be involved in an osteochondropathy, Kashin–Beck disease [60].
Since no data are available as to the current knowledge on the possible protective role of Se, and of Se-Met in particular, against Cd-induced toxicity in cartilage, the effects of the interaction between Se-Met and CdCl2 in human chondrocytes were explored.
Exposure of chondrocyte cultures to different concentrations of CdCl2 had a negative impact on cell viability, as demonstrated by the significant decrease in the number of viable cells showed by MTT and histological evaluation. Se-Met had a protective role, as it restored cell viability in chondrocytes pretreated with different concentrations of Se-Met before CdCl2, compared to those treated with CdCl2 alone, thus demonstrating a positive protective role in cell viability and an improvement of the structural organization of chondrocytes after the negative impact of PTEs, such as Cd.
Biochemical, molecular, and morphometric analyses were performed to confirm the positive role of Se-Met pretreatment in cell viability and structural organization in the adverse impact of Cd challenge. The CdCl2-induced increased apoptosis was significantly counteracted by the pretreatment with Se-Met, as demonstrated by the reduction in the intrinsic pathway of apoptosis, especially BAX and BAK1, involved in the permeabilization of mitochondrial membrane [61], and of the effector proteases, particularly CASP-3 and -9, responsible for initiating the degradation phase of apoptosis [62]. These antiapoptotic effects could also be partially justified by an overlapping between apoptosis and inflammation; in fact, a possible involvement of methionine in lowering induced inflammatory response and oxidative status has been previously demonstrated [63]. In turn, a lower percentage of TUNEL-positive cells was observed, indicating that Se-Met also has a significant positive role in modulating apoptosis in chondrocytes.
Overall, on the basis of the results of these experiments, the following strengths were highlighted. First, it is confirmed that trace elements, such as Se, have a crucial role in health and diseases [64]; indeed, in the present experimental model, the organic form Se-Met effectively modulated the apoptosis in human chondrocytes challenged with an extremely toxic industrial and environmental pollutant, such as Cd. Second, to our knowledge, this paper revealed for the first time a protective effect of Se-Met pretreatment against CdCl2 in human chondrocytes and better defined the dose–response effect; as for this purpose, it is important to underline, for a broad audience of readers and more in general for researchers interested to this specific topic, that, generally, the cartilage was not deeply investigated and, even more, after Cd exposure. As a matter of fact, our research group has demonstrated the effects of several compounds as a possible challenge against PTEs, such as Cd, in different organs [4,20,65], even if they do not represent common targets, showing the possible protective role against Cd toxicity, with a careful view to translational science.
Of course, in this context, it should be carefully focused, “keeping feet on the ground” and without generating easy enthusiasm, on a synergistic or antagonistic action between different micronutrients, such as Se, bioactive foods, and/or nutraceuticals of plant-based diets, on neuroendocrine immune system modulation and gut microbiota dysbiosis, with particular regard to the presence of environmental pollution caused by PTEs, such as Cd [66,67].
In the present experimental model, an additional limit is clearly represented by the fact that the results obtained in vitro are inevitably preliminary, even if undoubtedly encouraging, and open further investigations experimentally in vivo and in patients. Moreover, it is currently impossible to demonstrate whether the organic form Se-Met effectively modulates the apoptosis in response to CdCl2 challenge in cotreatment or in post-treatment, and the possible recommended doses to protect the human body.
Overall, a large body of literature, also including personal previous experimental observations [27,68], suggests that the effect of healthy dietary strategies, as well as the multifaceted mechanism of action of Se alone or in combination with other nutraceuticals/functional foods [26,27,64,69], could effectively counteract the detrimental molecular cascade in organ injury, also including the cartilage, caused by environmental PTEs, such as Cd.
It is hoped that the present experiments, despite the above limitations and the lack of clinical data available, could be helpful in improving the quality of life, as well as environmental and food sustainability, particularly in under-developed countries. The natural presence and/or the addition to the diet of compounds such as Se can be considered a new trustworthy medical tactic in subjects exposed to PTEs, in particular to those whose mechanisms of action are similar to Cd [70], keeping in mind that pathologies are multifactorial events which therefore require approaches aimed at different targets simultaneously. Moreover, another translational implication of the present data is to test whether the organic form of Se, Se-Met (alone or in combination with other compounds), may have prophylactic and/or therapeutic effects against the chondrocyte alterations caused by exposure to a number of cartilage-disrupting chemicals agents, such as organochlorine compounds, pesticides, bisphenol A, nitrates [71,72,73].
4. Materials and Methods
4.1. Cell Culture
Human primary articular chondrocytes were obtained from ScienceCellTM Research Laboratories (Carlsbad, CA, USA). Cells were cultured in 75 cm2 flasks containing 15 mL of a specific Chondrocyte Medium, with the addition of 2.5% fetal bovine serum (FBS), L-glutamine (2.0 mM), and penicillin/streptomycin (100 U mL−1, 100 mg mL−1). All products were provided by ScienceCellTM Research Laboratories (Carlsbad, CA, USA). Chondrocytes were incubated at 37 °C in humidified air with 5% CO2 [74]. Experiments were performed using chondrocyte cultures between the third and the fifth passage.
4.2. Cell Treatment
Chondrocytes were cultured in 24-well culture plates at a density of 5 × 104 cells/well. Twelve hours after plating (time 0), the culture medium was replaced with fresh medium containing 1% FBS and Se-Met (Sigma-Aldrich, Milan, Italy) was added at doses of 5, 10, and 20 μM [68]. After incubating overnight, CdCl2 was added at doses of 5, 7.5, and 10 μM [39]. Finally, 24 h later, chondrocytes were subjected to gene expression evaluation and MTT assay. To perform histological evaluation and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, further experiments were conducted using LabTechTM 8-Chamber Slide Systems (Thermo Fisher Scientific, Waltham, MA, USA), where chondrocytes were seeded at a density of 1 × 104 cells/well. The Se-Met and CdCl2 concentrations were established by preliminary tests assessing their effects on cell vitality at increasing concentrations (see Supplementary Materials).
4.3. MTT Assay
Cell viability was evaluated by the colorimetric assay that uses the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT) to measure cell metabolic activity [75]. The MTT is a yellowish solution and is converted to water-insoluble MTT-formazan of dark blue color by mitochondrial dehydrogenases of living cells. Twenty-four hours after incubation with CdCl2 in the presence/absence of Se-Met, MTT solution (5 mg/mL) was added to cultured cells and incubated for two hours at 37 °C. Subsequently the formazan crystals were dissolved with DMSO and absorbance was measured at 550 nm using a spectrophotometer (Biospectrometer basic, Eppendorf, Enfield, CT, USA). The viability was expressed as absorbance relative to control cells (% of untreated controls, considered 100%).
4.4. Histological Evaluation
Slides were fixed in 4% paraformaldehyde and then stained with hematoxylin and eosin (HE). They were then photographed with a Nikon Ci-L (Nikon Instruments, Tokyo, Japan) light microscope at a magnification of 200× with a digital camera Nikon DS-Ri2 [26].
4.5. Evidence of Apoptosis with TUNEL Assay
The TUNEL technique was performed with the apoptosis detection kit (In situ Apoptosis Detection kit, Abcam, Cambridge, UK) according to the manufacturer’s instructions. Briefly, the slides, once fixed in 4% paraformaldehyde and washed in PBS, were permeabilized with proteinase K. After blocking endogenous peroxidase activity with 3% H2O2 in methanol, the slides were incubated with terminal deoxynucleotidyl transferase, with biotin-labeled deoxynucleotides, with streptavidin-horseradish peroxidase conjugate, and lastly with diaminobenzidine solution. For a better evaluation of apoptotic cells, a rapid counterstaining with hematoxylin was performed. Slides were then photographed with the digital camera Nikon Ds-Ri2 of the Nikon Ci-L light microscope [27].
4.6. Morphometric Evaluation
Once saved as Tagged Image Format Files (TIFF), the images were blindly evaluated by two trained observers. As to the slides stained with HE, a Unit Area (UA = 250 × 250 μm) was chosen to calculate the mean number of cells/UA from 20 UAs for each group, including in the evaluation the cells in contact with the right and the top borders. On the contrary, the cells contacting the left and the bottom borders were not counted. For the evaluation of apoptosis, the mean number of TUNEL-positive cells/UA (UA = 250 × 250 μm) was obtained from 20 UAs for each group. The values of TUNEL-positive cells/UA were obtained as indicated above [27].
4.7. RNA Extraction and Quantitative Reverse Transcription-PCR (qRT-PCR)
Total RNA was isolated from the chondrocytes using Total Purification Plus Kit (Norgen Biotek Corporation, Thorold, ON, Canada). After quantification, total RNA was reverse transcribed using the high-capacity reverse transcription kit (Applied Biosystems, Foster City, CA, USA), following the manufacturer’s protocols. qRT-PCR reactions were performed by using PowerUp SYBR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) in order to evaluate gene expression of CASP-3, CASP-9, BAX, BAK1, and β-actin. Reactions were performed by using a 7500 Real-Time PCR System model 7500 (Applied Biosystems, Foster City, CA, USA). β-actin mRNA was used as an endogenous control to allow relative quantification of mRNAs. After normalization, the mean value of untreated chondrocyte target gene levels was chosen as the calibrator and the results were expressed according to the 2−ΔΔCt calculation, as fold change relative to normal controls [75]. Primers used for target and endogenous control genes are listed in Table 1. Specific primers were designed based on the published nucleotide sequence of the NCBI GenBank database.

Table 1.
Sequences of primers used for qRT PCR.
4.8. Statistical Analysis
All groups were evaluated with the one-way ANOVA followed by the Newman–Keuls post-test for intergroup comparisons. A p-value of ≤0.05 was considered statistically significant. Values are indicated as mean ± standard deviation (SD).
5. Conclusions
In conclusion, on the basis of these results, it is suggested that Se-Met might offer a new possible approach that, appropriately combined with good agricultural practice to abate Cd contamination in food crops and animals, could also provide an intriguing preventive and/or therapeutic strategy to counteract Cd-induced cartilage injury. Therefore, recent research on multifaceted approaches with plant-based diets, bioactive functional foods, nutraceuticals, micronutrients, and drugs can be considered a new therapeutic tool to prevent and treat NCDs and their comorbidities, even with randomized controlled clinical trials on widely exposed populations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ph17070936/s1, Figure S1: Dose-response effects of Se-Met on chondrocytes viability. Values show the mean ± S.D. of no less than five experiments and are expressed as % increase compared to controls. * p < 0.05 vs CTRL; Figure S2: Dose-response effects of CdCl2 on chondrocytes viability. Values show the mean ± S.D. of no less than five experiments and are expressed as % increase compared to controls. * p < 0.05 and *** p < 0.001 vs CTRL; Figure S3: Histological organization of chondrocytes exposed to different doses of CdCl2 and evaluated with hematoxylin-eosin stain (Scale bar: 100 μm). A: Control groups. Chondrocytes are regularly distributed and show uniform size, euchromatic nuclei and long processes. B: Chondrocytes challenged with 2.5 μM CdCl2. Cells show irregular size and, occasionally, foamy cytoplasm (arrow). C: Chondrocytes challenged with 5 μM CdCl2. Normal or necrotic (arrow) cells or cells with reduced size and heterochromatic nuclei (arrowhead) are present. D: Chondrocytes challenged with 7.5 μM CdCl2. Cells are reduced in number, and normal, necrotic (arrow) or small with heterochromatic nuclei (arrowhead) cells are evident. E: Chondrocytes challenged with 10 μM CdCl2. A lower number of cells is present. Few are normal, the other are small, with heterochromatic nuclei and thin processes (arrowhead). F: Histogram of chondrocytes/UA in the different groups (mean ± SD). * p < 0.05 versus CTRL.
Author Contributions
V.U.B., F.A., L.M. and A.D. conceived this project; V.U.B., F.A., J.F., A.P., E.G. and D.P. performed the experiments; H.R.M. and G.M.C. supervised the study; D.P., L.M., H.R.M. and A.D. analysed data and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pourret, O.; Hursthouse, A. It’s Time to Replace the Term “Heavy Metals” with “Potentially Toxic Elements” When Reporting Environmental Research. Int. J. Environ. Res. Public Health 2019, 16, 4446. [Google Scholar] [CrossRef] [PubMed]
- Rigby, H.; Smith, S.R. The significance of cadmium entering the human food chain via livestock ingestion from the agricultural use of biosolids, with special reference to the UK. Environ. Int. 2020, 143, 105844. [Google Scholar] [CrossRef] [PubMed]
- Peana, M.; Pelucelli, A.; Medici, S.; Cappai, R.; Nurchi, V.M.; Zoroddu, M.A. Metal Toxicity and Speciation: A Review. Curr. Med. Chem. 2021, 28, 7190–7208. [Google Scholar] [CrossRef]
- Marini, H.R.; Bellone, F.; Catalano, A.; Squadrito, G.; Micali, A.; Puzzolo, D.; Freni, J.; Pallio, G.; Minutoli, L. Nutraceuticals as Alternative Approach against Cadmium-Induced Kidney Damage: A Narrative Review. Metabolites 2023, 13, 722. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, M.; Jiang, L.; Song, L. New insight into molecular interaction of heavy metal pollutant—Cadmium (II) with human serum albumin. Environ. Sci. Pollut. Res. Int. 2016, 23, 4959. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef]
- Satarug, S.; Gobe, G.C.; Ujjin, P.; Vesey, D.A. A Comparison of the Nephrotoxicity of Low Doses of Cadmium and Lead. Toxics 2020, 8, 18. [Google Scholar] [CrossRef]
- Lee, W.K.; Thévenod, F. Cell organelles as targets of mammalian cadmium toxicity. Arch. Toxicol. 2020, 94, 1017–1049. [Google Scholar] [CrossRef]
- Charkiewicz, A.E.; Omeljaniuk, W.J.; Nowak, K.; Garley, M.; Nikliński, J. Cadmium Toxicity and Health Effects-A Brief Summary. Molecules 2023, 28, 6620. [Google Scholar] [CrossRef]
- Cui, Z.G.; Ahmed, K.; Zaidi, S.F.; Muhammad, J.S. Ins and outs of cadmium-induced carcinogenesis: Mechanism and prevention. Cancer Treat. Res. Commun. 2021, 27, 100372. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.H.; Lee, S.M.; Kim, N.H.; Moon, Y.G.; Tak, T.K.; Hyun, M.; Heo, J.D. Cadmium induces cytotoxicity in normal mouse renal MM55.K cells. Int. J. Environ. Health Res. 2022, 32, 131–140. [Google Scholar] [CrossRef]
- Zhuang, J.; Nie, G.; Yang, F.; Dai, X.; Cao, H.; Xing, C.; Hu, G.; Zhang, C. Cadmium induces cytotoxicity through oxidative stress-mediated apoptosis pathway in duck renal tubular epithelial cells. Toxicol. In Vitro 2019, 61, 104625. [Google Scholar] [CrossRef]
- Li, Z.; Chi, H.; Zhu, W.; Yang, G.; Song, J.; Mo, L.; Zhang, Y.; Deng, Y.; Xu, F.; Yang, J.; et al. Cadmium induces renal inflammation by activating the NLRP3 inflammasome through ROS/MAPK/NF-κB pathway in vitro and in vivo. Arch. Toxicol. 2021, 95, 3497–3513. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.J.; Cao, Q.J.; Xu, M.Y.; Yang, L.; Wu, Y.J. Individual and combined hepatocytotoxicity of DDT and cadmium in vitro. Toxicol. Ind. Health 2021, 37, 270–279. [Google Scholar] [CrossRef]
- Zou, H.; Wang, T.; Yuan, J.; Sun, J.; Yuan, Y.; Gu, J.; Liu, X.; Bian, J.; Liu, Z. Cadmium-induced cytotoxicity in mouse liver cells is associated with the disruption of autophagic flux via inhibiting the fusion of autophagosomes and lysosomes. Toxicol. Lett. 2020, 321, 32–43. [Google Scholar] [CrossRef]
- Del Pino, J.; Zeballos, G.; Anadón, M.J.; Moyano, P.; Díaz, M.J.; García, J.M.; Frejo, M.T. Cadmium-induced cell death of basal forebrain cholinergic neurons mediated by muscarinic M1 receptor blockade, increase in GSK-3β enzyme, β-amyloid and tau protein levels. Arch. Toxicol. 2016, 90, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, Y.; Zhao, S.; Chen, J.; Yang, J.; Wang, T.; Zou, H.; Wang, Y.; Gu, J.; Liu, X.; et al. Cadmium-induced apoptosis in neuronal cells is mediated by Fas/FasL-mediated mitochondrial apoptotic signaling pathway. Sci. Rep. 2018, 8, 8837. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Xu, M.; Zhang, W.; Song, R.; Zou, H.; Gu, J.; Liu, X.; Bian, J.; Liu, Z.; Yuan, Y. Cadmium induces mitochondrial dysfunction via SIRT1 suppression-mediated oxidative stress in neuronal cells. Environ. Toxicol. 2023, 38, 743–753. [Google Scholar] [CrossRef]
- Varmazyari, A.; Taghizadehghalehjoughi, A.; Sevim, C.; Baris, O.; Eser, G.; Yildirim, S.; Hacimuftuoglu, A.; Buha, A.; Wallace, D.R.; Tsatsakis, A.; et al. Cadmium sulfide-induced toxicity in the cortex and cerebellum: In vitro and in vivo studies. Toxicol. Rep. 2020, 7, 637–648. [Google Scholar] [CrossRef]
- Rinaldi, M.; Micali, A.; Marini, H.R.; Adamo, E.B.; Puzzolo, D.; Pisani, A.; Trichilo, V.; Altavilla, D.; Squadrito, F.; Minutoli, L. Cadmium, Organ Toxicity and Therapeutic Approaches: A Review on Brain, Kidney and Testis Damage. Curr. Med. Chem. 2017, 24, 3879–3893. [Google Scholar] [CrossRef]
- Wang, M.; Liu, J.; Zhu, G.; Chen, X. Low levels of cadmium exposure affect bone by inhibiting Lgr4 expression in osteoblasts and osteoclasts. J. Trace Elem. Med. Biol. 2022, 73, 127025. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, W.; Chen, Y.; Guo, Y.; Gao, M.; Chen, W.; Liu, Y.; Liu, S. Diagnostic significance of metallothionein members in recognizing cadmium exposure in various organs under low-dose exposure. Chemosphere 2019, 229, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Noorafshan, A.; Niazi, B.; Mohamadpour, M.; Hoseini, L.; Hoseini, N.; Owji, A.A.; Rafati, A.; Sadeghi, Y.; Karbalay-Doust, S. First and second order stereology of hyaline cartilage: Application on mice femoral cartilage. Ann. Anat. 2016, 208, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Afridi, H.I.; Kazi, T.G.; Kazi, N.; Talpur, F.N.; Shah, F.; Naeemullah-Arain, S.S.; Brahman, K.D. Evaluation of status of arsenic, cadmium, lead and zinc levels in biological samples of normal and arthritis patients of age groups (46–60) and (61–75) years. Clin. Lab. 2013, 59, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, A.; Lemire, D.; Driessnack, M.; Naderi, M.; Niyogi, S. Interactive effects of chronic dietary selenomethionine and cadmium exposure in rainbow trout (Oncorhynchus mykiss): A preliminary study. Chemosphere 2018, 197, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Marini, H.R.; Micali, A.; Freni, J.; Pallio, G.; Irrera, N.; Squadrito, F.; Altavilla, D.; Antonelli, A.; Ferrari, S.M.; et al. Protective Effects of Myo-Inositol and Selenium on Cadmium-Induced Thyroid Toxicity in Mice. Nutrients 2020, 12, 1222. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Micali, A.; Ieni, A.; Antonelli, A.; Fallahi, P.; Pallio, G.; Irrera, N.; Squadrito, F.; Picciolo, G.; Puzzolo, D.; et al. The Association of Myo-Inositol and Selenium Contrasts Cadmium-Induced Thyroid C Cell Hyperplasia and Hypertrophy in Mice. Front. Endocrinol. 2021, 12, 608697. [Google Scholar] [CrossRef]
- Satarug, S.; Gobe, G.C.; Vesey, D.A. Multiple Targets of Toxicity in Environmental Exposure to Low-Dose Cadmium. Toxics 2022, 10, 472. [Google Scholar] [CrossRef]
- Jamwal, A.; Naderi, M.; Niyogi, S. An in vitro examination of selenium-cadmium antagonism using primary cultures of rainbow trout (Oncorhynchus mykiss) hepatocytes. Metallomic 2016, 8, 218–227. [Google Scholar] [CrossRef]
- Yan, J.; Fei, Y.; Han, Y.; Lu, S. Selenoprotein O deficiencies suppress chondrogenic differentiation of ATDC5 cells. Cell. Biol. Int. 2016, 40, 1033–1040. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Morucci, G.; Maresca, M.; Tenci, B.; Cascella, R.; Paternostro, F.; Ghelardini, C.; Gulisano, M.; Di Cesare Mannelli, L.; Pacini, A. Selenium and zinc: Two key players against cadmium-induced neuronal toxicity. Toxicol. In Vitro 2018, 48, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 92, 1002. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Cheng, T.; Yu, X. The Impact of Trace Elements on Osteoarthritis. Front. Med. 2021, 8, 771297. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Torres, J.; Zamudio-Cuevas, Y.; Martínez-Nava, G.A.; Aztatzi-Aguilar, O.G.; Sierra-Vargas, M.P.; Lozada-Pérez, C.A.; Suárez-Ahedo, C.; Landa-Solís, C.; Olivos-Meza, A.; Del Razo, L.M.; et al. Impact of Cadmium Mediated by Tobacco Use in Musculoskeletal Diseases. Biol. Trace Elem. Res. 2022, 200, 2008–2015. [Google Scholar] [CrossRef] [PubMed]
- Babazadeh, D.; Ahmadi Simab, P. Methionine in Poultry Nutrition: A Review. J. World’s Poult. Sci. 2022, 1, 1–11. [Google Scholar] [CrossRef]
- Chwalba, A.; Orłowska, J.; Słota, M.; Jeziorska, M.; Filipecka, K.; Bellanti, F.; Dobrakowski, M.; Kasperczyk, A.; Zalejska-Fiolka, J.; Kasperczyk, S. Effect of Cadmium on Oxidative Stress Indices and Vitamin D Concentrations in Children. J. Clin. Med. 2023, 12, 1572. [Google Scholar] [CrossRef] [PubMed]
- Branca, J.J.V.; Carrino, D.; Paternostro, F.; Morucci, G.; Fiorillo, C.; Nicoletti, C.; Gulisano, M.; Ghelardini, C.; Di Cesare Mannelli, L.; Becatti, M.; et al. The Protection of Zinc against Acute Cadmium Exposure: A Morphological and Molecular Study on a BBB In Vitro Model. Cells. 2022, 11, 1646. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Torres, J.; Plata-Rodríguez, R.; Zamudio-Cuevas, Y.; Martínez-Nava, G.A.; Landa-Solís, C.; Mendoza-Soto, L.; Olivos-Meza, A.; Suárez-Ahedo, C.; Barbier, O.C.; Narváez-Morales, J.; et al. Effect of cadmium on the viability on monolayer cultures of synoviocytes, chondrocytes, and Hoffa: A preliminary study. Toxicol. Ind. Health 2020, 36, 940–945. [Google Scholar] [CrossRef]
- Zamudio-Cuevas, Y.E.; Martínez-Nava, G.; Reyes-Hinojosa, D.; Mendoza-Soto, L.; Fernández-Torres, J.; López-Reyes, A.; Olivos-Meza, A.; Armienta-Hernández, M.A.; Ruíz-Huerta, E.A.; de Jesús González-Guadarrama, M.; et al. Impact of cadmium toxicity on cartilage loss in a 3D in vitro model. Environ. Toxicol. Pharmacol. 2020, 74, 103307. [Google Scholar]
- Gu, J.; Li, S.; Wang, G.; Zhang, X.; Yuan, Y.; Liu, X.; Bian, J.; Tong, X.; Liu, Z. Cadmium Toxicity on Chondrocytes and the Palliative Effects of 1α, 25-Dihydroxy Vitamin D3 in White Leghorns Chicken’s Embryo. Front. Vet. Sci. 2021, 8, 637369. [Google Scholar] [CrossRef]
- Martínez-Nava, G.A.; Mendoza-Soto, L.; Fernández-Torres, J.; Zamudio-Cuevas, Y.; Reyes-Hinojosa, D.; Plata-Rodríguez, R.; Olivos-Meza, A.; Ruíz-Huerta, E.A.; Armienta-Hernández, M.A.; Hernández-Álvarez, E.; et al. Effect of cadmium on the concentration of essential metals in a human chondrocyte micromass culture. J. Trace Elem. Med. Biol. 2020, 62, 126614. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Ran, D.; Shi, X.; Zhao, H.; Liu, Z. Cadmium toxicity: A role in bone cell function and teeth development. Sci. Total Environ. 2021, 769, 144646. [Google Scholar] [CrossRef]
- Wang, Q.; Ying, L.; Wei, B.; Ji, Y.; Xu, Y. Effects of quercetin on apoptosis and extracellular matrix degradation of chondrocytes induced by oxidative stress-mediated pyroptosis. J. Biochem. Mol. Toxicol. 2022, 36, e22951. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Kondo, T.; Zhao, Q.L.; Li, F.J.; Tanabe, K.; Arai, Y.; Zhou, Z.C.; Kasuya, M. Apoptosis induced by cadmium in human lymphoma U937 cells through Ca2+-calpain and caspase-mitochondria- dependent pathways. J. Biol. Chem. 2000, 275, 39702–39709. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cheon, H.S.; Kim, S.Y.; Juhnn, Y.S.; Kim, Y.Y. Cadmium induces neuronal cell death through reactive oxygen species activated by GADD153. BMC Cell. Biol. 2013, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cheon, H.; Kim, S.M.; Kim, Y.Y. GSK-3β-mediated regulation of cadmium-induced cell death and survival. Cell. Mol. Biol. Lett. 2018, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, F.; Micali, A.; Rinaldi, M.; Irrera, N.; Marini, H.; Puzzolo, D.; Pisani, A.; Lorenzini, C.; Valenti, A.; Laurà, R.; et al. Polydeoxyribonucleotide, an Adenosine-A2A Receptor Agonist, Preserves Blood Testis Barrier from Cadmium-Induced Injury. Front. Pharmacol. 2017, 7, 537, Erratum in Front. Pharmacol. 2022, 13, 1073543. [Google Scholar] [CrossRef] [PubMed]
- Pallio, G.; Micali, A.; Benvenga, S.; Antonelli, A.; Marini, H.R.; Puzzolo, D.; Macaione, V.; Trichilo, V.; Santoro, G.; Irrera, N.; et al. Myo-inositol in the protection from cadmium-induced toxicity in mice kidney: An emerging nutraceutical challenge. Food Chem. Toxicol. 2019, 132, 110675. [Google Scholar] [CrossRef] [PubMed]
- Pérez Díaz, M.F.F.; Plateo Pignatari, M.G.; Filippa, V.P.; Mohamed, F.H.; Marchevsky, E.J.; Gimenez, M.S.; Ramirez, D.C. A soybean-based diet modulates cadmium-induced vascular apoptosis. J. Trace Elem. Med. Biol. 2019, 52, 239–246. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Micali, A.; Marini, H.R.; Freni, J.; Santoro, G.; Puzzolo, D.; Squadrito, F.; Pallio, G.; Navarra, M.; Cirmi, S.; et al. A Flavonoid-Rich Extract from Bergamot Juice, Alone or in Association with Curcumin and Resveratrol, Shows Protective Effects in a Murine Model of Cadmium-Induced Testicular Injury. Pharmaceuticals 2021, 14, 386. [Google Scholar] [CrossRef]
- Cirmi, S.; Maugeri, A.; Micali, A.; Marini, H.R.; Puzzolo, D.; Santoro, G.; Freni, J.; Squadrito, F.; Irrera, N.; Pallio, G.; et al. Cadmium-Induced Kidney Injury in Mice Is Counteracted by a Flavonoid-Rich Extract of Bergamot Juice, Alone or in Association with Curcumin and Resveratrol, via the Enhancement of Different Defense Mechanisms. Biomedicines 2021, 9, 1797. [Google Scholar] [CrossRef]
- Fang, J.; Xie, S.; Chen, Z.; Wang, F.; Chen, K.; Zuo, Z.; Cui, H.; Guo, H.; Ouyang, P.; Chen, Z.; et al. Protective Effect of Vitamin E on Cadmium-Induced Renal Oxidative Damage and Apoptosis in Rats. Biol. Trace Elem. Res. 2021, 199, 4675–4687. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ding, L.; Wang, K.; Huang, R.; Yu, W.; Yan, B.; Wang, H.; Zhang, C.; Yang, Z.; Liu, Z. Role of endoplasmic reticulum stress in cadmium-induced hepatocyte apoptosis and the protective effect of quercetin. Ecotoxicol. Environ. Saf. 2022, 241, 113772. [Google Scholar] [CrossRef] [PubMed]
- Al Kahtani, M.A. Effect of both selenium and biosynthesized nanoselenium particles on cadmium-induced neurotoxicity in albino rats. Hum. Exp. Toxicol. 2020, 39, 159–172. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, S.; Gavrilović, A.; Li, D.; Tang, R. Selenium alleviates cadmium-induced oxidative stress, endoplasmic reticulum stress, and apoptosis in L8824 cells. Ecotoxicol. Environ. Saf. 2023, 262, 115337. [Google Scholar] [CrossRef]
- Zwolak, I. The Role of Selenium in Arsenic and Cadmium Toxicity: An Updated Review of Scientific Literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef] [PubMed]
- Wan, N.; Xu, Z.; Liu, T.; Min, Y.; Li, S. Ameliorative Effects of Selenium on Cadmium-Induced Injury in the Chicken Ovary: Mechanisms of Oxidative Stress and Endoplasmic Reticulum Stress in Cadmium-Induced Apoptosis. Biol. Trace Elem. Res. 2018, 184, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Wang, S.; Zhang, C.; Hu, X.; Zhou, L.; Li, Y.; Xu, L. Selenium ameliorates cadmium-induced mouse leydig TM3 cell apoptosis via inhibiting the ROS/JNK/c-jun signaling pathway. Ecotoxicol. Environ. Saf. 2020, 192, 110266. [Google Scholar] [CrossRef]
- Sun, M.; Hussain, S.; Hu, Y.; Yan, J.; Min, Z.; Lan, X.; Guo, Y.; Zhao, Y.; Huang, H.; Feng, M.; et al. Maintenance of SOX9 stability and ECM homeostasis by selenium-sensitive PRMT5 in cartilage. Osteoarthr. Cartil. 2019, 7, 932–944. [Google Scholar] [CrossRef]
- Kurokawa, S.; Berry, M.J. Selenium. Role of the essential metalloid in health. Met. Ions Life Sci. 2013, 13, 499–534. [Google Scholar]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond—Mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Asadi, M.; Taghizadeh, S.; Kaviani, E.; Vakili, O.; Taheri-Anganeh, M.; Tahamtan, M.; Savardashtaki, A. Caspase-3: Structure, function, and biotechnological aspects. Biotechnol. Appl. Biochem. 2022, 69, 1633–1645. [Google Scholar] [CrossRef] [PubMed]
- Vailati-Riboni, M.; Xu, T.; Qadir, B.; Bucktrout, R.; Parys, C.; Loor, J.J. In vitro methionine supplementation during lipopolysaccharide stimulation modulates immunometabolic gene network expression in isolated polymorphonuclear cells from lactating Holstein cows. J. Dairy Sci. 2019, 102, 8343–8351. [Google Scholar] [CrossRef] [PubMed]
- Minutoli, L.; Squadrito, F.; Altavilla, D.; Marini, H. Chapter 21: Therapy with Selenium Cocktails and Co-Use of Lycopene and Selenium. In Selenium: Chemistry, Analysis, Function and Effects; Preedy, V.R., Ed.; Series: Food and Nutritional Components in Focus; Royal Society of Chemistry: London, UK, 2015; Volume 9, pp. 363–376. [Google Scholar] [CrossRef]
- Marini, H.R.; Micali, A.; Squadrito, G.; Puzzolo, D.; Freni, J.; Antonuccio, P.; Minutoli, L. Nutraceuticals: A New Challenge against Cadmium-Induced Testicular Injury. Nutrients 2022, 14, 663. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Rawal, R. Influence of heavy metal exposure on gut microbiota: Recent advances. J. Biochem. Mol. Toxicol. 2023, 37, e23485. [Google Scholar] [CrossRef] [PubMed]
- Porru, S.; Esplugues, A.; Llop, S.; Delgado-Saborit, J.M. The effects of heavy metal exposure on brain and gut microbiota: A systematic review of animal studies. Environ. Pollut. 2024, 348, 123732. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; D’Ascola, A.; Vicchio, T.M.; Campo, S.; Gianì, F.; Giovinazzo, S.; Frasca, F.; Cannavò, S.; Campennì, A.; Trimarchi, F. Selenium exerts protective effects against oxidative stress and cell damage in human thyrocytes and fibroblasts. Endocrine 2020, 68, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.R. Mediterranean Diet and Soy Isoflavones for Integrated Management of the Menopausal Metabolic Syndrome. Nutrients 2022, 14, 1550. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, F.; Mostafalou, S.; Bahadar, H.; Abdollahi, M. Review of endocrine disorders associated with environmental toxicants and possible involved mechanisms. Life Sci. 2016, 145, 265–273. [Google Scholar] [CrossRef]
- Holz, J.D.; Sheu, T.J.; Drissi, H.; Matsuzawa, M.; Zuscik, M.J.; Puzas, J.E. Environmental agents affect skeletal growth and development. Birth Defects Res. C Embryo Today 2007, 81, 41–50. [Google Scholar] [CrossRef]
- Huang, W.; Wang, X.; Zheng, S.; Wu, R.; Liu, C.; Wu, K. Effect of bisphenol A on craniofacial cartilage development in zebrafish (Danio rerio) embryos: A morphological study. Ecotoxicol. Environ. Saf. 2021, 212, 111991. [Google Scholar] [CrossRef] [PubMed]
- Deprouw, C.; Courties, A.; Fini, J.B.; Clerget-Froidevaux, M.S.; Demeneix, B.; Berenbaum, F.; Sellam, J.; Louati, K. Pollutants: A candidate as a new risk factor for osteoarthritis-results from a systematic literature review. RMD Open 2022, 8, e001983. [Google Scholar] [CrossRef] [PubMed]
- Scuruchi, M.; D’Ascola, A.; Avenoso, A.; Zappone, A.; Mandraffino, G.; Campo, S.; Campo, G.M. miR9 inhibits 6-mer HA-induced cytokine production and apoptosis in human chondrocytes by reducing NF-kB activation. Arch. Biochem. Biophys. 2022, 718, 109139. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Fu, M.; Bi, R.; Zheng, X.; Fu, B.; Tian, S.; Liu, C.; Li, Q.; Liu, J. Cadmium induced BEAS-2B cells apoptosis and mitochondria damage via MAPK signaling pathway. Chemosphere 2021, 263, 128346. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).