Oral Administration of Nacre Extract from Pearl Oyster Shells Has Anti-Aging Effects on Skin and Muscle, and Extends the Lifespan in SAMP8 Mice
Abstract
1. Introduction
2. Results
2.1. Composition of Nacre Extract (NE)
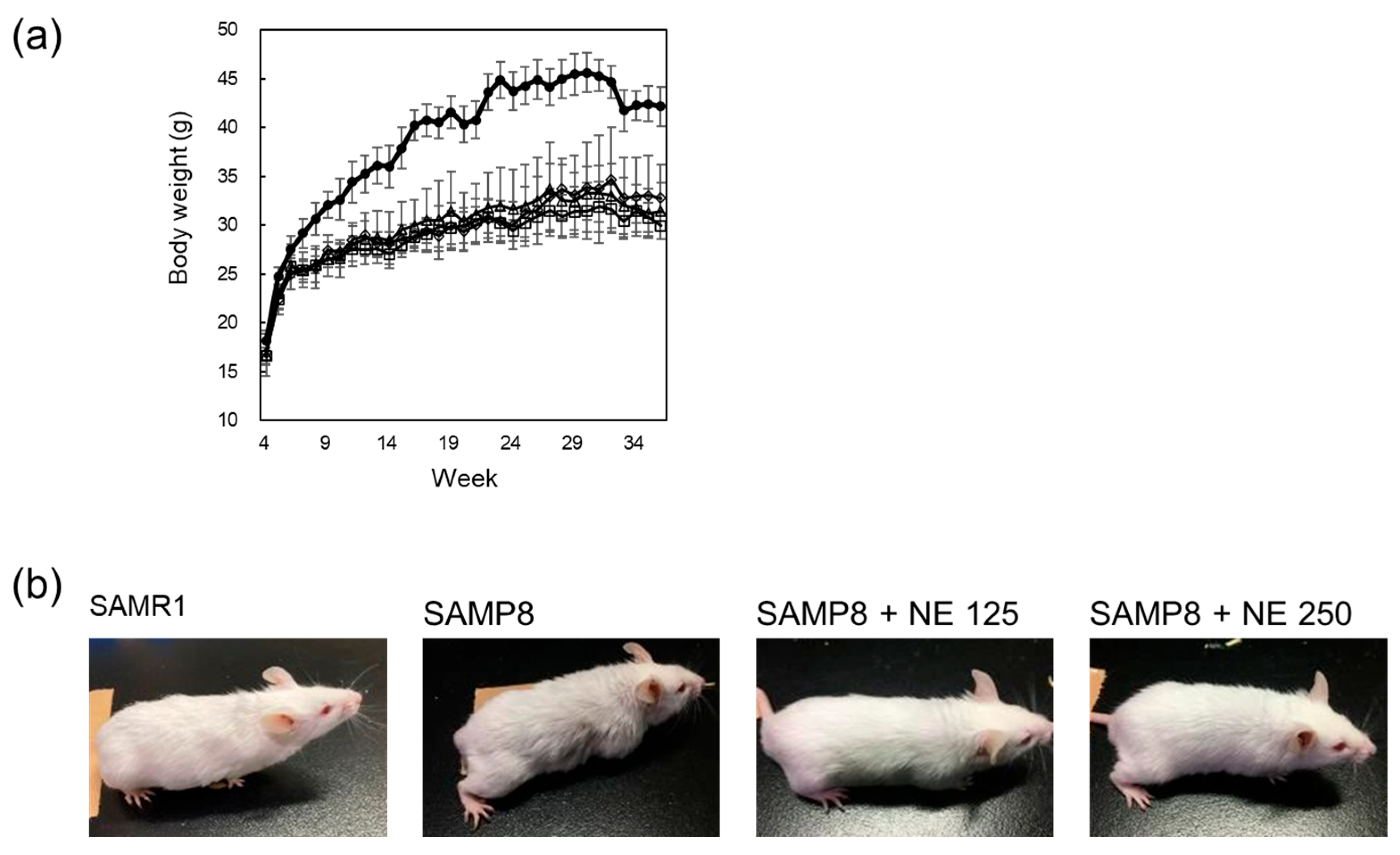
2.2. The Effects of Nacre Extract on Aging of SAMP8 Mice
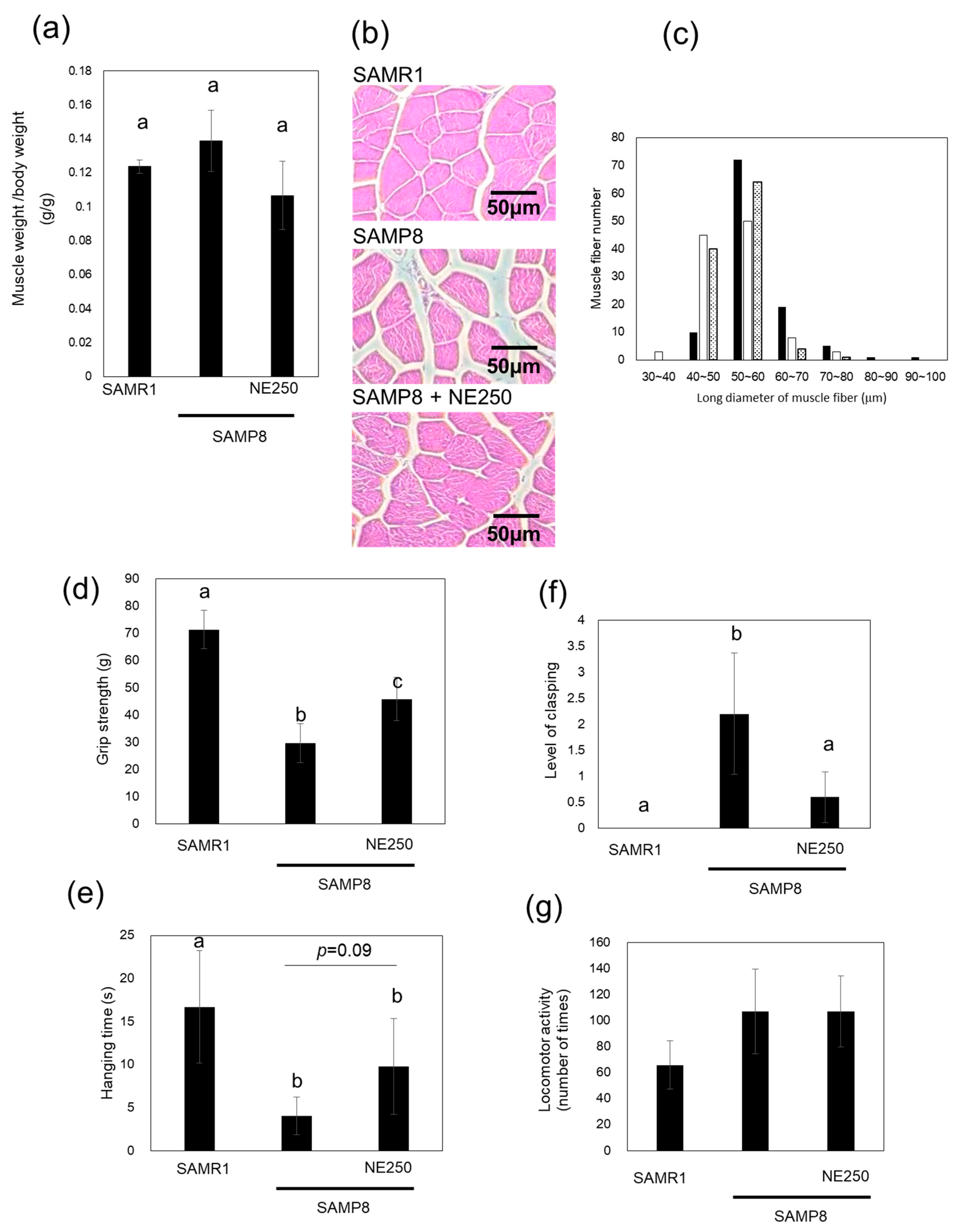
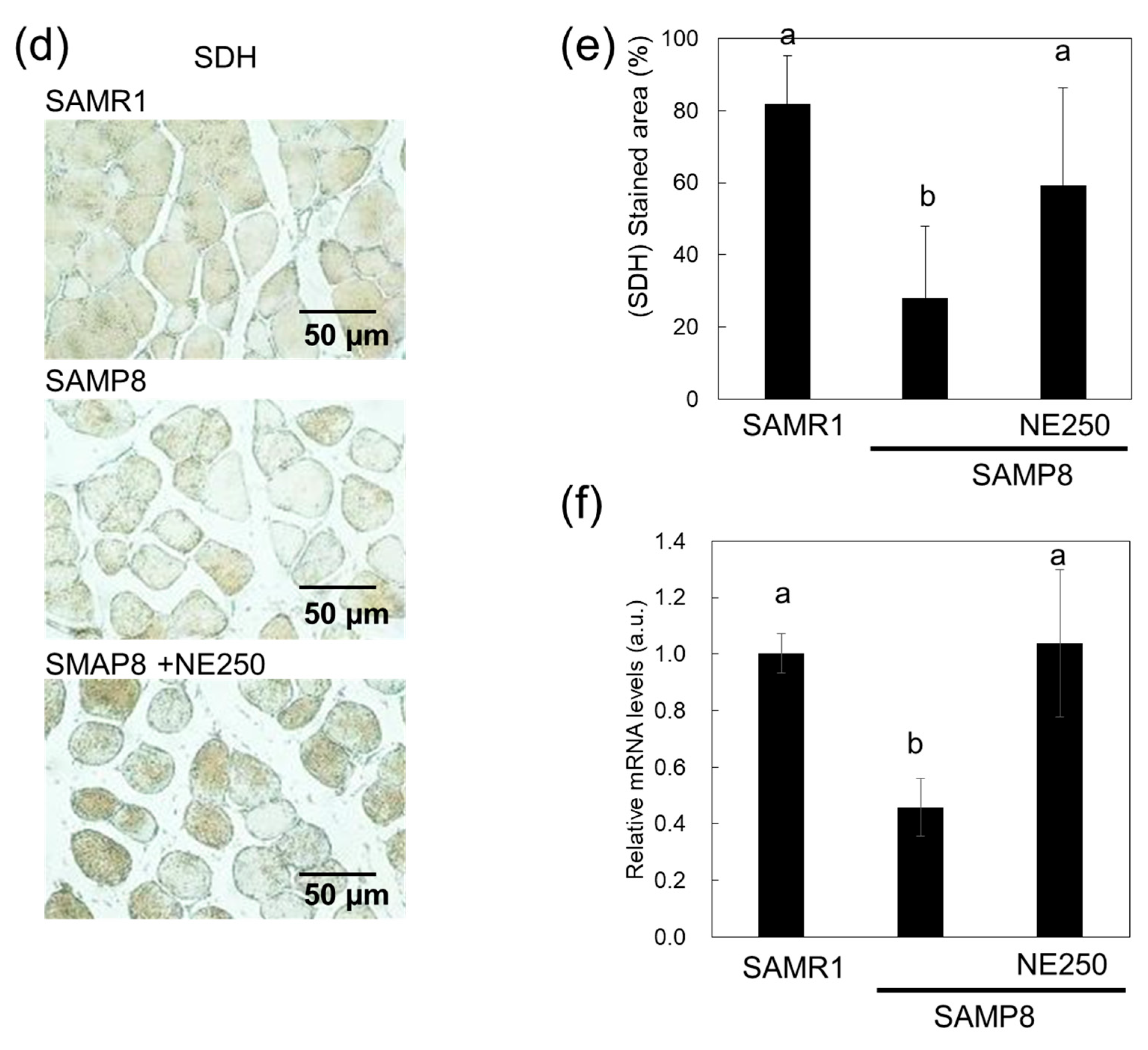
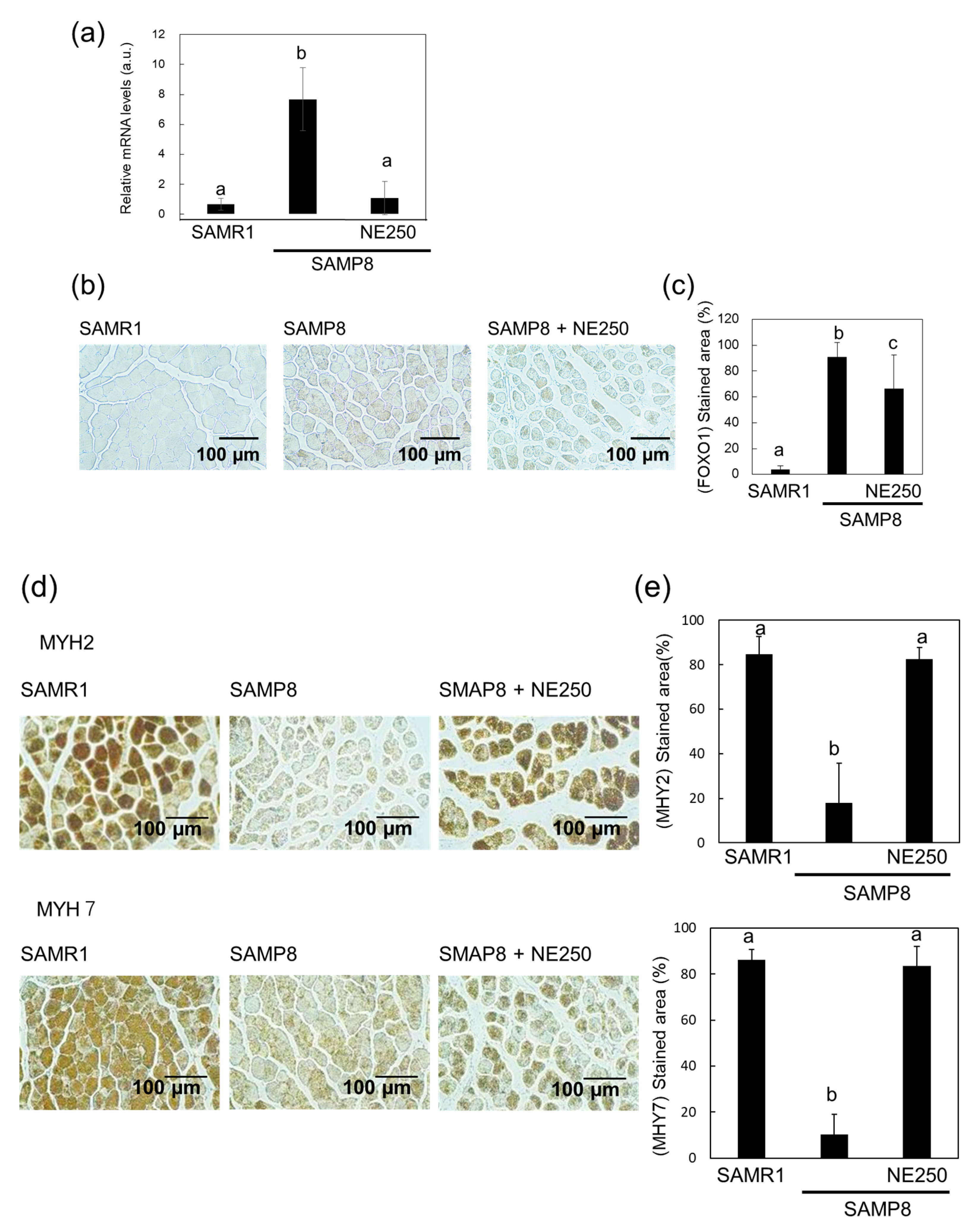
2.3. The Effects of Nacre Extract on Muscle Aging
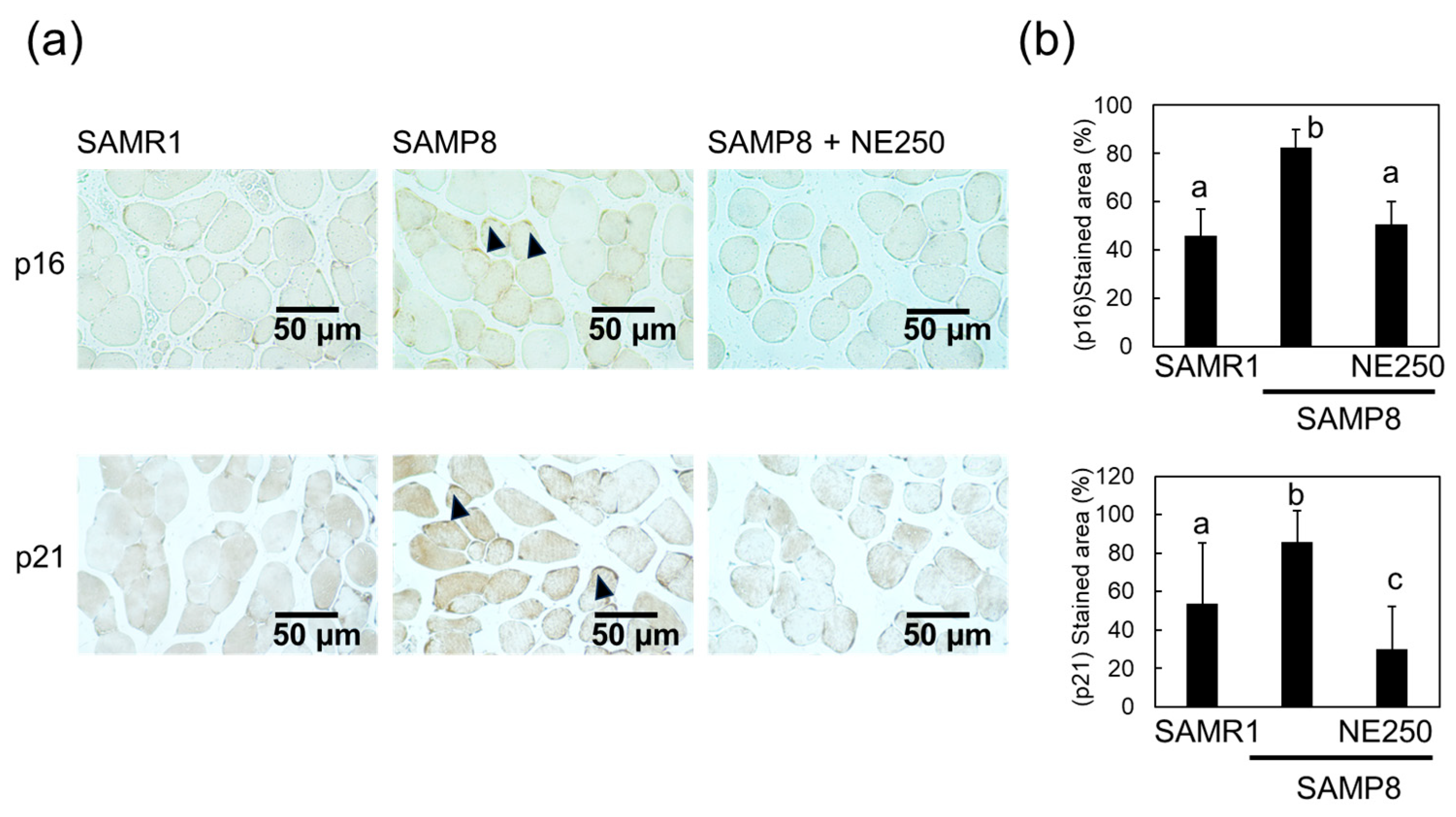
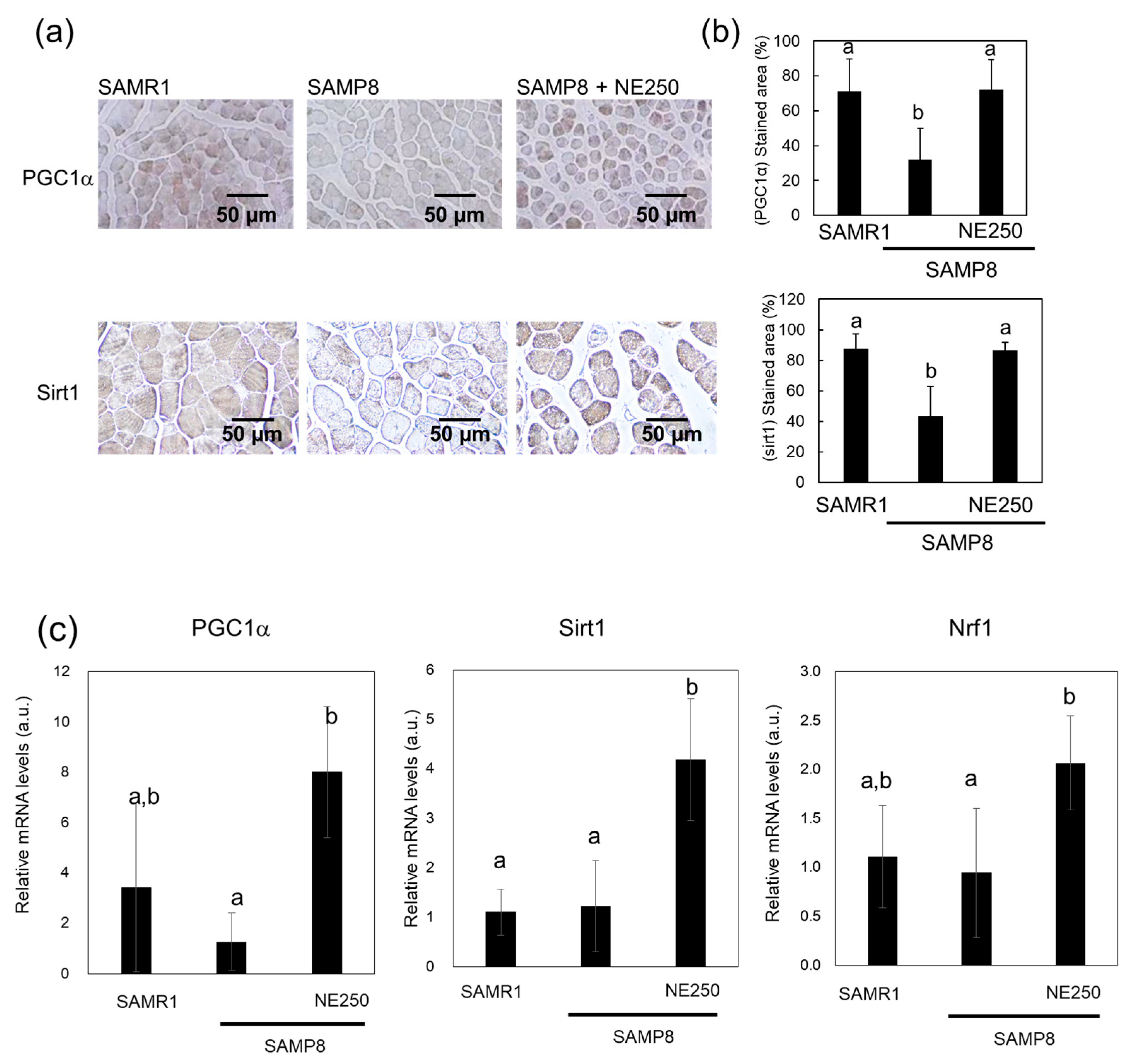
2.4. Action Mechanism of Nacre Extract in Skeletal Muscle
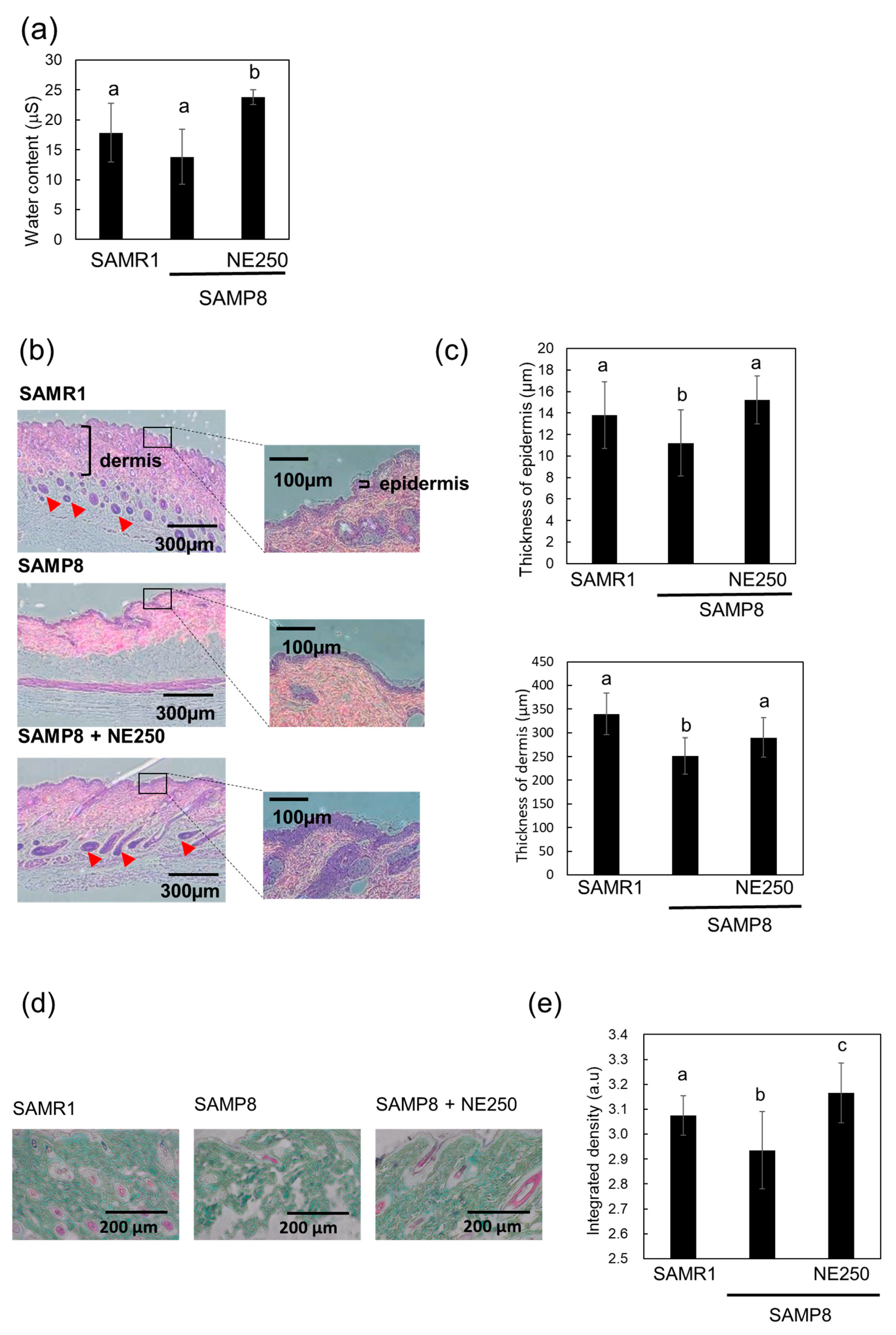
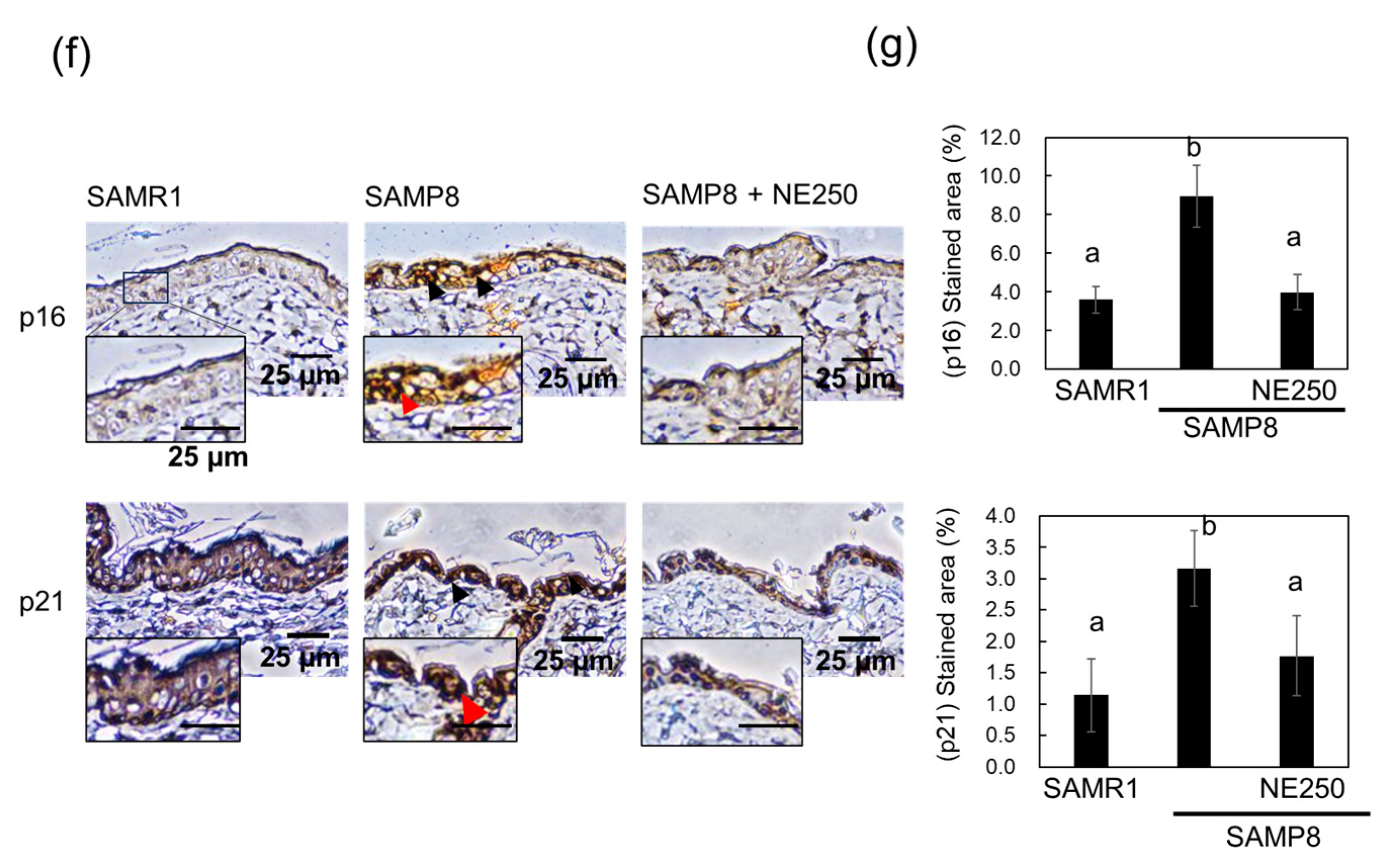
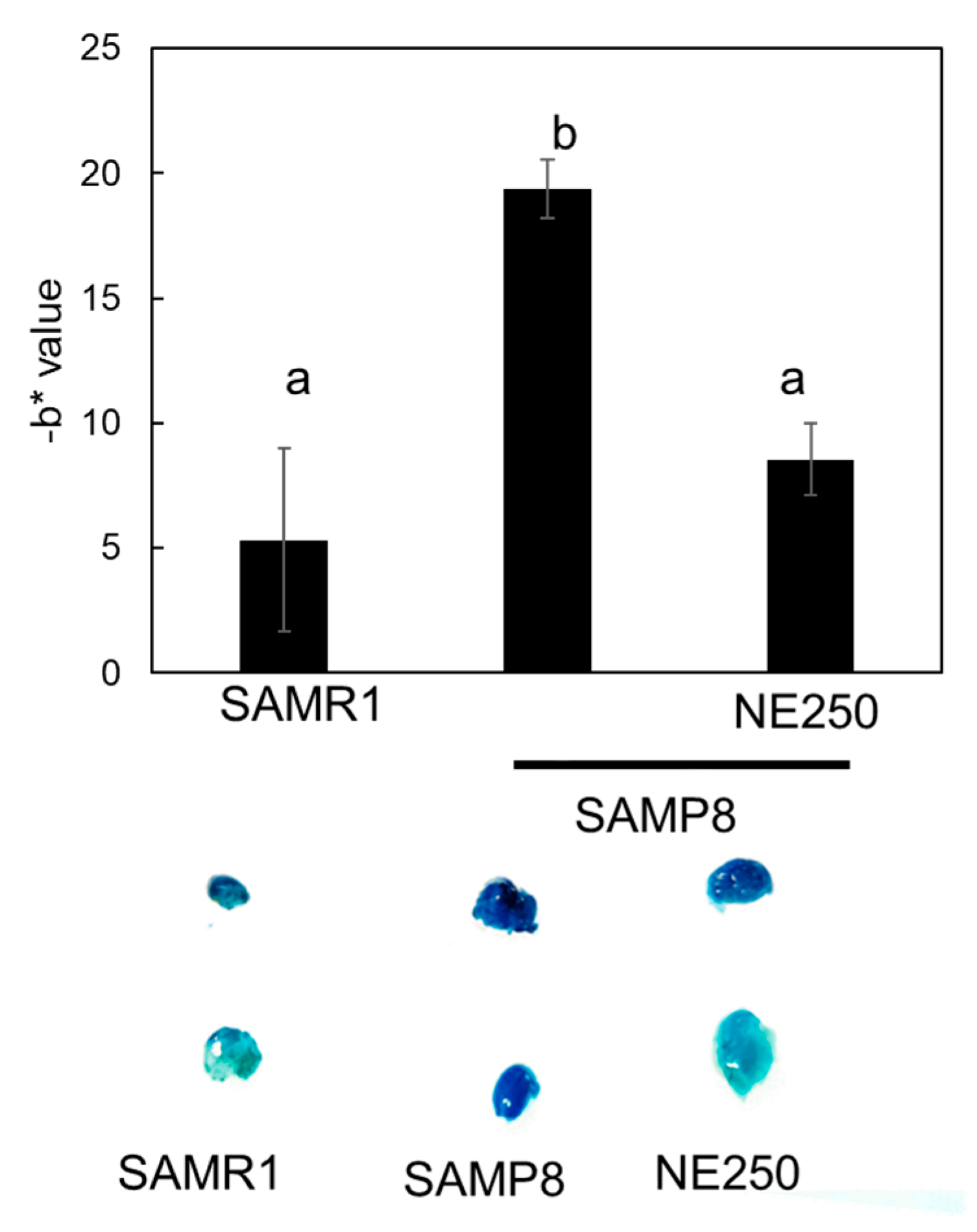
2.5. The Effects of Nacre Extract on Skin Aging in SAMP8 Mice
2.6. Effect of Nacre Extract on Adipose Aging
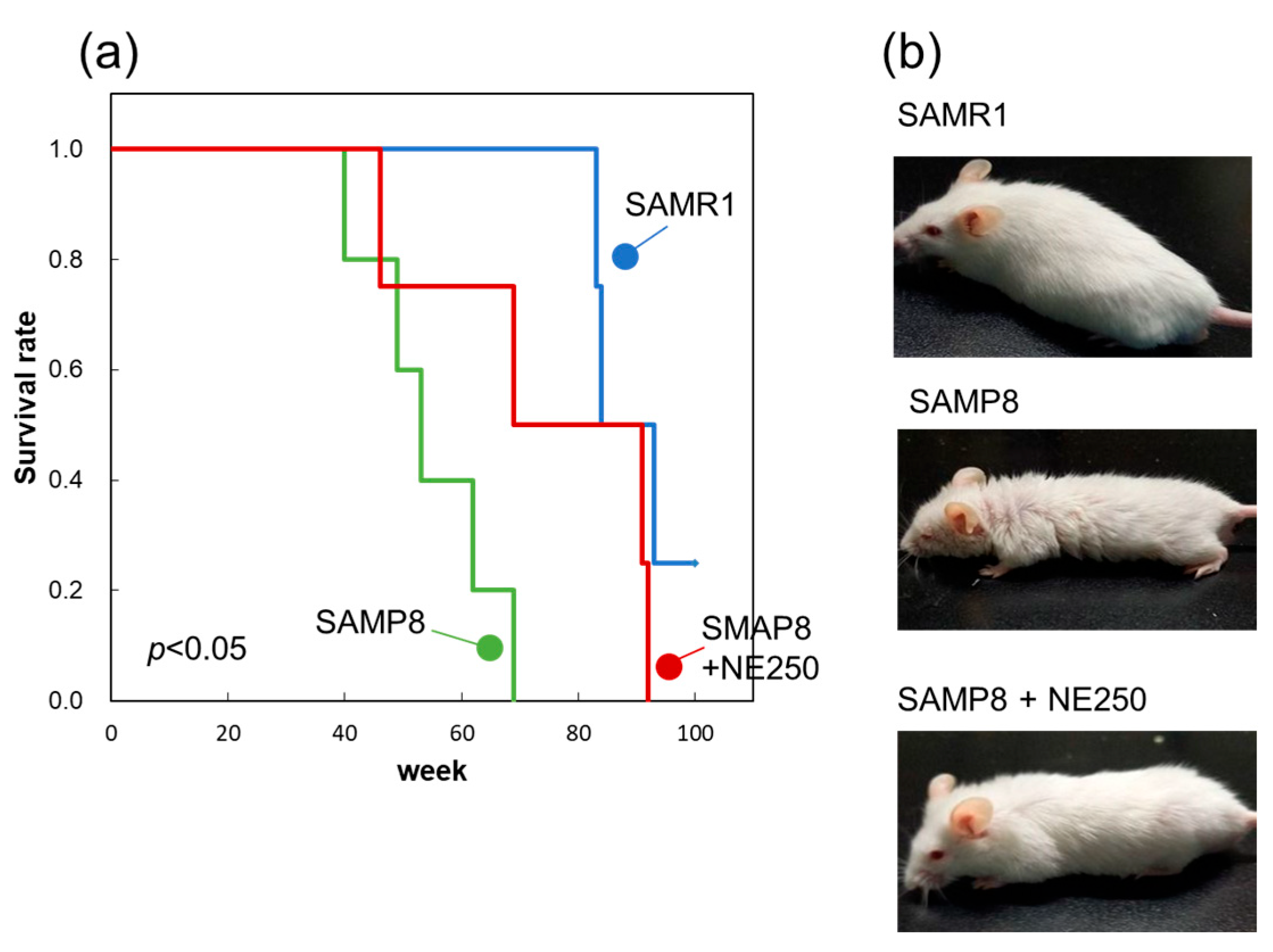
2.7. Effect of Nacre Extract on Lifespan
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Nacre Extract
4.3. SDS–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
4.4. Monosaccharide Analysis
4.5. Amino Acid Composition Analysis
4.6. Animals
4.7. Grading Score for Senescence
4.8. The Open Field Test
4.9. Grip Strength Measurement
4.10. The Wire-Hang Test
4.11. The Limb Clasping Test
4.12. Histochemistry
4.13. Senescence-Associated Beta-Galactosidase Activity of Adipose Tissue
4.14. Real-Time PCR
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, F.; Deng, C.M.; Fu, Z. GC-MS determination of microamounts of organic chemical compositions in nacre powder of Hyriopsis cumingii Lea. Phys. Test. Chem. Anal. 2010, 46, 300–302. [Google Scholar]
- He, J.F.; Deng, Q.; Pu, Y.H.; Liao, B.; Zeng, M. Amino acids composition analysis of pearl powder and pearl layer powder. Food Ind. 2016, 37, 270–273. [Google Scholar]
- Khaltourina, D.; Matveyev, Y.; Alekseev, A.; Cortese, F.; Ioviţă, A. Aging Fits the Disease Criteria of the International Classification of Diseases. Mech. Ageing Dev. 2020, 189, 111230. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.J.; Candau, R.B.; Bernardi, H. FoxO transcription factors: Their roles in the maintenance of skeletal muscle homeostasis. Cell. Mol. Life Sci. 2014, 71, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Gao, P.; Li, Z.; Dai, A.; Yang, M.; Chen, S.; Su, J.; Deng, Z.; Li, L. Forkhead Box O Signaling Pathway in Skeletal Muscle Atrophy. Am. J. Pathol. 2022, 192, 1648–1657. [Google Scholar] [CrossRef]
- Wu, J.; Ding, P.; Wu, H.; Yang, P.; Guo, H.; Tian, Y.; Meng, L.; Zhao, Q. Sarcopenia: Molecular regulatory network for loss of muscle mass and function. Front. Nutr. 2023, 10, 1037200. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.A.; McNeil, A.T.; Koh, T.J.; Brunskill, A.F.; Fantuzzi, G. Expression of genes in the skeletal muscle of individuals with cachexia/sarcopenia: A systematic review. PLoS ONE 2019, 14, e0222345. [Google Scholar] [CrossRef]
- Smith, I.J.; Alamdari, N.; O’Neal, P.; Gonnella, P.; Aversa, Z.; Hasselgren, P.O. Sepsis increases the expression and activity of the transcription factor Forkhead Box O 1 (FOXO1) in skeletal muscle by a glucocorticoid-dependent mechanism. Int. J. Biochem. Cell Biol. 2010, 42, 701–711. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, S.; Fergusson, M.M.; Finkel, T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J. Biol. Chem. 2005, 280, 16456–16460. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Ji, L.L. Role of PGC-1α in muscle function and aging. J. Sports Sci. 2013, 2, 81–86. [Google Scholar] [CrossRef]
- Ou, H.C.; Chu, P.M.; Huang, Y.T.; Cheng, H.C.; Chou, W.C.; Yang, H.L.; Chen, H.I.; Tsai, K.L. Low-level laser prevents doxorubicin-induced skeletal muscle atrophy by modulating AMPK/SIRT1/PCG-1α-mediated mitochondrial function, apoptosis and up-regulation of pro-inflammatory responses. Cell Biosci. 2021, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Petrocelli, J.J.; Drummond, M.J. PGC-1α-Targeted Therapeutic Approaches to Enhance Muscle Recovery in Aging. Int. J. Environ. Res. Public Health 2020, 17, 8650. [Google Scholar] [CrossRef] [PubMed]
- Arany, Z. PGC-1 coactivators and skeletal muscle adaptations in health and disease. Curr. Opin. Genet. Dev. 2008, 18, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Cai, B.; Nie, Q. PGC-1α affects skeletal muscle and adipose tissue development by regulating mitochondrial biogenesis. Mol. Genet. Genomics 2022, 297, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Pradhan, R.; Dey, S.; Kumar, R. The Role of Sirtuins in Sarcopenia and Frailty. Aging Dis. 2023, 14, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, J.H.; Lee, H.Y.; Min, K.J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.; Lagouge, L.; Noriega, J.C.; Milne, P.J.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Quan, T. Molecular insights of human skin epidermal and dermal aging. J. Dermatol. Sci. 2023, 112, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S.; Mills, S.J.; Ashworth, J.J. Ageing and wound healing. Biogerontology 2022, 3, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.; Xia, W.; He, T.; Calderone, K.; Bou-Gharios, G.; Voorhees, J.J.; Dlugosz, A.A.; Fisher, G.J. Matrix metalloproteinase-1 expression in fibroblasts accelerates dermal aging and promotes papilloma development in mouse skin. J. Investig. Dermatol. 2023, 143, 1700–1707. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Rosin, N.L.; Sparks, H.; Sinha, S.; Rahmani, W.; Sharma, N.; Workentine, M.; Abbasi, S.; Labit, E.; Stratton, J.A.; et al. Dysfunction of Hair Follicle Mesenchymal Progenitors Contributes to Age-Associated Hair Loss. Dev. Cell 2020, 53, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Almendariz-Palacios, C.; Mousseau, D.D.; Eskiw, C.H.; Gillespie, Z.E. Still Living Better through Chemistry: An Update on Caloric Restriction and Caloric Restriction Mimetics as Tools to Promote Health and Lifespan. Int. J. Mol. Sci. 2020, 21, 922. [Google Scholar] [CrossRef]
- Hwangbo, D.S.; Lee, H.Y.; Abozaid, L.S.; Min, K.J. Mechanisms of Lifespan Regulation by Calorie Restriction and Intermittent Fasting in Model Organisms. Nutrients 2020, 12, 1194. [Google Scholar] [CrossRef] [PubMed]
- Soo, S.K.; Rudich, P.D.; Traa, A.; Harris-Gauthier, N.; Shields, H.J.; Van-Raamsdonk, J.M. Compounds that extend longevity are protective in neurodegenerative diseases and provide a novel treatment strategy for these devastating disorders. Mech. Ageing Dev. 2020, 190, 111297. [Google Scholar] [CrossRef] [PubMed]
- Bene, M.; Salmon, A.B. Testing the evidence that lifespan-extending compound interventions are conserved across laboratory animal model species. GeroScience 2023, 45, 1401–1409. [Google Scholar] [CrossRef]
- Miller, R.A.; Harrison, D.E.; Astle, C.M.; Floyd, R.A.; Flurkey, K.; Hensley, K.L.; Javors, M.A.; Leeuwenburgh, C.; Nelson, J.F.; Ongini, E.; et al. An Aging Interventions Testing Program: Study design and interim report. Aging Cell 2007, 6, 565–575. [Google Scholar] [CrossRef]
- Fuji, T.; Inoue, T.; Hasegawa, Y. Nacre extract prevents scopolamine-induced memory deficits in rodents. Asian Pac. J. Trop. Med. 2018, 11, 202–208. [Google Scholar]
- Yamagami, H.; Fuji, T.; Wako, M.; Hasegawa, Y. Sulfated polysaccharide isolated from the nacre of pearl oyster improves scopolamine-induced memory impairment. Antioxidants 2021, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Yotsuya, Y.; Hasegawa, Y. Nacre extract from pearl oyster attenuates amyloid beta-induced memory impairment. J. Nat. Med. 2022, 76, 419–434. [Google Scholar] [CrossRef] [PubMed]
- Omachi, T.; Matsuyama, N.; Hasegawa, Y. Nacre extract from pearl oyster suppresses LPS-induced depression and anxiety. J. Funct. Foods 2023, 100, 105373. [Google Scholar] [CrossRef]
- Omachi, T.; Hasegawa, Y. Effect of nacre extract from pearl oyster shells against behavioral and psychological symptoms of dementia in senescence-accelerated mouse P8 (SAMP8). J. Funct. Foods 2024, 116, 106280. [Google Scholar] [CrossRef]
- Yamamoto, H.; Shimomura, N.; Oura, K.; Hasegawa, Y. Nacre Extract from Pearl Oyster Shell Prevents D-Galactose-Induced Brain and Skin Aging. Mar. Biotechnol. 2023, 25, 503–518. [Google Scholar] [CrossRef]
- Derave, W.; Eijnde, B.O.; Ramaekers, M.; Hespel, P. Soleus muscles of SAMP8 mice provide an accelerated model of skeletal muscle senescence. Exp. Gerontol. 2005, 40, 562–572. [Google Scholar]
- Romanello, V. The interplay between mitochondrial morphology and myomitokines in aging sarcopenia. Int. J. Mol. Sci. 2021, 22, 91. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H. Skin Aging and Dry Skin. J. Dermatol. 2004, 31, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Branchet, M.C.; Boisnic, S.; Frances, C.; Robert, A.M. Skin thickness changes in normal aging skin. Gerontology 1990, 36, 28–35. [Google Scholar] [CrossRef]
- Baker, D.J.; Childs, B.G.; Durik, M.; Wijers, M.E.; Sieben, C.J.; Zhong, J.; Saltness, R.A.; Jeganathan, K.B.; Casaclang-Verzosa, G.; Pezeshki, A.; et al. Naturally occurring p16 Ink4a -positive cells shorten healthy lifespan. Nature 2016, 530, 1–5. [Google Scholar] [CrossRef]
- Yun, G.A.; Leung, K.S.; Siu, P.M.F.; Qin, J.H.; Chow, S.K.H.; Qin, L.; Li, C.Y.; Cheung, H. Muscle mass, structural and functional investigations of senescence-accelerated mouse P8 (SAMP8). Exp. Anim. 2015, 64, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular senescence and tumor suppressor gene p16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef]
- Idda, M.L.; McClusky, W.G.; Lodde, V.; Munk, R.; Abdelmohsen, K.; Rossi, M.; Gorospe, M. Survey of senescent cell markers with age in human tissues. Aging 2020, 12, 4052. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.-D.; Wagner, N. The Senescence Markers p16INK4A, p14ARF/p19ARF, and p21 in Organ Development and Homeostasis. Cells 2022, 11, 1966. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; Muñoz-Cánoves, P. Regenerative Decline of Stem Cells in Sarcopenia. Mol. Aspects Med. 2016, 50, 109–117. [Google Scholar] [CrossRef]
- Ferri, E.; Marzetti, E.; Calvani, R.; Picca, A.; Cesari, M.; Arosio, B. Role of Age-Related Mitochondrial Dysfunction in Sarcopenia. Int. J. Mol. Sci. 2020, 21, 5236. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.W.; Chang, Y.C.; Chan, Y.C.; Hu, S.H.; Liu, M.Y.; Chang, S.J. Dysregulations of mitochondrial quality control and autophagic flux at an early age lead to progression of sarcopenia in SAMP8 mice. Biogerontology 2020, 21, 367–380. [Google Scholar] [CrossRef]
- Andreux, P.A.; van Diemen, M.P.J.; Heezen, M.R.; Auwerx, J.; Rinsch, C.; Groeneveld, G.J.; Singh, A. Mitochondrial function is impaired in the skeletal muscle of pre-frail elderly. Sci. Rep. 2018, 8, 8548. [Google Scholar] [CrossRef] [PubMed]
- Gerhart-Hines, Z.; Rodgers, J.T.; Bare, O.; Lerin, C.; Kim, S.H.; Mostoslavsky, R.; Alt, F.W.; Wu, Z.; Puigserver, P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007, 26, 1913–1923. [Google Scholar] [CrossRef]
- Chen, X.; Ji, Y.; Liu, R.; Zhu, X.; Wang, K.; Yang, X.; Liu, B.; Gao, Z.; Huang, Y.; Shen, Y.; et al. Mitochondrial dysfunction: Roles in skeletal muscle atrophy. J. Transl. Med. 2023, 21, 503. [Google Scholar] [CrossRef]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia: Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Miura, S.; Suzuki, M.; Kia, Y.; Mizulami, J.; Taniguchi, T.; Mochida, K.; Hata, T.; Matsuda, J.; Aburatani, H.; et al. Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated Type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J. Biol. Chem. 2004, 279, 41114–41123. [Google Scholar] [CrossRef] [PubMed]
- Green, C.L.; Lamming, D.W.; Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 2022, 23, 56–73. [Google Scholar] [CrossRef]
- Pifferi, F.; Aujard, F. Caloric restriction, longevity and aging: Recent contributions from human and non-human primate studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 95, 109702. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Min, K.J. Sirtuins and life span extension. In Sirtuin Biology in Medicine; Maiese, K., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 37–47. [Google Scholar]
- Zhang, J.X.; Li, S.R.; Yao, S.; Bi, Q.R.; Hou, J.J.; Cai, L.Y.; Han, S.M.; Wu, W.Y.; Guo, D.A. Anticonvulsant and sedative–hypnotic activity screening of pearl and nacre (mother of pearl). J. Ethnopharmacol. 2016, 181, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Van Deursen, J.M. Clearing senescent cells with targeted drugs could combat age-associated disease. Science 2019, 364, 636–637. [Google Scholar] [CrossRef] [PubMed]
- Robbins, P.D.; Jurk, D.; Khosla, S.; Kirkland, J.L.; LeBrasseur, N.K.; Miller, J.D.; Passos, J.F.; Pignolo, R.J.; Tchkonia, T.; Niedernhofer, L.J. Senolytic Drugs: Reducing Senescent Cell Viability to Extend Health Span. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 779–803. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Tuday, E.; Allen, S.; Kim, J.; Trott, D.W.; Holland, W.L.; Donato, A.J.; Lesniewski, L.A. Senolytic drugs, dasatinib and quercetin, attenuate adipose tissue inflammation, and ameliorate metabolic function in old age. Aging Cell 2023, 22, e13767. [Google Scholar] [CrossRef] [PubMed]
- Inotsuka, R.; Udono, M.; Yamatsu, A.; Kim, M.; Katakura, Y. Exosome-Mediated Activation of Neuronal Cells Triggered by γ-Aminobutyric Acid (GABA). Nutrients 2021, 13, 2544. [Google Scholar] [CrossRef]
- Sugihara, Y.; Onoue, S.; Tashiro, K.; Sato, M.; Hasegawa, T.; Katakura, Y. Carnosine Induces Intestinal Cells to Secrete Exosomes That Activate Neuronal Cells. PLoS ONE 2019, 14, e0217394. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Takahashi, K.; Ohkawa, C.; Sasaki, Y.; Hasegawa, Y. Identification of a resistant protein from scallop shell extract and its bile acid-binding activity. Fish. Sci. 2013, 79, 1017–1025. [Google Scholar] [CrossRef]
- Takahashi, K.; Hasegawa, Y. Glycoproteins isolated from scallop shells inhibit differentiation of 3T3-L1 preadipocyte cells. Fish. Sci. 2014, 80, 1301–1310. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Inoue, T.; Kawaminami, S.; Fujita, M. Effects of scallop shell extract on scopolamine-induced memory impairment and MK801-induced locomotor activity. Asian Pac. J. Trop. Med. 2016, 9, 662–667. [Google Scholar] [CrossRef]
- Girgis, C.M.; Cha, K.M.; Houweling, P.J.; Rao, R.; Mokbel, N.; Lin, M.; Clifton-Bligh, R.J.; Gunton, J.E. Vitamin D Receptor Ablation and Vitamin D Deficiency Result in Reduced Grip Strength, Altered Muscle Fibers, and Increased Myostatin in Mice. Calcif. Tissue Int. 2015, 97, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Takahashi, Y.; Murakami, S.; Wani, K.; Miyazaki, T.; Matsumoto, Y.; Okamoto, M.; Ishihara, T. The prevention of home-cage grid climbing affects muscle strength in mice. Sci. Rep. 2022, 12, 15263. [Google Scholar] [CrossRef] [PubMed]
- Haramipour, P.; Hassanpour, S.; Rezaei, A. Prenatal Exposure to L-Citrulline Has Positive Effects on Reflexive Motor Behavior in Newborn Mice. Arch. Razi. Inst. 2022, 77, 1961–1970. [Google Scholar]
- Christensen, D.Z.; Kraus, S.L.; Flohr, A.; Cotel, M.C.; Wirths, O.; Bayer, T.A. Transient intraneuronal Ab rather than extracellular plaque pathology correlates with neuron loss in the frontal cortex of APP/PS1KI mice. Acta Neuropathol. 2008, 116, 647–655. [Google Scholar] [CrossRef]
- Rouault, C.; Marcelin, G.; Adriouch, S.; Rose, C.; Genser, L.; Ambrosini, M.; Bichet, J.C.; Zhang, Y.; Marquet, F.; Aron-Wisnewsky, J.; et al. Senescence-associated β-galactosidase in subcutaneous adipose tissue associates with altered glycaemic status and truncal fat in severe obesity. Diabetologia 2021, 64, 240–254. [Google Scholar] [CrossRef]

| Amino Acid | Composition (mol%) |
|---|---|
| Aspartic acid and Asparagine | 27.5 |
| Threonin | 2.9 |
| Serine | 6.2 |
| Glutamic acid and Glutamine | 7.2 |
| Glycine | 25.4 |
| Alanine | 5.1 |
| Cystine | 0.4 |
| Valine | 3.0 |
| Methionine | 1.9 |
| Isoleucine | 1.9 |
| Leucine | 2.7 |
| Tyrosine | 2.3 |
| Phenylalanine | 1.7 |
| Lysine | 3.4 |
| Histidine | 1.3 |
| Argnine | 2.1 |
| Proline | 5.0 |
| Tryptophan | not determined |
| Monosaccharide | Composition (mol%) |
|---|---|
| D-Glucose | 24.3 |
| D-Galactose | 13.8 |
| D-Mannose | 12.8 |
| N-Acetyl-D-Glucosamine | 12.7 |
| N-Acetyl-D-Galactosamine | 9.5 |
| D-Rhamnose | 8.7 |
| D-Ribulose | 6.6 |
| D-Fucose | 3.6 |
| D-Xylose | 1.8 |
| D-Glucuronic acid | 1.8 |
| N-Acetyl-D-Mannosamine | 1.4 |
| D-Galacturonic acid | 1.3 |
| D-Arabinose | 1.2 |
| 2-deoxyglucose | 0.5 |
| SAMR1 | SAMP8 | SAMP8+NE125 | SAMP8+NE250 | |
|---|---|---|---|---|
| Passivity | 0 | 1.2 ± 0.75 | 0.20 ± 0.40 | 0 |
| Reactivity | 0 | 0.60 ± 0.49 | 0 | 0.40 ± 0.49 |
| Coat coarseness | 0.40 ± 0.49 | 1.8 ± 0.75 | 0.60 ± 0.49 | 0.40 ± 0.49 |
| Glossiness | 0 | 0.60 ± 0.49 | 0.20 ± 0.40 | 0.40 ± 0.49 |
| Hair loss | 0 | 1.4 ± 0.49 | 0.80 ± 0.40 | 0.40 ± 0.49 |
| Ulcers and cataracts | 0 | 0 | 0 | 0 |
| Overall average | 0.07 ± 0.08 a | 0.93 ± 0.27 b | 0.30 ± 0.12 a | 0.27 ± 0.17 a |
| Control Diet | NE125 Diet | NE250 Diet | |
|---|---|---|---|
| Casein | 20.00 | 20.00 | 20.00 |
| Corn starch | 15.00 | 15.00 | 15.00 |
| Cellulose | 5.00 | 5.00 | 5.00 |
| Mineral mix | 3.50 | 3.50 | 3.50 |
| Vitamin mix | 1.00 | 1.00 | 1.00 |
| L-cystine | 0.30 | 0.30 | 0.30 |
| Choline bitartrate | 0.20 | 0.20 | 0.20 |
| Soybean oil | 5.00 | 5.00 | 5.00 |
| Sucrose | 50.00 | 50.00 | 50.00 |
| Nacrre extract (NE) | 0.00 | 0.15 | 0.30 |
| Total | 100 | 100.15 | 100.3 |
| Gene Name | Sequence (5′ to 3′) |
|---|---|
| Peroxisome proliferator-activated receptor gamma coactivator (PGC)-1α | F-CGGAAATCATATCCAACCAG |
| R-TGAGAACCGCTAGCAAGTTTG | |
| Sirtuin1 | F-CTCCTGTTGACCGATGGACT |
| R-GCGGAGTCCAGTCACTAGAG | |
| Nuclear respiratory factor (Nrf)1 | F-AGCACGGAGTGACCCAAAC |
| R-TGTACGTGGCTACATGGACCT | |
| Succinate dehydrogenase (SDH) | F-AAGGCAAATGCTGGAGAAGA |
| R-TGGTTCTGCATCGACTTCTG | |
| Forkhead box O (FOXO)1 | F-GCAGCCAGGCATCTCATAAC |
| R-CAGATGTGTGAGGCATGGTG | |
| β-actin | F-CTTCTTGGGTATGGAATCCTGTG |
| R-ATGTCAACGTCACACTTCATGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, H.; Shimomura, N.; Hasegawa, Y. Oral Administration of Nacre Extract from Pearl Oyster Shells Has Anti-Aging Effects on Skin and Muscle, and Extends the Lifespan in SAMP8 Mice. Pharmaceuticals 2024, 17, 713. https://doi.org/10.3390/ph17060713
Yamamoto H, Shimomura N, Hasegawa Y. Oral Administration of Nacre Extract from Pearl Oyster Shells Has Anti-Aging Effects on Skin and Muscle, and Extends the Lifespan in SAMP8 Mice. Pharmaceuticals. 2024; 17(6):713. https://doi.org/10.3390/ph17060713
Chicago/Turabian StyleYamamoto, Hana, Nanami Shimomura, and Yasushi Hasegawa. 2024. "Oral Administration of Nacre Extract from Pearl Oyster Shells Has Anti-Aging Effects on Skin and Muscle, and Extends the Lifespan in SAMP8 Mice" Pharmaceuticals 17, no. 6: 713. https://doi.org/10.3390/ph17060713
APA StyleYamamoto, H., Shimomura, N., & Hasegawa, Y. (2024). Oral Administration of Nacre Extract from Pearl Oyster Shells Has Anti-Aging Effects on Skin and Muscle, and Extends the Lifespan in SAMP8 Mice. Pharmaceuticals, 17(6), 713. https://doi.org/10.3390/ph17060713








