The Genus Dacryodes Vahl.: Ethnobotany, Phytochemistry and Biological Activities
Abstract
1. Introduction
2. Literature Survey Databases
3. Description of Dacryodes Vahl. Species and Ethnobotany
3.1. Dacryodes klaineana (Pierre) H.J. Lam
3.2. Dacryodes buettneri (Engl.) H.J. Lam
3.3. Dacryodes rostrata (Blume) H.J. Lam
3.4. Dacryodes peruviana (Loes.) H.J. Lam
3.5. Dacryodes edulis (G. Don) H.J. Lam
3.5.1. Dacryodes edulis Uses as Food
3.5.2. Dacryodes edulis Miscellaneous Uses
3.5.3. Medicinal Purposes
3.5.4. Spiritual Purposes
3.5.5. Nutritional Value of D. edulis
4. Plant Distribution
5. Commercialization
6. Conservation of Dacryodes edulis Fruits
7. Bioactivity Studies
7.1. Antimicrobial Activity
7.2. Anticancer Activity
7.3. Antidiabetic Activity
7.4. Antioxidant and Free Radicals Scavenging Activity
7.5. Effect on Hepatocytes
7.6. Toxicity
8. Phytochemistry
9. Summary
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Petrovska, B.B. Historical review of medicinal plants′ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ononamadu, C.J.; Alhassan, A.J.; Ibrahim, A.; Imam, A.A.; Ihegboro, G.O.; Owolarafe, A.T.; Ezeigwe, O.C.; Atiku, M.K.; Sule, M.S. Toxicological study of aqueous-methanol solvent fraction of methanol extract of Dacryodes edulis leaves. Toxicol. Rep. 2020, 7, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Salmeron-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide Research Trends on Medicinal Plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef] [PubMed]
- Kunle, O.F.; Egharevba, H.O.; Ahmadu, P.O. Standardization of herbal medicines—A review. Int. J. Biodivers. Conserv. 2012, 4, 101–112. [Google Scholar] [CrossRef]
- Zu, Y.; Qin, L.; Zhan, F.; Wu, J.; Li, Y.; Chen, J.; Wang, J.; Hu, W. Intercropping of Sonchus asper and Vicia faba affects plant cadmium accumulation and root responses. Pedosphere 2020, 30, 457–465. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jenkins, C.N.; Abell, R.; Brooks, T.M.; Gittleman, J.L.; Joppa, L.N.; Raven, P.H.; Roberts, C.M.; Sexton, J.O. The biodiversity of species and their rates of extinction, distribution, and protection. Science 2014, 344, 1246752. [Google Scholar] [CrossRef]
- Ajibesin, K.K.; Ekpo, B.A.; Bala, D.N.; Essien, E.E.; Adesanya, S.A. Ethnobotanical survey of Akwa Ibom State of Nigeria. J. Ethnopharmacol. 2008, 115, 387–408. [Google Scholar] [CrossRef]
- Dike, I.P.; Obembe, O.O.; Adebiyi, F.E. Ethnobotanical survey for potential anti-malarial plants in south-western Nigeria. J. Ethnopharmacol. 2012, 144, 618–626. [Google Scholar] [CrossRef]
- Rasool Hassan, B.A. Medicinal Plants (Importance and Uses). Pharm. Anal. Acta 2012, 3, e139. [Google Scholar] [CrossRef]
- Kuete, V. Potential of Cameroonian Plants and Derived Products against Microbial Infections: A Review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef]
- Kuete, V.; Efferth, T. Cameroonian Medicinal Plants: Pharmacology and Derived Natural Products. Front. Pharmacol. 2010, 1, 123. [Google Scholar] [CrossRef]
- Tee, L.H.; Yang, B.; Nagendra, K.P.; Ramanan, R.N.; Sun, J.; Chan, E.-S.; Tey, B.T.; Azlan, A.; Ismail, A.; Lau, C.Y.; et al. Nutritional compositions and bioactivities of Dacryodes species: A review. Food Chem. 2014, 165, 247–255. [Google Scholar] [CrossRef]
- Olivier, T.T.; Moïse, F.; Jackson, S.A.; Francis, N.T. A Review on Traditional Uses, Phytochemical and Pharmacological Profiles, Spiritual and Economic Values, and Toxicity of Dacryodes edulis (G. Don) H.J. Lam. J. Drug Deliv. Ther. 2016, 6, 84–90. [Google Scholar] [CrossRef]
- Tsakem, B.; Ponou, B.K.; Toussie, B.T.; Tematio, R.F.; Noundou, X.S.; Krause, R.W.; Teponno, R.B.; Tapondjou, L.A. The Genus Olax: Traditional uses, phytochemistry and biological activities. In Natural Products Chemistry of Botanical Medicines from Cameroonian Plants, 1st ed.; Siwe Noundou, X., Ed.; CRC Press: Boca Raton, FL, USA, 2021; Volume 1, pp. 1–22. [Google Scholar] [CrossRef]
- Tee, L.H.; Yang, B.; Tey, B.T.; Chan, E.-S.; Azlan, A.; Ismail, A.; Sun, J.; Lau, C.Y.; Palanisamy, U.D.; Ramanan, R.N.; et al. Valorization of Dacryodes rostrata fruit through the characterization of its oil. Food Chem. 2017, 235, 257–264. [Google Scholar] [CrossRef]
- Rol, N.; Egbe, R.E.; Bechem, E.; Yengo, T.; Chia, E.L.; Eyenieh, N.M.; Asaha, S. Ethnobotanical study of commonly used medicinal plants of the Takamanda Rainforest South West, Cameroon. Afr. J. Plant Sci. 2013, 7, 21–34. [Google Scholar] [CrossRef]
- Onana, J.M. A synoptic revision of Dacryodes (Burseraceae) in Africa, with a new species from Central Africa. Kew Bull. 2008, 63, 385–400. [Google Scholar] [CrossRef]
- Obame, L.C.; Koudou, J.; Chalchat, J.; Boassole, I.; Edou, P.; Ouattara, A. Volatile components, antioxidant and antibacterial activities of Dacryodes buettneri H.J. Lam. essential oil from Gabon. Sci. Res. Essays 2007, 2, 491–495. [Google Scholar]
- Kong, K.W.; Chew, L.Y.; Prasad, K.N.; Lau, C.Y.; Ismail, A.; Sun, J.; Hosseinpoursarmadi, B. Nutritional constituents and antioxidant properties of indigenous kembayau (Dacryodes rostrata (Blume) H.J. Lam) fruits. Food Res. Int. 2011, 44, 2332–2338. [Google Scholar] [CrossRef]
- Moise, M.-M.; Benjamin, L.-M.; Etienne, M.; Thierry, G.; Dalida, K.N.; Doris, T.M.; Samy, W.M. Intake of Gnetum africanum and Dacryodes edulis, Imbalance of Oxidant/Antioxidant Status and Prevalence of Diabetic Retinopathy in Central Africans. PLoS ONE 2012, 7, e49411. [Google Scholar] [CrossRef]
- Obame, L.C.; Edou, P.; Bassolé, I.H.N.; Koudou, J.; Agnaniet, H.; Eba, F.; Traore, A.S. Chemical composition, antioxidant and antimicrobial properties of the essential oil of Dacryodes edulis (G. Don) H.J. Lam. from Gabon. Afr. J. Microbiol. Res. 2008, 2, 148–152. [Google Scholar]
- Onocha, P.A.; Oloyede, G.K.; Afolabi, Q.O. Cytotoxicity and Free Radical Scavenging Activities of Hexane Fractions of Nigeria Specie of African Pear (Dacryodes edulis). Int. J. Biol. Chem. 2011, 5, 143–149. [Google Scholar] [CrossRef]
- Ahmad, F.; Khalid, P.; Khan, M.M.; Rastogi, A.K.; Kidwai, J.R. Insulin like activity in (−) epicatechin. Acta Diabetol. Lat. 1989, 26, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Ezeonyi, E.I.; Blessing, O.O.; Chinelo, J.E.; Peter, O.I.; Chuks, O.E.; Ifeoma, I.C. Pharmacognostic and phytochemical properties of methanol crude extract and fractions of the leaves of Dacryodes klaineana (Pierre) H.J. Lam (Burseraceae). World J. Pharm. Res. 2022, 11, 209–222. [Google Scholar] [CrossRef]
- Van Damme, P. Plant Resources of Tropical Africa 7(1); Prota Foundation, Backhuys Publishers: Wageningen, The Netherlands, 2009; p. 186. [Google Scholar]
- Ogheneovo, O.R.; Oseigbokan, A.L.; Olabisi, R.B.; Oduola, L.I. Phytochemical screening and mineral analysis of the pulp of Dacryodes klaineana (Pierre) H.J. LAM. Am. J. Chem. Mater. Sci. 2019, 6, 21–24. [Google Scholar]
- Neuwinger, H.D. African Traditional Medicine: A Dictionary of Plant Use and Applications with Supplement: Search System for Diseases; Medpharm Scientific Publishers: Stuttgart, Germany, 2000. [Google Scholar]
- Useful Tropical Plants Home Page. Available online: https://tropical.theferns.info/viewtropical.php?id=Dacryodes+edulis (accessed on 1 January 2023).
- Burkill, H.M. The Useful Plants of West Tropical Africa, 2nd ed.; Royal Botanic Gardens: Kew, UK, 1985; Volume 1. [Google Scholar]
- Igoli, J.O.; Ogaji, O.G.; Tor-Ayiin, T.A.; Igoli, N.P. Traditional medicine practice amongst the Igede people of Nigeria. Part II. Afr. J. Tradit. Complement. Altern. Med. 2005, 2, 134–152. [Google Scholar] [CrossRef]
- Lim, T.K. Dacryodes rostrata. In Edible Medicinal and Non-Medicinal Plants: Volume 1; Lim, T.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 633–635. [Google Scholar] [CrossRef]
- Saw, L.G.; LaFrankie, J.V.; Kochummen, K.M.; Yap, S.K. Fruit trees in a Malaysian rain forest. Econ. Bot. 1991, 45, 120–136. [Google Scholar] [CrossRef]
- Tinggal, S.H. Brunei olive-Kembayau and Pinanasan. In Brunei Darussalam Fruits in Colour Brunei; Universiti Brunei Darussalam: Gadong, Brunei, 1992; pp. 102–109. [Google Scholar]
- Hoe, V.B.; Siong, K.H. The nutritional value of indigenous fruits and vegetables in Sarawak. Asia Pac. J. Clin. Nutr. 1999, 8, 24–31. [Google Scholar] [CrossRef]
- Latiff, A.; Zakri, A.H. Protection of traditional knowledge, innovations and practices: The Malaysian experience. In Proceedings of the UNCTAD Expert Meeting on Systems and National Experiences for Protecting Traditional Knowledge, Innovations and Practices, Geneva, Switzerland, 30 October–1 November 2000. [Google Scholar]
- Thavamoney, N.; Sivanadian, L.; Tee, L.H.; Khoo, H.E.; Prasad, K.N.; Kong, K.W. Extraction and recovery of phytochemical components and antioxidative properties in fruit parts of Dacryodes rostrata influenced by different solvents. J. Food Sci. Technol. 2018, 55, 2523–2532. [Google Scholar] [CrossRef]
- Espinoza, L.C.; Valarezo, E.; Fábrega, M.J.; Rodríguez-Lagunas, M.J.; Sosa, L.; Calpena, A.C.; Mallandrich, M. Characterization and In Vivo Anti-Inflammatory Efficacy of Copal (Dacryodes peruviana (Loes.) H.J. Lam) Essential Oil. Plants 2022, 11, 3104. [Google Scholar] [CrossRef]
- Valarezo, E.; Ojeda-Riascos, S.; Cartuche, L.; Andrade-González, N.; González-Sánchez, I.; Meneses, M.A. Extraction and Study of the Essential Oil of Copal (Dacryodes peruviana), an Amazonian Fruit with the Highest Yield Worldwide. Plants 2020, 9, 1658. [Google Scholar] [CrossRef]
- Trade Winds fruit Home Page. Available online: https://www.tradewindsfruit.com/dacryodes-rostrata-klasu-seeds (accessed on 8 May 2023).
- The Roaming Fork Home Page. Available online: https://theroamingfork.com/african-pear/ (accessed on 8 May 2023).
- Zofou, D.; Tematio, E.L.; Ntie-Kang, F.; Tene, M.; Ngemenya, M.N.; Tane, P.; Titanji, V.P.K. New Antimalarial Hits from Dacryodes edulis (Burseraceae)—Part I: Isolation, In Vitro Activity, In Silico “drug-likeness” and Pharmacokinetic Profiles. PLoS ONE 2013, 8, e79544. [Google Scholar] [CrossRef] [PubMed]
- Ogboru, R.O.; Okolie, P.L.; Agboje, I. Phytochemical Screening and Medicinal Potentials of the Bark of Dacryodes edulis (G. Don) H.J. Lam. J. Environ. Anal. Chem. 2015, 2, 2380–2391. [Google Scholar] [CrossRef]
- Okwu, D.; Nnamdi, F. Evaluation of The Chemical Composition of Dacryodes edulis And Raphia hookeri Mann and Wendl Exudates Used in Herbal Medicine In South Eastern Nigeria. Afr. J. Tradit. Complement. Altern. Med. 2008, 5, 192–200. [Google Scholar] [CrossRef]
- Dossou, B.R.; Missang, C.E.; Karou, S.D.; Ameyapoh, Y. Relationship between texture and cell-wall components of safou (Dacryodes edulis (G. Don) H.J. Lam) fruits at different storage conditions. J. Appl. Biosci. 2018, 125, 12566–12580. [Google Scholar] [CrossRef]
- Gérard, D.B.P.; Léonce, K.B.R.; Thierry, K.F.M.; Soumaïla, D. Effect of softening level on physico-chemical parameters of safou (Dacryodes edulis) pulp and oil grown in Côte d’Ivoire. J. Appl. Biosci. 2020, 145, 14852–14861. [Google Scholar] [CrossRef]
- Ikhuoria, E.; Maliki, U.M. Characterization of avocado pear (Persea americana) and African pear (Dacryodes edulis) extracts. Afr. J. Biotechnol. 2007, 6, 950–952. [Google Scholar] [CrossRef]
- Omonhinmin, C.A. Ethnobotany of Dacryodes edulis (G. Don) H.J. Lam in Southern Nigeria 1: Practices and applications among the Yoruba speaking people. Ethnobot. Res. Appl. 2012, 10, 175–184. [Google Scholar] [CrossRef]
- Dalziel, J.M. Flora of West Tropical Africa. Crown Agents for Overseas Governments; Milbank: London, UK, 1937; pp. 1–296. [Google Scholar]
- Ekong, D.; Okogun, J. Terpenoids of Dacryoides edulis. Phytochemistry 1969, 8, 669–671. [Google Scholar] [CrossRef]
- Ayuk, E.T.; Duguma, B.; Franzel, S.; Kengue, J.; Mollet, M.; Tiki-Manga, T.; Zekeng, P. Uses, management, and economic potential of Dacryodes edulis (Burseraceae) in the Humid Lowlands of Cameroon. Econ. Bot. 1999, 53, 292–301. [Google Scholar] [CrossRef]
- Ozgen, U.; Kaya, Y.; Coskun, M. Ethnobotanical studies in the villages of the District of Ilica (Province Erzurum) Turkey. Econ. Bot. 2004, 58, 691–696. [Google Scholar] [CrossRef]
- IFAD. The International Centre for Research in Agroforestry (ICRAF): Diversification of Smallholder Farming Systems in West and Central Africa through Cultivation of Indigenous Trees. Executive Board—Sixty-Seventh Session Rome, 8–9 September 1999; IFAD: Rome, Italy, 1999. [Google Scholar]
- Simons, A.; Leakey, R. Tree domestication in tropical agroforestry. Agrofor. Syst. 2004, 61, 167–181. [Google Scholar] [CrossRef]
- Okolo, C.A.; Ejere, V.C.; Chukwuka, C.O.; Ezeigbo, I.I.; Nwibo, D.D.; Okorie, A.N. Hexane Extract of Dacryodes edulis Fruits Possesses Anti-Diabetic and Hypolipidaemic Potentials in Alloxan Diabetes of Rats. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Ekpa, O. The use of Raphia hookeri and Pachylobus edulis in cosmetic formulation. Discov. Innov. 1993, 5, 313–316. [Google Scholar]
- Hassan-Olajokun, R.E.; Deji-Agboola, A.M.; Olasunkanmi, O.O.; Banjo, T.A.; Olaniran, O. Antimicrobial Activity of Fractioned Components from Dacryodes edulis: Invitro Study. Eur. J. Med. Plants 2020, 31, 71–82. [Google Scholar] [CrossRef]
- Jiofack, T.; Fokunang, C.; Guedje, N.; Kumeuze, V.; Fongnzossie, E. Ethnobotanical uses of medicinal plants of two ethnoecological regions of Cameroon. Int. J. Med. Sci. 2010, 2, 60–79. [Google Scholar]
- Ogunmoyole, T.; Kade, I.J.; Johnson, O.D.; Makun, O.J. Effect of boiling on the phytochemical constituents and antioxidant properties of African pear Dacryodes edulis seeds in vitro. Afr. J. Biochem. Res. 2012, 6, 105–114. [Google Scholar] [CrossRef]
- Afolayan, F.I.D.; Sulaiman, K.A.; Okunade, W.T. Ethnobotanical survey of plants used in cancer therapy in Iwo and Ibadan, South-Western of Nigeria. J. Pharm. Pharmacogn. Res. 2020, 8, 346–367. [Google Scholar]
- Hutchison, J.; Dalziel, J.M. Flora of West Africa II; Herpper, F.N., Ed.; Macmillian Publishers Ltd.: Lagos, Nigeria, 1963; Volume 11. [Google Scholar]
- Erukainure, O.L.; Mopuri, R.; Oyebode, O.A.; Koorbanally, N.A.; Islam, S. Dacryodes edulis enhances antioxidant activities, suppresses DNA fragmentation in oxidative pancreatic and hepatic injuries; and inhibits carbohydrate digestive enzymes linked to type 2 diabetes. Biomed. Pharmacother. 2017, 96, 37–47. [Google Scholar] [CrossRef]
- Jolayemi, O.S.; Olanrewaju, O.J.; Ogunwale, O. Exploring in Vitro Antioxidant Activity and Physicochemical Properties of Selected Under-Exploited Tropical Fruits. Acta Univ. Cibiniensis Ser. E Food Technol. 2020, 24, 165–174. [Google Scholar] [CrossRef]
- Makouate, H.F.; Lekagne, D.J.B. African Pear (Dacoryodes edulis (G. Don) H.J. Lam) Physical characteristics, nutritional properties and postharvest management: A Review. Agric. Conspec. Sci. 2022, 87, 1–10. [Google Scholar]
- Mbofung, C.M.; Silou, T.; Mouragadja, I. Chemical Characterisation of Safou (Dacryodes edulis) And Evaluation of its Potential as an Ingredient in Nutritious Biscuits. For. Trees Livelihoods 2002, 12, 105–117. [Google Scholar] [CrossRef]
- Kadji, B.R.L.; Kone, F.M.T.; Sika, A.E.; Dabonne, S. Physico-chemical properties of Safou (Dacryodes edulis) fruits grown in Côte d’Ivoire. J. Appl. Biosci. 2016, 105, 10103–10110. [Google Scholar] [CrossRef]
- Esiet, U.I.; Gloria Obuzor, G.; Jnr, M.H. Proximate and phytochemical studies of exudate of Dacryodes edulis. Res. J. Chem. Sci. 2016, 6, 1–9. [Google Scholar]
- Youmbi, E.; Mbeuyo, M.; Tchinda, N.D.; Amougou, A. Physico-chemical characterisation and classification of fruits of Dacryodes edulis from the major agro-ecological zones of Cameroon. Fruits 2010, 65, 355–365. [Google Scholar] [CrossRef]
- Oluwaniyi, O.O.; Nwosu, O.F.; Okoye, C.M. Comparative study of the constituents of the fruits pulps and seeds of Canarium ovatum, Persea americana and Dacryodes edulis. Jordan J. Chem. 2017, 12, 113–125. [Google Scholar]
- Sonwa, D.J.; Okafor, J.C.; Buyungu, P.M.; Weise, S.F.; Tchatat, M.; Adesina, A.A.; Nkongmeneck, A.B.; Ndoye, O.; Endamana, D. Dacryodes edulis, a Neglected Non-Timber Forest Species for the Agroforestry Systems of West and Central Africa. For. Trees Livelihoods 2002, 12, 41–55. [Google Scholar] [CrossRef]
- Kengue, J. Le safoutier (Dacryodes edulis) G. Don, H.J. Lam: Pemières Données sur la Morphologie et la Biologie. Ph.D. Thesis, University of Yaoundé, Yaoundé, Cameroon, 1990; pp. 4–7. [Google Scholar]
- Ajiwe, V.; Okeke, C.; Nnabuike, B.; Ogunleye, G.; Elebo, E. Applications of oils extracted from African star apple (Chrysophyllum africanum), horse eye bean (Mucuna sloanei) and African pear (Dacryodes edulis) seeds. Bioresour. Technol. 1997, 59, 259–261. [Google Scholar] [CrossRef]
- Widyanto, R.M.; Safira, L.; Sofian, N.F.; Mardhiyati, S.A.; Pradiptasari, P.; Dini, C.Y.; Proborini, W.D. Studies on the Antioxidant and Cytotoxic Potentials of the Peel Extract of Dacryodes rostrata. BIO Web Conf. 2021, 41, 07005. [Google Scholar] [CrossRef]
- Seah, W.W.; Chong, K.Y.; Neo, L.; Tan, S.Y.; Koh, C.Y.; Lim, R.C.J.; Loh, J.W.; Ng, W.Q.; Yee, A.T.K.; Hugh, T.W.; et al. Towards a field guide to the trees of the Nee Soon Swamp Forest (V): Burseraceae. Nat. Singap. 2019, 12, 15–36. [Google Scholar] [CrossRef]
- Daly, D.C.; Martinez-Habibe, M.C. Ten new species of Dacryodes from Amazonia and the Guianas. Studies in neotropical Burseraceae XXIII. Brittonia 2019, 71, 201–224. [Google Scholar] [CrossRef]
- Daly, D.C.; Neill, D.; Martínez-Habibe, M.C. An ecologically significant new species of Dacryodes from the northern Andes. Studies in neotropical Burseraceae XV. Brittonia 2012, 64, 49–56. [Google Scholar] [CrossRef]
- Idárraga-Piedrahita, A.; Ortiz, R.D.C.; Callejas-Posada, R.; Merello, M. Listado de las Plantas Vasculares del Departamento de Antioquia. In Flora de Antioquia. Catálogo de las Plantas Vasculares, Volume II; D’Vinni: Bogotá, Columbia, 2011; Volume 2, pp. 379–380. [Google Scholar]
- Martínez-Habibe, M.C.; Daly, D.C. Nine new species of Dacryodes from Andean South America. Studies in neotropical Burseraceae XXIV. Brittonia 2019, 71, 325–344. [Google Scholar] [CrossRef]
- Santamaría-Aguilar, D.; Aguilar, R. Dacryodes talamancensis (Burseraceae), la segunda especie del género para América Central. Phytoneuron 2017, 11, 1–6. [Google Scholar]
- Basnet, K. Effect of Topography on the Pattern of Trees in Tabonuco (Dacryodes excelsa) Dominated Rain Forest of Puerto Rico. Biotropica 1992, 24, 31–42. [Google Scholar] [CrossRef]
- Awono, A.; Ndoye, O.; Schreckenberg, K.; Tabuna, H.; Isseri, F.; Temple, L. Production and Marketing of Safou (Dacryodes edulis) in Cameroon and Internationally: Market Development Issues. For. Trees Livelihoods 2002, 12, 125–147. [Google Scholar] [CrossRef]
- Noumi, G.B.; Dandjouma, A.K.A.; Kapseu, C.; Parmentier, M. Le savoir-faire local dans la valorisation alimentaire des fruits du safoutier (Dacryodes edulis (G. Don) H.J. Lam) au Cameroun. Tropicultura 2006, 24, 58–62. [Google Scholar]
- Diakabana, P.; Dzondo, M.G.; Mvilia, L.G.S.; Gampoula, R.H.; Sompila, A.W.G.T. Mise au point d’un procédé de conservation de la pulpe de fruit de safou (Dacryodes edulis) par la méthode de déshydratation-imprégnation par immersion (DII). J. Food Stab. 2021, 4, 1–13. [Google Scholar] [CrossRef]
- Aponjolosun, B.S.; Fasola, T.R. Phytochemical, antimicrobial and toxicity assessment of Dacryodes edulis (G. Don.) H. J. Lam Leaf Extracts. Afr. J. Biomed. Res. 2022, 25, 101–106. [Google Scholar] [CrossRef]
- Oyetunji, O.O.; Opeyemi, A.P. Antibacterial and Antioxidant Activities of Dacryodes edulis Methanolic Leaf Extract. J. Adv. Med. Pharm. Sci. 2017, 14, 1–11. [Google Scholar] [CrossRef]
- Omosimua, R.O.; Macham, B.D.; Onanuga, A.; Thomas, B.T.; Ugbomor, C.O.; Lyappan, G.; Ramalingam, S.; Afolabi, A.S.; Thomas, S.A. Antioxidant and antimicrobial activities of flavonoids from the fruits extract of Dacryodes edulis. In Phytomedicine, 1st ed.; Afolabi, A., Ed.; CRC Press: Florida, FL, USA, 2020; pp. 1–13. [Google Scholar]
- Kamgaing, M.T.W.; Mvondo, M.A.; Kamani, S.L.P.; Essono, S.M.; Ngnokam, S.L.W. The Aqueous Extract of Dacryodes edulis (Burseraceae) Leaves Inhibits Cell Proliferation Induced by Estradiol on the Uterus and Vagina of Ovariectomized Female Wistar Rats. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8869281. [Google Scholar] [CrossRef]
- Lumachi, F.; Santeufemia, D.; Basso, S.M.M. Current medical treatment of estrogen receptor-positive breast cancer. World J. Biol. Chem. 2015, 6, 231–239. [Google Scholar] [CrossRef]
- Mvondo, M.A.; Kamgaing, M.T.W.; Ngnokam, S.L.W. Aqueous Extract of Dacryodes edulis (Burseraceae) Leaves Inhibited Tumor Growth in Female Wistar Rats with 7,12-Dimethylbenz[a]anthracene-Induced Breast Cancer. Evid. -Based Complement. Altern. Med. 2021, 2021, 9960950. [Google Scholar] [CrossRef]
- Ononamadu, C.J.; Alhassan, A.J.; Ibrahim++, A.; Imam, A.A.; Ihegboro, G.O.; Owolarafe, T.A.; Sule, M.S. Methanol-Extract/Fractions of Dacryodes edulis Leaves Ameliorate Hyperglycemia and Associated Oxidative Stress in Streptozotocin-Induced Diabetic Wistar Rats. J. Evid. -Based Integr. Med. 2019, 24, 1–12. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Ijomone, O.M.; Chukwuma, C.I.; Xiao, X.; Salau, V.F.; Islam, S. Dacryodes edulis (G. Don) H.J. Lam modulates glucose metabolism, cholinergic activities and Nrf2 expression, while suppressing oxidative stress and dyslipidemia in diabetic rats. J. Ethnopharmacol. 2020, 255, 112744. [Google Scholar] [CrossRef]
- Miguel, L.M.; Mokondjimobe, E.; Okiemy-Andissa, N.; Diatewa, M.; Moukassa, D.; Longo Mbenza, B.; Abena, A.A. Medicinal potentials of Dacryodes edulis (G. Don) HJ LAM. Literature review. Int. J. Curr. Res. 2017, 9, 63014–63018. [Google Scholar]
- Nwidu, L.L.; Alikwe, P.C.N.; Elmorsy, E.; Carter, W.G. An Investigation of Potential Sources of Nutraceuticals from the Niger Delta Areas, Nigeria for Attenuating Oxidative Stress. Medicines 2019, 6, 15. [Google Scholar] [CrossRef]
- Uhunmwangho, E.S.; Omoregie, E.S. Antioxidant properties associated with the biochemical changes in the development of African pear (Dacryodes edulis) Fruit. Saudi J. Biomed. Res. 2018, 3, 56–65. [Google Scholar] [CrossRef]
- Tee, L.H.; Ramanan, R.N.; Tey, B.T.; Chan, E.S.; Azrina, A.; Amin, I.; Bao, Y.; Lau, C.Y.; Prasad, K.N. Phytochemicals and antioxidant capacities from Dacryodes rostrata Fruits. Med. Chem. 2015, 5, 023–027. [Google Scholar]
- Orhue, N.E.J.; Adaikpoh, M.A.; Okuo, V.A.; Iseghohi, S.O. Ethanol extract of Dacryodes edulis seeds suppresses carbon tetrachloride-induced liver damage in wistar albino rats. NISEB J. 2015, 15, 1–6. [Google Scholar]
- Orhue, N.E.J.; Adaikpoh, M.A.; Odude, O.; Iseghohi, S.O. Prevention of Carbon Tetrachloride-induced Hepatic Steatosis and Cellular Damage by Aqueous Extract of Dacryodes edulis Seeds in Wistar Rats. Bio-Research 2015, 13, 885–888. [Google Scholar] [CrossRef]
- Eidangbe, G.O.; Obasi, I.O.; Okaka, A.C.; Eidangbe, R.C.; Olub, O.M. Attenuation of Carbon Tetrachloride—Induced Hepatoxicity by Dacryodes edulis Seeds Ethanolic Extract in Male Wistar Rats. Biointerface Res. Appl. Chem. 2015, 11, 9490–9500. [Google Scholar] [CrossRef]
- Dube, N.P.; Siwe-Noundou, X.; Krause, R.W.M.; Kemboi, D.; Tembu, V.J.; Manicum, A.-L. Review of the Traditional Uses, Phytochemistry, and Pharmacological Activities of Rhoicissus Species (Vitaceae). Molecules 2021, 26, 2306. [Google Scholar] [CrossRef] [PubMed]
- Enengedi, I.S.; Ekpa, D.; Akpabio, U.; Enengedi, I.; Ekpa, O. Utilization of the seeds of Nypa palm (Nypa fructicans) in Food Preparation. View project Cosmeceuticals View project Antioxidant and free radical scavenging properties of Dacryodes edulis leaf and bark extracts. Int. J. Herb. Med. 2019, 7, 36–44. Available online: https://www.researchgate.net/publication/343135097 (accessed on 26 December 2022).
- Atawodi, S.; Atawodi, J.; Idakwo, P.; Pfundstein, B.; Haubner, R.; Wurtele, G.; Spiegelhalder, B.; Bartsch, H.; Owen, R. Evaluation of the Polyphenol Composition and Antioxidant Activity of African Variety of Dacryodes edulis (G. Don) H.J Lam Fruit. J. Med. Food 2009, 12, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Ndem, J.I.; Bassey, U.E.; Etim, O.E. Hypolipidemic potential of raw seeds of Dacryodes edulis on Wistar rats. Eur. J. Biomed. Pharm. Sci. 2017, 4, 111–115. [Google Scholar]
- Tor-Anyiin, T.A.; Igoli, J.O.; Anyam, J.N. Studies on Dacryodes edulis III: Isolation and characterization of gallic acid from methanolic extract of raw (untreated) seeds of Dacryodes edulis and its antimicrobial properties. J. Chem. Soc. Niger. 2016, 41, 6–9. Available online: http://journals.chemsociety.org.ng/index.php/jcsn/article/view/38 (accessed on 26 December 2022).
- Sharma, N.; Tiwari, N.; Vyas, M.; Khurana, N.; Muthuraman, A.; Utreja, P. An overview of therapeutic effects of vanillic acid. Plant Arch. 2020, 20 (Suppl. S2), 3053–3059. [Google Scholar]
- Walton, N.J.; Mayer, M.J.; Narbad, A. Vanillin. Phytochemistry 2003, 63, 505–515. [Google Scholar] [CrossRef]
- Maia, M.; Resende, D.I.; Durães, F.; Pinto, M.M.; Sousa, E. Xanthenes in Medicinal Chemistry—Synthetic strategies and biological activities. Eur. J. Med. Chem. 2021, 210, 113085. [Google Scholar] [CrossRef]
- Pinto, M.M.M.; Sousa, M.E.; Nascimento, M.S. Xanthone Derivatives: New Insights in Biological Activities. Curr. Med. Chem. 2005, 12, 2517–2538. [Google Scholar] [CrossRef]
- Siswadi, S.; Saragih, G.S. Phytochemical analysis of bioactive compounds in ethanolic extract of Sterculia quadrifida R.Br. AIP Conf. Proc. 2021, 2353, 030098. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, A.; Ahmed, I.; Shill, M.C.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Babu, S.; Jayaraman, S. An update on β-sitosterol: A potential herbal nutraceutical for diabetic management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef]
- Nna, P.J. Isolation and characterisation of β-Amyrin from stem bark of Dacryodes edulis. Pharm Chem. J. 2021, 8, 102–106. [Google Scholar]
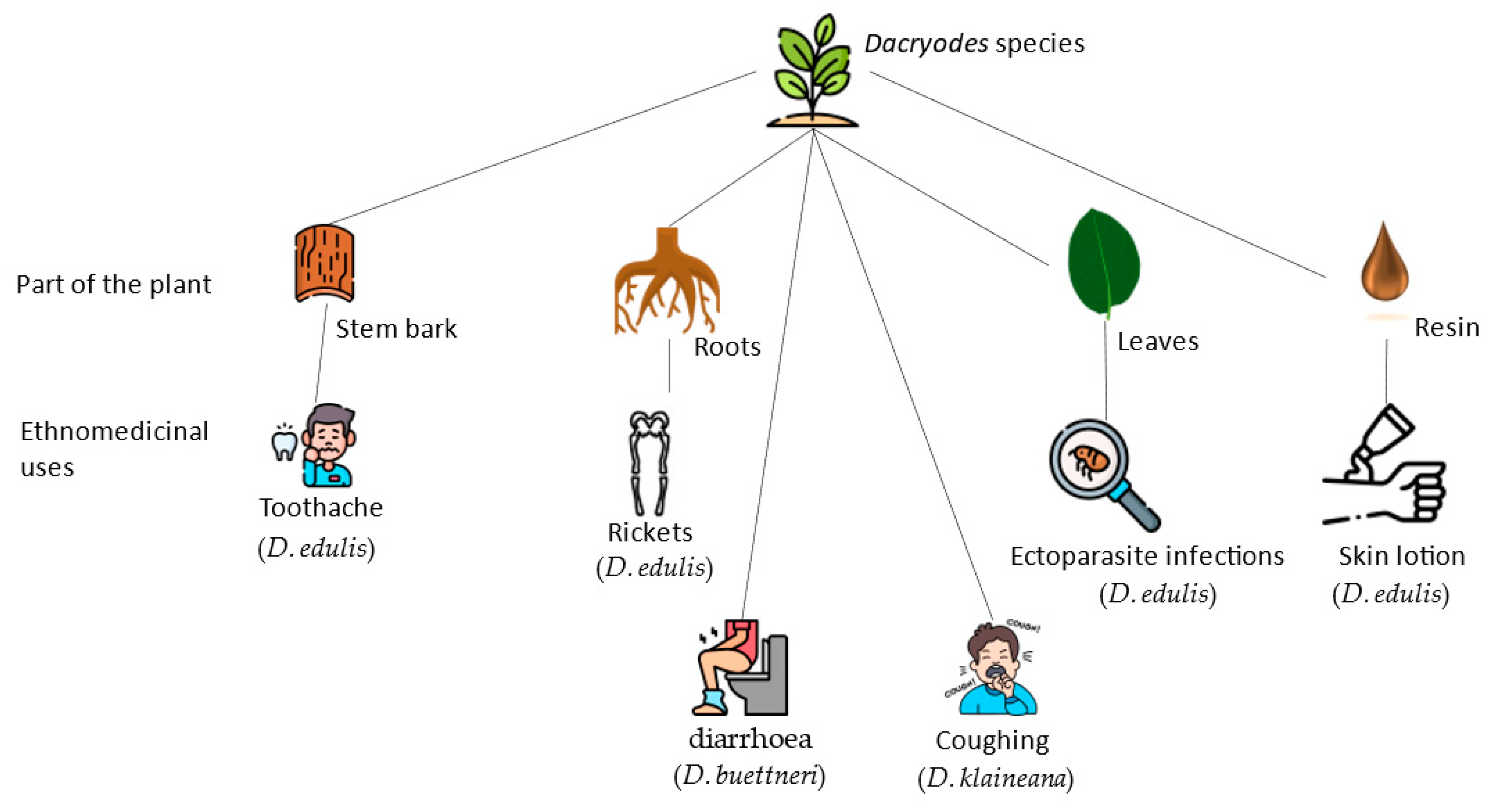
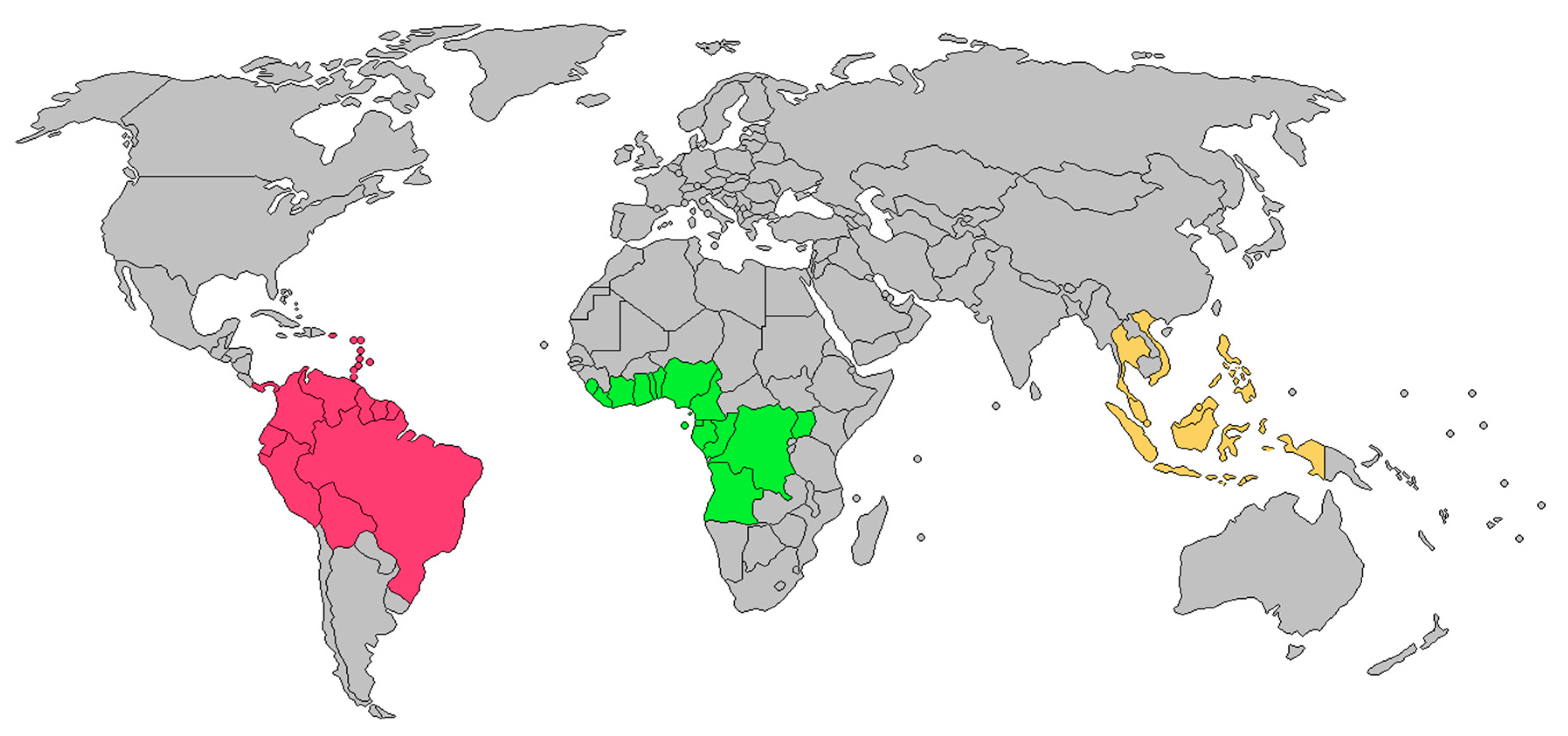
| Species | Plant Part | Ethnomedicinal Use | References |
|---|---|---|---|
| D. edulis | Stem bark | For toothache, gum problems, gargling, mouthwash, tonsillitis, and earache, bark or leaf decoction is applied. | [7,29,30] |
| Roots | Cooked with additional herbs and eaten to combat beriberi and rickets | [47] | |
| Leaves | Administered For skin diseases, ectoparasite infections, and disorders. | [43,48,60] | |
| Resin | Exudes is applied to cure skin diseases (ringworms, craw-craw and wounds). | [43,48,60] | |
| D. klaineana | Roots | Treatment of skin conditions by consuming them boiled. | [7,28] |
| Leaves | Remedy for painful menstruation; tachycardia and cough. | [25,29,30] | |
| D. buettneri | Not reported | Used for the treatment of diarrhea, microbiological infections, malaria, constipation, jaundice, and fever, its resin is used as an astringent and a disinfectant. | [18,31] |
| Plants (Organs) | Biological Activities | Mode of Assessment | References |
|---|---|---|---|
| D. edulis (Fruits) | Antidiabetic | In vivo | [53] |
| D. edulis (Fruits) | Antioxidant | In vitro | [93] |
| D. edulis (Fruits) | Antihyperglycemic | Ex vivo | [60] |
| D. edulis (Leaves) | Antibacterial | In vitro | [55,83,84] |
| D. edulis (Leaves) | Antiproliferative | In vivo | [86] |
| D. edulis (Leaves) | Antitumoral | In vivo | [88] |
| D. edulis (Leaves) | α-amylase and α-glucosidase inhibition | In vitro | [89] |
| D. edulis (Leaves) | Anti-hypoglycaemia | In vivo | [89] |
| D. edulis (Leaves) | Antidiabetic | In vivo | [90] |
| D. edulis (Leaves) | Antioxidant | In vitro | [89,91] |
| D. edulis (Pulps) | Antioxidant | In vitro | [61] |
| D. edulis (Seeds) | Radical scavenging | In vitro | [57] |
| D. edulis (Seeds) | Antioxidant | In vitro | [91] |
| D. edulis (Seeds) | Hepatoprotective | In vivo | [94,96] |
| D. peruviana (Fruits) | Antibacterial | In vitro | [38] |
| D. rostrata (Seeds, peels, Pulps) | Antioxidant | In vitro | [19,62] |
| D. rostrata (Peels) | Antioxidant | In vitro | [71] |
| D. rostrata (Fruits) | Antioxidant | In vitro | [30] |
| D. rostrata (Peels) | Cytotoxic | In vitro | [71] |
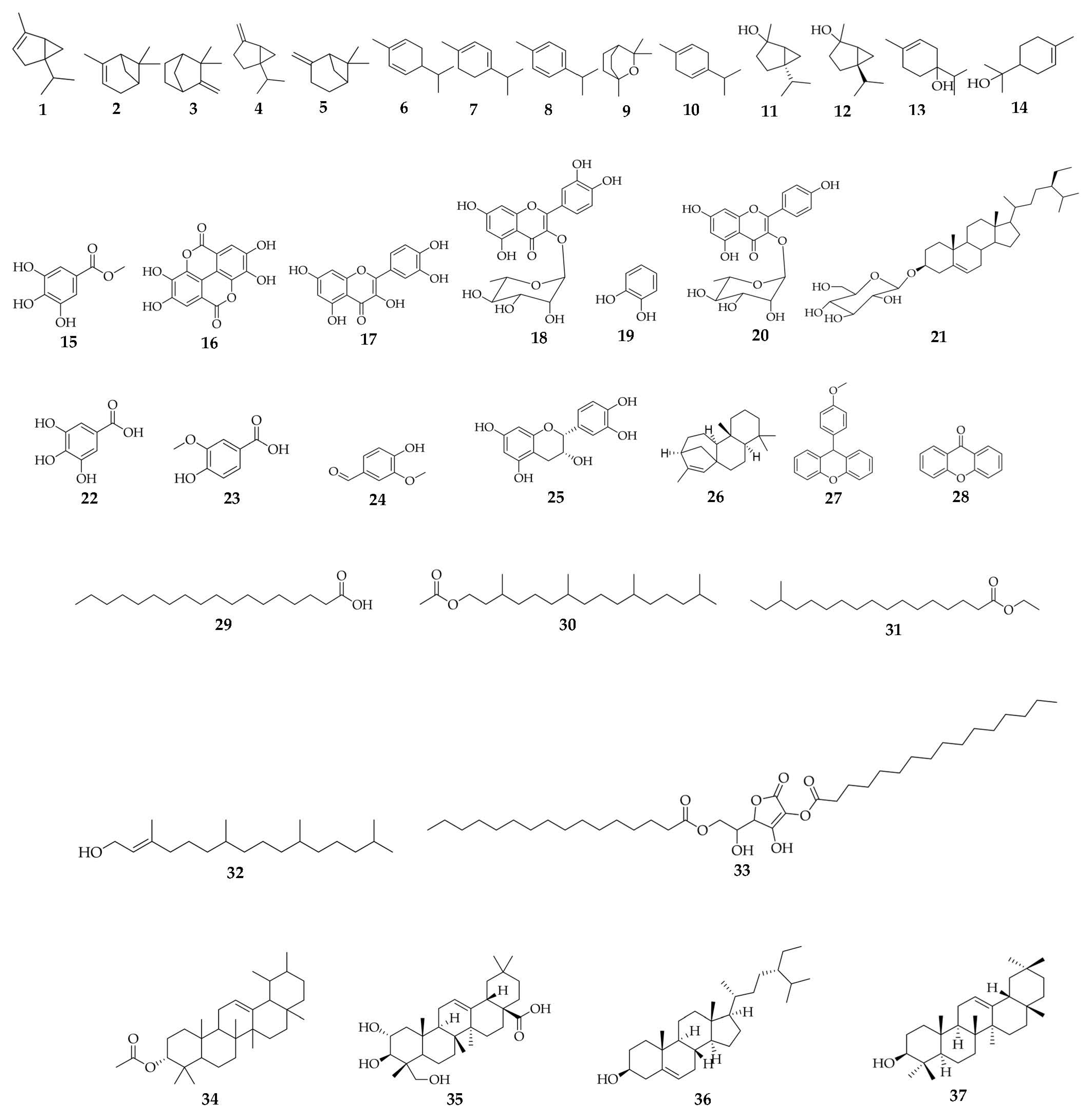
| Compound Name | Molecular Formula | Plant Part | Biological Activities | References |
|---|---|---|---|---|
| α-Thujene (1) | C10H16 | Resin | Antioxidant, antimalarial, antibacterial, antimicrobial, and herbicidal activities | [21] |
| α−Pinene (2) | C10H16 | Resin | Antimicrobial, apoptotic, antimetastatic, antibiotic, and antiinflammatory properties. | [21] |
| Camphene (3) | C10H16 | Resin | Antifungal potential against certain fungi when combined with sage oil and has the potential to act as an antioxidant when combined with vitamin C and citrus oils. | [21] |
| Sabinene (4) | C10H16 | Resin | Antifungal and antiinflammatory activities. | [21] |
| β-Pinene (5) | C10H16 | Resin | Fungicidal agent, antiviral and antimicrobial agents. | [21] |
| α−Phellandrene (6) | C10H16 | Resin | Reduce pain sensitivity and increase energy levels. Contains potential anticancer properties. | [21] |
| α-Terpinene (7) | C10H16 | Resin | Antioxidant, anticancer, anticonvulsant, antiulcer, antihypertensive, and antinociceptive | [21] |
| p- Cymene (8) | C10H14 | Resin | Used to prevent coughs and eliminate phlegm. | [21] |
| 1,8-Cineole (9) | C10H18O | Resin | The respiratory tract is mucolytic and spasmolytic. It has been shown to be beneficial in the treatment of inflammatory airway diseases such as asthma and chronic obstructive pulmonary disease (COPD). | [21] |
| γ−Terpinene (10) | C10H16 | Resin | Antioxidant | [21] |
| cis Sabinene hydrate (11) | C10H18O | Resin | Antiinflammatory and antifungal properties. | [21] |
| trans Sabinene hydrate (12) | C10H18O | Resin | Antiinflammatory and antifungal properties. | [21] |
| Terpinen-4-ol (13) | C10H18O | Resin | Antiinflammatory, anticancer, and antioxidant agents | [21] |
| α−Terpineol (14) | C10H18O | Resin | Antioxidant, anticancer, anticonvulsant, antiulcer, antihypertensive, antinociceptive activities | [21] |
| Methyl gallate (15) | C8H8O5 | Fruits | Antioxidant activity | [98,100] |
| Ellagic acid (16) | C14H6O8 | Fruits | Cancer prevention, antiviral and antibacterial activities | [99,100] |
| Quercetin (17) | C15H10O7 | Fruits | Antioxidant and anticancer properties | [41,100] |
| Quercetin 3-O-α-l-rhamnoside (18) | C21H20O11 | Fruits | Antiinflammatory and antioxidant effects that might assist to control blood sugar, kill cancer cells, lessen swelling, and help to prevent heart disease. | [100] |
| Catechol (19) | C6H6O2 | Fruits | Antioxidant, Antiinflammatory, and antimicrobial agent | [100] |
| Afzelin (20) | C21H20O10 | Stem bark | Protect human keratinocytes from the harmful effects of UV irradiation via its biological properties (Antioxidant, Antiinflammatory and DNA-protective) and also act as a UV absorber. | [41,98] |
| Sitosterol-3-O-β-d-glucopyranoside sterol (21) | C35H60O6 | Stem bark | Treat endotoxemia and inflammation accompanied by the overproduction of nitric oxide. | [41,98] |
| Gallic acid/3, 4, 5-trihydroxybenzoic acid (22) | C7H6O5 | Seeds | Antibacterial and antifungal properties | [101,102] |
| Vanillic acid (23) | C8H8O4 | Leaves | Antioxidant, hepatoprotective, cardioprotective, and antiapoptotic activities | [84,90,103] |
| Vanillin (24) | C8H8O3 | Leaves | Antioxidant and antimicrobial properties | [90,103,104] |
| (−)-epicatechin (25) | C15H14O6 | Leaves | Insulinogenic | [23,90] |
| Kaur-15-ene (26) | C20H32 | Leaves | Not reported | [61] |
| 9-(4-methoxyphenyl) xanthene (27) | C20H16O3 | Leaves | Xanthene derivatives display neuroprotector, antitumor, antimicrobial properties | [61,105] |
| Xanthone (28) | C13H8O2 | Leaves | Antitumor | [61,106] |
| Octadecanoic acid (29) | C18H36O2 | Leaves | Antiinflammatory | [61,107] |
| Phytol acetate (30) | C22H42O2 | Leaves | Phyton derivatives display cytotoxic, antianxiety, antioxidant, metabolism-modulating, autophagy- and apoptosis-inducing, antinociceptive, antimicrobial effects, antiinflammatory, immune-modulating. | [61,108] |
| Ethyl 15-methylheptadecanoate (31) | C20H40O2 | Leaves | Not reported | [61] |
| trans Phytol (32) | C20H40O | Leaves | Phytol derivatives display cytotoxic, metabolism-modulating antianxiety, antioxidant, antinociceptive, immune-modulating antiinflammatory, autophagy- and apoptosis-inducing, and antimicrobial effects | [61,108] |
| Ascorbic acid 2,6- dihexadecanoate (33) | C38H68O8 | Leaves | Not reported | [61] |
| Urs-12-ene-3-ol acetate (34) | C32H52O2 | Leaves | Not reported | [61] |
| 2,3,23-trihydroxyolean-12-en-28-oic acid methyl ester (35) | C30H48O5 | Leaves | Not reported | [61] |
| Sitosterol (36) | C29H50O | Leaves | Antinociceptive, analgesic, immunomodulatory, antimicrobial, anticancer, anxiolytic & sedative effects, antioxidant, and antidiabetic activity, antiinflammatory, hypolipidemic, respiratory disease protection, wound healing effect, hepatoprotective. | [61,109] |
| β-amyrin (37) | C30H50O | Stem bark | Not reported | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swana, L.; Tsakem, B.; Tembu, J.V.; Teponno, R.B.; Folahan, J.T.; Kalinski, J.-C.; Polyzois, A.; Kamatou, G.; Sandjo, L.P.; Chamcheu, J.C.; et al. The Genus Dacryodes Vahl.: Ethnobotany, Phytochemistry and Biological Activities. Pharmaceuticals 2023, 16, 775. https://doi.org/10.3390/ph16050775
Swana L, Tsakem B, Tembu JV, Teponno RB, Folahan JT, Kalinski J-C, Polyzois A, Kamatou G, Sandjo LP, Chamcheu JC, et al. The Genus Dacryodes Vahl.: Ethnobotany, Phytochemistry and Biological Activities. Pharmaceuticals. 2023; 16(5):775. https://doi.org/10.3390/ph16050775
Chicago/Turabian StyleSwana, Leseho, Bienvenu Tsakem, Jacqueline V. Tembu, Rémy B. Teponno, Joy T. Folahan, Jarmo-Charles Kalinski, Alexandros Polyzois, Guy Kamatou, Louis P. Sandjo, Jean Christopher Chamcheu, and et al. 2023. "The Genus Dacryodes Vahl.: Ethnobotany, Phytochemistry and Biological Activities" Pharmaceuticals 16, no. 5: 775. https://doi.org/10.3390/ph16050775
APA StyleSwana, L., Tsakem, B., Tembu, J. V., Teponno, R. B., Folahan, J. T., Kalinski, J.-C., Polyzois, A., Kamatou, G., Sandjo, L. P., Chamcheu, J. C., & Siwe-Noundou, X. (2023). The Genus Dacryodes Vahl.: Ethnobotany, Phytochemistry and Biological Activities. Pharmaceuticals, 16(5), 775. https://doi.org/10.3390/ph16050775










