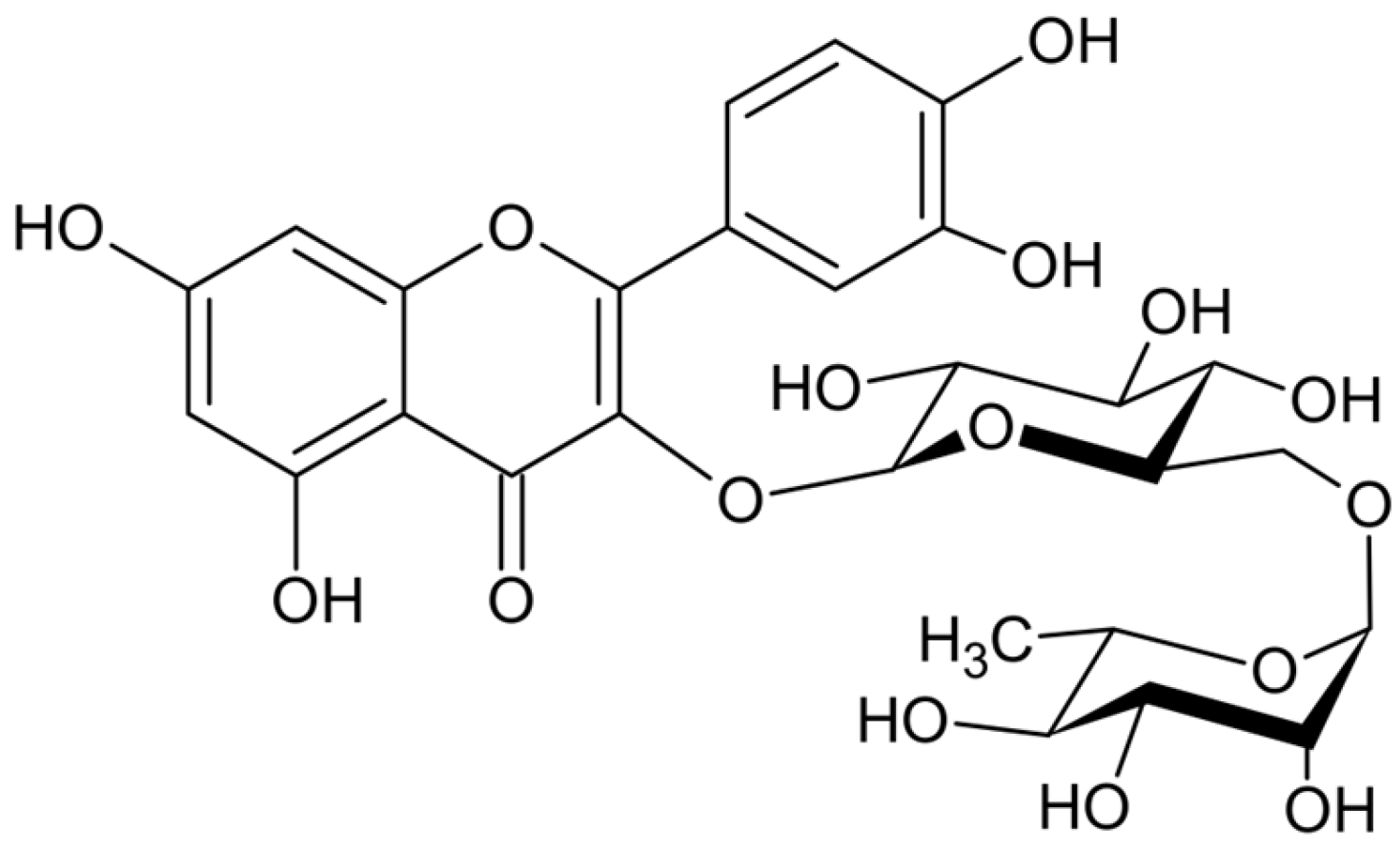
Rutin Gel with Bone Graft Accelerates Bone Formation in a Rabbit Model by Inhibiting MMPs and Enhancing Collagen Activities
Abstract
1. Introduction
2. Results
2.1. Gene Expression
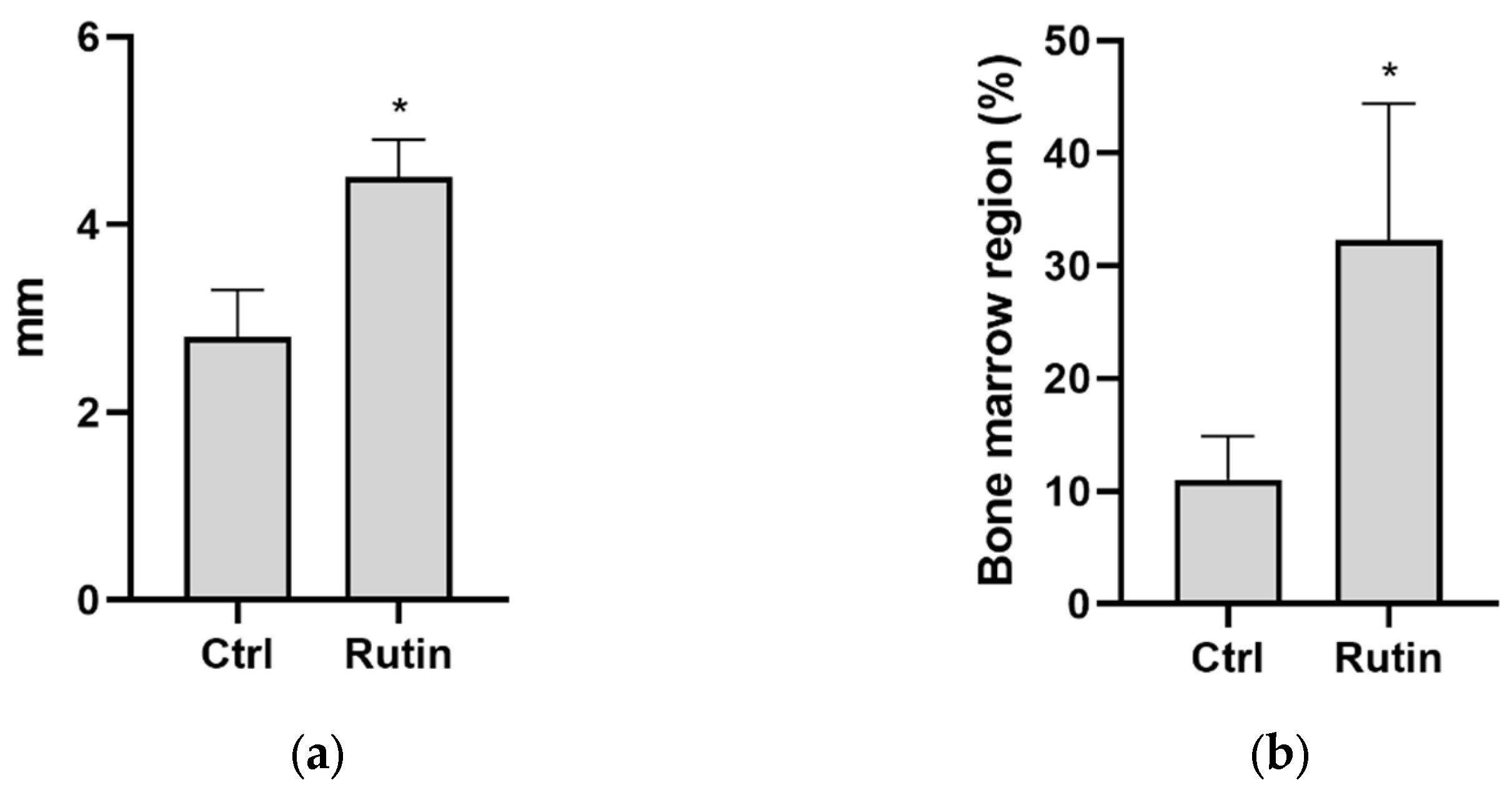
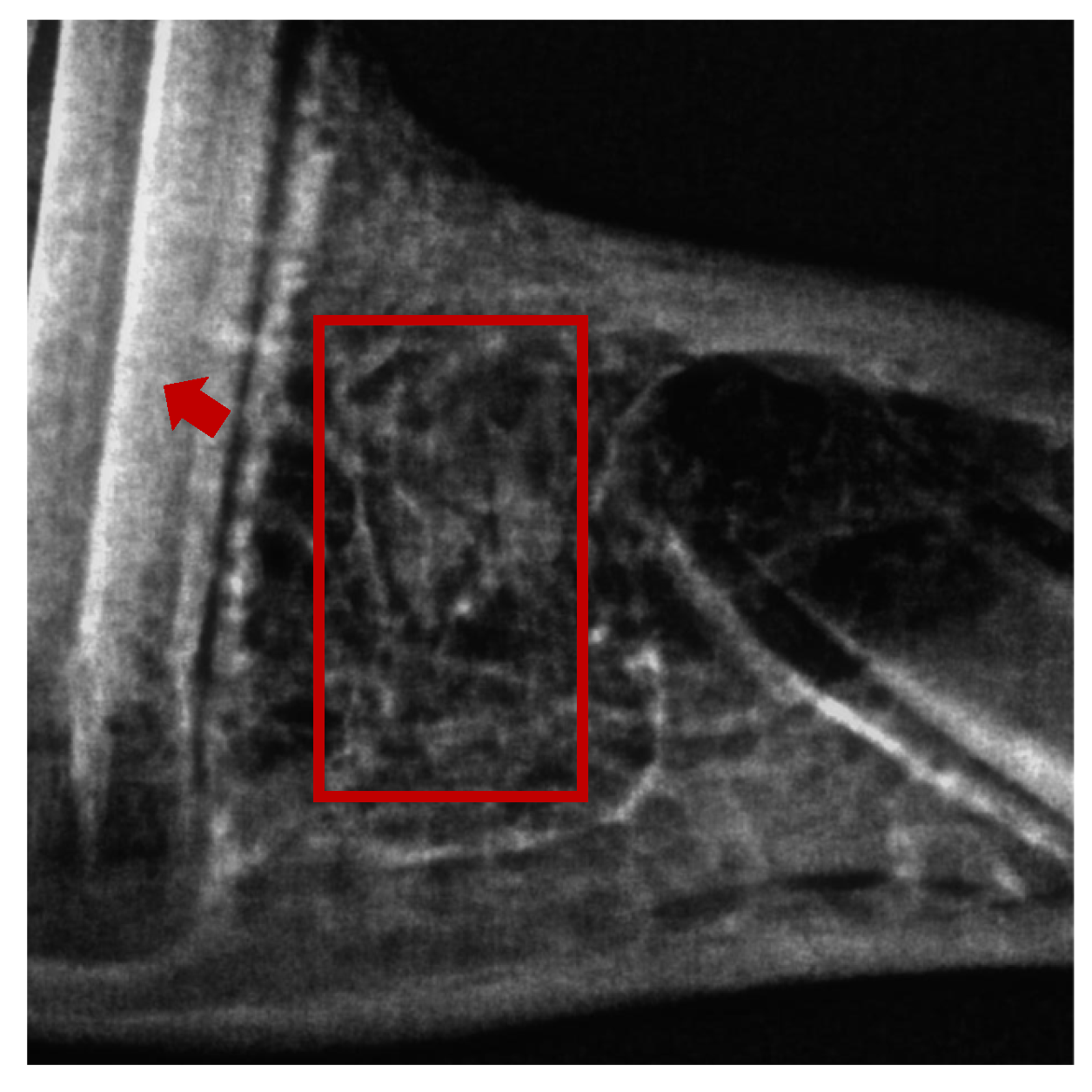
2.2. Histomorphometry
3. Discussion
4. Materials and Methods
4.1. Preparation of the Rutin Gel
4.2. In Vivo Experiments
4.3. Surgical Procedure
4.4. Sample Collection
4.5. RNA Extraction and cDNA Synthesis
4.6. Quantification of mRNA Expression
4.7. Histomorphometry
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Titsinides, S.; Agrogiannis, G.; Karatzas, T. Bone grafting materials in dentoalveolar reconstruction: A comprehensive review. Jpn. Dent. Sci. Rev. 2019, 55, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Turek, T.J.; Blake, C.A.; D’Augusta, D.; Li, X.; Stevens, M.; Seeherman, H.J.; Wozney, J.M. Recombinant Human Bone Morphogenetic Protein-2 Accelerates Healing in a Rabbit Ulnar Osteotomy Model. J. Bone Joint Surg. Am. 2001, 83, 1219–1230. [Google Scholar] [CrossRef]
- El Bialy, I.; Jiskoot, W.; Reza Nejadnik, M. Formulation, Delivery and Stability of Bone Morphogenetic Proteins for Effective Bone Regeneration. Pharm. Res. 2017, 34, 1152–1170. [Google Scholar] [CrossRef] [PubMed]
- Shimono, K.; Oshima, M.; Arakawa, H.; Kimura, A.; Nawachi, K.; Kuboki, T. The effect of growth factors for bone augmentation to enable dental implant placement: A systematic review. Jpn. Dent. Sci. Rev. 2010, 46, 43–53. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Kusano, K.; Miyaura, C.; Inada, M.; Tamura, T.; Ito, A.; Nagase, H.; Kamoi, K.; Suda, T. Regulation of Matrix Metalloproteinases (MMP-2, -3, -9, and -13) by Interleukin-1 and Interleukin-6 in Mouse Calvaria: Association of MMP Induction with Bone Resorption. Endocrinology 1998, 139, 1338–1345. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Elgezawi, M.; Haridy, R.; Almas, K.; Abdalla, M.A.; Omar, O.; Abuohashish, H.; Elembaby, A.; Christine Wölfle, U.; Siddiqui, Y.; Kaisarly, D. Matrix Metalloproteinases in Dental and Periodontal Tissues and Their Current Inhibitors: Developmental, Degradational and Pathological Aspects. Int. J. Mol. Sci. 2022, 23, 8929. [Google Scholar] [CrossRef]
- Cheng, P.; Li, D.; Gao, Y.; Cao, T.; Jiang, H.; Wang, J.; Li, J.; Zhang, S.; Song, Y.; Liu, B.; et al. Prevascularization promotes endogenous cell-mediated angiogenesis by upregulating the expression of fibrinogen and connective tissue growth factor in tissue-engineered bone grafts. Stem Cell. Res. Ther. 2018, 9, 176–189. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Jiang, Z.; Hu, C.; Gao, Y.; Zhou, Q. Efficacy of total flavonoids of Rhizoma drynariae on the blood vessels and the bone graft in the induced membrane. Phytomedicine 2022, 99, 153995–154003. [Google Scholar] [CrossRef]
- Benjamin, M.M.; Khalil, R.A. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. Exp. Suppl. 2012, 103, 209–279. [Google Scholar] [CrossRef]
- Kumar, G.B.; Nair, B.G.; Perry, J.J.P.; Martin, D.B.C. Recent insights into natural product inhibitors of matrix metalloproteinases. Medchemcomm 2019, 10, 2024–2037. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Sharma, S.; Ali, A.; Ali, J.; Sahni, J.K.; Baboota, S. Rutin: Therapeutic potential and recent advances in drug delivery. Expert Opin. Investig. Drugs 2013, 22, 1063–1079. [Google Scholar] [CrossRef]
- Younis, T.; Jabeen, F.; Hussain, A.; Rasool, B.; Raza Ishaq, A.; Nawaz, A.; El-Nashar, H.A.S.; El-Shazly, M. Antioxidant and Pulmonary Protective Potential of Fraxinus xanthoxyloides Bark Extract against CCl4-Induced Toxicity in Rats. Chem. Biodivers. 2023, 20, e202200755. [Google Scholar] [CrossRef]
- Abdelghffar, E.A.R.; Mostafa, N.M.; El-Nashar, H.A.S.; Eldahshan, O.A.; Singab, A.N.B. Chilean pepper (Schinus polygamus) ameliorates the adverse effects of hyperglycaemia/dyslipidaemia in high fat diet/streptozotocin-induced type 2 diabetic rat model. Ind. Crops Prod. 2022, 183, 114953. [Google Scholar] [CrossRef]
- Pinzaru, I.; Tanase, A.; Enatescu, V.; Coricovac, D.; Bociort, F.; Marcovici, I.; Watz, C.; Vlaia, L.; Soica, C.; Dehelean, C. Proniosomal Gel for Topical Delivery of Rutin: Preparation, Physicochemical Characterization and In Vitro Toxicological Profile Using 3D Reconstructed Human Epidermis Tissue and 2D Cells. Antioxidants 2021, 10, 85. [Google Scholar] [CrossRef]
- Liu, L.L.; Zhang, Y.; Zhang, X.F.; Li, F.H. Influence of rutin on the effects of neonatal cigarette smoke exposure-induced exacerbated MMP-9 expression, Th17 cytokines and NF-κB/iNOS-mediated inflammatory responses in asthmatic mice model. Korean J. Physiol. Pharmacol. 2018, 22, 481–491. [Google Scholar] [CrossRef]
- Chen, X.; Yu, M.; Xu, W.; Zou, L.; Ye, J.; Liu, Y.; Xiao, Y.; Luo, J. Rutin inhibited the advanced glycation end products-stimulated inflammatory response and extra-cellular matrix degeneration via targeting TRAF-6 and BCL-2 proteins in mouse model of osteoarthritis. Aging 2021, 13, 22134–22147. [Google Scholar] [CrossRef]
- Her, Y.; Lee, T.K.; Kim, J.D.; Kim, B.; Sim, H.; Lee, J.C.; Ahn, J.H.; Park, J.H.; Lee, J.W.; Hong, J.; et al. Topical Application of Aronia melanocarpa Extract Rich in Chlorogenic Acid and Rutin Reduces UVB-Induced Skin Damage via Attenuating Collagen Disruption in Mice. Molecules 2020, 25, 4577. [Google Scholar] [CrossRef]
- Mitchell, P.G.; Magna, H.A.; Reeves, L.M.; Lopresti-Morrow, L.L.; Yocum, S.A.; Rosner, P.J.; Geoghegan, K.F.; Hambor, J.E. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J. Clin. Investig. 1996, 97, 761–768. [Google Scholar] [CrossRef]
- Soundia, A.; Hadaya, D.; Esfandi, N.; Gkouveris, I.; Christensen, R.; Dry, S.M.; Bezouglaia, O.; Pirih, F.; Nikitakis, N.; Aghaloo, T.; et al. Zoledronate Impairs Socket Healing after Extraction of Teeth with Experimental Periodontitis. J. Dent. Res. 2018, 97, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, T.; Huang, H.; Cheng, W.; Lai, Y.; Bai, X.; Chen, J.; Yue, Y.; Zheng, Z.; Guo, C.; et al. Fracture healing in a collagen-induced arthritis rat model: Radiology and histology evidence. J. Orthop. Res. 2018, 36, 2876–2885. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P. The Role of Collagen Organization on the Properties of Bone. Calcif. Tissue Int. 2015, 97, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Fattah Baraka, N.; Fathallah Ahmed, N.; Ismail Hussein, S. The effect of Rutin hydrate on Glucocorticoids induced osteoporosis in mandibular alveolar bone in Albino rats (Radiological, histological and histochemical study). Saudi Dent. J. 2022, 34, 464–472. [Google Scholar] [CrossRef]
- Lee, H.-H.; Jang, J.-W.; Lee, J.-K.; Park, C.-K. Rutin Improves Bone Histomorphometric Values by Reduction of Osteoclastic Activity in Osteoporosis Mouse Model Induced by Bilateral Ovariectomy. J. Korean Neurosurg. Soc. 2020, 63, 433–443. [Google Scholar] [CrossRef]
- Chen, X.; Hu, C.; Wang, G.; Li, L.; Kong, X.; Ding, Y.; Jin, Y. Nuclear factor-κB modulates osteogenesis of periodontal ligament stem cells through competition with β-catenin signaling in inflammatory microenvironments. Cell Death Dis. 2013, 4, 510–518. [Google Scholar] [CrossRef]
- Xiao, Y.; Wei, R.; Yuan, Z.; Lan, X.; Kuang, J.; Hu, D.; Song, Y.; Luo, J. Rutin suppresses FNDC1 expression in bone marrow mesenchymal stem cells to inhibit postmenopausal osteoporosis. Am. J. Transl. Res. 2019, 11, 6680–6690. [Google Scholar]
- Moreira, C.A.; Dempster, D.W.; Baron, R. Anatomy and ultrastructure of bone–histogenesis, growth and remodeling. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Colnot, C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J. Bone Miner. Res. 2009, 24, 274–282. [Google Scholar] [CrossRef]
- Petrescu, H.P.; Dinu, G.; Nodiţi, G.; Berceanu-Văduva, M.; Bratu, D.C.; Vermeşan, D. Experimental morphologic and radiologic study of the integration of bone grafts into the host tissue and of the dynamics of the graft-receptor interface. Rom. J. Morphol. Embryol. 2014, 55, 607–612. [Google Scholar]
- da Silva, J.; Herrmann, S.M.; Heuser, V.; Peres, W.; Possa Marroni, N.; González-Gallego, J.; Erdtmann, B. Evaluation of the genotoxic effect of rutin and quercetin by comet assay and micronucleus test. Food Chem. Toxicol. 2002, 40, 941–947. [Google Scholar] [CrossRef]
- Cristina Marcarini, J.; Ferreira Tsuboy, M.S.; Cabral Luiz, R.; Regina Ribeiro, L.; Beatriz Hoffmann-Campo, C.; Ségio Mantovani, M. Investigation of cytotoxic, apoptosis-inducing, genotoxic and protective effects of the flavonoid rutin in HTC hepatic cells. Exp. Toxicol. Pathol. 2011, 63, 459–465. [Google Scholar] [CrossRef]
- Saulnier, N.; Viguier, E.; Perrier-Groult, E.; Chenu, C.; Pillet, E.; Roger, T.; Maddens, S.; Boulocher, C. Intra-articular administration of xenogeneic neonatal Mesenchymal Stromal Cells early after meniscal injury down-regulates metalloproteinase gene expression in synovium and prevents cartilage degradation in a rabbit model of osteoarthritis. Osteoarthr. Cartil. 2015, 23, 122–133. [Google Scholar] [CrossRef]
- Inoue, H.; Arai, Y.; Kishida, T.; Terauchi, R.; Honjo, K.; Nakagawa, S.; Tsuchida, S.; Matsuki, T.; Ueshima, K.; Fujiwara, H.; et al. Hydrostatic pressure influences HIF-2 alpha expression in chondrocytes. Int. J. Mol. Sci. 2015, 16, 1043–1050. [Google Scholar] [CrossRef]
- Ishibashi, H.; Tonomura, H.; Ikeda, T.; Nagae, M.; Sakata, M.; Fujiwara, H.; Tanida, T.; Mastuda, K.-I.; Kawata, M.; Kubo, T. Hepatocyte growth factor/c-met promotes proliferation, suppresses apoptosis, and improves matrix metabolism in rabbit nucleus pulposus cells in vitro. J. Orthop. Res. 2016, 34, 709–716. [Google Scholar] [CrossRef]
- González, J.C.; López, C.; Álvarez, M.E.; Pérez, J.E.; Carmona, J.U. Autologous leukocyte-reduced platelet-rich plasma therapy for Achilles tendinopathy induced by collagenase in a rabbit model. Sci. Rep. 2016, 6, 19623–19633. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Okada, M.; Matsuura, T.; Akizuki, T.; Hoshi, S.; Shujaa Addin, A.; Fukuba, S.; Izumi, Y. Ridge preservation of extraction sockets with buccal bone deficiency using poly lactide-co-glycolide coated β-tricalcium phosphate bone grafts: An experimental study in dogs. J. Periodontol. 2019, 90, 1014–1022. [Google Scholar] [CrossRef]
- Hamad, A.M.; Ahmed, H.G. Association of some carbohydrates with estrogen expression in breast lesions among Sudanese females. J. Histotechnol. 2018, 41, 2–9. [Google Scholar] [CrossRef]
- Hamad, A.; Ahmed, H. Association of connective tissue fibers with estrogen expression in breast lesions among Sudanese females. Int. Clin. Pathol. J 2016, 2, 97–102. [Google Scholar] [CrossRef]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Theory and Practice of Histological Techniqueseighth; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]






| Histological Parameters | Control Group | Rutin-Treated Group |
|---|---|---|
| Coronal width (mm) | 1.7 ± 0.2 | 1.2 ± 0.3 |
| Middle width (mm) | 0.8 ± 0.32 | 1.3 ± 0.05 |
| Apical width (mm) | 4.5 ± 0.4 | 6.3 ± 0.4 * |
| Bone formation area diameter (mm) | 2.8 ± 0.5 | 4.5 ± 0.4 * |
| Mineralized bone region (%) | 26.6 ± 6.4 | 34.2 ± 4.5 |
| Bone marrow region (%) | 11 ± 3.9 | 32.3 ± 12.1 * |
| Connective tissue region (%) | 46.9 ± 10.9 | 33.0 ± 8.7 |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| MMP1 | CCTGATGTGGCTCAGTTCGT | GTCCACATCTGCCCTTGACA |
| MMP3 | TGGACCTGGAAATGTTTTGG | ATCAAAGTGGGCATCTCCAT |
| MMP9 | ACGGCCGACTATGACACC | TTGCCGTCCTGGGTGTAG |
| MMP13 | CCTCTTCTTCTCCGGAAACC | GGTAGTCTTGGTCCATGGTATGA |
| COL3A1 | GCAGGGACTCCAGGTCTTAGAGG | CGTGTTCACCTCTCTCTCCCAGGG |
| GAPDH | CACAGTTTCCATCCCAGACC | TGGTTTCATGACAAGGTAGGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albaqami, F.F.; Althurwi, H.N.; Alharthy, K.M.; Hamad, A.M.; Awartani, F.A. Rutin Gel with Bone Graft Accelerates Bone Formation in a Rabbit Model by Inhibiting MMPs and Enhancing Collagen Activities. Pharmaceuticals 2023, 16, 774. https://doi.org/10.3390/ph16050774
Albaqami FF, Althurwi HN, Alharthy KM, Hamad AM, Awartani FA. Rutin Gel with Bone Graft Accelerates Bone Formation in a Rabbit Model by Inhibiting MMPs and Enhancing Collagen Activities. Pharmaceuticals. 2023; 16(5):774. https://doi.org/10.3390/ph16050774
Chicago/Turabian StyleAlbaqami, Fahad F., Hassan N. Althurwi, Khalid M. Alharthy, Abubaker M. Hamad, and Fatin A. Awartani. 2023. "Rutin Gel with Bone Graft Accelerates Bone Formation in a Rabbit Model by Inhibiting MMPs and Enhancing Collagen Activities" Pharmaceuticals 16, no. 5: 774. https://doi.org/10.3390/ph16050774
APA StyleAlbaqami, F. F., Althurwi, H. N., Alharthy, K. M., Hamad, A. M., & Awartani, F. A. (2023). Rutin Gel with Bone Graft Accelerates Bone Formation in a Rabbit Model by Inhibiting MMPs and Enhancing Collagen Activities. Pharmaceuticals, 16(5), 774. https://doi.org/10.3390/ph16050774







