Abstract
Natural polysaccharides have been widely exploited in drug delivery and tissue engineering research. They exhibit excellent biocompatibility and fewer adverse effects; however, it is challenging to assess their bioactivities to that of manufactured synthetics because of their intrinsic physicochemical characteristics. Studies showed that the carboxymethylation of polysaccharides considerably increases the aqueous solubility and bioactivities of inherent polysaccharides and offers structural diversity, but it also has some limitations that can be resolved by derivatization or the grafting of carboxymethylated gums. The swelling ratio, flocculation capacity, viscosity, partition coefficient, metal absorption properties, and thermosensitivity of natural polysaccharides have been improved as a result of these changes. In order to create better and functionally enhanced polysaccharides, researchers have modified the structures and properties of carboxymethylated gums. This review summarizes the various ways of modifying carboxymethylated gums, explores the impact that molecular modifications have on their physicochemical characteristics and bioactivities, and sheds light on various applications for the derivatives of carboxymethylated polysaccharides.
1. Introduction
Polysaccharides, which are requisite polymers found in plants, animals, fungi, and bacteria, are one of the leading trends in modern medicine [1,2]. Due to their nontoxicity, affordability, ease of availability, biosafety, biodegradability, and widespread regulatory approval, natural polysaccharides outperform synthetic ones in many ways [3,4,5]. Natural polysaccharides exhibit adequate biological properties but are less suitable as drug carriers than synthetic polymers, as their structure and property correlation revealed certain drawbacks. Some polysaccharides do not possess bioactivity due to their particular structure and physicochemical properties [6]. Bioactive polysaccharides have higher molecular weight, making cell membrane penetration challenging [7,8]. The bioactivities of some polysaccharides are quite low. Many of them have an uncontrolled hydration rate, viscosity loss, and thickening during storage, and are microbially sensitive [9]. They require some modifications because the structure of polysaccharides affects their bioactivities and physicochemical properties. The characteristic features and functional groups of polysaccharides facilitate the selective chemical or biochemical modifications of polymeric backbone that expands their applications [10,11,12].
Molecular modification is the process of altering the structure of a substance using physical, chemical, or biological methods to produce a variety of structural variants. With the right techniques, structural changes can be exploited to impact physicochemical and bioactive properties. The chemical modification of polysaccharides to boost their bioactivity has been highlighted in several recent studies [13,14,15,16].
It has already been revealed that there are various molecular modification strategies, such as sulfation [17], sialylation [18], phosphorylation [19], acetylation, alkylation, and others, including the most widely utilized carboxymethylation [20]. Modifying carboxyl, amino, or hydroxyl terminal groups with sulfate produced sulfated polysaccharides with improved biological activity [21]. Due to the limited variety of phosphate mono- and polysaccharides in nature, phosphate polysaccharides have few biological functions [22]. It has been discovered that monosaccharides such as fructose, glucose, and others lack intrinsic bioactivities that may be induced through phosphorylation modification. Under specific circumstances, selenite or selenious acid is frequently utilized to produce selenite–ester bonds with hydroxyl (-OH) groups on polysaccharides. By combining selenium with polysaccharide, one can create an organic complex that has the bioactivities of both substances while also being simple for the body to absorb and use [23]. Branches of polysaccharide molecules are primarily treated via acetylation modification, which results in a significant increase in polysaccharide solubility [24]. Acetic anhydride is converted into a positive electrophilic reagent capable of attacking polysaccharide molecules in this process. Decreased viscosity and improved solubility are two important factors in enhancing bioactivity, and these can both be achieved through alkylation modification at the main chain′s terminal end.
A polysaccharide chain is carboxymethylated (CM) by adding a carboxymethyl group to it. The Williamson ether synthesis serves as the foundation for the carboxymethylation reaction, in which carboxymethyl groups are etherified with primary or secondary alcohol groups of polysaccharides [25]. It is most typically used to improve the water solubility, viscosity, swellability, and bioactivity of polysaccharides. It provides structural diversity and a degree of crystallinity, enhances surface irregularity, imparts an anionic property, and even adds new bioactivities [26,27]. The low cost of chemical reagents, ease of processing, and nontoxicity of the products are the key advantages of this reaction [28]. For carboxymethylation, aqueous medium and organic solvent techniques are frequently employed [29]. Homogeneity in alkalization, minimal side reactions, a high etherification reagent usage rate, faster main reactions and etherification reactions, and process stability are all advantages of the solvent approach over the aqueous medium method. Isopropanol has the disadvantage of being both costly and harmful [30]. Carboxymethylation improves the polysaccharides’ solubility in water and decreases the viscosity, which enhances the free-radical initiator and monomer penetration during the grafting reaction, boosts mucoadhesiveness, and enhances reactivity.
Natural gums are polysaccharides comprising many joined sugar units that build large molecules [31,32]. Plants produce them as part of their injury recovery mechanisms. Gums are an industrial staple and superior to synthetic polymers because of their biosafety and biocompatibility [33]. Naturally occurring gums from plants, microorganisms, and seaweed present a remarkable potential for chemical derivatization and modification to create unique, cutting-edge biomaterials [34,35]. Among the carboxymethylated polysaccharides, carboxymethylated gums (CMGs) are most widely explored for their different functional characteristics and diverse medicinal applications. CMGs are used to formulate hydrogels [36], nanoparticles [37], tablets [38,39], nanohybrids [40], films [41], and microparticles [42] and also find applications in tissue engineering [43]. The structural and physicochemical characteristics of CMGs contribute to their capabilities in tissue engineering and drug delivery systems [44]. However, there are several issues with the carboxymethylation of natural gums that can be resolved by derivatization. These include the following factors: (1) Early gastrointestinal fluid erosion hinders the use of CMGs in sustained-release preparations for a longer time. Derivatization synergistically increases the sustaining capability [45]. (2) Derivatization increases thermostability as well as storage stability [46]. (3) An increase in the proportion of grafting contributes to a greater reduction in bacterial and fungal development [47]. (4) Derivatization increases the water affinity, swellability, and flocculation capability of the polymers [48,49]. (5) Additionally, CMGs have some limitations, including biodegradability, which severely limits their applications. With derivatization, these shortcomings can be overcome, giving the polymer framework novel properties (Table 1). Badwaik et al. explored the challenges and issues with the carboxymethylation of natural gum polysaccharides [44]. No review has been published to date that explores the derivatization of carboxymethylated gum derivatization technologies and their implications in drug delivery and other biological sectors. As a result, in the present review, we offer a systematic update on studies involving the chemical functionalization of carboxymethylated polysaccharides and their application in drug delivery, gene delivery, and tissue engineering.

Table 1.
An overview of how derivatization affects CMG characteristics.
2. Derivatives of Carboxymethylated Gums (CMGs)
CMGs have been modified to create biomaterials with superior characteristics. These alterations increase their partition coefficient, swelling capacity, thermosensitivity, flocculation ability, viscosity, and metal sorption properties, while others decrease viscosity and thermal stability. The derivatization of CMGs involves graft copolymerization, cross-linking, conjugation, and polyelectrolyte complexation.
2.1. Graft Copolymerization
The chemical modification of CMGs via graft copolymerization has prompted a great deal of interest in recent years and has made a significant contribution to the development of more effective applications in industrial and biological spheres. Graft copolymerization adds stearic bulk and protects the matrix and carbohydrate backbone [71]. Carboxymethylation improves gum grafting, as the carboxymethyl group of gums allows the monomer and initiator to diffuse. Additionally, the negative charge along the gum chain resulting from the ionizing tendency of carboxyl groups attracts initiators toward the gum, leading to the formation of additional active sites accessible to monomers and enhancing the gum’s reactivity. To initiate the grafting of monomers such as acrylonitrile, N-isopropylacrylamide, 4-vinyl pyridine, methoxy poly(ethylene glycol) amine (mPEG-A), methacrylic acid, acrylamide, 2-acrylamidoglycolic acid (2-AA-GA), etc., on carboxymethyl gums, initiator systems such as APS (ammonium persulphate), CAN (ceric ammonium nitrate), 1-ethyl-3-[3-(dimethylamino)propyl]-carbamide (E-DC) and N-HS (N-hydroxysuccimide), TMEDA (N,N,N,N′-tetramerthlene diamine) redox pair, bromated or thiourea redox pair, peoxymonosulfate (PMS) or thiourea redox pair, etc., have been produced. Graft copolymerization with microwaves has also been described.
2.1.1. Graft Copolymerization Initiated by Free Radicals
Sand et al. grafted 2-AA-GA onto partially carboxymethylated guar gum (CM-GG) utilizing a redox pair, PMS, or thiourea (Figure 1) [58]. R1-SH (a protonated species) is generated when hydrogen ion interacts with thiourea, which further forms a complex via interaction with PMS. Dissociation produces R1-S and SO4−, which are responsible for abstracting hydrogen from CM-GG molecules. Monomer molecules acquire CM-GG radicals near the reaction site, enabling chain initiation, and they transfer free radicals to surrounding molecules, thus producing macroradicals. A graft copolymer is formed by connecting grafted chains. In comparison to CM-GG, grafted 2-AA-GA is more thermostable and has better metal ion sorption, swelling, and flocculation, properties. The long pendant chains of 2-AA-GA boost the graft’s swelling ratio. Access to coal particles is improved by dangling chains of 2-AA-GA.
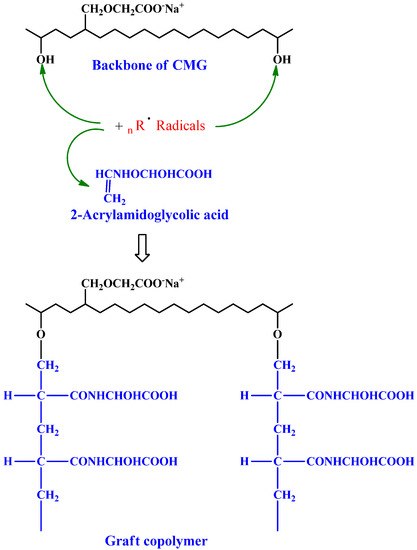
Figure 1.
2-Acrylamidoglycholic acid graft partially copolymerized carboxymethylated guar gum. Reproduced with permission from [58]. Copyright© 2011, Elsevier Ltd.
Xia et al. produced KGM-g-mPEG (konjac glucomannan grafted methoxy polyethylene glycol) via the reaction of CM-KGM (carboxymethylated konjac glucomannan) with mPEG-A in the presence of E-DC and N-HS. For connecting mPEG to KGM, its hydroxyl group must be changed to an amino group [52]. From mPEG-OCOCH2NH-Boc, the Boc group was removed to make mPEG-OCOCH2NH2. E-DC permitted the nucleophilic attack of PEG’s primary amines on O-acylisourea to produce an amide bond. KGM-g-mPEG solubility increases with increased PEG content. The synthesized compound showed more than a 50% increment in the solubility of KGM (highest solubility; 17.80 g/100 mL in water). PEG-grafted KGM decreases solution viscosity at low shear rates. Li et al. produced KGM-g-mPEG, following the procedure described by Xia et al. [52], and formulated nanospheres utilizing α-cyclodextrin (α-CD).
Tripathy et al. grafted 4-vinyl pyridine onto CM-GG and examined its flocculation behavior, swelling, and metal ion sorption characteristics [50]. The generated isothiocarbamide radicals form thiourea and abstract hydrogen ions from the CM backbone of GGs to form free radicals. The graft copolymer chain is built by receiving CM-GG radicals, initiating the chain, and after propagation, finally leads to graft copolymer formation. The graft copolymerized CM-GG is thermally more stable (due to strong covalent connections between the grafted chains) and has better metal ion sorption ability (due to the poly-pendent chain). The coiling chain of poly(4-vinyl pyridine) absorbs more water due to the hydrophilic 4-vinyl pyridine monomer. H-partially CM-GG-g-methacrylamide (H-partially carboxymethylated guar-gum-grafted methacrylamide) was produced by Yadav et al. [49]. Deoxygenated potassium peroxy monosulfate was utilized to initiate the process. For alkaline hydrolysis, graft copolymers are reacted with varied concentrations of NaOH (1.5–3.5 N) [72]. Silver and potassium peroxy monosulfate are utilized to generate radicals [73]. N,N′-MB-AA (N,N′-methylene bisacrylamide) cross-links the grafted copolymer during the chemical process. Trivedi et al. performed acrylamide graft copolymerization utilizing CAN as a redox activator on the sodium salt of partially CM-GG [74]. Pal et al. produced polyacrylamide-grafted CM-GG using a free-radical activator, potassium persulfate [54]. The stimuli-responsive graft copolymer polysaccharides are formed by coupling (pN-IPAA) ((poly(N-isopropyl-acrylamide)) onto (CM-HP-GG) (O-carboxymethyl-O-hydroxypropyl-guar gum) in an aqueous solution, employing potassium persulfate and TMEDA as an initiating system [59].
For water-soluble polysaccharides or polymers, carbodiimide chemistry may be used in an aqueous solution, employing a coupling agent (E-DC) [75]. Thermosensitive graft copolymers with a CM-GG backbone and poly(N-isopropyl-acrylamide) (pNIP-AA) and pNIP-AA side chains were synthesized [49]. By combining CM-GG and pNIP-AA-NH2 with E-DC, CM-GG-g-pNIP-AA (carboxymethylated guar-gum-grafted poly(N-isopropyl-acrylamide) copolymers were made. By employing a chain transfer agent (2-amino-ethane-thiol hydrochloride), semi-telechelic pNPAA-NH2 homopolymers with a reactive group at one end were produced. CM-GG viscosity was elevated via hydrophobic modification using pNIP-AA. Researchers observed that E-DC alone has low coupling yields; therefore, adding N-HS improves efficiency [55]. The coupling reaction with the carboxyl groups of polysaccharides (carboxymethyl tamarind gum and CM-GG) and PEPO terminal amine in cold water utilizing E-DC or N-HS as coupling reagents was reported by Gupta et al. [60].
Soliman et al. produced amphoteric CM-CH-g-pAA (carboxymethyl chitosan-graft-polyacrylamide) copolymer using the peroxy graft copolymerization of acrylamide (AA) onto carboxymethyl chitosan (CM-CH) using potassium persulfate as initiator [56]. By employing potassium persulfate as an initiator, poly(N-vinyl imidazole) (pN-VI) grafting was carried out onto CM-CH in an aqueous solution [47]. Increasing potassium persulfate concentration reduces grafting yields. This could be due to initiator–chain-transfer agent competition or initiator–radical coupling. %G and %GE reach a maximum at a concentration of 1 mol/L, after which they further decline, suggesting that higher N-vinyl-imidazole (N-VI) concentrations do not stimulate additional grafting. Bamford and Schofield hypothesized a radical chain-transfer mechanism and degradative chain transfer to N-VI [76]. Furthermore, grafting a huge portion of polymer onto CM-CH generates a steric barrier for subsequent grafting. Sabaa et al. produced a novel superabsorbent polymer by graft copolymerizing 4-vinyl pyridine onto CM-CH chains in an aqueous solution using potassium persulfate as an initiator. Due to PVP’s basic pyridine group, grafted copolymers swell in acidic pH conditions. CM-CH-g-p4-VP (carboxymethylated chitosan-grafted poly(4-vinylpyridine)) has both basic (pyridine rings) and acidic (COOH) functionality; hence, at neutral pH, swelling is negligible. The presence of the -COOH group in CMCH’s major chains allows it to absorb Maxilon blue with better efficacy than Congo red.
2.1.2. Radiation-Initiated Graft Copolymerization
Microwave (a radiation source) and potassium persulfate (an initiator) were used to produce polyacrylamide-grafted CM-GG [54]. Small-polar molecules such as water generate heat when microwaved due to the rotation of molecules. No free radicals are created. Whole-molecule rotation is prevented by larger molecules or macromolecules. When this happens, polar groups (such as the -OH groups attached to CMG molecules) absorb the microwave and are anchored to an immobile raft. Bonds collapse, creating sites for free radicals. Additionally, the microwave energy that is swiftly transmitted from water molecules to acrylamide molecules causes dielectric heating, which breaks double bonds and creates additional free radicals. The monomer and free radicals (produced on the polar -OH groups of the CMG backbone) recombine to create graft copolymers through chain initiation, propagation, and termination steps. Ammonium persulfate was used as a free radical activator in the microwave-assisted synthesis of carboxymethyl xanthan-gum-grafted polyacrylamide (CM-XG-g-pAA) copolymer. Synthesized nontoxic graft copolymers have better antibacterial efficacy than CM-XG [57].
Patra et al. grafted sodium carboxymethylated okra gum (Na-CM-OG) with polymethacrylamide (pMAA) using free-radical initiation and microwave radiation. The study used a redox free-radical initiator, CAN. When CAN is split up into Ce4+ ions, oxygen free radicals are created on the OG backbone, striking the -OH groups of the anomeric -CHOH groups of okra gum and removing the hydrogen atom via a redox process. pMAA radical is generated by combining pMAA with MAA free radicals. The study shows that carboxymethylation and graft copolymerization synergize okra gum’s sustaining capacity compared with either alone.
2.1.3. Photo-Induced Graft Copolymerization
The graft copolymerization of the sodium salt of partially carboxymethylated guar gum (Na-PCM-GG) and acrylonitrile was carried out, utilizing ceric ammonium nitrate as the photoinitiator in an aqueous medium to create Na-PCM-GG-pMMA: a new graft copolymer [61]. The effects of various variables of synthesis were investigated, including the photoinitiator (CAN), monomer (AN), and nitric acid concentrations; in addition, the effects of the substrate amount, temperature, and reaction time on grafting yields were also investigated to determine the reaction conditions for the best possible photo-induced grafting. During alkaline hydrolysis, the nitrile groups of the appropriately synthesized graft copolymer Na-PCM-GG-g-pMMA could be rapidly converted into water-soluble carboxamide and carboxylate groups, accompanied by the in situ cross-linking of the grafted pMMA chains, yielding the superabsorbent hydrogel H-Na-PCM-GG-g-pMMA.
2.2. Cross-Linking
Cross-linking reduces polymer segment mobility and creates a three-dimensional network [77]. Carboxymethylation improves gums’ hydrophilicity and swelling in dissolution liquid and regulates drug release. Cross-linking reduces the gum’s swelling since the drug could leak out before reaching the absorption site [31]. Cross-linked polymers are sturdier than natural polymers. Cross-linking occurs when cross-linkers react with functional groups (-OH, -COOH, and -NH2) [66].
2.2.1. Covalent Cross-Linking
Silva et al. created carboxymethylated cashew gum (CM-CSG) and cross-linked cashew gum using epichlorohydrin [78]. NaOH forms polysaccharide alkoxide in the epichlorohydrin–polysaccharide reaction. Epichlorohydrin opens the epoxy ring, forming a new epoxy macromolecular. Cross-linking forms a three-dimensional network and reduces polymer segment mobility [56]. Carboxymethylation and cross-linking improved swelling by 23%. Cross-linking carboxymethylated tragacanth gum (CM-TG) polysaccharide backbones with glutaraldehyde (GA) produced CM-TG-GA hydrogels [79]. Figure 2 shows a probable process of CM-TG-GA synthesis. GA interacted with CM-TG hydroxyls to create hemiacetal cross-links. Finally, a three-dimensional superabsorbent polymer was developed. Cross-linking often results in unstable hemiacetal and acetal linkages between CM-TG and GA. This can form a semi-IPN (interpenetrating polymer networks) hydrogel. This instability aids in the biodegradation of superabsorbents after use.
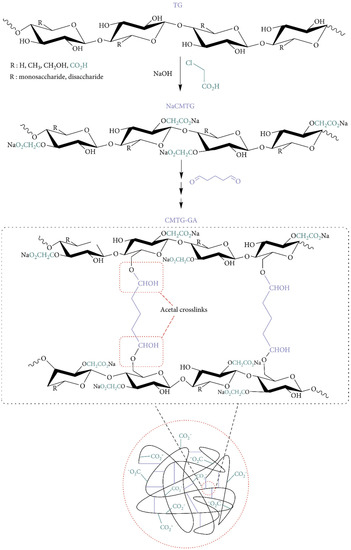
Figure 2.
Proposed mechanism for synthesis of CM-TG-GA. Reproduced from [79]. Copyright© 2021, Yahya Bachra et al.
The pH-sensitive delivery vehicle genipin-cross-linked O-CM-CH-GAR (O-carboxymethyl chitosan–gum Arabic) was explored by Guo-Qing Huang et al. [80]. Genipin, an aglycone formed from geniposide hydrolysis, reacts with the primary amine groups in CH. Due to its natural genesis, low toxicity, excellent biocompatibility, and cross-linking ability, genipin has been widely used in the cross-linking of targeted delivery systems [81]. O-CM-CH-GAR coacervates were cross-linked with genipin at pH 3.0, 4.5, and 6.0. Genipin cross-linking increased coacervate stability against pH sensitivity in simulated gastric fluid. In the simulated gastric solution, coacervates swelled more than in simulated colon and intestinal solutions (swelling was high at pH 4.5 and 6.0 than at pH 3.0). After 2 h of cross-linking, the cumulative percentages of the release of bovine serum albumin in a simulated gastric solution at pH 3.0, 4.5, and 6.0 microcapsules were 79.79%, 55.23%, and 17.14%, respectively.
The same authors created GA-cross-linked O-carboxymethyl chitosan–gum Arabic (O-CM-CH-GAR) coacervates and examined the effect of coacervation acidity on GA cross-linking behavior and cross-linking products. Cross-linking and GA sensitivity decreased from pH 3.0 to 6.0. GA cross-linking improved the flexibility and stability of O-CM-CH-GAR coacervates and acidity, thus affecting the swelling in simulated gastric fluid [82]. Coacervation acidity affected GA cross-linking in O-CM-CH–GAR coacervates and could be exploited for the intestine-targeted administration of sensitive substances. Polycation-to-polyanion ratios increased with an increase in coacervation pH, due to electrostatic interactions [83]. Different coacervate compositions may explain the variation in GA cross-linking activity.
2.2.2. Ionic Cross-Linking
Ionic cross-linking is safer than covalent cross-linking since it is a simple and mild process. Cross-linker and gum derivatives interact ionically. Multivalent counter ions (e.g., calcium, barium, and aluminum) or anionic substances (e.g., sodium trimetapol phosphate) are needed for CMG ionic cross-linking. Anionic molecules produce electrostatic interactions, while metallic ions contribute to the establishment of coordinate covalent bonds.
Mocanu et al. [65] cross-linked carboxymethyl pullulan gum with sodium trimetaphosphate (Na-TMP) or epichlorohydrin. The Na-TMP cross-linked sample had highly acidic phosphate groups and weakly acidic carboxymethyl groups, while epichlorohydrin had just weakly acidic carboxymethyl groups. Cross-linking with Na-TMP improved hydrophilicity more than epichlorohydrin. Maity et al. synthesized and examined the influence of Ca2+ on the erosion, swelling, and mechanism of drug release from Ca-CM-XG matrices [84]. Ca-CM-XG matrices with more cross-linking swelled less and eroded more than CM-XG matrices. This is owing to calcium coordination with CM-XG carboxyl groups that increase gel layer viscosity. The simultaneous pH-dependent swelling and erosion affected drug release. Until reaching the critical calcium ion concentration, increased Ca2+ concentration enhances the drug release from matrices. Borax was successfully cross-linked with CM-GG. The borax cross-linked CM-GG solution (1% w/v) became viscous as a result of the cross-linking. In every testing medium, borax cross-linked CM-GG had a lower swelling index than CM-GG. Borax cross-linked CM-GG and CM-GG were employed as binders in the formulation of ibuprofen and paracetamol. Borax is really a well-known effective cross-linker for polymers with hydroxyl groups [85].
2.2.3. Dual Cross-Linking
Ciprofloxacin HCl was incorporated into carboxymethyl sago pulp (CM-SGP). CM-SGP discs were created via cross-linking and irradiation [86]. To assess their potential as drug delivery systems, drug-loaded CM-SGP discs were characterized for size and weight uniformity, entrapment efficiency, drug loading, DSC (differential scanning calorimetric) studies, FTIR (Fourier transform infrared) spectroscopy, TGA (thermogravimetric analysis), X-ray diffraction, and FE-SEM (field-emission scanning electron microscopy). The swelling dynamics, in vitro release, and antimicrobial efficacy were determined. After exposure to radiation, ciprofloxacin′s antibacterial activity persisted; thus, CM-SGP discs have enormous potential as a method of administering medications to the eyes.
Carboxymethyl chitosan (CM-CH) and carboxymethyl cellulose (CM-C) were cross-linked with CaSO4 and genipin to make a hydrogel film via ionic and covalent cross-linking. Figure 3 depicts the mechanism of cross-linking. Being a nucleophilic reagent, genipin first reacted with amino groups of CMCS through a nucleophilic attack to produce the heterocyclic amino compound, the intermediate 1. Following hydroxyl group elimination, another heterocyclic amino compound, the intermediate 2 was produced. These two intermediates subsequently covalently conjugated to dimers 3 and 4, through the reaction of intermediate 1 with 2 and both intermediate 2, respectively. After crosslinking with genipin, the CMCS amino groups were partly transformed as heterocyclic amines. Cross-linked CM-CH/CMC hydrogel films displayed swelling–deswelling characteristics with two major peaks at pH 3 and 7. The hydrogel films’ mechanical characteristics were also tested. Ionic and covalent cross-linking processes were found to affect the load and toughness of cross-linked hydrogels. The biocompatibility of cross-linked CM-CH/CMC films was shown by cells cultivated on cross-linked hydrogels and negative and positive controls [87].
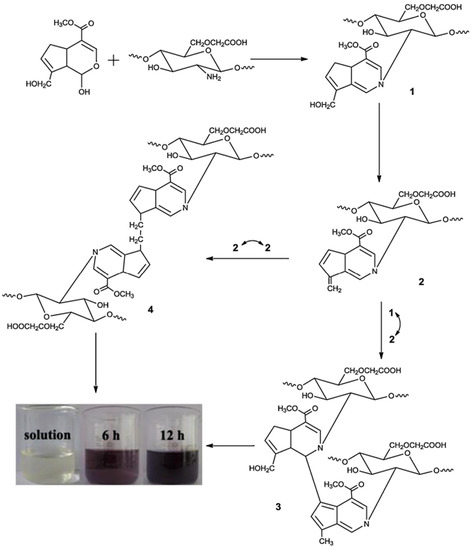
Figure 3.
Mechanisms of CM-CGS–genipin cross-linking. Reproduced with permission from [87]. Copyright© 2014, Elsevier Ltd.
Mitra et al. developed hydrogel beads via ionic and dual cross-linking by utilizing sequential and simultaneous techniques [88]. The mean dissolution time and drug diffusion coefficient demonstrate that the sequential method produces smaller beads with greater drug entrapment performance and prolonged drug release. Drug release decreased by increasing the cross-linking times and cross-linker concentrations. Drug release in the acid solution was accelerated by the solubility of the drug and the swelling of the matrices. Drug content, dissolution profiles, and FTIR demonstrated 3-month drug stability in the beads. Muniyandy et al. [89] combined CM-SGP with gelatin for extended drug delivery. GA-saturated toluene and aqueous aluminum chloride were cross-linkers for this study. GA has two aldehyde groups that cross-link hydroxyl polymers [82]. Coacervates encapsulated ibuprofen with 29–56% w/w loading and 85–93% w/w entrapment efficiency. Fresh drug-loaded coacervates were 10.8 ± 1.93 µm in size. Dual-cross-linked microcapsules released medication slowly over 6 h [82].
2.3. Conjugation
Ha et al. developed a novel cholesterol conjugate of carboxymethyl konjac glucomannan (CM-KGM) [63]. In aqueous media, these polymeric amphiphiles can aggregate by themselves. The CAC scores of the conjugates were lesser than 5.89 × 10−3 mg/mL, demonstrating the high aggregate-forming efficiency of the cholesterol residue. These self-aggregate display properties that depend on the pH and ionic strength. By using FTIR, 1HNMR, XRD, and zeta potential studies, the carboxymethylated guar-gum-grafted polyethyleneimine (CM-GG-g-PEI) copolymer was produced and described by Jana et al. [90]. When the plasmid DNA and polymer weight ratio was greater than 1:10, CM-GG-g-PEI showed strong binding to the plasmid DNA and generated complexes with diameters between 150 and 200 nm. The GG-grafted LMW-b-PEI (coupling low-molecular-weight branched PEI) copolymer was produced with CM-GG with LMW-b-PEI (weight 800 Da) using carbodiimide chemistry and N-HS as a coupling agent. Figure 4 depicts the CM-GG-g-PEI reaction scheme.
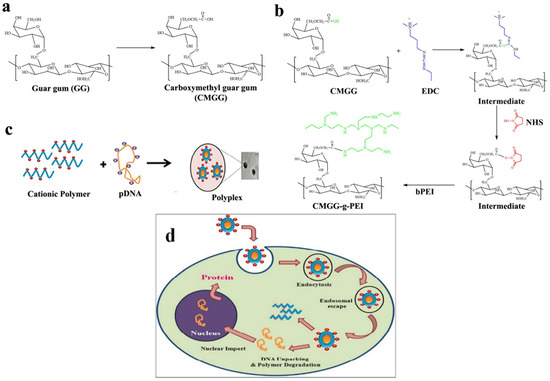
Figure 4.
(a) Illustration of the production of CM-GG from guar gum; (b) CM-GG-g-PEI synthesis mechanism; (c) polymer–pDNA complex preparation; (d) schematic illustration of encapsulation of the polymer/DNA complex. Reproduced with permission from [90]. Copyright© 2011, Royal Society of Chemistry.
Researchers created a novel copolymer (carboxymethylated mesquite-gum-grafted polyethyleneimine (CM-MQG-g-PEI)) [91]. Initially, MQG carboxymethylation was carried out to form carboxymethylated mesquite gum (CM-MQG). The cross-linking of CM-MQG and b-PEI was then accomplished using the cross-linking agents EDC and N-HS. Carboxymethyl chitosan-conjugated magnetite (Fe3O4) nanoparticles (CM-CH-MG-NPs) were produced by covalently attaching CM-CH to the surface of MG-NPs through carbodiimide conversion in a medium of paraffin acetic acid [92]. CM-CH-MG-NPs had a spherical shape, with a diameter of 15 nm and a high CM-CH binding percentage (24.7 wt%). The capacity of magnetic sorbents to remove the heavy metal Pb(II) from aqueous systems was evaluated. Pb (II) sorption increased with pH (3.0–6.0) but reduced with CNaNO3 (0.001–0.500 M) or T (25–55 °C). CM-CH-MG-NPs sorb Pb(II) with a substantial Cs-effect. The Langmuir and Freundlich isotherms explain sorption equilibrium at each Cs (sorbent dosages). Langmuir-SCA and Freundlich-SCA isotherms described Cs effect data. The Cs effect was unaffected by pH, CNaNO3, and T.
2.4. Polyelectrolyte Complexation (PECs)
PECs are oppositely charged particle complexes (polymer/drug, polymer/polymer, and polymer/drug/polymer) and are formed when oppositely charged polyions interact electrostatically. Such contact may result in the precipitation, coacervation, or formation of the gel. This reduces the toxicity and other consequences of chemical cross-linking agents. These PECs meet biocompatible polymer system standards and can be tailored to produce active substance components and carrier substances. The Flory–Huggins theory concerning the free polyelectrolyte energy and electrostatic forces resulting from mixing has been postulated to explain PEC formation. Two polymers’ backbones typically repel one another, but how they interact depends on their charge fraction. The Flory interaction parameter, i.e., polymer backbone repulsion, prevails when the charge fraction is low, causing the solution to separate into two phases. At high charge fractions, polymers form a complex due to electrostatic interactions.
The carboxylic group gives the gum its anionic properties and enables the formation of PECs with cationic polymers. Chitosan has an overall positive charge because of the amino groups it contains, making it simple to combine with polymers that have a negative charge. The reaction of CMG and CH PECs can be illustrated by:
In a previous study, chitosan (CH) and carboxymethyl gum katira (CM-GK) interaction resulted in the development of PECs that were modified using ofloxacin as the reference drug [93]. The results of that study demonstrated that increasing the ratio of CM-GK and CH in the CM-GK-CH-PECs (carboxymethyl gum katira–chitosan PECs) decreased the percentage yield while increasing the percentage of drug entrapment. Drug loading at 50% (%w/w) and polymer ratio (CM-GK/CH) 2.13 were the best calculation parameters. The optimized batch of the CM-GK-CH-PECs had an ofloxacin entrapment efficiency of 84.86% and a yield of 69.04%. Furthermore, using Higuchi’s square-root kinetics, the improved batch of CM-GK-CH-PECs released ofloxacin at 84.32%. The conclusion drawn from this work is that the polyelectrolyte complex of CM-GK and CH offers significant promise as a polymer-based method for sustained drug administration.
Chitosan and carboxymethyl cashew gum compounds were developed, and their thermal stability was assessed by Maciel et al. [94]. Carboxymethyl konjac glucomannan [95], carboxymethyl tamarind kernel powder [96], carboxymethyl fenugreek gum [97], carboxymethyl guar gum [98], carboxymethyl gum kondagogu [99], and cashew gum have been complexed with chitosan to form PECs and utilized in various drug delivery system.
2.5. Esterification
Using thioglycolic acid, More et al. esterified carboxymethyl gellan gum (CM-GLG). The hydroxyl group of the glucuronic acid portion of CM-GLG is esterified with the carboxyl group of thioglycolic acid during the esterification reaction [64]. As shown in the scheme in Figure 5, thiolated carboxymethyl chitosan-β-cyclodextrin was produced by covalently attaching thioglycolic acid to the primary amino groups of carboxymethyl chitosan-β-cyclodextrin (CM-CH-β-CD) [100]. Another study used a two-step process to synthesize thiolated CM-CH-β-CD. In the first stage, EDC and N-HS were used to form an amide link between the main amino groups of CM-CH and β-CD. The carboxyl groups of the resulting CM-CH-β-CD were coupled with the amino groups of cysteine methyl ester-hydrochloride in the second step [70].
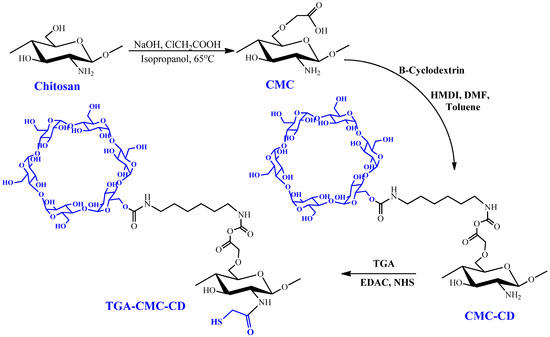
Figure 5.
Chitosan modification and production of the TGA-CM-CH-CD polymer. Reproduced with permission from [100]. Copyright© 2013, Springer Science Business Media, New York.
3. Applications of Derivatized Carboxymethylated Gums
3.1. Carrier for Drug Delivery
Drug delivery systems that involve derivatized CMGs include oral, ocular, and transdermal delivery (Table 2). Derivatized CMGs have been studied in a variety of therapeutic oral delivery systems that are commonly used for drug administration, either as controlled or extended-release matrices or as useful biomaterials. Derivatized CMGs have been used to transport medications to a particular site, including the colon, small intestine, stomach, and mouth.

Table 2.
Summary of derivatized CMGs utilized as drug delivery carriers.
3.1.1. Oral Drug Delivery
The genipin-cross-linked O-CMC-GA coacervate was investigated for a pH-sensitive delivery system. Both the acidity of coacervate and cross-linking duration impacted O-CMC–GA pH responsiveness and BSA releases [80]. Due to their stability and gradual expansion in simulated gastrointestinal fluids; GA-cross-linked O-CMC-GAR coacervates potentially carry sensitive substances to the intestine. Coacervation acidity affected GA cross-linking behavior, and the product attributes are also changed by modifying the coacervate composition [82].
Patra et al. produced a prolonged-release tablet matrix for sodium carboxymethyl okra-gum-grafted polymethacrylamide (Na-CM-OG-g-PMA) copolymer [45]. The diclofenac sodium tablet formulation with S-CMOG-g-PMA with DCS-0.604 and %G-423.4 exhibited remarkable prolonged drug release (90% for 11.7 h) and a 72.0 similarity factor. The copolymer proved advantageous in terms of biodegradability and biocompatibility, rendering it a sensible semi-synthetic biopolymer. Carboxymethyl fenugreek galactomannan–gellan gum–calcium silicate (CMC-Fg-Gm-GG-CS) hydrogel beads were made to transport glimepiride in a controlled manner. Ionotropic gelation with Al+3/Zn+2/Ca+2 ions produced glimepiride-loaded hybrids. Formulations showed sustained drug release (62–94% for Q8hrs) and good drug encapsulation efficiency (EE; 48–97%) [97].
In order to generate CM-XG-g-pAA, Badwaik et al. synthesized carboxymethyl xanthan gum and then further grafted it with polyacrylamide. Wet-granulated tablets release the drug gradually. The release profile followed Korsmeyer–Peppas equation and showed drug transport via erosion and diffusion [105]. Bajpai and Sharma formulated vitamin-B12-loaded barium ions in sodium alginate CMC-GG hydrogel beads. Encapsulation efficiency was 50%, and the release of drug in the simulated gastric fluid was 20% (in 3 h), and that in the simulated intestinal fluid was 70% (in 7 h) with vitamin-B12-loaded beads [106].
Colon-specific delivery systems are important for treating ulcerative colitis and irritable bowel disease, as well as for the systemic injection of protein and peptide drugs [98,109]. Maity and Sa investigated cross-linked carboxymethyl xanthan gum tablets for prednisolone delivery. Ca2+ ion modulation regulates carboxymethyl xanthan gum drug release from matrix tablets [84]. Cross-linking limits polymer chain flexibility by generating a gel layer around the tablet’s surface. Increased cross-linking agent density enhances gel strength and reduces macromolecular mesh size, reducing drug diffusion through the gel layer. When placed in the colon, the optimized formulation released the drug in 10 h [109].
In another work, Kumar et al. produced a fluticasone colon-specific tablet matrix using cross-linked chitosan and CM-GG/IPC (interpolymer complexes). Chitosan:CMG (50:50) tablets encapsulated with IPC inhibited drug release in the small intestine and stomach, and it also delivered the drug to the colon [98]. Singh et al. produced metronidazole matrix tablets by cross-linking CM-GG with Ca2+ ions. Ca2+ ion concentration improved gel layer viscosity and inhibited water entry, minimizing tablet swelling and drug release. After a certain concentration of Ca2+ ions, the gel layer’s viscosity decreased as a result of matrix erosion. After a certain Ca2+ concentration, matrix erosion reduced the gel layer’s viscosity [104]. Randhawa et al. produced colon-specific targeted tamoxifen pills by covering them with chitosan and carboxymethyl fenugreek gum (CM-FG) or chitosan and CM-GG. The medication was released from the coated tablets at pH 6.8 while being protected from absorption in the stomach and small intestine [39].
A significant amount of effort has been put towards enhancing the mucoadhesive properties of polymeric carriers to boost drug absorption [110]. Because mucoadhesive delivery systems remain at the site of drug absorption for a longer period of time, dosage frequency can be reduced, thus increasing patient compliance. Mucoadhesive polymers rely on hydrogen bonding and ionic interactions with mucus [106]. Thiol-containing polymers have greater adhesion. Thiomers engage cysteine-rich mucus glycoproteins via disulfide exchange. Poor interactions between thiolated polymers and hydrophobic drugs lead to rapid drug release, limiting their clinical usefulness. Grafting produces thiolated carboxymethylated gums with modulated drug release [100].
Prabaharan et al. synthesized thiolated carboxymethyl chitosan-g-β-cyclodextrin (Th-CM-CH-g-β-CD) for ketoprofen [70]. Similarly, thiolated carboxymethyl chitosan-g-β-cyclodextrin nanoparticles (Th-CM-CH-g-β-CD) with maximum mucoadhesion and albendazole entrapment efficiency were prepared using an aqueous solution containing tripolyphosphate (1 wt%) and the polymer (115.65 lmol/g) of grafted thiol groups for the controlled release of hydrophobic drugs [95]. Anionic gelation with sodium tripolyphosphate produced TGA-CMC-CD nanoparticles for mucosal albendazole delivery. More et al. studied the mucoadhesive capability of carboxymethyl gellan gum–thioglycolic acid (CM-GG-TGA) conjugate and its physicochemical properties [69].
3.1.2. Ocular Delivery
Ciprofloxacin HCl is the preferred medication for treating corneal ulcers and bacterial conjunctivitis, but its usability is limited by a relatively shorter elimination half-life [96]. Ordinary ophthalmic ointments and drops require multiple administrations to maintain plasma concentration. Therefore, ciprofloxacin HCl in a controlled-release dosage form may increase ocular bioavailability, lessen the administration frequency, and prolong the amount of time the drug remains in the precorneal tissue. Carboxymethyl sago pulp discs show potential in the ophthalmic delivery of ciprofloxacin because they can enhance ocular bioavailability by prolonging the time the drug is interacting with corneal tissues and by removing the need for recurring administration, as is necessary for conventional eye drops. The administered dose also satisfies the sterilizing condition, a necessity for ocular drug administration [86].
3.1.3. Transdermal Delivery
Multiwalled carbon nanotube (MW-CNT) in situ composite membranes complexed with 2-hydroxyethyl methacrylate (HEMA)-grafted CM-GG were used to develop a transdermal device for the controlled release of diclofenac sodium. Supreme matrix interactions with 0.5 and 1 wt% MW-CNT resulted in a superior copolymer-adsorbed fibrillar alignment of MW-CNT compared with 2 and 3 wt% MW-CNT. These formulations improved encapsulation efficiency and prolonged drug release (65% vs. 17% at 1 wt%). It was determined that each strategy significantly lengthened the drug molecules’ half-lives (from 2.5 h at 1 wt% to 47 h). The microstructure wrapped in a copolymer is graphically depicted in Figure 6 [111]. Similarly, Giri et al. produced hydrogels consisting of chemically modified multiwalled carbon nanotubes (MW-CNTs) and carboxymethyl guar gum (CM-GG) for prolonged and consistent release of diclofenac sodium transdermally [107].
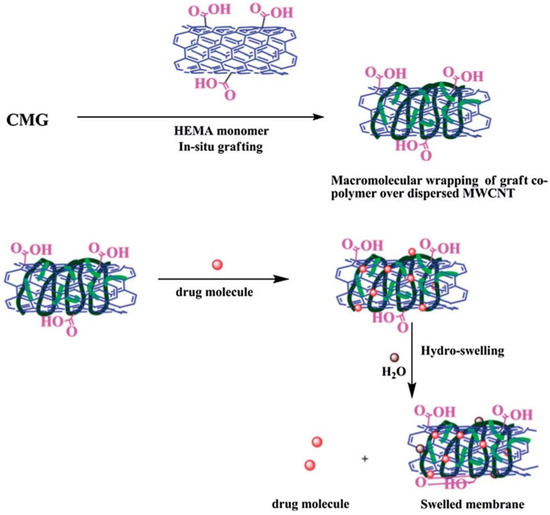
Figure 6.
Schematic presentation of HEMA-grafted CMG (wrapping over the anisotropic MW-CNT units to produce an effective encapsulate for the drug and ensure its steady release over a prolonged time). Reproduced with permission from [111]. Copyright© 2014, Royal Society of Chemistry.
3.1.4. Vaccine Delivery
In an interesting study, Chen et al. chemically modified konjac glucomannan (KGM) to generate anionic CM-KGM and cationic quaternized konjac glucomannan (Q-KGM). Two different types of nanoparticles (NPs), namely sodium tripolyphosphate/Q-KGM and CM-KGM/Q-KGM, were developed. To further investigate the impact of NPs on the immune response in mice, the prepared NPs were loaded with ovalbumin (OVA). Figure 7 shows a diagrammatic representation and TEM images of the formulated NPs. The NPs had an asymmetrical spherical morphology and excellent sustained-release characteristics. It was also discovered that the OVA-loaded NPs were not toxic to cells. Additionally, the nanoparticles produced from modified KGM could boost immune system activity and improve both the humoral and the cellular immune response [101]. Shi et al. also fabricated nanospheres from CM-KGM and 2-hydroxypropyl trimethylammonium chloride chitosan (HP-TmAC) loaded with OVA for vaccine delivery [102].
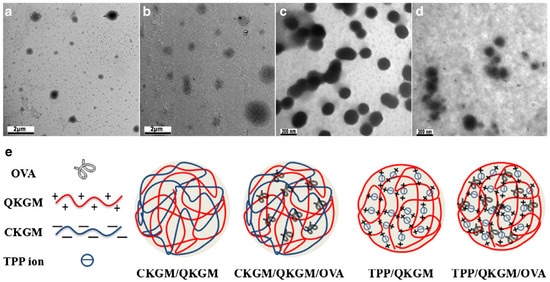
Figure 7.
TEM images of blank and modified konjac glucomannan NPs and respective OVA-loaded NPs: (a) blank CM-KGM/Q-KGM/OVA NPs; (b) blank TPP/Q-KGM NPs; (c) TPP/Q-KGM/OVA NPs; (d) image of various kinds of NPs; (e) the schematic illustration of various kinds of NPs. Reproduced with permission from [101]. Copyright© 2018, Springer Science Business Media, LLC, part of Springer Nature.
3.1.5. Enzyme Encapsulation
Grafted CMGs are frequently used to create nanospheres as carriers for delivering drugs. To enclose the enzyme glucose oxidase in an aqueous solution, Li et al. produced self-assembled rod-coil carboxymethyl konjac-glucomannan-grafted poly(ethyleneglycol) (CM-KGM-g-PEG) and α-CD (α-cyclodextrin) complexes [46]. In comparison to free, unencapsulated glucose oxidase, the entrapped form had superior storage stability, optimum bioactivity over a broader pH range, and better thermostability. Additionally, it demonstrated in vitro biocompatibility. Similar research was conducted by Mocanu et al. on the interactions of biologically active compounds, including drugs (propranolol and quinidine), enzymes (lysozyme), and carboxymethyl pullulan microparticles cross-linked with siloxane [103]. The same group of authors produced the side chains of Jeffamine-[polyoxy-alkyleneamines (polyethylene-oxide)] units in cross-linked carboxymethyl pullulan hydrogels. These hydrogels have thermoassociated capabilities because of the Jeff units and pH-sensitive features because of the anionic functional groups. Interactions between proteins such as bovine serum albumin and lysozyme, as well as antioxidants such as lutein, and these hydrogels were induced to determine their suitability for use in drug delivery.
3.2. As a Gene/Vaccine Delivery Vehicle
Gene delivery vehicles can be either viral or nonviral vectors. Interest in nonviral gene delivery vehicles has increased due to the potential infectivity, sophisticated and complex production, immunogenicity, and carcinogenic effects of viral vectors. To increase effectiveness and lessen the cytotoxicity of gene vector transfection, the cross-linking of low-molecular-weight units with degradable connections has been studied [112]. A nonviral vector with high buffering capacity and transfection effectiveness is low-molecular-weight branched polyethyleneimine (LMW b-PEI) [113]. This polymer has high charge density, the potential to destabilize membranes, and the capacity to shield endocytosed plasmid DNA from enzymatic deterioration, rendering it ideal for encasing plasmid DNA in nanoparticles for gene therapy. PEI’s toxicity increases with molecule weight and thus is considered a risk for gene delivery [114,115]. These polymers do not break down in the body and pose a long-term threat [116]. To alleviate this limitation, a GG-grafted LMW b-PEI (800 Da) copolymer was produced and tested. CMGG-g-PEI buffered better than GG. The produced copolymer condensed pDNA by producing positively charged polyplexes, and it shielded pDNA from DNase I digestion and allowed it to enter the cell via its higher electron sponge effect (Figure 4). In in vitro experiments, the transfection of A549 cells using CMGG-g-PEI/plasmid DNA complexes was superior to that using LMW b-PEI (800 Da)/pDNA complexes and inferior to that using b-PEI (25 kDa)/plasmid DNA complexes. When compared to PEI, the graft copolymer showed much lower cytotoxicity. Hence, it has been established that the nonviral gene therapy vector CM-GG-g-PEI is a reliable and effective treatment option [90].
3.3. Therapeutic Applications
Sabaa et al. developed antibacterial CM-CH-g-PNVI copolymers [47]. Gram-negative (E. coli) and gram-positive bacteria (S. aureus) were exposed to CMCh-g-PNVI and chitosan. S. aureus viable cell count was inhibited more by chitosan-g-PNVI (G-107%) than by CM-CH-g-pNVI (G-99%), with 61.7% vs. 46.7% inhibition. Chitosan-g-PNVI inhibited E. coli by 74.1%, whereas CM-CH-g-pNVI inhibited it by 93.1%. Increasing the graft percentage led to an increase in the inhibition of E. coli and S. aureus viable cell numbers. Chitosan and its high-molecular-weight derivatives cover cell surfaces and inhibit intracellular leakage [117]. Likewise, chitosan-g-PNVI and CM-CH-g-pNVI analogues were tested on A. fumigatus and F. oxysporum. The 99% PNVI graft on CMCh inhibited F. oxysporum and A. fumigatus by 48.6% and 38.5%, respectively. Increasing the graft proportion improves inhibitory activity. CM-CH-g-pNVI (G-197%) inhibited F. oxysporum and A. fumigatus by 63.3% and 54.0%, respectively. Orsu et al. created nanofiber films from the biopolymer carboxymethyl tamarind gum (CM-TG), which was potentiated with reduced graphene oxide (rGO) and polyvinyl alcohol for the potential proliferation of neural cells. The results showed exceptional neuronal growth through an additive effect, which is important for replicating the extracellular matrix (ECM) and can be clinically tested for a variety of neurodegenerative illnesses [118].
In another investigation, the antibacterial activity of XG, CM-XG, CM-XG-g-PAAm, and m(CM-XG-g-PAAm) against S. aureus and E. coli was studied [84]. The carboxymethylation of XG improves water solubility. Antibacterial CM-XG may release H+ ions that penetrate the bacterial cell walls and induce cell death. Acrylamide grafting improves CMXG’s solubility and cationic centers. Synthesized graft copolymer’s antibacterial activity may be owing to the interactions between CM-XG-g-PAAm’s positive charge and E. coli (lipopolysaccharide) negative charge. Grafted carboxymethylated mesquite gum demonstrated improved hemocompatibility over b-PEI and carboxymethylated mesquite gum (CBX-MG). A hemolysis test was designed to assess polymer erythrocyte lysis [111]. The outcomes demonstrated nonhemolytic properties at doses less than 0.1 µg/mL (CBX-MG-PEI). The copolymer’s physicochemical and hemocompatibility potentials render it useful in formulating nanoparticles, transfection, and biomaterials [91].
3.4. Tissue Engineering
Tissue engineering can repair organs and tissues. Patient cells are seeded and cultivated in vitro to generate a new organ or tissue. An injectable, biodegradable hydrogel system including the conjugates of chondroitin sulfate tyramine (CDS-TY) and carboxymethyl pullulan tyramine (CM-PL-TY) was produced under physiological circumstances with horseradish peroxidase (HR-PX) and hydrogen peroxide (H2O2) for cartilage tissue engineering (CT-TE). CT-TE is a viable therapeutic strategy for cartilage regeneration and has advantages over existing cartilage treatment methods [119]. For regenerative medicine, this injectable method is effective, intrusive, and readily adaptable. The physicochemical features of a stable hydrogel system can be modified by adjusting the polymer weight ratio and cross-linking reagent concentrations. Porcine auricular chondrocytes encapsulated in CMP-TA and CS-TA hydrogels showed high cytocompatibility. CMP-TA and CS-TA composite hydrogels showed improved cartilaginous ECM deposition and cell proliferation, which facilitates chondrogenesis. A mouse subcutaneous implantation model’s histological analysis confirmed the hydrogel system’s tissue compatibility. Injectable pullulan/chondroitin sulfate composite hydrogels may be effective for rebuilding cartilage tissue. Bao et al. created new hydrogel films from CMCS and CMC, which were double-cross-linked using genipin and CaSO4 as ionic and covalent cross-linkers, respectively [87]. The main effects on the cross-linked hydrogels’ toughness and maximum load were caused, respectively, by ionic and covalent cross-linking. The biocompatibility of the cross-linked hydrogel films manifested in cultured cells, as well as negative and positive controls. Due to their advantageous characteristics, the double-cross-linked hydrogel films could be used as a biomaterial for skin tissue engineering.
4. Conclusions
Current developments and research tactics are focused on functionalizing established materials. One of the most studied conversions that lead to the discovery of newer biomaterials with extremely potential applications is modifying carboxymethylated gums. However, the application of carboxymethylated gum has encountered some challenges. Early gastrointestinal fluid erosion hinders the use of CMG in sustained-release preparations for a longer time. Derivatization, however, synergistically increases the sustaining capability. Thermostability and storage stability are also increased by derivatization. An increase in the proportion of grafting contributes to a greater reduction in bacterial and fungal development. Derivatization increases the water affinity, swellability, and flocculation capability of polymers. Additionally, CMG has some limitations including biodegradability, which severely limits its applications. With derivatization, these shortcomings can be overcome, providing the polymer framework with novel properties.
The prospective implications of modified carboxymethylated gums with respect to physiochemical characteristics and their biomedical and pharmaceutical applications are discussed in this review, with an emphasis on drug delivery, in vitro cell culture, and tissue engineering. For these uses, the biocompatibility of modified carboxymethylated gums and their simplicity in producing derivatives with novel characteristics are their most intriguing qualities. Almost all types of bioactive compounds can be prepared using modified carboxymethylated gums for prolonged and controlled release. Altogether, the data on the uses of derivatized carboxymethylated gums are promising. More research is needed before derivatized carboxymethyl-gum-based carriers may be used in clinics.
Author Contributions
Conceptualization, H.B. and T.K.G.; writing—original draft preparation, M.B., K.S., S.M. and T.K.G.; writing—review and editing, K.T.N., C.S., Y.A. and S.O.; supervision, H.B. and S.G.; funding acquisition, S.O. All authors have read and agreed to the published version of the manuscript.
Funding
The authors sincerely acknowledge the research grant support (Research Grants # 12R121 and 12R104 to Shreesh Ojha) from United Arab Emirates University, United Arab Emirates.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dalei, G.; Das, S. Carboxymethyl guar gum: A review of synthesis, properties and versatile applications. Eur. Polym. J. 2022, 176, 111433. [Google Scholar] [CrossRef]
- Patel, J.; Maji, B.; Moorthy, N.S.H.N.; Maiti, S. Xanthan gum derivatives: Review of synthesis, properties and diverse applications. RSC Adv. 2020, 10, 27103–27136. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Dutta, P.; Giri, T.K. Al3+/Ca2+ cross-linked hydrogel matrix tablet of etherified tara gum for sustained delivery of tramadol hydrochloride in gastrointestinal milieu. Int. J. Biol. Macromol. 2023, 232, 123448. [Google Scholar] [CrossRef] [PubMed]
- Khushbu; Warkar, S.G. Potential applications and various aspects of polyfunctional macromolecule- carboxymethyl tamarind kernel gum. Eur. Polym. J. 2020, 140, 110042. [Google Scholar] [CrossRef]
- Rai, N.; Bal, T.; Swain, S. In vitro evaluations of biodegradable polyacrylamide grafted moringa bark gum graft copolymer (MOG-g-PAAM) as biomedical and controlled drug delivery device synthesized by microwave accelerated free radical synthesis. Indian J. Pharm. Educ. Res. 2020, 54, 385–396. [Google Scholar] [CrossRef]
- Ofoedu, C.E.; You, L.; Osuji, C.M.; Iwouno, J.O.; Kabuo, N.O.; Ojukwu, M.; Agunwah, I.M.; Chacha, J.S.; Muobike, O.P.; Agunbiade, A.O.; et al. Hydrogen peroxide effects on natural sourced polysacchrides: Free radical formation/production, degradation process, and reaction mechanism—A critical synopsis. Foods 2021, 10, 699. [Google Scholar] [CrossRef]
- Alban, S.; Franz, G. Characterization of the anticoagulant actions of a semisynthetic curdlan sulfate. Thromb. Res. 2000, 99, 377–388. [Google Scholar] [CrossRef]
- Hirsh, J.; Levine, M.N. Low molecular weight Heparin. Blood 1992, 79, 1–17. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Modification of sterculia gum with methacrylic acid to prepare a novel drug delivery system. Int. J. Biol. Macromol. 2008, 43, 142–150. [Google Scholar] [CrossRef]
- Singh, M.; Raorane, C.J.; Alka; Shastri, D.; Raj, V.; Kim, S.-C.; Tuteja, M. Recent Progress on Modified Gum Katira Polysaccharides and Their Various Potential Applications. Polymers 2022, 14, 3648. [Google Scholar] [CrossRef]
- Aanisah, N.; Wardhana, Y.W.; Chaerunisaa, A.Y.; Budiman, A. Review on Modification of Glucomannan as an Excipient in Solid Dosage Forms. Polymers 2022, 14, 2550. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Kuchina, Y.A. Intermolecular Interactions in the Formation of Polysaccharide-Gelatin Complexes: A Spectroscopic Study. Polymers 2022, 14, 2777. [Google Scholar] [CrossRef]
- Xu, Y.; Ji Wu, Y.; Sun, P.; Zhang, F.; Linhardt, R.J.; Zhang, A. Chemically modified polysaccharides: Synthesis, characterization, structure activity relationships of action. Int. J. Biol. Macromol. 2019, 132, 970–977. [Google Scholar] [CrossRef]
- Li, S.; Xiong, Q.; Lai, X.; Li, X.; Wan, M.; Zhang, J.; Yan, Y.; Cao, M.; Lu, L.; Guan, J.; et al. Molecular Modification of Polysaccharides and Resulting Bioactivities. Compr. Rev. Food Sci. Food Saf. 2016, 15, 237–250. [Google Scholar] [CrossRef]
- Kumar, D.; Pandey, J.; Raj, V.; Kumar, P. A Review on the Modification of Polysaccharide Through Graft Copolymerization for Various Potential Applications. Open Med. Chem. J. 2017, 11, 109–126. [Google Scholar] [CrossRef]
- Chakka, V.P.; Zhou, T. Carboxymethylation of polysaccharides: Synthesis and bioactivities. Int. J. Biol. Macromol. 2020, 165, 2425–2431. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Yao, Z.; Zhao, M.; Qi, H. Sulfation, anticoagulant and antioxidant activities of polysaccharide from green algae Enteromorpha linza. Int. J. Biol. Macromol. 2013, 58, 225–230. [Google Scholar] [CrossRef]
- Qiu, S.; Chen, J.; Chen, X.; Fan, Q.; Zhang, C.; Wang, D.; Li, X.; Chen, X.; Liu, C.; Gao, Z.; et al. Optimization of selenylation conditions for lycium barbarum polysaccharide based on antioxidant activity. Carbohydr. Polym. 2014, 103, 148–153. [Google Scholar] [CrossRef]
- Ye, M.; Yuan, R.Y.; He, Y.L.; Du, Z.Z.; Ma, X.J. Phosphorylation and anti-tumor activity of exopolysaccharide from Lachnum YM120. Carbohydr. Polym. 2013, 97, 690–694. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Wang, Y.; Nie, S.; Li, C.; Xie, M. Acetylation and carboxymethylation of the polysaccharide from Ganoderma atrum and their antioxidant and immunomodulating activities. Food Chem. 2014, 156, 279–288. [Google Scholar] [CrossRef]
- Li, X.L.; Xiao, J.J.; Zha, X.Q.; Pan, L.H.; Asghar, M.N.; Luo, J.P. Structural identification and sulfated modification of an antiglycation Dendrobium huoshanense polysaccharide. Carbohydr. Polym. 2014, 106, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Wan, Z.J.; Shi, L.; Lu, X.X. Preparation and antiherpetic activities of chemically modified polysaccharides from Polygonatum cyrtonema Hua. Carbohydr. Polym. 2011, 83, 737–742. [Google Scholar] [CrossRef]
- Fan, J.M.; Zhang, J.S.; Tang, Q.J.; Liu, Y.F.; Zhang, A.Q.; Pan, Y.J. Structural elucidation of a neutral fucogalactan from the mycelium of Coprinus comatus. Carbohydr. Res. 2006, 341, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhang, J.; Lv, Z.; Ye, L.; Yang, Y.; Tang, Q. Chemical modification of an acidic polysaccharide (TAPA1) from Tremella aurantialba and potential biological activities. Food Chem. 2014, 143, 336–340. [Google Scholar] [CrossRef]
- De Nooy, A.E.J.; Rori, V.; Masci, G.; Dentini, M.; Crescenzi, V. Synthesis and prelimiary characterisation of charged derivatives and hydrogels from scleroglucan. Carbohydr. Res. 2000, 324, 116–126. [Google Scholar] [CrossRef]
- Abhishek, R.; Ahuja, M. Evaluation of carboxymethyl moringa gum as nanometric carrier. Carbohydr. Polym. 2017, 174, 896–903. [Google Scholar]
- Badwaik, H.R.; Al Hoque, A.; Kumari, L.; Sakure, K.; Baghel, M.; Giri, T.K. Moringa gum and its modified form as a potential green polymer used in biomedical field. Carbohydr. Polym. 2020, 249, 116893. [Google Scholar] [CrossRef]
- Verraest, D.L.; Peters, J.A.; Batelaan, J.G.; Van Bekkum, H. Carboxymethylation of inulin. Carbohydr. Res. 1995, 271, 101–112. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, T.; Wei, H.; Zhang, M.; Zou, Y.; Mao, V.; Wu, X. Carboxymethylation of polysaccharides from Auricularia auricula and their antioxidant activities in vitro. Int. J. Biol. Macromol. 2011, 49, 1124–1130. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, G.; Li, J.; Surhio, M.M.; Ye, M. Structure characterization, modifi cation through carboxymethylation and sulfation, and in vitro antioxidant and hypoglycemic activities of a polysaccharide from Lachnum sp. Process Biochem. 2018, 72, 177–187. [Google Scholar] [CrossRef]
- Rana, V.; Rai, P.; Tiwary, A.K.; Singh, R.S.; Kennedy, J.F.; Knill, C.J. Modified gums: Approaches and applications in drug delivery. Carbohydr. Polym. 2011, 83, 1031–1047. [Google Scholar] [CrossRef]
- Devi, G.; Kaur, M.; Nagpal, M.; Sharma, A.; Puri, V.; Dhingra, G.A.; Arora, M. Advanced dosage form design: Role of modified natural gums. Asian J. Chem. 2021, 33, 1–9. [Google Scholar] [CrossRef]
- Murthy, H.N. Chemical Constituents and Applications of Gums, Resins, and Latexes of Plant Origin. Ref. Ser. Phytochem. 2022, 1, 3–23. [Google Scholar]
- Sharma, D.; Sharma, P. Synergistic studies of Cassia tora gum with xanthan and guar gum: Carboxymethyl synthesis of cassia gum-xanthan synergistic blend and characterization. Carbohydr. Res. 2023, 523, 108723. [Google Scholar] [CrossRef]
- Tanwar, M.; Gupta, R.K.; Rani, A. Natural gums and their derivatives based hydrogels: In biomedical, environment, agriculture, and food industry. Crit. Rev. Biotechnol. 2023; online ahead of print. [Google Scholar]
- Mocanu, G.; Mihaï, D.; Dulong, V.; Picton, L.; Le, D. New anionic crosslinked multi-responsive pullulan hydrogels. Carbohydr. Polym. 2012, 87, 1440–1446. [Google Scholar] [CrossRef]
- Gupta, A.P.; Verma, D.K. Preparation and characterization of carboxymethyl guar gum nanoparticles. Int. J. Biol. Macromol. 2014, 68, 247–250. [Google Scholar] [CrossRef]
- Giri, T.K.; Choudhary, C.; Alexander, A.; Ajazuddin, A.; Badwaik, H.; Tripathy, M.; Tripathi, D.K. Sustained release of diltiazem hydrochloride from cross-linked biodegradable IPN hydrogel beads of pectin and modified xanthan gum. Indian J. Pharm. Sci. 2013, 75, 619–627. [Google Scholar]
- Randhawa, R.; Bassi, P.; Kaur, G. In vitro, in vivo evaluation of inter polymer complexes between carboxymethyl fenugreek gum and chitosan or carboxymethyl guar gum and chitosan for colon delivery of tamoxifen. Asian Pac. J. Trop. Dis. 2012, 2, S202–S207. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, P. Carboxymethyl tamarind gum-silica nanohybrids for effective immobilization of amylase. J. Mol. Catal. B Enzym. 2011, 70, 67–73. [Google Scholar] [CrossRef]
- Zhu, G.; Sheng, L.; Tong, Q. Preparation and characterization of carboxymethyl-gellan and pullulan blend films. Food Hydrocoll. 2014, 35, 341–347. [Google Scholar] [CrossRef]
- Rafigh, S.M.; Vaziri Yazdi, A.; Safekordi, A.A.; Heydari Nasab, A.; Ardjmand, M.; Naderi, F.; Mozafari, H. Protein adsorption using novel carboxymethyl-curdlan microspheres. Int. J. Biol. Macromol. 2016, 87, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Das, A.; Basu, A.; Ghosh, D.; Datta, P.; Mukherjee, A. Carboxymethyl guar gum synthesis in homogeneous phase and macroporous 3D scaffolds design for tissue engineering. Carbohydr. Polym. 2018, 191, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Badwaik, H.R.; Kumari, L.; Maiti, S.; Sakure, K.; Ajazuddin; Nakhate, K.T.; Tiwari, V.; Giri, T.K. A review on challenges and issues with carboxymethylation of natural gums: The widely used excipients for conventional and novel dosage forms. Int. J. Biol. Macromol. 2022, 209, 2197–2212. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Nath, N.; Nandi, G. Synthesis, characterization and fabrication of sodium carboxymethyl-okra-gum-grafted-polymethacrylamide into sustained release tablet matrix. Int. J. Biol. Macromol. 2020, 164, 3885–3900. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xia, B.; Branham, M.; Ha, W.; Wu, H.; Peng, S.L.; Ding, L.S.; Li, B.J.; Zhang, S. Self-assembly of carboxymethyl konjac glucomannan-g-poly(ethylene glycol) and (α-cyclodextrin) to biocompatible hollow nanospheres for glucose oxidase encapsulation. Carbohydr. Polym. 2011, 86, 120–126. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mohamed, N.A.; Mohamed, R.R.; Khalil, N.M.; Abd El Latif, S.M. Synthesis, characterization and antimicrobial activity of poly (N-vinyl imidazole) grafted carboxymethyl chitosan. Carbohydr. Polym. 2010, 79, 998–1005. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mohamed, N.A.; Mohamed, R.R.; Abd El Latif, S.M. Chemically induced graft copolymerization of 4-vinyl pyridine onto carboxymethyl chitosan. Polym. Bull. 2011, 67, 693–707. [Google Scholar] [CrossRef]
- Yadav, M.; Mishra, D.K.; Behari, K. Synthesis of partially hydrolyzed graft copolymer (H-partially carboxymethylated guar gum-g-methacrylic acid): A superabsorbing material. Carbohydr. Polym. 2011, 85, 29–36. [Google Scholar] [CrossRef]
- Tripathy, J.; Mishra, D.K.; Srivastava, A.; Mishra, M.M.; Behari, K. Synthesis of partially carboxymethylated guar gum-g-4-vinyl pyridine and study of its water swelling, metal ion sorption and flocculation behaviour. Carbohydr. Polym. 2008, 72, 462–472. [Google Scholar] [CrossRef]
- Elgamal, A.M.; Abu Elella, M.H.; Saad, G.R.; Abd El-Ghany, N.A. Synthesis, characterization and swelling behavior of high-performance antimicrobial biocompatible copolymer based on carboxymethyl xanthan. Mater. Today Commun. 2022, 33, 104209. [Google Scholar]
- Xia, B.; Ha, W.; Meng, X.W.; Govender, T.; Peng, S.L.; Ding, L.S.; Li, B.J.; Zhang, S. Preparation and characterization of a poly(ethylene glycol) grafted carboxymethyl konjac glucomannan copolymer. Carbohydr. Polym. 2010, 79, 648–654. [Google Scholar] [CrossRef]
- Gupta, N.R.; Ghute, P.P.; Badiger, M.V. Synthesis and characterization of thermo-sensitive graft copolymer of carboxymethyl guar and poly(N-isopropylacrylamide). Carbohydr. Polym. 2011, 83, 74–80. [Google Scholar] [CrossRef]
- Pal, S.; Ghorai, S.; Dash, M.K.; Ghosh, S.; Udayabhanu, G. Flocculation properties of polyacrylamide grafted carboxymethyl guar gum (CMG-g-PAM) synthesised by conventional and microwave assisted method. J. Hazard. Mater. 2011, 192, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Staros, J.V.; Wright, R.W.; Swingle, D.M. Enhancement by N-hydroxysulfosuccinimide of water-soluble carbodiimide-mediated coupling reactions. Anal. Biochem. 1986, 156, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Soliman, E.A.; Mansour, E.S.M.E.S.; Hassan, H.A.M.; Hassan, N.A.M. Optimization of Graft Polymerization and Performance of Carboxymethyl Chitosan/Polyacrylamide Flocculants. J. Res. Dev. Chem. 2014, 2014, 351498. [Google Scholar] [CrossRef]
- Badwaik, H.R.; Alexander, A.; Sakure, K. Understanding the Significance of Microwave Radiation for the Graft Copolymerization of Acrylamide on Carboxymethyl Xanthan Gum. Curr. Microw. Chem. 2019, 6, 13–22. [Google Scholar] [CrossRef]
- Sand, A.; Yadav, M.; Mishra, M.M.; Tripathy, J.; Behari, K. Studies on graft copolymerization of 2-acrylamidoglycolic acid on to partially carboxymethylated guar gum and physico-chemical properties. Carbohydr. Polym. 2011, 83, 14–21. [Google Scholar] [CrossRef]
- Shi, H.Y.; Zhang, L.M. New grafted polysaccharides based on O-carboxymethyl-O-hydroxypropyl guar gum and N-isopropylacrylamide: Synthesis and phase transition behavior in aqueous media. Carbohydr. Polym. 2007, 67, 337–342. [Google Scholar] [CrossRef]
- Gupta, N.R.; Arun Torris, A.T.; Wadgaonkar, P.P.; Rajamohanan, P.R.; Ducouret, G.; Hourdet, D.; Creton, C.; Badiger, M.V. Synthesis and characterization of PEPO grafted carboxymethyl guar and carboxymethyl tamarind as new thermo-associating polymers. Carbohydr. Polym. 2015, 117, 331–338. [Google Scholar] [CrossRef]
- Trivedi, J.H.; Thaker, M.D.; Trivedi, H.C. Photo-induced graft copolymerization of acrylonitrile onto sodium salt of partially carboxymethylated guar gum. J. Appl. Polym. Sci. 2015, 132, 41371. [Google Scholar] [CrossRef]
- Malik, R.; Warkar, S.G.; Saxena, R. Carboxy-methyl tamarind kernel gum based bio-hydrogel for sustainable agronomy. Mater. Today Commun. 2023, 35, 105473. [Google Scholar] [CrossRef]
- Ha, W.; Wu, H.; Wang, X.L.; Peng, S.L.; Ding, L.S.; Zhang, S.; Li, B.J. Self-aggregates of cholesterol-modified carboxymethyl konjac glucomannan conjugate: Preparation, characterization, and preliminary assessment as a carrier of etoposide. Carbohydr. Polym. 2011, 86, 513–519. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Saxena, S.K.; Sharma, S. Swelling behavior of barium ions-crosslinked bipolymeric sodium alginate—Carboxymethyl guar gum blend beads. J. React. Funct. Polym. 2006, 66, 659–666. [Google Scholar] [CrossRef]
- Mocanu, G.; Mihai, D.; LeCerf, D.; Picton, L.; Muller, G. Synthesis of new associative gel microspheres from carboxymethyl pullulan and their interactions with lysozyme. Eur. Polym. J. 2004, 40, 283–289. [Google Scholar] [CrossRef]
- Giri, T.K.; Thakur, D.; Ajazuddin; Badwaik, H.; Tripathi, D.K. Alginate based Hydrogel as a Potential Biopolymeric Carrier for Drug Delivery and Cell Delivery Systems: Present Status and Applications. Curr. Drug Deliv. 2012, 9, 539–555. [Google Scholar] [CrossRef]
- Pezron, E.; Leibler, L.; Lafuma, F. Complex Formation in Polymer-Ion Solutions. 2. Polyelectrolyte Effects. Macromolecules 1989, 22, 2656–2662. [Google Scholar] [CrossRef]
- Mali, K.K.; Ghorpade, V.S.; Dias, R.J.; Dhawale, S.C. Synthesis and characterization of citric acid crosslinked carboxymethyl tamarind gum-polyvinyl alcohol hydrogel films. Int. J. Biol. Macromol. 2023, 236, 123969. [Google Scholar] [CrossRef]
- More, M.P.; Bhamare, M.S.; Bhavsar, C.J.; Patil, P.O.; Deshmukh, P.K. Development of novel thiolated carboxymethyl-gellan gum as potential mucoadhesive polymer: Application of DoE. Adv. Mater. Sci. 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Prabaharan, M.; Gong, S. Novel thiolated carboxymethyl chitosan-g-β-cyclodextrin as mucoadhesive hydrophobic drug delivery carriers. Carbohydr. Polym. 2008, 73, 117–125. [Google Scholar] [CrossRef]
- Badwaik, H.; Giri, T.; Nakhate, K.; Kashyap, P.; Tripathi, K.D. Xanthan Gum and Its Derivatives as a Potential Bio-polymeric Carrier for Drug Delivery System. Curr. Drug Deliv. 2013, 10, 587–600. [Google Scholar] [CrossRef]
- Toti, U.S.; Aminabhavi, T.M. Modified guar gum matrix tablet for controlled release of diltiazem hydrochloride. J. Control. Release. 2004, 95, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Srivastava, A.; Srivastava, A.; Behari, K. Synthesis and characterization of xanthan gum-g-N-vinyl formamide with a potassium monopersulfate/Ag(I) system. J. Appl. Polym. Sci. 2006, 101, 1637–1645. [Google Scholar] [CrossRef]
- Trivedi, J.H.; Kalia, K.; Patel, N.K.; Trivedi, H.C. Ceric-induced grafting of acrylonitrile onto sodium salt of partially carboxymethylated guar gum. Carbohydr. Polym. 2005, 60, 117–125. [Google Scholar] [CrossRef]
- Kast, C.E.; Bernkop-Schnürch, A. Thiolated polymers—Thiomers: Development and in vitro evaluation of chitosan-thioglycolic acid conjugates. Biomaterials 2001, 22, 2345–2352. [Google Scholar] [CrossRef]
- Bamford, C.H.; Schofield, E. Non-classical free-radical polymerization: Degradative addition to monomer in the polymerization of 1-vinylimidazole. Polymer 1981, 22, 1227–1235. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Silva, D.A.; Feitosa, J.P.A.; Maciel, J.S.; Paula, H.C.B.; de Paula, R.C.M. Characterization of crosslinked cashew gum derivatives. Carbohydr. Polym. 2006, 66, 16–26. [Google Scholar] [CrossRef]
- Bachra, Y.; Grouli, A.; Damiri, F.; Talbi, M.; Berrada, M. A Novel Superabsorbent Polymer from Crosslinked Carboxymethyl Tragacanth Gum with Glutaraldehyde: Synthesis, Characterization, and Swelling Properties. Int. J. Biomater. 2021, 2021, 5008833. [Google Scholar] [CrossRef]
- Huang, G.Q.; Cheng, L.Y.; Xiao, J.X.; Wang, S.Q.; Han, X.N. Genipin-crosslinked O-carboxymethyl chitosan-gum Arabic coacervate as a pH-sensitive delivery system and microstructure characterization. J. Biomater. Appl. 2016, 31, 193–204. [Google Scholar] [CrossRef]
- Manickam, B.; Sreedharan, R.; Elumalai, M. ‘Genipin’—The Natural Water Soluble Cross-linking Agent and Its Importance in the Modified Drug Delivery Systems: An Overview. Curr. Drug Deliv. 2014, 11, 139–145. [Google Scholar] [CrossRef]
- Huang, G.Q.; Han, X.N.; Xiao, J.X. Glutaraldehyde-crosslinked O-carboxymethyl chitosan–gum Arabic coacervates: Characteristics versus complexation acidity. J. Dispers. Sci. Technol. 2017, 38, 1607–1612. [Google Scholar] [CrossRef]
- Yang, Y.; Anvari, M.; Pan, C.H.; Chung, D. Characterisation of interactions between fish gelatin and gum arabic in aqueous solutions. Food Chem. 2012, 135, 555–561. [Google Scholar] [CrossRef]
- Maity, S.; Sa, B. Ca-carboxymethyl xanthan gum mini-matrices: Swelling, erosion and their impact on drug release mechanism. Int. J. Biol. Macromol. 2014, 68, 78–85. [Google Scholar] [CrossRef]
- Patel, J.J.; Karve, M.; Patel, V.A.; Patel, N.K. Synthesis, characterization and application of borax cross-linked carboxymethyl guar gum. Chem. Pharm. Res. 2015, 7, 628–637. [Google Scholar]
- Lam, Y.L.; Muniyandy, S.; Kamaruddin, H.; Mansor, A.; Janarthanan, P. Radiation cross-linked carboxymethyl sago pulp hydrogels loaded with ciprofloxacin: Influence of irradiation on gel fraction, entrapped drug and in vitro release. Radiat. Phys. Chem. 2015, 106, 213–222. [Google Scholar] [CrossRef]
- Bao, D.; Chen, M.; Wang, H.; Wang, J.; Liu, C.; Sun, R. Preparation and characterization of double crosslinked hydrogel films from carboxymethylchitosan and carboxymethylcellulose. Carbohydr. Polym. 2014, 110, 113–120. [Google Scholar] [CrossRef]
- Mitra, S.; Maity, S.; Sa, B. Effect of different cross-linking methods and processing parameters on drug release from hydrogel beads. Int. J. Biol. Macromol. 2015, 74, 489–497. [Google Scholar] [CrossRef]
- Muniyandy, S.; Sathasivam, T.; Veeramachineni, A.K.; Janarthanan, P. Dual cross-linked carboxymethyl sago pulp-gelatine complex coacervates for sustained drug delivery. Polymers 2015, 7, 1088–1105. [Google Scholar] [CrossRef]
- Jana, P.; Sarkar, K.; Mitra, T.; Chatterjee, A.; Gnanamani, A.; Chakraborti, G.; Kundu, P.P. Synthesis of a carboxymethylated guar gum grafted polyethyleneimine copolymer as an efficient gene delivery vehicle. RSC Adv. 2016, 6, 13730–13741. [Google Scholar] [CrossRef]
- Pinilla-Torres, A.M.; Carrión-García, P.Y.; Sánchez-Domínguez, C.N.; Gallardo-Blanco, H.; Sánchez-Domínguez, M. Modification of branched polyethyleneimine using mesquite gum for its improved hemocompatibility. Polymers 2021, 13, 2766. [Google Scholar] [CrossRef]
- Lu, S.; Li, H.; Zhang, F.; Du, N.; Hou, W. Sorption of Pb(II) on carboxymethyl chitosan-conjugated magnetite nanoparticles: Application of sorbent dosage-dependent isotherms. Colloid Polym. Sci. 2016, 294, 1369–1379. [Google Scholar] [CrossRef]
- Minkal; Ahuja, M.; Bhatt, D.C. Polyelectrolyte complex of carboxymethyl gum katira-chitosan: Preparation and characterization. Int. J. Biol. Macromol. 2018, 106, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Maciel, J.S.; Silva, D.A.; Paula, H.C.B.; De Paula, R.C.M. Chitosan/carboxymethyl cashew gum polyelectrolyte complex: Synthesis and thermal stability. Polymer 2005, 41, 2726–2733. [Google Scholar] [CrossRef]
- Du, J.; Sun, R.; Zhang, S.; Zhang, L.F.; Xiong, C.D.; Peng, Y.X. Novel polyelectrolyte carboxymethyl konjac glucomannan-chitosan nanoparticles for drug delivery. I. Physicochemical characterization of the carboxymethyl konjac glucomannan-chitosan nanoparticles. Biopolymers 2005, 78, 1–8. [Google Scholar] [CrossRef]
- Kaur, G.; Jain, S.; Tiwary, A.K. Chitosan-carboxymethyl tamarind kernel powder interpolymer complexation: Investigations for colon drug delivery. Sci. Pharm. 2010, 78, 57–78. [Google Scholar] [CrossRef]
- Bera, H.; Mothe, S.; Maiti, S.; Vanga, S. Composite beads for glimepiride delivery. Int. J. Biol. Macromol. 2018, 107, 604–614. [Google Scholar] [CrossRef]
- Kumar, V.; Tiwary, A.K.; Kaur, G. Investigations on chitosan-carboxymethyl guar gum complexes interpolymer complexes for colon delivery of fluticasone. Int. J. Drug Deliv. 2010, 2, 242–250. [Google Scholar] [CrossRef]
- Kumar, A.; Ahuja, M. Carboxymethyl gum kondagogu-chitosan polyelectrolyte complex nanoparticles: Preparation and characterization. Int. J. Biol. Macromol. 2013, 62, 80–84. [Google Scholar] [CrossRef]
- Alamdarnejad, G.; Sharif, A.; Taranejoo, S.; Janmaleki, M.; Kalaee, M.R.; Dadgar, M.; Khakpour, M. Synthesis and characterization of thiolated carboxymethyl chitosan-graft-cyclodextrin nanoparticles as a drug delivery vehicle for albendazole. J. Mater. Sci. Mater. Med. 2013, 24, 1939–1949. [Google Scholar] [CrossRef]
- Chen, N.; Zhu, P.; Du, T.; Han, K.; Wang, D.; Ye, J.; Xiao, S.; Ye, X.; Wang, Y. Preparation of Modified Konjac Glucomannan Nanoparticles and their Application as Vaccine Adjuvants to Promote Ovalbumin-Induced Immune Response in Mice. Pharm. Res. 2018, 35, 105. [Google Scholar] [CrossRef]
- Shi, C.; Zhu, P.; Chen, N.; Ye, X.; Wang, Y.; Xiao, S. Preparation and sustainable release of modified konjac glucomannan/chitosan nanospheres. Int. J. Biol. Macromol. 2016, 91, 609–614. [Google Scholar] [CrossRef]
- Mocanu, G.; Mihai, D. Microparticles Crosslinked with Siloxane: Interactions with Biologically Active substances. J. Bioact. Compat. Polym. 2008, 23, 82–94. [Google Scholar] [CrossRef]
- Singh, R.; Maity, S.; Sa, B. Effect of ionic crosslink on the release of metronidazole from partially carboxymethylated guar gum tablet. Carbohydr. Polym. 2014, 106, 414–421. [Google Scholar] [CrossRef]
- Badwaik, H.R.; Sakure, K.; Alexander, A.; Ajazuddin; Dhongade, H.; Tripathi, D.K. Synthesis and characterisation of poly(acryalamide) grafted carboxymethyl xanthan gum copolymer. Int. J. Biol. Macromol. 2016, 85, 361–369. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Sharma, S. Investigation of pH-sensitive swelling and drug release behavior of barium alginate/carboxymethyl guar gum hydrogel beads. J. Macromol. Sci. Part A Pure Appl. Chem. 2006, 43, 1513–1521. [Google Scholar] [CrossRef]
- Giri, A.; Bhowmick, M.; Pal, S.; Bandyopadhyay, A. Polymer hydrogel from carboxymethyl guar gum and carbon nanotube for sustained trans-dermal release of diclofenac sodium. Int. J. Biol. Macromol. 2011, 49, 885–893. [Google Scholar] [CrossRef]
- Rani, I.; Warkar, S.G.; Kumar, A. Nano ZnO embedded poly (ethylene glycol) diacrylate cross-linked carboxymethyl tamarind kernel gum (CMTKG)/poly (sodium acrylate) composite hydrogels for oral delivery of ciprofloxacin drug and their antibacterial properties. Mater. Today Commun. 2023, 35, 105635. [Google Scholar] [CrossRef]
- Maestrelli, F.; Cirri, M.; Corti, G.; Mennini, N.; Mura, P. Development of enteric-coated calcium pectinate microspheres intended for colonic drug delivery. Eur. J. Pharm. Biopharm. 2008, 69, 508–518. [Google Scholar] [CrossRef]
- Duchene, D.; Touchard, F.; Peppas, N.A. Pharmaceutical and Medical Aspects of Bioadhesive Systems for Drug Administration. Drug Dev. Ind. Pharm. 1988, 14, 283–318. [Google Scholar] [CrossRef]
- Giri, A.; Bhunia, T.; Mishra, S.R.; Goswami, L.; Panda, A.B.; Bandyopadhyay, A. A transdermal device from 2-hydroxyethyl methacrylate grafted carboxymethyl guar gum-multi-walled carbon nanotube composites. RSC Adv. 2014, 4, 13546–13556. [Google Scholar] [CrossRef]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Lu, H.; Dai, Y.; Lv, L.; Zhao, H. Chitosan-graft-polyethylenimine/DNA nanoparticles as novel non-viral gene delivery vectors targeting osteoarthritis. PLoS ONE 2014, 9, e84703. [Google Scholar] [CrossRef] [PubMed]
- Grandinetti, G.; Ingle, N.P.; Reineke, T.M. Interaction of poly(ethylenimine)-DNA polyplexes with mitochondria: Implications for a mechanism of cytotoxicity. Mol. Pharm. 2011, 8, 1709–1719. [Google Scholar] [CrossRef] [PubMed]
- Roacho-p, J.A.; Rodr, K.O.; Gallardo-blanco, H.L.; Velazco-campos, M.R.; Sosa-cruz, K.V.; Garc, P.E.; Rojas-patl, L.; Margarita, S.; Rivas-estilla, A.M.; Chapa-gonzalez, V.G.C.; et al. A Full Set of In Vitro Assays in Chitosan/Tween 80 Microspheres Loaded with Magnetite Nanoparticles. Polymers 2021, 13, 400. [Google Scholar] [CrossRef]
- Ahuja, M.; Kumar, A.; Singh, K. Synthesis, characterization and in vitro release behavior of carboxymethyl xanthan. Int. J. Biol. Macromol. 2012, 51, 1086–1090. [Google Scholar] [CrossRef]
- Rahman, M.M.; Roy, S.; Das, S.C.; Jha, M.K.; Ahsan, M.Q.; Shahparan, M.; Reza, M.S. Formulation and evaluation of hydroxypropylmethylcellulose based matrix systems as oral sustained release drug delivery systems for ciprofloxacin hydrochloride. Int. J. Pharm. Sci. Rev. Res. 2011, 6, 34–41. [Google Scholar]
- Orsu, P.; Koyyada, A.; Naidu, K.L.; Yadav, S. Nanofibers of carboxymethyl tamarind gum / reduced graphene oxide composite for neuronal cell proliferation. J. Drug Deliv. Sci. Technol. 2021, 66, 1–6. [Google Scholar] [CrossRef]
- Chen, F.; Yu, S.; Liu, B.; Ni, Y.; Yu, C.; Su, Y.; Zhu, X.; Yu, X.; Zhou, Y.; Yan, D. An Injectable Enzymatically Crosslinked Carboxymethylated Pullulan/Chondroitin Sulfate Hydrogel for Cartilage Tissue Engineering. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).