Improved Solubility and Activity of Natural Product in Nanohydrogel
Abstract
:1. Introduction
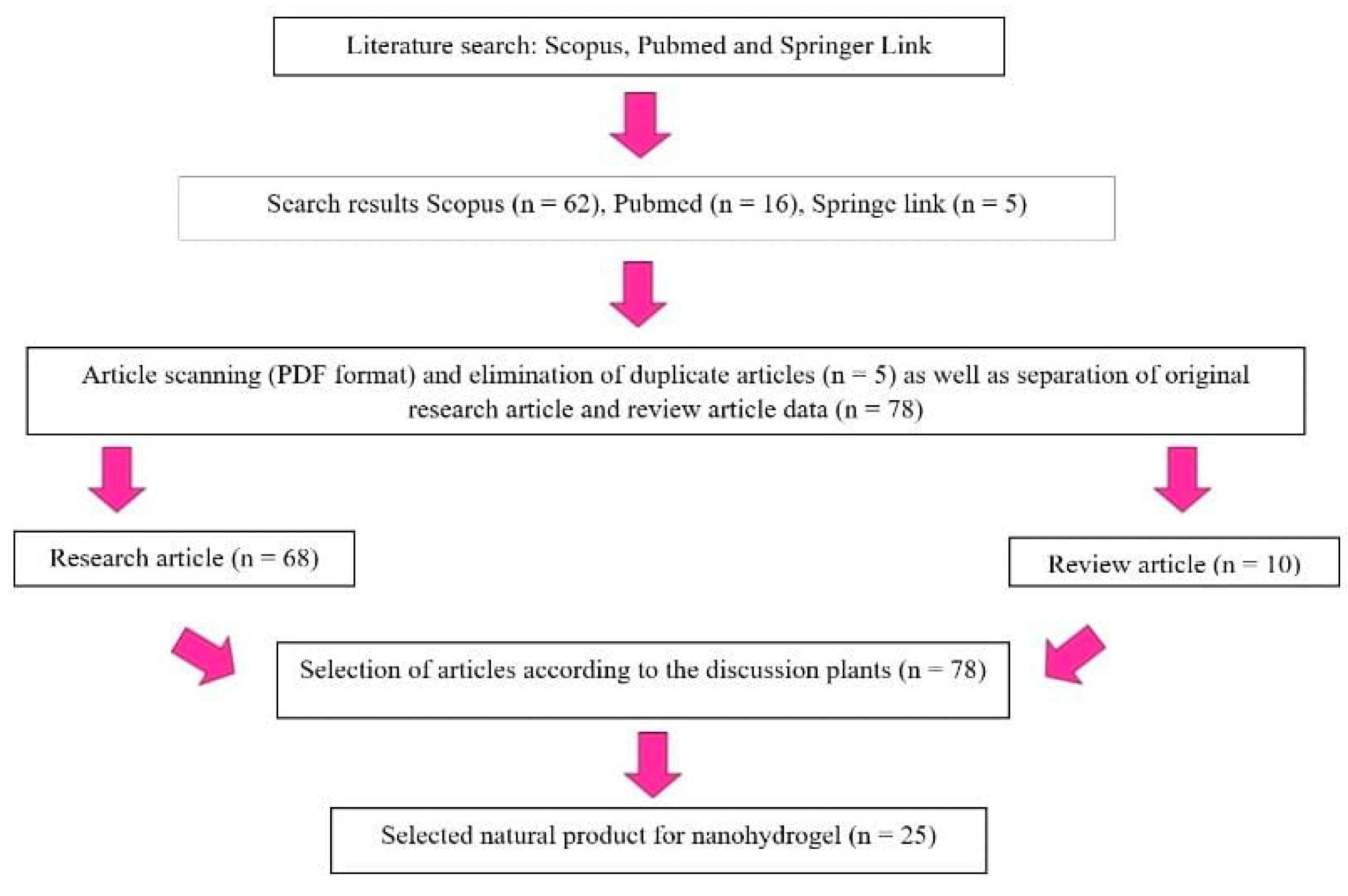
2. Methods
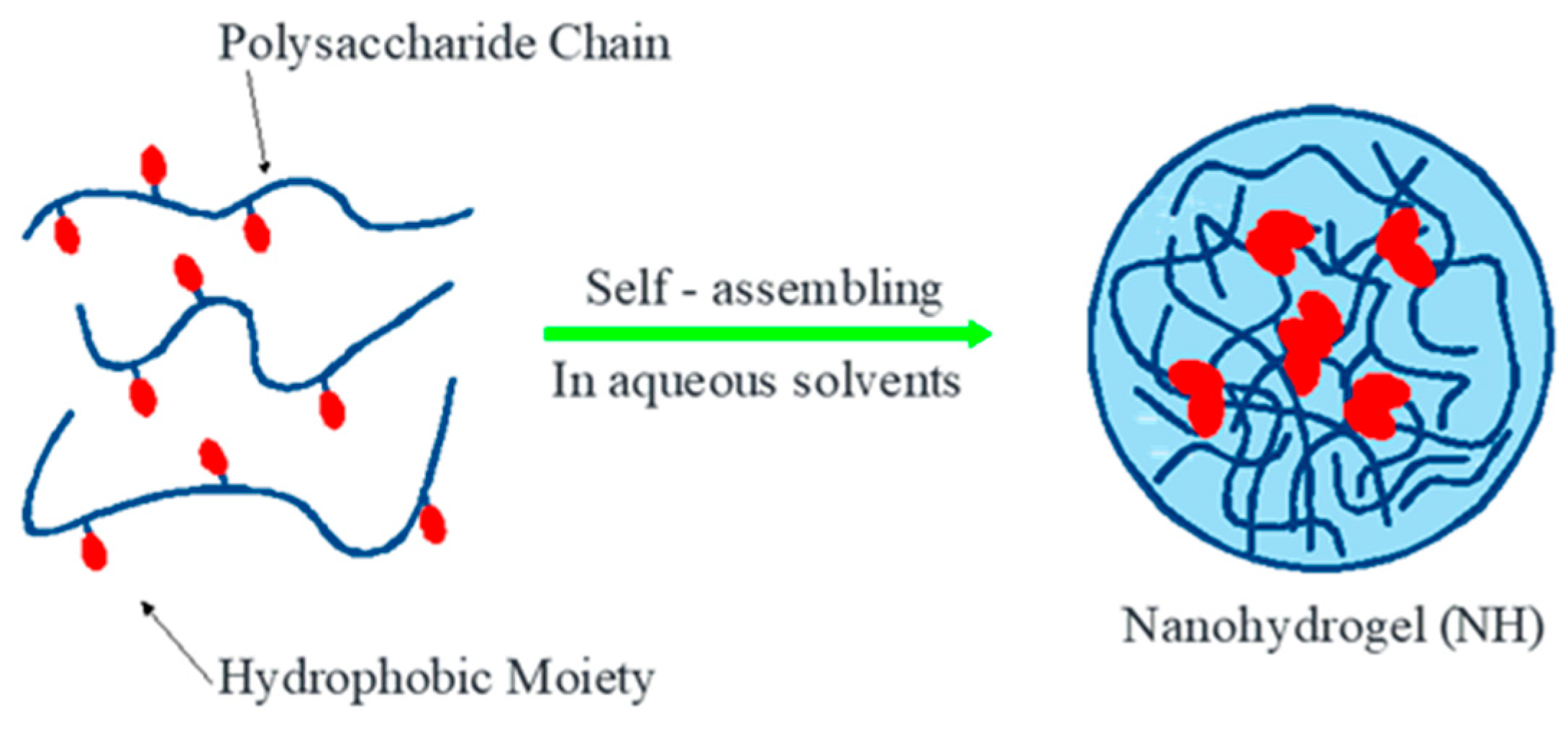
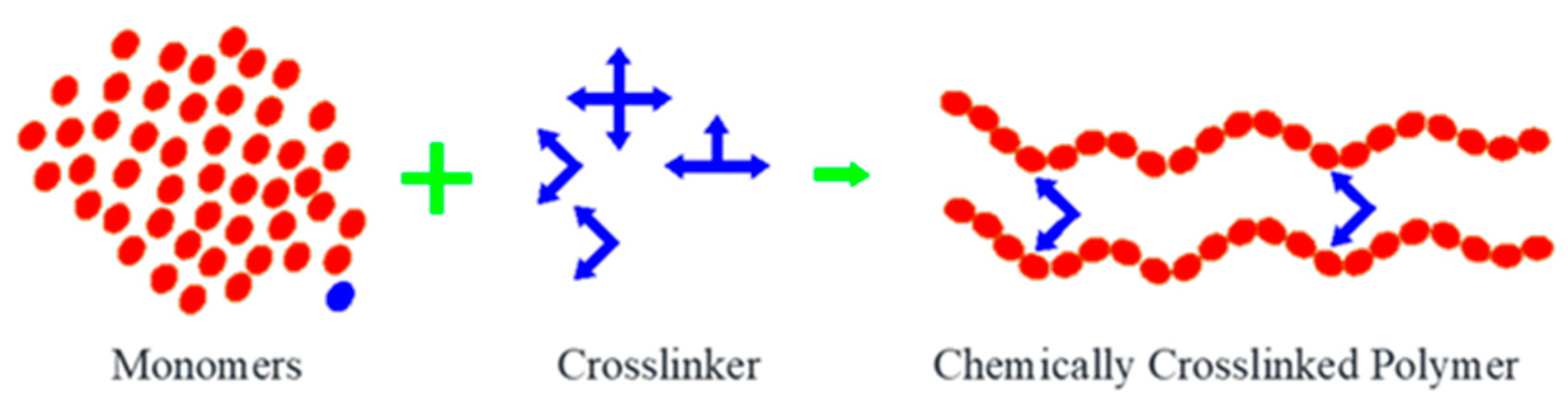
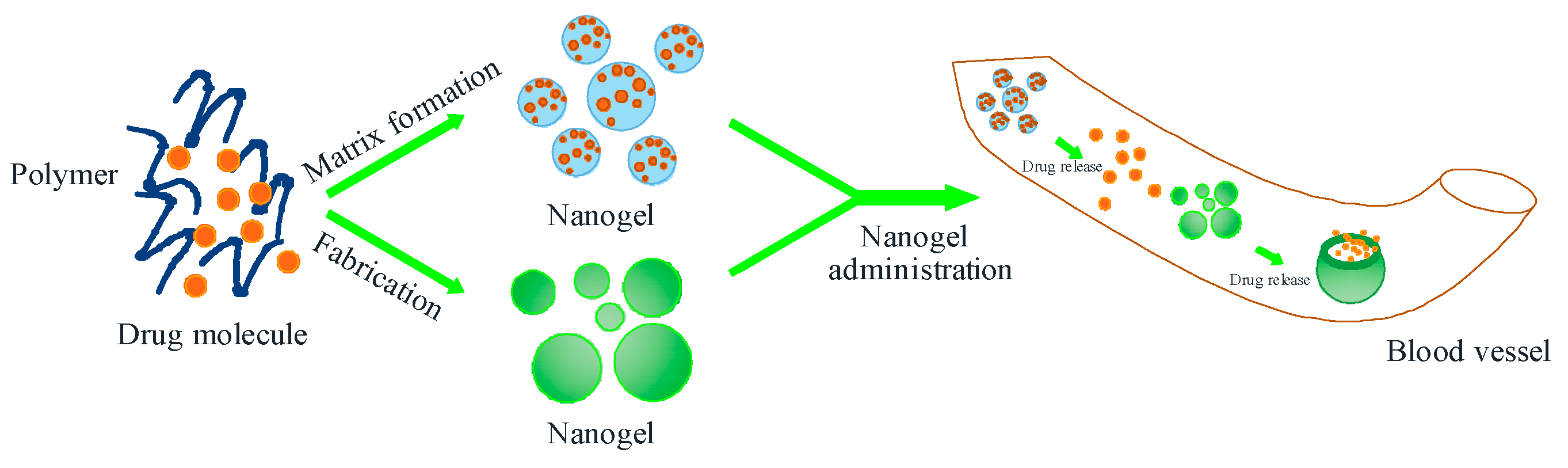
3. Nanohydrogel
4. Nanohydrogel-Based Drug Delivery System for Natural Product Compounds
4.1. Nanohydrogel Natural Ingredients Increase the Activity of Compounds
4.2. Nanohydrogel Natural Ingredients Increase the Solubility of Compounds
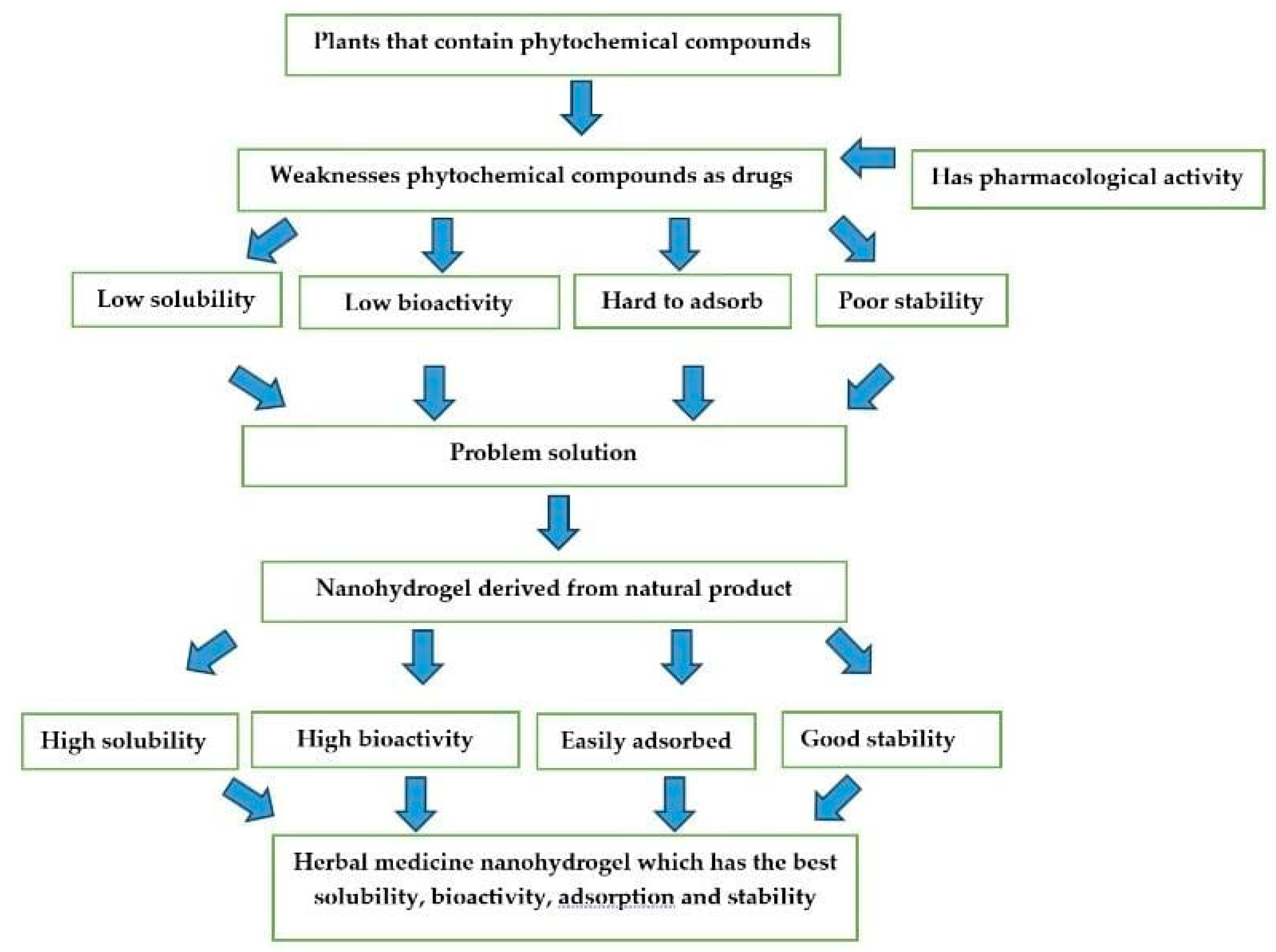
5. The Challenge of Nanohydrogel Formulations Comes from Natural Ingredients
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kishore, N.; Kumar, P.; Shanker, K.; Verma, A.K. Human disorders associated with inflammation and the evolving role of natural products to overcome. Eur. J. Med. Chem. 2019, 179, 272–309. [Google Scholar] [CrossRef]
- Sharma, K.; Mishra, V.; Ranjan, K.; Yadav, N.; Sharma, M. A Methodological Approach of Plant Essential Oils and their Isolated Bioactive Components for Antiviral Activities. In Essential Oils: Extraction Methods and Applications; Wiley: Hoboken, NJ, USA, 2023; pp. 1–29. [Google Scholar]
- Luo, H.; Vong, C.T.; Chen, H. Naturally occurring anti- cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar]
- WHO. Progress Report by the Director General; Report No.:444/20-22; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
- Sumaiya, S.; Siddiqui, A.; Chaudhary, S.; Aslam, M.; Ahmad, S.; Ansari, M. Isolation and characterization of bioactive components from hydroalcoholic extract of Cymbopogon jwarancusa (Jones) Schult. to evaluate its hepatoprotective activity. J. Ethnopharmacol. 2024, 31930, 117185. [Google Scholar] [CrossRef]
- Chavda, V.P.; Ertas, Y.N.; Walhekar, V.; Modh, D.; Doshi, A.; Shah, N. Advanced Computational Methodologies Used in the Discovery of New Natural Anticancer Compounds. Front. Pharmacol. 2021, 12, 702611. [Google Scholar] [CrossRef]
- Ouoba, K.; Lehmann, H.; Zongo, A.; Semde, R.; Pabst, J. Phytopharmaceutical practices of traditional health practitioners in Burkina Faso: A cross-sectional study. BMC Complement. Med. Ther. 2023, 23, 215. [Google Scholar] [CrossRef]
- Purwitasari, N.; Siswodiharjo, S.; Alhoot, M.; Agi, M. Pharmacological Potential of Some Indonesian Medicinal Plants as Promising Options for COVID-19 During the Pandemic Era: A Literature Review. J. Med. Chem. Sci. 2023, 6, 2735–2749. [Google Scholar]
- Muhaimin, M.; Chaerunisaa, A.Y.; Rostinawati, T.; Amalia, E.; Hazrina, A.; Nurhasanah, S. A review on Nanoparticles of Moringa oleifera Extract: Preparation, Characterization, and Activity. Int. J. App Pharm. 2023, 15, 43–51. [Google Scholar] [CrossRef]
- Hussein, R.A.; El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. In Herbal Medicine; IntechOpen: London, UK, 2019; pp. 1–21. [Google Scholar]
- Mendoza, N.; Silva, E.M.E. Introduction to Phytochemicals: Secondary Metabolites from Plants with Active Principles for Pharmacological Importance. In Phytochemicals-Source of Antioxidants and Role in Disease Prevention; InTech: London, UK, 2018; pp. 1–24. [Google Scholar]
- Engelhardt, L.; Pohnl, T.; Alhussein, M.; John, M.; Neugart, S. Changes in bioactive compounds and antioxidant activity of three Amaranthus L. genotypes from a model to household processing. Food Chem. 2023, 42915, 136891. [Google Scholar] [CrossRef]
- Dini, I.; Laneri, S. The new challenge of green cosmetics: Natural food ingredients for cosmetic formulations. Molecules 2021, 26, 3921. [Google Scholar] [CrossRef]
- Xiaowei, C.; Wei, W.; Hong, G.; Hui, C.; Xiaofei, Z.; Haonan, W.; Yumeng, W.; Xuelan, Z.; Chunchao, H. Review of Polygonatum sibiricum: A new natural cosmetic ingredient. Pharmazie 2019, 74, 513–519. [Google Scholar]
- Lestari, U.; Muhaimin, M.; Chaerunisaa, A.Y.; Sujarwo, W. Anti-Aging Potential of Plants of the Anak Dalam Tribe, Jambi, Indonesia. Pharmaceuticals 2023, 16, 1300. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Chatterjee, N.; Chakraborty, A.; Banerjee, A.; Haiti, S.; Datta, S.; Chattopadhyay, H.; Dhar, P. Fabrication of rice bran oil nanoemulsion and conventional emulsion with Mustard Protein Isolate as a novel excipient: Focus on shelf-life stability, lipid digestibility and cellular bioavailability. Food Hydrocoll. Health 2023, 4, 100143. [Google Scholar] [CrossRef]
- Maleki, D.; Alipour, M.; Dalir, A.; Ahmadian, E.; Eftekhari, A.; Forouhandeh, A.; Rahbar, S.; Syarifi, S.; Zununi, V. Curcumin nanoformulations: Beneficial nanomedicine against cancer. Phytother. Res. 2022, 36, 1156–1181. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, M.; Wang, D.; Mujumdar, A.; Deng, D. Development of quinoa (Chenopodium quinoa Willd) protein isolate-gum Arabic conjugates via ultrasound-assisted wet heating for spice essential oils emulsification: Effects on water solubility, bioactivity, and sensory stimulation. Food Chem. 2024, 431, 137001. [Google Scholar] [CrossRef] [PubMed]
- Jiaxing, C.; Zhaosong, S.; Tianzhu, Y.; Jia, Z.; Bo, Y.; Xiali, L. Encapsulation of hyperoside with acyclic cucurbiturils: Supramolecular binding behavior, water solubility and in vitro antioxidant activity. J. Mol. Struct. 2023, 1294, 136342. [Google Scholar]
- Arpa, M.; Okur, N.; Gok, M.; Ozgumus, S.; Cevher, E. Chitosan-based buccal mucoadhesive patches to enhance the systemic bioavailability of tizanidine. Int. J. Pharm. 2023, 642, 123168. [Google Scholar] [CrossRef]
- Li, J.; Huang, G.; Qian, H.; Pi, F. Fabrication of soy protein isolate—High methoxyl pectin composite emulsions for improving the stability and bioavailability of carotenoids. Food Biosci. 2023, 53, 102738. [Google Scholar] [CrossRef]
- Wang, L.; Mao, J.; Zhou, Q.; Deng, Q.; Zheng, L.; Sji, J. A curcumin oral delivery system based on sodium caseinate and carboxymethylpachymaran nanocomposites. Int. J. Biol. Macromol. 2023, 253, 126698. [Google Scholar] [CrossRef]
- Quintriqueo-Cid, A.; Gimenez, B.; Romero-Hasler, P.; Soto-Bustamante, E.; Lozano-Sanchez, P.; Robert, P. Influence of the crystallinity on the physicochemical properties of spray-dried quercetin-inulin microparticles and their performance during in vitro digestion. Food Chem. 2024, 434, 137325. [Google Scholar] [CrossRef]
- Eid, R.; Zaki, M.; Al-Shraim, M.; Eldeen, M.; Massoud, E.; Shati, A.; Kamar, S.; Hadara, M. Silymarin’s defensive role against hepatotoxicity induced by amiodarone in albino rats. Int. J. Morphol. 2021, 39, 407–415. [Google Scholar] [CrossRef]
- Prabhakar, O. Naringenin attenuates cerebral ischemia-reperfusion injury through inhibiting oxidative stress and inflammation in diabetic rats. Res. J. Pharm. Technol. 2021, 14, 3751–3756. [Google Scholar] [CrossRef]
- Yimaz, A.; Ozturk, S.; Salih, B.; Ayyala, R.; Sahiner, N. ESI-IM-MS characterization of cyclodextrin complexes and their chemically cross-linked alpha (α-), beta (β-) and gamma (γ-) cyclodextrin particles as promising drug delivery materials with improved bioavailability. Colloids Surf. B Biointerfaces 2023, 230, 113522. [Google Scholar]
- Hu, G.; Batool, Z.; Cai, Z.; Liu, Y.; Ma, M.; Sheng, L.; Jin, Y. Production of self-assembling acylated ovalbumin nanogels as stable delivery vehicles for curcumin. Food Chem. 2021, 355, 129635. [Google Scholar]
- Thakur, S.; Bains, A.; Sridhar, K.; Khausik, R.; Gupta, V.; Chawla, P.; Sharma, M. Gum arabic/guar gum based biopolymeric nanohydrogel for shelf-life enhancement of grapes and photocatalytic dye reduction. Ind. Crops Prod. 2023, 2031, 117114. [Google Scholar]
- Keskin, D.; Zu, G.; Forson, A.; Tromp, L.; Sjollema, J.; Van-Rijn, P. Nanogels: A novel approach in antimicrobial delivery systems and antimicrobial coatings. Bioact. Mater. 2021, 6, 3634–3657. [Google Scholar]
- Chung, F.; Huang, C.; Chen, C.; Chen, Y. Natural nanogels crosslinked with S-benzyl-L-cysteine exhibit potent antibacterial activity. Biomater. Adv. 2023, 153, 213551. [Google Scholar]
- Kim, H.; Kim, S.; Kang, J.; Shin, U. Positively and Negatively Charged Collagen Nanohydrogels: pH-responsive Drug-releasing Characteristics. Bull. Korean Chem. Soc. 2018, 39, 477–482. [Google Scholar]
- Gadhave, D.; Rasal, N.; Sonawane, R.; Sekar, M.; Kokare, C. Nose-to-brain delivery of teriflunomide-loaded lipid-based carbopol-gellan gum nanogel for glioma: Pharmacological and in vitro cytotoxicity studies. Int. J. Biol. Macromol. 2021, 167, 906–920. [Google Scholar]
- Sourour, I.; Takwa, B.; Yousef, M.; Nashiru, B. Curcumin and Derivatives in Nanoformulations with Therapeutic Potential on Colorectal Cancer. AAPS PharmSciTech. 2022, 23, 115. [Google Scholar]
- Subhash, C.; Kulkarni, G.T.; Neerupma, D.; Harsha, K. Protein-Based Nanohydrogels for Bioactive Delivery. Front. Chem. 2021, 9, 573748. [Google Scholar]
- Saini, S.; Nanda, S.; Dhiman, A. Chitosan Nanoparticles: An Approbative System for the Delivery of Herbal Bioactives. Nat. Prod. J. 2022, 12, e160921188333. [Google Scholar] [CrossRef]
- Kaur, N.; Bains, A.; Khausik, R.; Dhull, S.; Melinda, F.; Chawula, P. A review on antifungal efficiency of plant extracts entrenched polysaccharide-based nanohydrogels. Nutrients 2021, 13, 2055. [Google Scholar] [PubMed]
- Yapar, E.A. Herbal cosmetics and novel drug delivery systems. Indian. J. Pharm. Educ. Res. 2017, 51, 3. [Google Scholar] [CrossRef]
- Desu, P.K.; Karmakar, B.; Kondi, V.; Tiwari, O.N.; Halder, G. Optimizing formulation of green tea extract-loaded chitosan nanogel. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Gupta, R.; Wadhera, G.; Kumar, P. Modern Herbal Nanogels: Formulation, Delivery Methods, and Applications. Gels 2022, 8, 97. [Google Scholar] [CrossRef]
- Parhi, R.; Sahoo, S.; Das, A. Applications of polysaccharides in topical and transdermal drug delivery: A recent update of literature. Braz. J. Pharm. Sci. 2022, 58, e20802. [Google Scholar]
- Baloch, H.; Siddiqua, A.; Nawaz, A.; Latif, M.S.; Zahra, S.Q.; Alomar, S.Y.; Ahmad, N.; Elsayed, T.M. Synthesis and Characterization of Sulfur Nanoparticles of Citrus limon Extract Embedded in Nanohydrogel Formulation: In Vitro and In Vivo Studies. Gels 2023, 9, 284. [Google Scholar] [CrossRef]
- Sandhiya, V.; Ubaidulla, U. A Review on Herbal Drug Loaded into Pharmaceutical Carrier Techniques and Its Evaluation Process. Futur. J. Pharm. Sci. 2020, 6, 51. [Google Scholar]
- Paroha, S.; Dewangan, R.P.; Sahoo, P.K. Pharmaceutical Technology for Improving the Bioavailability of Natural Products. In Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2020; Volume 43. [Google Scholar]
- Lv, S.; Zhang, S.; Zuo, J.; Liang, S.; Yang, J.; Wang, J.; Wei, D. Progress in preparation and properties of chitosan-based hydrogels. Int. J. Biol. Macromol. 2023, 242, 124915. [Google Scholar]
- Wang, X.N.; Wang, Z.J.; Zhao, Y.; Wang, H.; Xiang, M.L.; Liu, Y.Y.; Zhao, L.X.; Luo, X.D. Antifungal alkaloids from Mahonia fortunei against pathogens of postharvest fruit. Nat. Prod. Bioprospect. 2023, 13, 10. [Google Scholar]
- Ho, S.-H.N.; Trinh, N.-T.; Nguyen, T.-H.T.; Le, K.M.; Van Nguyen, T.-D.; Tran, C.T.; Van Vo, T.; Vong, L.B. Silica–Containing Redox Nanoparticles Improve the Antioxidant Activity of Curcumin and its Gastrointestinal Biodistribution Evaluation. Biointerface Res. Appl. Chem. 2023, 13, 566. [Google Scholar]
- Ratan, C.; Arian, A.; Rajendran, R.; Jayakumar, R.; Masson, M.; Mangalatillam, S. Nano-based formulations of curcumin: Elucidating the potential benefits and future prospects in skin cancer. Biomed. Mater. 2023, 18, 052008. [Google Scholar]
- Quazi, M.Z.; Park, N. Nanohydrogels: Advanced Polymeric Nanomaterials in the Era of Nanotechnology for Robust Functionalization and Cumulative Applications. Int. J. Mol. Sci. 2022, 23, 1943. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhou, M.; Wang, H.; Guo, Y.; Cheng, X.; Zhou, T.; Wang, H.; Zhang, Y.; Ma, Y.; Tao, W. Exosome-sheathed ROS-responsive nanogel to improve targeted therapy in perimenopausal depression. J. Nanobiotechnol. 2023, 21, 261. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Ganeshpurkar, A.; Shrotriya, S.; Sawant, P.; Mulgund, S. Small size silver nanoparticles loaded with glycoside rich portion of Boerhavia Diffusa Linn. promotes wound healing: In-silico and in-vivo studies. Colloids Surf. B Biointerfaces 2023, 230, 113483. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Ahmad, I.; Irfan, S.; Ahmad, M.; Usmani, S.; Soaib, A.; Ahmad, W. Hydrogel: An Encouraging Nanocarrier System for the Delivery of Herbal Bioactive Compounds. Curr. Nanosci. 2021, 17, 797–807. [Google Scholar] [CrossRef]
- Rajurkar, A.; Gogri, D.; Jamdade, N.; Pathak, A. Green Synthesis of Silver Nanoparticles: Their Characterization, Antimicrobial, Antioxidant Activity and Nanogel Formulation. Nano Biomed. Eng. 2023, 15, 42–50. [Google Scholar] [CrossRef]
- Kuo, H.; Tong, S.; Chao, M.; Tseng, C. Ganoderma tsugae prevents cognitive impairment and attenuates oxidative damage in d-galactose-induced aging in the rat brain. PLoS ONE 2022, 17, 0266331. [Google Scholar] [CrossRef]
- Eryaman, Z.; Hizal, J.; Yilmazoglu, L.; Daban, U.; Mert, H.; Kanmaz, N. The performance of hypochlorous acid modified Ag nanoparticle-based assay in the determination of total antioxidant capacity of Boswellia Serrata and Aronia. Talanta 2024, 267, 125218. [Google Scholar] [CrossRef]
- Peng, L.; Liu, S.; Xu, S.; Chen, L.; Shan, Y.; Wei, W.; Liang, W.; Gao, J. Inhibitory effects of salidroside and paeonol on tyrosinase activity and melanin synthesis in mouse B16F10 melanoma cells and ultraviolet B-induced pigmentation in guinea pig skin. Phytomedicine 2013, 20, 1082–1087. [Google Scholar] [CrossRef]
- Esmaeili, A.; Behzadi, S. Performance comparison of two herbal and industrial medicines using nanoparticles with a starch/cellulose shell and alginate core for drug delivery: In vitro studies. Colloids Surf. B Biointerfaces 2017, 158, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Takke, A.; Shende, P. Nanotherapeutic silibinin: An insight of phytomedicine in healthcare reformation, Nanomedicine: Nanotechnology. Biol. Med. 2019, 21, 102057. [Google Scholar]
- Atarian, M.; Rajaei, A.; Tabatabaei, M.; Mohsenifar, A.; Bodaghi, H. Formulation of Pickering sunflower oil-in-water emulsion stabilized by chitosan-stearic acid nanogel and studying its oxidative stability. Carbohydr. Polym. 2019, 210, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Koliada, A.; Zayachkivska, A.; Lushchak, O. Nanodelivery of Natural Antioxidants: An Anti-aging Perspective. Front. Bioeng. Biotechnol. 2020, 7, 447. [Google Scholar] [CrossRef]
- Esentürk-Güzel, İ.; Topuzoğlu, S.; Abdo, L.; Gürer, E.S.; Algin Yapar, E. Ammi visnaga L. and Nanocarrier Approaches in the Treatment of Skin Diseases. J. Res. Pharm. 2022, 26, 820–827. [Google Scholar]
- Chaerunisaa, A.Y.; Susilawati, Y.; Muhaimin, M.; Milanda, T.; Hendriani, R.; Subarnas, A. Antibacterial activity and subchronic toxicity of Cassia fistula L. Barks in rats. Toxicol. Rep. 2020, 7, 649–657. [Google Scholar] [CrossRef]
- Zhan, J.; He, F.; Chen, S.; Poudel, A.; Yang, Y.; Xiao, L.; Xiang, F.; Li, S. Preparation and antibacterial activity of thermo-responsive nanohydrogels from qiai essential oil and pluronic F108. Molecules 2021, 26, 5771. [Google Scholar] [CrossRef]
- Ashouri, F.; Beyranvand, F.; Beigi, B.; Tavafi, M.; Sheikhian, A.; Varzi, A.; Shahrokhi, S. Macrophage polarization in wound healing: Role of aloe vera/chitosan nanohydrogel. Drug Deliv. Transl. Res. 2019, 9, 1027–1042. [Google Scholar] [CrossRef]
- Mohamad, S.; Abdullah, S.; Alhakamy, N.; Shaik, R.; Ansari, A.; Riadi, Y.; Ahmad, J.; Ali, R.; Gorain, B.; Karim, S. Sustained-release ginseng/sodium alginate nano hydrogel formulation, characterization, and in vivo assessment to facilitate wound healing. J. Drug Deliv. Sci. Technol. 2022, 74, 03565. [Google Scholar]
- Tong, Y.; Zhang, Y.; Quarcoo, F.; Miao, C.; Xiao, X.; Ju, X.; Li, W. Polyphenol zwitterionic nanohydrogel modified polyvinylidene difluoride membrane for separating oil-in-water emulsions with superior cycle stability. J. Environ. Chem. Eng. 2023, 11, 110811. [Google Scholar] [CrossRef]
- Fathi, R.; Mohammadi, R. Preparation of pH-responsive magnetic nanocomposite hydrogels based on k-carrageenan/chitosan/silver nanoparticles: Antibacterial carrier for potential targeted anticancer drug delivery. Int. J. Biol. Macromol. 2023, 246, 125546. [Google Scholar] [CrossRef] [PubMed]
- Bains, A.; Sharma, P.; Kaur, S.; Yadav, R.; Kumar, A.; Sridhar, K.; Chawla, P.; Sharma, M. Gum arabic/guar gum stabilized Hydnocarpus wightiana oil nanohydrogel: Characterization, antimicrobial, anti-inflammatory, and anti-biofilm activities. Int. J. Biol. Macromol. 2023, 239, 124341. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Hou, Y.; Zue, Z.; Bai, L.; Wang, W.; Chen, H.; Yang, H.; Yang, L.; Wei, D. Soy Protein Isolate/Genipin-Based Nanoparticles for the Stabilization of Pickering Emulsion to Design Self-Healing Guar Gum-Based Hydrogels. Biomacromolecules 2023, 24, 2087–2099. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.; Elneklawi, M.; Mohammad, E. Aloe Vera coated Dextran Sulfate/Chitosan nanoparticles (Aloe Vera @ DS/CS) encapsulating Eucalyptus essential oil with antibacterial potent property. J. Biomater. Sci. Polym. Ed. 2023, 34, 810–827. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, P.; Bains, A.; Chawla, P.; Sridhar, K.; Sharma, M.; Inbaraj, B. Antimicrobial and Anti-Inflammatory Activity of Low-Energy Assisted Nanohydrogel of Azadirachta indica Oil. Gels 2022, 8, 434. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Liu, J.; Liu, J. A temperature-responsive selenium nanohydrogel for strawberry grey mould management. J. Mater. Chem. 2022, 10, 5231–5241. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Devi, K.; Sharma, S.; Naushad, M.; Ghfar, A.; Ahamad, T.; Stadler, F. Guar gum-crosslinked-Soya lecithin nanohydrogel sheets as effective adsorbent for the removal of thiophanate methyl fungicide. Int. J. Biol. Macromol. 2018, 114, 295–305. [Google Scholar] [CrossRef]
- El-kady, A.; Mahmoud, E.; Sayed, M.; Kamel, S.; Naga, S. In-vitro and in-vivo evaluation for the bio-natural Alginate/nano-Hydroxyapatite (Alg/n-HA) injectable hydrogel for critical size bone substitution. Int. J. Biol. Macromol. 2023, 25331, 126618. [Google Scholar] [CrossRef]
- Medina, R.; Mier, A.; Moroni, E.; Merlier, F.; Gheber, L.; Vago, R.; Maffuci, I.; Tse-Sum, B.; Haupt, K. Molecularly imprinted polymer nanogels targeting the HAV motif in cadherins inhibit cell-cell adhesion and migration. J. Mater. Chem. B 2022, 10, 6688–6697. [Google Scholar] [CrossRef]
- Morsi, R.; Mansour, D.; Mousa, A. Ameliorative potential role of Rosmarinus officinalis extract on toxicity induced by etoposide in male albino rats. Braz. J. Biol. 2024, 84, e258234. [Google Scholar] [CrossRef]
- Quazi, M.; Park, N. DNA Hydrogel-Based Nanocomplexes with Cancer-Targeted Delivery and Light-Triggered Peptide Drug Release for Cancer-Specific Therapeutics. Biomacromolecules 2023, 24, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Otalvaro, C.; Coburn, J.M. Fabrication Methods and Form Factors of Gellan Gum-Based Materials for Drug Delivery and Anti-Cancer Applications. ACS Biomater. Sci. Eng. 2023, 9, 3832–3842. [Google Scholar] [CrossRef] [PubMed]
- Dewi, M.K.; Chaerunisaa, A.Y.; Muhaimin, M.; Joni, I.M. Improved Activity of Herbal Medicines through Nanotechnology. Nanomaterials 2022, 12, 4073. [Google Scholar] [CrossRef] [PubMed]
| No | Bioactive Compounds | Solubility Bioactive Compounds | Nanohydrogel Preparation Method | Dose/Distribution/Toxicity/Loading Efficiency | Improved Activity | Improved Solubility | Nanohydrogel Characteristics | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Apigenin isolate | Apigenin is hydrophobic (difficult to dissolve in water). | Stearate and chitosan polymers | The cytotoxic effect of apigenin on K562 cells had an IC50 of 20 mmol, whereas the IC50 for apigenin, when delivered with nanohydrogel, was 10 mmol. | Apigenin nanohydrogel can suppress the growth of K562 cells. | Nanohydrogel apigenin is hydrophilic (easily soluble in water). | In materials engineering, apigenin could be used as a thermal stabilizer of starch. | [30] |
| 2 | Epigallocatechin-3-gallate from C. sinensis extract | Green tea extract exhibits high solubility in water. | Chitosan polymer | The concentration of epigallocatechin-3-gallate used was 1%. | Nanohydrogel enhances chemotherapeutic properties and reduces blood cholesterol levels. | Nanohydrogel with epigallocatechin-3-gallate shows excellent water solubility. | The prepared chitosan nanohydrogel, NG15, demonstrates a % encapsulation efficiency of 99.87%. | [38] |
| 3 | Curcumin from C. domestica Val. extract | Curcumin has poor water solubility. | Synthesized with modifications using a method where a cholesteryl-amine linker is created through the reaction of cholesteryl chloroformate | Curcumin was tested at 5, 10, 15, and 20 µg/mL concentrations. | A nanodrug containing curcumin enhances stability, cellular permeability, and anticancer activity. | Nanohydrogel with curcumin demonstrates excellent solubility, surpassing the solubility of pure curcumin compounds in water. | Nanohydrogel with curcumin consists of particles measuring 20 nm in size. | [46] |
| 4 | Essential Oils from C. cyminum extract | Essential oils are hydrophobic (not readily soluble in water). | The aggregation phenomenon | Concentrations of essential oils were 650 and 350 ppm. | Chitosan nanohydrogel essential oils enhance antimicrobial activity. | Nanohydrogel with essential oils is hydrophilic (easily soluble in water). | For the IR spectroscopic analysis, 2 mg of C. cyminum (Ci), 2 mg of chitosan (CS), and 100 µL of CS–Ci nanogel are used. | [52] |
| 5 | Salidroside and paeonol isolates | Paeonol, a lipophilic compound, has a significantly higher solubility than salidroside. | The nanosphere-gel system | The concentrations of salidroside and paeonol were both 0.2 mg. | The nanosphere-gel system significantly enhances the anti-melanogenesis effect. | Paeonol in the nanosphere-gel dissolves more easily in oil than pure paeonol compounds. | The nanosphere-gel size is 286 nm, with a PDI of 0.397, a zeta potential of −22 mV, and paeonol encapsulation of 65.33%. | [53] |
| 6 | Sacchachitin from G. tsugae extract | Sacchachitin is sparingly soluble in water. | The ball milling method | The concentration of sacchachitin was 200–400 µg/mL. | Nanohydrogel formed from micronized sacchachitin shows enhanced corneal burn repair compared to Ganoderma fruit extract. | Nanohydrogel sacchachitin is more water-soluble than pure sacchachitin. | Nanohydrogel sacchachitin has particles ranging in size from 10 to 50 μm and possess a negative charge of approximately −22.59 mV. | [54] |
| 7 | Boswellic acid from B. serrata extract | Boswellic acid exhibits high solubility in isopropyl myristate and low-to-moderate solubility in oil. | Involving a high-speed homogenizer at 6000 RPM for up to 10 min | The concentration of Boswellia serrata extract was 133.2 mg. | Nanohydrogel boswellic acid enhances bioavailability as an anti-inflammatory. | Nanohydrogel boswellic acid dissolves more readily in oil than pure boswellic acid. | The particle size of nanohydrogel boswellic acid is 0.22 μm. | [55] |
| 8 | Calcitonin from A. retroflexus extract | Calcitonin is highly soluble in water. | The nanocomposite is in gel form | The distribution of A. retroflexus extract was 80.1% over 7 days. | Nanohydrogel A. retroflexus extract enhances anticancer activity. | Nanohydrogel calcitonin is easily soluble in water. | Chitin-poly (L-lactic acid) composite nanohydrogels in PBS achieve an efficiency of 61.73%. Nanohydrogel sizes range from 28.48 to 176.7 nm, with a nanoparticle size of 25.6 nm at pH 4. | [56] |
| 9 | Silymarin and silibinin from S. marianum extract | Silymarin and silibinin have low solubility in water. | Chitosan polymer | Nanoparticles had a loading efficiency of 85–95%. | Nanohydrogel chitosan, silymarin, and silibinin show enhanced antimicrobial activity. | Nanohydrogel silymarin and silibinin are more water-soluble than pure compounds. | The particle size of nanohydrogel silibinin is approximately 20 nm, with a loading efficiency of 85–95%. Nanohydrogel silibinin is spherical, homogeneous, and has a dense surface. | [57] |
| 10 | Fatty acids and methyl esters from sunflower extract | Fatty acids and methyl esters are poorly soluble in water. | Chitosan-stearic acid polymer | The most suitable was the nanohydrogel with chitosan polymer and stearic acid in a 20:1 ratio. | Nanohydrogel A. retroflexus extract enhances anticancer activity. | Nanohydrogel methyl esters are soluble at low pH values and more soluble in water than pure methyl esters. | Methyl ester-chitosan-stearate nanogel at pH 8 with a ratio of 0.5:1 has a particle size ranging from 12.0 to 38.1 nm. In comparison, the fatty oil nanogel with a ratio of 20:1 at pH 10 has a particle size ranging from 15.6 to 45.3 nm. | [58] |
| 11 | Curcumin from C. longa isolate | Curcumin exhibits limited solubility in water. | Chitosan polymer | The concentration of curcumin used ranged from 50 ppm to 200 ppm. | Nanohydrogel curcumin can effectively inhibit the growth of K562 cells. | Nanohydrogel with curcumin demonstrates excellent solubility and is more water-soluble than pure curcumin. | The particle size of nanohydrogel curcumin is 20 nm. | [59] |
| 12 | Khellin from A. visnaga isolate | Khellin exhibits poor solubility in water. | Chitosan polymer | The concentration of khellin used was 22.6 mg. | This treatment shows promise for skin diseases such as psoriasis, eczema, alopecia areata, and vitiligo. | Nanohydrogel khellin is more water-soluble than pure khellin. | The nanohydrogel khellin is a carrier system with an average diameter of ≤100 nm. | [60] |
| 13 | p-Cymene, thymol, and 1,8-cineole from T. vulgaris extract | p-Cymene, thymol, and 1,8-cineole are insoluble in water. | Copper chitosan for a nanocomposite hydrogel | Polymeric nanohydrogels were preferred for their biodegradability, biocompatibility, inexhaustibility, non-toxicity, cost-effectiveness, and wide applications. | Thymol nanohydrogel enhances antifungal activity, effective against Aspergillus flavus. | The nanohydrogel form of thymol is more water-soluble. | Nanohydrogels exhibit minimal drug-loading efficiency. | [61] |
| 14 | Qiai essential oil (QEO) | Essential oils are known for their poor aqueous solubility and high volatility. | Nanohydrogels | The cumulative release rate reached 95% within 35 h. | The nanohydrogel Qiai essential oil enhances antibacterial activity against S. aureus and E. coli compared to free QEO. | The nanohydrogel Qiai essential oil is more water-soluble. | The encapsulation efficiency and loading capacity of the nanohydrogel Qiai essential oil reaches 80.2% and 6.8%, respectively. | [62] |
| 15 | A. vera extract | It is a natural polysaccharide with water-soluble characteristics. | Nanohydrogel with chitosan polymers | Salmonella typhi was the most sensitive bacterium to all tested nanohydrogel formulations of AV and Ch (except for AV-Ch (3:1 v/w)). Growth of Candida albicans was also inhibited by nanohydrogel AV-Ch (1:1 v/w) at MIC = 500. | It enhanced wound recovery response by harnessing the potential healing properties of nanohydrogel Aloe vera-chitosan. | Nanohydrogel Aloe vera-chitosan is highly water-soluble. | Nanohydrogel Aloe vera-chitosan exhibits uniform shapes and sizes smaller than 100 nm. | [63] |
| 16 | Ginseng extract | Ginseng extract is not soluble in water. | Nanohydrogel with sodium alginate polymer | The diffusion study revealed extended ginseng diffusion from the nanohydrogel for over 20 h. | The ginseng nanohydrogel (NHG) enhanced the potential for wound healing. | The ginseng nanohydrogel (NHG) is highly water-soluble. | The optimized nanoformulation exhibits a particle size of 420.11 ± 5.21 nm, a PDI of 0.424 ± 0.013, a zeta potential of 0.006 ± 0.002 mV, and an encapsulation efficiency of 89.051 ± 0.022%. | [64] |
| 17 | Plant polyphenols | Polyphenols are hydrophobic. | Polyphenol zwitterionic nanohydrogel | This nanohydrogel displayed an excellent oil rejection rate (99%) and flux (2100 L·m−2·h−1·bar−1) for up to 30 separation cycles. | Significant improvement in the separation of oily wastewater was achieved. | The polyphenol zwitterionic nanohydrogel is highly water-soluble. | Characterization using Fourier transform infrared spectroscopy and X-ray photoelectron spectroscopy confirms the successful fabrication of the PVDF-Poly(DMA-co-SPP) membrane. | [65] |
| 18 | The Mentha plant extract | The Mentha plant extract is insoluble in water. | Nanocomposite hydrogels with k-carrageenan (k-Cr) and chitosan (CS) polymers | These nanocomposite hydrogels showed high toxicity (about 22% at a concentration of 20 μg/mL) against HeLa cells. | They significantly improved antibacterial activity against S. aureus (an MIC value of 39.06 μg/mL) and E. coli (an MIC value of >19.53). | The nanocomposite hydrogels with k-carrageenan (k-Cr) and chitosan (CS) polymers are highly water-soluble. | These nanocomposite hydrogels exhibit a loading capacity of about 98%. | [66] |
| 19 | Hydnocarpus wightiana oil | Hydnocarpus wightiana oil is hydrophobic and insoluble in water. | Nanohydrogel-based Hydnocarpus wightiana oil | This nanohydrogel had the capability for target-specific drug absorption and was biocompatible. | It significantly improved antibacterial and antifungal activity against pathogenic bacteria and fungi, with MIC values ranging from 0.78 to 1.56 μL/mL and effectiveness ranging from 70.29% to 83.62%. | Nanohydrogel-based Hydnocarpus wightiana oil is highly water-soluble. | The nanohydrogel exhibits an average droplet size of 103.6 nm with a surface charge of −17.6 mV. | [67] |
| 20 | Soy protein isolate | Soy protein isolate is hydrophobic and insoluble in water. | Soy protein isolate nanoparticles (SPI NPs) using Genipin | These nanoparticles showed high self-healing efficiency, reaching 91.6% within 10 h, and exhibited favorable mechanical properties with a tensile strength of 0.89 MPa and strain of 853.2%. | The nanoparticles hydrogel (SPI NPs) by Genipin improved wound healing. | The nanoparticle hydrogel (SPI NPs) by Genipin is highly water-soluble. | An oil-in-water (O/W) Pickering emulsion is formed by SPI NPs encapsulating linseed oil. | [68] |
| 21 | Aloe vera and Eucalyptus essential oil | Eucalyptus essential oil is hydrophobic and insoluble in water. | Nanoparticle hydrogel with Eucalyptus essential oil, incorporating E. staigeriana, loaded with Aloe vera, and coated with Dextran Sulfate/Chitosan | The release profile of E. staigeriana from the prepared nanoparticles was studied in vitro at a pH of 7.4. | The E. staggering-loaded Aloe vera-coated Dextran Sulfate/Chitosan hydrogel could be an effective dressing material to accelerate wound healing. | The nanoparticle hydrogel Eucalyptus essential oil is highly water-soluble. | Aloe Vera-coated Dextran Sulfate/Chitosan nanoparticles encapsulated with E. staigeriana inhibit bacteria by 47.27%. | [69] |
| 22 | Azadirachta indica oil | Azadirachta indica oil is hydrophobic and insoluble in water. | Nanohydrogel Azadirachta indica oil | Chromatography analysis quantified the presence of gallic acid (0.0076 ppm), caffeic acid (0.077 ppm), and syringic acid (0.0129 ppm) in the oil. | Enhanced antimicrobial and anti-inflammatory activities were observed. | Nanohydrogel Azadirachta indica oil is highly water-soluble. | The nanohydrogel exhibits a droplet size of 104.1 nm and a zeta potential of −19.3 mV. | [70] |
| 23 | Agarose | Agarose is hydrophobic and insoluble in water. | Agarose with selenium nanohydrogel | These nanohydrogels demonstrated good biocompatibility and the ability to enhance photosynthetic efficiency and plant growth. | The nanohydrogels exhibited excellent effects in controlling strawberry gray mold and extending fruit storage time. | Agarose with selenium nanohydrogel is highly water-soluble. | Measurement of fluorescence parameters shows that the maximum photochemical efficiency (Fv/Fm) of plant leaves in the inoculated group (B. cinerea) is 0.58. In contrast, the Fv/Fm value of the nanohydrogel group exceeds 0.8. | [71] |
| 24 | Guar gum | Guar gum is hydrophobic and insoluble in water. | Guar gum-crosslinked-Soya lecithin nanohydrogel | It achieved a maximum adsorption capacity of 59.205 mg/g. | The improved spontaneous and endothermic adsorption process was observed. | Guar gum-crosslinked lecithin nanohydrogel is highly water-soluble. | It is observed with a 20 mg GG-crosslinked-SY NHS and a 25 ppm thiophanate methyl solution concentration, as calculated from the Langmuir isotherm. | [72] |
| 25 | Alginate (Alg) | Alginate is insoluble in water. | Injectable nanohydrogel paste based on natural alginate (Alg) derived from brown sea algae as a polysaccharide polymer | The nanohydrogel paste reduced healing time and fully restored well-mature bone tissue, similar to natural bone. | Injectable nanohydrogels Alginate/n-Hydroxyapatite (Alg/n-HA) exhibited appreciable biodegradability and bioactivity. | Nanohydrogel Alginate/n-Hydroxyapatite (Alg/n-HA) is highly water-soluble. | The viscosity and mechanical properties of the paste are investigated, as well as in-vitro studies related to water absorption and biodegradability in PBS. | [73] |
| Phytochemical Compounds | Method | IC50 Extract/Fraction/Isolate | IC50 Nanohydrogel | Ref. | ||
|---|---|---|---|---|---|---|
| Curcumin | MTT Test | In 4T1 cells, it is 2 µg/mL. | In MiaPaCa-2 cells, it is 9 µg/mL. | In 4T1 cells, it is 5 µg/mL. | In MiaPaCa-2 cells, it is 18 μg/mL. | [46] |
| Apigenin | MTT Test and Trypan Blue Staining | K562 cells increased by 20 mmol after 48 h. | 48 h = 20 ± 1.66 µmol/mL; 48 h = 20 ± 0.82 µmol/mL. | K562 cells increased by 10 mmol after 48 h. | 48 h = 10 ± 0.53 µmol/mL; 48 h = 10 ± 1.98 µmol/mL. | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lestari, U.; Muhaimin, M.; Chaerunisaa, A.Y.; Sujarwo, W. Improved Solubility and Activity of Natural Product in Nanohydrogel. Pharmaceuticals 2023, 16, 1701. https://doi.org/10.3390/ph16121701
Lestari U, Muhaimin M, Chaerunisaa AY, Sujarwo W. Improved Solubility and Activity of Natural Product in Nanohydrogel. Pharmaceuticals. 2023; 16(12):1701. https://doi.org/10.3390/ph16121701
Chicago/Turabian StyleLestari, Uce, Muhaimin Muhaimin, Anis Yohana Chaerunisaa, and Wawan Sujarwo. 2023. "Improved Solubility and Activity of Natural Product in Nanohydrogel" Pharmaceuticals 16, no. 12: 1701. https://doi.org/10.3390/ph16121701
APA StyleLestari, U., Muhaimin, M., Chaerunisaa, A. Y., & Sujarwo, W. (2023). Improved Solubility and Activity of Natural Product in Nanohydrogel. Pharmaceuticals, 16(12), 1701. https://doi.org/10.3390/ph16121701







