Spices as Sustainable Food Preservatives: A Comprehensive Review of Their Antimicrobial Potential
Abstract
:1. Introduction
2. Methodology
2.1. Flora Nomenclature
2.2. Antimicrobial Activity of Spices
2.3. Inclusion and Exclusion Criteria
3. Results and Discussion
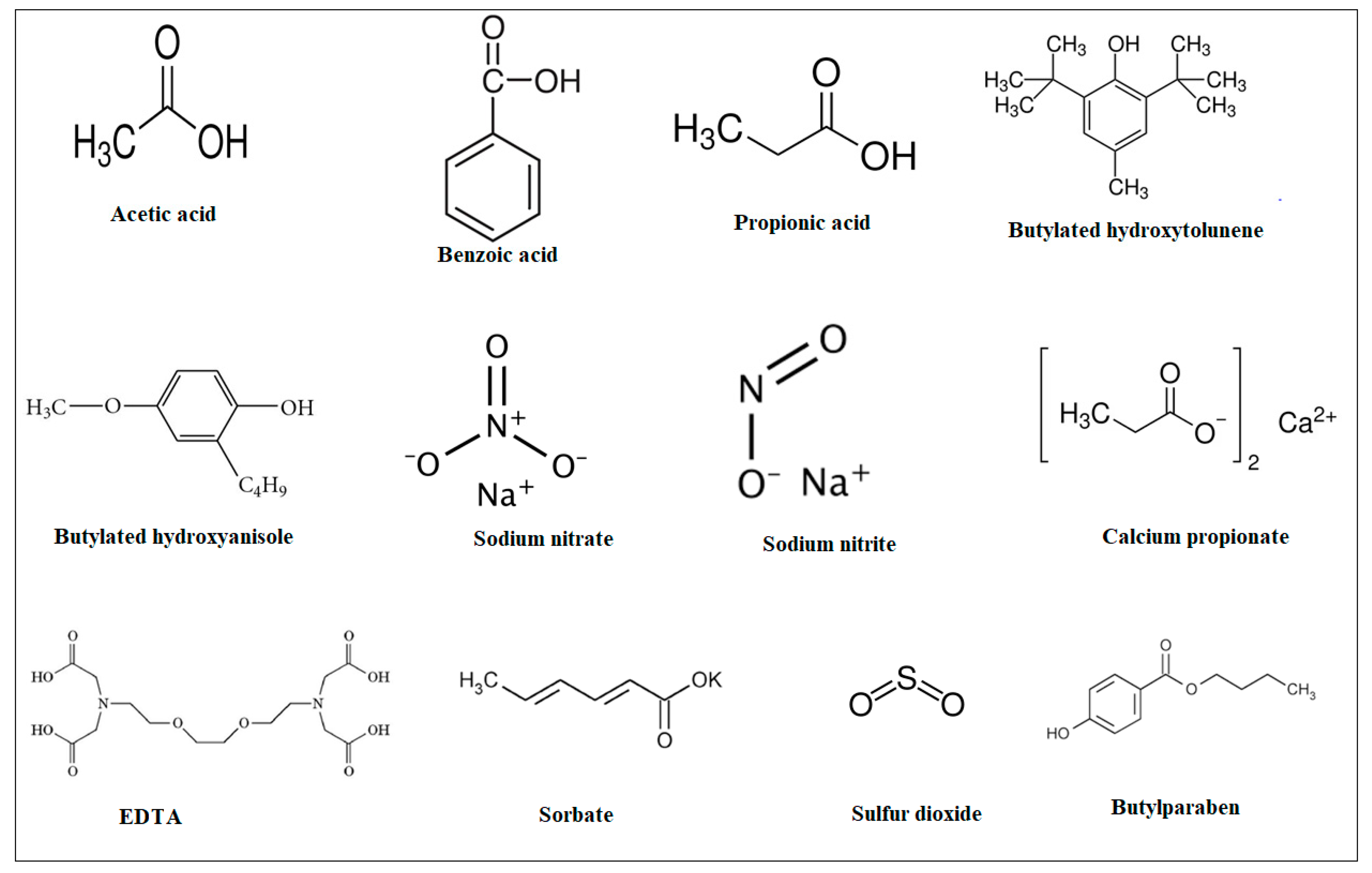
3.1. Artificial Food Preservatives
- (i)
- Organic acids, such as ascorbic acid, acetic acid, and benzoic acid, are widely used in acidic food products to prevent microbial growth [13];
- (ii)
- Nitrites and nitrates are commonly applied in cured meat products to inhibit the growth of Clostridium botulinum and provide color stabilization [13];
- (iii)
- Sulfites are utilized in dried fruits, wines, and juices to control microbial activity and prevent browning [14];
- (iv)
- Antioxidants, including butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT), are used to extend the shelf life of products containing fats and oils [15];
- (v)
- Antibiotics: nisin and natamycin are examples of antibiotics used to preserve a variety of food items [16].
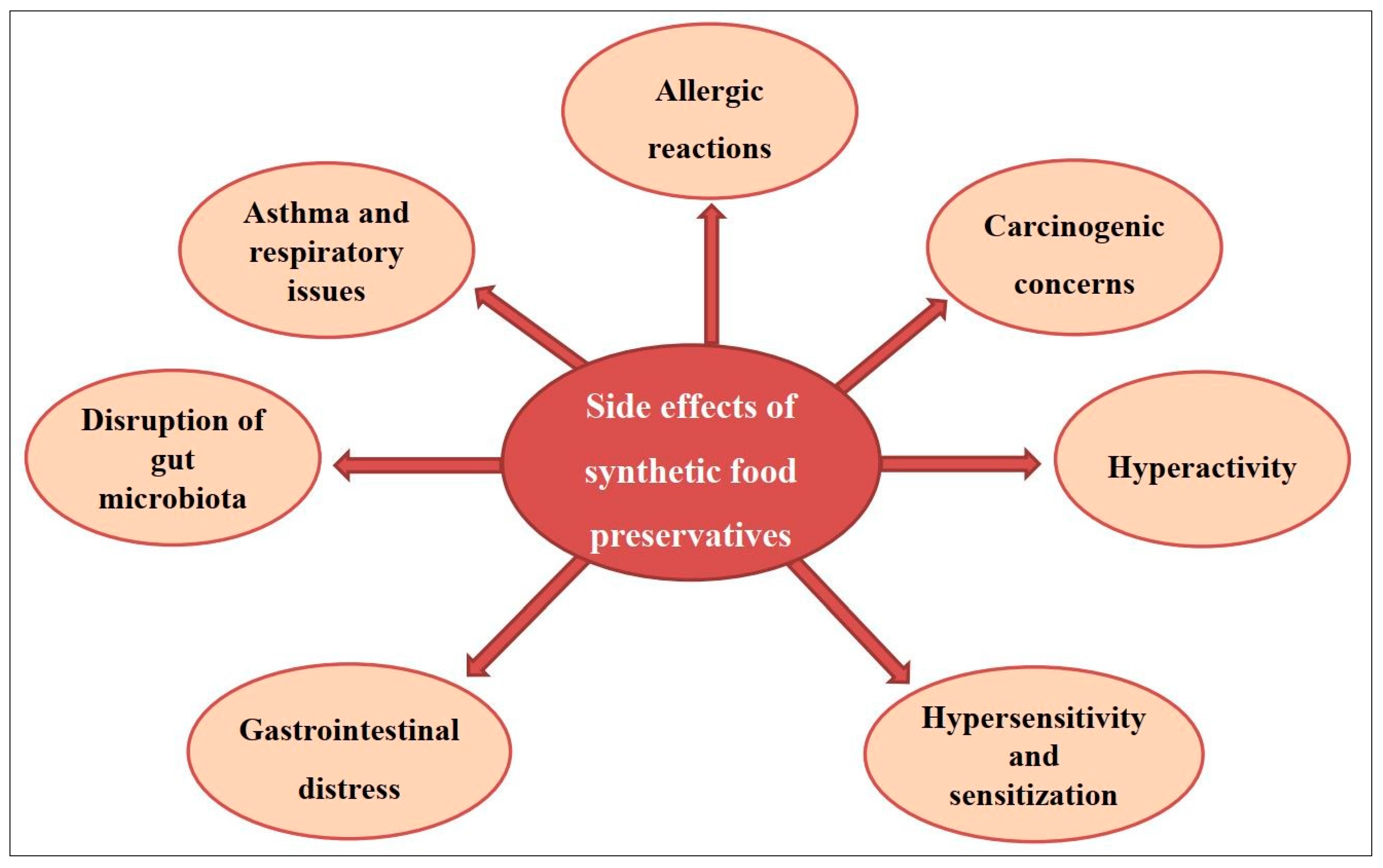
3.2. Side Effects of Artificial Food Preservatives
3.3. Food Spoilage Microorganisms
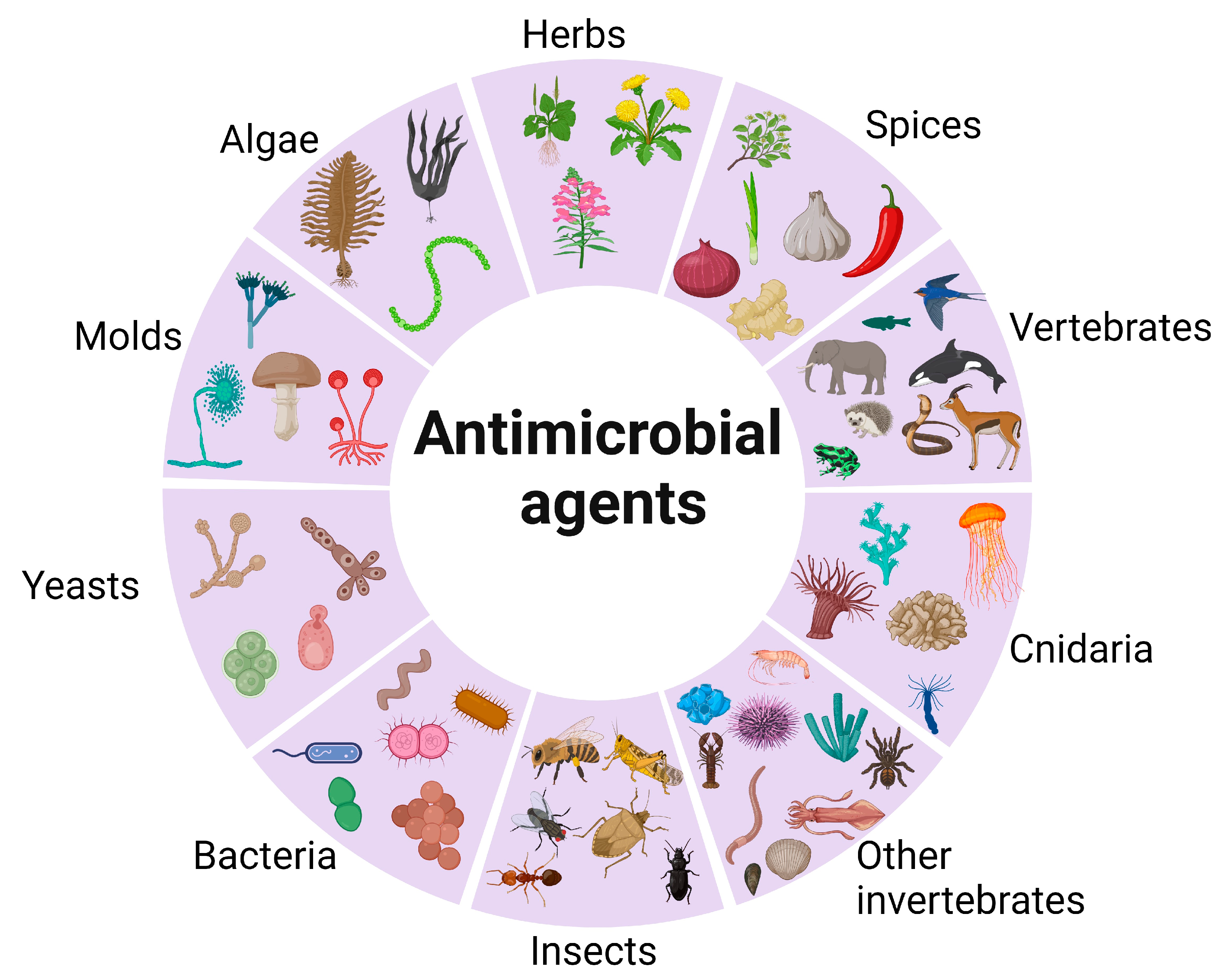
3.4. Natural Antimicrobial Agents
3.5. Spices as a Source of Natural Antimicrobial Agents
3.6. Suggested Antimicrobial Spices for Food Preservation
3.6.1. Allspice
3.6.2. Anise
3.6.3. Basil
3.6.4. Bell Pepper
3.6.5. Black Pepper
3.6.6. Black Seeds
3.6.7. Caraway
3.6.8. Cardamom
3.6.9. Cinnamon
3.6.10. Clove
3.6.11. Coriander
3.6.12. Cumin
3.6.13. Dill
3.6.14. Fennel
3.6.15. Fenugreek
3.6.16. Garlic
3.6.17. Ginger
3.6.18. Mastic
3.6.19. Nutmeg
3.6.20. Parsley
3.6.21. Rosemary
3.6.22. Saffron
3.6.23. Thyme
3.6.24. Turmeric
3.6.25. Vanilla
| No. | Spice | Scientific Name | Botanical Family | Major Bioactive Compound | Chemical Structure | Antimicrobial Activity | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Allspice | Pimenta dioica (L.) Merr. | Myrtaceae | Eugenol | 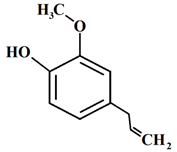 | Listeria monocytogenes CECT 933, Vibrio vulnificus CECT 529, Salmonella enterica CECT 443, Shigella flexeneri CECT 4804, Escherichia coli ATCC 35218, Staphylococcus aureus ATCC 6538, and Aspergillus flavus. | [58,59] |
| 2 | Anise | Pimpinella anisum L. | Apiaceae | Anethole | 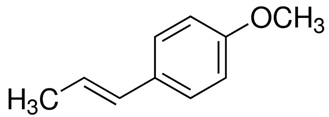 | Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853, Streptococcus pyogenes ATCC 19615, and Candida albicans ATCC 10231. | [61,62] |
| 3 | Basil | Ocimum basilicum L. | Lamiaceae | Methyl cinnamate | 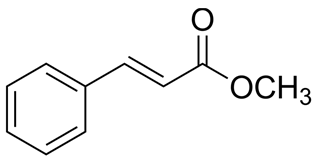 | Staphylococcus epidermidis MTCC 435, Streptococcus mutans MTCC 890, Escherichia coli MTCC 723, Candida kefyr ATCC 204093, and Candida albicans ATCC 14053. | [66,67] |
| 4 | Bell pepper | Capsicum annuum L. | Solanaceae | Capsaicinoids | 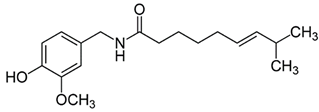 | Listeria monocytogenes, Salmonella typhimurium, Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Proteus mirabilis, Lactobacillus plantarum, Lactobacillus acidophilus, and Cochliobolus spp. | [70,71] |
| 5 | Black Pepper | Piper nigrum L. | Piperaceae | Piperine | 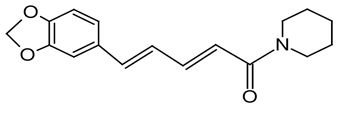 | Klebsiella pneumonia ATCC 27853, Escherichia coli ATCC 25922, Salmonella enterica ATCC 43972, Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29122, Staphylococcus epidermidis ATCC 14990, Bacillus subtilis ATCC 6633, and Aspergillus flavus CGMCC 3.06434. | [75,76] |
| 6 | Black seeds | Nigella sativa L. | Ranunculaceae | Thymoquinone | 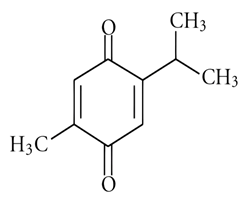 | Escherichia coli, Klebsiella pneumoniae, Staphylococcus aurous, Enterobacter aerogenes, Fusarium Solani, Candida albicans AUMC 1299, Aspergillus flavus AUMC 1276, Fusarium oxysporum AUMC 215, Scopulariopsis brevicaulis AUMC 1653, Geotrichum candidum AUMC 226, and Trichophyton rubrum AUMC 1804. | [81,82] |
| 7 | Caraway | Carum carvi L. | Apiaceae | Carvone |  | Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Bacillus cereus, and Aspergillus flavus. | [86,87,88] |
| 8 | Cardamom | Elettaria cardamomum (L.) Maton | Zingiberaceae | Cardamonin | 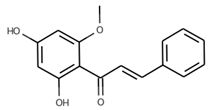 | Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus (ATCC 43300), Salmonella typhimurium (ATCC 14023), and Escherichia coli (ATCC 25922). | [90,92,93] |
| 9 | Cinnamon | Cinnamomum verum J.Presl | Lauraceae | Cinnamaldehyde | 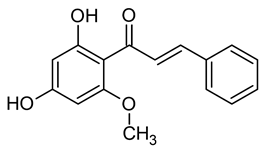 | Micrococcus luteus, Bacillus subtilis, Bacillus cereus, Klebsiella aerogenes, Escherichia coli, Salmonella enterica, Penicillium expansum, Candida albicans, and Candida tropicalis. | [95,96] |
| 10 | Clove | Syzygium aromaticum (L.) Merr. & L.M.Perry | Myrtaceae | Eugenol | 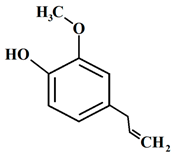 | Staphylococcus aureus, Listeria innocua, Pseudomonas aeruginosa. Serratia marcescens, Bacillus subtilis, Penicillium commune, Penicillium expansum, Penicillium glabrum, and Penicillium chrysogenum | [99,100,101] |
| 11 | Coriander | Coriandrum sativum L. | Apiaceae | Linalool | 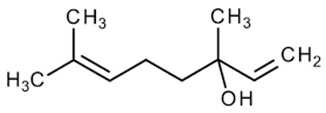 | Bacillus subtilis, Stenotrophomonas Penicillium expansum, Streptococcus pyogenes, Listeria monocytogenes, Enterobacter aerogenes, Salmonella typhimurium, and Shigella dysenteriae. | [104,105,106] |
| 12 | Cumin | Cuminum cyminum L. | Apiaceae | Cuminaldehyde | 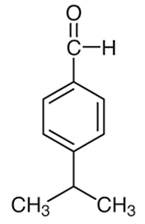 | Staphylococcus aureus, Bacillus cereus, Escherichia coli, Salmonella typhi, Botrytis cinerea, Penicillium expansum, and Aspergillus niger. | [108,109,110] |
| 13 | Dill | Anethum graveolens L. | Apiaceae | Carvone | 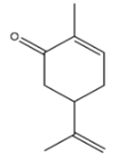 | Escherichia coli, Staphylococcus aureus, Yersinia enterocolitica, Geotrichum candidum, Salmonella typhimurium, Rhodotorula glutinis, Saccharomyces cerevisiae, and Candida albicans. | [112,113] |
| 14 | Fennel | Foeniculum vulgare Mill. | Apiaceae | Anethole | 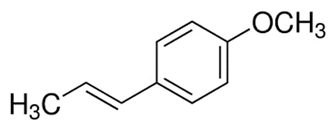 | Staphylococcus albus, Bacillus subtilis, Salmonella typhimurium, Shigella dysenteriae, Escherichia coli, Bacillus cereus, Staphylococcus aureus, Candida albicans, and Aspergillus flavus. | [117,118,119] |
| 15 | Fenugreek | Trigonella foenum-graecum L. | Fabaceae | Sotolone | 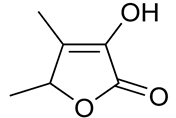 | Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, Bacillus subtilis NCTC 8236, Pseudomonas aeruginosa ATCC 27853, Aspergillus niger ATCC 9763, and Candida albicans ATCC 7596. | [121,122] |
| 16 | Garlic | Allium sativum L. | Amaryllidaceae | Allicin | 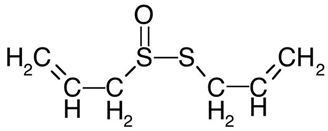 | Staphylococcus saprophyticus, Staphylococcus aureus, Staphylococcus epidermidis, Bacillus cereus, Streptococcus pneumoniae, Shigella flexneri, Proteus vulgaris, Klebsiella pneumoniae, Escherichia coli, Aspergillus versicolor, Penicillium expansum, Penicillium citrinum, and Candida albicans. | [125,127,128] |
| 17 | Ginger | Zingiber officinale Roscoe | Zingiberaceae | Gingerol | 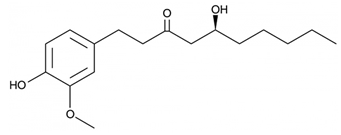 | Staphylococcus epidermidis, Staphylococcus aureus, Enterococcus faecalis, Streptococcus faecalis, Bacillus subtilis, Bacillus megaterium, Bacillus cereus, Escherichia coli, Klebsiella pneumoniae, Salmonella typhimurium, Salmonella typhi, Pseudomonas aeruginosa, Proteus spp., Aspergillus niger, Aspergillus flavus, Penicillium expansum, Alternaria alternata, Fusarium oxysporum, Mucor hemalis, Penicillium notatum, Candida albicans, and Fusarium oxysporum. | [130,132,133] |
| 18 | Mastic | Pistacia lentiscus L. | Anacardiaceae | α-pinene | 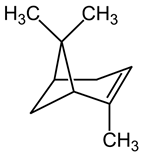 | Escherichia coli, Staphylococcus aureus, Bacillus subtilis, Helicobacter pylori, Streptococcus mutans, Microsporum canis, Trichophyton mentagrophytes, and Trichophyton violaceum. | [135,136] |
| 19 | Nutmeg | Myristica fragrans Houtt. | Myristicaceae | myristicin | 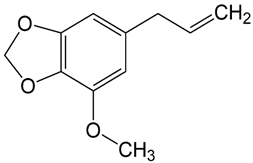 | Staphylococcus aureus MTCC 737, Bacillus subtilis MTCC 441, Pseudomonas putida MTCC 1072, Pseudomonas aeruginosa MTCC 7903, Listeria monocytogenes, Aspergillus flavus MTCC 277, Aspergillus niger MTCC 282, and Aspergillus fumigatus MTCC 343. | [140,141,142] |
| 20 | Parsley | Petroselinum crispum (Mill.) Fuss | Apiaceae | Apigenin | 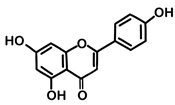 | Salmonella enterica, Staphylococcus aureus, Listeria monocytogenes, Penicillium ochrochloron, and Trichoderma viride | [144,146] |
| 21 | Rosemary | Rosmarinus officinalis L. | Lamiaceae | Rosmarinic acid | 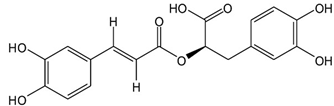 | Bacillus cereus, Staphylococcus aureus, Salmonella choleraesuis, Clostridium perfringens, Aeromonas hydrophila, Escherichia coli, Listeria monocytogenes, and Brochothrix thermosphacta. | [149,150] |
| 22 | Saffron | Crocus sativus L. | Iridaceae | Crocin | 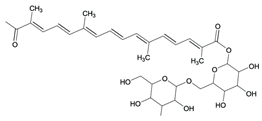 | Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus vulgaris, Staphylococcus aureus, Escherichia coli, Candida albicans, Aspergillus fumigatus, and Aspergillus niger. | [152,154] |
| 23 | Thyme | Thymus vulgaris L. | Lamiaceae | Thymol | 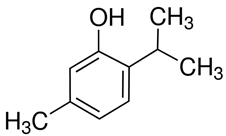 | Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, Salmonella typhimurium ATCC 14028, Klebsiella pneumoniae ATCC 13882, Enterococcus faecalis ATCC 29212, Escherichia coli ATCC 25922, and Candida albicans ATCC 10231. | [156,157] |
| 24 | Turmeric | Curcuma longa L. | Zingiberaceae | Curcumin |  | Staphylococcus aureus ATCC 25923, Staphylococcus epidermis ATCC 12228, Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 10031, Vibrio harveyi, Vibrio cholerae, Bacillus subtilis, Bacillus cereus, Aeromonas hydrophila, Staphylococcus intermedius, Edwardsiella tarda, Streptococcus agalactiae, Cryptococcus neoformans, Candida albicans, and Fusarium solani. | [161,162] |
| 25 | Vanilla | Vanilla planifolia Andrews | Orchidaceae | Vanillin | 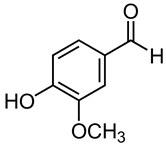 | Staphylococcus epidermidis, Staphylococcus saprophyticus, Staphylococcus aureus, Streptococcus pyogenes, Enterococcus faecalis, Enterobacter hormaechei, Enterobacter cloacae, Klebsiella pneumoniae, Salmonella typhimurium, Escherichia coli, and Pseudomonas aeruginosa. | [165,166] |
4. Future Prospective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019; pp. 2–13. [Google Scholar]
- de Almeida Roger, J.; Magro, M.; Spagnolo, S.; Bonaiuto, E.; Baratella, D.; Fasolato, L.; Vianello, F. Antimicrobial and magnetically removable tannic acid nanocarrier: A processing aid for Listeria monocytogenes treatment for food industry applications. Food Chem. 2018, 267, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S. Food Spoilage: Microorganisms and their prevention. Asian J. Plant Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Sevindik, M.; Uysal, I. Food spoilage and Microorganisms. Turk. J. Agric. Food Sci. Technol. 2021, 9, 1921–1924. [Google Scholar] [CrossRef]
- in’t Veld, J.H.H. Microbial and biochemical spoilage of foods: An overview. Int. J. Food Microbiol. 1996, 33, 1–18. [Google Scholar] [CrossRef]
- Koutchma, T.; Ezzatpanah, H. Frontiers: Current Challenges in Food Process Design and Engineering. Front. Food Sci. Technol. 2021, 1, 780013. [Google Scholar] [CrossRef]
- Zhou, R.; Rezaeimotlagh, A.; Zhou, R.; Zhang, T.; Wang, P.; Hong, J.; Soltani, B.; Mai-Prochnow, A.; Liao, X.; Ding, T. In-package plasma: From reactive chemistry to innovative food preservation technologies. Trends Food Sci. Technol. 2022, 120, 59–74. [Google Scholar] [CrossRef]
- Sharma, S. Food preservatives and their harmful effects. Int. J. Sci. Res. Publ. 2015, 5, 1–2. [Google Scholar]
- Kaptan, B.; Kayısoglu, S. Consumers’ attitude towards food additives. Am. J. Food Sci. Nutr. Res. 2015, 2, 21–25. [Google Scholar]
- Zugravu, C.A.; Pogurschi, E.N.; Pătrașcu, D.; Iacob, P.-D.; Nicolae, C.G. Attitudes towards food additives: A pilot study. Ann. Univ. Dunarea De Jos Galati. Fascicle VI-Food Technol. 2017, 41, 50–61. [Google Scholar]
- Martínez-Graciá, C.; González-Bermúdez, C.A.; Cabellero-Valcárcel, A.M.; Santaella-Pascual, M.; Frontela-Saseta, C. Use of herbs and spices for food preservation: Advantages and limitations. Curr. Opin. Food Sci. 2015, 6, 38–43. [Google Scholar] [CrossRef]
- Mirza, S.K.; Asema, U.; Kasim, S.S. To study the harmful effects of food preservatives on human health. J. Med. Chem. Drug Discov. 2017, 2, 610–616. [Google Scholar]
- Branen, A.L.; Davidson, P.M.; Salminen, S.; Thorngate, J. Food Additives; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Yang, W.H.; Purchase, E.C. Adverse reactions to sulfites. CMAJ Can. Med. Assoc. J. 1985, 133, 865. [Google Scholar]
- Foulke, J.E. A Fresh Look at Food Preservatives; Department of Health and Human Services, Public Health Service, Food and Drug Administration: Silver Spring, MD, USA, 1993.
- Silva, M.M.; Lidon, F. Food preservatives–An overview on applications and side effects. Emir. J. Food Agric. 2016, 28, 366–373. [Google Scholar] [CrossRef]
- Smoley, C. Everything Added to Food in the United States; US Food and Drug Administration: Silver Spring, MD, USA, 1993; Volume 31.
- Davidson, P.M.; Taylor, T.M.; Schmidt, S.E. Chemical preservatives and natural antimicrobial compounds. In Food Microbiology: Fundamentals and Frontiers; Wiley: Hoboken, NJ, USA, 2012; pp. 765–801. [Google Scholar]
- Carocho, M.; Morales, P.; Ferreira, I.C. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Gupta, R.; Yadav, R.K. Impact of chemical food preservatives on human health. Palarch’s J. Archaeol. Egypt/Egyptol. 2021, 18, 811–818. [Google Scholar]
- Mandal, D. Food preservative chemistry: Effects and side effects. J. Indian Chem. Soc. 2019, 96, 1519–1528. [Google Scholar]
- Azuma, S.; Quartey, N.-A.; Ofosu, I. Sodium benzoate in non-alcoholic carbonated (soft) drinks: Exposure and health risks. Sci. Afr. 2020, 10, e00611. [Google Scholar] [CrossRef]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E. Food additives and hyperactive behaviour in 3-year-old and 8/9-year-old children in the community: A randomised, double-blinded, placebo-controlled trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Gultekin, F.; Doguc, D.K. Allergic and immunologic reactions to food additives. Clin. Rev. Allergy Immunol. 2013, 45, 6–29. [Google Scholar] [CrossRef]
- Zaknun, D.; Schroecksnadel, S.; Kurz, K.; Fuchs, D. Potential role of antioxidant food supplements, preservatives and colorants in the pathogenesis of allergy and asthma. Int. Arch. Allergy Immunol. 2012, 157, 113–124. [Google Scholar] [CrossRef]
- Bozoglu, F. Food allergies, intolerances and food-borne intoxications. In Strategies for Achieving Food Security in Central Asia; Springer: Berlin/Heidelberg, Germany, 2012; pp. 93–108. [Google Scholar]
- Molognoni, L.; Daguer, H.; Motta, G.E.; Merlo, T.C.; Lindner, J.D.D. Interactions of preservatives in meat processing: Formation of carcinogenic compounds, analytical methods, and inhibitory agents. Food Res. Int. 2019, 125, 108608. [Google Scholar] [CrossRef] [PubMed]
- Tobacman, J.K. Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ. Health Perspect. 2001, 109, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, H.; Qin, N.; Ren, X.; Zhu, B.; Xia, X. Impact of food additives on the composition and function of gut microbiota: A review. Trends Food Sci. Technol. 2020, 99, 295–310. [Google Scholar] [CrossRef]
- Witkowski, M.; Grajeta, H.; Gomułka, K. Hypersensitivity reactions to food additives—Preservatives, antioxidants, flavor enhancers. Int. J. Environ. Res. Public Health 2022, 19, 11493. [Google Scholar] [CrossRef]
- Schmidt, M.; Zannini, E.; Arendt, E.K. Recent advances in physical post-harvest treatments for shelf-life extension of cereal crops. Foods 2018, 7, 45. [Google Scholar] [CrossRef]
- Tzachor, A.; Richards, C.E. Future Foods for Urban Food Production; Brears, R., Ed.; University of Cambridge: Cambridge, UK, 2021. [Google Scholar]
- Onyeaka, H.N.; Nwabor, O.F. Food Preservation and Safety of Natural Products; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Wei, Q.; Wang, X.; Sun, D.-W.; Pu, H. Rapid detection and control of psychrotrophic microorganisms in cold storage foods: A review. Trends Food Sci. Technol. 2019, 86, 453–464. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Li, Y.; Liu, T.; Flint, S.; Zhang, G.; Yuan, L.; Pei, Z.; He, G. The heat resistance and spoilage potential of aerobic mesophilic and thermophilic spore forming bacteria isolated from Chinese milk powders. Int. J. Food Microbiol. 2016, 238, 193–201. [Google Scholar] [CrossRef]
- Pothakos, V.; Devlieghere, F.; Villani, F.; Björkroth, J.; Ercolini, D. Lactic acid bacteria and their controversial role in fresh meat spoilage. Meat Sci. 2015, 109, 66–74. [Google Scholar] [CrossRef]
- Hernández, A.; Pérez-Nevado, F.; Ruiz-Moyano, S.; Serradilla, M.; Villalobos, M.; Martín, A.; Córdoba, M. Spoilage yeasts: What are the sources of contamination of foods and beverages? Int. J. Food Microbiol. 2018, 286, 98–110. [Google Scholar] [CrossRef]
- Dagnas, S.; Membré, J.-M. Predicting and preventing mold spoilage of food products. J. Food Prot. 2013, 76, 538–551. [Google Scholar] [CrossRef]
- Petruzzi, L.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Microbial spoilage of foods: Fundamentals. In The Microbiological Quality of Food; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–21. [Google Scholar]
- Hayashi, M.A.; Bizerra, F.C.; Junior, P.I.D.S. Antimicrobial Compounds from Natural Sources; Frontiers E-books: Lausanne, Switzerland, 2014. [Google Scholar]
- Rahman, M.S. Handbook of Food Preservation; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Gould, G.W. Emerging technologies in food preservation and processing in the last 40 years. In Innovations in Food Processing; CRC Press: Boca Raton, FL, USA, 2000; pp. 1–11. [Google Scholar]
- Abdallah, E.M. Plants: An alternative source for antimicrobials. J. Appl. Pharm. Sci. 2011, 1, 16–20. [Google Scholar]
- Mashabela, M.N.; Ndhlovu, P.T.; Mbeng, W.O. Herbs and Spices’ Antimicrobial Properties and Possible Use in the Food Sector. In Herbs and Spices; IntechOpen: London, UK, 2022. [Google Scholar]
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on major food-borne zoonotic bacterial pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef]
- Saeed, F.; Afzaal, M.; Tufail, T.; Ahmad, A. Use of natural antimicrobial agents: A safe preservation approach. In Active Antimicrobial Food Packaging; Books on Demand: Norderstedt, Germany, 2019; Volume 18. [Google Scholar]
- Sachan, A.; Kumar, S.; Kumari, K.; Singh, D. Medicinal uses of spices used in our traditional culture: Worldwide. J. Med. Plants Stud. 2018, 6, 116–122. [Google Scholar]
- Food and Drug Administration. CPG Sec 525.750 Spices-Definitions; FDA: Silver Spring, MD, USA, 2020.
- Opara, E.I.; Chohan, M. Culinary herbs and spices: Their bioactive properties, the contribution of polyphenols and the challenges in deducing their true health benefits. Int. J. Mol. Sci. 2014, 15, 19183–19202. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Fresno, R.; Rosana, A.R.R.; Sajed, T.; Onookome-Okome, T.; Wishart, N.A.; Wishart, D.S. Herbs and spices-biomarkers of intake based on human intervention studies—A systematic review. Genes Nutr. 2019, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Peter, K.; Shylaja, M. Introduction to herbs and spices: Definitions, trade and applications. In Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–24. [Google Scholar]
- Ortega-Lozano, A.J.; Hernández-Cruz, E.Y.; Gómez-Sierra, T.; Pedraza-Chaverri, J. Antimicrobial Activity of Spices Popularly Used in Mexico against Urinary Tract Infections. Antibiotics 2023, 12, 325. [Google Scholar] [CrossRef]
- Husain, F. Trends in the international spice trade. In Proceedings of the International Trade Forum, Geneva, Switzerland, 16 April 1996; International Trade Centre: Geneva, Switzerland, 1996; p. 14. [Google Scholar]
- Mayekar, V.M.; Ali, A.; Alim, H.; Patel, N. A review: Antimicrobial activity of the medicinal spice plants to cure human disease. Plant Sci. Today 2021, 8, 629–646. [Google Scholar] [CrossRef]
- Peter, K.; Ravindran, P.; Nirmal Babu, K.; Divakaran, M. Breeding of Spice Crops (Black Pepper, Cardamom, Ginger and Turmeric); Academia: Tainan, Taiwan, 2008. [Google Scholar]
- Gottardi, D.; Bukvicki, D.; Prasad, S.; Tyagi, A.K. Beneficial effects of spices in food preservation and safety. Front. Microbiol. 2016, 7, 1394. [Google Scholar] [CrossRef]
- Mérida-Reyes, M.S.; Muñoz-Wug, M.A.; Oliva-Hernández, B.E.; Gaitán-Fernández, I.C.; Simas, D.L.R.; Ribeiro da Silva, A.J.; Pérez-Sabino, J.F. Composition and antibacterial activity of the essential oil from Pimenta dioica (L.) Merr. from Guatemala. Medicines 2020, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Sarathambal, C.; Rajagopal, S.; Viswanathan, R. Mechanism of antioxidant and antifungal properties of Pimenta dioica (L.) leaf essential oil on Aspergillus flavus. J. Food Sci. Technol. 2021, 58, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- ALrashidi, A.A.; Noumi, E.; Snoussi, M.; Feo, V.D. Chemical composition, antibacterial and anti-quorum sensing activities of Pimenta dioica L. essential oil and its major compound (eugenol) against foodborne pathogenic bacteria. Plants 2022, 11, 540. [Google Scholar] [CrossRef] [PubMed]
- Andallu, B.; Rajeshwari, C. Aniseeds (Pimpinella anisum L.) in health and disease. In Nuts and Seeds in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2011; pp. 175–181. [Google Scholar]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Dubey, N.K. Nanostructured Pimpinella anisum essential oil as novel green food preservative against fungal infestation, aflatoxin B1 contamination and deterioration of nutritional qualities. Food Chem. 2021, 344, 128574. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, E.; Muselin, F.; Tîrziu, E.; Folescu, M.; Dumitrescu, C.S.; Orboi, D.M.; Cristina, R.T. Pimpinella anisum L. Essential Oil a Valuable Antibacterial and Antifungal Alternative. Plants 2023, 12, 2428. [Google Scholar] [CrossRef] [PubMed]
- Sayadi, M.; Mojaddar Langroodi, A.; Jafarpour, D. Impact of zein coating impregnated with ginger extract and Pimpinella anisum essential oil on the shelf life of bovine meat packaged in modified atmosphere. J. Food Meas. Charact. 2021, 15, 5231–5244. [Google Scholar] [CrossRef]
- Javanmardi, J.; Khalighi, A.; Kashi, A.; Bais, H.; Vivanco, J. Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. J. Agric. Food Chem. 2002, 50, 5878–5883. [Google Scholar] [CrossRef]
- Kathirvel, P.; Ravi, S. Chemical composition of the essential oil from basil (Ocimum basilicum Linn.) and its in vitro cytotoxicity against HeLa and HEp-2 human cancer cell lines and NIH 3T3 mouse embryonic fibroblasts. Nat. Prod. Res. 2012, 26, 1112–1118. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Attia, F.A.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Goswami, P.; Singh, V.R.; Verma, S.K.; Darokar, M.P.; Singh, N.; Saikia, D.; Chanotiya, C.S. Essential Oil Composition and Antimicrobial Activity of Methyl cinnamate-Linalool Chemovariant of Ocimum basilicum L. from Indi Rajendra Chandra Padalia, Ram Swaroop Verma, Amit Chauhan, Prakash Goswami, Ved Ram Singh, Sajendra Kumar Verma, Mahendra Pandurang Darokar, Alka kurmi, Nandan Singh, Dharmendra Saikia and Chandan Singh Chanotiya. Rec. Nat. Prod. 2017, 11, 193. [Google Scholar]
- Nadeem, H.R.; Akhtar, S.; Ismail, T.; Qamar, M.; Sestili, P.; Saeed, W.; Azeem, M.; Esatbeyoglu, T. Antioxidant Effect of Ocimum basilicum Essential Oil and Its Effect on Cooking Qualities of Supplemented Chicken Nuggets. Antioxidants 2022, 11, 1882. [Google Scholar] [CrossRef]
- Khan, F.A.; Mahmood, T.; Ali, M.; Saeed, A.; Maalik, A. Pharmacological importance of an ethnobotanical plant: Capsicum annuum L. Nat. Prod. Res. 2014, 28, 1267–1274. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A. Biological properties, bioactive constituents, and pharmacokinetics of some Capsicum spp. and capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Esparza, L.M.; Mora, Z.V.-d.l.; Vázquez-Paulino, O.; Ascencio, F.; Villarruel-López, A. Bell peppers (Capsicum annum L.) losses and wastes: Source for food and pharmaceutical applications. Molecules 2021, 26, 5341. [Google Scholar] [CrossRef] [PubMed]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2019, 274, 872–885. [Google Scholar] [CrossRef]
- Srinivasan, K. Black pepper and its pungent principle-piperine: A review of diverse physiological effects. Crit. Rev. Food Sci. Nutr. 2007, 47, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, E.M.; Abdalla, W.E. Black pepper fruit (Piper nigrum L.) as antibacterial agent: A mini-review. J. Bacteriol. Mycol. Open Access 2018, 6, 141–145. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.; Pandian, A.; Warkentin, T.D. Phytochemistry and therapeutic potential of black pepper [Piper nigrum (L.)] essential oil and piperine: A review. Clin. Phytosci. 2021, 7, 52. [Google Scholar] [CrossRef]
- Zarai, Z.; Boujelbene, E.; Salem, N.B.; Gargouri, Y.; Sayari, A. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT-Food Sci. Technol. 2013, 50, 634–641. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, J.; Famous, E.; Pan, S.; Peng, X.; Tian, J. Antioxidant, hepatoprotective and antifungal activities of black pepper (Piper nigrum L.) essential oil. Food Chem. 2021, 346, 128845. [Google Scholar] [CrossRef]
- Nakatani, N.; Inatani, R.; Ohta, H.; Nishioka, A. Chemical constituents of peppers (Piper spp.) and application to food preservation: Naturally occurring antioxidative compounds. Environ. Health Perspect. 1986, 67, 135–142. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z.; Ahmad, H.; Hayat, S.S.S.; Inayat, N.; Siyyar, S. Nigella sativa L.: Uses in traditional and contemporary medicines—An overview. Acta Ecol. Sin. 2021, 41, 253–258. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Manap, A.; Yazid, M.; Tan, C.P.; Muhialdin, B.J.; Alhelli, A.M.; Meor Hussin, A.S. The effects of different extraction methods on antioxidant properties, chemical composition, and thermal behavior of black seed (Nigella sativa L.) oil. Evid. Based Complement. Altern. Med. 2016, 2016, 6273817. [Google Scholar] [CrossRef] [PubMed]
- Al-Ameedy, T.H.; Omran, R. Antimicrobial Activity of Nigella Sativa Extract Against some Bacterial and Fungal Species. J. Univ. Babylon Pure Appl. Sci. 2019, 27, 277–286. [Google Scholar]
- Elsharkawy, E.R.; Abdallah, E.M.; Markb, A.A. Potential Cytotoxic, Antifungal, and Antioxidant Activity of Dithymoquinone and Thymoquinone. J. Hunan Univ. Nat. Sci. 2021, 48, 90–99. [Google Scholar]
- Foroughi, A.; Pournaghi, P.; Tahvilian, R.; Zangeneh, M.M.; Zangeneh, A.; Moradi, R. Ethnomedicinal plants: Study on the chemical composition and antibacterial activity of the Nigella sativa (Black seed) oil’s. Int. J. Pharm. Clin. Res. 2016, 8, 1528–1532. [Google Scholar]
- Sachan, A.K.; Das, D.R.; Kumar, M. Carum carvi-An important medicinal plant. J. Chem. Pharm. Res. 2016, 8, 529–533. [Google Scholar]
- Raal, A.; Arak, E.; Orav, A. The content and composition of the essential oil found in Carum carvi L. commercial fruits obtained from different countries. J. Essent. Oil Res. 2012, 24, 53–59. [Google Scholar]
- Lizarazo, C.I.; Lampi, A.-M.; Mäkelä, P.S. Can foliar-applied nutrients improve caraway (Carum carvi L.) seed oil composition? Ind. Crops Prod. 2021, 170, 113793. [Google Scholar] [CrossRef]
- Bharti, S.; Pathak, V.; Alam, T.; Arya, A.; Singh, V.; Verma, A.; Rajkumar, V. Starch bio-based composite active edible film functionalized with Carum carvi L. essential oil: Antimicrobial, rheological, physic-mechanical and optical attributes. J. Food Sci. Technol. 2022, 59, 456–466. [Google Scholar] [CrossRef]
- Lasram, S.; Zemni, H.; Hamdi, Z.; Chenenaoui, S.; Houissa, H.; Tounsi, M.S.; Ghorbel, A. Antifungal and antiaflatoxinogenic activities of Carum carvi L., Coriandrum sativum L. seed essential oils and their major terpene component against Aspergillus flavus. Ind. Crops Prod. 2019, 134, 11–18. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Murugan, M.; Dhanya, M.; Warkentin, T.D. Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton]—A critical review. J. Ethnopharmacol. 2020, 246, 112244. [Google Scholar] [CrossRef]
- Goncalves, L.M.; Valente, I.M.; Rodrigues, J.A. An overview on cardamonin. J. Med. Food 2014, 17, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Tandon, S.; Ahmad, J.; Yadav, A.; Kahol, A. Essential oil composition of seed and fruit coat of Elettaria cardamomum from South India. J. Essent. Oil Bear. Plants 2005, 8, 204–207. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Ramadhani, A.M.; Erwa, I.Y. Phytochemical screening, chemical composition and antibacterial activity of essential oil of cardamom. World J. Pharm. Res 2019, 8, 1166–1175. [Google Scholar]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Alkhelaif, A.S. In vitro antimicrobial potency of Elettaria cardamomum ethanolic extract against multidrug resistant of food poisoning bacterial strains. J. King Saud Univ. Sci. 2022, 34, 102167. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Abdur, R.; MM, S.A. Cinnamon bark as antibacterial agent: A mini-review. GSC Biol. Pharm. Sci. 2020, 10, 103–108. [Google Scholar] [CrossRef]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid. Based Complement. Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; Mrabti, H.N.; El Hachlafi, N.; El Kamili, T.; Elbouzidi, A.; Abdallah, E.M.; Flouchi, R.; Assaggaf, H.; Qasem, A.; Zengin, G. Integrated analysis of antimicrobial, antioxidant, and phytochemical properties of Cinnamomum verum: A comprehensive In vitro and In silico study. Biochem. Syst. Ecol. 2023, 110, 104700. [Google Scholar] [CrossRef]
- Senhaji, O.; Faid, M.; Kalalou, I. Inactivation of Escherichia coli O157: H7 by essential oil from Cinnamomum zeylanicum. Braz. J. Infect. Dis. 2007, 11, 234–236. [Google Scholar] [CrossRef]
- Bhakta, S.; Das, S.K. In praise of the phytogenic medicinal plant Syzygium aromaticum: A review. Turk. J. Agric.-Food Sci. Technol. 2021, 9, 1863–1868. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove essential oil (Syzygium aromaticum L. Myrtaceae): Extraction, chemical composition, food applications, and essential bioactivity for human health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef]
- Behbahani, B.A.; Noshad, M.; Falah, F. Study of Chemical Structure, Antimicrobial, Cytotoxic and Mechanism of Action of Syzygium aromaticum Essential Oil on Foodborne Pathogens. Potravinarstvo 2019, 13, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Galovičová, L.; Borotová, P.; Valková, V.; Ďúranová, H.; Kowalczewski, P.Ł.; Said-Al Ahl, H.A.; Hikal, W.M.; Vukic, M.; Savitskaya, T. Chemical composition, in vitro and in situ antimicrobial and antibiofilm activities of Syzygium aromaticum (Clove) essential oil. Plants 2021, 10, 2185. [Google Scholar] [CrossRef]
- Idowu, S.; Adekoya, A.E.; Igiehon, O.O.; Idowu, A.T. Clove (Syzygium aromaticum) spices: A review on their bioactivities, current use, and potential application in dairy products. J. Food Meas. Charact. 2021, 15, 3419–3435. [Google Scholar] [CrossRef]
- Mahleyuddin, N.N.; Moshawih, S.; Ming, L.C.; Zulkifly, H.H.; Kifli, N.; Loy, M.J.; Sarker, M.M.R.; Al-Worafi, Y.M.; Goh, B.H.; Thuraisingam, S. Coriandrum sativum L.: A review on ethnopharmacology, phytochemistry, and cardiovascular benefits. Molecules 2021, 27, 209. [Google Scholar] [CrossRef] [PubMed]
- Khani, A.; Rahdari, T. Chemical composition and insecticidal activity of essential oil from Coriandrum sativum seeds against Tribolium confusum and Callosobruchus maculatus. Int. Sch. Res. Not. 2012, 2012, 263517. [Google Scholar] [CrossRef]
- Kačániová, M.; Galovičová, L.; Ivanišová, E.; Vukovic, N.L.; Štefániková, J.; Valková, V.; Borotová, P.; Žiarovská, J.; Terentjeva, M.; Felšöciová, S. Antioxidant, antimicrobial and antibiofilm activity of coriander (Coriandrum sativum L.) essential oil for its application in foods. Foods 2020, 9, 282. [Google Scholar] [CrossRef]
- Noshad, M.; Behbahani, B.A.; Nikfarjam, Z.; Zargari, F. Antimicrobial activity between Coriandrum sativum seed and Cuminum cyminum essential oils against foodborne pathogens: A multi-ligand molecular docking simulation. LWT 2023, 185, 115217. [Google Scholar] [CrossRef]
- Singh, R.P.; Gangadharappa, H.; Mruthunjaya, K. Cuminum cyminum—A popular spice: An updated review. Pharmacogn. J. 2017, 9, 292–301. [Google Scholar] [CrossRef]
- Sowbhagya, H. Chemistry, technology, and nutraceutical functions of cumin (Cuminum cyminum L.): An overview. Crit. Rev. Food Sci. Nutr. 2013, 53, 1–10. [Google Scholar] [CrossRef]
- Wongkattiya, N.; Sanguansermsri, P.; Fraser, I.H.; Sanguansermsri, D. Antibacterial activity of cuminaldehyde on food-borne pathogens, the bioactive component of essential oil from Cuminum cyminum L. collected in Thailand. J. Complement. Integr. Med. 2019, 16, 20180195. [Google Scholar] [CrossRef]
- Ghasemi, G.; Fattahi, M.; Alirezalu, A.; Ghosta, Y. Antioxidant and antifungal activities of a new chemovar of cumin (Cuminum cyminum L.). Food Sci. Biotechnol. 2019, 28, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Shekhawat, G.S. Anethum graveolens: An Indian traditional medicinal herb and spice. Pharmacogn. Rev. 2010, 4, 179. [Google Scholar] [PubMed]
- Dimov, M.; Dobreva, K.; Stoyanova, A. Chemical composition of the dill essential oils (Anethum graveolens L.) from Bulgaria. Bulg. Chem. Commun. 2019, 51, 214–216. [Google Scholar]
- Chahal, K.; Kumar, A.; Bhardwaj, U.; Kaur, R. Chemistry and biological activities of Anethum graveolens L. (dill) essential oil: A review. J. Pharmacogn. Phytochem. 2017, 6, 295–306. [Google Scholar]
- Najaran, Z.T.; Hassanzadeh, M.K.; Nasery, M.; Emami, S.A. Dill (Anethum graveolens L.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 405–412. [Google Scholar]
- He, W.; Huang, B. A review of chemistry and bioactivities of a medicinal spice: Foeniculum vulgare. J. Med. Plants Res. 2011, 5, 3595–3600. [Google Scholar]
- Tognolini, M.; Ballabeni, V.; Bertoni, S.; Bruni, R.; Impicciatore, M.; Barocelli, E. Protective effect of Foeniculum vulgare essential oil and anethole in an experimental model of thrombosis. Pharmacol. Res. 2007, 56, 254–260. [Google Scholar] [CrossRef]
- Abdollahi, M.R.; Kianersi, F.; Moosavi, S.S.; Dastan, D.; Asadi, S. Identification and Expression Analysis of Two Genes Involved in the Biosynthesis of t-Anethole in Fennel (Foeniculum vulgare Mill.) and Their Up-Regulation in Leaves in Response to Methyl Jasmonate Treatments. J. Plant Growth Regul. 2023, 42, 759–770. [Google Scholar] [CrossRef]
- Diao, W.-R.; Hu, Q.-P.; Zhang, H.; Xu, J.-G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.-H.; Khalel, K.I. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.). Ind. Crops Prod. 2013, 44, 437–445. [Google Scholar] [CrossRef]
- Sarwar, S.; Hanif, M.A.; Ayub, M.A.; Boakye, Y.D.; Agyare, C. Fenugreek. In Medicinal Plants of South Asia; Elsevier: Amsterdam, The Netherlands, 2020; pp. 257–271. [Google Scholar]
- Nagulapalli Venkata, K.C.; Swaroop, A.; Bagchi, D.; Bishayee, A. A small plant with big benefits: Fenugreek (Trigonella foenum-graecum Linn.) for disease prevention and health promotion. Mol. Nutr. Food Res. 2017, 61, 1600950. [Google Scholar] [CrossRef]
- ElNour, M.E.; Ali, A.M.; Saeed, B. Antimicrobial activities and phytochemical screening of callus and seeds extracts of fenugreek (Trigonella foenum-graecum). Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 147–157. [Google Scholar]
- Hatab, S.; Lin, K.; Miao, W.; Chen, M.; Lin, J.; Deng, S. Potential utilization of green tea leaves and fenugreek seeds extracts as natural preservatives for pacific white shrimp during refrigerated storage. Foodborne Pathog. Dis. 2018, 15, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Londhe, V.; Gavasane, A.; Nipate, S.; Bandawane, D.; Chaudhari, P. Role of garlic (Allium sativum) in various diseases: An overview. Angiogenesis 2011, 12, 129–134. [Google Scholar]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; Wasef, L.G.; Elewa, Y.H.; Al-Sagan, A.; Abd El-Hack, M.E.; Taha, A.E.; M. Abd-Elhakim, Y.; Prasad Devkota, H. Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef]
- Najjaa, H.; Chekki, R.; Elfalleh, W.; Tlili, H.; Jaballah, S.; Bouzouita, N. Freeze-dried, oven-dried, and microencapsulation of essential oil from Allium sativum as potential preservative agents of minced meat. Food Sci. Nutr. 2020, 8, 1995–2003. [Google Scholar] [CrossRef]
- Abdallah, E.M. In vitro antibacterial evaluation of fresh garlic juice (Allium sativum L.) cultivated in Sudan. Acad. J. Life Sci. 2017, 3, 89–93. [Google Scholar]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Dhanik, J.; Arya, N.; Nand, V. A review on Zingiber officinale. J. Pharmacogn. Phytochem. 2017, 6, 174–184. [Google Scholar]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Zhang, Y. Research progress on chemical constituents of Zingiber officinale Roscoe. BioMed Res. Int. 2019, 2019, 5370823. [Google Scholar] [CrossRef]
- Abdalla, W.E.; Abdallah, E.M. Antibacterial activity of ginger (Zingiber officinale Rosc.) rhizome: A mini review. Int. J. Pharmacogn. Chin. Med. 2018, 2, 000142. [Google Scholar] [CrossRef]
- Beristain-Bauza, S.D.C.; Hernández-Carranza, P.; Cid-Pérez, T.S.; Ávila-Sosa, R.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. Antimicrobial activity of ginger (Zingiber officinale) and its application in food products. Food Rev. Int. 2019, 35, 407–426. [Google Scholar] [CrossRef]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): A review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef]
- Tabanca, N.; Nalbantsoy, A.; Kendra, P.E.; Demirci, F.; Demirci, B. Chemical characterization and biological activity of the mastic gum essential oils of Pistacia lentiscus var. chia from Turkey. Molecules 2020, 25, 2136. [Google Scholar] [CrossRef] [PubMed]
- Landau, S.; Muklada, H.; Markovics, A.; Azaizeh, H. Traditional uses of Pistacia lentiscus in veterinary and human medicine. In Medicinal and Aromatic Plants of the Middle-East; Springer: Dordrecht, The Netherlands, 2014; pp. 163–180. [Google Scholar]
- Mitropoulou, G.; Bardouki, H.; Vamvakias, M.; Panas, P.; Paraskevas, P.; Kourkoutas, Y. Assessment of Antimicrobial Efficiency of Pistacia lentiscus and Fortunella margarita Essential Oils against Spoilage and Pathogenic Microbes in Ice Cream and Fruit Juices. Microbiol. Res. 2022, 13, 667–680. [Google Scholar] [CrossRef]
- Periasamy, G.; Karim, A.; Gibrelibanos, M.; Gebremedhin, G. Nutmeg (Myristica fragrans Houtt.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 607–616. [Google Scholar]
- Asgarpanah, J.; Kazemivash, N. Phytochemistry and pharmacologic properties of Myristica fragrans Hoyutt.: A review. Afr. J. Biotechnol. 2012, 11, 12787–12793. [Google Scholar] [CrossRef]
- Marzuki, I.; Joefrie, B.; Aziz, S.A.; Agusta, H.; Surahman, M. Physico-chemical characterization of Maluku nutmeg oil. Int. J. Sci. Eng. 2014, 7, 61–64. [Google Scholar] [CrossRef]
- Gupta, A.D.; Bansal, V.K.; Babu, V.; Maithil, N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J. Genet. Eng. Biotechnol. 2013, 11, 25–31. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, X.; Zhao, C.; Cui, H. Liposome containing nutmeg oil as the targeted preservative against Listeria monocytogenes in dumplings. RSC Adv. 2016, 6, 978–986. [Google Scholar] [CrossRef]
- Agyare, C.; Appiah, T.; Boakye, Y.D.; Apenteng, J.A. Petroselinum crispum: A review. In Medicinal Spices and Vegetables from Africa; Academic Press: Cambridge, MA, USA, 2017; pp. 527–547. [Google Scholar]
- Poureini, F.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Comparative study on the extraction of apigenin from parsley leaves (Petroselinum crispum L.) by ultrasonic and microwave methods. Chem. Pap. 2020, 74, 3857–3871. [Google Scholar] [CrossRef]
- Snoussi, M.; Dehmani, A.; Noumi, E.; Flamini, G.; Papetti, A. Chemical composition and antibiofilm activity of Petroselinum crispum and Ocimum basilicum essential oils against Vibrio spp. strains. Microb. Pathog. 2016, 90, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Linde, G.; Gazim, Z.; Cardoso, B.; Jorge, L.; Tešević, V.; Glamočlija, J.; Soković, M.; Colauto, N. Antifungal and antibacterial activities of Petroselinum crispum essential oil. Genet. Mol. Res. 2016, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, C.; Abrams, T.R.; Brigham, A.; Ceurvels, J.; Clubb, J.; Curtiss, W.; Kirkwood, C.D.; Giese, N.; Hoehn, K.; Iovin, R. An evidence-based systematic review of rosemary (Rosmarinus officinalis) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2010, 7, 351–413. [Google Scholar] [CrossRef] [PubMed]
- Al-Sereiti, M.; Abu-Amer, K.; Sena, P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J. Exp. Biol. 1999, 37, 124–130. [Google Scholar] [PubMed]
- Xie, J.; VanAlstyne, P.; Uhlir, A.; Yang, X. A review on rosemary as a natural antioxidation solution. Eur. J. Lipid Sci. Technol. 2017, 119, 1600439. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and antimicrobial properties of rosemary (Rosmarinus officinalis, L.): A review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Srivastava, R.; Ahmed, H.; Dixit, R.K.; Saraf, S. Crocus sativus L.: A comprehensive review. Pharmacogn. Rev. 2010, 4, 200. [Google Scholar] [CrossRef]
- Samarghandian, S.; Borji, A. Anticarcinogenic effect of saffron (Crocus sativus L.) and its ingredients. Pharmacogn. Res. 2014, 6, 99. [Google Scholar] [CrossRef]
- Ahmed, N.; Anwar, S.; Al-Sokari, S.S.; Ansari, S.Y.; Wagih, M.E. Saffron crocus (Crocus sativus) oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 705–713. [Google Scholar]
- Muzaffar, S.; Rather, S.A.; Khan, K.Z. In vitro bactericidal and fungicidal activities of various extracts of saffron (Crocus sativus L.) stigmas from Jammu & Kashmir, India. Cogent Food Agric. 2016, 2, 1158999. [Google Scholar]
- Dauqan, E.M.; Abdullah, A. Medicinal and functional values of thyme (Thymus vulgaris L.) herb. J. Appl. Biol. Biotechnol. 2017, 5, 17–22. [Google Scholar]
- Pirbalouti, A.G.; Hashemi, M.; Ghahfarokhi, F.T. Essential oil and chemical compositions of wild and cultivated Thymus daenensis Celak and Thymus vulgaris L. Ind. Crops Prod. 2013, 48, 43–48. [Google Scholar] [CrossRef]
- Borugă, O.; Jianu, C.; Mişcă, C.; Goleţ, I.; Gruia, A.; Horhat, F. Thymus vulgaris essential oil: Chemical composition and antimicrobial activity. J. Med. Life 2014, 7, 56. [Google Scholar] [PubMed]
- Gonçalves, N.D.; de Lima Pena, F.; Sartoratto, A.; Derlamelina, C.; Duarte, M.C.T.; Antunes, A.E.C.; Prata, A.S. Encapsulated thyme (Thymus vulgaris) essential oil used as a natural preservative in bakery product. Food Res. Int. 2017, 96, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.; Leon, L. Biological activities of Curcuma longa L. Memórias Inst. Oswaldo Cruz 2001, 96, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Ilyas, S.; Parveen, Z.; Javed, S. Chemical analysis of essential oils from turmeric (Curcuma longa) rhizome through GC-MS. Asian J. Chem. 2010, 22, 3153. [Google Scholar]
- Abdeldaiem, M. Use of yellow pigment extracted from turmeric (Curcuma longa) rhizomes powder as natural food preservative. Am. J. Food Sci. Technol 2014, 2, 36–47. [Google Scholar]
- Zorofchian Moghadamtousi, S.; Abdul Kadir, H.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A review on antibacterial, antiviral, and antifungal activity of curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Bythrow, J.D. Vanilla as a medicinal plant. Semin. Integr. Med. 2005, 3, 129–131. [Google Scholar] [CrossRef]
- De Guzman, C.; Zara, R. Vanilla. In Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2012; pp. 547–589. [Google Scholar]
- Olatunde, A.; Mohammed, A.; Ibrahim, M.A.; Tajuddeen, N.; Shuaibu, M.N. Vanillin: A food additive with multiple biological activities. Eur. J. Med. Chem. Rep. 2022, 5, 100055. [Google Scholar] [CrossRef]
- Maisch, N.A.; Bereswill, S.; Heimesaat, M.M. Antibacterial effects of vanilla ingredients provide novel treatment options for infections with multidrug-resistant bacteria—A recent literature review. Eur. J. Microbiol. Immunol. 2022, 12, 53–62. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulieman, A.M.E.; Abdallah, E.M.; Alanazi, N.A.; Ed-Dra, A.; Jamal, A.; Idriss, H.; Alshammari, A.S.; Shommo, S.A.M. Spices as Sustainable Food Preservatives: A Comprehensive Review of Their Antimicrobial Potential. Pharmaceuticals 2023, 16, 1451. https://doi.org/10.3390/ph16101451
Sulieman AME, Abdallah EM, Alanazi NA, Ed-Dra A, Jamal A, Idriss H, Alshammari AS, Shommo SAM. Spices as Sustainable Food Preservatives: A Comprehensive Review of Their Antimicrobial Potential. Pharmaceuticals. 2023; 16(10):1451. https://doi.org/10.3390/ph16101451
Chicago/Turabian StyleSulieman, Abdel Moneim E., Emad M. Abdallah, Naimah Asid Alanazi, Abdelaziz Ed-Dra, Arshad Jamal, Hajo Idriss, Abdullah Sulaiman Alshammari, and Sohair A. M. Shommo. 2023. "Spices as Sustainable Food Preservatives: A Comprehensive Review of Their Antimicrobial Potential" Pharmaceuticals 16, no. 10: 1451. https://doi.org/10.3390/ph16101451
APA StyleSulieman, A. M. E., Abdallah, E. M., Alanazi, N. A., Ed-Dra, A., Jamal, A., Idriss, H., Alshammari, A. S., & Shommo, S. A. M. (2023). Spices as Sustainable Food Preservatives: A Comprehensive Review of Their Antimicrobial Potential. Pharmaceuticals, 16(10), 1451. https://doi.org/10.3390/ph16101451









