Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome—Review of Classical and New Compounds: Part-I
Abstract
1. Introduction
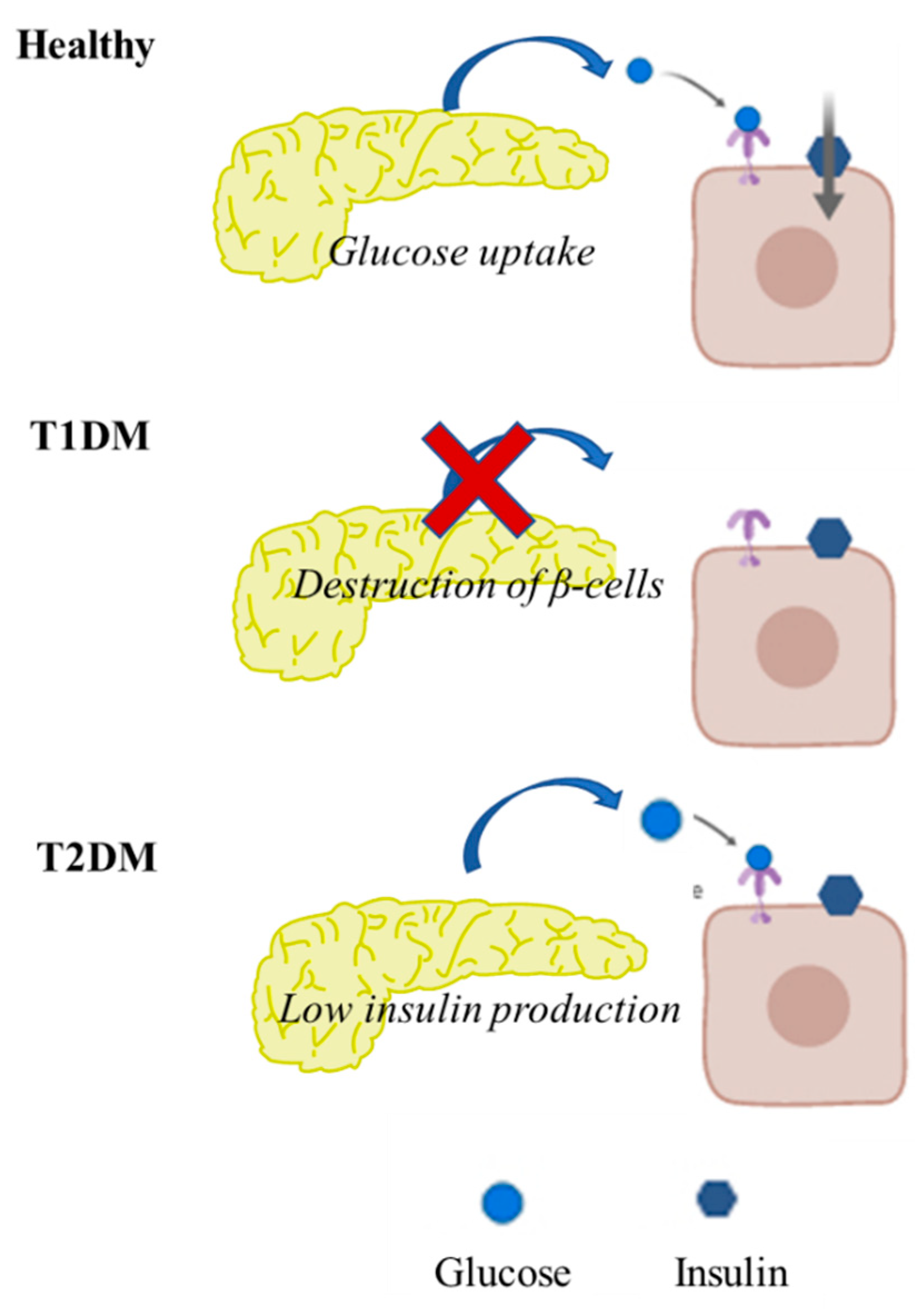
2. Pathophysiology of T2DM
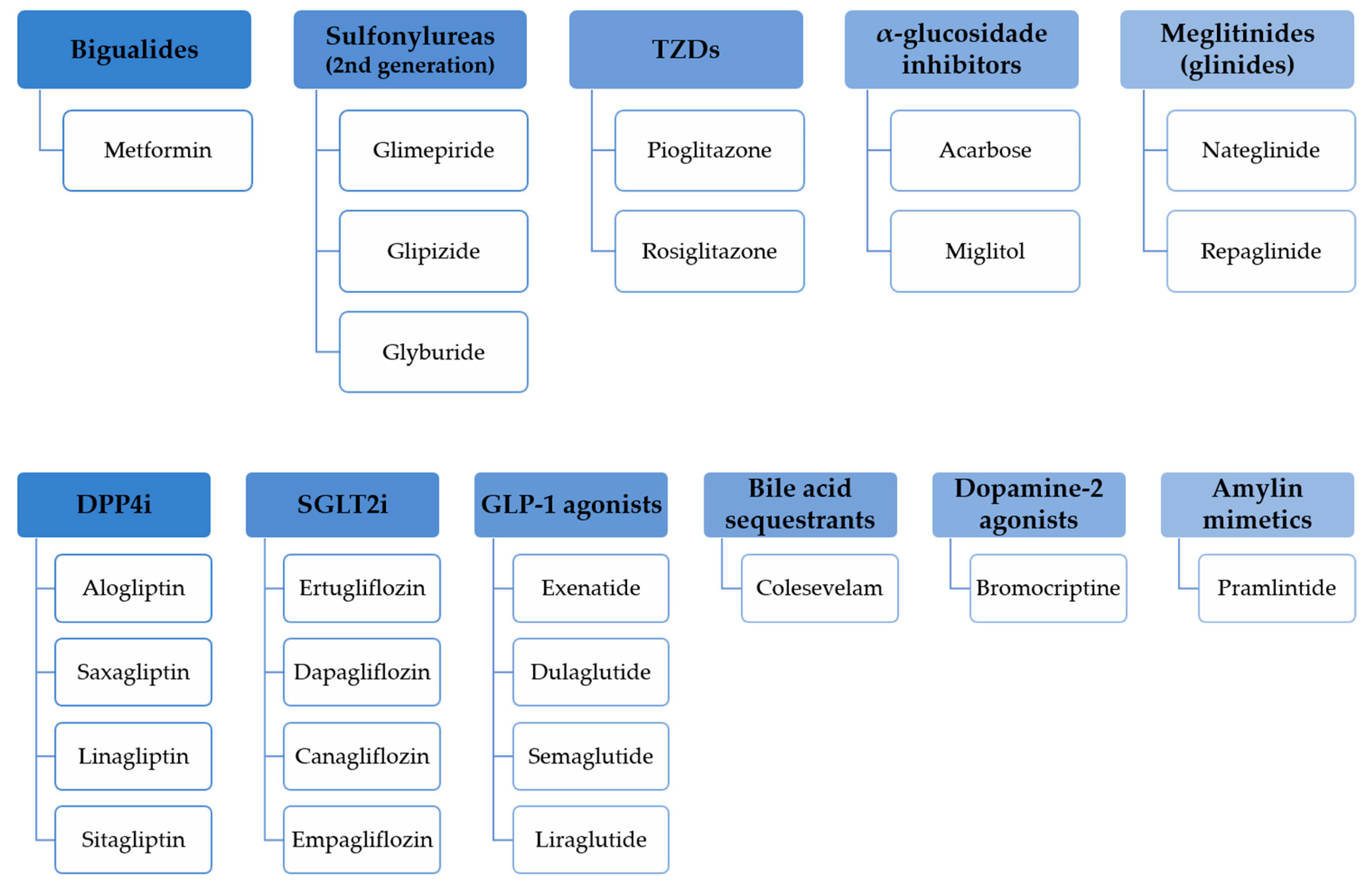
3. Oral and Injectable Hypoglycemic Drugs Overview
3.1. Registered Approaches
3.1.1. Sulphonylureas
3.1.2. Biguanides
3.1.3. Thiazolidinediones
3.1.4. α-Glucosidase Inhibitors
3.1.5. Sodium-Glucose Co-Transporter Type 2 (SGLT2) Inhibitors
3.1.6. Dual SGLT1/SGLT2 Agonists
3.1.7. Glucagon-Like Peptide-1 amide (GLP-1) Receptor Agonists
3.1.8. Dipeptidyl-peptidase-4 (DPP4) Inhibitors
3.1.9. Bile Acid Sequestrants
3.1.10. Dopamine Type 2 Receptor Agonists
3.1.11. Amylin Mimetics
3.2. Novel Approaches
3.2.1. Mangiferin-Berberine Salt
3.2.2. β-tigogenin Cellobioside (Tiqueside)
3.2.3. Incretin-Based Therapies
3.2.4. TGR 5 Agonists
3.2.5. β-Cell Acting Compounds
3.2.6. Small-Molecule Insulin Releasers
3.2.7. Glucokinase Activators
3.2.8. Fatty Acid Receptor Agonists
3.2.9. Imeglimin
3.2.10. Adipokine-Based Treatments
3.2.11. Selective Peroxisome Proliferator-Activated Receptor (PPAR) Modulators
3.2.12. β-hydroxysteroid Dehydrogenase 1 (11βHSD1) Inhibitors
3.2.13. Antiobesity Drugs
3.2.14. 55.P0110
3.2.15. Antiosteoporotic Trace Minerals: Silicon (Si) and Strontium (Sr)
3.2.16. Hybrid Molecules and Hybrid Natural Products
3.2.17. Bis(α-furancarboxylato)oxovanadium (IV) (BFOV)
3.2.18. LR Compounds: LR-9 and LR-74
3.2.19. Insulinomimetic Zinc (II) Complexes with Natural Products
3.2.20. 4-(2,2-dimethyl-L-oxopropyl)benzoic Acid
3.2.21. Boronated Nucleosides and Nucleotides
4. Natural Sugar Lowering Compounds
4.1. Diplacone and Mimulone
4.2. Ethanolic Extract of Semecarpus Anacardium (Linn.) Bark
4.3. Catalpol
4.4. Apigenin-6-C-2”-O-α-L-rhamnopyranosyl)-β-L-fucopyranoside
4.5. Sophora japonica L.
4.6. Water-Soluble Chitosan
4.7. Ginseng
4.8. Momordica charantia (Bitter Melon)
4.9. Trigonella foenum graecum (Fenugreek)
4.10. Gymnema sylvestre (Gurmar)
4.11. Allium cepa and Allium sativum
4.12. Pterocarpus marsupium and other Epicatechin-Containing Plants
4.13. Vaccinium myrtillus (Bilberry)
4.14. Atriplex halimus (Salt Bush)
4.15. Aloe vera
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Porth, C.M. Essentials of Pathophysiology: Concepts of Altered Health States, 3rd ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 15 October 2010; Volume 3, ISBN-10 1582557241; ISBN-13 978-1582557243. [Google Scholar]
- Timper, K.; Donath, M.Y. Diabetes mellitus Type 2—The new face of an old lady. Swiss Med. Wkly. 2012, 142, w13635. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.; Souto, S.B.; Vinha, E.; Braga, D.C.; Carvalho, D. Oral glucose lowering drugs in type 2 diabetic patients with chronic kidney disease. Hormones (Athens) 2013, 12, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Souto, S.B.; Souto, E.B.; Braga, D.C.; Medina, J.L. Prevention and current onset delay approaches of type 2 diabetes mellitus (T2DM). Eur. J. Clin. Pharmacol. 2011, 67, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.; Souto, S.B.; Sanchez-Lopez, E.; Machado, A.L.; Severino, P.; Jose, S.; Santini, A.; Silva, A.M.; Fortuna, A.; Garcia, M.L.; et al. Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome-Strategies for In Vivo Administration: Part-II. J. Clin. Med. 2019, 8, 1332. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.; Del Buono, M.G.; Ozemek, C.; Lavie, C.J. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog. Cardiovasc. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Wahed, M.I.; Khatune, N.A.; Rahman, B.M.; Barman, R.K.; Islam, M.R. Antidiabetic and antioxidant activities of ethanolic extract of Semecarpus anacardium (Linn.) bark. BMC Complement. Altern. Med. 2015, 15, 138. [Google Scholar] [CrossRef]
- Bebernitz, G.R.; Dain, J.G.; Deems, R.O.; Otero, D.A.; Simpson, W.R.; Strohschein, R.J. Reduction in Glucose Levels in STZ Diabetic Rats by 4-(2,2-Dimethyl-1-oxopropyl)benzoic Acid: A Prodrug Approach for Targeting the Liver. J. Med. Chem. 2001, 44, 512–523. [Google Scholar] [CrossRef]
- Scheen, A.J. Pharmacodynamics, Efficacy and Safety of Sodium–Glucose Co-Transporter Type 2 (SGLT2) Inhibitors for the Treatment of Type 2 Diabetes Mellitus. Drugs 2015, 75, 33–59. [Google Scholar] [CrossRef]
- Vivot, K.; Pasquier, A.; Goginashvili, A.; Ricci, R. Breaking Bad and Breaking Good: Beta-Cell Autophagy Pathways in Diabetes. J. Mol. Biol. 2019. [Google Scholar] [CrossRef]
- Purcell, A.W.; Sechi, S.; DiLorenzo, T.P. The Evolving Landscape of Autoantigen Discovery and Characterization in Type 1 Diabetes. Diabetes 2019, 68, 879–886. [Google Scholar] [CrossRef]
- Smolenski, S.; George, N.M. Management of ketosis-prone type 2 diabetes mellitus. J. Am. Assoc. Nurse Pract. 2019, 31, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Cavazzana, I.; Piantoni, S.; Sciatti, E.; Fredi, M.; Taraborelli, M.; Bonadei, I.; Airo, P.; Metra, M.; Tincani, A.; Franceschini, F.; et al. Relationship between endothelial dysfunction, videocapillaroscopy and circulating CD3+CD31+CXCR4+ lymphocytes in systemic lupus erythematosus without cardiovascular risk factors. Lupus 2019, 28, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Z.; Jiang, Z.; Sun, L.; Ji, J.; Miao, J.; Zhang, X.; Li, X.; Huang, S.; Wang, T.; et al. Hypoglycemic effect of catalpol on high-fat diet/streptozotocin-induced diabetic mice by increasing skeletal muscle mitochondrial biogenesis. Acta Biochim. Biophys. Sin. 2014, 46, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Zaccardi, F.; Webb, D.R.; Yates, T.; Davies, M.J. Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgrad. Med. J. 2016, 92, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Taskinen, M.-R.; Borén, J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis 2015, 239, 483–495. [Google Scholar] [CrossRef]
- Von Ah Morano, A.E.; Dorneles, G.P.; Peres, A.; Lira, F.S. The role of glucose homeostasis on immune function in response to exercise: The impact of low or higher energetic conditions. J. Cell. Physiol. 2019. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Wu, H. Metabolic Stress and Cardiovascular Disease in Diabetes Mellitus: The Role of Protein O-GlcNAc Modification. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1911–1924. [Google Scholar] [CrossRef]
- Al-Massadi, O.; Ferno, J.; Dieguez, C.; Nogueiras, R.; Quinones, M. Glucagon Control on Food Intake and Energy Balance. Int. J. Mol. Sci. 2019, 20, 3905. [Google Scholar] [CrossRef]
- Wen, S.; Wang, C.; Gong, M.; Zhou, L. An overview of energy and metabolic regulation. Sci. China Life Sci. 2019, 62, 771–790. [Google Scholar] [CrossRef]
- Weerawatanakorn, M.; Asikin, Y.; Takahashi, M.; Tamaki, H.; Wada, K.; Ho, C.-T.; Chuekittisak, R. Physico-chemical properties, wax composition, aroma profiles, and antioxidant activity of granulated non-centrifugal sugars from sugarcane cultivars of Thailand. J. Food Sci. Technol. 2016, 53, 4084–4092. [Google Scholar] [CrossRef]
- Figarola, J.L.; Scott, S.; Loera, S.; Xi, B.; Synold, T.; Rahbar, S. Renoprotective and Lipid-Lowering Effects of LR Compounds, Novel Advanced Glycation End Product Inhibitors, in Streptozotocin-Induced Diabetic Rats. Ann. N. Y. Acad. Sci. 2005, 1043, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Khunti, K.; Chatterjee, S.; Gerstein, H.C.; Zoungas, S.a.; Davies, M.J. Do sulphonylureas still have a place in clinical practice? Lancet Diabetes Endocrinol. 2018, 6, 821–832. [Google Scholar] [CrossRef]
- Jeppesen, P.B.; Gregersen, S.; Poulsen, C.R.; Hermansen, K. Stevioside acts directly on pancreatic β cells to secrete insulin: Actions independent of cyclic adenosine monophosphate and adenosine triphosphate— sensitivie K+-channel activity. Metab. Clin. Exp. 2000, 49, 208–214. [Google Scholar] [CrossRef]
- Sun, X.; Narciso, J.; Wang, Z.; Ference, C.; Bai, J.; Zhou, K. Effects of Chitosan-Essential Oil Coatings on Safety and Quality of Fresh Blueberries. J. Food Sci. 2014, 79, M955–M960. [Google Scholar] [CrossRef] [PubMed]
- Mildner-Szkudlarz, S.; Siger, A.; Szwengiel, A.; Bajerska, J. Natural compounds from grape by-products enhance nutritive value and reduce formation of CML in model muffins. Food Chem. 2015, 172, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Simental-Mendia, L.E.; Barreto, G.E.; Sahebkar, A. Metabolic effects of antidiabetic drugs on adipocytes and adipokine expression. J. Cell. Physiol. 2019, 234, 16987–16997. [Google Scholar] [CrossRef]
- Wroblewski, A.; Strycharz, J.; Swiderska, E.; Drewniak, K.; Drzewoski, J.; Szemraj, J.; Kasznicki, J.; Sliwinska, A. Molecular Insight into the Interaction between Epigenetics and Leptin in Metabolic Disorders. Nutrients 2019, 11, 1872. [Google Scholar] [CrossRef]
- Fappi, A.; Mittendorfer, B. Different physiological mechanisms underlie an adverse cardiovascular disease risk profile in men and women. Proc. Nutr. Soc. 2019. [Google Scholar] [CrossRef]
- White, U.; Ravussin, E. Dynamics of adipose tissue turnover in human metabolic health and disease. Diabetologia 2019, 62, 17–23. [Google Scholar] [CrossRef]
- Taskinen, M.-R. Diabetic dyslipidemia. Atheroscler. Suppl. 2002, 3, 47–51. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Oral and Injectable (Non-insulin) Pharmacological Agents for Type 2 Diabetes. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., Dungan, K., Grossman, A., Hershman, J.M., Kaltsas, G., Koch, C., Kopp, P., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Sola, D.; Rossi, L.; Schianca, G.P.C.; Maffioli, P.; Bigliocca, M.; Mella, R.; Corlianò, F.; Fra, G.P.; Bartoli, E.; Derosa, G. Sulfonylureas and their use in clinical practice. Arch. Med. Sci. 2015, 11, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Hundal, R.S.; Krssak, M.; Dufour, S.; Laurent, D.; Lebon, V.; Chandramouli, V.; Inzucchi, S.E.; Schumann, W.C.; Petersen, K.F.; Landau, B.R.; et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 2000, 49, 2063–2069. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Olefsky, J.M. Thiazolidinediones in the Treatment of Insulin Resistance and Type II Diabetes. Diabetes 1996, 45, 1661–1669. [Google Scholar] [CrossRef]
- Despopoulos, A.; Silbernagl, S. Color Atlas of Physiology, 7th ed.; Thieme Publisher Stuttgart: Stuttgart, Germany, 13 May 2015; ISBN-10 3135450074, ISBN-13 978-3135450070. [Google Scholar]
- Tonelli, J.; Li, W.; Kishore, P.; Pajvani, U.B.; Kwon, E.; Weaver, C.; Scherer, P.E.; Hawkins, M. Mechanisms of Early Insulin-Sensitizing Effects of Thiazolidinediones in Type 2 Diabetes. Diabetes 2004, 53, 1621–1629. [Google Scholar] [CrossRef]
- Hussein, Z.; Wentworth, J.M.; Nankervis, A.J.; Proietto, J.; Colman, P.G. Effectiveness and side effects of thiazolidinediones for type 2 diabetes: Real-life experience from a tertiary hospital. Med. J. Aust. 2004, 181, 536–539. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, J.D.; Wu, W.; Kong, W.J. The Compound of Mangiferin-Berberine Salt Has Potent Activities in Modulating Lipid and Glucose Metabolisms in HepG2 Cells. BioMed Res. Int. 2016, 2016, 8753436. [Google Scholar] [CrossRef]
- Schoonjans, K.; Auwerx, J. Thiazolidinediones: An update. Lancet 2000, 355, 1008–1010. [Google Scholar] [CrossRef]
- Scheen, A.J. Is There a Role for α-Glucosidase Inhibitors in the Prevention of Type 2 Diabetes Mellitus? Drugs 2003, 63, 933–951. [Google Scholar] [CrossRef]
- Dhatariya, K. Diabetes: The place of new therapies. Ther. Adv. Endocrinol. Metab. 2019. [Google Scholar] [CrossRef] [PubMed]
- Braga, T.; Kraemer-Aguiar, L.G.; Docherty, N.G.; Le Roux, C.W. Treating prediabetes: Why and how should we do it? Minerva Med. 2019, 110, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Tahrani, A.A.; Barnett, A.H. Future glucose-lowering drugs for type 2 diabetes. Lancet Diabetes Endocrinol. 2016, 4, 350–359. [Google Scholar] [CrossRef]
- Rendell, M.S. Sotagliflozin: A combined SGLT1/SGLT2 inhibitor to treat diabetes. Expert Rev. Endocrinol. Metab. 2018, 13, 333–339. [Google Scholar] [CrossRef]
- Cariou, B.; Charbonnel, B. Sotagliflozin as a potential treatment for type 2 diabetes mellitus. Expert Opin. Investig. Drugs 2015, 24, 1647–1656. [Google Scholar] [CrossRef]
- Zambrowicz, B.; Ding, Z.M.; Ogbaa, I.; Frazier, K.; Banks, P.; Turnage, A.; Freiman, J.; Smith, M.; Ruff, D.; Sands, A.; et al. Effects of LX4211, a dual SGLT1/SGLT2 inhibitor, plus sitagliptin on postprandial active GLP-1 and glycemic control in type 2 diabetes. Clin. Ther. 2013, 35, 273–285. [Google Scholar] [CrossRef]
- Rosenstock, J.; Cefalu, W.T.; Lapuerta, P.; Zambrowicz, B.; Ogbaa, I.; Banks, P.; Sands, A. Greater dose-ranging effects on A1C levels than on glucosuria with LX4211, a dual inhibitor of SGLT1 and SGLT2, in patients with type 2 diabetes on metformin monotherapy. Diabetes Care 2015, 38, 431–438. [Google Scholar] [CrossRef]
- Torekov, S.S.; Holst, J.J.; Ehlers, M.R. Dose response of continuous subcutaneous infusion of recombinant glucagon-like peptide-1 in combination with metformin and sulphonylurea over 12 weeks in patients with type 2 diabetes mellitus. Diabetes Obes. Metab. 2014, 16, 451–456. [Google Scholar] [CrossRef]
- Melino, S.; Leo, S.; Toska Papajani, V. Natural Hydrogen Sulfide Donors from Allium sp. as a Nutraceutical Approach in Type 2 Diabetes Prevention and Therapy. Nutrients 2019, 11, 1581. [Google Scholar] [CrossRef]
- Hansen, M.; Sonne, D.P.; Mikkelsen, K.H.; Gluud, L.L.; Vilsboll, T.; Knop, F.K. Effect of bile acid sequestrants on glycaemic control: Protocol for a systematic review with meta-analysis of randomised controlled trials. BMJ Open 2012. [Google Scholar] [CrossRef]
- Hansen, M.; Sonne, D.P.; Mikkelsen, K.H.; Gluud, L.L.; Vilsboll, T.; Knop, F.K. Bile acid sequestrants for glycemic control in patients with type 2 diabetes: A systematic review with meta-analysis of randomized controlled trials. J. Diabetes Complicat. 2017, 31, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.S.H.; Vinutha, M.B.; Pradeep, A.N.; Aithal, S.; Baleed, S.R.; Patil, U.N. Bromocriptine, a Dopamine (d2) Receptor Agonist, Used Alone and in Combination with Glipizide in Sub-Therapeutic Doses to Ameliorate Hyperglycaemia. J. Clin. Diagn. Res. 2013, 7, 1904–1907. [Google Scholar] [CrossRef]
- Mahajan, R. Bromocriptine mesylate: FDA-approved novel treatment for type-2 diabetes. Indian J. Pharmacol. 2009, 41, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Lopez Vicchi, F.; Luque, G.M.; Brie, B.; Nogueira, J.P.; Garcia Tornadu, I.; Becu-Villalobos, D. Dopaminergic drugs in type 2 diabetes and glucose homeostasis. Pharm. Res. 2016, 109, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hoskins, A.; Verma, N.; Bers, D.M.; Despa, S.; Despa, F. Amylin and diabetic cardiomyopathy—Amylin-induced sarcolemmal Ca2+ leak is independent of diabetic remodeling of myocardium. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1923–1930. [Google Scholar] [CrossRef]
- Boyle, C.N.; Lutz, T.A.; Le Foll, C. Amylin—Its role in the homeostatic and hedonic control of eating and recent developments of amylin analogs to treat obesity. Mol. Metab. 2018, 8, 203–210. [Google Scholar] [CrossRef]
- Association, A.D. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S90–S102. [Google Scholar] [CrossRef]
- Harwood, H.J.; Chandler, C.E.; Pellarin, L.D.; Bangerter, F.W.; Wilkins, R.W.; Long, C.A.; Cosgrove, P.G.; Malinow, M.R.; Marzetta, C.A.; Pettini, J.L. Pharmacologic consequences of cholesterol absorption inhibition: Alteration in cholesterol metabolism and reduction in plasma cholesterol concentration induced by the synthetic saponin beta-tigogenin cellobioside (CP-88818; tiqueside). J. Lipid Res. 1993, 34, 377–395. [Google Scholar]
- Yuan, H.-D.; Kim, J.T.; Kim, S.H.; Chung, S.H. Ginseng and diabetes: The evidences from in vitro, animal and human studies. J. Ginseng Res. 2012, 36, 27–39. [Google Scholar] [CrossRef]
- Kazakos, K. Incretin effect: GLP-1, GIP, DPP4. Diabetes Res. Clin. Pract. 2011, 93, S32–S36. [Google Scholar] [CrossRef]
- Matschinsky, F.M. Assessing the potential of glucokinase activators in diabetes therapy. Nat. Rev. Drug Discov. 2009, 8, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Terauchi, Y. Present status of clinical deployment of glucokinase activators. J. Diabetes Investig. 2015, 6, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-K.; Ahima, R.S. Physiology of leptin: Energy homeostasis, neuroendocrine function and metabolism. Metab. Clin. Exp. 2015, 64, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Belgardt, B.F.; Brüning, J.C. CNS leptin and insulin action in the control of energy homeostasis. Ann. N. Y. Acad. Sci. 2010, 1212, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.-S.; Wu, J.A.; Lowell, T.; Gu, M. Gut and Brain Effects of American Ginseng Root on Brainstem Neuronal Activities in Rats. Am. J. Chin. Med. 1998, 26, 47–55. [Google Scholar] [CrossRef]
- Brunmair, B.; Lehner, Z.; Stadlbauer, K.; Adorjan, I.; Frobel, K.; Scherer, T.; Luger, A.; Bauer, L.; Fürnsinn, C. 55P0110, a Novel Synthetic Compound Developed from a Plant Derived Backbone Structure, Shows Promising Anti-Hyperglycaemic Activity in Mice. PLoS ONE 2015, 10, e0126847. [Google Scholar] [CrossRef]
- Pham, H.T.T.; Hoang, M.C.; Ha, T.K.Q.; Dang, L.H.; Tran, V.O.; Nguyen, T.B.T.; Lee, C.H.; Oh, W.K. Discrimination of different geographic varieties of Gymnema sylvestre, an anti-sweet plant used for the treatment of type 2 diabetes. Phytochemistry 2018, 150, 12–22. [Google Scholar] [CrossRef]
- Kumar, S.; Misra, N.; Raj, K.; Srivastava, K.; Puri, S.K. Novel class of hybrid natural products derived from lupeol as antimalarial agents. Nat. Prod. Res. 2008, 22, 305–319. [Google Scholar] [CrossRef]
- Maehira, F.; Ishimine, N.; Miyagi, I.; Eguchi, Y.; Shimada, K.; Kawaguchi, D.; Oshiro, Y. Anti-diabetic effects including diabetic nephropathy of anti-osteoporotic trace minerals on diabetic mice. Nutrition 2011, 27, 488–495. [Google Scholar] [CrossRef]
- Dembinska-Kiec, A.; Mykkänen, O.; Kiec-Wilk, B.; Mykkänen, H. Antioxidant phytochemicals against type 2 diabetes. Br. J. Nutr. 2008, 99, ES109–ES117. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, W.; Tian, C.; Xie, M.; Gao, L.; Chen, Z.; Chen, X.; Li, L. Effects of bis(alpha-furancarboxylato)oxovanadium(IV) on glucose metabolism in fat-fed/streptozotocin-diabetic rats. Eur. J. Pharmacol. 2007, 572, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Treviño, S.; Díaz, A.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Yoshikawa, Y.; Ueda, E.; Ueda, R.; Yamamoto, S.; Kumekawa, K.; Yanagihara, N.; Sakurai, H. Insulinomimetic Zinc(II) Complexes with Natural Products: In Vitro Evaluation and Blood Glucose Lowering Effect in KK-Ay Mice with Type 2 Diabetes Mellitus. Chem. Pharm. Bull. 2003, 51, 1006–1008. [Google Scholar] [CrossRef] [PubMed]
- Hall, I.H.; Burnham, B.S.; Rajendran, K.G.; Chen, S.Y.; Sood, A.; Spielvogel, B.F.; Shaw, B.R. Hypolipidemic activity of boronated nucleosides and nucleotides in rodents. Biomed. Pharmacother. 1993, 47, 79–87. [Google Scholar] [CrossRef]
- Khan, H.B.H.; Vinayagam, K.S.; Moorthy, B.T.; Palanivelu, S.; Panchanatham, S. Anti-inflammatory and anti-hyperlipidemic effect of Semecarpus anacardium in a High fat diet: STZ-induced Type 2 diabetic rat model. Inflammopharmacology 2013, 21, 37–46. [Google Scholar] [CrossRef]
- Shieh, J.-P.; Cheng, K.-C.; Chung, H.-H.; Kerh, Y.-F.; Yeh, C.-H.; Cheng, J.-T. Plasma Glucose Lowering Mechanisms of Catalpol, an Active Principle from Roots of Rehmannia glutinosa, in Streptozotocin-Induced Diabetic Rats. J. Agric. Food Chem. 2011, 59, 3747–3753. [Google Scholar] [CrossRef]
- Sundaram, R.; Shanthi, P.; Sachdanandam, P. Effect of iridoid glucoside on plasma lipid profile, tissue fatty acid changes, inflammatory cytokines, and GLUT4 expression in skeletal muscle of streptozotocin-induced diabetic rats. Mol. Cell. Biochem. 2013, 380, 43–55. [Google Scholar] [CrossRef]
- Wang, C.-F.; Li, D.-Q.; Xue, H.-Y.; Hu, B. Oral supplementation of catalpol ameliorates diabetic encephalopathy in rats. Brain Res. 2010, 1307, 158–165. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Folador, P.; Moresco, H.H.; Brighente, I.M.C.; Pizzolatti, M.G.; Silva, F.R.M.B. Mechanism of action of the stimulatory effect of apigenin-6-C-(2″-O-α-l-rhamnopyranosyl)-β-l-fucopyranoside on 14C-glucose uptake. Chem. Biol. Interact. 2009, 179, 407–412. [Google Scholar] [CrossRef]
- Park, K.W.; Lee, J.-E.; Park, K.-m. Diets containing Sophora japonica L. prevent weight gain in high-fat diet-induced obese mice. Nutr. Res. 2009, 29, 819–824. [Google Scholar] [CrossRef]
- Naaz, A.; Yellayi, S.; Zakroczymski, M.A.; Bunick, D.; Doerge, D.R.; Lubahn, D.B.; Helferich, W.G.; Cooke, P.S. The Soy Isoflavone Genistein Decreases Adipose Deposition in Mice. Endocrinology 2003, 144, 3315–3320. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Yang, J.-Y.; Ambati, S.; Della-Fera, M.A.; Hausman, D.B.; Rayalam, S.; Baile, C.A. Combined Effects of Genistein, Quercetin, and Resveratrol in Human and 3T3-L1 Adipocytes. J. Med. Food 2008, 11, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Anraku, M.; Fujii, T.; Furutani, N.; Kadowaki, D.; Maruyama, T.; Otagiri, M.; Gebicki, J.M.; Tomida, H. Antioxidant effects of a dietary supplement: Reduction of indices of oxidative stress in normal subjects by water-soluble chitosan. Food Chem. Toxicol. 2009, 47, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Wissing, S.A.; Barbosa, C.M.; Muller, R.H. Evaluation of the physical stability of SLN and NLC before and after incorporation into hydrogel formulations. Eur. J. Pharm. Biopharm. 2004, 58, 83–90. [Google Scholar] [CrossRef]
- Jose, S.; Prema, M.T.; Chacko, A.J.; Thomas, A.C.; Souto, E.B. Colon specific chitosan microspheres for chronotherapy of chronic stable angina. Colloids Surf. B Biointerfaces 2011, 83, 277–283. [Google Scholar] [CrossRef]
- Jose, S.; Fangueiro, J.F.; Smitha, J.; Cinu, T.A.; Chacko, A.J.; Premaletha, K.; Souto, E.B. Cross-linked chitosan microspheres for oral delivery of insulin: Taguchi design and in vivo testing. Colloids Surf. B Biointerfaces 2012, 92, 175–179. [Google Scholar] [CrossRef]
- Severino, P.; de Oliveira, G.G.G.; Ferraz, H.G.; Souto, E.B.; Santana, M.H.A. Preparation of gastro-resistant pellets containing chitosan microspheres for improvement of oral didanosine bioavailability. J. Pharm. Anal. 2012, 2, 188–192. [Google Scholar] [CrossRef]
- Jose, S.; Ansa, C.R.; Cinu, T.A.; Chacko, A.J.; Aleykutty, N.A.; Ferreira, S.V.; Souto, E.B. Thermo-sensitive gels containing lorazepam microspheres for intranasal brain targeting. Int. J. Pharm. 2013, 441, 516–526. [Google Scholar] [CrossRef]
- Jose, S.; Fangueiro, J.F.; Smitha, J.; Cinu, T.A.; Chacko, A.J.; Premaletha, K.; Souto, E.B. Predictive modeling of insulin release profile from cross-linked chitosan microspheres. Eur. J. Med. Chem. 2013, 60, 249–253. [Google Scholar] [CrossRef]
- Severino, P.; Souto, E.B.; Pinho, S.C.; Santana, M.H. Hydrophilic coating of mitotane-loaded lipid nanoparticles: Preliminary studies for mucosal adhesion. Pharm. Dev. Technol. 2013, 18, 577–581. [Google Scholar] [CrossRef]
- Severino, P.; Da Silva, C.F.; Dalla Costa, T.C.; Silva, H.; Chaud, M.V.; Santana, M.H.; Souto, E.B. In vivo absorption of didanosine formulated in pellets composed of chitosan microspheres. In Vivo 2014, 28, 1045–1050. [Google Scholar] [PubMed]
- Andreani, T.; Miziara, L.; Lorenzon, E.N.; de Souza, A.L.; Kiill, C.P.; Fangueiro, J.F.; Garcia, M.L.; Gremiao, P.D.; Silva, A.M.; Souto, E.B. Effect of mucoadhesive polymers on the in vitro performance of insulin-loaded silica nanoparticles: Interactions with mucin and biomembrane models. Eur. J. Pharm. Biopharm. 2015, 93, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Ferreira da Silva, C.; Severino, P.; Martins, F.; Santana, M.H.; Souto, E.B. Didanosine-loaded chitosan microspheres optimized by surface-response methodology: A modified “Maximum Likelihood Classification” approach formulation for reverse transcriptase inhibitors. Biomed. Pharmacother. 2015, 70, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, G.P.; Debone, H.S.; Severino, P.; Souto, E.B.; da Silva, C.F. Design and characterization of chitosan/zeolite composite films—Effect of zeolite type and zeolite dose on the film properties. Mater. Sci Eng. C 2016, 60, 246–254. [Google Scholar] [CrossRef]
- Cefali, L.C.; Ataide, J.A.; Eberlin, S.; da Silva Goncalves, F.C.; Fernandes, A.R.; Marto, J.; Ribeiro, H.M.; Foglio, M.A.; Mazzola, P.G.; Souto, E.B. In vitro SPF and Photostability Assays of Emulsion Containing Nanoparticles with Vegetable Extracts Rich in Flavonoids. AAPS PharmSciTech 2018, 20, 9. [Google Scholar] [CrossRef]
- Andreani, T.; Fangueiro, J.F.; Severino, P.; Souza, A.L.R.; Martins-Gomes, C.; Fernandes, P.M.V.; Calpena, A.C.; Gremiao, M.P.; Souto, E.B.; Silva, A.M. The Influence of Polysaccharide Coating on the Physicochemical Parameters and Cytotoxicity of Silica Nanoparticles for Hydrophilic Biomolecules Delivery. Nanomaterials 2019. [Google Scholar] [CrossRef]
- Lü, J.-M.; Yao, Q.; Chen, C. Ginseng compounds: An update on their molecular mechanisms and medical applications. Curr. Vasc. Pharmacol. 2009, 7, 293–302. [Google Scholar] [CrossRef]
- Szczuka, D.; Nowak, A.; Zakłos-Szyda, M.; Kochan, E.; Szymańska, G.; Motyl, I.; Blasiak, J. American Ginseng (Panax quinquefolium L.) as a Source of Bioactive Phytochemicals with Pro-Health Properties. Nutrients 2019, 11, 1041. [Google Scholar] [CrossRef]
- Cui, S.; Wu, J.; Wang, J.; Wang, X. Discrimination of American ginseng and Asian ginseng using electronic nose and gas chromatography-mass spectrometry coupled with chemometrics. J. Ginseng Res. 2017, 41, 85–95. [Google Scholar] [CrossRef]
- Yue, P.Y.K.; Mak, N.K.; Cheng, Y.K.; Leung, K.W.; Ng, T.B.; Fan, D.T.P.; Yeung, H.W.; Wong, R.N.S. Pharmacogenomics and the Yin/Yang actions of ginseng: Anti-tumor, angiomodulating and steroid-like activities of ginsenosides. Chin. Med. 2007, 2, 6. [Google Scholar] [CrossRef]
- Peter, E.L.; Kasali, F.M.; Deyno, S.; Mtewa, A.; Nagendrappa, P.B.; Tolo, C.U.; Ogwang, P.E.; Sesaazi, D. Momordica charantia L. lowers elevated glycaemia in type 2 diabetes mellitus patients: Systematic review and meta-analysis. J. Ethnopharmacol. 2019, 231, 311–324. [Google Scholar] [CrossRef]
- Soliman, A.M.; Teoh, S.L.; Ghafar, N.A.; Das, S. Molecular Concept of Diabetic Wound Healing: Effective Role of Herbal Remedies. Mini Rev. Med. Chem. 2019, 19, 381–394. [Google Scholar] [CrossRef]
- Bortolotti, M.; Mercatelli, D.; Polito, L. Momordica charantia, a Nutraceutical Approach for Inflammatory Related Diseases. Front. Pharmacol. 2019, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Kodumuri, P.K.; Thomas, C.; Jetti, R.; Pandey, A.K. Fenugreek seed extract ameliorates cognitive deficits in streptozotocin-induced diabetic rats. J. Basic Clin. Physiol. Pharmacol. 2019. [Google Scholar] [CrossRef]
- Banerjee, S.; Bhattacharjee, P.; Kar, A.; Mukherjee, P.K. LC-MS/MS analysis and network pharmacology of Trigonella foenum-graecum—A plant from Ayurveda against hyperlipidemia and hyperglycemia with combination synergy. Phytomed. Int. J. Phytother. Phytopharm. 2019, 60, 152944. [Google Scholar] [CrossRef]
- Neha, S.; Anand, K.; Sunanda, P. Administration of Fenugreek Seed Extract Produces Better Effects in Glibenclamide-Induced Inhibition in Hepatic Lipid Peroxidation: An in vitro Study. Chin. J. Integr. Med. 2019, 25, 278–284. [Google Scholar] [CrossRef]
- Verma, N.; Usman, K.; Patel, N.; Jain, A.; Dhakre, S.; Swaroop, A.; Bagchi, M.; Kumar, P.; Preuss, H.G.; Bagchi, D. A multicenter clinical study to determine the efficacy of a novel fenugreek seed (Trigonella foenum-graecum) extract (Fenfuro™) in patients with type 2 diabetes. Food Nutr. Res. 2016, 60, 32382. [Google Scholar] [CrossRef]
- Fuller, S.; Stephens, J.M. Diosgenin, 4-hydroxyisoleucine, and fiber from fenugreek: Mechanisms of actions and potential effects on metabolic syndrome. Adv. Nutr. (Bethesda Md.) 2015, 6, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Lemon, C.H.; Imoto, T.; Smith, D.V. Differential Gurmarin Suppression of Sweet Taste Responses in Rat Solitary Nucleus Neurons. J. Neurophysiol. 2003, 90, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, K.; Ahamath, B.K.; Shanmugasundaram, K.R.; Shanmugasundaram, E.R.B. Antidiabetic effect of a leaf extract from Gymnema sylvestre in non-insulin-dependent diabetes mellitus patients. J. Ethnopharmacol. 1990, 30, 295–305. [Google Scholar] [CrossRef]
- Pradeep, S.R.; Barman, S.; Srinivasan, K. Attenuation of diabetic nephropathy by dietary fenugreek (Trigonella foenum-graecum) seeds and onion (Allium cepa) via suppression of glucose transporters and renin-angiotensin system. Nutrition 2019, 67–68, 110543. [Google Scholar] [CrossRef] [PubMed]
- Showande, S.J.; Fakeye, T.O.; Kajula, M.; Hokkanen, J.; Tolonen, A. Potential inhibition of major human cytochrome P450 isoenzymes by selected tropical medicinal Herbs-Implication for herb-drug interactions. Food Sci. Nutr. 2019, 7, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Bhanot, A.; Shri, R. A comparative profile of methanol extracts of Allium cepa and Allium sativum in diabetic neuropathy in mice. Pharmacogn. Res. 2010, 2, 374–384. [Google Scholar] [CrossRef]
- Otoom, S.A.; Al-Safi, S.A.; Kerem, Z.K.; Alkofahi, A. The use of medicinal herbs by diabetic Jordanian patients. J. Herb Pharmacother. 2006, 6, 31–41. [Google Scholar] [CrossRef]
- Sheela, C.G.; Kumud, K.; Augusti, K.T. Anti-diabetic effects of onion and garlic sulfoxide amino acids in rats. Planta Med. 1995, 61, 356–357. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K.; Chen, S. Spice plant Allium cepa: Dietary supplement for treatment of type 2 diabetes mellitus. Nutrition 2014, 30, 1128–1137. [Google Scholar] [CrossRef]
- Manickam, M.; Ramanathan, M.; Farboodniay Jahromi, M.A.; Chansouria, J.P.N.; Ray, A.B. Antihyperglycemic Activity of Phenolics from Pterocarpus marsupium. J. Nat. Prod. 1997, 60, 609–610. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Noor, M.H.M.; Abdullah, R.; Saad, M.Z.; Taufiq-Yap, Y.H. Therapeutic uses of epicatechin in diabetes and cancer. Vet. World 2017, 10, 869–872. [Google Scholar] [CrossRef]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular Mechanisms and Therapeutic Effects of (-)-Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxid. Med. Cell. Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Andreani, T.; Fernandes, L.; Garcia, M.L.; Egea, M.A.; Silva, A.M.; Souto, E.B. Physicochemical characterization of epigallocatechin gallate lipid nanoparticles (EGCG-LNs) for ocular instillation. Colloids Surf. B Biointerfaces 2014, 123, 452–460. [Google Scholar] [CrossRef]
- Fangueiro, J.F.; Silva, A.M.; Garcia, M.L.; Souto, E.B. Current nanotechnology approaches for the treatment and management of diabetic retinopathy. Eur. J. Pharm. Biopharm. 2015, 95, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Fangueiro, J.F.; Calpena, A.C.; Clares, B.; Andreani, T.; Egea, M.A.; Veiga, F.J.; Garcia, M.L.; Silva, A.M.; Souto, E.B. Biopharmaceutical evaluation of epigallocatechin gallate-loaded cationic lipid nanoparticles (EGCG-LNs): In vivo, in vitro and ex vivo studies. Int. J. Pharm. 2016, 502, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Martins-Gomes, C.; Fangueiro, J.F.; Andreani, T.; Souto, E.B. Comparison of antiproliferative effect of epigallocatechin gallate when loaded into cationic solid lipid nanoparticles against different cell lines. Pharm. Dev. Technol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Banjari, I.; Misir, A.; Pavlic, M.; Herath, P.N.; Waisundara, V.Y. Traditional Herbal Medicines for Diabetes Used in Europe and Asia: Remedies from Croatia and Sri Lanka. Altern. Ther. Health Med. 2019, 25, 40–52. [Google Scholar]
- Shi, M.; Loftus, H.; McAinch, A.J.; Su, X.Q. Blueberry as a source of bioactive compounds for the treatment of obesity, type 2 diabetes and chronic inflammation. J. Funct. Foods 2017, 30, 16–29. [Google Scholar] [CrossRef]
- Chikhi, I.; Allali, H.; Dib, M.E.A.; Medjdoub, H.; Tabti, B. Antidiabetic activity of aqueous leaf extract of Atriplex halimus L. (Chenopodiaceae) in streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Dis. 2014, 4, 181–184. [Google Scholar] [CrossRef]
- Kalderon, B.; Gutman, A.; Levy, E.; Shafrir, E.; Adler, J.H. Characterization of stages in development of obesity-diabetes syndrome in sand rat (Psammomys obesus). Diabetes 1986, 35, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Shakib, Z.; Shahraki, N.; Razavi, B.M.; Hosseinzadeh, H. Aloe vera as an herbal medicine in the treatment of metabolic syndrome: A review. Phytother. Res. 2019. [Google Scholar] [CrossRef]
- Bunyapraphatsara, N.; Yongchaiyudha, S.; Rungpitarangsi, V.; Chokechaijaroenporn, O. Antidiabetic activity of Aloe vera L. juice II. Clinical trial in diabetes mellitus patients in combination with glibenclamide. Phytomed. Int. J. Phytother. Phytopharm. 1996, 3, 245–248. [Google Scholar] [CrossRef]
- Tanaka, M.; Misawa, E.; Ito, Y.; Habara, N.; Nomaguchi, K.; Yamada, M.; Toida, T.; Hayasawa, H.; Takase, M.; Inagaki, M.; et al. Identification of Five Phytosterols from Aloe Vera Gel as Anti-Diabetic Compounds. Biol. Pharm. Bull. 2006, 29, 1418–1422. [Google Scholar] [CrossRef]
- Devaraj, S.; Yimam, M.; Brownell, L.A.; Jialal, I.; Singh, S.; Jia, Q. Effects of Aloe vera supplementation in subjects with prediabetes/metabolic syndrome. Metab. Syndr. Relat. Disord. 2013, 11, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Vogler, B.K.; Ernst, E. Aloe vera: A systematic review of its clinical effectiveness. Br. J. Gen. Pract. 1999, 49, 823–828. [Google Scholar] [PubMed]
| Drug Targets | Hyperglycemic Effect Observed with: |
|---|---|
| Insulin secretion | ↗ Incretins ↗ Meglitinides ↗ Sulfonylureas |
| Glucagon secretion | ↓ incretins ↓ amylin |
| Gastrointestinal tract | Incretins Alpha-glucosidase inhibitors Amylin Sequestrant of bile salt |
| Hepatic glucose output | ↓ Metformin ↓ Thiazolidinediones |
| Lipotoxicity | Thiazolidinediones |
| Control of appetite | Incretins Amylin |
| Neurotransmitter Dysfunction | Bromocriptine |
| Glucose reabsorption | ↓ Gliflozins inhibitors |
| Glucose uptake and use | ↗ Metformin ↗ Thiazolidinediones |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, R.; Souto, S.B.; Sánchez-López, E.; López Machado, A.; Severino, P.; Jose, S.; Santini, A.; Fortuna, A.; García, M.L.; Silva, A.M.; et al. Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome—Review of Classical and New Compounds: Part-I. Pharmaceuticals 2019, 12, 152. https://doi.org/10.3390/ph12040152
Vieira R, Souto SB, Sánchez-López E, López Machado A, Severino P, Jose S, Santini A, Fortuna A, García ML, Silva AM, et al. Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome—Review of Classical and New Compounds: Part-I. Pharmaceuticals. 2019; 12(4):152. https://doi.org/10.3390/ph12040152
Chicago/Turabian StyleVieira, Raquel, Selma B. Souto, Elena Sánchez-López, Ana López Machado, Patricia Severino, Sajan Jose, Antonello Santini, Ana Fortuna, Maria Luisa García, Amelia M. Silva, and et al. 2019. "Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome—Review of Classical and New Compounds: Part-I" Pharmaceuticals 12, no. 4: 152. https://doi.org/10.3390/ph12040152
APA StyleVieira, R., Souto, S. B., Sánchez-López, E., López Machado, A., Severino, P., Jose, S., Santini, A., Fortuna, A., García, M. L., Silva, A. M., & Souto, E. B. (2019). Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome—Review of Classical and New Compounds: Part-I. Pharmaceuticals, 12(4), 152. https://doi.org/10.3390/ph12040152










