Abstract
Glioblastoma (GBM) is the most common and aggressive primary brain tumor. Currently, treatment is ineffective and the median overall survival is 20.9 months. The poor prognosis of GBM is a consequence of several altered signaling pathways that favor the proliferation and survival of neoplastic cells. One of these pathways is the deregulation of phosphodiesterases (PDEs). These enzymes participate in the development of GBM and may have value as therapeutic targets to treat GBM. Methylxanthines (MXTs) such as caffeine, theophylline, and theobromine are PDE inhibitors and constitute a promising therapeutic anti-cancer agent against GBM. MTXs also regulate various cell processes such as proliferation, migration, cell death, and differentiation; these processes are related to cancer progression, making MXTs potential therapeutic agents in GBM.
1. Introduction
Glioblastoma (GBM) is the most aggressive and most frequent primary malignant tumor of the central nervous system (CNS) [1]. It occurs more frequently in men and in people older than 55 years [2]. Pathological characteristics of GBM include cellular heterogeneity, angiogenesis, high proliferation rate, and increased migratory capacity [3]. The treatment of GBM consists of surgical resection, radiotherapy, chemotherapy, and novel therapies such as alternating electrical fields [4]. Nevertheless, even with these novel therapeutic approaches, the overall survival of patients is around 20.9 months [5].
Activation of aberrant signaling pathways in GBM may promote the survival of neoplastic cells [6] and can provide new therapeutic targets. For example, phosphodiesterase (PDE) dysregulation in GBM leads to the survival, proliferation, and dedifferentiation of neoplastic cells [7]. Targeting this pathway could therefore be a valuable tool therapy for GBM.
Due to the ineffectiveness of the current GBM therapy, diverse natural compounds have been evaluated as chemotherapeutic agents, for example, resveratrol, curcumin, epigallocatechin 3-gallate gallate (EGCC), and others. These agents can be used as adjuvant therapy to improve standard treatments [8]. Curcumin is effective alone and combined with standard therapy in glioma cells; it also induces neural differentiation of human pluripotent embryonal carcinoma cells [9]. The EGCC in green tea induces cell death and reduces cellular proliferation and invasion in diverse glioma cell lines. It also enhances the efficacy of chemotherapy and radiotherapy in GBM [10]. Among these agents are the methylxanthines (MXTs); these are natural compounds with anti-cancer properties, the induction of apoptosis, the reduction of cellular migration, the arrest of the cell cycle, and the inhibition of PDEs. All these properties constitute therapeutic targets against GBM. Information about the effectiveness and mechanisms of the action of MTXs on GBM is summarized in the present review.
There are several natural or synthetic compounds that inhibit the activity of PDEs, such as MXTs, which are natural PDE-inhibitors and are currently used as therapy for several non-neoplastic diseases, with adequate pharmacokinetic, pharmacodynamic, and security profiles [11]. Due to their pharmacological properties, MXTs could become an adjuvant therapy against GBM [12,13]. However, their potential effectiveness against GBM remains controversial (Table 1). In the following paragraphs, we will discuss the current status of natural MXTs as therapy for GBM in vitro and in vivo (Figure 1).

Table 1.
Effect of methylxanthines in glioma.
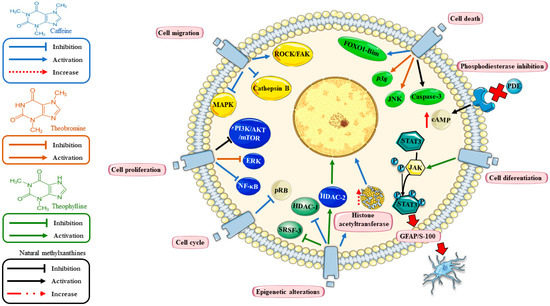
Figure 1.
Mechanisms of action of methylxanthine (MTXs) in glioblastoma (GBM). The figure shows common pathways regulated by MTXs (black arrows) including inhibition of phosphodiesterases (PDEs) (reducing the levels of cyclic Adenosine Monophosphate (cAMP)); regulation of cell proliferation (PI3K/AKT-mTOR); and cell death (caspase 3). Particularly, caffeine (blue arrows) promotes cell death (FOXO1-Bim); cell migration (by these proteins ROCK/FAK pathway, MAPK and cathepsin B); cell proliferation (NF-kB); cell cycle (pRB) and epigenetic mechanisms (HAT‒histone acetyltransferases and HDAC-1). Theobromine (orange arrow) regulates cell proliferation (ERK) and apoptosis (JNK and p38). Theophylline (green arrow) regulates cell differentiation (JAK/STAT3 pathway), the epigenetic mechanism (HDAC-2) and alternative splicing (SRSF3). The figure was designed using Servier Medical Art ©.
2. Phosphodiesterases in Glioblastoma (GBM)
PDEs are enzymes that hydrolyze the phosphodiester bond in the cyclic Guanidine Monophosphate (cGMP) and cyclic Adenosine Monophosphate (cAMP), lowering their intracellular concentration. These nucleotides act as second intracellular messengers and participate in cellular metabolism, cell growth, differentiation, and proliferation. They also participate in functions such as reproduction, cardiac function, vision, inflammation, and oncogenesis [24].
The PDE gene family has 11 different members (PDE1 to PDE11) with 21 genes that encode up to 100 different subtypes of proteins. They are grouped by their substrate specificity: cAMP-specific are PDE4, PDE7, and PDE8; cGMP-specific are PDE5, PDE6, and PDE9; the rest use both cAMP and cGMP [12,24].
Alterations in PDE activity contribute to tumorigenesis, reducing the levels of the cyclic nucleotides, which are described in malignant cells [25,26]. In GBM, overexpression of PDE4A1 correlates with reduced levels of cAMP [27,28]. On the other hand, PDE5 expression was associated with prolonged overall survival and the inhibition of PDE5 induced a more aggressive phenotype in vitro [7]. Breast and colon cancer cells treated with PDE inhibitors elevate their intracellular cAMP levels, which induce apoptosis, decreased cell migration, and growth arrest [12].
There is not enough information to define the role of cyclic nucleotides or PDE in GBM (Table 2). Some reports point to cyclic nucleotides as pro-oncogenic signals, and the regulation of PDEs constitutes a therapeutic alternative [12]. However, there are also reports that show that PDEs are related to an overall benefit to the patient. It is important to elucidate the exact role of cyclic nucleotides to propose their inhibitors as candidates for adjuvant therapy in GBM.

Table 2.
Role of phosphodiesterases on glioblastoma.
3. Methylxanthines
MXTs are natural alkaloids and secondary metabolites in various botanical species (Camellia sinensis L., Theobroma cacao, and Coffea sp.) such as caffeine, theophylline, and theobromine. They occur in dietary products like tea, coffee, chocolate, and energy drinks [60,61].
MXTs are clinically used in the treatment of various diseases, such as obesity, hyperlipidemia, chronic obstructive pulmonary disease, asthma, peripheral vascular disease, and apnea of prematurity [62]. Their use is under investigation in the treatment of cancer and neurodegenerative diseases. Recently, their potential role as antineoplastic has gained attention [12,60,63].
MXTs bind to adenosine receptors [64], which participate in several cellular functions, and their expression correlates with tumor survival, chemoresistance, grade, and cancer cell survival [65,66].
Adverse effects (AE) of MTXs are dizziness, irritability, nervousness, tremors, sleep difficulty, diarrhea, nausea, and vomiting [67]. The most relevant AEs are cardiovascular, arrhythmias and cardiac arrest. Caffeine and theophylline show similar AEs, whereas theobromine requires higher doses to become toxic [60]. For caffeine, 15 mg/L is the threshold for toxicity in humans; doses up to 20 g can lead to death secondary to cardiac arrhythmias, ventricular fibrillation, or kidney failure [68]. Administration of MTXs to pregnant women requires special attention [67,69].
3.1. Caffeine
Caffeine (1,3,7-trimethylxanthine) is extracted from the Coffea sp. and from the leaves of some teas. The physiological effects of caffeine include central nervous system (CNS) stimulation, smooth muscle relaxation, and tachycardia [70]. Caffeine has a wide absorption via the digestive system, easy passage of the blood-brain barrier (BBB), and good systemic distribution. Once absorbed, 95% of caffeine is metabolized to paraxanthine (85%), theobromine (10%), and theophylline (5%) [71,72].
Caffeine is used for treatment of apnea of prematurity in infants between 28 and 33 weeks of gestational age; it is effective in other pathologies such as fatigue [73]; headache; migraine [72,74]; orthostatic hypotension [75]; and other neurological diseases such as depression [76], brain injury [77], anxiety [78], and Alzheimer’s [79] and Parkinson’s disease [80].
Another promising clinical application of caffeine is in neuro-oncology. The population with the greatest caffeine consumption showed a reduced prevalence of gliomas [81]. Caffeine has been tested as a therapeutic adjuvant in the treatment of gliomas; Stewart et al. showed the beneficial effect of the combination of caffeine plus cytosine arabinoside and cisplatin [19]. Caffeine potentiates cisplatin-induced and camptothecin-induced apoptosis (G2 phase shortening) in glioma cell lines [20], increasing the subG1 stage [21].
Sinn et al. [22] showed that caffeine can sensitize radiation-resistant cells (U87MG, T98G, and U373MG), due to the activation of the checkpoint in the G1 phase. This effect may be explained by the capability of caffeine to inhibit phosphoinositide 3-kinase (PI3K), down-regulating the PI3K/Akt pathway and inducing apoptosis.
Caffeine can induce apoptosis and cell cycle arrest via different mechanisms; apoptosis can be induced by inhibition of PI3K, by activation of caspase-3, poly(ADP-ribose) polymerase (PARP), or forkhead box protein O1 (FoxO1) [23,82,83]. Caffeine in combination with tetrandrine (a natural alkaloid isolated from the root of the plant Radix Stephania Tetrandrae) induces apoptosis, independent of caspase activation [84]. Additionally, epigenetic modifications such as decreased activity of histone deacetylase 1 (HDAC1) and increased activity of histone acetyltransferase have been related to induction apoptosis by caffeine [85]. The MXT arrest the cell cycle in G0/G1 suppressing Rb phosphorylation [82], whereas caffeine also induces shortening of the G2 phase [20].
An important aspect of the malignancy in GBM is its ability to infiltrate healthy brain parenchyma. It has been proposed that caffeine can induce a cytotoxic effect, preventing the invasive behavior of glioma cells. These effects may result of interactions of caffeine with intracellular calcium. They can be due to the inhibition of the inositol triphosphate receptor type 3 (IP3R3), which has been associated with longer survival in a mouse xenograft model of GBM [86].
Focal adhesion complexes are used by GBM cells to invade and migrate. This complex is regulated by Rho-associated protein kinase (ROCK); caffeine inhibits migration of glioma cells, apparently through phosphorylation of p21, glycogen synthetase kinase 3 beta, and the ROCK pathway [23]. This decreased migration correlates with an experimental reduction of tumor growth [21,87].
Another important feature of GBM is angiogenesis. Hypoxia induces the expression of hypoxia-inducible factor 1 alpha (HIF-1a) protein, which promotes the expression of vascular endothelial growth factor (VEGF), inducing the formation of new blood vessels. Caffeine inhibits the expression of HIF-1α and VEGF [88].
In summary, caffeine can block several pathological pathways in GBM. Caffeine induces apoptosis, blocks the cell cycle, prevents migration and invasion of neoplastic cells, and inhibits angiogenesis.
3.2. Theophylline
Theophylline (1,3-dimethylxanthine) is extracted mainly from the tea plant (Camellia sinensis L.) and yerba mate (Ilex Paraguariensis) [89,90]. Theophylline stimulates the CNS and induces bronchodilatation [91,92]. Theophylline decreases metastasis, inflammation, and therapy-resistance in cancer cells. The role of theophylline has been related to the inhibition of PI3K, an activated pathway in cancer that favors metastasis and resistance to treatment [93]. In addition, it acts as an immunomodulator due to the activation of the HDAC-2 protein, which suppresses the expression of inflammatory genes [63,91,94]. This drug reduces survival and proliferation of A-172 and U87MG glioma cells [14]. Theophylline has been tested in other types of cancer, such as lung cancer. Domvri et al. showed the synergic effect between PDE inhibitors and chemotherapeutic agents (docetaxel, cisplatin, and carboplatin) [95]. In cervical and breast cancer cells, theophylline mediates alternative splicing through suppression of serine/arginine-rich splicing factor 3 (SRSF3) and its target genes, these effects alter the status of p53 isoforms, which contributes to cancer progression in these cells [96].
3.3. Theobromine
Theobromine (3,7-dimethylxanthine) is derived from Theobroma cacao, which is a metabolite from caffeine and is found in chocolate, tea, and some species of Camellia sinensis L. [60]. It has been shown that cocoa and its metabolites are antioxidant and cardiovascular protectors; theobromine might also possess anti-tumor and anti-inflammatory effects mediated by the elevation of intracellular cAMP levels due to the non-selective inhibition of PDE4A1 [18].
Theobromine passes the BBB and placental barriers, inducing neurophysiological effects [97] such as inhibition of adenosine receptors in the CNS [90]. However, as it lacks a methyl group, theobromine has a modest metabolic activity, being limited in its distribution in the CNS [60].
In GBM, Sugimoto et al. reported that theobromine inhibits proliferation and promotes cell death in the U87MG cell line, through inhibition of AKT/mTOR and PDE4. It was also shown that cell death induced by theobromine was related to the switch between the activity of ERK, JNK, and p38, inducing apoptosis; theobromine may prevent tumor progression by inhibiting NF-kB-phosphorylation [18].
4. Methylxanthines and Cellular Differentiation
The effect of theophylline as a modulator of differentiation was evaluated in GBM by Nagai et al. They reported that theophylline induces morphological changes in human glioblastoma and in malignant glioma cells induced by methylcholanthrene in mouse C57, to a less malignant phenotype [15]. Sato et al. also reported that theophylline induces glial-like morphological changes and expression of a protein marker of glial cells (S-100 protein) after five days of exposure in a mouse glioma cell line. From the sixth day of exposure, viability of this cell population was decreased [16], which could be interpreted as programmed death of differentiated cells.
Glioma cell treatment with N6,2′-O-dibutyryl cAMP (Bt2AMP) and theophylline caused delayed phosphorylation of the signal transducer and the activator of transcription-3 (STAT3) as well as expression of an astrocyte marker, glial fibrillary acidic protein (GFAP) [17].
There are few publications specifically describing differentiation induced by caffeine. The first clue about a possible role in differentiation of caffeine was mentioned by Kreider et al., in 1973; the authors found that non-toxic doses of caffeine can induce morphological changes of mature cells in melanoma cells [98]. In 1986, an epidemiological study in patients with breast cancer showed that high caffeine consumption correlated with an increased degree of cell differentiation in the tissue [99]. The morphological differentiation of neoplastic cells can be induced by the addition of caffeine, in a time-dependent manner, to sensitize the cancer cells to antineoplastic therapies like that with cisplatin, a widely used drug against various types of cancer, including GBM [100].
As can be speculated, information about the role of MTX as a differentiating drug is promising; nevertheless, research about this is limited.
5. Conclusions
The design of novel therapies for the treatment of GBM is urgently needed. However, the creation of novel therapeutic substances implies a large investment of time and money. In various fields of pharmacotherapy, there is an alternative—namely, drug repurposing. This is a process in which drugs that are clinically used are explored for other clinical indications. MXTs have beneficial effects in different GBM cell types and in animal models. However, there is limited knowledge about the specificity of these drugs to inhibit PDEs, such as the potential therapeutic use of MTXs against malignant properties of GBM.
MXTs regulate various cell processes such as proliferation, migration, cell death, and differentiation, all of them related to cancer progression, which are potential therapeutic targets in GBM (Figure 1). More research should be conducted on the role of different PDEs in GBM, as well as on the specificity of MXTs for different PDEs, which can lead to defining potential therapeutic applications of MXTs against GBM.
MXTs are classical pharmacological substances, with defined effectiveness and safety profiles, so the repurpose of these drugs is a fair possibility. All MXTs have similar toxicity profiles; side effects are related to the dose, so in order to prevent them, it is important to define the target mechanisms and the different dosages to achieve them.
There are several gaps in our knowledge of the pathological or physiological effects related to specific PDEs and the potential benefits of different MXTs.
Author Contributions
R.M.-M., I.R.-V., E.G.C.-C., D.P.-P., and J.S. contributed equally to the editing of the present work; R.M.-M. worked on generalities of MTX and PDEs in GBM; I.R.-V. worked on the design of the figure and the role of theophylline in GBM, E.G.C.-C. in theobromine, and D.P.-P. in caffeine.
Funding
This research received no external funding.
Acknowledgments
D.P.-P. and I.R.-V. receive a scholarship of the National Council of Science and Technology of México (CONACyT, Scholarships number 622940 and 734300, respectively).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol. 2017, 19, v1–v88. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, G.S.; Dzhenkov, D.; Ghenev, P.; Iliev, B.; Enchev, Y.; Tonchev, A.B. Cell biology of glioblastoma multiforme: From basic science to diagnosis and treatment. Med. Oncol. 2018, 35, 27. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs. Maintenance Temozolomide Alone on Survival in Patients with Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Otani, Y.; Kurozumi, K.; Date, I. Phenotypic Transition as a Survival Strategy of Glioma. Neurol. Med. Chir. 2016, 56, 387–395. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cesarini, V.; Martini, M.; Vitiani, L.R.; Gravina, G.L.; Di Agostino, S.; Graziani, G.; D’Alessandris, Q.G.; Pallini, R.; Larocca, L.M.; Rossi, P.; et al. Type 5 phosphodiesterase regulates glioblastoma multiforme aggressiveness and clinical outcome. Oncotarget 2017, 8, 13223–13239. [Google Scholar] [CrossRef]
- Vengoji, R.; Macha, M.A.; Batra, S.K.; Shonka, N.A. Natural products: A hope for glioblastoma patients. Oncotarget 2018, 9, 22194–22219. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Zangui, M.; Lotfi, M.; Ghayour-Mobarhan, M.; Ghorbani, A.; Jaliani, H.Z.; Sadeghnia, H.R.; Sahebkar, A. Therapeutic Potential of Curcumin in the Treatment of Glioblastoma Multiforme. Curr. Pharm. Des. 2019, 25, 333–342. [Google Scholar] [CrossRef]
- Le, C.T.; Leenders, W.P.J.; Molenaar, R.J.; van Noorden, C.J.F. Effects of the Green Tea Polyphenol Epigallocatechin-3-Gallate on Glioma: A Critical Evaluation of the Literature. Nutr. Cancer 2018, 70, 317–333. [Google Scholar] [CrossRef]
- Arnaud, M.J. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2011; pp. 33–91. [Google Scholar]
- Azevedo, M.F.; Faucz, F.R.; Bimpaki, E.; Horvath, A.; Levy, I.; de Alexandre, R.B.; Ahmad, F.; Manganiello, V.; Stratakis, C.A. Clinical and molecular genetics of the phosphodiesterases (PDEs). Endocr. Rev. 2014, 35, 195–233. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, S.; Hadjighassem, M.; Vousooghi, N.; Parvaresh, M.; Arbabi, F.; Amini, N.; Joghataei, M.T. The Role of Protein Kinase B Signaling Pathway in Anti-Cancer Effect of Rolipram on Glioblastoma Multiforme: An In Vitro Study. Basic Clin. Neurosci. 2017, 8, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.-Y.; Lee, G.-H.; Lee, M.-S.; Kim, H.-M.; Lee, J.-W. Phosphodiesterase inhibitors control A172 human glioblastoma cell death through cAMP-mediated activation of protein kinase A and Epac1/Rap1 pathways. Life Sci. 2012, 90, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Teraoka, A.; Matsutani, M.; Machiyama, N.; Tsuchida, T.; Hatanaka, H.; Sano, K. B-6. Effect of Cyclic AMP on the Malignant Brain Tumor Cells. Neurol. Med. Chir. 1971, 11, 231. [Google Scholar] [CrossRef]
- Sato, S.; Sugimura, T.; Yoda, K.; Fujimura, S. Morphological Differentiation of Cultured Mouse Glioblastoma Cells Induced by Dibutyryl Cyclic Adenosine Monophosphate. Cancer Res. 1975, 35, 2494–2499. [Google Scholar]
- Takanaga, H.; Yoshitake, T.; Hara, S.; Yamasaki, C.; Kunimoto, M. cAMP-induced astrocytic differentiation of C6 glioma cells is mediated by autocrine interleukin-6. J. Biol. Chem. 2004, 279, 15441–15447. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, N.; Miwa, S.; Hitomi, Y.; Nakamura, H.; Tsuchiya, H.; Yachie, A. Theobromine, the primary methylxanthine found in Theobroma cacao, prevents malignant glioblastoma proliferation by negatively regulating phosphodiesterase-4, extracellular signal-regulated kinase, Akt/mammalian target of rapamycin kinase, and nuclear fact. Nutr. Cancer 2014, 66, 419–423. [Google Scholar] [CrossRef]
- Stewart, D.J.; Hugenholtz, H.; DaSilva, V.; Benoit, B.; Richard, M.; Russell, N.; Maroun, J.; Verma, S. Cytosine arabinoside plus cisplatin and other drugs as chemotherapy for gliomas. Semin. Oncol. 1987, 14, 110–115. [Google Scholar]
- Janss, A.J.; Levow, C.; Bernhard, E.J.; Muschel, R.J.; McKenna, W.G.; Sutton, L.; Phillips, P.C. Caffeine and staurosporine enhance the cytotoxicity of cisplatin and camptothecin in human brain tumor cell lines. Exp. Cell Res. 1998, 243, 29–38. [Google Scholar] [CrossRef]
- Chen, Y.; Chou, W.C.; Ding, Y.M.; Wu, Y.C. Caffeine inhibits migration in glioma cells through the ROCK-FAK pathway. Cell. Physiol. Biochem. 2014, 33, 1888–1898. [Google Scholar] [CrossRef]
- Sinn, B.; Tallen, G.; Schroeder, G.; Grassl, B.; Schulze, J.; Budach, V.; Tinhofer, I. Caffeine Confers Radiosensitization of PTEN-Deficient Malignant Glioma Cells by Enhancing Ionizing Radiation-Induced G1 Arrest and Negatively Regulating Akt Phosphorylation. Mol. Cancer Ther. 2010, 9, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Ku, B.M.; Lee, Y.K.; Jeong, J.Y.; Ryu, J.; Choi, J.; Kim, J.S.; Cho, Y.W.; Roh, G.S.; Kim, H.J.; Cho, G.J.; et al. Caffeine inhibits cell proliferation and regulates PKA/GSK3β pathways in U87MG human glioma cells. Mol. Cells 2011, 31, 275–279. [Google Scholar] [CrossRef] [PubMed]
- Kleppisch, T. Phosphodiesterases in the central nervous system. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 71–92. [Google Scholar]
- Yarla, N.S.; Gali, H.; Pathuri, G.; Smriti, S.; Farooqui, M.; Panneerselvam, J.; Kumar, G.; Madka, V.; Rao, C.V. Targeting the paracrine hormone-dependent guanylate cyclase/cGMP/phosphodiesterases signaling pathway for colorectal cancer prevention. Semin. Cancer Biol. 2019, 56, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Murray, F.; Zahno, A.; Kanter, J.R.; Chou, D.; Suda, R.; Fenlon, M.; Rassenti, L.; Cottam, H.; Kipps, T.J.; et al. Cyclic nucleotide phosphodiesterase profiling reveals increased expression of phosphodiesterase 7B in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2008, 105, 19532–19537. [Google Scholar] [CrossRef] [PubMed]
- Vatter, S.; Pahlke, G.; Deitmer, J.W.; Eisenbrand, G. Differential phosphodiesterase expression and cytosolic Ca2+ in human CNS tumour cells and in non-malignant and malignant cells of rat origin. J. Neurochem. 2005, 93, 321–329. [Google Scholar] [CrossRef]
- Goldhoff, P.; Warrington, N.M.; Limbrick, D.D., Jr.; Hope, A.; Woerner, B.M.; Jackson, E.; Perry, A.; Piwnica-Worms, D.; Rubin, J.B. Targeted inhibition of cyclic AMP phosphodiesterase-4 promotes brain tumor regression. Clin. Cancer Res. 2008, 14, 7717–7725. [Google Scholar] [CrossRef] [PubMed]
- Rowther, F.B.; Wei, W.; Dawson, T.P.; Ashton, K.; Singh, A.; Madiesse-Timchou, M.P.; Thomas, D.G.T.; Darling, J.L.; Warr, T. Cyclic nucleotide phosphodiesterase-1C (PDE1C) drives cell proliferation, migration and invasion in glioblastoma multiforme cells in vitro. Mol. Carcinog. 2016, 55, 268–279. [Google Scholar] [CrossRef]
- Michibata, H.; Yanaka, N.; Kanoh, Y.; Okumura, K.; Omori, K. Human Ca2+/calmodulin-dependent phosphodiesterase PDE1A: Novel splice variants, their specific expression, genomic organization, and chromosomal localization. Biochim. Biophys. Acta 2001, 1517, 278–287. [Google Scholar] [CrossRef]
- Reed, T.M.; Browning, J.E.; Blough, R.I.; Vorhees, C.V.; Repaske, D.R. Genomic structure and chromosome location of the murine PDE1B phosphodiesterase gene. Mamm. Genome 1998, 9, 571–576. [Google Scholar] [CrossRef]
- Loughney, K.; Martins, T.J.; Harris, E.A.; Sadhu, K.; Hicks, J.B.; Sonnenburg, W.K.; Beavo, J.A.; Ferguson, K. Isolation and characterization of cDNAs corresponding to two human calcium, calmodulin-regulated, 3′,5′-cyclic nucleotide phosphodiesterases. J. Biol. Chem. 1996, 271, 796–806. [Google Scholar] [CrossRef]
- Rosman, G.J.; Martins, T.J.; Sonnenburg, W.K.; Beavo, J.A.; Ferguson, K.; Loughney, K. Isolation and characterization of human cDNAs encoding a cGMP-stimulated 3′,5′-cyclic nucleotide phosphodiesterase. Gene 1997, 191, 89–95. [Google Scholar] [CrossRef]
- Doecke, J.D.; Wang, Y.; Baggerly, K. Co-localized genomic regulation of miRNA and mRNA via DNA methylation affects survival in multiple tumor types. Cancer Genet. 2016, 209, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Meacci, E.; Taira, M.; Moos, M.; Smith, C.J.; Movsesian, M.A.; Degerman, E.; Belfrage, P.; Manganiello, V. Molecular cloning and expression of human myocardial cGMP-inhibited cAMP phosphodiesterase. Proc. Natl. Acad. Sci. USA 1992, 89, 3721–3725. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, S.; Akram, B.H.; Hainsworth, A.H.; Kruuse, C. Cyclic nucleotide phosphodiesterases (PDEs) and endothelial function in ischaemic stroke. A review. Cell. Signal. 2019, 61, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Taira, M.; Hockman, S.; Shimada, F.; Lieman, J.; Napolitano, M.; Ward, D.; Taira, M.; Makino, H.; Manganiello, V.C. Characterization of the cDNA and gene encoding human PDE3B, the cGIP1 isoform of the human cyclic GMP-inhibited cyclic nucleotide phosphodiesterase family. Genomics 1996, 36, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Houslay, M.D.; Baillie, G.S.; Maurice, D.H. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: A molecular toolbox for generating compartmentalized cAMP signaling. Circ. Res. 2007, 100, 950–966. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Richter, W.; Mehats, C.; Livera, G.; Park, J.-Y.; Jin, C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J. Biol. Chem. 2003, 278, 5493–5496. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, S.; Vousooghi, N.; Kapourchali, F.R.; Hadjighasem, M.; Hayat, P.; Amini, N.; Joghataei, M.T. Rolipram potentiates bevacizumab-induced cell death in human glioblastoma stem-like cells. Life Sci. 2017, 173, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Feng, Y.; Wang, H.; Zhang, C.; Sun, L.; Yan, Z.; Liu, Q.; Guo, T.; Li, M.; Yang, X.; et al. Integrated analysis using methylation and gene expression microarrays reveals PDE4C as a prognostic biomarker in human glioma. Oncol. Rep. 2014, 32, 250–260. [Google Scholar] [CrossRef]
- Lin, C.-S.; Chow, S.; Lau, A.; Tu, R.; Lue, T.F. Human PDE5A gene encodes three PDE5 isoforms from two alternate promoters. Int. J. Impot. Res. 2002, 14, 15–24. [Google Scholar] [CrossRef]
- Corton, M.; Blanco, M.J.; Torres, M.; Sanchez-Salorio, M.; Carracedo, A.; Brion, M. Identification of a novel mutation in the human PDE6A gene in autosomal recessive retinitis pigmentosa: Homology with the nmf28/nmf28 mice model. Clin. Genet. 2010, 78, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, V.K.L.; Takiuti, J.T.; Jauregui, R.; Lima, L.H.; Tsang, S.H. Structural disease progression in PDE6-associated autosomal recessive retinitis pigmentosa. Ophthalmic Genet. 2018, 39, 610–614. [Google Scholar] [CrossRef]
- Piriev, N.I.; Viczian, A.S.; Ye, J.; Kerner, B.; Korenberg, J.R.; Farber, D.B. Gene structure and amino acid sequence of the human cone photoreceptor cGMP-phosphodiesterase alpha’ subunit (PDEA2) and its chromosomal localization to 10q24. Genomics 1995, 28, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Brooks, M.D.; Jackson, E.; Warrington, N.M.; Luo, J.; Forys, J.T.; Taylor, S.; Mao, D.D.; Leonard, J.R.; Kim, A.H.; Piwnica-Worms, D.; et al. PDE7B is a novel, prognostically significant mediator of glioblastoma growth whose expression is regulated by endothelial cells. PLoS ONE 2014, 9, e107397. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Fletcher, C.F.; Copeland, N.G.; Jenkins, N.A.; Yaremko, L.M.; Michaeli, T. Assignment of the mouse Pde7A gene to the proximal region of chromosome 3 and of the human PDE7A gene to chromosome 8q13. Genomics 1998, 48, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Hetman, J.M.; Soderling, S.H.; Glavas, N.A.; Beavo, J.A. Cloning and characterization of PDE7B, a cAMP-specific phosphodiesterase. Proc. Natl. Acad. Sci. USA 2000, 97, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Murakawa, M.; Kadoshima-Yamaoka, K.; Tanaka, Y.; Inoue, H.; Murafuji, H.; Hayashi, Y.; Miura, K.; Nakatsuka, T.; Nagahira, K.; et al. Phosphodiesterase 7A inhibitor ASB16165 suppresses proliferation and cytokine production of NKT cells. Cell. Immunol. 2009, 258, 147–151. [Google Scholar] [CrossRef]
- Wang, P.; Wu, P.; Egan, R.W.; Billah, M.M. Human phosphodiesterase 8A splice variants: Cloning, gene organization, and tissue distribution. Gene 2001, 280, 183–194. [Google Scholar] [CrossRef]
- Hayashi, M.; Shimada, Y.; Nishimura, Y.; Hama, T.; Tanaka, T. Genomic organization, chromosomal localization, and alternative splicing of the human phosphodiesterase 8B gene. Biochem. Biophys. Res. Commun. 2002, 297, 1253–1258. [Google Scholar] [CrossRef]
- Basole, C.P.; Nguyen, R.K.; Lamothe, K.; Vang, A.; Clark, R.; Baillie, G.S.; Epstein, P.M.; Brocke, S. PDE8 controls CD4+ T cell motility through the PDE8A-Raf-1 kinase signaling complex. Cell. Signal. 2017, 40, 62–72. [Google Scholar] [CrossRef]
- Wang, H.; Sun, T.; Hu, J.; Zhang, R.; Rao, Y.; Wang, S.; Chen, R.; McLendon, R.E.; Friedman, A.H.; Keir, S.T.; et al. miR-33a promotes glioma-initiating cell self-renewal via PKA and NOTCH pathways. J. Clin. Investig. 2014, 124, 4489–4502. [Google Scholar] [CrossRef] [PubMed]
- Guipponi, M.; Scott, H.S.; Kudoh, J.; Kawasaki, K.; Shibuya, K.; Shintani, A.; Asakawa, S.; Chen, H.; Lalioti, M.D.; Rossier, C.; et al. Identification and characterization of a novel cyclic nucleotide phosphodiesterase gene (PDE9A) that maps to 21q22.3: Alternative splicing of mRNA transcripts, genomic structure and sequence. Hum. Genet. 1998, 103, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Dorner-Ciossek, C.; Kroker, K.S.; Rosenbrock, H. Role of PDE9 in Cognition. Adv. Neurobiol. 2017, 17, 231–254. [Google Scholar] [PubMed]
- Fujishige, K.; Kotera, J.; Michibata, H.; Yuasa, K.; Takebayashi, S.; Okumura, K.; Omori, K. Cloning and characterization of a novel human phosphodiesterase that hydrolyzes both cAMP and cGMP (PDE10A). J. Biol. Chem. 1999, 274, 18438–18445. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, V.; Zhong, S.; Lin, H.; Xu, W.; Bradaia, A.; Steidl, E.; Gleyzes, M.; Wadel, K.; Buisson, B.; Padovan-Neto, F.E.; et al. Phosphodiesterase 10A Inhibition Improves Cortico-Basal Ganglia Function in Huntington’s Disease Models. Neuron 2016, 92, 1220–1237. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Ogawa, S.; Kawamata, N.; Tunici, P.; Finocchiaro, G.; Eoli, M.; Ruckert, C.; Huynh, T.; Liu, G.; Kato, M.; et al. High-resolution genomic copy number profiling of glioblastoma multiforme by single nucleotide polymorphism DNA microarray. Mol. Cancer Res. 2009, 7, 665–677. [Google Scholar] [CrossRef]
- Peng, T.; Gong, J.; Jin, Y.; Zhou, Y.; Tong, R.; Wei, X.; Bai, L.; Shi, J. Inhibitors of phosphodiesterase as cancer therapeutics. Eur. J. Med. Chem. 2018, 150, 742–756. [Google Scholar] [CrossRef]
- Monteiro, J.P.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Structure-Bioactivity Relationships of Methylxanthines: Trying to Make Sense of All the Promises and the Drawbacks. Molecules 2016, 21, 974. [Google Scholar] [CrossRef]
- Ashihara, H.; Mizuno, K.; Yokota, T.; Crozier, A. Xanthine Alkaloids: Occurrence, Biosynthesis, and Function in Plants. Prog. Chem. Org. Nat. Prod. 2017, 105, 1–88. [Google Scholar]
- Basnet, R.M.; Zizioli, D.; Guarienti, M.; Finazzi, D.; Memo, M. Methylxanthines induce structural and functional alterations of the cardiac system in zebrafish embryos. BMC Pharmacol. Toxicol. 2017, 18, 72. [Google Scholar] [CrossRef]
- Morfin Maciel, B.M.; Castillo Morfin, B.M. Theophylline, a new look to an old drug. Rev. Alerg. Mex. 2010, 57, 112–122. [Google Scholar] [PubMed]
- Chen, J.F.; Eltzschig, H.K.; Fredholm, B.B. Adenosine receptors as drug targets—What are the challenges? Nat. Rev. Drug Discov. 2013, 12, 265–286. [Google Scholar] [CrossRef]
- Fishman, P.; Bar-Yehuda, S.; Synowitz, M.; Powell, J.D.; Klotz, K.N.; Gessi, S.; Borea, P.A. Adenosine receptors and cancer. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 399–441. [Google Scholar]
- Torres, A.; Vargas, Y.; Uribe, D.; Jaramillo, C.; Gleisner, A.; Salazar-Onfray, F.; Lopez, M.N.; Melo, R.; Oyarzun, C.; San Martin, R.; et al. Adenosine A3 receptor elicits chemoresistance mediated by multiple resistance-associated protein-1 in human glioblastoma stem-like cells. Oncotarget 2016, 7, 67373–67386. [Google Scholar] [CrossRef]
- Durrant, K.L. Known and hidden sources of caffeine in drug, food, and natural products. J. Am. Pharm. Assoc. 2002, 42, 625–637. [Google Scholar] [CrossRef]
- Jones, A.W. Review of Caffeine-Related Fatalities along with Postmortem Blood Concentrations in 51 Poisoning Deaths. J. Anal. Toxicol. 2017, 41, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef]
- Durrant, K.L.; Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L.; Astorino, T.A.; Liu, S.; Alkadhi, K.A.; Addicott, M.; Farah, A.; et al. Coffee, Caffeine, and Health Outcomes: An Umbrella Review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar]
- Cappelletti, S.; Daria, P.; Sani, G.; Aromatario, M. Caffeine: Cognitive and Physical Performance Enhancer or Psychoactive Drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef]
- Lipton, R.B.; Diener, H.C.; Robbins, M.S.; Garas, S.Y.; Patel, K. Caffeine in the management of patients with headache. J. Headache Pain 2017, 18, 107. [Google Scholar] [CrossRef]
- Temple, J.L.; Hostler, D.; Martin-Gill, C.; Moore, C.G.; Weiss, P.M.; Sequeira, D.J.; Condle, J.P.; Lang, E.S.; Higgins, J.S.; Patterson, P.D. Systematic Review and Meta-analysis of the Effects of Caffeine in Fatigued Shift Workers: Implications for Emergency Medical Services Personnel. Prehosp. Emerg. Care 2018, 22, 37–46. [Google Scholar] [CrossRef]
- Baratloo, A.; Rouhipour, A.; Forouzanfar, M.M.; Safari, S.; Amiri, M.; Negida, A. The Role of Caffeine in Pain Management: A Brief Literature Review. Anesthesiol. Pain Med. 2016, 6, e33193. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.C.; Ramirez, C.E.; Choi, L.; Okamoto, L.E.; Gamboa, A.; Diedrich, A.; Raj, S.R.; Robertson, D.; Biaggioni, I.; Shibao, C. Combination ergotamine and caffeine improves seated blood pressure and presyncopal symptoms in autonomic failure. Front. Physiol. 2014, 5, 270. [Google Scholar] [CrossRef] [PubMed]
- Szopa, A.; Poleszak, E.; Wyska, E.; Serefko, A.; Wośko, S.; Wlaź, A.; Pieróg, M.; Wróbel, A.; Wlaź, P. Caffeine enhances the antidepressant-like activity of common antidepressant drugs in the forced swim test in mice. Naunyn-Schmiedebergs Arch. Pharmacol. 2016, 389, 211–221. [Google Scholar] [CrossRef]
- Ning, Y.L.; Yang, N.; Chen, X.-Y.; Zhao, Z.A.; Zhang, X.Z.; Chen, X.Y.; Li, P.; Zhao, Y.; Zhou, Y.G. Chronic caffeine exposure attenuates blast-induced memory deficit in mice. Chin. J. Traumatol. 2015, 18, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Sachse, K.T.; Jackson, E.K.; Wisniewski, S.R.; Gillespie, D.G.; Puccio, A.M.; Clark, R.S.; Dixon, C.E.; Kochanek, P.M. Increases in Cerebrospinal Fluid Caffeine Concentration are Associated with Favorable Outcome after Severe Traumatic Brain injury in Humans. J. Cereb. Blood Flow Metab. 2008, 28, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Geiger, N.H.; Soliman, M.L.; Hui, L.; Geiger, J.D.; Chen, X. Caffeine, Through Adenosine A3Receptor-Mediated Actions, Suppresses Amyloid-β Protein Precursor Internalization and Amyloid-β Generation. J. Alzheimers Dis. 2015, 47, 73–83. [Google Scholar] [CrossRef]
- Khadrawy, Y.A.; Salem, A.M.; El-Shamy, K.A.; Ahmed, E.K.; Fadl, N.N.; Hosny, E.N. Neuroprotective and Therapeutic Effect of Caffeine on the Rat Model of Parkinson’s Disease Induced by Rotenone. J. Diet. Suppl. 2017, 14, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, Z.; Jin, Y.; Guo, J. Association between tea and coffee consumption and brain cancer risk: An updated meta-analysis. World J. Surg. Oncol. 2019, 17, 51. [Google Scholar] [CrossRef]
- Jiang, J.; Lan, Y.Q.; Zhang, T.; Yu, M.; Liu, X.Y.; Li, L.H.; Chen, X.P. The in vitro effects of caffeine on viability, cycle cycle profiles, proliferation, and apoptosis of glioblastomas. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3201–3207. [Google Scholar]
- Sun, F.; Han, D.F.; Cao, B.Q.; Wang, B.; Dong, N.; Jiang, D.H. Caffeine-induced nuclear translocation of FoxO1 triggers Bim-mediated apoptosis in human glioblastoma cells. Tumor Biol. 2016, 37, 3417–3423. [Google Scholar] [CrossRef]
- Chen, J.C.; Hwang, J.H.; Chiu, W.H.; Chan, Y.C. Tetrandrine and Caffeine Modulated Cell Cycle and Increased Glioma Cell Death via Caspase-Dependent and Caspase-Independent Apoptosis Pathways. Nutr. Cancer 2014, 66, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Hwang, J.H. Effects of caffeine on cell viability and activity of histone deacetylase 1 and histone acetyltransferase in glioma cells. Tzu Chi Med. J. 2016, 28, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Han, K.S.; Ku, B.M.; Lee, Y.K.; Hong, J.; Shin, H.Y.; Almonte, A.G.; Woo, D.H.; Brat, D.J.; Hwang, E.M.; et al. Caffeine-mediated inhibition of calcium release channel inositol 1,4,5-trisphosphate receptor subtype 3 blocks glioblastoma invasion and extends survival. Cancer Res. 2010, 70, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Ding, Y.M.; Hueng, D.Y.; Chen, J.Y.; Chen, Y. Caffeine suppresses the progression of human glioblastoma via cathepsin B and MAPK signaling pathway. J. Nutr. Biochem. 2016, 33, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Amico, A.G.; Rasà, D.M.; Saccone, S.; Federico, C.; Magro, G.; Cavallaro, S.; D’Agata, V. Caffeine Effect on HIFs/VEGF Pathway in Human Glioblastoma Cells Exposed to Hypoxia. Anti-Cancer Agents Med. Chem. 2018, 18, 1432–1439. [Google Scholar] [CrossRef]
- Conde, V.R.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Tea (Camellia sinensis (L.)): A putative anticancer agent in bladder carcinoma? Anti-Cancer Agents Med. Chem. 2015, 15, 26–36. [Google Scholar] [CrossRef]
- Onatibia-Astibia, A.; Franco, R.; Martinez-Pinilla, E. Health benefits of methylxanthines in neurodegenerative diseases. Mol. Nutr. Food Res. 2017, 61, 1600670. [Google Scholar] [CrossRef]
- Barnes, P.J. Theophylline in chronic obstructive pulmonary disease: New horizons. Proc. Am. Thorac. Soc. 2005, 2, 334–339. [Google Scholar] [CrossRef]
- Barnes, P.J. Theophylline. Pharmaceuticals 2010, 3, 725–747. [Google Scholar] [CrossRef]
- Koul, D.; Wang, S.; Wu, S.; Saito, N.; Zheng, S.; Gao, F.; Kaul, I.; Setoguchi, M.; Nakayama, K.; Koyama, K.; et al. Preclinical therapeutic efficacy of a novel blood-brain barrier-penetrant dual PI3K/mTOR inhibitor with preferential response in PI3K/PTEN mutant glioma. Oncotarget 2017, 8, 21741–21753. [Google Scholar] [CrossRef]
- Gallelli, L.; Falcone, D.; Cannataro, R.; Perri, M.; Serra, R.; Pelaia, G.; Maselli, R.; Savino, R.; Spaziano, G.; D’Agostino, B. Theophylline action on primary human bronchial epithelial cells under proinflammatory stimuli and steroidal drugs: A therapeutic rationale approach. Drug Des. Dev. Ther. 2017, 11, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Domvri, K.; Zarogoulidis, K.; Zogas, N.; Zarogoulidis, P.; Petanidis, S.; Porpodis, K.; Kioseoglou, E.; Hohenforst-Schmidt, W. Potential synergistic effect of phosphodiesterase inhibitors with chemotherapy in lung cancer. J. Cancer 2017, 8, 3648–3656. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.L.; Hsu, Y.J.; Chen, Y.; Wang, Y.W.; Huang, S.M. Theophylline exhibits anti-cancer activity via suppressing SRSF3 in cervical and breast cancer cell lines. Oncotarget 2017, 8, 101461–101474. [Google Scholar] [CrossRef] [PubMed]
- Rusconi, M.; Conti, A. Theobroma cacao L., the Food of the Gods: A scientific approach beyond myths and claims. Pharmacol. Res. 2010, 61, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kreider, J.W.; Rosenthal, M.; Lengle, N. Cyclic adenosine 3′,5′-monophosphate in the control of melanoma cell replication and differentiation. J. Natl. Cancer Inst. 1973, 50, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Pozner, J.; Papatestas, A.E.; Fagerstrom, R.; Schwartz, I.; Saevitz, J.; Feinberg, M.; Aufses, A.H., Jr. Association of tumor differentiation with caffeine and coffee intake in women with breast cancer. Surgery 1986, 100, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Tsuchiya, H. Enhancement of cytocidal and antitumor effect of cisplatin by caffeine in human osteosarcoma. Clin. Ther. 1989, 11, 43–52. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).