Biocompatible and Hermetic Encapsulation of PMUTs: Effects of Parylene F-VT4 and ALD Stacks on Membrane Vibration and Acoustic Performance
Abstract
1. Introduction
2. Devices and Methods
2.1. PMUT Description
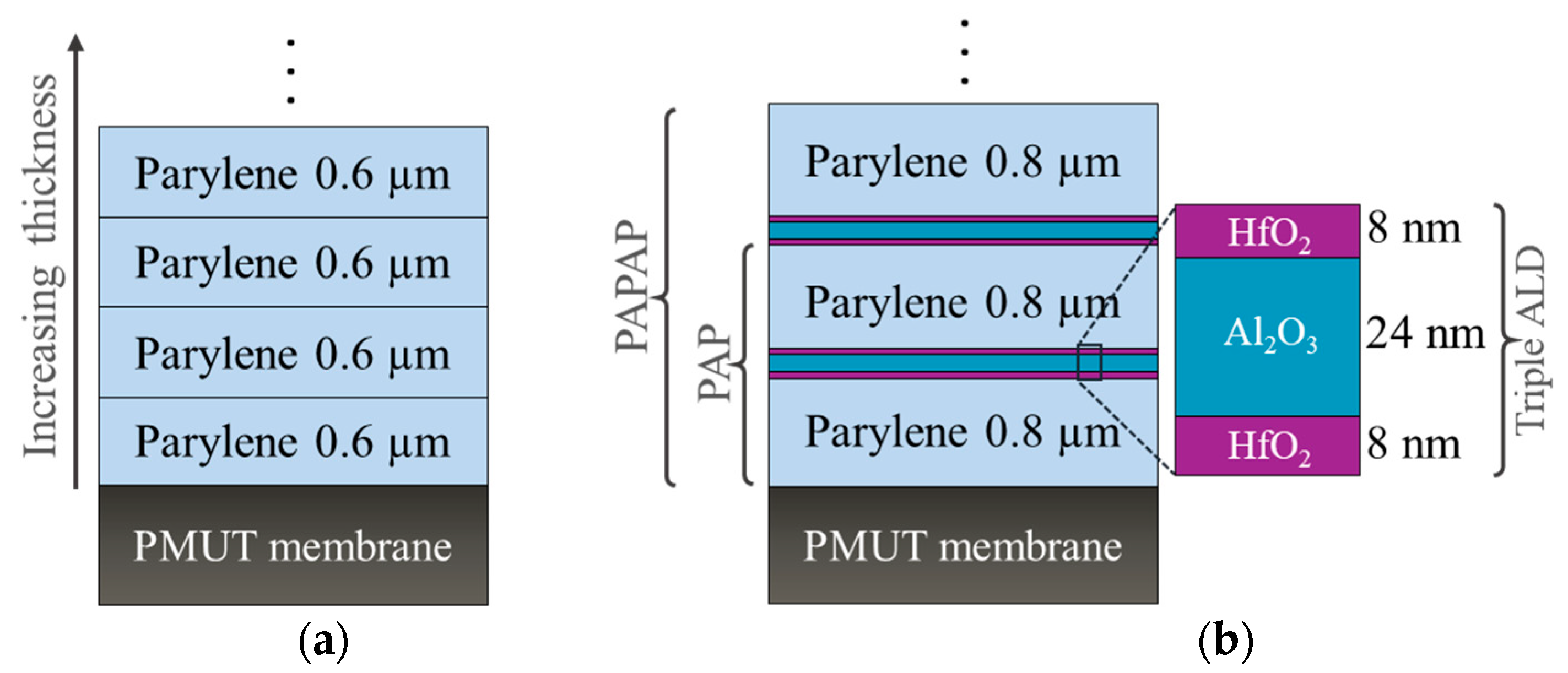
2.2. Encapsulation Layer Deposition
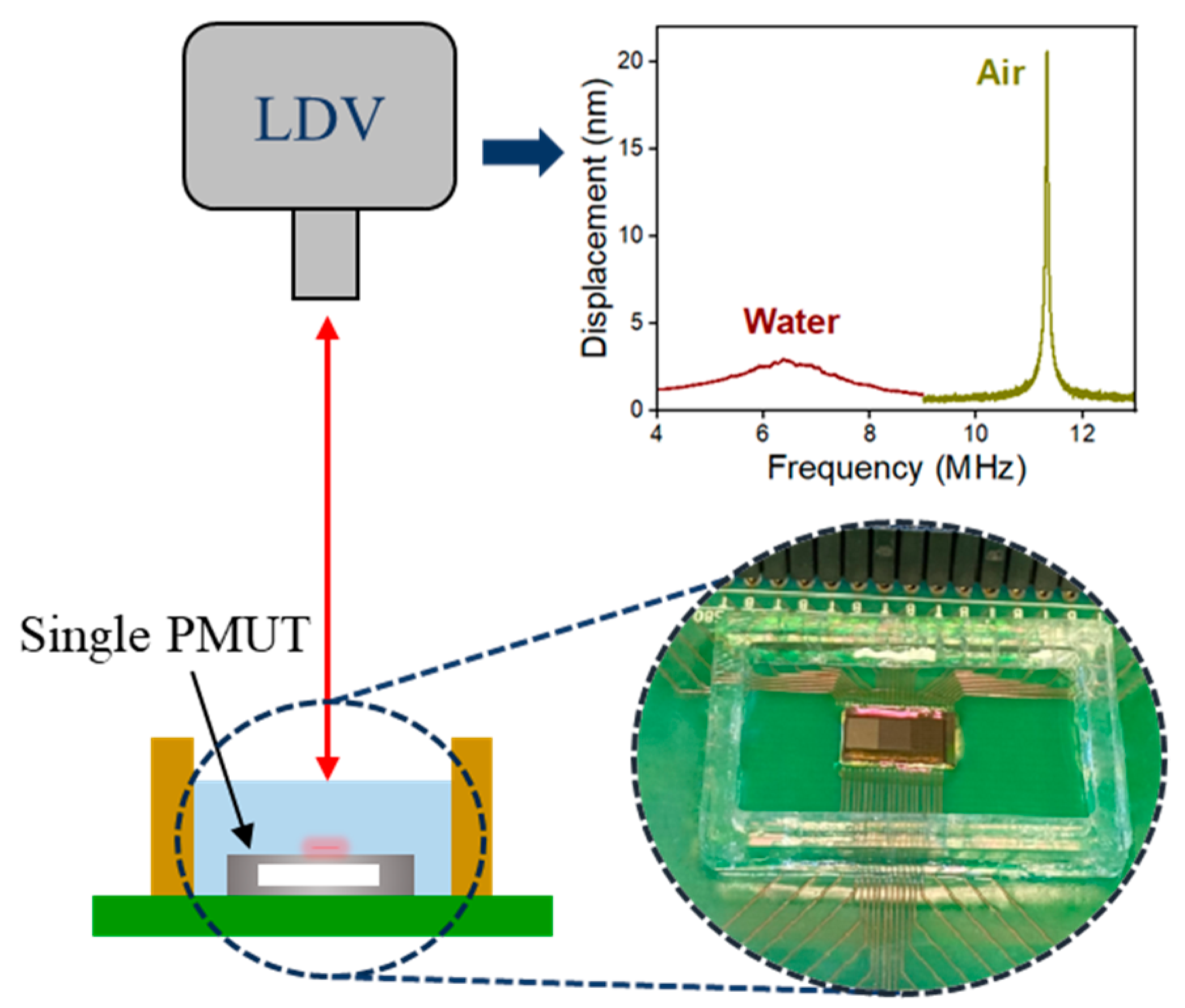
2.3. Vibration Measurement
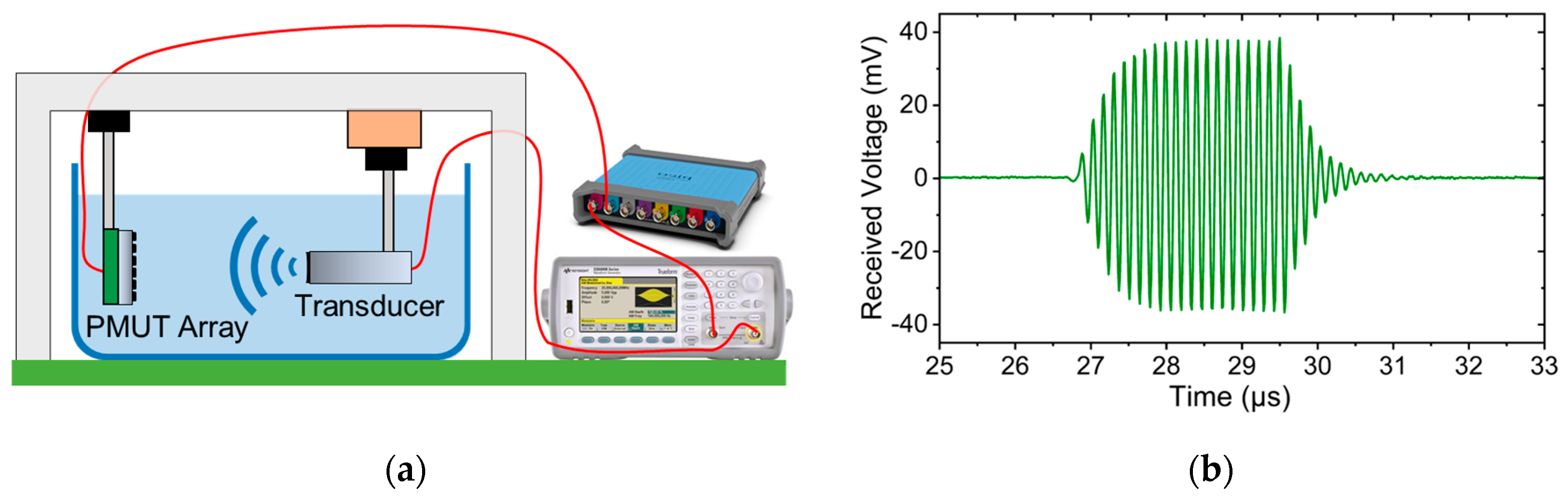
2.4. Acoustic Measurement
3. Modeling and Numerical Simulation
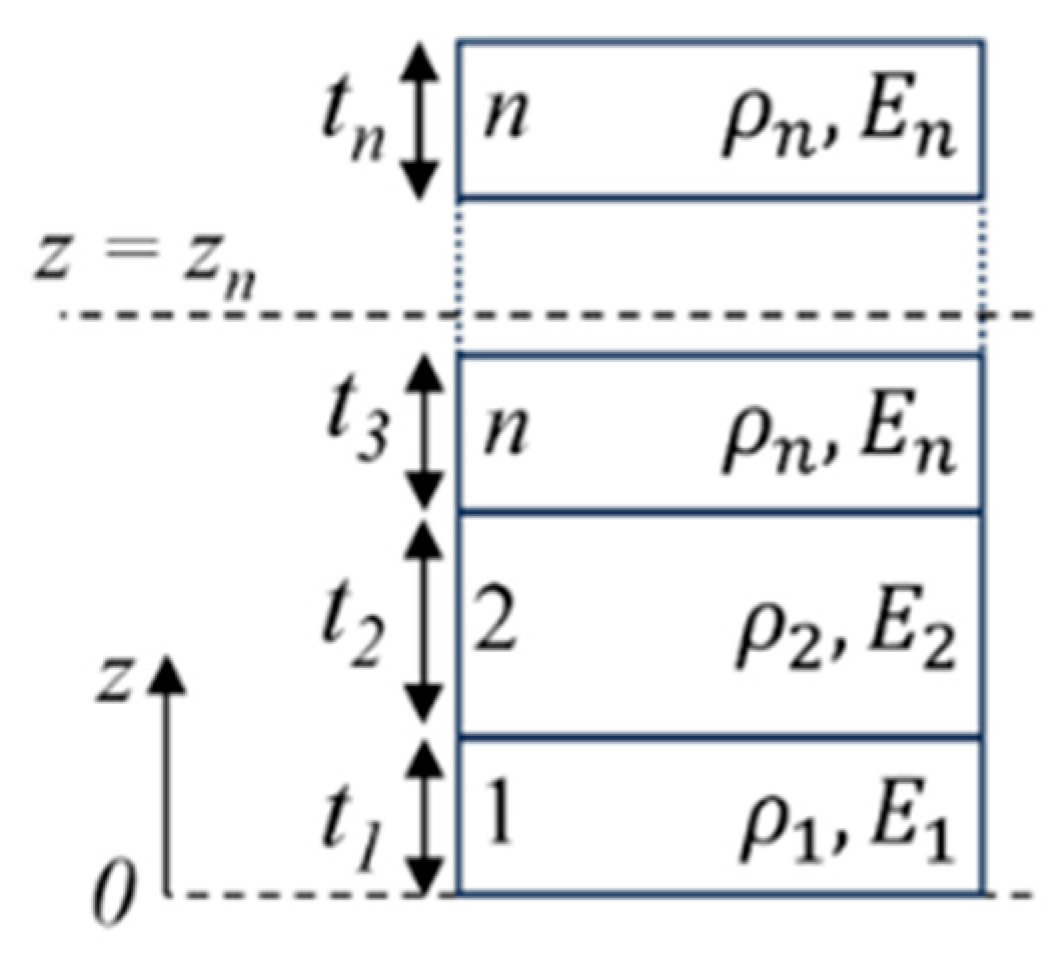
3.1. Resonance Frequency of a Circular Multilayered Plate
3.2. Fluid Loading Effect on the Resonance Frequency
3.3. Effect of Encapsulation on Resonance Frequency
3.4. Effect of Encapsulation on Displacement
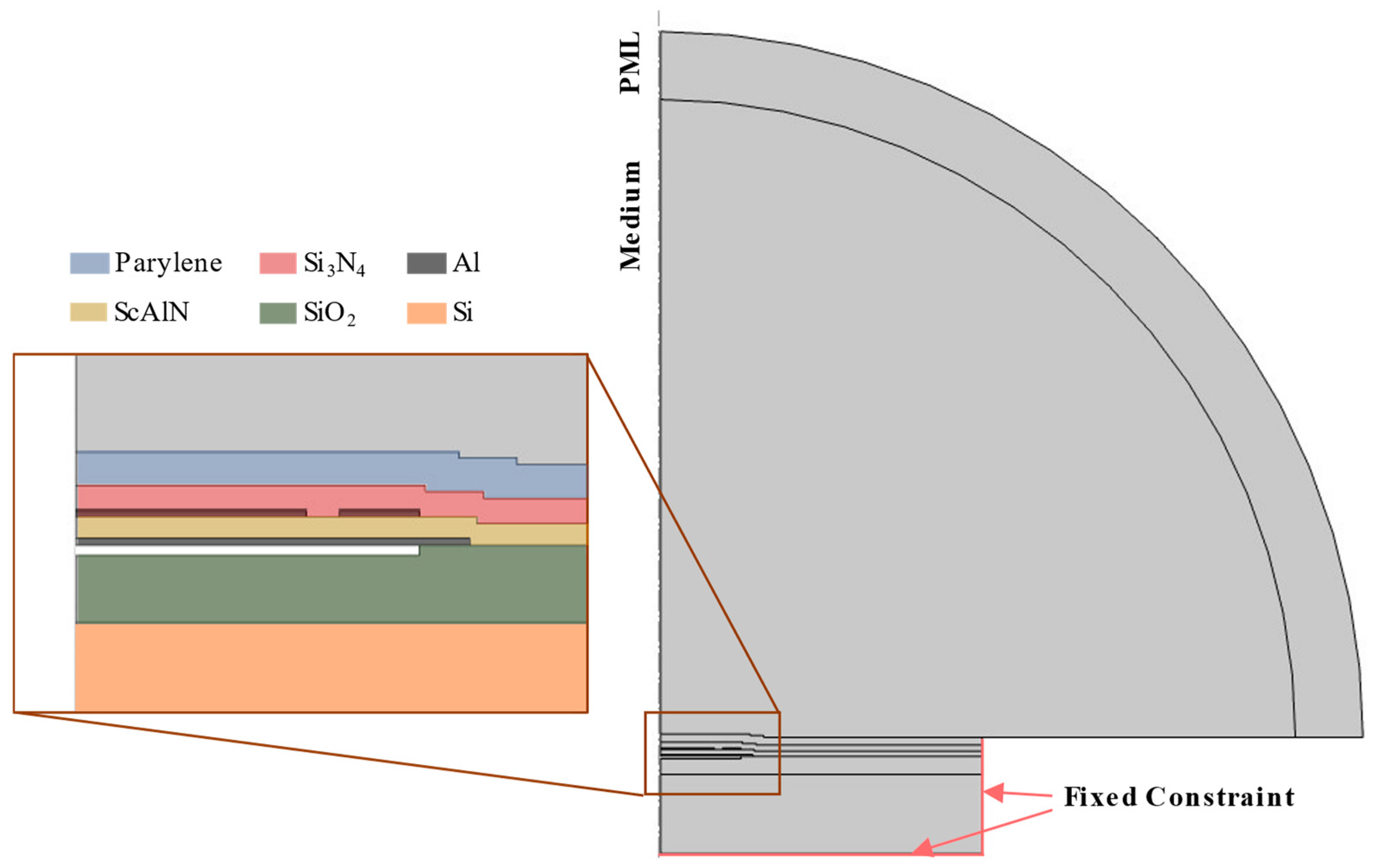
3.5. FEM Simulation Using COMSOL
4. Results and Discussion
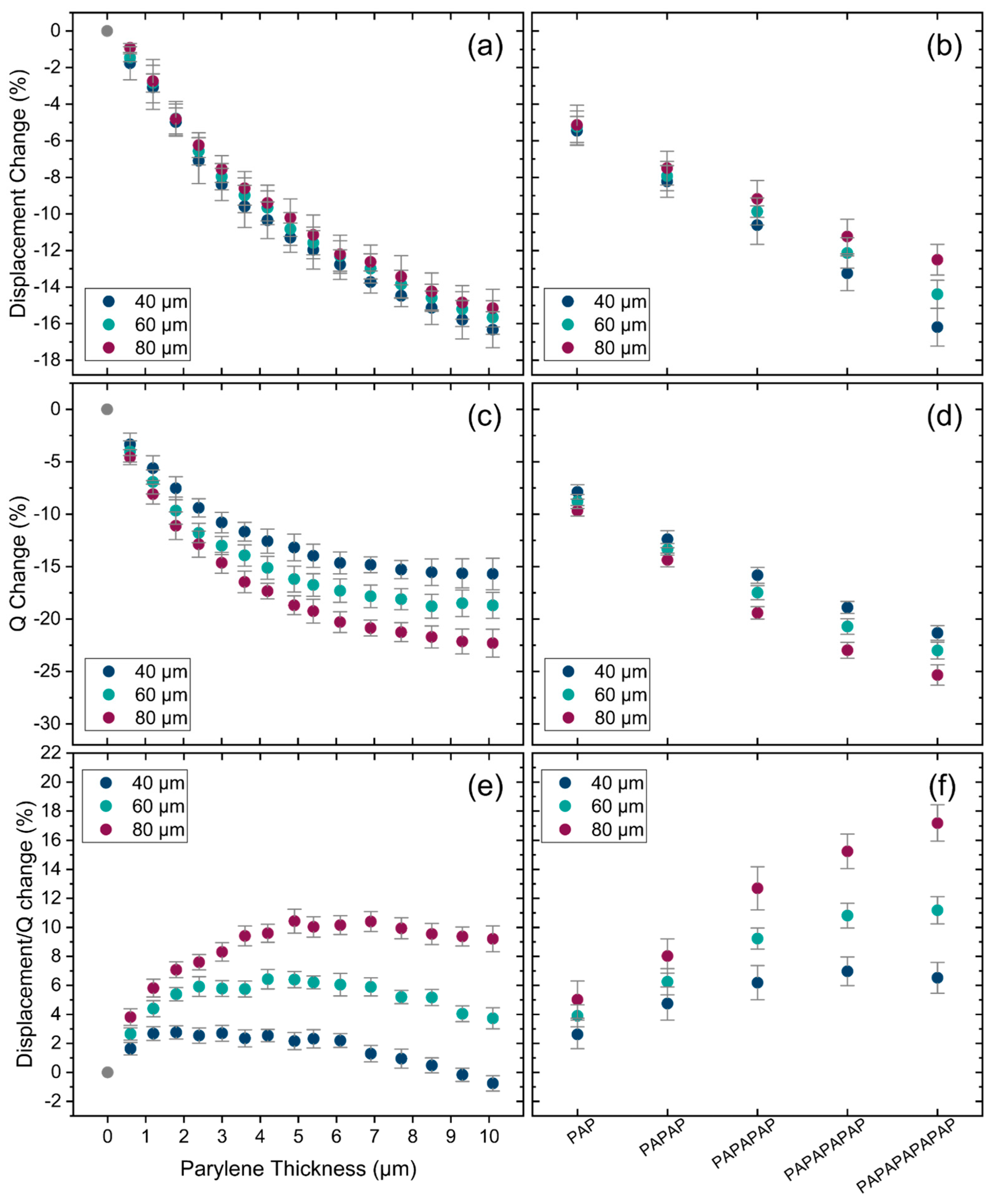
4.1. Impact of Encapsulation on Single PMUTs in Air
4.2. Impact of Encapsulation on Single PMUTs in Water
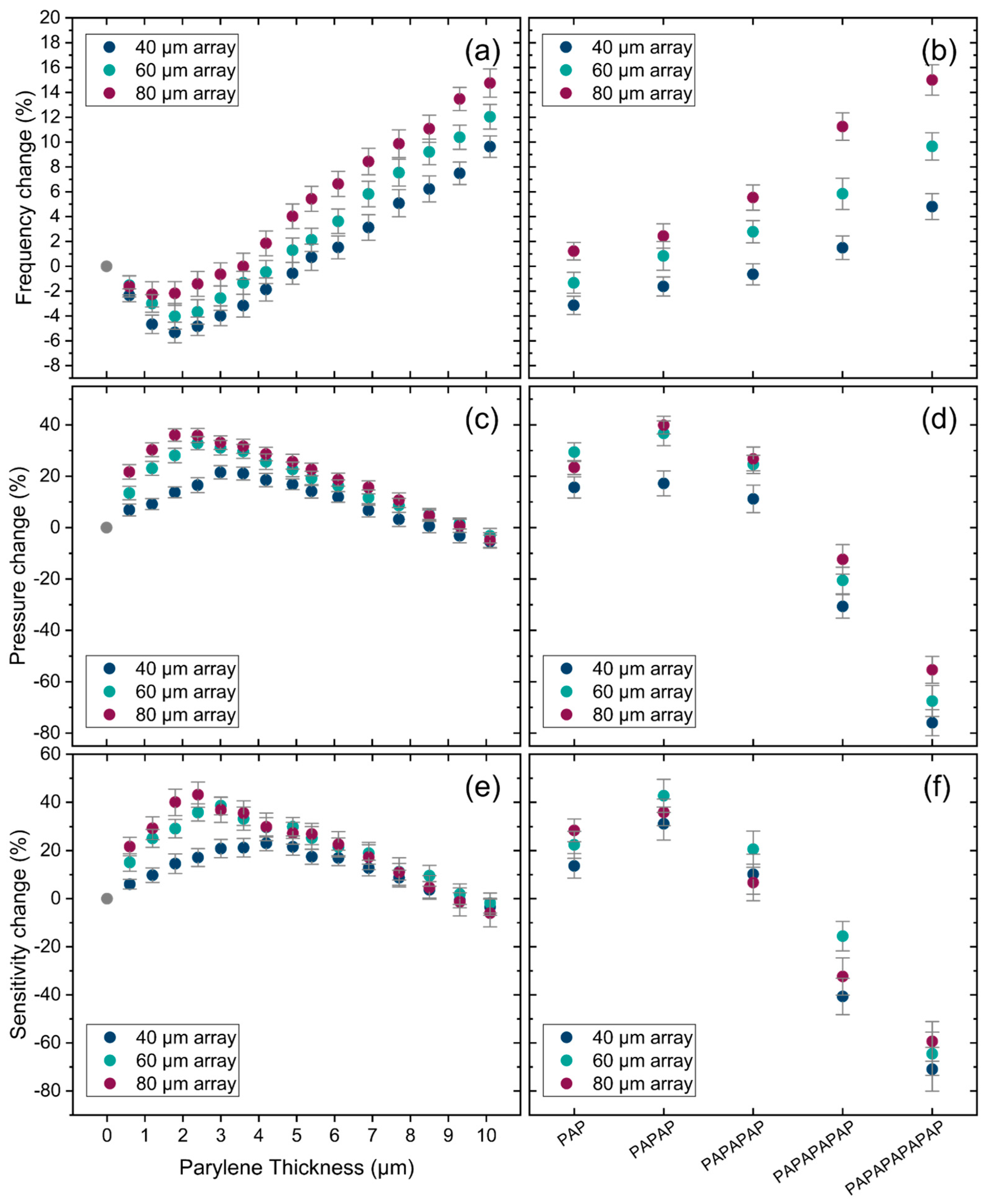
4.3. Impact of Water Mass Loading on Resonance Frequency
4.4. Acoustic Transmisson and Sensitivity of PMUT Arrays
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joung, Y.-H. Development of Implantable Medical Devices: From an Engineering Perspective. Int. Neurourol. J. 2013, 17, 98. [Google Scholar] [CrossRef] [PubMed]
- Yakovlev, A.; Kim, S.; Poon, A. Implantable Biomedical Devices: Wireless Powering and Communication. IEEE Commun. Mag. 2012, 50, 152–159. [Google Scholar] [CrossRef]
- Teshome, A.K.; Kibret, B.; Lai, D.T.H. A Review of Implant Communication Technology in WBAN: Progress and Challenges. IEEE Rev. Biomed. Eng. 2018, 12, 88–99. [Google Scholar] [CrossRef]
- Islam, M.N.; Yuce, M.R. Review of Medical Implant Communication System (MICS) Band and Network. ICT Express 2016, 2, 188–194. [Google Scholar] [CrossRef]
- Wegmueller, M.S.; Huclova, S.; Froehlich, J.; Oberle, M.; Felber, N.; Kuster, N.; Fichtner, W. Galvanic Coupling Enabling Wireless Implant Communications. IEEE Trans. Instrum. Meas. 2009, 58, 2618–2625. [Google Scholar] [CrossRef]
- Serhani, M.A.; El Kassabi, H.T.; Ismail, H.; Nujum Navaz, A. ECG Monitoring Systems: Review, Architecture, Processes, and Key Challenges. Sensors 2020, 20, 1796. [Google Scholar] [CrossRef]
- Caylor, J.; Reddy, R.; Yin, S.; Cui, C.; Huang, M.; Huang, C.; Rao, R.; Baker, D.G.; Simmons, A.; Souza, D. Spinal Cord Stimulation in Chronic Pain: Evidence and Theory for Mechanisms of Action. Bioelectron. Med. 2019, 5, 12. [Google Scholar] [CrossRef]
- Khanna, V.K.; Khanna, V.K. Wireless Communications and Powering of Implants. In Implantable Medical Electronics: Prosthetics, Drug Delivery, and Health Monitoring; Springer: Cham, Switzerland, 2016; pp. 185–207. [Google Scholar]
- Agarwal, K.; Jegadeesan, R.; Guo, Y.-X.; Thakor, N. V Wireless Power Transfer Strategies for Implantable Bioelectronics. IEEE Rev. Biomed. Eng. 2017, 10, 136–161. [Google Scholar] [CrossRef]
- Bos, T.; Dehaene, W.; Verhelst, M. Ultrasound In-Body Communication with OFDM through Multipath Realistic Channels. In Proceedings of the 2019 IEEE Biomedical Circuits and Systems Conference (BioCAS), Nara, Japan, 17–19 October 2019; IEEE: New York, NY, USA, 2019; pp. 1–4. [Google Scholar]
- Demirors, E.; Alba, G.; Santagati, G.E.; Melodia, T. High Data Rate Ultrasonic Communications for Wireless Intra-Body Networks. In Proceedings of the 2016 IEEE International Symposium on Local and Metropolitan Area Networks (LANMAN), Rome, Italy, 13–15 June 2016; IEEE: New York, NY, USA, 2016; pp. 1–6. [Google Scholar]
- Taalla, R.V.; Arefin, M.S.; Kaynak, A.; Kouzani, A.Z. A Review on Miniaturized Ultrasonic Wireless Power Transfer to Implantable Medical Devices. IEEE Access 2018, 7, 2092–2106. [Google Scholar] [CrossRef]
- Oevermann, J.; Weber, P.; Tretbar, S.H. Encapsulation of Capacitive Micromachined Ultrasonic Transducers (CMUTs) for the Acoustic Communication between Medical Implants. Sensors 2021, 21, 421. [Google Scholar] [CrossRef]
- Narvaez, F.; Hosseini, S.; Farkhani, H.; Moradi, F. Analysis and Design of a PMUT-Based Transducer for Powering Brain Implants. arXiv 2021, arXiv:2101.04443. [Google Scholar]
- Herrera, B.; Simeoni, P.; Giribaldi, G.; Colombo, L.; Rinaldi, M. Scandium-Doped Aluminum Nitride Pmut Arrays for Wireless Ultrasonic Powering of Implantables. IEEE Open J. Ultrason. Ferroelectr. Freq. Control. 2022, 2, 250–260. [Google Scholar] [CrossRef]
- Proto, A.; Rufer, L.; Basrour, S.; Penhaker, M. Modeling and Measurement of an Ultrasound Power Delivery System for Charging Implantable Devices Using an ALN-Based PMUT as Receiver. Micromachines 2022, 13, 2127. [Google Scholar] [CrossRef]
- Khuri-Yakub, B.T.; Oralkan, Ö. Capacitive Micromachined Ultrasonic Transducers for Medical Imaging and Therapy. J. Micromech. Microeng. 2011, 21, 054004. [Google Scholar] [CrossRef] [PubMed]
- Salim, M.S.; Abd Malek, M.F.; Heng, R.B.W.; Juni, K.M.; Sabri, N. Capacitive Micromachined Ultrasonic Transducers: Technology and Application. J. Med. Ultrasound 2012, 20, 8–31. [Google Scholar] [CrossRef]
- Oralkan, O.; Ergun, A.S.; Johnson, J.A.; Karaman, M.; Demirci, U.; Kaviani, K.; Lee, T.H.; Khuri-Yakub, B.T. Capacitive Micromachined Ultrasonic Transducers: Next-Generation Arrays for Acoustic Imaging? IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2002, 49, 1596–1610. [Google Scholar] [CrossRef]
- Gross, D.; Legros, M.; Vince, P.; Certon, D. Evaluation of an Ultrasound-Guided Focused Ultrasound Cmut Probe for Targeted Therapy Applications. Open J. Appl. Sci. 2018, 8, 25. [Google Scholar] [CrossRef][Green Version]
- Demirci, U.; Ergun, A.S.; Oralkan, O.; Karaman, M.; Khuri-Yakub, B.T. Forward-Viewing CMUT Arrays for Medical Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2004, 51, 887–895. [Google Scholar] [CrossRef]
- Moisello, E.; Novaresi, L.; Sarkar, E.; Malcovati, P.; Costa, T.L.; Bonizzoni, E. PMUT and CMUT Devices for Biomedical Applications: A Review. IEEE Access 2024, 12, 18640–18657. [Google Scholar] [CrossRef]
- Herickhoff, C.D.; van Schaijk, R. CMUT Technology Developments. Z. Med. Phys. 2023, 33, 256–266. [Google Scholar] [CrossRef]
- Qiu, Y.; Gigliotti, J.V.; Wallace, M.; Griggio, F.; Demore, C.E.M.; Cochran, S.; Trolier-McKinstry, S. Piezoelectric Micromachined Ultrasound Transducer (PMUT) Arrays for Integrated Sensing, Actuation and Imaging. Sensors 2015, 15, 8020–8041. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Lee, W.; Kang, W.; Shin, E.; Ryu, J.; Choi, H. Review of Piezoelectric Micromachined Ultrasonic Transducers and Their Applications. J. Micromech. Microeng. 2017, 27, 113001. [Google Scholar] [CrossRef]
- Moretti, E. Numerical Modelling and Simulation of Large PMUTs Arrays for Medical Applications. Ph.D. Thesis, Politecnico di Milano, Milan, Italy, 2023. [Google Scholar]
- Bazaka, K.; Jacob, M.V. Implantable Devices: Issues and Challenges. Electronics 2012, 2, 1–34. [Google Scholar] [CrossRef]
- Li, C.; Cauwe, M.; Mader, L.; Schaubroeck, D.; Op de Beeck, M. Accelerated Hermeticity Testing of Biocompatible Moisture Barriers Used for the Encapsulation of Implantable Medical Devices. Coatings 2019, 10, 19. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Xie, X.; Rieth, L.; Caldwell, R.; Diwekar, M.; Tathireddy, P.; Sharma, R.; Solzbacher, F. Long-Term Bilayer Encapsulation Performance of Atomic Layer Deposited Al2O3 and Parylene C for Biomedical Implantable Devices. IEEE Trans. Biomed. Eng. 2013, 60, 2943–2951. [Google Scholar]
- Kahouli, A.; Sylvestre, A.; Laithier, J.-F.; Lutsen, L.; Pairis, S.; André, E.; Garden, J.-L. Structural and Dielectric Properties of Parylene-VT4 Thin Films. Mater. Chem. Phys. 2014, 143, 908–914. [Google Scholar] [CrossRef]
- Afshari, E.; Verplancke, R.; Cauwe, M.; de Beeck, M. Quality and Lifetime of Thin Parylene F-VT4 Coatings for Hermetic Encapsulation of Implantable Medical Devices. Coatings 2025, 15, 648. [Google Scholar] [CrossRef]
- Li, C.; Cauwe, M.; Yang, Y.; Schaubroeck, D.; Mader, L.; Op de Beeck, M. Ultra-Long-Term Reliable Encapsulation Using an Atomic Layer Deposited HfO2/Al2O3/HfO2 Triple-Interlayer for Biomedical Implants. Coatings 2019, 9, 579. [Google Scholar] [CrossRef]
- Zhuang, X.; Nikoozadeh, A.; Beasley, M.A.; Yaralioglu, G.G.; Khuri-Yakub, B.T.; Pruitt, B.L. Biocompatible Coatings for CMUTs in a Harsh, Aqueous Environment. J. Micromech. Microeng. 2007, 17, 994–1001. [Google Scholar] [CrossRef]
- Lin, D.-S.; Zhuang, X.; Wong, S.H.; Kupnik, M.; Khuri-Yakub, B.T. Encapsulation of Capacitive Micromachined Ultrasonic Transducers Using Viscoelastic Polymer. J. Microelectromechanical Syst. 2010, 19, 1341–1351. [Google Scholar] [CrossRef]
- Afshari, E.; Houri, S.; Verplancke, R.; Rochus, V.; Cauwe, M.; Gijsenbergh, P.; Rottenberg, X.; de Beeck, M.O. Biocompatible Hermetic Encapsulation of PMUTs for Usage in Implantable Medical Devices. In Proceedings of the 2024 IEEE Ultrasonics, Ferroelectrics, and Frequency Control Joint Symposium (UFFC-JS), Taipei, Taiwan, 22–26 September 2024; IEEE: New York, NY, USA, 2024; pp. 1–4. [Google Scholar]
- Velea, A.I.; Panskus, R.; Szabo, B.; Oppelt, V.A.-L.; Holzapfel, L.; Karuthedath, C.B.; Sebastian, A.T.; Stieglitz, T.; Savoia, A.S.; Giagka, V. Effects of Soft Encapsulation on the Receive Performance of PMUTs for Implantable Devices. bioRxiv 2025. [Google Scholar] [CrossRef]
- Available online: https://Www.Silterra.Com/Technology/Piezoelectric-Micromachined-Ultrasonic-Transducer-Pmut/ (accessed on 26 June 2025).
- Billen, M.; Ferrer, E.M.; Pandian, M.S.; Rottenberg, X.; Rochus, V. Mid-Air Haptic Feedback Enabled by Aluminum Nitride PMUTs. In Proceedings of the 2022 IEEE 35th International Conference on Micro Electro Mechanical Systems Conference (MEMS), Tokyo, Japan, 9–13 January 2022; IEEE: New York, NY, USA, 2022; pp. 247–250. [Google Scholar]
- Fox, C.H.J.; Chen, X.; McWilliam, S. Analysis of the Deflection of a Circular Plate with an Annular Piezoelectric Actuator. Sens. Actuators A Phys. 2007, 133, 180–194. [Google Scholar] [CrossRef]
- Chare, C.; Gijsenbergh, P.; Jeong, Y.; Heremans, P.; Cheyns, D.; Genoe, J. Electromechanical Equivalent Circuit Model for Axisymmetric PMUTs with Elastic Boundary Conditions. J. Microelectromechanical Syst. 2022, 31, 457–472. [Google Scholar] [CrossRef]
- Alf, M.E.; Asatekin, A.; Barr, M.C.; Baxamusa, S.H.; Chelawat, H.; Ozaydin-Ince, G.; Petruczok, C.D.; Sreenivasan, R.; Tenhaeff, W.E.; Trujillo, N.J. Chemical Vapor Deposition of Conformal, Functional, and Responsive Polymer Films. Adv. Mater. 2010, 22, 1993–2027. [Google Scholar] [CrossRef]
- Lee, H.; Cho, J. Development of Conformal PDMS and Parylene Coatings for Microelectronics and MEMS Packaging. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Orlando, FL, USA, 5–11 November 2005; Volume 42177, pp. 279–283. [Google Scholar]
- Sherman, C.H.; Butler, J.L. Transducers and Arrays for Underwater Sound; Springer: New York, NY, USA, 2007; Volume 4. [Google Scholar]
- Muralt, P.; Ledermann, N.; Paborowski, J.; Barzegar, A.; Gentil, S.; Belgacem, B.; Petitgrand, S.; Bosseboeuf, A.; Setter, N. Piezoelectric Micromachined Ultrasonic Transducers Based on PZT Thin Films. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2005, 52, 2276–2288. [Google Scholar] [CrossRef]
- Woinowsky-Krieger, S.; Timoshenko, S. Theory of Plates and Shells; McGraw-Hill: New York, NY, USA, 1959; ISBN 0070858209. [Google Scholar]
- Smyth, K.; Bathurst, S.; Sammoura, F.; Kim, S.-G. Analytic Solution for N-Electrode Actuated Piezoelectric Disk with Application to Piezoelectric Micromachined Ultrasonic Transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2013, 60, 1756–1767. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.K.; Kim, K.C. Axisymmetric Vibration of Circular Plates in Contact with Fluid. J. Sound Vib. 1991, 146, 381–389. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Chaudhri, M.M.; Enomoto, Y. Accurate Determination of the Mechanical Properties of Thin Aluminum Films Deposited on Sapphire Flats Using Nanoindentations. J. Mater. Res. 1999, 14, 2314–2327. [Google Scholar] [CrossRef]
- Mayrhofer, P.M.; Euchner, H.; Bittner, A.; Schmid, U. Circular Test Structure for the Determination of Piezoelectric Constants of ScxAl1−XN Thin Films Applying Laser Doppler Vibrometry and FEM Simulations. Sens. Actuators A Phys. 2015, 222, 301–308. [Google Scholar] [CrossRef][Green Version]
- Weekers, B.; Billen, M.; Haouari, R.; Rochus, V. Design of a PMUT Array for Multifrequency Imaging. In Proceedings of the 2021 22nd International Conference on Thermal, Mechanical and Multi-Physics Simulation and Experiments in Microelectronics and Microsystems (EuroSimE), St. Julian, Malta, 19–21 April 2021; IEEE: New York, NY, USA, 2021; pp. 1–5. [Google Scholar]
- Available online: https://Comelec.Ch/Wp-Content/Uploads/2020/11/Comelec_parylene_Properties.Pdf (accessed on 26 June 2025).
- Zhang, Q.; Xiao, X.; Cheng, Y.-T.; Verbrugge, M.W. A Non-Destructive Method for Measuring the Mechanical Properties of Ultrathin Films Prepared by Atomic Layer Deposition. Appl. Phys. Lett. 2014, 105, 061901. [Google Scholar] [CrossRef]
- Zizka, J.; King, S.; Every, A.G.; Sooryakumar, R. Mechanical Properties of Low-and High-k Dielectric Thin Films: A Surface Brillouin Light Scattering Study. J. Appl. Phys. 2016, 119, 144102. [Google Scholar] [CrossRef]
- Bhugra, H.; Piazza, G. Piezoelectric MEMS Resonators; Springer: Cham, Switzerland, 2017; ISBN 3319286889. [Google Scholar]
- Young, W.C.; Budynas, R.G.; Sadegh, A.M. Roark’s Formulas for Stress and Strain; McGraw-Hill: New York, NY, USA, 2002; Volume 7. [Google Scholar]
- Magrab, E.B. Mindlin-Reissner Plates. In Vibrations of Elastic Systems: With Multiphysics Applications; Springer: Cham, Switzerland, 2024; pp. 409–433. [Google Scholar]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afshari, E.; Houri, S.; Verplancke, R.; Rochus, V.; Cauwe, M.; Gijsenbergh, P.; Op de Beeck, M. Biocompatible and Hermetic Encapsulation of PMUTs: Effects of Parylene F-VT4 and ALD Stacks on Membrane Vibration and Acoustic Performance. Sensors 2025, 25, 4074. https://doi.org/10.3390/s25134074
Afshari E, Houri S, Verplancke R, Rochus V, Cauwe M, Gijsenbergh P, Op de Beeck M. Biocompatible and Hermetic Encapsulation of PMUTs: Effects of Parylene F-VT4 and ALD Stacks on Membrane Vibration and Acoustic Performance. Sensors. 2025; 25(13):4074. https://doi.org/10.3390/s25134074
Chicago/Turabian StyleAfshari, Esmaeil, Samer Houri, Rik Verplancke, Veronique Rochus, Maarten Cauwe, Pieter Gijsenbergh, and Maaike Op de Beeck. 2025. "Biocompatible and Hermetic Encapsulation of PMUTs: Effects of Parylene F-VT4 and ALD Stacks on Membrane Vibration and Acoustic Performance" Sensors 25, no. 13: 4074. https://doi.org/10.3390/s25134074
APA StyleAfshari, E., Houri, S., Verplancke, R., Rochus, V., Cauwe, M., Gijsenbergh, P., & Op de Beeck, M. (2025). Biocompatible and Hermetic Encapsulation of PMUTs: Effects of Parylene F-VT4 and ALD Stacks on Membrane Vibration and Acoustic Performance. Sensors, 25(13), 4074. https://doi.org/10.3390/s25134074





