Wearable Technology for Monitoring Electrocardiograms (ECGs) in Adults: A Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Identifying Research Questions
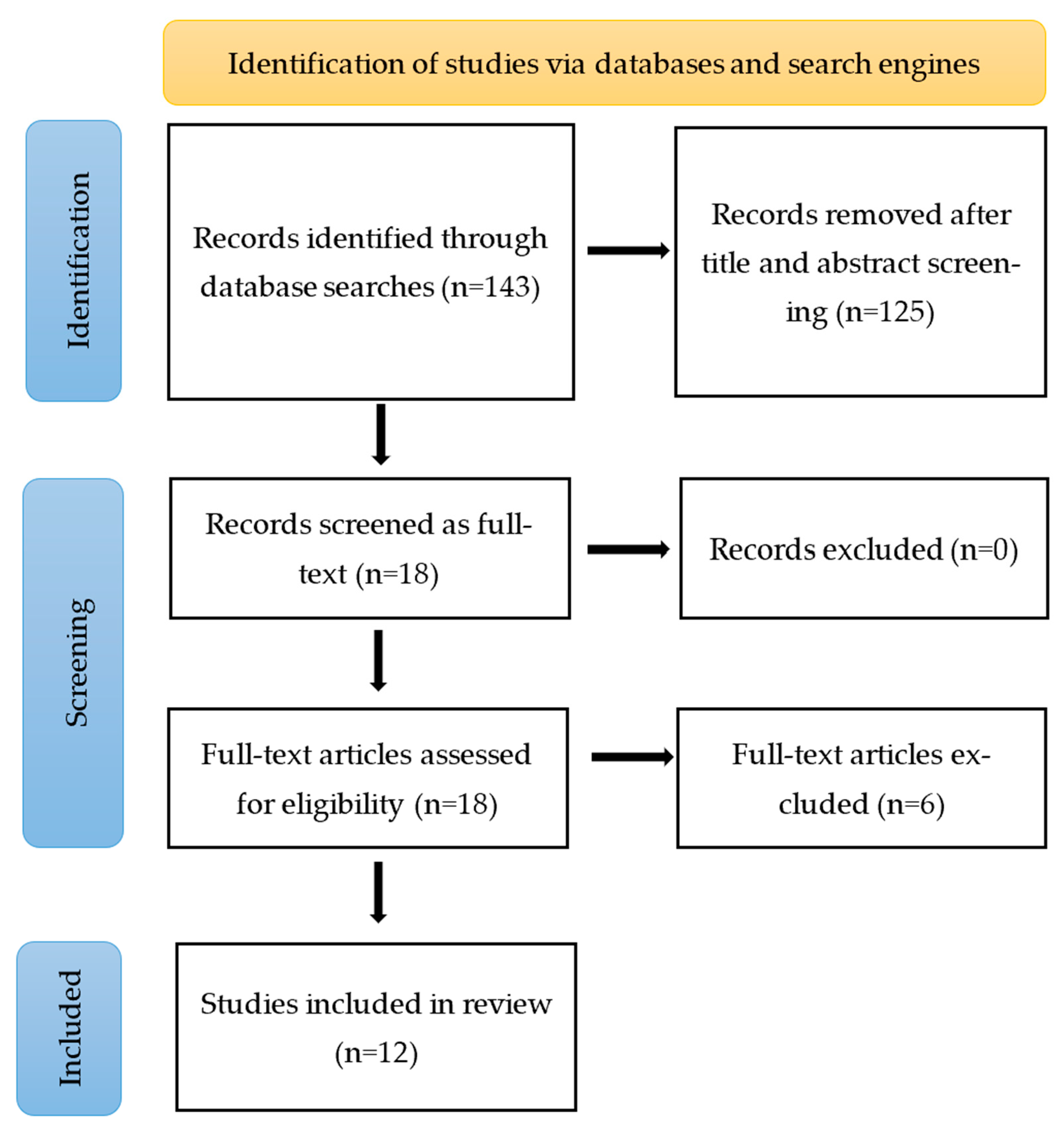
2.2. Identifying the Relevant Studies
- Population: adults (>19 years), all genders, with or without cardiac irregularities.
- Intervention: Multichannel biopotential wearable device (Datalogger).
- Context/comparison: 12-lead ECGs or other variants of ECGs.
- Outcome (endpoints): ECG and heart rate variability (HRV).
2.3. Selecting the Studies
2.4. Charting the Data
2.5. Clinical Data Appraisal, Collating, Summarizing, and Reporting
3. Results
3.1. Study Characteristics
3.2. Appraisal of the Clinical Data
3.3. Evaluation of Performance Outcomes
3.4. Evaluation of Safety Outcomes
4. Discussion
Future Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deaton, C.; Froelicher, E.S.; Wu, L.H.; Ho, C.; Shishani, K.; Jaarsma, T. The global burden of cardiovascular disease. Eur. J. Cardiovasc. Nurs. 2011, 10 (Suppl. S2), S5–S13. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Krishnamurthi, R.V.; Barker-Collo, S.; McPherson, K.M.; Barber, P.A.; Parag, V.; Arroll, B.; Bennett, D.A.; Tobias, M.; Jones, A. 30-year trends in stroke rates and outcome in Auckland, New Zealand (1981–2012): A multi-ethnic population-based series of studies. PLoS ONE 2015, 10, e0134609. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; De Ferranti, S.; Després, J.-P.; Fullerton, H.J.; Howard, V.J. Heart disease and stroke statistics—2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed]
- Delano, M.K. A Long Term Wearable Electrocardiogram (ECG) Measurement System. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2012. [Google Scholar]
- Kalra, A.; Lowe, A.; Al-Jumaily, A. Critical review of electrocardiography measurement systems and technology. Meas. Sci. Technol. 2018, 30, 012001. [Google Scholar] [CrossRef]
- Rafie, N.; Kashou, A.H.; Noseworthy, P.A. ECG interpretation: Clinical relevance, challenges, and advances. Hearts 2021, 2, 505–513. [Google Scholar] [CrossRef]
- Stracina, T.; Ronzhina, M.; Redina, R.; Novakova, M. Golden standard or obsolete method? Review of ECG applications in clinical and experimental context. Front. Physiol. 2022, 13, 867033. [Google Scholar] [CrossRef]
- Serhani, M.A.; El Kassabi, H.T.; Ismail, H.; Nujum Navaz, A. ECG Monitoring Systems: Review, Architecture, Processes, and Key Challenges. Sensors 2020, 20, 1796. [Google Scholar] [CrossRef]
- Drew, B.J.; Califf, R.M.; Funk, M.; Kaufman, E.S.; Krucoff, M.W.; Laks, M.M.; Macfarlane, P.W.; Sommargren, C.; Swiryn, S.; Van Hare, G.F. Practice standards for electrocardiographic monitoring in hospital settings: An American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: Endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004, 110, 2721–2746. [Google Scholar]
- Khunti, K. Accurate interpretation of the 12-lead ECG electrode placement: A systematic review. Health Educ. J. 2014, 73, 610–623. [Google Scholar] [CrossRef]
- Lilly, L.S. Pathophysiology of Heart Disease: A Collaborative Project of Medical Students and Faculty; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Abuwarda, Z.; Mostafa, K.; Oetomo, A.; Hegazy, T.; Morita, P. Wearable devices: Cross benefits from healthcare to construction. Autom. Constr. 2022, 142, 104501. [Google Scholar] [CrossRef]
- Vinetti, G.; Lopomo, N.F.; Taboni, A.; Fagoni, N.; Ferretti, G. The current use of wearable sensors to enhance safety and performance in breath-hold diving: A systematic review. Diving Hyperb. Med. 2020, 50, 54. [Google Scholar] [CrossRef] [PubMed]
- Hayward, J.; Chansin, G. Wearable Sensors 2015–2025: Market Forecasts, Technologies, Players; IDTechEx: Cambridge, UK, 2015. [Google Scholar]
- Prieto-Avalos, G.; Cruz-Ramos, N.A.; Alor-Hernández, G.; Sánchez-Cervantes, J.L.; Rodríguez-Mazahua, L.; Guarneros-Nolasco, L.R. Wearable devices for physical monitoring of heart: A review. Biosensors 2022, 12, 292. [Google Scholar] [CrossRef] [PubMed]
- Bayoumy, K.; Gaber, M.; Elshafeey, A.; Mhaimeed, O.; Dineen, E.H.; Marvel, F.A.; Martin, S.S.; Muse, E.D.; Turakhia, M.P.; Tarakji, K.G. Smart wearable devices in cardiovascular care: Where we are and how to move forward. Nat. Rev. Cardiol. 2021, 18, 581–599. [Google Scholar] [CrossRef] [PubMed]
- Cosoli, G.; Spinsante, S.; Scardulla, F.; D’Acquisto, L.; Scalise, L. Wireless ECG and cardiac monitoring systems: State of the art, available commercial devices and useful electronic components. Measurement 2021, 177, 109243. [Google Scholar] [CrossRef]
- Sequeira, L.; Perrotta, S.; LaGrassa, J.; Merikangas, K.; Kreindler, D.; Kundur, D.; Courtney, D.; Szatmari, P.; Battaglia, M.; Strauss, J. Mobile and wearable technology for monitoring depressive symptoms in children and adolescents: A scoping review. J. Affect. Disord. 2020, 265, 314–324. [Google Scholar] [CrossRef]
- Yan, L.; Yoo, J.; Kim, B.; Yoo, H.-J. A 0.5-μ Vrms 12-μ W Wirelessly Powered Patch-Type Healthcare Sensor for Wearable Body Sensor Network. IEEE J. Solid-State Circuits 2010, 45, 2356–2365. [Google Scholar]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A. Epidermal electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Lin, W.Y.; Ke, H.L.; Chou, W.C.; Chang, P.C.; Tsai, T.H.; Lee, M.Y. Realization and Technology Acceptance Test of a Wearable Cardiac Health Monitoring and Early Warning System with Multi-Channel MCGs and ECG. Sensors 2018, 18, 3538. [Google Scholar] [CrossRef]
- Boudreaux, B.D.; Hebert, E.P.; Hollander, D.B.; Williams, B.M.; Cormier, C.L.; Naquin, M.R.; Gillan, W.W.; Gusew, E.E.; Kraemer, R.R. Validity of Wearable Activity Monitors during Cycling and Resistance Exercise. Med. Sci. Sports Exerc. 2018, 50, 624–633. [Google Scholar] [CrossRef]
- Bumgarner, J.M.; Lambert, C.T.; Hussein, A.A.; Cantillon, D.J.; Baranowski, B.; Wolski, K.; Lindsay, B.D.; Wazni, O.M.; Tarakji, K.G. Smartwatch Algorithm for Automated Detection of Atrial Fibrillation. J. Am. Coll. Cardiol. 2018, 71, 2381–2388. [Google Scholar] [CrossRef]
- Castelletti, S.; Dagradi, F.; Goulene, K.; Danza, A.I.; Baldi, E.; Stramba-Badiale, M.; Schwartz, P.J. A wearable remote monitoring system for the identification of subjects with a prolonged QT interval or at risk for drug-induced long QT syndrome. Int. J. Cardiol. 2018, 266, 89–94. [Google Scholar] [CrossRef]
- Pereira, R.D.A.; Alves, J.L.D.B.; Silva, J.H.D.C.; Costa, M.D.S.; Silva, A.S. Validity of a Smartphone Application and Chest Strap for Recording RR Intervals at Rest in Athletes. Int. J. Sports Physiol. Perform. 2020, 15, 896–899. [Google Scholar] [CrossRef]
- Fontana, P.; Martins, N.R.A.; Camenzind, M.; Rossi, R.M.; Baty, F.; Boesch, M.; Schoch, O.D.; Brutsche, M.H.; Annaheim, S. Clinical Applicability of a Textile 1-Lead ECG Device for Overnight Monitoring. Sensors 2019, 19, 2436. [Google Scholar] [CrossRef]
- Lin, I.M. Effects of a cardiorespiratory synchronization training mobile application on heart rate variability and electroencephalography in healthy adults. Int. J. Psychophysiol. 2018, 134, 168–177. [Google Scholar] [CrossRef]
- Peng, S.; Xu, K.; Chen, W. Comparison of Active Electrode Materials for Non-Contact ECG Measurement. Sensors 2019, 19, 3585. [Google Scholar] [CrossRef]
- Reverberi, C.; Rabia, G.; De Rosa, F.; Bosi, D.; Botti, A.; Benatti, G. The RITMIA™ Smartphone App for Automated Detection of Atrial Fibrillation: Accuracy in Consecutive Patients Undergoing Elective Electrical Cardioversion. BioMed Res. Int. 2019, 2019, 4861951. [Google Scholar] [CrossRef]
- Steinberg, C.; Philippon, F.; Sanchez, M.; Fortier-Poisson, P.; O’Hara, G.; Molin, F.; Sarrazin, J.F.; Nault, I.; Blier, L.; Roy, K.; et al. A Novel Wearable Device for Continuous Ambulatory ECG Recording: Proof of Concept and Assessment of Signal Quality. Biosensors 2019, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, Y.T.; Tokita, M.; Murata, H.; Hirasawa, Y.; Yodogawa, K.; Iwasaki, Y.K.; Asai, K.; Shimizu, W.; Kasai, N.; Nakashima, H.; et al. Validation of wearable textile electrodes for ECG monitoring. Heart Vessel. 2019, 34, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Roetker, N.S.; Folsom, A.R.; Alonso, A.; Heckbert, S.R.; Chen, L.Y. Feasibility of using a leadless patch monitor in community cohort studies: The Multi-ethnic Study of Atherosclerosis. Pacing Clin. Electrophysiol. 2018, 41, 1389–1390. [Google Scholar] [CrossRef] [PubMed]
- Fontana, P.; Martins, N.R.A.; Camenzind, M.; Boesch, M.; Baty, F.; Schoch, O.D.; Brutsche, M.H.; Rossi, R.M.; Annaheim, S. Applicability of a Textile ECG-Belt for Unattended Sleep Apnoea Monitoring in a Home Setting. Sensors 2019, 19, 3367. [Google Scholar] [CrossRef] [PubMed]
- Word Health Organization. Physical Activity. Available online: https://www.who.int/en/news-room/fact-sheets/detail/physical-activity (accessed on 31 July 2021).
- Bai, Y.; Welk, G.J.; Nam, Y.H.; Lee, J.A.; Lee, J.-M.; Kim, Y.; Meier, N.F.; Dixon, P.M. Comparison of consumer and research monitors under semistructured settings. Med. Sci. Sports Exerc. 2016, 48, 151–158. [Google Scholar] [CrossRef] [PubMed]


| Company/Brand | Product | FDA Status |
|---|---|---|
| Watches | ||
| Adidas | miCoach Fit Smart | NA |
| Apple | Apple Watch series | A |
| Biobeat | BB-613WP | A |
| Fitbit | Flex, One, Charge, Sense, Versa, Luxe, Inspire | A |
| Garmin | Epix Pro, Fenix 7 pro, Venu, Tactix 7 | A |
| Pixle Watch | NA | |
| Huawei | Huawei Watch GT, Ultimate, Huawei Band | NA |
| Karacus | DIONE, TRITON | NA |
| Omron | HeartGuide | A |
| Samsung | Galaxy Watch 3, 4, 5, 6 | A |
| SmartCardia | INYU | NA |
| Tom Tom | TomTom Spark | NA |
| Withings | Steel HR, Move, ScanWatch Horizon | A |
| Bands/bracelets | ||
| AliveCor | Kardiaband | A |
| BIOSTRAP | Armband HRM | NA |
| Fitbit | Charge 4 | A |
| HEALBE | GoBe3 | U |
| Microsoft | Microsoft Band | NA |
| MOCACARE | MOC cuff | A |
| Under Armour | UA Band | NA |
| Visi Mobile | The Visi Mobile System | A |
| Xiaomi | Mi Smart Band 5 | U |
| Patches | ||
| BardyDx | Zio Patch | A |
| BioTelemetry | Bio Tel Heart | A |
| Corventis Inc | Nuvant MCT | A |
| Huinno | MEMO Patch | NA |
| iRhythm | Zio Patch | A |
| MediBioSense | MediBio Sense MBS HealthStream | A |
| Preventice Solutions | BodyGuardian | A |
| Samsung | S-Patch Ex | A |
| Clothes | ||
| HealthWatch Technologies (smart garments) | Master Caution | A |
| Hexoskin (smart shirt) | Astroskin | NA |
| Medtronic (chest strap) | Zephyr | A |
| Polar (chest strap) | Polar H7 Strap | |
| Sleeplay (smart sock) | Owlet Smart Sock 3 | NA |
| Spire Health Tag | Spire | NA |
| Vivometrics (smart shirt) | The LifeShirt System | A |
| Zoll (vest) | LifeVest | A |
| Miscellaneous | ||
| AliveCor (phone attachment) | KardiaMobile | A |
| Personal Activity Intelligence (phone attachment) | PAI Health | U |
| Motiv (ring) | Motiv Ring | NA |
| Oura (finger ring) | Oura Ring | NA |
| FreeWavz (smart earphones) | FreeWavz-Blue | U |
| BioSensive Technologies (earrings) | Joule Earrings | NA |
| SonoHealth | EK Graph | NA |
| Jabra (headphones) | Sports Pulse Wireless Headphone | NA |
| Appraisal Criteria for Suitability | ||
| Criteria | Description | Grading System |
| Appropriate device | Were the data generated from the device in question? | D1: Actual device |
| D2: Equivalent device | ||
| D3: Other device | ||
| Appropriate device application | Was the device used for the same intended use? | A1: Same use |
| A2: Minor deviation | ||
| A3: Major deviation | ||
| Appropriate patient group | Where were the data generated from a patient group that was representative of the intended treatment population and clinician condition? | P1: Applicable |
| P2: Limited | ||
| P3: Different population | ||
| Appropriate report/data collation | Did the reports or collations of data contain sufficient information to be able to undertake a rational and objective assessment? | R1: High quality |
| R2: Minor deficiencies | ||
| R3: Insufficient information | ||
| Appraisal Criteria for Data Contribution | ||
| Criteria | Description | Grading System |
| Data source type | Was the design of the study appropriate? | T1: Yes |
| T2: No | ||
| Outcome measures | Did the outcome measures report reflect the intended performance of the device? | O1: Yes |
| O: No | ||
| Long-term monitoring | Was the duration of monitoring long enough to assess the duration of the treatment’s effects and identify complications? | L1: Yes |
| L2: No | ||
| L3: NA/other studies | ||
| Statistical significance | Was a statistical analysis of the data provided and was it appropriate? | S1: Yes |
| S2: No | ||
| Clinical significance | Was the magnitude of the treatment effect observed clinically significant? | C1: Yes |
| C2: No | ||
| C3: NA | ||
| References | Appraisal Grading |
|---|---|
| Boudreaux et al., 2018 [23] | D2/A1/P1/R1/T1/O1/L3/S1/C3 |
| Bumgarner et al., 2018 [24] | D2/A1/P3/R1/T1/O1/L3/S1/C3 |
| Castelletti et al., 2018 [25] | D2/A1/P1/R1/T1/O1/L3/S1/C3 |
| I. M. Lin, 2018 [28] | D2/A1/P1/R1/T1/O1/L3/S1/C3 |
| W. Y. Lin et al., 2018 [22] | D2/A1/P1/R1/T1/O1/L3/S1/C3 |
| Zhang et al., 2018 [33] | D2/A1/P1/R1/T1/O1/L1/S1/C3 |
| Fontana et al., 2019 [34] | D2/A2/P1/R1/T1/O1/L1/S1/C3 |
| Peng et al., 2019 [29] | D2/A1/P1/R1/T1/O1/L3/S1/C3 |
| Reverberi et al., 2019 [30] | D2/A2/P1/R1/T1/O1/L3/S1/C3 |
| Steinberg et al., 2019 [31] | D2/A1/P1/R1/T1/O1/L1/S1/C3 |
| Tsukada et al., 2019 [32] | D2/A1/P1/R1/T1/O1/L1/S1/C3 |
| Pereira, Alves, Silva, Costa, and Silva, 2020 [26] | D2/A2/P1/R1/T1/O1/L3/S1/C3 |
| References | Wearable Devices | Number of Participants (N), Group, and Methodology | Results | Conclusion | Comment on Performance and Safety |
|---|---|---|---|---|---|
| Fontana et al., 2019 [34] | A wearable textile ECG belt compared against lab polysomnography (PSG). | N = 12 patients with sleep apnea, aged 48–59; BMI: 28–35.5; sleep monitoring for 28 nights at home; measurements compared to clinical data. | Artifact percent: home (9.7% +/− 14.7%) and clinical (7.5% +/− 10.8%); comparable SNR in both settings. | Textile ECG belt: home vs. clinical signal quality comparison: no reduction; signal quality improved compared to clinical PSG. | Long-term monitoring; no adverse effects reported. Grade: 1 Rank: 1 |
| Steinberg et al., 2019 [31] | OM signal system: single-lead wearable ECG sensors vs. three-lead ECG Holter. | N = 15 healthy subjects; garment with three silicone electrodes recorded single-lead ECG for 24 h; signal quality assessed by three electrophysiologists for PQRST distinction. | Signal quality and accuracy matched Holter monitoring (84% vs. 93% electrophysiologists rating, p = 0.06); Noise level comparable to Holter recording. | OM garments (shirt and bra): novel wearable ECG sensors; rich signals for rhythm analysis; ease of use, validated against standard Holter recordings. | Evidence on performance and safety in long-term monitoring provided. Grade: 1 Rank: 2 |
| Tsukada et al., 2019 [32] | Conductive textile vs. single-lead ECG Holter system. | N = 66 healthy adults tested textile electrode pads in sportswear for comfort, conductivity. Motion artifacts and noise compared with conventional electrodes. | PQRST patterns same. Conventional electrodes: louder signal. Twisting: noise. Textile stays conductive after 50 washes. | Single-lead textile electrodes in inner garment: feasible for continuous ECG monitoring, except during vigorous trunk movement. | No skin irritation reported. Article meets performance and safety criteria. Grade: 1 Rank: 3 |
| Peng et al., 2019 [29] | Active electrode-based ECG with flexible materials: textile, copper tape, and flexible circuit. Passive Ag/AgCl electrodes used for validation. | N = 10 healthy subjects; created hardware for active electrodes, signal processing, and data transmission; Measured ECG through clothes; evaluated quality in three postures. | Effective, clear ECG waves with all materials; FPC best quality signals (p < 0.05); supine position best signals due to good contact; side lying worst quality. | Detects R waves accurately; calculated SNR compares material quality, not true SNR; FPC material produces clear PQRST waves in sitting and supine positions. | Active electrode ECG system tested with Ag/AgCl electrodes; safety not checked; no bad effects with non-contact sensor. Grade: 2 Rank: 4 |
| Zhang et al., 2018 [33] | Zio patch (Zio). | N = 45; patients aged 65+ with AF; group 1 self-applied; group 2 in-office; ECG signals measured; wear time compared. | No difference in the mean wear time (p = 0.76) between groups; skin irritation most common adverse reaction (N = 3); self-application equivalent to in-office application. | Zio’s small, leadless, self-contained form with easy installation ensures high self-application success; smaller sample size limited understanding of ethnicity and other patient factors. | Editorial version of feasibility study covers performance and safety; small sample size limits applicability. Grade 2 Rank: 5 |
| W. Y. Lin et al., 2018 [22] | Multi-channel MCG/ECG compared to single-lead ECG bio-amplifier. | N = 48; framework designed for MCG/ECG data acquisition; implemented as wearable smart clothing for cardiac monitoring; usability study conducted (N = 48, age > 20 years) to understand users’ behavioral intention. | ECG from monitoring circuit comparable to Bio Amp ECG with standard electrodes; validation of MCGs and ECG smart monitoring clothes. | Unique, validated smart clothes for cardiac health monitoring designed; capacity for long-term, continuous monitoring; integrated with smart clothing for real-time data analysis. | Wearable device: research prototype; paper covers design, development, processing, assessment; validated in patients. Grade: 1 Rank: 6 |
| I. M. Lin, 2018 [28] | CRST: Zephyr BioHarness chest belt + Bluetooth/mobile; RT: MioAlpha HR watch + Bluetooth/mobile; both compared to ProComp Infiniti biofeedback device EC. | N = 96; healthy adults: CRST, RT, and control; study: psychological questionnaires (depression and anxiety); pre and post-test: ECG, EEG, and breathing rates; CRST: pace breathing; RT: muscle relaxation. | CRST group: higher HRV, lower breathing rates post-test than RT, C groups; no significant EEG effect pre- and post-test in all groups. | The use of a CRST mobile application increased balance in the autonomic nervous system at the resting state. | Zephyr BioHarness compared to ECG; objective: compare training programs; no safety assessment evidence. Grade: 2; rank: 7 |
| Castelletti et al., 2018 [25] | BodyGuardian™ (BG) unit made of electrode gel, sensor, and adhesive layer. This was compared against a 12-lead Holter ECG. | N = 36; healthy and LQTS patients; validation study; Bland–Altman plot compared remote automated QTc (BGM) with manual monitoring (MM). | In all 36 subjects, QTc: MM 446 ± 41 ms, BGM 445 ±47 ms; mean ± SE BAp for QTc: all subjects −1.4 ± 1.8 ms, controls 8.3 ± 2.3 ms, LQTS −7.2 ± 2.5 ms; disagreement < 15 ms: all subjects, controls, LQTS 57%, 63%, and 54%. | This wearable monitoring system reliably identifies a prolonged QT interval and probably also subjects at risk for drug-induced LQTS. | Article compared new automated system reliability; suggested application for identifying drug-induced LQTS; no focus on BG device safety. Grade: 2; rank: 8 |
| Bumgarner et al., 2018 [24] | Kardia Band (KB) used with an Apple watch recorded single-channel ECG. This was compared against a 12-lead ECG. | N = 100; AF patients for cardioversion; observational/validation study; pre-CV ECG, KB recording; post-CV ECG, KB recording if CV; sensitivity, specificity, and K coefficient compared with ECG; physician interpretation. | Compared with ECG, the KB interpreted AF with 93% sensitivity, 84% specificity, and a K coefficient of 0.77. | The KB algorithm for AF detection supported by physician review can accurately differentiate AF from sinus rhythm. | Tested for sinus vs. atrial fibrillation; high sensitivity, specificity; discusses safe, durable platform for recording review, storage; FDA-approved, safety reviewed. Grade: 2; rank: 9 |
| Boudreaux et al., 2018 [23] | Eight wearable devices compared to the gold standard for HR (6-lead ECG) and metabolic analyzer for EE; devices: AWS2, FB, FC2, GVHR, TT, PA360, PH7, and BSP headphones. | N = 50; healthy subjects (age: 18–35); validation study; graded cycling trials; three sets of four resistance exercises at 10-rep max loads; HR, EE recorded; validity established with MAPE ≤ 10%. | Polar H7, BSP valid for both exercise modes (cycling: MAPE = 6.87%, R = 0.79; resistance: MAPE = 6.31%, R = 0.83); Apple Watch Series 2 most valid for cycling (MAPE = 4.14%, R = 0.80); BSP most accurate for resistance exercise (MAPE = 6.24%, R = 0.86); no device valid for EE in any exercise. | Across all devices, as the exercise intensity increased, there was a greater underestimation of HR. EE estimation was inaccurate during cycling or resistance exercise. | Most devices use PPG principle (n = 7); polar H7 focused in review; polar H7 validated against standard ECG, no significant difference; paper lacks specific safety mention. Grade: 2 Rank: 10 |
| Pereira et al., 2020 [26] | Heart rate variability (HRV) Expert (CardioMood) smartphone app connected with a chest strap (Polar H10) was compared against a 5-lead conventional ECG. | N = 31; Healthy male runners (mean age: 36.1 ± 6.3); RR intervals recorded by smartphone app, conventional ECG for 5 min; HRV assessed supine, sitting; time-domain indices, frequency-domain indices, and non-linear indices recorded. | No statistically significant difference in both positions (p > 0.05) was observed; a strong correlation coefficient was observed between the heart rate variability indexes and all variables (r = 1.0; p = 0.00). | Smartphone app, chest strap provided excellent ECG compliance; all variables in time, frequency domain, nonlinear indices assessed; regardless of position; app replaces ECG for HRV analysis in runners. | The objective was to evaluate the accuracy of the smartphone app. The device used (polar H10) is a validated device; no strong evidence on performance and safety. Grade: 4 Rank: 11 |
| Reverberi et al., 2019 [30] | A wearable chest-strap BT HR monitor combined with RITMIA app was compared against the 12- lead ECG interpreted by the physician. | N = 95; patients with atrial fibrillation; ECG recorded with 12-lead, chest-strap; before, after elective cardioversion (ECV) procedure; two cardiologists reviewed and compared the data; the feasibility, sensitivity, specificity, and K coefficient for RITMIA diagnosis were calculated. | The RITMIA app correctly detected AF with 97% sensitivity, 95.6% specificity, and a K coefficient of 0.93; no discomfort while wearing the chest-belt HR sensor was reported. | The RITIMA app algorithm was very accurate in differentiating AF from sinus rhythm as compared to any other commercial chest-strap ECG monitor. | The objective was to establish the RITIMA app used with a wireless chest strap. The recording was conducted for 10 min that did not assess the performance and safety per se. Grade: 4; rank: 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dahiya, E.S.; Kalra, A.M.; Lowe, A.; Anand, G. Wearable Technology for Monitoring Electrocardiograms (ECGs) in Adults: A Scoping Review. Sensors 2024, 24, 1318. https://doi.org/10.3390/s24041318
Dahiya ES, Kalra AM, Lowe A, Anand G. Wearable Technology for Monitoring Electrocardiograms (ECGs) in Adults: A Scoping Review. Sensors. 2024; 24(4):1318. https://doi.org/10.3390/s24041318
Chicago/Turabian StyleDahiya, Ekta Singh, Anubha Manju Kalra, Andrew Lowe, and Gautam Anand. 2024. "Wearable Technology for Monitoring Electrocardiograms (ECGs) in Adults: A Scoping Review" Sensors 24, no. 4: 1318. https://doi.org/10.3390/s24041318
APA StyleDahiya, E. S., Kalra, A. M., Lowe, A., & Anand, G. (2024). Wearable Technology for Monitoring Electrocardiograms (ECGs) in Adults: A Scoping Review. Sensors, 24(4), 1318. https://doi.org/10.3390/s24041318









