Spatial Distribution Pattern of the Mesozooplankton Community in Ross Sea Region Marine Protected Area (RSR MPA) during Summer
Abstract
1. Introduction
2. Materials and Methods
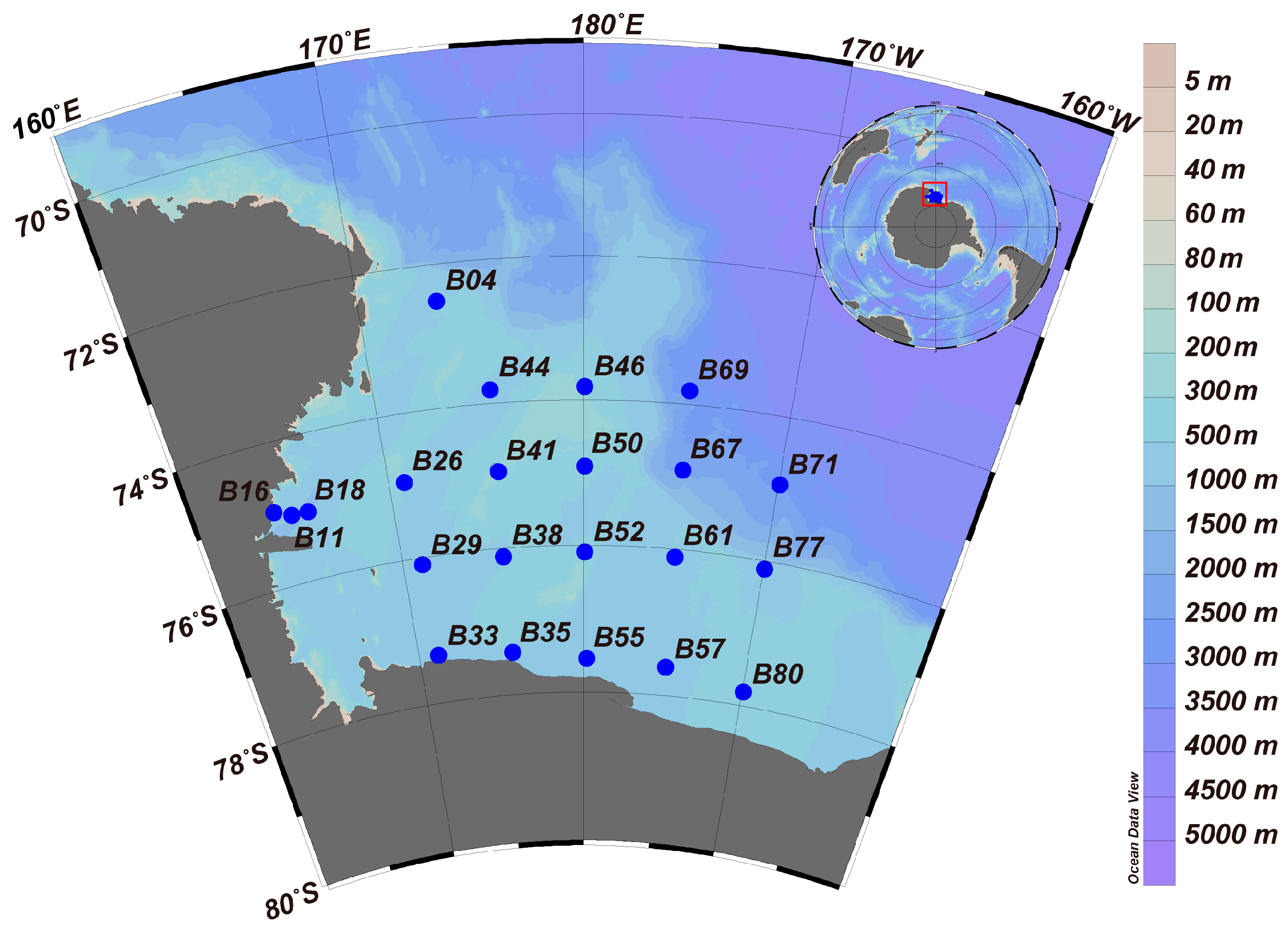
2.1. Data Sampling and Sample Processing
2.2. Data Processing
3. Results
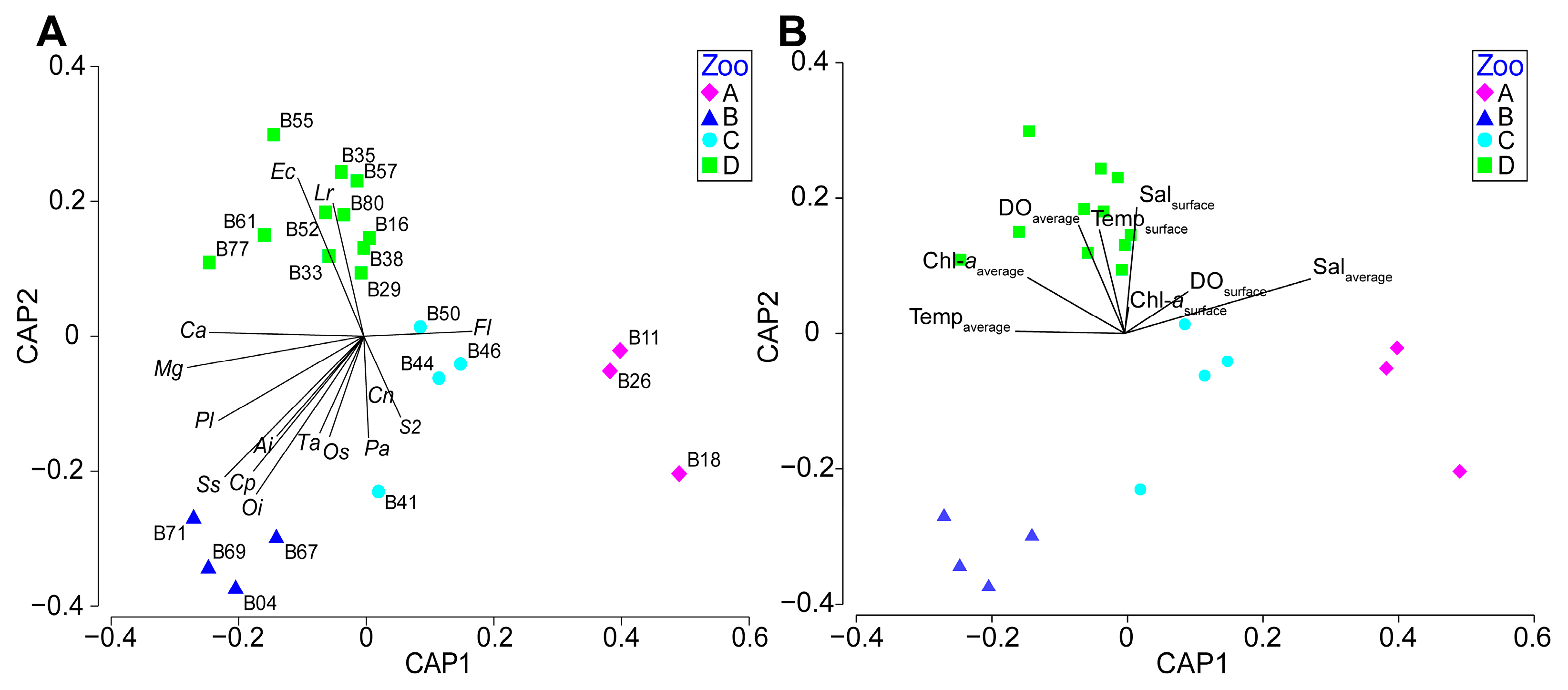
3.1. Spatial Variation in the Environmental Parameters
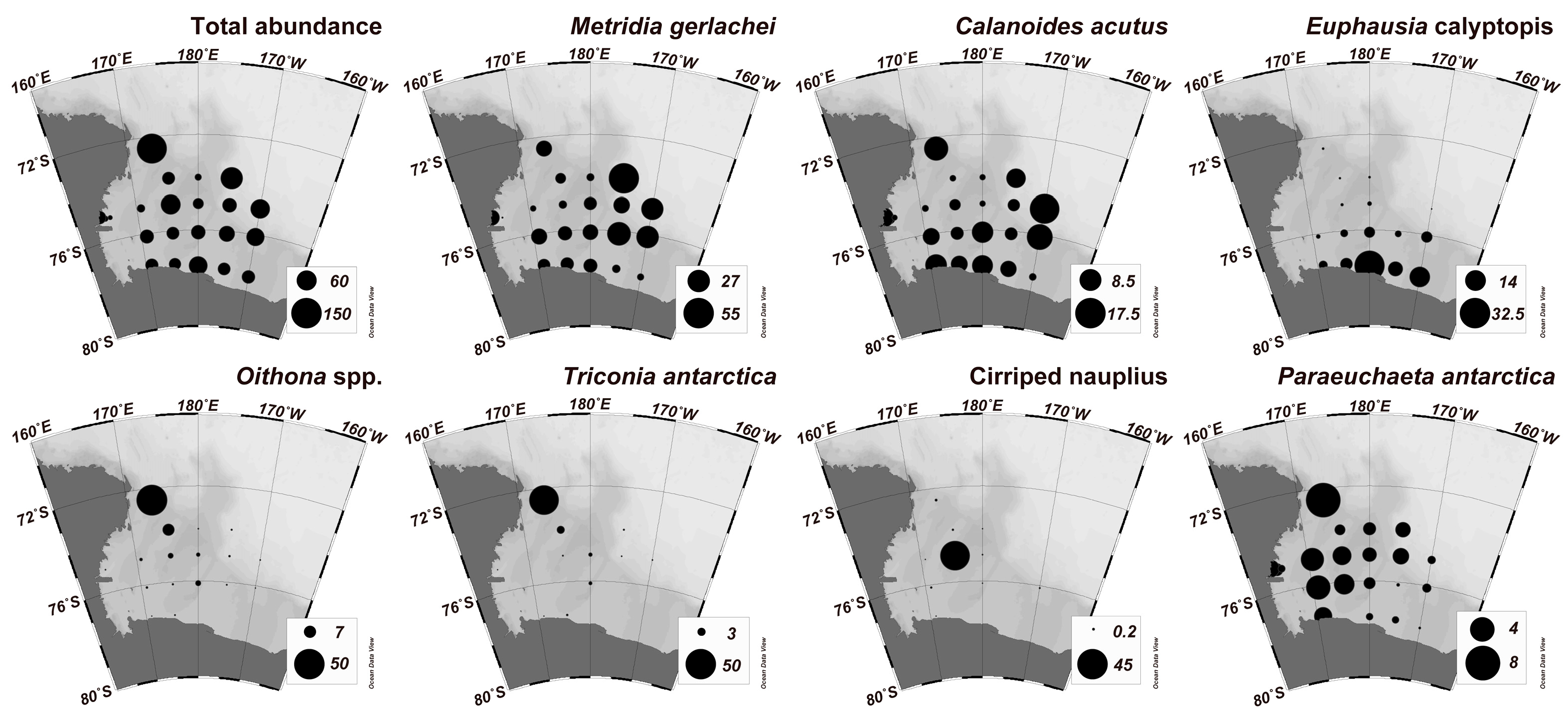
3.2. Mesozooplankton Community Composition
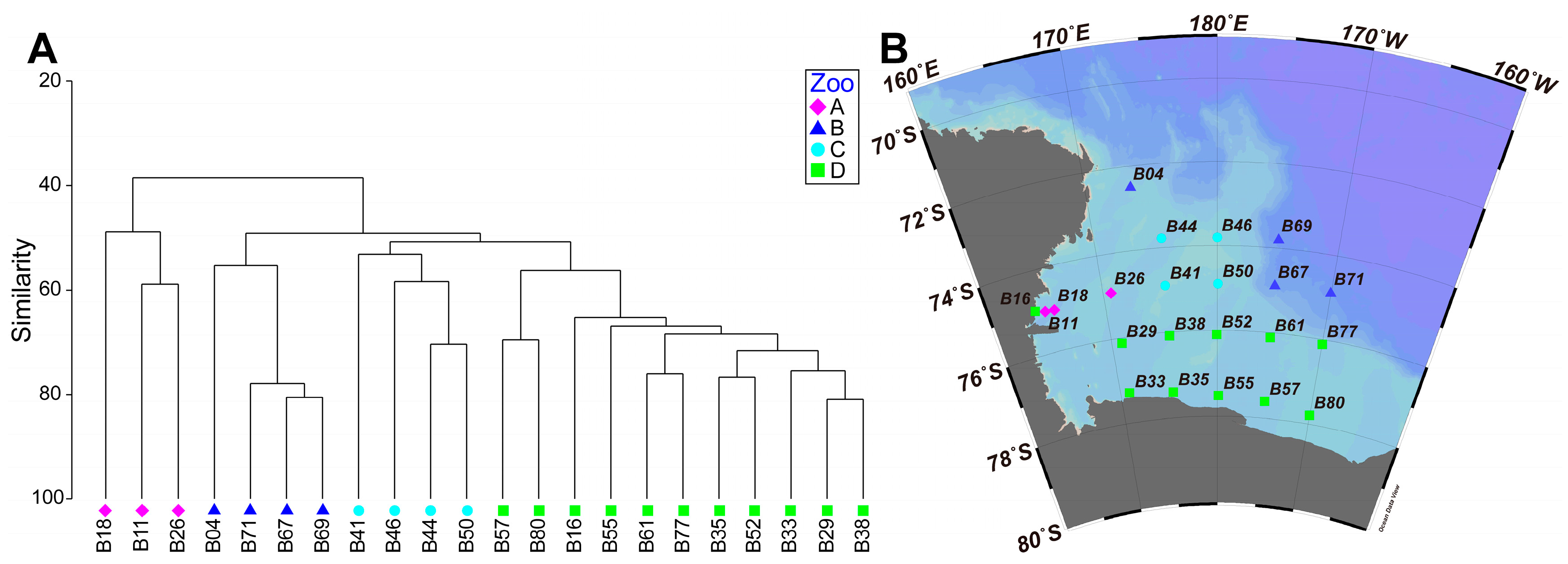
3.3. Spatial Pattern of the Mesozooplankton Community
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ohshima, K.I.; Fukamachi, Y.; Williams, G.D.; Nihashi, S.; Roquet, F.; Kitade, Y.; Tamura, T.; Hirano, D.; Herraiz-Borreguero, L.; Field, I. Antarctic Bottom Water production by intense sea-ice formation in the Cape Darnley polynya. Nat. Geosci. 2013, 6, 235–240. [Google Scholar] [CrossRef]
- Ainley, D.G. A history of the exploitation of the Ross Sea, Antarctica. Polar Rec. 2010, 46, 233–243. [Google Scholar] [CrossRef]
- Smith, W.O.; Sedwick, P.N.; Arrigo, K.R.; Ainley, D.G.; Orsi, A.H. The Ross Sea in a sea of change. Oceanography 2012, 25, 90–103. [Google Scholar] [CrossRef]
- Smith, W.; Dinniman, M.S.; Hofmann, E.E.; Klinck, J.M. The effects of changing winds and temperatures on the oceanography of the Ross Sea in the 21st century. Geophys. Res. Lett. 2014, 41, 1624–1631. [Google Scholar] [CrossRef]
- Orsi, A.H.; Wiederwohl, C.L. A recount of Ross Sea waters. Deep Sea Res. Part II Top. Stud. Oceanogr. 2009, 56, 778–795. [Google Scholar] [CrossRef]
- Fragoso, G.M.; Smith, W.O. Influence of hydrography on phytoplankton distribution in the Amundsen and Ross Seas, Antarctica. J. Mar. Syst. 2012, 89, 19–29. [Google Scholar] [CrossRef]
- Smith, W.O.; Gordon, L.I. Hyperproductivity of the Ross Sea (Antarctica) polynya during austral spring. Geophys. Res. Lett. 1997, 24, 233–236. [Google Scholar] [CrossRef]
- Kang, M.; Fajaryanti, R.; Son, W.; Kim, J.-H.; La, H.S. Acoustic detection of krill scattering layer in the Terra Nova Bay polynya, Antarctica. Front. Mar. Sci. 2020, 7, 584550. [Google Scholar] [CrossRef]
- Wilson, S.E.; Swalethorp, R.; Kjellerup, S.; Wolverton, M.A.; Ducklow, H.W.; Yager, P.L. Meso-and macro-zooplankton community structure of the Amundsen Sea Polynya, Antarctica (Summer 2010–2011) Zooplankton community structure of the Amundsen Sea Polynya, Antarctica. Elem. Sci. Anthr. 2015, 3, 000033. [Google Scholar] [CrossRef]
- Smith, W.O.; Ainley, D.G.; Arrigo, K.R.; Dinniman, M.S. The oceanography and ecology of the Ross Sea. Annu. Rev. Mar. Sci. 2014, 6, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, E.A.; Pshenichnov, L.K.; Krot, A.; Paramonov, V.; Slypko, I.; Zabroda, P. Zooplankton Distribution and Community Structure in the Pacific and Atlantic Sectors of the Southern Ocean during Austral Summer 2017–18: A Pilot Study Conducted from Ukrainian Long-Liners. J. Mar. Sci. Eng. 2020, 8, 488. [Google Scholar] [CrossRef]
- Picco, P.; Schiano, M.E.; Pensieri, S.; Bozzano, R. Time-frequency analysis of migrating zooplankton in the Terra Nova Bay polynya (Ross Sea, Antarctica). J. Mar. Syst. 2017, 166, 172–183. [Google Scholar] [CrossRef]
- Smith, W.; Delizo, L.; Herbolsheimer, C.; Spencer, E. Distribution and abundance of mesozooplankton in the Ross Sea, Antarctica. Polar Biol. 2017, 40, 2351–2361. [Google Scholar] [CrossRef]
- Guglielmo, L.; Zagami, G.; Saggiomo, V.; Catalano, G.; Granata, A. Copepods in spring annual sea ice at Terra Nova Bay (Ross Sea, Antarctica). Polar Biol. 2007, 30, 747–758. [Google Scholar] [CrossRef]
- Stevens, C.J.; Pakhomov, E.A.; Robinson, K.V.; Hall, J.A. Mesozooplankton biomass, abundance and community composition in the Ross Sea and the Pacific sector of the Southern Ocean. Polar Biol. 2015, 38, 275–286. [Google Scholar] [CrossRef]
- Carli, A.; Pane, L.; Stocchino, C. Planktonic copepods in Terra Nova Bay (Ross Sea): Distribution and relationship with environmental factors. In Ross Sea Ecology; Springer: Berlin/Heidelberg, Germany, 2000; pp. 309–321. [Google Scholar]
- Elliott, D.T.; Tang, K.W.; Shields, A.R. Mesozooplankton beneath the summer sea ice in McMurdo Sound, Antarctica: Abundance, species composition, and DMSP content. Polar Biol. 2009, 32, 113–122. [Google Scholar] [CrossRef]
- Hopkins, T. Midwater food web in McMurdo Sound, Ross Sea, Antarctica. Mar. Biol. 1987, 96, 93–106. [Google Scholar] [CrossRef]
- Grillo, M.; Cecchetto, M.; Giallain, M.; Granata, A.; Guglielmo, L.; Pane, L.; Schiaparelli, S. Distributional records of Ross Sea (Antarctica) planktic Copepoda from bibliographic data and samples curated at the Italian National Antarctic Museum (MNA): Checklist of species collected in the Ross Sea sector from 1987 to 1995. ZooKeys 2020, 969, 1–22. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, B.K.; Lee, B.; Son, W.; Jo, N.; Lee, J.; Lee, S.H.; Ha, S.-Y.; Kim, J.-H.; La, H.S. Distribution of the Mesozooplankton Community in the Western Ross Sea Region marine protected area during late summer bloom. Front. Mar. Sci. 2022, 9, 860025. [Google Scholar] [CrossRef]
- Sertorio, T.Z.; Licandro, P.; Ossola, C.; Artegiani, A. Copepod communities in the Pacific Sector of the Southern Ocean in early summer. In Ross Sea Ecology; Springer: Berlin/Heidelberg, Germany, 2000; pp. 291–307. [Google Scholar]
- Cummings, V.J.; Bowden, D.A.; Pinkerton, M.H.; Halliday, N.J.; Hewitt, J.E. Ross Sea Benthic Ecosystems: Macro-and Mega-faunal Community Patterns From a Multi-environment Survey. Front. Mar. Sci. 2021, 8, 629787. [Google Scholar] [CrossRef]
- CCAMLR. Conservation Measure 91-05 (2016): Ross Sea Region Marine Protected Area; CCAMLR: Hobart, TAS, Australia, 2016. [Google Scholar]
- Hall, A.E.; Herbert, R.J.; Stafford, R. Temporal and spatial variation in adult and juvenile mobile fauna associated with natural and artificial coastal habitats. Mar. Biol. 2021, 168, 19. [Google Scholar] [CrossRef]
- Clarke, K.; Gorley, R. PRIMER v7: User Manual/Tutorial; Primer-E Ltd.: Plymouth, UK, 2015; p. 296. [Google Scholar]
- Clarke, K.R.; Gorley, R.N.; Somerfield, P.J.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E: Plymouth, UK, 2014. [Google Scholar]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Valesini, F.; Tweedley, J.; Clarke, K.; Potter, I. The importance of regional, system-wide and local spatial scales in structuring temperate estuarine fish communities. Estuaries Coasts 2014, 37, 525–547. [Google Scholar] [CrossRef]
- Tonelli, M.; Wainer, I.; Curchitser, E. A modelling study of the hydrographic structure of the Ross Sea. Ocean Sci. Discuss. 2012, 9, 3431–3449. [Google Scholar]
- Arrigo, K.R.; DiTullio, G.R.; Dunbar, R.B.; Robinson, D.H.; VanWoert, M.; Worthen, D.L.; Lizotte, M.P. Phytoplankton taxonomic variability in nutrient utilization and primary production in the Ross Sea. J. Geophys. Res. Ocean. 2000, 105, 8827–8846. [Google Scholar] [CrossRef]
- Pane, L.; Feletti, M.; Francomacaro, B.; Mariottini, G.L. Summer coastal zooplankton biomass and copepod community structure near the Italian Terra Nova Base (Terra Nova Bay, Ross Sea, Antarctica). J. Plankton Res. 2004, 26, 1479–1488. [Google Scholar] [CrossRef]
- Skjoldal, H.R.; Wiebe, P.H.; Postel, L.; Knutsen, T.; Kaartvedt, S.; Sameoto, D.D. Intercomparison of zooplankton (net) sampling systems: Results from the ICES/GLOBEC sea-going workshop. Prog. Oceanogr. 2013, 108, 1–42. [Google Scholar] [CrossRef]
- Hosie, G.W.; Cochran, T.G.; Pauly, T.; Beaumont, K.L.; Wright, S.; Kitchener, J. Zooplankton community structure of Prydz Bay. In Proceedings of the 18th NIPR Symposium on Polar Biology, Tokyo, Japan, 19–21 August 1997; pp. 90–133. [Google Scholar]
- Yang, G.; Li, C.; Sun, S. Inter-annual variation in summer zooplankton community structure in Prydz Bay, Antarctica, from 1999 to 2006. Polar Biol. 2011, 34, 921–932. [Google Scholar] [CrossRef]
- Ward, P.; Hirst, A. Oithona similis in a high latitude ecosystem: Abundance, distribution and temperature limitation of fecundity rates in a sac spawning copepod. Mar. Biol. 2007, 151, 1099–1110. [Google Scholar] [CrossRef]
- Pakhomov, E.A.; Froneman, P.W.; Perissinotto, R. Salp/krill interactions in the Southern Ocean: Spatial segregation and implications for the carbon flux. Deep Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 1881–1907. [Google Scholar] [CrossRef]
- Azzali, M.; Leonori, I.; De Felice, A.; Russo, A. Spatial–temporal relationships between two euphausiid species in the Ross Sea. Chem. Ecol. 2006, 22, S219–S233. [Google Scholar] [CrossRef]
- Taki, K.; Yabuki, T.; Noiri, Y.; Hayashi, T.; Naganobu, M. Larval development and spawning ecology of euphausiids in the Ross Sea and its adjacent waters in 2004/05. Plankton Benthos Res. 2009, 4, 135–146. [Google Scholar] [CrossRef]
- Taki, K.; Yabuki, T.; Noiri, Y.; Hayashi, T.; Naganobu, M. Horizontal and vertical distribution and demography of euphausiids in the Ross Sea and its adjacent waters in 2004/2005. Polar Biol. 2008, 31, 1343–1356. [Google Scholar] [CrossRef]


| Min | Max | Av | SD | |
|---|---|---|---|---|
| Tempsurface (°C) | −1.61 | 0.44 | −0.59 | 0.46 |
| Tempaverage (°C) | −1.82 | −1.03 | −1.42 | 0.21 |
| Salsurface (PSU) | 33.72 | 34.3 | 34.08 | 0.16 |
| Salaverage (PSU) | 34.08 | 34.72 | 34.40 | 0.18 |
| DOsurface (mg/L) | 342.52 | 401.38 | 370.36 | 16.68 |
| DOaverage (mg/L) | 288.64 | 335.43 | 310.59 | 10.87 |
| Chl-asurface (μg/m3) | 0.17 | 2.08 | 0.75 | 0.43 |
| Chl-aaverage (μg/m3) | 0.23 | 1.01 | 0.66 | 0.19 |
| Source | df | SS | MS | Pseudo-F | Significance Level (p) |
|---|---|---|---|---|---|
| Groups | 3 | 13301 | 4433.6 | 5.9198 | 0.001 |
| Residual | 18 | 13481 | 748.94 | ||
| Total | 21 | 26782 | |||
| Pairwise tests | df | t | Significance levels (p) | ||
| Group A and B | 5 | 2.7391 | 0.029 | ||
| Group A and C | 5 | 1.9677 | 0.025 | ||
| Group A and D | 12 | 2.6507 | 0.003 | ||
| Group B and C | 6 | 2.0928 | 0.033 | ||
| Group B and D | 13 | 2.6494 | 0.004 | ||
| Group C and D | 13 | 2.2354 | 0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Son, W.; Kim, J.-H.; La, H.S. Spatial Distribution Pattern of the Mesozooplankton Community in Ross Sea Region Marine Protected Area (RSR MPA) during Summer. Diversity 2024, 16, 174. https://doi.org/10.3390/d16030174
Kim SH, Son W, Kim J-H, La HS. Spatial Distribution Pattern of the Mesozooplankton Community in Ross Sea Region Marine Protected Area (RSR MPA) during Summer. Diversity. 2024; 16(3):174. https://doi.org/10.3390/d16030174
Chicago/Turabian StyleKim, Sung Hoon, Wuju Son, Jeong-Hoon Kim, and Hyoung Sul La. 2024. "Spatial Distribution Pattern of the Mesozooplankton Community in Ross Sea Region Marine Protected Area (RSR MPA) during Summer" Diversity 16, no. 3: 174. https://doi.org/10.3390/d16030174
APA StyleKim, S. H., Son, W., Kim, J.-H., & La, H. S. (2024). Spatial Distribution Pattern of the Mesozooplankton Community in Ross Sea Region Marine Protected Area (RSR MPA) during Summer. Diversity, 16(3), 174. https://doi.org/10.3390/d16030174








