Abstract
Assessing the effectiveness of artificial structures as a monitoring tool for benthic diversity in temperate reefs is crucial to determining their relevance in reef conservation and management. In this study, we utilized Autonomous Reef Monitoring Structures (ARMS) to evaluate sessile benthic communities that colonized ARMS units after 12 and 34 months of immersion within distinct habitats (coral-dominated and macroalgae-dominated habitats) in Jeju Island, Korea. We used two methods: image analysis of the ARMS plates and DNA metabarcoding of the ARMS units. We found significant differences in the sessile benthic community between the plate faces, installation periods, and habitats. DNA metabarcoding also revealed differences in sessile benthic diversity among habitats. Additionally, we identified the Lithophyllum genus within the crustose coralline algae community, whose dominance might trigger a transition to coral-dominated habitats in Jeju Island. We recommend integrating ARMS image analysis with DNA metabarcoding to enhance and complement studies focusing on benthic diversity. By utilizing ARMS, this study provides valuable information for understanding sessile benthic communities and biodiversity, contributing to an enhanced understanding of the responses of ecological communities to climate change.
1. Introduction
The settlement of benthic organisms is significantly influenced by hydrodynamic and biological factors, including light, nutrients, surface orientation, color, depth, and biochemical cues [1]. To assess the communities, it is important to understand the influences of environmental conditions [2], mechanisms of colonization, and succession of benthic assemblages [3]. However, evaluating their impacts on benthic ecosystems remains challenging owing to the absence of universally standardized monitoring tools [1]. Therefore, the monitoring and assessment of various descriptors encompassing biological components, such as community composition and biodiversity, demand the utilization of innovative methods and approaches [4,5,6,7,8,9].
Recent monitoring studies showed a significant decline in the populations of kelp species [10,11] and an expansion of crustose coralline algae (CCA) species [11,12,13], scleractinian corals [12,13,14,15], and zoantharian species [16] in the benthic community of Jeju Island, the Republic of Korea. These phenomena cause changes in the benthic ecosystems, resulting in negative impacts on local habitats and biodiversity [17]. Currently, monitoring efforts are directed toward assessing the abundance of particular species and making observations of benthic communities, primarily in response to the influence of global climate change. Moreover, numerous monitoring studies focusing on diversity patterns in rocky reefs have primarily relied on visual censuses of the exposed areas alone; thus, conventional approaches typically overlook cryptic benthic species that are unidentified during traditional reef surveys [18] owing to their small size and/or hidden locations, resulting in an inaccurate picture of community compositions.
Artificial settlement plates offer a straightforward and effective approach for assessing the recruitment and growth of benthic communities [18]. Autonomous reef monitoring structures (ARMS) are an internationally standardized technique for monitoring hard benthic substrates in various regions to efficiently gather data on marine organisms while reducing the sampling effort, lowering costs, and minimizing habitat destruction [19]. ARMS have recently been introduced as a sampling tool to assess cryptic reef biodiversity [20,21] and provide a standardized method that has been used since 2006 to conduct visual censuses of species abundance and estimate biodiversity via molecular analyses [21]. The ARMS technique consists of layered PVC settlement plates specifically designed to emulate the intricate three-dimensional structure found in coral reef habitats [7,22]. It has been deployed in the Indo-Pacific and Caribbean [22], the Atlantic coast of the US [20], and European seas and the Red Sea [7]. David et al. [7] evaluated the ARMS technique among the distinct plate faces between sites (within seas) and seas using photo analysis as a rapid and efficient method for sessile benthic diversity monitoring. Leray and Knowlton [20] utilized the DNA barcoding approach to assess the ARMS technique across a range of geographic scales comprising a temperate location and a subtropical location.
The aim of this study was to test the potential of image analysis and DNA metabarcoding of sessile benthic communities using the ARMS technique as a screening tool across diverse environmental conditions, including variations in plate faces, installation periods, habitat, and layers. To achieve this objective, we analyzed the benthic communities that colonized the ARMS units after 12 and 34 months of immersion within distinct habitats (coral-dominated and macroalgae-dominated habitats) at two sites in Jeju Island, Korea. Our general goal was to assess the relevance of image analysis and DNA metabarcoding for benthic biodiversity monitoring. We first tested whether the community compositions captured in photographs were significantly different between the distinct plate surfaces of the ARMS, plate faces, installation periods, habitats, and layers. Additionally, we investigated the feasibility of detecting sessile benthic diversity using DNA metabarcoding across different installation periods and habitats. Furthermore, the ARMS were used to establish a foundational dataset of qualitative and quantitative information to enhance our understanding of sessile benthic communities and cryptic biodiversity. This information is vital for comprehending the impacts of climate change on benthic ecosystems on Jeju Island and in other regions.
2. Materials and Methods
2.1. Study Area
This study was conducted in Seogwipo on the southern coast of Jeju Island. Rising seawater temperatures have considerably affected coastal benthic communities in the Seogwipo area, resulting in observable changes [10,13]. Specifically, seawater temperatures on the southern coast of Jeju Island are warmer than those on the northern and eastern coasts [10,23]. From 1981 to 2020, the average annual sea surface temperature (SST) recorded in Seogwipo was 18.7 ± 0.7 °C, exhibiting a seasonal range characterized by a minimum monthly average of 14.4 ± 0.8 °C in March and a maximum monthly average of 24.6 ± 1.4 °C in August [10].
ARMS units were deployed at depths of 12–13 m in two subtidal sites in Seogwipo: Bomok (BM) and Gangjeong (GJ) (Table 1 and Figure 1). Information regarding the deployment dates and sites is provided in Table 1. The sites were selected based on information on each benthic community. Scleractinian corals (Alveopora japonica, Montipora millepora, and Psamocora profundacella) dominated BM, whereas turf-forming algae (geniculate coralline algae and filamentous turf algae) with a low abundance of canopy-forming brown algae (Ecklonia cava) dominated GJ on the subtidal rocky bottom (Figure 1).

Table 1.
ARMS unit information deployed at GJ and BM survey sites around Jeju Island, Korea.
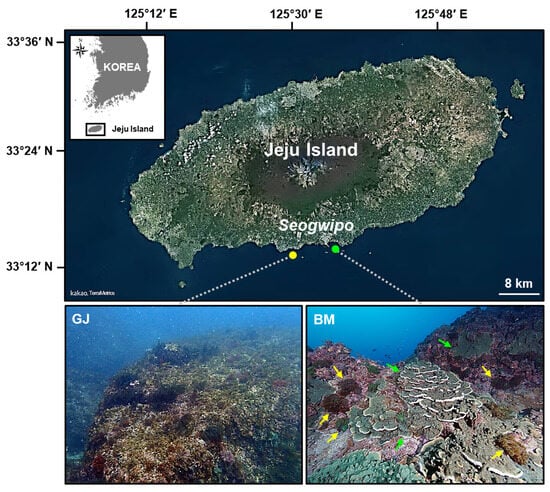
Figure 1.
Map showing the locations of two survey sites (Gangjeong (GJ) and Bomok (BM)) situated off the southern coast of Jeju Island, Korea, and the foreground autonomous reef monitoring structure (ARMS) photo (map source: https://map.kakao.com; accessed on 30 June 2023). The colonies of scleractinian coral Montipora millepora (green arrow) and Alveopora japonica (yellow arrow) were observed at the BM site.
2.2. ARMS Deployment and Recovery
The ARMS units were installed by scuba divers (Figure 2) and submerged for 12 months (1-year) and 34 months (3-years) at BM and GJ, respectively, following which they were retrieved and transported to the laboratory for subsequent processing and analysis. During the dismantling of the ARMS, each plate (nine plates per ARMS) was carefully brushed to remove mobile organisms without detaching sessile organisms from the filtered seawater. The plates were subsequently immersed in aerated seawater until photographs were obtained (Figure 3).
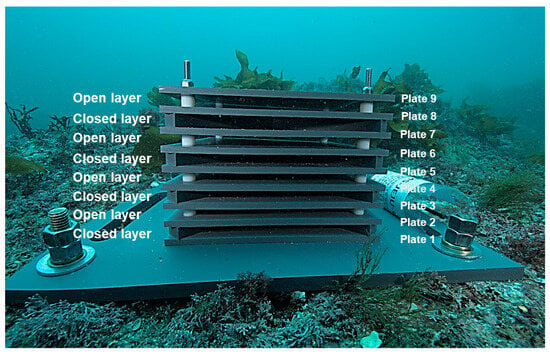
Figure 2.
Newly deployed ARMS unit at a depth of 13 m in GJ-2. The ARMS unit consists of nine plates and spacers stacked in an alternating series of open and closed formats.

Figure 3.
Retrieved samples of ARMS plate faces from GJ-2. The cross markings on the plates are a result of the cross spacers that alternated between some plates.
The top (T) and bottom (B) surfaces of each plate were individually analyzed (17 plate faces were analyzed per ARMS unit). After all plates were photographed, they were scraped clean to remove sessile organisms. The sessile organisms from all plates were combined, and the sessile fraction was blended. The samples were preserved in 50 mL centrifuge tubes filled with dimethyl sulfoxide (DMSO) and stored at −20 °C until DNA extraction.
2.3. Image Analysis
The photographs of the plates were analyzed using PhotoQuad® software (version 1.4), and the percentage cover of the benthic categories was determined using the random point count method in the image analysis software. In total, 64 allocated points were randomly spawned on each 22.5 cm × 22.5 cm plate. They were categorized as “not alive” when the allocated points coincided with the crosses or screws on the plate. All sessile benthic species were identified at the family level, except CCA and filamentous turf algae. The final merged categories included Porifera, Mollusca, Annelida, Bryozoa, Chordata, Rhodophyta, CCA, and turf-forming algae, along with categories labeled as “benthic biofilm”, “not alive”, and “undetermined”. To refine our analysis, we computed relative cover calculations and performed statistical evaluations on sessile benthic categories, deliberately omitting “benthic biofilm”, “not alive”, and “undetermined” from our dataset to ensure clarity and precision in our findings.
2.4. DNA Extraction and Metabarcoding
Metabarcoding analysis of the sessile fraction samples was performed using the COI gene. The samples were forwarded to Macrogene, Inc. (Seoul, the Republic of Korea), where DNA was extracted using the DNeasy PowerMax Soil Kit (Qiagen, Hilden, Germany). Following the Illumina Metagenomic Sequencing Library protocols, sequencing libraries were prepared to amplify the LCO-1490 and HCO-2198 regions. PCR amplification involved 5 ng of input gDNA, 5× reaction buffer, 1 mM of dNTP mix, 500 nM of each universal F/R PCR primer, and Herculase II fusion DNA polymerase (Agilent Technologies, Santa Clara, CA, USA). The cycle for the first PCR comprised 3 min of heat activation at 95 °C, followed by 25 cycles of 30 s each at 95 °C, 44.7 °C, and 72 °C, and concluding with a final 5 min extension at 72 °C. The utilized universal primer pair with Illumina adapter overhang sequences was as follows: the LCO-1490 Amplicon PCR forward primer (5′GGTCAACAAATCATAAAGATATTGG3′) and the HCO-2198 Amplicon PCR reverse primer (5′TAAACTTCAGGGTGACCAAAAAATCA3′).
The first PCR product underwent purification using AMPure beads (Agencourt Bioscience, Beverly, MA, USA). Subsequently, 10 µL of the first PCR product was further PCR-amplified to construct the final library and indexed using NexteraXT Indexed Primers. The thermal conditions for the second PCR were the same as those for the first PCR, except for 10 cycles. Post-amplification, the PCR product was again purified with AMPure beads. The final, purified product was then quantified using qPCR according to the qPCR Quantification Protocol Guide (KAPA Library Quantification kits for Illumina Sequencing platforms) and qualified using TapeStation D1000 ScreenTape (Agilent Technologies, Waldbronn, Germany). Finally, sequencing was performed on the MiSeq™ platform (Illumina, San Diego, CA, USA).
Subsequently, the demultiplexed FASTQ files were processed with the DADA2 pipeline to extract Amplicon Sequence Variants (ASVs) for further taxonomic investigation. These ASVs were then subjected to BLASTn searches against the NCBI GenBank database to identify their closest matches, retrieve relevant contextual sequences for tree construction, and ensure accurate sequence identification. The metabarcoding analysis facilitated the enumeration and taxonomic categorization of sessile benthic species into groups such as Porifera, Cnidaria, Mollusca, Annelida, Arthropoda, Bryozoa, Chordata, Chlorophyta, Phaeophyta, Rhodophyta, and CCA.
2.5. Statistical Analyses
All analyses were conducted after standardization and square root transformation of the percentage cover of the sessile benthic categories. Bray–Curtis similarity was used for all statistical analyses. Variations in the percentage cover of the benthic categories were compared between plate faces (top and bottom), installation periods (1-year and 3-years), habitats (macroalgae-dominated habitats and coral-dominated habitats), and layers (open and closed) using analysis of similarity (ANOSIM). Non-metric multidimensional scaling (nMDS) was used to evaluate the variability in the benthic community composition according to plate faces, installation periods, and habitats. The contributions of the benthic categories to the dissimilarity in the sessile benthic communities within and between the plate faces, installation periods, and habitats were determined using similarity percentages (SIMPER). The ANOSIM, nMDS, and SIMPER analyses were performed using PRIMER, version 7 (PRIMER-e Ltd., Auckland, New Zealand).
3. Results
3.1. Sessile Benthic Community Composition
Photographs of 68 plate faces were analyzed to understand the composition of the sessile benthic community, which was identified at the family level (Table S1). A summary of the sessile benthic species and their respective percentage covers on each ARMS plate is shown in Table S2. Overall, the percentage cover of the ARMS area colonized by identifiable taxonomic categories ranged from 40 to 53% in GJ and 49 to 60% in BM. During the installation periods, the 3-year ARMS units (GJ-2 and BM-2) were more heavily colonized than the 1-year ARMS units (GJ-1 and BM-1), with 53% and 40% colonization rates for GJ and 60% and 49% for BM, respectively (Table S1).
The dominant groups found on the plates included Annelida, Bryozoa, Chordata, Rhodophyta, and CCA (Figure 4). In contrast, Porifera, Mollusca, and turf-forming algae exhibited low abundance and distribution on the plates. Members of the phylum Bryozoa dominated colonization, with 63.1% in BM-2, 45.0% in BM-1, 42.8% in GJ-2, and 42.4% in GJ-1. Chordata was more abundant in the 3-year ARMS units than in the 1-year ARMS units, with 7.3% and 0.3% for GJ and 6.7% and 0.8% for BM, respectively. In contrast, CCA was more abundant in the 1-year ARMS units than in the 3-year ARMS units, with 43.1% and 24.6% in GJ and 30.9% and 15.0% in BM, respectively (Figure 4).
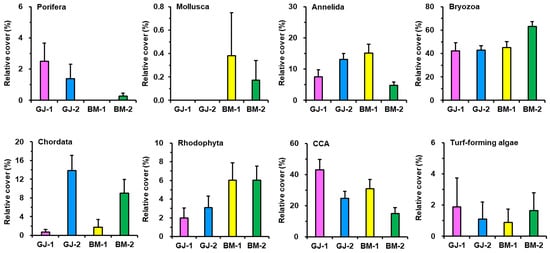
Figure 4.
Bar graphs showing the mean relative covers of major sessile benthic categories on the ARMS units. The relative cover is followed by the standard error.
ANOSIM revealed significant differences in the sessile benthic composition between the plate faces (R = 0.437; p = 0.001), installation periods (R = 0.178; p = 0.001), and habitats (R = 0.064 and p = 0.011; Table 2). However, no significant differences were observed between layers (R = 0.018, p = 0.164). The nMDS revealed a distinct separation composition according to the plate faces, installation periods, and habitats of the ARMS plates in the sessile benthic compositions (Figure 5). The contributions of the most abundant groups differed between the top and bottom faces of the plates according to the SIMPER analysis on the plate faces (Table 3). Specifically, the CCA and Rhodophyta groups were more frequently found on the top faces, whereas the members of Bryozoa, Annelida, and Chordata were typically found on the bottom faces. The groups that made the most substantial contributions to the dissimilarity are listed below in decreasing order of significance: CCA (27.19%), Bryozoa (17.43%), Annelida (17.17%), Chordata (12.10%), and Rhodophyta (13.86%). These taxonomic groups together accounted for up to 90% of the total dissimilarity observed between the top and bottom faces of the ARMS plates. The contributions of the most abundant groups differed between the 1-year and 3-year ARMS plates according to the SIMPER analysis in the installation periods (Table 3). CCA were more frequently found on 1-year ARMS units, whereas Chordata were typically found on 3-year ARMS units. The contributions of the most abundant groups also differed between the macroalgae-dominated habitats and coral-dominated habitats of the ARMS plates according to the SIMPER analysis in the habitats.

Table 2.
Analysis of similarity results comparing benthic community composition according to the plate face, installation period, habitat, and layer factors.
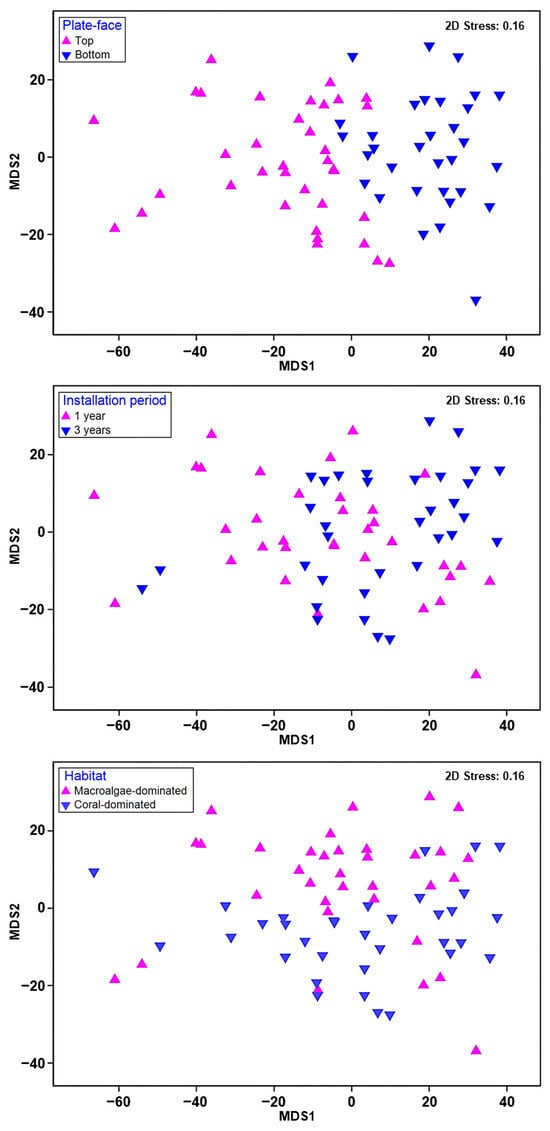
Figure 5.
Non-metric multidimensional scaling (nMDS) of sessile benthic categories for the plate faces, installation periods, and habitats of ARMS units. Each point represents a replicate (ARMS plate).

Table 3.
Similarity percentage analysis of dissimilarity between the plate faces, installation periods, and habitats of ARMS plates.
3.2. Composition of Sessile Benthic Community Using DNA Metabarcoding
PCR amplification and COI gene sequencing were successful for all ARMS units. The total number of raw paired-end reads for all samples was 918,102, with an average of 229,526 reads per ARMS unit (Table 4). After merging paired-end reads, filtering, and identifying sessile benthic species, 187,870 target reads (20.5%) were retained. The target reads were generated as follows: 67,731 reads (31.7%) in GJ-1, 73,537 reads (27.6%) in GJ-2, 34,522 reads (14.8%) in BM-1, and 12,080 reads (5.9%) in BM-2.

Table 4.
Summary of metabarcoding results in ARMS units.
In total, 39 orders, 161 genera, and 173 species were identified across the four ARMS units sampled from the two sites (Table S3). Specifically, 144 species were identified at GJ, whereas 93 species were found at BM (Table 5). Rhodophyta encompassed the highest number of sessile benthic categories. The numbers of Rhodophyta and CCA species in GJ were higher than those in BM, with 43 and 9 species in GJ and 23 and 2 species in BM, respectively (Table 5).

Table 5.
Number of sessile benthic species identified using DNA metabarcoding analysis.
The most abundant taxon in the ARMS biomass samples across the dataset was the phylum Cnidaria, except for BM-1, followed by Bryozoa, Rhodophyta, and Porifera. Porifera and Chordata were more abundant in the 3-year ARMS units (GJ-2 and BM-2) than in the 1-year ARMS units (GJ-1 and BM-1). In contrast, members of Rhodophyta and CCA were more abundant in the 1-year ARMS units than in the 3-year ARMS units (Figure 6).
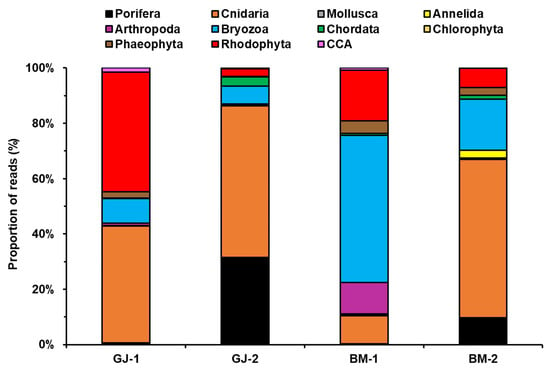
Figure 6.
Compositions of assemblages in metabarcoding analysis of sessile benthic categories.
3.3. Comparison of Image Analysis and DNA Metabarcoding
We observed disparities in the results when comparing the outcomes of the image analysis and DNA metabarcoding (Figure 4 and Figure 6). Bryozoa species were most prevalent according to the image analysis, whereas Cnidarian species exhibited the highest abundance in the DNA metabarcoding, despite their absence in the visual census. However, the CCA and Annelida species showed high abundances in the image analysis but were comparatively low in the DNA metabarcoding. Several species were identified using DNA metabarcoding; however, certain species, including geniculate coralline algae (Amphiroa sp. and Jania sp.), Mollusca (Isognomon sp.), and Chordata (Herdmania sp.; Table S1), were observed in the visual census but not detected using DNA metabarcoding.
4. Discussion
Enhancing survey protocols for biodiversity assessment is critically needed [21] considering the current rate of biodiversity loss [24,25,26]. We aimed to assess the environmental conditions in sessile benthic communities using ARMS units deployed at two sites with identical installation periods and depths. Despite classification being the best in high taxonomic categories, ARMS image analysis is a powerful method to compare sessile benthic community compositions. Our results indicate that the plate faces, installation periods, and habitats significantly affected the compositions and dynamics of the sessile benthic communities developing on the ARMS units in the Jeju Island reef environment (Table 2). Moreover, DNA metabarcoding analysis revealed differences in the number of sessile benthic species between the two sites (Table 5).
Benthic community composition data are more promising than single-taxon abundance data for detecting changes and, consequently, monitoring ecological status [7,27]. In this study, the plate faces showed the strongest variation in sessile benthic composition, in agreement with previous studies that demonstrated the importance of the plate surface in the sessile benthic community composition [7]. For example, Steyaert et al. [28] reported that the plate faces (top or bottom) had a significant impact on the percentage covers of various organisms, including bryozoans, gastropods, soft and calcified tube worms, CCA, and fleshy red, brown, and green encrusting macroalgae on ARMS. Our results show that the high occurrences of CCA and Rhodophyta on the top faces, coupled with the high occurrences of Bryozoa, Annelida, and Chordata on the bottom faces, distinctly illustrate a pattern that is likely influenced by light exposure. Regarding the dissimilarity in the sessile benthic taxa between the top and bottom groups, CCA contributed the most to this dissimilarity. The top plates placed on the ARMS attracted a high CCA cover in this study and previous studies specifically focusing on CCA [18] and benthic community compositions [7]. The reduced CCA cover on the bottom plates could be explained by differences in light availability, supply, survivorship, and sedimentation [18]. Future studies should consider these environmental factors (light, nutrition, surface orientation, depth, and biochemical cues) when assessing sessile benthic community compositions, particularly when focusing on specific taxa.
The collective results from the image analysis and DNA metabarcoding data in this study suggest that the installation period had a strong effect on the occurrence of Chordata (tunicate) communities (Figure 3 and Figure 6; Table 3), indicating limited settlement and dispersion between the 1-year and 3-year installation periods in the present study. Carter and Prekel [29] documented that biotic cover and diversity progressively increased in artificial reefs from 9 to 36 months post-deployment. Similarly, this study observed a higher abundance of Chordata species in the 3-year ARMS units than in the 1-year ARMS units. These results indicate that discrepancies between 1-year and 3-year sessile benthic composition on ARMS may reflect differences in settlement, survivorship, or succession [18]. Therefore, each specific time point should be carefully considered in future ARMS studies, to obtain a comprehensive understanding of each benthic community in reefs.
Habitat factors are strongly associated with relationships within benthic communities, including functional roles, spatial arrangements, competition, and niche habitation [1,18]. Before installing the ARMS units and during their retrieval at the two distinct sites, BM was characterized as a habitat dominated by Scleractinia corals, whereas GJ was dominated by macroalgal species. Distinct habitats within a specific sea region are likely to share a common pool of potentially colonizing species, indicating the influence of distinct environmental conditions [7]. For example, our findings are consistent with those of David et al. [7], which reported significant differences in the ARMS communities between sites in four sea regions. Moreover, DNA metabarcoding analysis clearly revealed differences in the sessile benthic composition between sites, indicating enhanced sensitivity in detecting environmental effects on species composition.
Traditional image analysis approaches primarily target the superficial layers of the benthic community, which may lead to insufficient detection of specific organisms, an underestimation of diversity, and ultimately inaccurate assessments [7,23]. However, DNA barcoding and metabarcoding data capture both presence–absence and relative abundance information, which ensures greater accuracy in monitoring marine benthic diversity [7,20,22]. Consequently, biodiversity assessment methodologies are now rapidly shifting to DNA metabarcoding, driven by advancements in sequencing technologies [30]. For example, Pearman et al. [21] reported that DNA metabarcoding detected differences in the benthic community composition between sites, whereas morphological approaches did not reveal a significant difference in ARMS reef monitoring. The present study also revealed a higher level of sessile benthic diversity using DNA metabarcoding than via image analysis. However, certain species of geniculate coralline algae (Amphiroa sp. and Jania sp.), Mollusca (Isognomon sp.), and Chordata (Herdmania sp.) were observed in the visual census but were undetected using DNA metabarcoding. Therefore, it is likely that species-level identification is not feasible owing to limitations in the classification of molecular data and the absence of reference sequences [21]. Consequently, DNA metabarcoding applications may be limited by the low availability of DNA from cryptobenthic organisms, which hinders the ability to identify significant differences in sessile benthic composition at low taxonomic levels [31,32]. To address these limitations, we suggest that incorporating visual census techniques as complementary approaches to DNA metabarcoding techniques can be a valuable strategy to enhance and complement studies focused on benthic diversity [21,27,33].
The benthic ecosystem of Jeju Island is undergoing rapid changes, characterized by species of scleractinia corals, such as Alveopora japonica or Montipora millepora, opportunistically colonizing benthic substrates covered by CCA following the decline of kelp forests, exerting a profound influence on local biodiversity [10,12,13,17,23,34,35,36]. This study revealed that the BM site is dominated by corals and exhibited lower sessile biodiversity than the GJ site (Table 5), which is consistent with the study by Kang et al. [36], which reported that coral populations have likely existed for a long evolutionary period in this area, rather than following a poleward migration from subtropical environments owing to recent climate change. Therefore, the dominance of scleractinian corals may be attributed to alterations in the interactions between benthic species from environmental changes [13,35].
CCA play an essential role in reef systems by providing suitable substrates and structural support for benthic communities [37]. This study successfully identified CCA species that remained unidentified in the visual census using DNA metabarcoding, with nine species distributed across all surveyed sites (Table S3). The number of CCA species in GJ was higher than that in BM, with four genera (Harveylithon, Lithophyllum, Mesophyllum, and Synarthrophyton), whereas BM had only one genus (Lithophyllum). Vieira et al. [35] suggested that scleractinian corals (A. japonica) combined with the abundance of available space on barren grounds where CCA cover the rocky bottom may have facilitated an increase in its population along the coastline of Jeju Island. However, the diversity and relationships between the CCA communities and scleractinian corals on Jeju Island were not examined. An understanding of the diversity, distribution, and ecological characteristics of CCA communities must be garnered to understand the interactions between CCA and coral species [38]. Specific CCA play a crucial role in facilitating the settlement of particular coral species, with their chemical cues influencing the larval settlement of corals [39]. Abdul Wahab et al. [40] found that the Lithophyllaceae family was the best inducer overall and across most coral species. The presence of the Lithophyllaceae family member Lithophyllum at the BM site could potentially trigger a transition in the benthic ecosystem from macroalgae-dominated to coral-dominated habitats. However, additional research and monitoring should be conducted to understand the ecological implications of the observed changes.
The habitat consistently exhibited a dominance of scleractinian corals before installing the ARMS units and during their retrieval from the BM. However, corals were unidentified in the image analysis, and they had a low abundance in the DNA metabarcoding. The shape and structural complexity of the substrate are crucial factors influencing the colonization preferences of benthic communities [18,41]. Consequently, relying solely on ARMS may be inadequate for investigating the diversity of certain benthic communities [42]. For example, Pearman et al. [21] reported that ARMS plates might be unsuitable for coral larval recruitment, owing to reasons such as incompatible light regimes, substrate characterization, and competition from other biotic groups. Furthermore, the sandwich-like structure of an ARMS, with its relatively small size, seems to be a disadvantage for coral recruitment compared with natural substrates with complex 3D structures [21]. Corals might require additional time to establish themselves in the benthic substrate, as observed for Chordata communities in this study [43].
David et al. [7] recommended deploying three ARMS units per site and expanding the analysis to encompass multiple sites within a specific sea region to investigate the environmental effects more comprehensively. However, scuba diving can be expensive, and it is essential to consider the long-term expenses associated with its deployment and retrieval when selecting a site that requires diving. Thus, we suggest deploying one ARMS unit to test protocols, acquire practical experience, and assess the potential of a candidate site or habitat [44] prior to conducting full monitoring programs. This study showed that deploying one ARMS unit can reveal differences in sessile benthic communities between sites. We plan to deploy additional ARMS units in subsequent years.
5. Conclusions
This study used ARMS image analysis and DNA metabarcoding to assess the influences of various factors on the composition and biodiversity of the sessile benthic communities in the reef environment of Jeju Island. The sessile benthic communities were notably influenced by plate faces, installation periods, and habitats, revealing the significance of considering these factors in comprehensive assessments of community composition. Moreover, DNA metabarcoding revealed differences in sessile diversity between different habitats, demonstrating the efficacy of ARMS in comparing sessile benthic communities and biodiversity. Therefore, DNA metabarcoding has certain benefits, such as the ability to capture presence–absence and relative abundance information to assess benthic diversity, although species-level identification is not feasible. Furthermore, we identified the Lithophyllum genus within the CCA community, and its dominance can potentially trigger a shift from macroalgae-dominated to coral-dominated habitats. The insights from this study enhance our understanding of Jeju Island’s sessile benthic communities and provide valuable support for biodiversity assessment and conservation, particularly in the face of the ongoing biodiversity loss challenges within these communities.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/d16020083/s1. Table S1: List of taxa and the percentage cover in data from two sites prior to merging taxonomic categories; Table S2: Percentage cover of each taxonomic category for each sample; Table S3: Proportion of DNA sequences analyzed for sessile benthic species in sampling units in the two sites.
Author Contributions
Conceptualization, D.-H.K. and H.-S.Y.; methodology and formal analysis, K.-T.L., T.K., G.-H.P., C.O., H.-S.K. and H.-S.Y.; software, K.-T.L., G.-H.P. and H.-S.K.; validation, C.O., H.-S.P., D.-H.K. and H.-S.Y.; investigation and resources, K.-T.L., T.K. and H.-S.Y.; data curation, K.-T.L. and H.-S.Y.; writing and visualization, K.-T.L. and H.-S.Y.; project administration and funding acquisition, C.O., H.-S.K. and H.-S.Y. We confirm that this manuscript and data are original and have not been previously published or considered elsewhere. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Korea Institute of Ocean Science and Technology Project (PEA0111; PEA0125) and was partly supported by a research grant funded by the National Institute of Fisheries Science, the Ministry of Oceans and Fisheries, Korea (R2023035).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Acknowledgments
We would like to express our appreciation for the administrative and technical support from the Jeju Research Center, KIOST.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Levy, N.; Simon-Blecher, N.; Ben-Ezra, S.; Yuval, M.; Doniger, T.; Leray, M.; Karako-Lampert, S.; Tarazi, E.; Levy, O. Evaluating biodiversity for coral reef reformation and monitoring on complex 3D structures using environmental DNA (eDNA) metabarcoding. Sci. Total Environ. 2023, 856, 159051. [Google Scholar] [CrossRef]
- Suding, K.N.; Lavorel, S.; Chapin, F.S.; Cornelissen, J.H.C.; Díaz, S.; Garnier, E.; Goldberg, D.; Hooper, D.U.; Jackson, S.T.; Navas, M.L. Scaling environmental change through the community-level: A trait-based response-and-effect framework for plants. Glob. Chang. Biol. 2008, 14, 1125–1140. [Google Scholar] [CrossRef]
- Higgins, E.; Scheibling, R.E.; Desilets, K.M.; Metaxas, A. Benthic community succession on artificial and natural coral reefs in the northern Gulf of Aqaba, Red Sea. PLoS ONE 2019, 14, e0212842. [Google Scholar] [CrossRef] [PubMed]
- Mermillod-Blondin, F.; François-Carcaillet, F.; Rosenberg, R. Biodiversity of benthic invertebrates and organic matter processing in shallow marine sediments: An experimental study. J. Exp. Mar. Biol. Ecol. 2005, 315, 187–209. [Google Scholar] [CrossRef]
- Brown, E.A.; Chain, F.J.J.; Zhan, A.; MacIsaac, H.J.; Cristescu, M.E. Early detection of aquatic invaders using metabarcoding reveals a high number of non-indigenous species in Canadian ports. Divers. Distrib. 2016, 22, 1045–1059. [Google Scholar] [CrossRef]
- Danovaro, R.; Carugati, L.; Berzano, M.; Cahill, A.E.; Carvalho, S.; Chenuil, A.; Corinaldesi, C.; Cristina, S.; David, R.; Dell’Anno, A.; et al. Implementing and innovating marine monitoring approaches for assessing marine environmental status. Front. Mar. Sci. 2016, 3, 213. [Google Scholar] [CrossRef]
- David, R.; Uyarra, M.C.; Carvalho, S.; Anlauf, H.; Borja, A.; Cahill, A.E.; Carugati, L.; Danovaro, R.; De Jode, A.; Feral, J.P.; et al. Lessons from photo analyses of Autonomous Reef Monitoring Structures as tools to detect (bio-)geographical, spatial, and environmental effects. Mar. Pollut. Bull. 2019, 141, 420–429. [Google Scholar] [CrossRef] [PubMed]
- van der Heyde, M.; Bunce, M.; Wardell-Johnson, G.; Fernandes, K.; White, N.E.; Nevill, P. Testing multiple substrates for terrestrial biodiversity monitoring using environmental DNA metabarcoding. Mol. Ecol. Resour. 2020, 20, 732–745. [Google Scholar] [CrossRef]
- Bae, S.; Kim, P.; Yi, C.H. Biodiversity and spatial distribution of ascidian using environmental DNA metabarcoding. Mar. Environ. Res. 2023, 185, 105893. [Google Scholar] [CrossRef]
- Ribas-Deulofeu, L.; Loubeyres, M.; Denis, V.; De Palmas, S.; Hwang, S.-J.; Woo, S.; Song, J.-I.; Chen, C.A. Jeju Island: A sentinel for tracking ocean warming impacts on high-latitude benthic communities. Coral Reefs 2023, 42, 1097–1112. [Google Scholar] [CrossRef]
- Chung, H.; Cho, K.W.; Chung, K.H.; Kim, J.H.; Shin, J.; Seo, Y.; Kang, J.S.; Lee, I.K. Ecological characteristics of algal whitening in coastal zone of Seogwipo area, Cheju Island. Algae 1998, 13, 361–374. [Google Scholar]
- Denis, V.; Ribas-Deulofeu, L.; Loubeyres, M.; De Palmas, S.; Hwang, S.-J.; Woo, S.; Song, J.-I.; Chen, C.A. Recruitment of the subtropical coral Alveopora japonica in the temperate waters of Jeju Island, South Korea. Bull. Mar. Sci. 2014, 91, 85–96. [Google Scholar] [CrossRef]
- Lee, K.T.; Lee, H.M.; Subramaniam, T.; Yang, H.S.; Park, S.R.; Kang, C.K.; Keshavmurthy, S.; Choi, K.S. Dominance of the scleractinian coral Alveopora japonica in the barren subtidal hard bottom of high-latitude Jeju Island off the south coast of Korea assessed by high-resolution underwater images. PLoS ONE 2022, 17, e0275244. [Google Scholar] [CrossRef]
- Hong, H.-K.; Keshavmurthy, S.; Kang, C.-K.; Hwang, K.; Park, S.R.; Cho, S.-H.; Choi, K.-S. Alveopora japonica repopulation of a bare substrate off Jeju Island, Korea. Bull. Mar. Sci. 2015, 91, 477–478. [Google Scholar] [CrossRef]
- Kim, T.; Kang, D.-H. An encrusting hard coral enclosing soft coral in the high-latitude Asia–Pacific marginal distribution zone. Diversity 2022, 14, 856. [Google Scholar] [CrossRef]
- Yang, H.-S.; Cho, Y.-G.; Kim, T.; Heo, S.-J. First report with molecular confirmation of the colonial sphenopid Palythoa mutuki (Cnidaria: Anthozoa: Zoantharia: Sphenopidae) forming massive colonies in Southern Jeju Island, Korea. J. Mar. Sci. Eng. 2023, 11, 574. [Google Scholar] [CrossRef]
- Kim, T.; Kim, T.; Yang, H.-S.; Choi, S.K.; Son, Y.B.; Kang, D.-H. Alveopora japonica conquering temperate reefs despite massive coral bleaching. Diversity 2022, 14, 86. [Google Scholar] [CrossRef]
- Kennedy, E.V.; Ordoñez, A.; Lewis, B.E.; Diaz-Pulido, G. Comparison of recruitment tile materials for monitoring coralline algae responses to a changing climate. Mar. Ecol. Prog. Ser. 2017, 569, 129–144. [Google Scholar] [CrossRef]
- Yang, H.-S.; Kim, T.; Lee, K.-T.; Kim, T.; Baker, D.M.; Kang, D.-H. Use of autonomous reef monitoring structures to monitor changes in the marine environment in Jeju, South Korea: A brief review. Ocean Sci. J. 2023, 58, 17. [Google Scholar] [CrossRef]
- Leray, M.; Knowlton, N. DNA bar coding and metabarcoding of standardized samples reveal patterns of marine benthic diversity. Proc. Natl. Acad. Sci. USA 2015, 112, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Pearman, J.K.; Anlauf, H.; Irigoien, X.; Carvalho, S. Please mind the gap—Visual census and cryptic biodiversity assessment at central Red Sea coral reefs. Mar. Environ. Res. 2016, 118, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, L.; Caley, M.J.; Brainard, R.E.; Knowlton, N. The diversity of coral reefs: What are we missing? PLoS ONE 2011, 6, e25026. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-T.; Perrois, G.; Yang, H.-S.; Kim, T.; Choi, S.K.; Kang, D.-H.; Kim, T. Impact of super typhoon ‘Hinnamnor’ on density of kelp forest and associated benthic communities in Jeju Island, Republic of Korea. J. Mar. Sci. Eng. 2023, 11, 1035. [Google Scholar] [CrossRef]
- Folke, C.; Carpenter, S.; Walker, B.; Scheffer, M.; Elmqvist, T.; Gunderson, L.; Holling, C.S. Regime shifts, resilience, and biodiversity in ecosystem management. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 557–581. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.I.; Lambin, E.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. Planetary boundaries: Exploring the safe operating space for humanity. Ecol. Soc. 2009, 14, 32. [Google Scholar] [CrossRef]
- Richardson, K.; Steffen, W.; Lucht, W.; Bendtsen, J.; Cornell, S.E.; Donges, J.F.; Drüke, M.; Fetzer, I.; Bala, G.; von Bloh, W.; et al. Earth beyond six of nine planetary boundaries. Sci. Adv. 2023, 9, eadh2458. [Google Scholar] [CrossRef] [PubMed]
- Borja, A.; Elliott, M.; Andersen, J.H.; Berg, T.; Carstensen, J.; Halpern, B.S.; Heiskanen, A.-S.; Korpinen, S.; Lowndes, J.S.S.; Martin, G.; et al. Overview of integrative assessment of marine systems: The ecosystem approach in practice. Front. Mar. Sci. 2016, 3, 20. [Google Scholar] [CrossRef]
- Steyaert, M.; Lindhart, M.; Khrizman, A.; Dunbar, R.B.; Bonsall, M.B.; Mucciarone, D.A.; Ransome, E.; Santodomingo, N.; Winslade, P.; Head, C.E.I. Remote reef cryptobenthic diversity: Integrating autonomous reef monitoring structures and in situ environmental parameters. Front. Mar. Sci. 2022, 9, 932375. [Google Scholar] [CrossRef]
- Carter, A.; Prekel, S. Benthic colonization and ecological successional patterns on a planned nearshore artificial reef system in Broward County, SE Florida. In Proceedings of the 11th International Coral Reef Symposium, Lauderdale, FL, USA, 7–11 July 2008; pp. 1209–1213. [Google Scholar]
- Fredericq, S.; Krayesky-Self, S.; Sauvage, T.; Richards, J.; Kittle, R.; Arakaki, N.; Hickerson, E.; Schmidt, W.E. The critical importance of rhodoliths in the life cycle completion of both macro- and microalgae, and as holobionts for the establishment and maintenance of marine biodiversity. Front. Mar. Sci. 2019, 5, 502. [Google Scholar] [CrossRef]
- Carugati, L.; Corinaldesi, C.; Dell’Anno, A.; Danovaro, R. Metagenetic tools for the census of marine meiofaunal biodiversity: An overview. Mar. Genom. 2015, 24, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Rshaidat, M.M.; Snider, A.; Rosebraugh, S.; Devine, A.M.; Devine, T.D.; Plaisance, L.; Knowlton, N.; Leray, M. Deep COI sequencing of standardized benthic samples unveils overlooked diversity of Jordanian coral reefs in the northern Red Sea. Genome 2016, 59, 724–737. [Google Scholar] [CrossRef]
- West, K.M.; Adam, A.A.S.; White, N.; Robbins, W.D.; Barrow, D.; Lane, A.; Richards, T. The applicability of eDNA metabarcoding approaches for sessile benthic surveying in the Kimberley region, north-western Australia. Environ. DNA 2022, 4, 34–49. [Google Scholar] [CrossRef]
- Sugihara, K.; Yamano, H.; Choi, K.-S.; Hyeong, K. Zooxanthellate scleractinian corals of Jeju Island, Republic of Korea. In Integrative Observations and Assessments; Springer: Berlin/Heidelberg, Germany, 2014; pp. 111–130. [Google Scholar] [CrossRef]
- Vieira, C.; Keshavmurthy, S.; Ju, S.-J.; Hyeong, K.; Seo, I.; Kang, C.-K.; Hong, H.-K.; Chen, C.A.; Choi, K.-S. Population dynamics of a high-latitude coral Alveopora japonica Eguchi from Jeju Island, off the southern coast of Korea. Mar. Freshw. Res. 2016, 67, 594–604. [Google Scholar] [CrossRef]
- Kang, J.H.; Jang, J.E.; Kim, J.H.; Kim, S.; Keshavmurthy, S.; Agostini, S.; Reimer, J.D.; Chen, C.A.; Choi, K.-S.; Park, S.R.; et al. The origin of the subtropical coral Alveopora japonica (Scleractinia: Acroporidae) in high-latitude environments. Front. Ecol. Evol. 2020, 8, 12. [Google Scholar] [CrossRef]
- McCoy, S.J.; Kamenos, N.A. Coralline algae (Rhodophyta) in a changing world: Integrating ecological, physiological, and geochemical responses to global change. J. Phycol. 2015, 51, 6–24. [Google Scholar] [CrossRef]
- Schils, T. Branching Lithophyllum coralline algae: Dominant reef builders on herbivory-depressed tropical reefs after high coral mortality. Diversity 2023, 15, 1025. [Google Scholar] [CrossRef]
- Gómez-Lemos, L.A.; Doropoulos, C.; Bayraktarov, E.; Diaz-Pulido, G. Coralline algal metabolites induce settlement and mediate the inductive effect of epiphytic microbes on coral larvae. Sci. Rep. 2018, 8, 17557. [Google Scholar] [CrossRef]
- Abdul Wahab, M.A.; Ferguson, S.; Snekkevik, V.K.; McCutchan, G.; Jeong, S.; Severati, A.; Randall, C.J.; Negri, A.P.; Diaz-Pulido, G. Hierarchical settlement behaviours of coral larvae to common coralline algae. Sci. Rep. 2023, 13, 5795. [Google Scholar] [CrossRef] [PubMed]
- Leite, B.R.; Duarte, S.; Troncoso, J.S.; Costa, F.O. Artificial seaweed substrates complement ARMS in DNA metabarcoding-based monitoring of temperate coastal macrozoobenthos. Diversity 2023, 15, 657. [Google Scholar] [CrossRef]
- Palomino-Alvarez, L.A.; Vital, X.G.; Castillo-Cupul, R.E.; Suárez-Mozo, N.Y.; Ugalde, D.; Cervantes-Campero, G.; Muciño-Reyes, M.R.; Homá-Canché, P.; Hernández-Díaz, Y.Q.; Sotelo-Casas, R.; et al. Evaluation of the use of Autonomous Reef Monitoring Structures (ARMS) for describing the species diversity of two coral reefs in the Yucatan Peninsula, Mexico. Diversity 2021, 13, 579. [Google Scholar] [CrossRef]
- Edmunds, P.J. Patterns in the distribution of juvenile corals and coral reef community structure in St. John, US Virgin Islands. Mar. Ecol. Prog. Ser. 2000, 202, 113–124. [Google Scholar] [CrossRef]
- Obst, M.; Exter, K.; Allcock, A.L.; Arvanitidis, C.; Axberg, A.; Bustamante, M.; Cancio, I.; Carreira-Flores, D.; Chatzinikolaou, E.; Chatzigeorgiou, G.; et al. A marine biodiversity observation network for genetic monitoring of hard-bottom communities (ARMS-MBON). Front. Mar. Sci. 2020, 7, 572680. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).