Effects of Freshwater Inflow during the Rainy Season on the Benthic Polychaete Community in the Geum River Estuary, South Korea
Abstract
1. Introduction
2. Materials and Methods
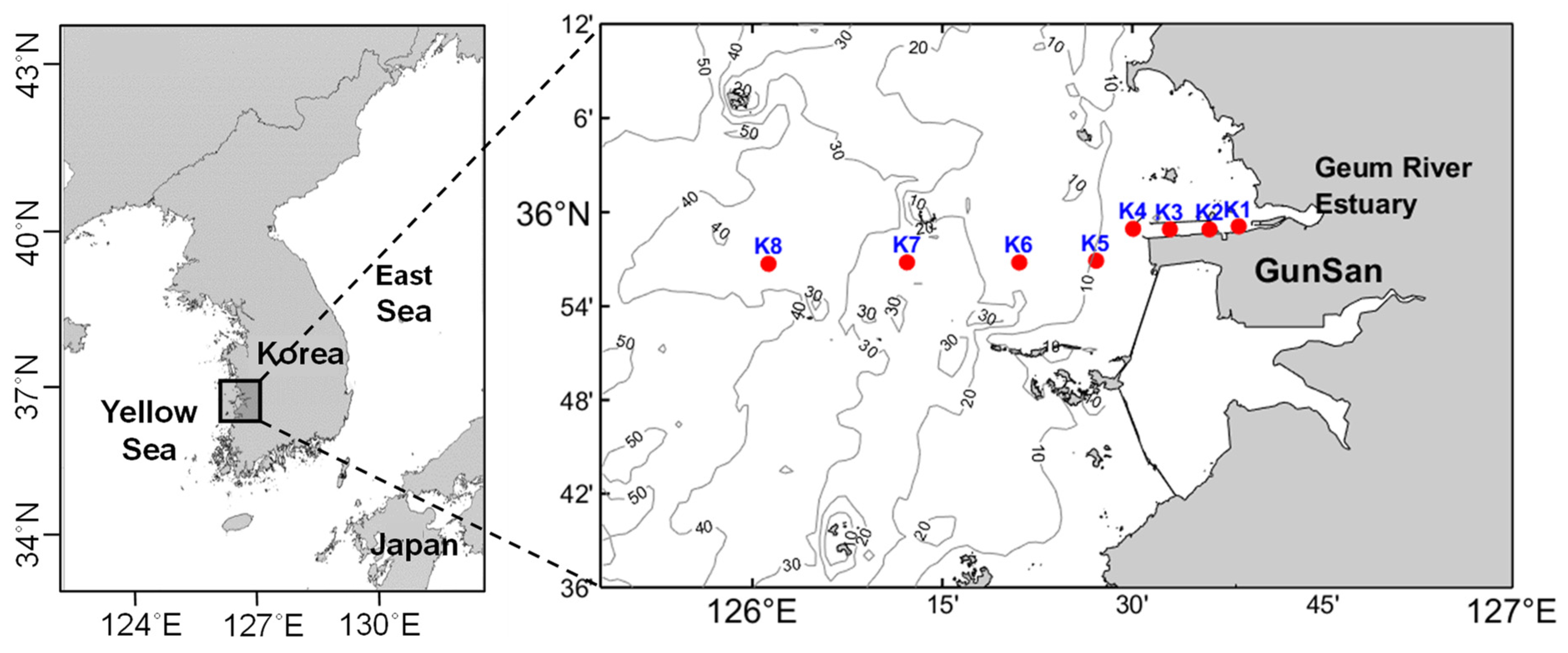
2.1. Study Area
2.2. Sample Processing
2.3. Data Processing
2.4. Feeding Guilds
3. Results
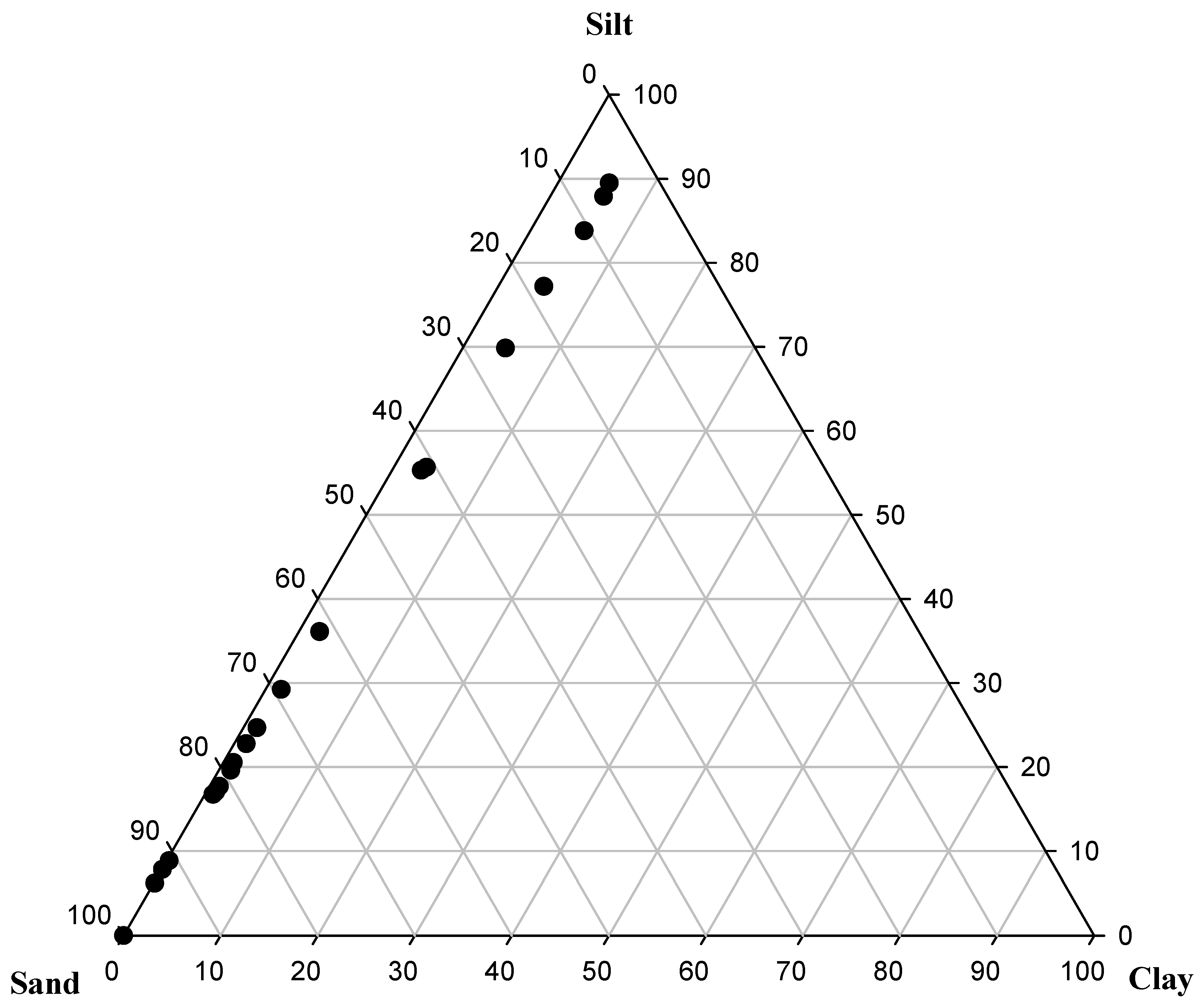
3.1. Environmental Variability
3.2. Macrobenthos
3.3. Dominant Species
3.4. Ecological Indices
3.5. Polychaete Community Characteristics
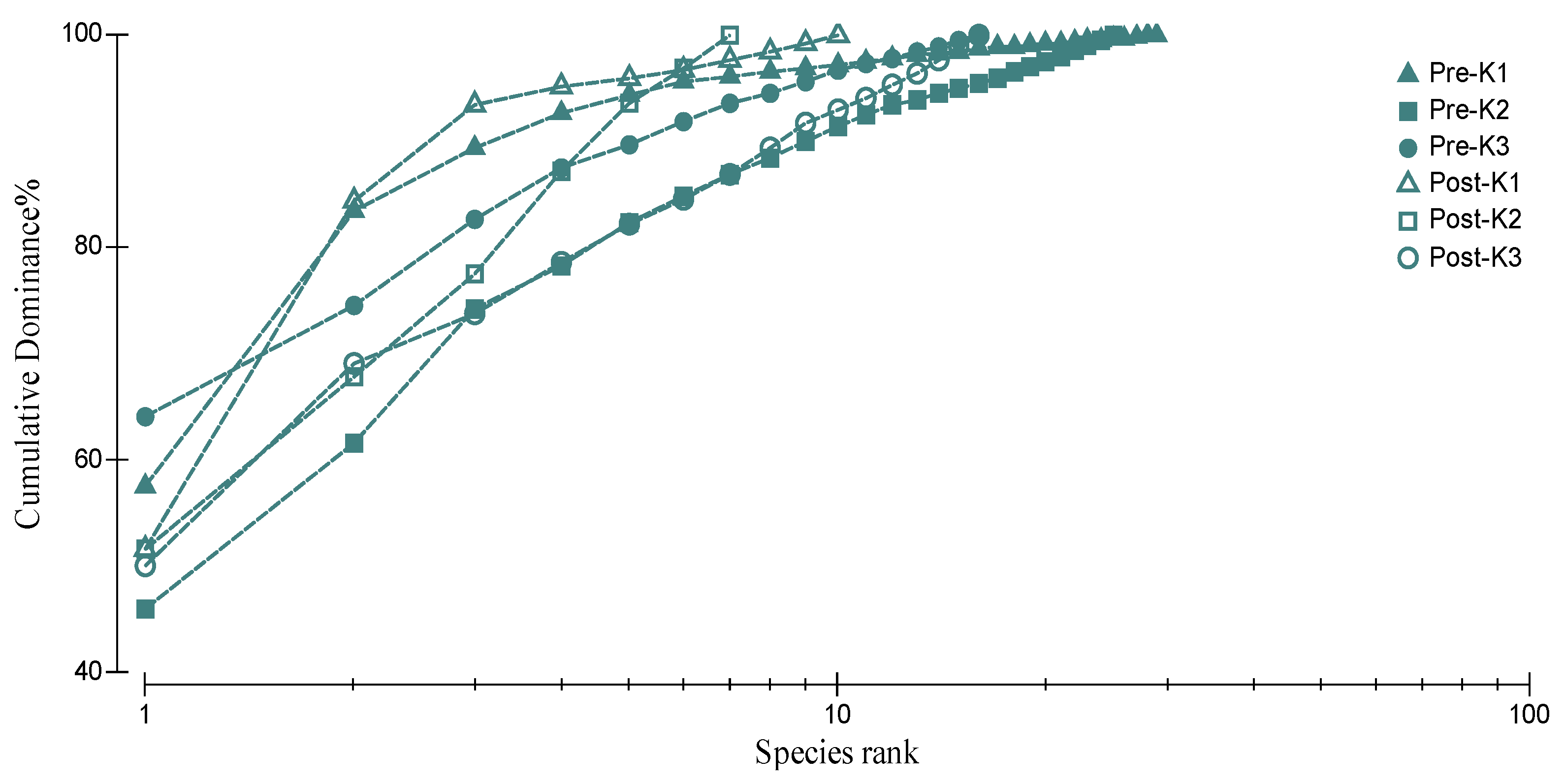
3.6. Polychaete Species K-Dominance Curve
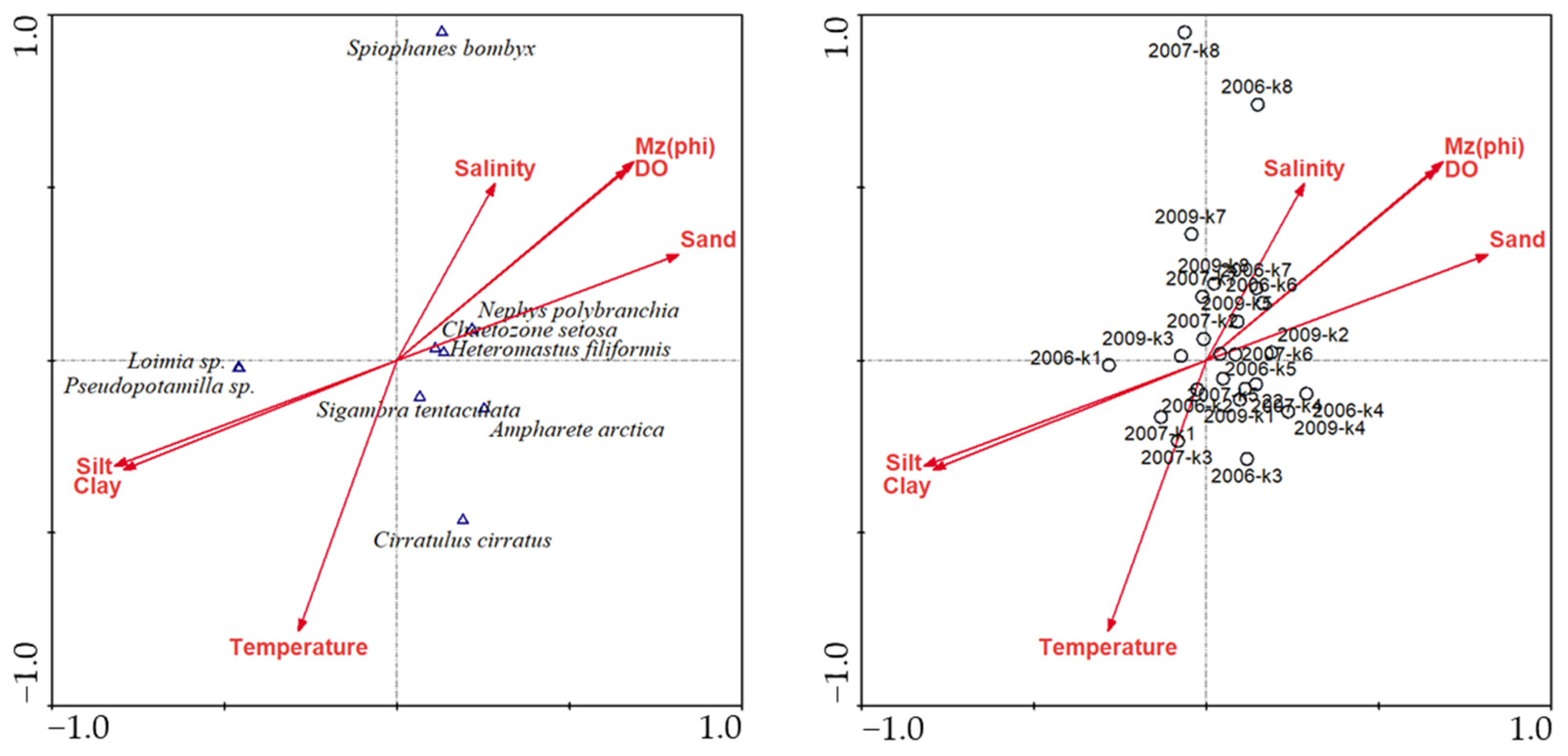
3.7. Environmental Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, C.; Kang, I. The variability of precipitation in Korea. J. Korean Meteorol. Soc. 1988, 24, 38–48. [Google Scholar]
- Kang, I.S.; Ho, C.H.; Lim, Y.K.; Lau, K.M. Principal modes of climatological seasonal and intraseasonal variations of the Asian summer monsoon. Mon. Weather Rev. 1999, 127, 322–340. [Google Scholar] [CrossRef]
- Ho, C.H.; Lee, J.Y.; Ahn, M.H.; Lee, H.S. A sudden change in summer rainfall characteristics in Korea during the late 1970s. Int. J. Climatol. 2003, 23, 117–128. [Google Scholar] [CrossRef]
- Seo, K.; Son, J.; Lee, J. A new look at Changma. Atmosphere 2011, 21, 109–121. [Google Scholar]
- Lee, J.Y.; Kwon, M.H.; Yun, K.S.; Min, S.K.; Park, I.H.; Ham, Y.G.; Jin, E.K.; Kim, J.H.; Seo, K.H.; Kim, W.M.; et al. The long-term variability of Changma in the East Asian summer monsoon system: A review and revisit. Asia-Pac. J. Atmos. Sci. 2017, 53, 257–272. [Google Scholar] [CrossRef]
- Filho, J.S.R.; Aviz, D. Macrobenthic communities of an Amazonian estuary (Guajará Bay, Brazil): Temporal and spatial changes. J. Coast. Res. 2013, 65, 123–128. [Google Scholar] [CrossRef]
- Palmer, T.A.; Montagna, P.A.; Pollack, J.B.; Kalke, R.D.; DeYoe, H.R. The role of freshwater inflow in lagoons, rivers, and bays. Hydrobiologia 2011, 667, 49–67. [Google Scholar] [CrossRef]
- Costanza, R.; D’Arge, R.; de Groot, R.; Farber, S.; Grasso, M.; Hannon, B.; Limburg, K.; Naeem, S.; O’Neill, R.V.; Paruelo, J.; et al. The value of the world’s ecosystem services and natural capital. Nature 1997, 387, 253–260. [Google Scholar] [CrossRef]
- Whitfield, A.K.; Elliott, M.; Basset, A.; Blaber, S.J.M.; West, R.J. Paradigms in estuarine ecology—A review of the Remane diagram with a suggested revised model for estuaries. Estuar. Coast. Shelf Sci. 2012, 97, 78–90. [Google Scholar] [CrossRef]
- Dias, E.; Morais, P.; Cotter, A.M.; Antunes, C.; Hoffman, J.C. Estuarine consumers utilize marine, estuarine and terrestrial organic matter and provide connectivity among these food webs. Mar. Ecol. Prog. Ser. 2016, 554, 21–34. [Google Scholar] [CrossRef]
- Davenport, J.; Ezgeta-Balić, D.; Peharda, M.; Skejić, S.; Ninčević-Gladan, Ž.; Matijević, S. Size-differential feeding in Pinna nobilis L. (Mollusca: Bivalvia): Exploitation of detritus, phytoplankton and zooplankton. Estuar. Coast. Shelf Sci. 2011, 92, 246–254. [Google Scholar] [CrossRef]
- Rossi, F.; Baeta, A.; Marques, J.C. Stable isotopes reveal habitat-related diet shifts in facultative deposit-feeders. J. Sea Res. 2015, 95, 172–179. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kwon, H.-K.; Choi, H.-Y.; Yang, J.-S.; Choi, J.-Y. Physical Environment Changes in the Keum River Estuary by the Dyke Gate Operation: II. Salinity Structure and Estuary Type. Korean J. Environ. Biol. 1999, 4, 255–265. [Google Scholar]
- Figueroa, S.M.; Lee, G.H.; Shin, H.J. The effect of periodic stratification on floc size distribution and its tidal and vertical variability: Geum Estuary, South Korea. Mar. Geol. 2019, 412, 187–198. [Google Scholar] [CrossRef]
- Kim, D.; Hong, S.; Choi, H.; Choi, B.; Kim, J.; Khim, J.S.; Park, H.; Shin, K.H. Multimedia distributions, bioaccumulation, and trophic transfer of microcystins in the Geum River Estuary, Korea: Application of compound-specific isotope analysis of amino acids. Environ. Int. 2019, 133, 105194. [Google Scholar] [CrossRef]
- Nunes, M.; Coelho, J.P.; Cardoso, P.G.; Pereira, M.E.; Duarte, A.C.; Pardal, M.A. The macrobenthic community along a mercury contamination in a temperate estuarine system (Ria de Aveiro, Portugal). Sci. Total Environ. 2008, 405, 186–194. [Google Scholar] [CrossRef]
- Fauchald, K.; Jumars, P.A. The diet of worms: A study of polychaete feeding guilds. Oceanogr. Mar. Biol. Annu. Rev. 1979, 17, 193–284. [Google Scholar]
- Vijapure, T.; Sukumaran, S.; Neetu, S.; Chandel, K. Macrobenthos at marine hotspots along the northwest Indian inner shelf: Patterns and drivers. Mar. Environ. Res. 2019, 144, 111–124. [Google Scholar] [CrossRef]
- Domínguez Castanedo, N.; Hernández Alcántara, P.; Solís-Weiss, V.; Granados Barba, A. Distribution of polychaete feeding guilds in sedimentary environments of the Campeche Bank, Southern Gulf of Mexico. Helgol. Mar. Res. 2012, 66, 469–478. [Google Scholar] [CrossRef]
- Musale, A.S.; Desai, D.V. Distribution and abundance of macrobenthic polychaetes along the South Indian coast. Environ. Monit. Assess. 2011, 178, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Sivadas, S.; Ingole, B.; Nanajkar, M. Benthic polychaetes as good indicators of anthropogenic impact. Indian J. Mar. Sci. 2010, 39, 201–211. [Google Scholar]
- Jumars, P.A.; Dorgan, K.M.; Lindsay, S.M. Diet of worms emended: An update of polychaete feeding guilds. Ann. Rev. Mar. Sci. 2015, 7, 497–520. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, W.F.; Barros, F. Structural and functional approaches to describe polychaete assemblages: Ecological implications for estuarine ecosystems. Mar. Freshw. Res. 2011, 62, 918–926. [Google Scholar] [CrossRef]
- Han, Q.; Jiang, X.; Wang, X. The polychaete feeding guild composition in the Sishili Bay, the northern Yellow Sea, China. J. Mar. Biol. Assoc. UK 2016, 96, 1083–1092. [Google Scholar] [CrossRef]
- Carvalho, S.; Pereira, P.; Pereira, F.; de Pablo, H.; Vale, C.; Gaspar, M.B. Factors structuring temporal and spatial dynamics of macrobenthic communities in a eutrophic coastal lagoon (Óbidos lagoon, Portugal). Mar. Environ. Res. 2011, 71, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, H.; De Montaudouin, X.; Chardy, P.; Bachelet, G. Structuring factors and recent changes in subtidal macrozoobenthic communities of a coastal lagoon, Arcachon Bay (France). Estuar. Coast. Shelf Sci. 2005, 64, 561–576. [Google Scholar] [CrossRef]
- Borja, A.; Josefson, A.B.; Miles, A.; Muxika, I.; Olsgard, F.; Phillips, G.; Rodríguez, J.G.; Rygg, B. An approach to the intercalibration of benthic ecological status assessment in the North Atlantic ecoregion, according to the European Water Framework Directive. Mar. Pollut. Bull. 2007, 55, 42–52. [Google Scholar] [CrossRef]
- Silva, A.C.F.; Tavares, P.; Shapouri, M.; Stigter, T.Y.; Monteiro, J.P.; Machado, M.; Cancela da Fonseca, L.; Ribeiro, L. Estuarine biodiversity as an indicator of groundwater discharge. Estuar. Coast. Shelf Sci. 2012, 97, 38–43. [Google Scholar] [CrossRef]
- Hughes, B.D. The influence of factors other than pollution on the value of Shannon’s diversity index for benthic macro-invertebrates in streams. Water Res. 1978, 12, 359–364. [Google Scholar] [CrossRef]
- Labrune, C.; Amouroux, J.M.; Sarda, R.; Dutrieux, E.; Thorin, S.; Rosenberg, R.; Grémare, A. Characterization of the ecological quality of the coastal Gulf of Lions (NW Mediterranean). A comparative approach based on three biotic indices. Mar. Pollut. Bull. 2006, 52, 34–47. [Google Scholar] [CrossRef]
- Patrício, J.; Neto, J.M.; Teixeira, H.; Salas, F.; Marques, J.C. The robustness of ecological indicators to detect long-term changes in the macrobenthos of estuarine systems. Mar. Environ. Res. 2009, 68, 25–36. [Google Scholar] [CrossRef]
- Choi, J.W.; Koh, C.H. Macrobenthos Community in Keum-Mankyung-Dongjin Estuaries and its adjacent Coastal Region, Korea. J. Korean Soc. Oceanogr. 1994, 29, 304–318. [Google Scholar]
- Yoon, S.J.; Hong, S.; Kwon, B.O.; Ryu, J.; Lee, C.H.; Nam, J.; Khim, J.S. Distributions of persistent organic contaminants in sediments and their potential impact on macrobenthic faunal community of the Geum River Estuary and Saemangeum Coast, Korea. Chemosphere 2017, 173, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Ryu, J. Short-term Variations in Spatial Distribution of the Macrozoobenthic Community near the Geum River Estuary, Korea. J. Korean Soc. Mar. Environ. Energy 2018, 21, 368–380. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER V6: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2006. [Google Scholar]
- Borja, A.; Tunberg, B.G. Assessing benthic health in stressed subtropical estuaries, eastern Florida, USA using AMBI and M-AMBI. Ecol. Indic. 2011, 11, 295–303. [Google Scholar] [CrossRef]
- Rouse, G.W.; Fauchald, K. Cladistics and Polychaetes. Zool. Scr. 1997, 26, 139–204. [Google Scholar] [CrossRef]
- Macdonald, T.A.; Burd, B.J.; Macdonald, V.I.; Van Roodselaar, A. Taxonomic and Feeding Guild Classification for the Marine Benthic Macroinvertebrates of the Strait of Georgia, British Columbia Canadian Technical Report of Fisheries and Aquatic Sciences 2874. Can. Tech. Rep. Fish. Aquat. Sci. 2010, 2874, 1–69. [Google Scholar]
- Hong, J.; Yoo, J. Salinity and sediment types as sources of variability in the distribution of the benthic macrofauna in Han estuary and Kyonggi Bay, Korea. J. Korean Soc. Oceanogr. 1996, 31, 217–231. [Google Scholar]
- Ritter, C.; Montagna, P.A.; Applebaum, S. Short-term succession dynamics of macrobenthos in a salinity-stressed estuary. J. Exp. Mar. Bio. Ecol. 2005, 323, 57–69. [Google Scholar] [CrossRef]
- Yu, O.H.; Lee, H.G.; Lee, J.H. Influence of Environmental Variables on the Distribution of Macrobenthos in the Han River Estuary, Korea. Ocean Sci. J. 2012, 47, 519–528. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, B.; Xue, Y. The response of the diets of four carnivorous fishes to variations in the Yellow Sea ecosystem. Deep. Res. Part II Top. Stud. Oceanogr. 2010, 57, 996–1000. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, J.; Qing, W.; Feng, Y.; Tang, J. Speleothem-derived Asian Summer Monsoon variations in Central China, 54-46ka. J. Quat. Sci. 2011, 26, 781–790. [Google Scholar] [CrossRef]
- Kim, J.-C.; Park, K.-W.; Yoo, K.-D.; Jung, S.-Y. Spatial and Temporal Distribution of Macrobenthos in Intertidal Hard Bottoms in Dokdo Island. Korean J. Environ. Ecol. 2015, 29, 221–227. [Google Scholar] [CrossRef]
- Gardel, A.; Anthony, E.J.; dos Santos, V.F.; Huybrechts, N.; Lesourd, S.; Sottolichio, A.; Maury, T.; Jolivet, M. Fluvial sand, Amazon mud, and sediment accommodation in the tropical Maroni River estuary: Controls on the transition from estuary to delta and chenier plain. Reg. Stud. Mar. Sci. 2021, 41, 101548. [Google Scholar] [CrossRef]
- Kang, S.-K.; Lee, D.-R.; Moon, J.-W.; Choi, S.-J. Effects of Dams and Water Use on Flow Regime Alteration of the Geum River Basin. J. Korea Water Resour. Assoc. 2010, 43, 325–336. [Google Scholar] [CrossRef]
- Soares-Gomes, A.; Mendes, C.L.T.; Tavares, M.; Santi, L. Taxonomic sufficiency of polychaete taxocenes for estuary monitoring. Ecol. Indic. 2012, 15, 149–156. [Google Scholar] [CrossRef]
- Tavares, A.S.; Santos Sousa, L.K.; Passos Ribeiro, R.; De Oliveira, V.M.; da Silva De Almeida, Z. Structure of a polychaete community in a mangrove in the northern coast of Brazil [Estructura de la comunidad de poliquetos en un manglar de la costa norte de Brasil]. Acta Biol. Colomb. 2018, 23, 286–294. [Google Scholar] [CrossRef]
- Aviz, D.; de Carvalho, I.L.R.; Filho, J.S.R. Cambios espaciales y temporales en las comunidades de macroinvertebrados bentónicos en la zona costera del Amazonas (estuario del guajará, Brasil), causada por los vertidos de efluentes urbanos. Sci. Mar. 2012, 76, 381–390. [Google Scholar] [CrossRef]
- Wang, W.; Sui, J.; Kou, Q.; Li, X.Z. Review of the genus Loimia Malmgren, 1866 (Annelida, Terebellidae) from China seas with recognition of two new species based on integrative taxonomy. PeerJ 2020, 2020, e9491. [Google Scholar] [CrossRef]
- Seitz, R.D.; Schaffner, L.C. Population ecology and secondary production of the polychaete Loimia medusa (Terebellidae). Mar. Biol. 1995, 121, 701–711. [Google Scholar] [CrossRef]
- Pagliosa, P.R. Another diet of worms: The applicability of polychaete feeding guilds as a useful conceptual framework and biological variable. Mar. Ecol. 2005, 26, 246–254. [Google Scholar] [CrossRef]
- Choi, H.K.; Yoon, S.M. First record of Heteromastus filiformis (Polychaeta: Capitellidae) from Korean waters, with a taxonomic note on the genus. J. Species Res. 2016, 5, 264–271. [Google Scholar] [CrossRef]
- Pearson, T.; Rosenberg, H. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. Annu. Rev. 1978, 16, 229–311. [Google Scholar]
- Park, J.; Song, S.J.; Ryu, J.; Kwon, B.O.; Hong, S.; Bae, H.; Choi, J.W.; Khim, J.S. Macrozoobenthos of Korean tidal flats: A review on species assemblages and distribution. Ocean Coast. Manag. 2014, 102, 483–492. [Google Scholar] [CrossRef]
- Jayachandran, P.R.; Bijoy Nandan, S.; Jima, M.; Joseph, P.; Don Xavier, N.D.; Sreedevi, O.K.; Prabhakaran, M.P.; Joseph, K.J. Macrobenthic functional feeding groups in a microtidal monsoonal estuary (Kodungallur–Azhikode estuary, India). Reg. Stud. Mar. Sci. 2019, 25, 100444. [Google Scholar] [CrossRef]



| Major Mode | Motility Pattern | Morphological Structure | ||
|---|---|---|---|---|
| Motile (M) | Sessile (S) | Discretely Motile (D) | ||
| Carnivores (C) | Unarmed pharynx (X) | CMX | ||
| Jawed pharynx (J) | CMJ | CDJ | ||
| Herbivores (H) | Unarmed pharynx (X) | HMX | ||
| Jawed pharynx (J) | HMJ | HDJ | ||
| Filter feeders (F) | Tentaculate (T) | FST | FDT | |
| Pumping (P) | FSP | FDP | ||
| Surface deposit feeders (S) | Unarmed pharynx (X) | SMX | SDX | |
| Jawed pharynx (J) | SMJ | SDJ | ||
| Tentaculate (T) | SMT | SDT | ||
| Burrowers (B) | Unarmed pharynx (X) | BMX | BSX | BDX |
| (Subsurface deposit feeders) | Jawed pharynx (J) | BMJ | ||
| Tentaculate (T) | BMT | |||
| Month | Site | Mz (µm) | Clay (%) | Silt (%) | Sand (%) | As (ppm) | Cr (ppm) | Cu (ppm) | Pb (ppm) | Zn (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|
| June | K1 | 19.4 | 5.0 | 83.5 | 11.5 | 11.1 | 70.3 | 22.5 | 29.6 | 102.5 |
| June | K2 | 21.8 | 4.7 | 77.2 | 18.1 | 9.1 | 66.7 | 19.1 | 27.4 | 90.4 |
| June | K3 | 35.8 | 3.4 | 55.6 | 41.0 | 9.8 | 43.5 | 10.0 | 25.8 | 52.2 |
| June | K4 | 149.4 | 1.0 | 17.7 | 81.3 | 6.5 | 34.9 | 3.7 | 21.6 | 32.4 |
| June | K5 | 132.0 | 1.6 | 29.2 | 69.1 | 6.8 | 30.2 | 5.2 | 21.9 | 38.1 |
| June | K6 | 185.9 | 0.4 | 10.9 | 88.7 | 7.3 | 24.2 | 3.2 | 21.6 | 33.8 |
| June | K7 | 163.0 | 1.0 | 20.6 | 78.4 | 5.2 | 21.0 | 3.9 | 20.2 | 26.9 |
| June | K8 | 278.2 | 0.1 | 6.2 | 93.7 | 5.0 | 12.3 | 2.2 | 21.1 | 17.6 |
| July | K1 | 40.1 | 3.0 | 55.3 | 41.7 | 11.5 | 42.9 | 11.7 | 29.7 | 63.0 |
| July | K2 | 17.0 | 5.5 | 87.9 | 6.6 | 11.8 | 71.0 | 26.6 | 32.9 | 116.9 |
| July | K3 | 36.4 | 3.3 | 57.9 | 38.8 | 7.4 | 46.1 | 9.5 | 20.6 | 51.7 |
| July | K4 | 133.6 | 1.4 | 24.7 | 73.9 | 5.7 | 33.8 | 4.8 | 20.7 | 32.9 |
| July | K5 | 140.9 | 1.3 | 24.8 | 73.9 | 7.1 | 31.2 | 4.8 | 21.9 | 34.9 |
| July | K6 | 186.2 | 0.3 | 8.9 | 90.9 | 7.4 | 24.0 | 3.0 | 22.2 | 31.9 |
| July | K7 | 178.6 | 1.0 | 17.1 | 82.0 | 6.2 | 22.4 | 3.6 | 21.0 | 31.3 |
| July | K8 | 320.0 | 0.0 | 0.0 | 100.0 | 4.1 | 8.8 | 1.7 | 21.6 | 11.9 |
| September | K1 | 23.1 | 4.4 | 69.8 | 25.7 | 13.2 | 76.5 | 31.1 | 36.6 | 131.1 |
| September | K2 | 18.3 | 5.6 | 83.8 | 10.6 | 10.4 | 68.8 | 23.9 | 31.7 | 106.7 |
| September | K3 | 16.8 | 5.3 | 89.5 | 5.2 | 10.9 | 72.4 | 22.7 | 31.4 | 104.1 |
| September | K4 | 137.7 | 1.2 | 19.6 | 79.1 | 4.8 | 30.5 | 4.3 | 21.0 | 30.9 |
| September | K5 | 111.9 | 2.2 | 36.1 | 61.7 | 6.4 | 31.5 | 5.8 | 21.7 | 39.6 |
| September | K6 | 160.2 | 0.9 | 16.7 | 82.4 | 6.7 | 25.0 | 3.5 | 22.1 | 32.4 |
| September | K7 | 240.3 | 0.1 | 7.8 | 92.1 | 5.1 | 17.7 | 2.5 | 19.3 | 20.2 |
| September | K8 | 185.9 | 1.3 | 22.8 | 76.0 | 5.2 | 18.3 | 3.4 | 19.6 | 23.8 |
| Environmental Variables | Pre-Rainy Season | Mid-Rainy Season | Post-Rainy Season |
|---|---|---|---|
| Surface water temperature (°C) | 20.26 ± 1.61 | 20.67 ± 0.30 | 23.15 ± 0.75 |
| Bottom water temperature (°C) | 18.40 ± 2.55 | 19.06 ± 2.42 | 20.89 ± 1.88 |
| Surface salinity (psu) | 30.38 ± 1.20 | 18.93 ± 8.72 | 25.03 ± 5.25 |
| Bottom salinity (psu) | 31.19 ± 0.58 | 31.00 ± 0.80 | 30.25 ± 1.20 |
| Surface dissolved oxygen (mg/L) | 5.50 ± 0.46 | 5.63 ± 0.29 | 5.17 ± 0.10 |
| Bottom dissolved oxygen (mg/L) | 5.74 ± 0.60 | 5.40 ± 0.24 | 5.23 ± 0.15 |
| Sand content (%) | 60.22 ± 30.20 | 63.47 ± 29.50 | 54.11 ± 32.58 |
| Silt content (%) | 37.62 ± 28.40 | 34.56 ± 27.78 | 43.28 ± 30.56 |
| Clay content (%) | 2.16 ± 1.80 | 1.97 ± 1.73 | 2.61 ± 2.02 |
| Taxon | Pre-Rainy Season | Mid-Rainy Season | Post-Rainy Season | |
|---|---|---|---|---|
| Density | Polychaete | 1443.13 ± 1472.96 | 1169.50 ± 822.62 | 998.86 ± 985.87 |
| Mollusca | 1258.75 ± 2011.05 | 798.32 ± 212.64 | 569.49 ± 1236.92 | |
| Crustacea | 725.63 ± 1024.36 | 593.61 ± 401.85 | 545.19 ± 654.95 | |
| Echinodermata | 21.25 ± 44.91 | 50.62 ± 136.53 | 104.76 ± 264.66 | |
| Others | 66.25 ± 56.10 | 48.77 ± 17.31 | 47.64 ± 45.49 | |
| Total | 3515.00 ± 3547.42 | 2601.80 ± 1207.83 | 2212.22 ± 2279.40 | |
| Species | Polychaete | 23.63 ± 4.56 | 20.01 ± 8.25 | 20.09 ± 8.51 |
| Mollusca | 8.00 ± 4.69 | 6.48 ± 2.87 | 6.18 ± 4.01 | |
| Crustacea | 11.38 ± 4.95 | 9.41 ± 7.05 | 10.35 ± 7.35 | |
| Echinodermata | 1.13 ± 0.78 | 1.22 ± 1.65 | 1.31 ± 1.22 | |
| Others | 3.00 ± 2.00 | 2.61 ± 0.66 | 2.47 ± 1.46 | |
| Total | 47.13 ± 14.11 | 39.57 ± 19.42 | 40.26 ± 19.80 |
| No | Family | Feeding Type | Number of Species | Average Density (m2) |
|---|---|---|---|---|
| 1 | Ampharetidae | Surface deposit feeder | 4 | 132 |
| 2 | Capitellidae | Subsurface deposit feeder | 4 | 215 |
| 3 | Chaetopteridae | Filter feeder | 2 | 8 |
| 4 | Chrysopetalidae | Carnivores | 1 | 0.2 |
| 5 | Cirratulidae | Surface deposit feeder | 4 | 65 |
| 6 | Dorvilleidae | Herbivores | 2 | 6 |
| 7 | Eunicidae | Carnivores | 3 | 1 |
| 8 | Flabelligeridae | Filter feeder | 2 | 4 |
| 9 | Glyceridae | Carnivores | 6 | 29 |
| 10 | Hesionidae | Carnivores | 4 | 8 |
| 11 | Lacydoniidae | Carnivores | 1 | 14 |
| 12 | Lumbrineridae | Subsurface deposit feeder | 2 | 6 |
| 13 | Magelonidae | Surface deposit feeder | 2 | 12 |
| 14 | Maldanidae | Subsurface deposit feeder | 1 | 1 |
| 15 | Nephtyidae | Carnivores | 3 | 30 |
| 16 | Nereididae | Subsurface deposit feeder | 1 | 1 |
| 17 | Oenonidae | Carnivores | 1 | 2 |
| 18 | Onuphidae | Subsurface deposit feeder | 2 | 7 |
| 19 | Opheliidae | Subsurface deposit feeder | 1 | 0.4 |
| 20 | Orbiniidae | Subsurface deposit feeder | 1 | 6 |
| 21 | Oweniidae | Filter feeder | 3 | 7 |
| 22 | Paraonidae | Surface deposit feeder | 3 | 22 |
| 23 | Pectinariidae | Filter feeder | 1 | 1 |
| 24 | Phyllodocidae | Carnivores | 6 | 15 |
| 25 | Pilargidae | Carnivores | 2 | 43 |
| 26 | Poecilochaetidae | Surface deposit feeder | 1 | 8 |
| 27 | Polynoidae | Carnivores | 3 | 17 |
| 28 | Sabellidae | Filter feeder | 5 | 76 |
| 29 | Sigalionidae | Carnivores | 3 | 3 |
| 30 | Spionidae | Filter feeder | 16 | 86 |
| 31 | Sternaspidae | Subsurface deposit feeder | 1 | 14 |
| 32 | Syllidae | Carnivores | 2 | 1 |
| 33 | Terebellidae | Surface deposit feeder | 3 | 125 |
| 34 | Trichobranchidae | Surface deposit feeder | 1 | 0.4 |
| Species | Family | Density (ind./m2) | Frequency (%) | Pre-Rainy Season | Mid-Rainy Season | Post-Rainy Season | Feeding Guilds |
|---|---|---|---|---|---|---|---|
| Heteromastus filiformis | Capitellidae | 210 | 79 | * | * | * | BDX, SMX |
| Ampharete cf. finmarchica | Ampharetidae | 128 | 50 | * | * | * | SST |
| Loimia sp. | Terebellidae | 123 | 4 | * | SST | ||
| Pseudopotamilla sp. | Sabellidae | 56 | 8 | * | FST | ||
| Sigambra tentaculata | Pilargidae | 42 | 63 | * | * | * | CMJ |
| Chaetozone setosa | Cirratulidae | 36 | 88 | * | * | * | SMT, SDT |
| Spiophanes bombyx | Spionidae | 31 | 29 | * | * | * | FDT, SDT |
| Nephtys polybranchia | Nephtyidae | 26 | 79 | * | * | * | BMJ |
| Cirratulus cirratus | Cirratulidae | 25 | 4 | * | SMT, SDT |
| Month | Site | N | S | ES(100) | H’ | AMBI | M-AMBI | Status |
|---|---|---|---|---|---|---|---|---|
| June | K1 | 5140 | 29 | 9.4 | 1.339 | 0.659 | 0.757 | Good |
| June | K2 | 990 | 25 | 15.9 | 1.97 | 3.705 | 0.593 | Good |
| June | K3 | 920 | 16 | 11.3 | 1.422 | 4.068 | 0.428 | Moderate |
| June | K4 | 1475 | 28 | 16.1 | 1.804 | 0.867 | 0.787 | High |
| June | K5 | 375 | 24 | 21.2 | 2.777 | 1.845 | 0.797 | High |
| June | K6 | 1735 | 28 | 15.1 | 1.438 | 4.047 | 0.526 | Moderate |
| June | K7 | 330 | 21 | 19 | 2.494 | 2.391 | 0.689 | Good |
| June | K8 | 580 | 18 | 13.6 | 1.716 | 2.533 | 0.552 | Good |
| July | K1 | 70 | 7 | 7 | 1.673 | 3.500 | 0.414 | Moderate |
| July | K2 | 370 | 13 | 11.5 | 1.474 | 4.014 | 0.411 | Moderate |
| July | K3 | 770 | 16 | 12.8 | 2.171 | 3.188 | 0.570 | Good |
| July | K4 | 2495 | 34 | 15 | 1.625 | 1.270 | 0.794 | High |
| July | K5 | 405 | 18 | 16 | 2.145 | 3.241 | 0.581 | Good |
| July | K6 | 1880 | 25 | 12.5 | 1.212 | 3.957 | 0.481 | Moderate |
| July | K7 | 345 | 21 | 19.2 | 2.67 | 2.642 | 0.693 | Good |
| July | K8 | 255 | 9 | 8.8 | 1.469 | 2.794 | 0.455 | Moderate |
| September | K1 | 610 | 10 | 7.4 | 1.227 | 4.303 | 0.338 | Poor |
| September | K2 | 155 | 7 | 7 | 1.486 | 3.968 | 0.362 | Poor |
| September | K3 | 420 | 16 | 13.9 | 1.797 | 3.821 | 0.486 | Moderate |
| September | K4 | 1415 | 34 | 21.2 | 2.504 | 1.570 | 0.873 | High |
| September | K5 | 495 | 22 | 18.4 | 2.516 | 2.449 | 0.710 | Good |
| September | K6 | 805 | 26 | 20.6 | 2.782 | 2.349 | 0.769 | Good |
| September | K7 | 535 | 36 | 28.6 | 3.062 | 2.505 | 0.879 | High |
| September | K8 | 490 | 23 | 20.1 | 2.767 | 3.082 | 0.675 | Good |
| Clay (%) | Silt (%) | Sand (%) | Temperature (°C) | Salinity (psu) | Dissolved Oxygen (mg/L) | Heteromatus filiformis | Loimia sp. | Pseudopotamilla sp. | Sigambra tentaculata | Chaetozone setosa | Spiophanes bombyx | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean grain size (phi) | −0.97 *** | −0.97 *** | 0.97 *** | −0.83 *** | 0.85 *** | 0.76 *** | −0.32 | −0.26 | −0.14 | −0.40 | 0.44 * | 0.73 *** |
| Clay (%) | 0.99 *** | −0.99 *** | 0.78 *** | −0.80 *** | −0.72 *** | 0.26 | 0.26 | 0.10 | 0.42 * | −0.44 * | 0.66 *** | |
| Silt (%) | −1 *** | 0.76 *** | −0.79 *** | −0.71 *** | 0.26 | 0.26 | 0.10 | 0.44 * | −0.45 * | −0.65 *** | ||
| Sand (%) | −0.76 *** | 0.79 *** | 0.71 *** | −0.26 *** | −0.26 | −0.10 | −0.44 * | 0.45 * | 0.65 *** | |||
| Temp (°C) | −0.97 *** | −0.93 *** | 0.37 | 0.17 | 0.11 | 0.20 | −0.26 | −0.80 *** | ||||
| Sal (psu) | 0.89 *** | −0.42 * | −0.17 | −0.14 | −0.29 | 0.34 | 0.80 *** | |||||
| DO (mg/L) | −0.37 | −0.35 | −0.17 | −0.13 | 0.14 | 0.76 *** | ||||||
| Heteromastus filiformis | −0.29 | −0.26 | 0.67 *** | −0.08 | −0.49 * | |||||||
| Loimia sp. | 0.72 *** | −0.23 | −0.09 | −0.13 | ||||||||
| Pseudopotamilla sp. | −0.21 | −0.21 | −0.19 | |||||||||
| Sigambra tentaculata | −0.30 | −0.41 * | ||||||||||
| Chaetozone setosa | 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.L.; Oh, K.-H.; Ra, K.; Yu, O.H. Effects of Freshwater Inflow during the Rainy Season on the Benthic Polychaete Community in the Geum River Estuary, South Korea. Diversity 2024, 16, 180. https://doi.org/10.3390/d16030180
Kim SL, Oh K-H, Ra K, Yu OH. Effects of Freshwater Inflow during the Rainy Season on the Benthic Polychaete Community in the Geum River Estuary, South Korea. Diversity. 2024; 16(3):180. https://doi.org/10.3390/d16030180
Chicago/Turabian StyleKim, Sang Lyeol, Kyung-Hee Oh, Kongtae Ra, and Ok Hwan Yu. 2024. "Effects of Freshwater Inflow during the Rainy Season on the Benthic Polychaete Community in the Geum River Estuary, South Korea" Diversity 16, no. 3: 180. https://doi.org/10.3390/d16030180
APA StyleKim, S. L., Oh, K.-H., Ra, K., & Yu, O. H. (2024). Effects of Freshwater Inflow during the Rainy Season on the Benthic Polychaete Community in the Geum River Estuary, South Korea. Diversity, 16(3), 180. https://doi.org/10.3390/d16030180







