Structure from Motion Photogrammetry as an Effective Nondestructive Technique to Monitor Morphological Plasticity in Benthic Organisms: The Case Study of Sarcotragus foetidus Schmidt, 1862 (Porifera, Demospongiae) in the Portofino MPA
Abstract
1. Introduction
2. Materials and Methods
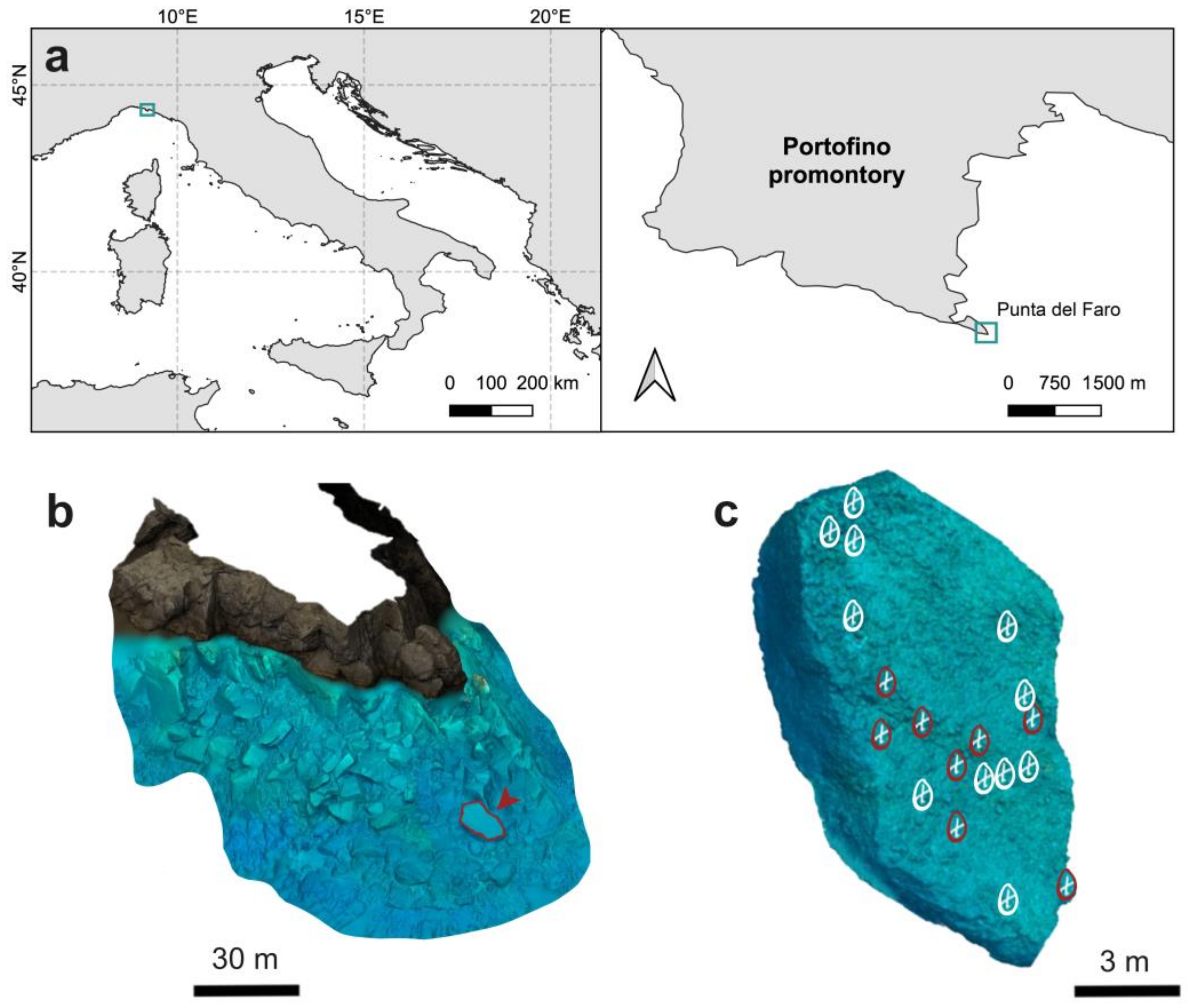
2.1. Study Area, Image Acquisition, and Photogrammetric Processing
2.2. Volume and Biomass Estimations
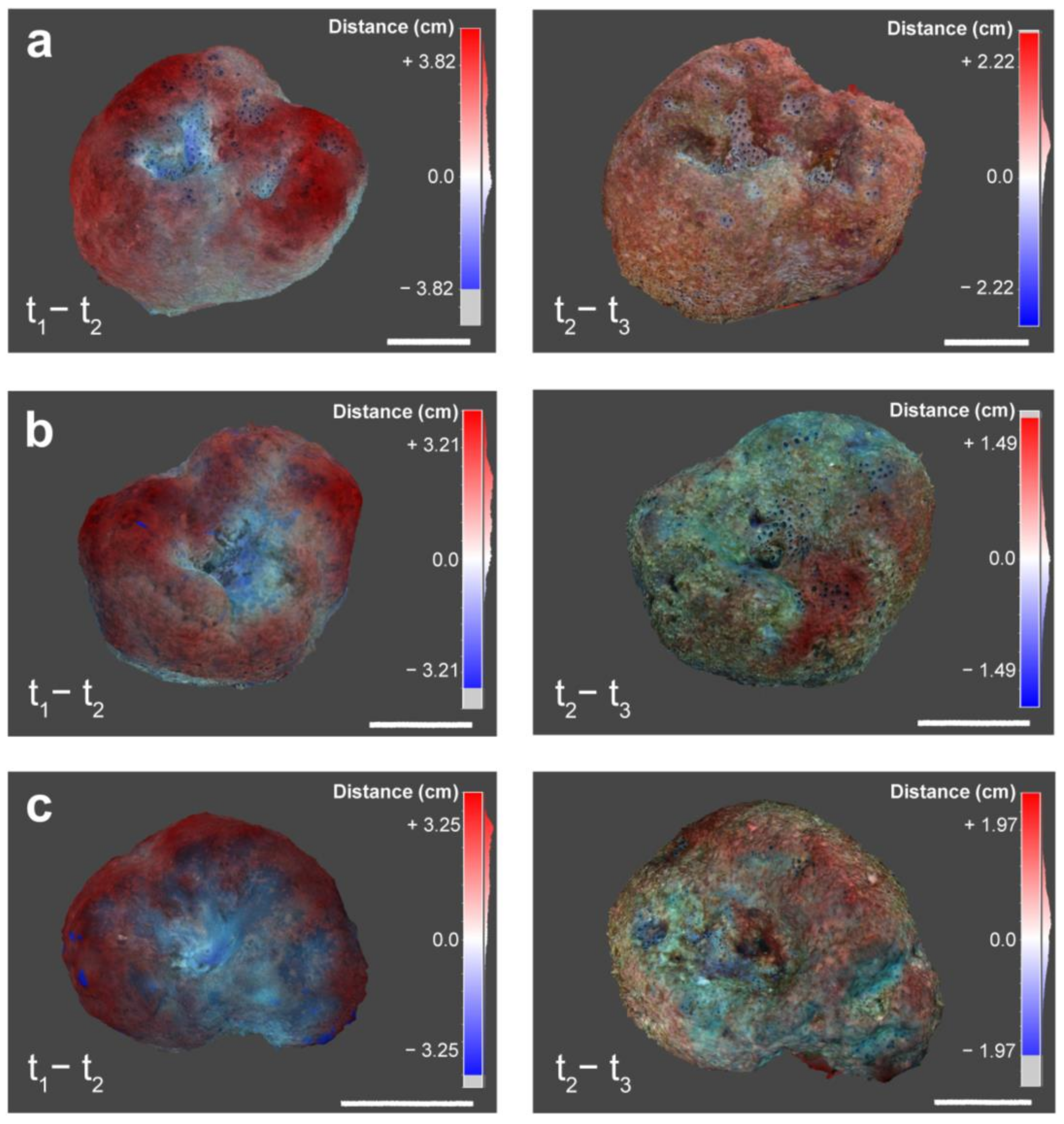
2.3. Analysis of S. foetidus Morphological Changes
2.4. Sea Surface Temperature (SST) Data and Marine Heat Wave (MHW) Detection
2.5. Data Analyses
3. Results
3.1. SfM-Photogrammetry Assessment
3.2. Volume and Biomass Changes over Time
3.3. Temperature Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, J.J. The functional roles of marine sponges. Estuar. Coast. Shelf Sci. 2008, 79, 341–353. [Google Scholar] [CrossRef]
- Van Soest, R.W.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; De Voogd, N.J.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J.N. Global diversity of sponges (Porifera). PLoS ONE 2012, 7, e35105. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Aguilar, R.; Bannister, R.; Bell, J.; Conway, J.; Dayton, P.; Díaz, C.; Gutt, J.; Kelly, M.; Kenchington, E.; et al. Sponge Grounds as Key Marine Habitats: A Synthetic Review of Types, Structure, Functional Roles, and Conservation Concerns. In Marine Animal Forests: The Ecology of Benthic Biodiversity Hotspots; Rossi, S., Bramanti, L., Gori, A., Orejas, S.d.V.C., Eds.; Springer: Cham, Switzerland, 2017; pp. 1–39. [Google Scholar] [CrossRef]
- Weisz, J.B.; Lindquist, N.; Martens, C.S. Do associated microbial abundances impact marine demosponge pumping rates and tissue densities? Oecologia 2008, 155, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Ribes, M.; van Duyl, F.C. Nutrient fluxes through sponges: Biology, budgets, and ecological implications. Adv. Mar. Biol. 2012, 62, 113–182. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.K.; Murillo, F.J.; Lirette, C.; Maldonado, M.; Colaço, A.; Ottaviani, D.; Kenchington, E. Removal of deep-sea sponges by bottom trawling in the Flemish Cap area: Conservation, ecology and economic assessment. Sci. Rep. 2019, 9, 15843. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Palma Esposito, F.; Sangiovanni, E.; Pagano, E.; Mannucci, C.; Polini, B.; Ghelardini, C.; Dell’Agli, M.; Izzo, A.A.; Calapai, G.; et al. Pharmacological activities of extracts and compounds isolated from mediterranean sponge sources. Pharmaceuticals 2021, 14, 1329. [Google Scholar] [CrossRef] [PubMed]
- Roveta, C.; Annibaldi, A.; Afghan, A.; Calcinai, B.; Di Camillo, C.G.; Gregorin, C.; Illuminati, S.; Pulido Mantas, T.; Truzzi, C.; Puce, S. Biomonitoring of heavy metals: The unexplored role of marine sessile taxa. Appl. Sci. 2021, 11, 580. [Google Scholar] [CrossRef]
- Garrabou, J.; Zabala, M. Growth dynamics in four Mediterranean demosponges. Estuar. Coast. Shelf Sci. 2001, 52, 293–303. [Google Scholar] [CrossRef]
- Riesgo, A.; Maldonado, M. Differences in reproductive timing among sponges sharing habitat and thermal regime. Invertebr. Biol. 2008, 127, 357–367. [Google Scholar] [CrossRef]
- Pronzato, R.; Bavestrello, G.; Cerrano, C. Morpho-functional adaptations of three species of Spongia (Porifera, Demospongiae) from a Mediterranean vertical cliff. Bull. Mar. Sci. 1998, 63, 317–328. [Google Scholar]
- Gökalp, M.; Kooistra, T.; Rocha, M.S.; Silva, T.H.; Osinga, R.; Murk, A.J.; Wijgerde, T. The effect of depth on the morphology, bacterial clearance, and respiration of the Mediterranean sponge Chondrosia reniformis (Nardo, 1847). Mar. Drugs 2020, 18, 358. [Google Scholar] [CrossRef]
- Olinger, L.K.; Scott, A.R.; McMurray, S.E.; Pawlik, J.R. Growth estimates of Caribbean reef sponges on a shipwreck using 3D photogrammetry. Sci. Rep. 2019, 9, 18398. [Google Scholar] [CrossRef]
- Roche, S.; Ronziere, M.C.; Herbage, D.; Freyria, A.M. Native and DPPA cross-linked collagen sponges seeded with fetal bovine epiphyseal chondrocytes used for cartilage tissue engineering. Biomaterials 2000, 22, 9–18. [Google Scholar] [CrossRef]
- Çelik, İ.; Cirik, Ş.; Altιnağaç, U.; Ayaz, A.; Çelik, P.; Tekeşoğlu, H.; Yιlmaz, H.; Öztekin, A. Growth performance of bath sponge (Spongia officinalis Linnaeus, 1759) farmed on suspended ropes in the Dardanelles (Turkey). Aquac. Res. 2011, 42, 1807–1815. [Google Scholar] [CrossRef]
- De Caralt, S.; Uriz, M.J.; Wijffels, R.H. Grazing, differential size-class dynamics and survival of the Mediterranean sponge Corticium candelabrum. Mar. Ecol. Prog. Ser. 2008, 360, 97–106. [Google Scholar] [CrossRef]
- Pulido Mantas, T.; Roveta, C.; Calcinai, B.; Di Camillo, C.G.; Gambardella, C.; Gregorin, C.; Coppari, M.; Marrocco, T.; Puce, S.; Riccardi, A.; et al. Photogrammetry, from the Land to the Sea and Beyond: A Unifying Approach to Study Terrestrial and Marine Environments. J. Mar. Sci. Eng. 2023, 11, 759. [Google Scholar] [CrossRef]
- Koopmans, M.; Wijffels, R.H. Seasonal growth rate of the sponge Haliclona oculata (Demospongiae: Haplosclerida). Mar. Biotechnol. 2008, 10, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, S.; Airoldi, L.; Ballesteros, E.; Benedetti-Cecchi, L.; Boero, F.; Bulleri, F.; Cebrian, E.; Cerrano, C.; Claudet, J.; Colloca, F.; et al. Mediterranean rocky reefs in the Anthropocene: Present status and future concerns. Adv. Mar. Biol. 2021, 89, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, M.; Cerrano, C.; Bavestrello, G.; Carella, M.; Pansini, M.; Calcinai, B. Diversity of Porifera in the Mediterranean coralligenous accretions, with description of a new species. ZooKeys 2013, 336, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Di Camillo, C.G.; Cerrano, C. Mass mortality events in the NW Adriatic Sea: Phase shift from slow-to fast-growing organisms. PLoS ONE 2015, 10, e0126689. [Google Scholar] [CrossRef] [PubMed]
- Garrabou, J.; Gómez-Gras, D.; Ledoux, J.B.; Linares, C.; Bensoussan, N.; López-Sendino, P.; Bazairi, H.; Espinosa, F.; Ramdani, M.; Grimes, S.; et al. Collaborative database to track mass mortality events in the Mediterranean Sea. Front. Mar. Sci. 2019, 6, 478167. [Google Scholar] [CrossRef]
- Garrabou, J.; Gómez-Gras, D.; Medrano, A.; Cerrano, C.; Ponti, M.; Schlegel, R.; Bensoussan, N.; Turicchia, E.; Sini, M.; Gerovasileiou, V.; et al. Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Glob. Change Biol. 2022, 28, 5708–5725. [Google Scholar] [CrossRef] [PubMed]
- Gerovasileiou, V.; Chintiroglou, C.C.; Konstantinou, D.; Voultsiadou, E. Sponges as “living hotels” in Mediterranean marine caves. Sci. Mar. 2016, 80, 279–289. [Google Scholar] [CrossRef]
- Pola, L.; Cerrano, C.; Del Sette, G.; Torsani, F.; Calcinai, B. Seasonal trend of the macrofauna inhabiting two Mediterranean species of Sarcotragus (Porifera, Demospongiae). In Proceedings of the 53rd European Marine Biology Symposium, Ostend, Belgium, 17–21 September 2018. [Google Scholar]
- Çinar, M.E.; Bakir, K.; Doğan, A.; Acik, S.; Kurt, G.; Katağan, T.; Öztürk, B.; Dağli, E.; Özcan, T.; Kirkim, F.; et al. Macro-benthic invertebrates associated with the black sponge Sarcotragus foetidus (Porifera) in the Levantine and Aegean Seas, with special emphasis on alien species. Estuarine. Coast. Shelf Sci. 2019, 227, 106306. [Google Scholar] [CrossRef]
- Goren, L.; Idan, T.; Shefer, S.; Ilan, M. Macrofauna inhabiting massive demosponges from shallow and mesophotic habitats along the Israeli Mediterranean coast. Front. Mar. Sci. 2021, 7, 612779. [Google Scholar] [CrossRef]
- Gerovasileiou, V.; Dailianis, T.; Sini, M.; Otero, M.D.M.; Numa, C.; Katsanevakis, S.; Voultsiadou, E. Assessing the regional conservation status of sponges (Porifera): The case of the Aegean ecoregion. Mediterr. Mar. Sci. 2018, 19, 526–537. [Google Scholar] [CrossRef]
- Chaudhari, S.; Kumar, M.S. Marine sponges Sarcotragus foetidus, Xestospongia carbonaria and Spongia obscura constituents ameliorate IL-1 β and IL-6 in lipopolysaccharide-induced RAW 264.7 macrophages and carrageenan-induced oedema in rats. Inflammopharmacology 2020, 28, 1091–1119. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Tassara, E.; Dodero, A.; Castellano, M.; Vicini, S.; Ferrando, S.; Aicardi, S.; Cavallo, D.; Bertolino, M.; Petrenko, I.; et al. Potential biomedical applications of collagen filaments derived from the marine demosponges Ircinia oros (Schmidt, 1864) and Sarcotragus foetidus (Schmidt, 1862). Mar. Drugs 2021, 19, 563. [Google Scholar] [CrossRef]
- UNEP/MAP-SPA/RAC. SAP/RAC: SPA-BD Protocol—Annex II: List of Endangered or Threatened Species. 2018. Available online: https://www.rac-spa.org/sites/default/files/annex/annex_2_en_20182.pdf (accessed on 6 March 2024).
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.A.X.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Manca Zeichen, M.; Finoia, M.G.; Locritani, M.; Ruggeri, N.; Tunesi, L.; Gasparini, G.P.; Bassetti, M.; Grandi, V.; Cattaneo-Vietti, R.; Povero, P. A preliminary analysis of in situ and remotely sensed environmental variables in the coastal region of the Portofino Marine Protected Area. Chem. Ecol. 2008, 24, 57–66. [Google Scholar] [CrossRef]
- Bayley, D.T.; Mogg, A.O. A protocol for the large-scale analysis of reefs using Structure from Motion photogrammetry. Methods Ecol. Evol. 2020, 11, 1410–1420. [Google Scholar] [CrossRef]
- Çinar, M.E.; Katagan, T.; Ergen, Z.; Sezgin, M. Zoobenthos-inhabiting Sarcotragus muscarum (Porifera: Demospongiae) from the Aegean Sea. Hydrobiologia 2002, 482, 107–117. [Google Scholar] [CrossRef]
- Özcan, T.; Katağan, T. Decapod Crustaceans associated with the sponge Sarcotragus muscarum Schmidt, 1864 (Porifera: Demospongiae) from the Levantine coasts of Turkey. Iran. J. Fish. Sci. 2011, 10, 286–293. [Google Scholar]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.; Benthuysen, A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef]
- Hobday, A.J.; Oliver, E.C.J.; Sen Gupta, A.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Holbrook, N.J.; Moore, P.J.; Thomsen, M.S.; Wernberg, T.; et al. Categorising and naming marine heatwaves. Oceanography 2018, 31, 162–173. [Google Scholar] [CrossRef]
- Schlegel, R.W.; Smit, A.J. Heatwaver: A central algorithm for the detection of heatwaves and cold-spells. J. Open Source Softw. 2018, 3, 821. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 6 March 2024).
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Gerovasileiou, V.; Voultsiadou, E. Marine caves of the Mediterranean Sea: A sponge biodiversity reservoir within a biodiversity hotspot. PLoS ONE 2012, 7, e39873. [Google Scholar] [CrossRef] [PubMed]
- Pansini, M. Petrosia pulitzeri n. sp. (Porifera, Demospongiae) from Mediterranean caves. Ital. J. Zool. 1996, 63, 169–172. [Google Scholar] [CrossRef]
- Bertolino, M.; Calcinai, B.; Cattaneo-Vietti, R.; Cerrano, C.; Lafratta, A.; Pansini, M.; Pica, D.; Bavestrello, G. Stability of the sponge assemblage of Mediterranean coralligenous concretions along a millennial time span. Mar. Ecol. 2014, 35, 149–158. [Google Scholar] [CrossRef]
- Azzola, A.; Bavestrello, G.; Bertolino, M.; Bianchi, C.N.; Bo, M.; Enrichetti, F.; Morri, C.; Oprandi, A.; Toma, M.; Montefalcone, M. You cannot conserve a species that has not been found: The case of the marine sponge Axinella polypoides in Liguria, Italy. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 737–747. [Google Scholar] [CrossRef]
- Abdo, D.A.; Seager, J.W.; Harvey, E.S.; McDonald, J.I.; Kendrick, G.A.; Shortis, M.R. Efficiently measuring complex sessile epibenthic organisms using a novel photogrammetric technique. J. Exp. Mar. Biol. Ecol. 2006, 339, 120–133. [Google Scholar] [CrossRef]
- Rodríguez-Basalo, A.; Prado, E.; Sánchez, F.; Ríos, P.; Gómez-Ballesteros, M.; Cristobo, J. High resolution spatial distribution for the hexactinellid sponges Asconema setubalense and Pheronema carpenteri in the Central Cantabrian Sea. Front. Mar. Sci. 2021, 8, 612761. [Google Scholar] [CrossRef]
- Ríos, P.; Prado, E.; Carvalho, F.C.; Sánchez, F.; Rodríguez-Basalo, A.; Xavier, J.R.; Ibarrola, T.P.; Cristobo, J. Community composition and habitat characterization of a rock sponge aggregation (Porifera, Corallistidae) in the Cantabrian Sea. Front. Mar. Sci. 2020, 7, 578. [Google Scholar] [CrossRef]
- Prado, E.; Cristobo, J.; Rodríguez-Basalo, A.; Ríos, P.; Rodríguez-Cabello, C.; Sánchez, F. In situ growth rate assessment of the hexactinellid sponge Asconema setubalense using 3D photogrammetric reconstruction. Front. Mar. Sci. 2021, 8, 26. [Google Scholar] [CrossRef]
- Idan, T.; Goren, L.; Shefer, S.; Ilan, M. Sponges in a changing climate: Survival of Agelas oroides in a warming Mediterranean Sea. Front. Mar. Sci. 2020, 7, 603593. [Google Scholar] [CrossRef]
- Olinger, L.K.; Chaves-Fonnegra, A.; Enochs, I.C.; Brandt, M.E. Three competitors in three dimensions: Photogrammetry reveals rapid overgrowth of coral during multispecies competition with sponges and algae. Mar. Ecol. Prog. Ser. 2021, 657, 109–121. [Google Scholar] [CrossRef]
- Schönberg, C.H.L. No taxonomy needed: Sponge functional morphologies inform about environmental conditions. Ecol. Indic. 2021, 129, 107806. [Google Scholar] [CrossRef]
- Enrichetti, F.; Bavestrello, G.; Betti, F.; Coppari, M.; Toma, M.; Pronzato, R.; Canese, S.; Bertolino, M.; Costa, G.; Pansini, M.; et al. Keratose-dominated sponge grounds from temperate mesophotic ecosystems (NW Mediterranean Sea). Mar. Ecol. 2020, 41, e12620. [Google Scholar] [CrossRef]
- Turon, X.; Garriga, A.; Erwin, P.M. Lights and shadows: Growth patterns in three sympatric and congeneric sponges (Ircinia spp.) with contrasting abundances of photosymbionts. Mar. Biol. 2013, 160, 2743–2754. [Google Scholar] [CrossRef]
- Perez-Lopez, P.; Ledda, F.D.; Bisio, A.; Feijoo, G.; Perino, E.; Pronzato, R.; Manconi, R.; Moreira, M.T. Life cycle assessment of in situ mariculture in the Mediterranean Sea for the production of bioactive compounds from the sponge Sarcotragus spinosulus. J. Clean. Prod. 2017, 142, 4356–4368. [Google Scholar] [CrossRef]
- Garate, L.; Blanquer, A.; Uriz, M.J. Contrasting biological features in morphologically cryptic Mediterranean sponges. PeerJ 2017, 5, e3490. [Google Scholar] [CrossRef][Green Version]
- Webster, N.; Pantile, R.; Botte, E.; Abdo, D.; Andreakis, N.; Whalan, S. A complex life cycle in a warming planet: Gene expression in thermally stressed sponges. Mol. Ecol. 2013, 22, 1854–1868. [Google Scholar] [CrossRef] [PubMed]
- Koutsouveli, V.; Manousaki, T.; Riesgo, A.; Lagnel, J.; Kollias, S.; Tsigenopoulos, C.S.; Arvanitidis, C.; Dounas, C.; Magoulas, A.; Dailianis, T. Gearing up for warmer times: Transcriptomic response of risk of mortality outbreaks associated with thermal stress in NW Mediterranean coastal areas. Ocean Dyn. 2020, 64, 103–115. [Google Scholar] [CrossRef]
- Dinçtürk, E.; Öndes, F.; Leria, L.; Maldonado, M. Mass mortality of the keratose sponge Sarcotragus foetidus in the Aegean Sea (Eastern Mediterranean) correlates with proliferation of Vibrio bacteria in the tissues. Front. Microbiol. 2023, 14, 1272733. [Google Scholar] [CrossRef] [PubMed]
- Betti, F.; Bavestrello, G.; Bo, M.; Enrichetti, F.; Cattaneo-Vietti, R. Effects of the 2018 exceptional storm on the Paramuricea clavata (Anthozoa, Octocorallia) population of the Portofino Promontory (Mediterranean Sea). Reg. Stud. Mar. Sci. 2020, 34, 101037. [Google Scholar] [CrossRef]
- Cebrian, E.; Uriz, M.J.; Garrabou, J.; Ballesteros, E. Sponge mass mortalities in a warming Mediterranean Sea: Are cyanobacteria-harboring species worse off? PLoS ONE 2011, 6, e20211. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Lachs, L.; Pygas, D.R.; Humanes, A.; Sommer, B.; Figueira, W.F.; Edwards, A.J.; Bythell, J.C.; Guest, J.R. Photogrammetry as a tool to improve ecosystem restoration. Trends Ecol. Evol. 2021, 36, 1093–1101. [Google Scholar] [CrossRef]
- Raoult, V.; David, P.A.; Dupont, S.F.; Mathewson, C.P.; O’Neill, S.J.; Powell, N.N.; Williamson, J.E. GoPros™ as an underwater photogrammetry tool for citizen science. PeerJ 2016, 4, e1960. [Google Scholar] [CrossRef]
- Garrabou, J.; Bensoussan, N.; di Franco, A.; Boada, J.; Cebrian, E.; Santamaria, J.; Guala, I.; Grech, D.; Cerrano, C.; Pulido Mantas, T.; et al. Monitoring Climate-Related Responses in Mediterranean Marine Protected Areas and Beyond: Eleven Standard Protocols; Institute of Marine Sciences, Spanish Research Council ICM-CSIC: Barcelona, Spain, 2022; 74p. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulido Mantas, T.; Roveta, C.; Calcinai, B.; Benelli, F.; Coppari, M.; Di Camillo, C.G.; Pantaleo, U.; Puce, S.; Cerrano, C. Structure from Motion Photogrammetry as an Effective Nondestructive Technique to Monitor Morphological Plasticity in Benthic Organisms: The Case Study of Sarcotragus foetidus Schmidt, 1862 (Porifera, Demospongiae) in the Portofino MPA. Diversity 2024, 16, 175. https://doi.org/10.3390/d16030175
Pulido Mantas T, Roveta C, Calcinai B, Benelli F, Coppari M, Di Camillo CG, Pantaleo U, Puce S, Cerrano C. Structure from Motion Photogrammetry as an Effective Nondestructive Technique to Monitor Morphological Plasticity in Benthic Organisms: The Case Study of Sarcotragus foetidus Schmidt, 1862 (Porifera, Demospongiae) in the Portofino MPA. Diversity. 2024; 16(3):175. https://doi.org/10.3390/d16030175
Chicago/Turabian StylePulido Mantas, Torcuato, Camilla Roveta, Barbara Calcinai, Fabio Benelli, Martina Coppari, Cristina Gioia Di Camillo, Ubaldo Pantaleo, Stefania Puce, and Carlo Cerrano. 2024. "Structure from Motion Photogrammetry as an Effective Nondestructive Technique to Monitor Morphological Plasticity in Benthic Organisms: The Case Study of Sarcotragus foetidus Schmidt, 1862 (Porifera, Demospongiae) in the Portofino MPA" Diversity 16, no. 3: 175. https://doi.org/10.3390/d16030175
APA StylePulido Mantas, T., Roveta, C., Calcinai, B., Benelli, F., Coppari, M., Di Camillo, C. G., Pantaleo, U., Puce, S., & Cerrano, C. (2024). Structure from Motion Photogrammetry as an Effective Nondestructive Technique to Monitor Morphological Plasticity in Benthic Organisms: The Case Study of Sarcotragus foetidus Schmidt, 1862 (Porifera, Demospongiae) in the Portofino MPA. Diversity, 16(3), 175. https://doi.org/10.3390/d16030175









