Abstract
Darwin’s finches are a classic example of adaptive radiation involving differential use of dietary resources among sympatric species. Here, we apply stable isotope (δ13C, δ15N, and δ2H) analyses of feathers to examine ecological segregation among eight Darwin’s finch species in Santa Cruz Island, Galápagos collected from live birds and museum specimens (1962–2019). We found that δ13C values were higher for the granivorous and herbivorous foraging guilds, and lower for the insectivorous finches. Values of δ15N were similar among foraging guilds but values of δ2H were higher for insectivores, followed by granivores, and lowest for herbivores. The herbivorous guild generally occupied the largest isotopic standard ellipse areas for all isotopic combinations and the insectivorous guild the smallest. Values of δ2H provided better trophic discrimination than those of δ15N possibly due to confounding influences of agricultural inputs of nitrogen. Segregation among guilds was enhanced by portraying guilds in three-dimensional isotope (δ13C, δ15N, and δ2H) space. Values of δ13C and δ15N were higher for feathers of museum specimens than for live birds. We provide evidence that Darwin’s finches on Santa Cruz Island tend to be generalists with overlapping isotopic niches and suggest that dietary overlap may also be more considerable than previously thought.
1. Introduction
Darwin’s finches of the Galápagos Islands are a classic example of adaptive radiation and ecological segregation [1,2]. Currently, 17 species that derived from a common ancestor are recognized and they occupy a variety of individual ecological niches. It has been suggested that behavioral flexibility and innovation were the main drivers in the radiation of Darwin’s finches [3,4,5]. Today, ground finches (Geospiza spp.) feed primarily on seeds, while tree finches (Camarhynchus spp.) feed on fruits and arthropods in trees, the single vegetarian finch (Platyspiza crassirostris) on leaves and fruits, and warbler finches (Certhidea spp.) on arthropods [1,6]. These differences in diet are also famously associated with differences in beak size and shape. Geospiza finches show temporal and spatial variation in their diets and there is dietary overlap among species [6,7], but in general, tree and ground finches show opportunistic resource use across seasons and years [6,8,9]. Ground finches represent an adaptive radiation, where they may be characterized as being ‘imperfect generalists’ that have evolved to use a variety of overlapping resources, and are mostly opportunistic feeders [6]. However, like many avian groups, information on precise diets is lacking. The frequent environmental changes and climatic variation likely drive the use of versatile feeding strategies and dietary overlap among Darwin’s finches [6,10].
Measurements of naturally occurring stable isotope ratios in consumer tissues have become a useful tool to reconstruct aspects of dietary, ecological, and environmental histories in terrestrial and marine ecosystems [11,12,13,14,15]. Such measurements can be used to trace movements of nutrients, compounds, particles and organisms across landscapes, and provide information on the source of nutrients to foodwebs and trophic positions of consumers [12], thus augmenting more conventional approaches to studying diets.
Isotope studies of diet and habitat use are based on the principle that isotope values of consumer tissues reflect those of diet and drinking water [16]. In particular, stable-carbon (δ13C) and nitrogen (δ15N) isotope ratios have been used to evaluate source of feeding and trophic level, respectively. Stable carbon isotope ratios are particularly useful in evaluating relative contributions to foodwebs from plants with different photosynthetic pathways (i.e., C3, C4 or CAM) [17,18]. In addition to providing such direct information on diets, these two isotopes are also linked to climate and anthropogenic landuse practices [19,20,21,22]. Stable hydrogen (δ2H) isotope ratios in consumer tissues can reflect those of diet and drinking water [16,23]. Stable hydrogen isotopes in precipitation also show well-known and predictable continental patterns, and so have been used to track animal movements [21,24,25], but tissue δ2H values can also be used to investigate diet and habitat use at more local scales [26].
Birds in the Galápagos Islands have access to both terrestrial and marine nutrients. Marine nutrients can be derived directly through foraging in intertidal or beach zones or indirectly through cascading effects of nutrient transfer from marine sources to terrestrial foodwebs. Consumers that rely on marine resources typically have higher δ13C, δ15N and δ2H values compared to those consuming terrestrial foods [27]. Within terrestrial systems, changes in altitude can result in different vegetation zones due to associated changes in precipitation and temperature and these influence local foodweb stable isotope values [28,29,30,31]. Despite the potential isotopic complexity of foodwebs of birds on the Galápagos Islands, the use of a multi-isotope approach to examining avian diets in this system can be informative (e.g., [32,33,34]). Moreover, the development of multidimensional isotopic niches in ecology has allowed the examination of interspecific niche overlap and segregation thus allowing insight into how animal communities are structured [13]. While isotopic niches are not necessarily true ecological niches [35,36], they nonetheless provide a “first look” into potential evolutionary patterns resulting in coexisting species communities [37,38].
Here, we build upon the extensive foundation of studies on Darwin’s finches, by introducing the use of stable isotope analyses of feathers to examine mechanisms of ecological segregation among these iconic sympatric species. We hypothesized that variation in environmental conditions and nutritional sources throughout different habitats on Santa Cruz Island, will be generally reflected in the isotopic signals of feathers but that individual species’ isotopic niches would reveal strong segregation at each community level. We used a three-isotope (δ13C, δ15N, and δ2H) approach to examine feathers collected from both contemporary field campaigns and long-term museum collections, which also allowed us to examine niche characteristics through time [16,39].
2. Materials and Methods
2.1. Field Collections
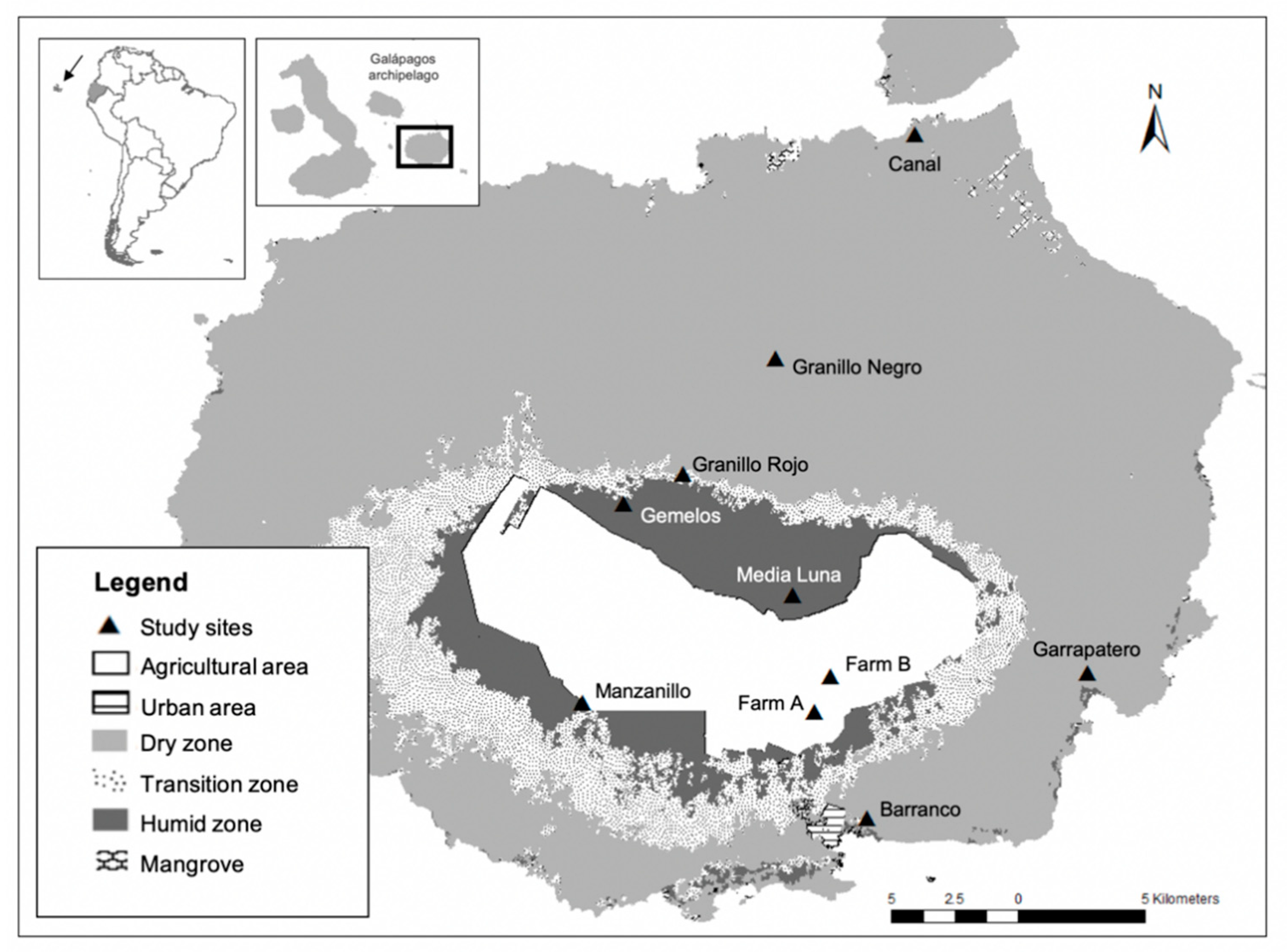
Santa Cruz Island is the second largest island in the Galápagos archipelago (985 km2, maximum altitude 864 m; Figure 1) [29,30]. In June 2019, we collected feather samples at ten study sites (Figure 1), in the following habitats [29,30,31,40]: (1) Dry zone (up to ~120 m above sea level), comprised of deciduous forest, shrubland and grassland; (2) Transition zone (~120–300 masl), comprised of evergreen (seasonal) forest and shrubland; (3) Humid zone, (>300 masl), comprised of three distinctive vegetation areas: Scalesia forest, Miconia shrubland and fern area (>650 masl); and (4) agricultural lands, located within the transition and humid zones (Figure 1). We also coordinated with the road-kill mortality project PC-09-18 conducted by the Fundación Charles Darwin–Dirección del Parque Nacional Galápagos (FCD-DPNG), in which wing flight feather samples were collected by G.J.U. at roadside margins in 2018.
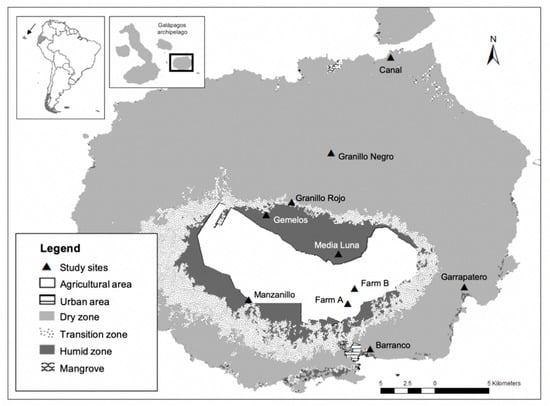
Figure 1.
Location of study sites in Santa Cruz Island, Galápagos, Ecuador. Dry zone (4 sites): Barranco, Garrapatero, Granillo Negro and Canal; Transition zone (2 sites): Granillo Rojo and Manzanillo; Humid zone (2 sites): Gemelos and Media Luna; and Agricultural lands (2 sites): Farm A and Farm B (map modified from shapefiles kindly provided by Rivas-Torres et al. [31]).
At each site, birds were captured using an array of six mistnets (6 m and 12 m) operated for two days (0600 h-1130 h). Nets were checked continuously. Birds were removed from mist nets and placed in individual cloth bags in a shaded area until processed. Birds were weighed (nearest 0.1 g) using a digital or string Pesola scale and morphometric measurements (head-bill length, exposed culmen, tarsus) were taken with a Vernier dial caliper with a precision of 0.02 mm. A measurement of the unflattened, right wing-chord was taken with a wing ruler (1 mm). Individuals were banded with a uniquely coded aluminum band before release. From each individual, we sampled 2–3 secondary wing coverts; all feathers from one individual were stored in a single paper coin envelope. Envelopes were stored in a plastic box containing silica gel, and kept at room temperature.
Feathers were sampled from 231 live birds, belonging to eight species (Table 1 and Supplementary Figure S1): Small Ground Finch (SGF, Geospiza fuliginosa); Medium Ground Finch (MGF, Geospiza fortis); Large Ground Finch (LGF, Geospiza magnirostris); Common Cactus Finch (CF, Geospiza scandens); Small Tree Finch (STF, Camarhynchus parvulus); Woodpecker Finch (WF, Camarhynchus pallidus); Green Warbler Finch (GWF, Certhidea olivacea); Vegetarian Finch (VF, Platyspiza crassirostris). We assigned all species to three foraging guilds (following [6,41]). Guilds were: (1) granivorous ground finches (i.e., opportunist feeders primarily on seeds) which included two species, SGF and MGF; (2) herbivorous finches (i.e., feeders on large seeds, leaves, fruits, and nectar), which included LGF, CF and VF; and (3) insectivorous finches (i.e., feeding primarily on small invertebrates), which included STF, GWF and WF (Table 1, Tables S1 and S2).

Table 1.
Isotopic values (means and standard deviations in ‰) in feathers of live birds and museum specimens (see Table S2 for details on museum specimens). Differences per species, foraging guilds and habitats were examined with ANOVAs with Tukey’s post-hoc tests (different superscripts represent statistical differences at p < 0.05).
2.2. Museum Collections
We collected wing secondary coverts from 19 museum specimens stored at the Vertebrate Collection of the Charles Darwin Research Station (VCCDRS) in Puerto Ayora, Santa Cruz (specimens ranged from 1962 to 2011; Table S2). From these specimens, we pulled 2-3 secondary wing coverts feathers per specimen. All feathers from one specimen were placed in a single coin envelope, and stored as described above.
2.3. Laboratory Analyses
All feather samples were cleaned of surface contaminants using a 2:1 chloroform:methanol rinse and air dried overnight in a fume hood. Feather δ13C and δ15N assays were processed at the stable isotope laboratory of Environment and Climate Change Canada in Saskatoon, Canada. Subsamples (0.5 ± 0.02 mg) of vane material were weighed into tin capsules and combusted at 1030 °C in a Eurovector 3000 elemental analyser (Milan, Italy). The resulting N2 and CO2 were separated chromatographically and introduced to an Elementar Isoprime (Manchester, UK) or a Nu Instruments Horizon (Wrexham, UK) isotope ratio mass spectrometer. We used two internal reference materials to calibrate results to Vienna Pee Dee Belemnite (VPDB: δ13C) and AIR (δ15N), BWBIII (baleen) keratin (δ13C = −20.18, δ15N = +14.31‰) and the commercial gelatin PRCgel (δ13C = −13.64, δ15N = +5.07‰). Within-run (n = 5) precisions as determined from both reference and sample duplicate analyses were ±0.1‰for both δ13C and δ15N.
For the δ2H measurements of feathers (δ2Hw), 0.35 ± 0.02 mg of feather vane was weighed into silver 3.5 × 5 mm capsules, and analyzed using a Eurovector Uniprep autosampler (Milan, Italy) carousel attached to a Eurovector 3000 Elemental Analyzer, coupled to a Thermo Delta V Plus (Bremen, Germany) isotope-ratio mass spectrometer in continuous-flow mode with helium carrier gas. After the samples were loaded, the Uniprep autosampler (heated to 60 °C) was vacuum evacuated and subsequently flushed with dry helium twice to remove adsorbed atmospheric moisture from the crushed silver capsules. The autosampler was then held under positive helium pressure for the duration of the analytical run. Two USGS keratin standards, EC-01 (formerly CBS: Caribou Hoof Standard) and EC-02 (formerly KHS: Kudu Horn Standard of Environment Canada) were included every ten samples. An internal laboratory standard, powdered keratin (MP Biomedicals Inc., Cat No. 90211, Lot No.9966H) was included to monitor instrument drift and provide a check on accuracy over the course of each analytical session. Samples were combusted at 1350 °C using glassy carbon. Values of δ2H of non-exchangeable hydrogen were derived using the comparative equilibration approach of Wassenaar and Hobson [42] and calibrated to VSMOW using EC-01 (±1.9‰ 1 SD, n = 18, accepted δ2H = −197.0‰) and EC-02 (±1.6‰, n = 17, accepted δ2H = −54.1‰). Overall measurement error for EC-01 and EC-02 δ2H was ~2‰.
2.4. Statistical Analyses
Our response variables were feather δ13C, δ15N and δ2H values and the explanatory variables used were Species, Habitat, Site, Altitude, Foraging guild and Mass. We conducted separate ANOVAs and Tukey’s post-hoc tests to examine differences in feather isotopic values among categories of interest such as species, foraging guilds and habitats. To evaluate the importance of these explanatory variables on feather isotope values, we used generalized linear models, with a Gaussian distribution and the “identity” link function. Given that δ15N values had a non-normal distribution, skewed to the lower values, we applied a “log-link” to δ15N values rather than the “identity” link function. Best models were selected and compared using Akaike Information Criterion scores corrected for small sample sizes (AICc). The model with the lowest AICc was considered to have the best fit. Model sets were conducted separately for: (1) live birds, which were the majority (eight species); and (2) species sampled from museum specimens and live birds (only CF, LGF and STF) for which we used Origin (i.e., museum or live) as an explanatory variable instead of Mass. We also examined individual relationships among feather δ13C, δ15N and δ2H values and with each of the explanatory variables considered using simple general linear models. In addition, with general linear models we tested whether feather isotope values differed among guilds in each of the habitats.
Isotopic niche was evaluated among foraging guilds using standard ellipse areas (SEA) with the correction for small sample sizes (SEAc), estimated using a Bayesian approach in the SIBER package [43]. We also used this Bayesian approach in SIBER to estimate niche overlap between ellipses of sampling categories. In order to graph the isotopic niche in a three-dimensional space, we followed the model and code proposed by Rossman et al. [33], which represents an extension of Jackson et al. [43] and measures standard ellipse volumes instead of areas. These approaches allowed us to make a comparative analysis of isotopic niches among species and category levels of the different explanatory variables, and allowed a characterization of niche overlap and segregation among Darwin’s finch species. All statistical analyses were conducted in R (Version 4.0.3, [44]).
3. Results
Feather δ13C, δ15N and δ2H values are summarized in Table 1. Values of δ13C were lowest for GWF, highest for CF and SGF, and intermediate for MGF, LGF, STF and WF (Table 1). Foraging guilds differed for all isotopes, whereby the insectivorous guild had the lowest δ13C values and highest δ15N and δ2H values (Table 1). Habitat groupings differed only in δ13C values (Table 1).
For live birds, the model with the lowest AICc score and strongest support both for δ13C and δ2H values included Mass, Species and Site as explanatory variables (Table 2 and Supplementary Table S3). Values of δ15N did not differ among species (Table 1). For δ15N values, the model with lowest AICc score and strongest support included Mass, Foraging guild and Habitat (Table 2 and Supplementary Table S3). Values of δ2H were highest for WF, CF, STF and GWF and lowest for LGF (Table 1) and lowest for sites San José farm, Gemelos and Granillo Rojo (Supplementary Table S3).

Table 2.
Summary of results from the best generalized linear models (GLM) explaining the response variables (i.e., δ13C, δ15N and δ2H values) in each dataset analyzed: (A) Live birds (N = 231), and (B) Species with live and museum data (N = 85).
When analyzing the species dataset including both live birds and museum specimens, models with lowest AICc scores and highest support were (1) for δ13C values, the best model included Species and Habitat as explanatory variables; (2) for δ15N values the best model included Origin and Species; and (3) for δ2H values, the best model included Species and Habitat (Table 2 and Supplementary Table S3). Museum specimens had higher δ13C and δ15N values than live individuals (Table 3). Values of δ15N were highest in the humid zone and for museum specimens, and were lowest for LGF (Supplementary Table S3). Values of δ2H were lowest for LGF and at the farm habitat and were highest at the transition zone (Supplementary Table S3).

Table 3.
Simple linear relationships between feather δ13C, δ15N and δ2H values and each of the explanatory variables, for each dataset analyzed: (A) Live birds (N = 231), and (B) Species with live and museum data (N = 85). Significant models are in bold.
When examining feather isotope values among guilds within each habitat separately, we found significant differences in all isotope values, especially for the insectivorous foraging guild in the humid habitat (Supplementary Table S4).
Isotopic Niche Size, Overlap and Trophic Position
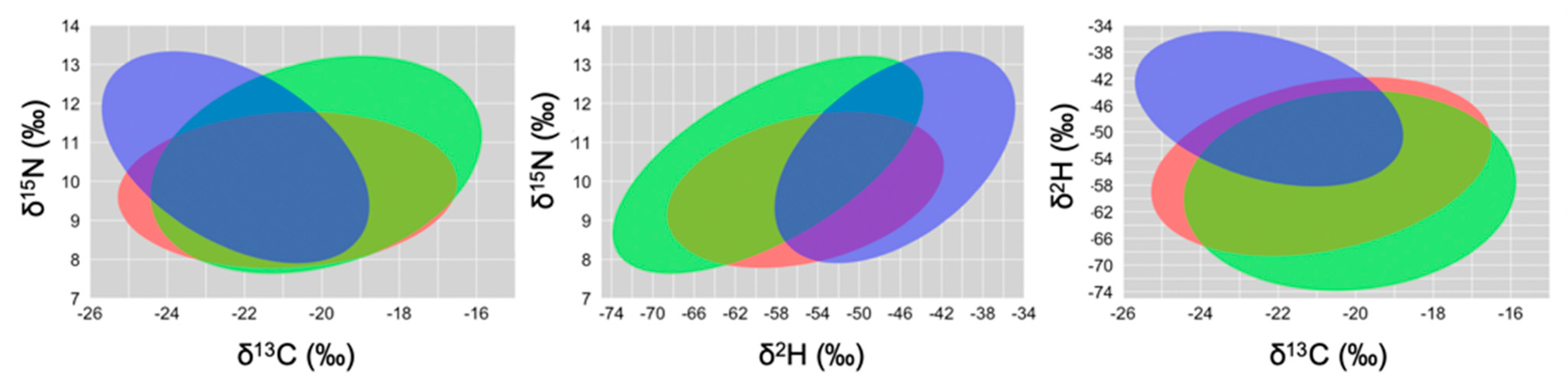
The herbivorous foraging guild had the largest SEA in all isotope biplots, followed by the granivorous guild, and the insectivorous guild had the smallest SEA (Figure 2, Table 4). Isotopic niche overlap between the insectivorous and each of the other two foraging guilds decreased when using δ2H values instead of δ15N (Table 4); segregation among foraging guilds (Figure 2 and Supplementary Figure S1) and among species (Supplementary Figure S2) was better when using δ2H values as well.
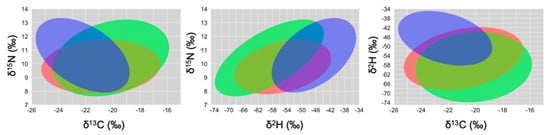
Figure 2.
Two-dimensional graphs based on δ13C~δ15N (left), δ2H~δ15N (center) and δ13C~δ2H (right), showing the isotopic niches of foraging guilds: granivorous finches (red; SGF and MGF), herbivorous finches (green/yellow; LGF, CF and VF), and insectivorous finches (blue; STF, GWF and WF). We followed the code proposed by Rossman et al. [33].

Table 4.
Standard ellipse areas (SEA) and ellipse areas corrected for small sample size (SEAc), and pairwise isotopic niche overlap between these foraging guilds.
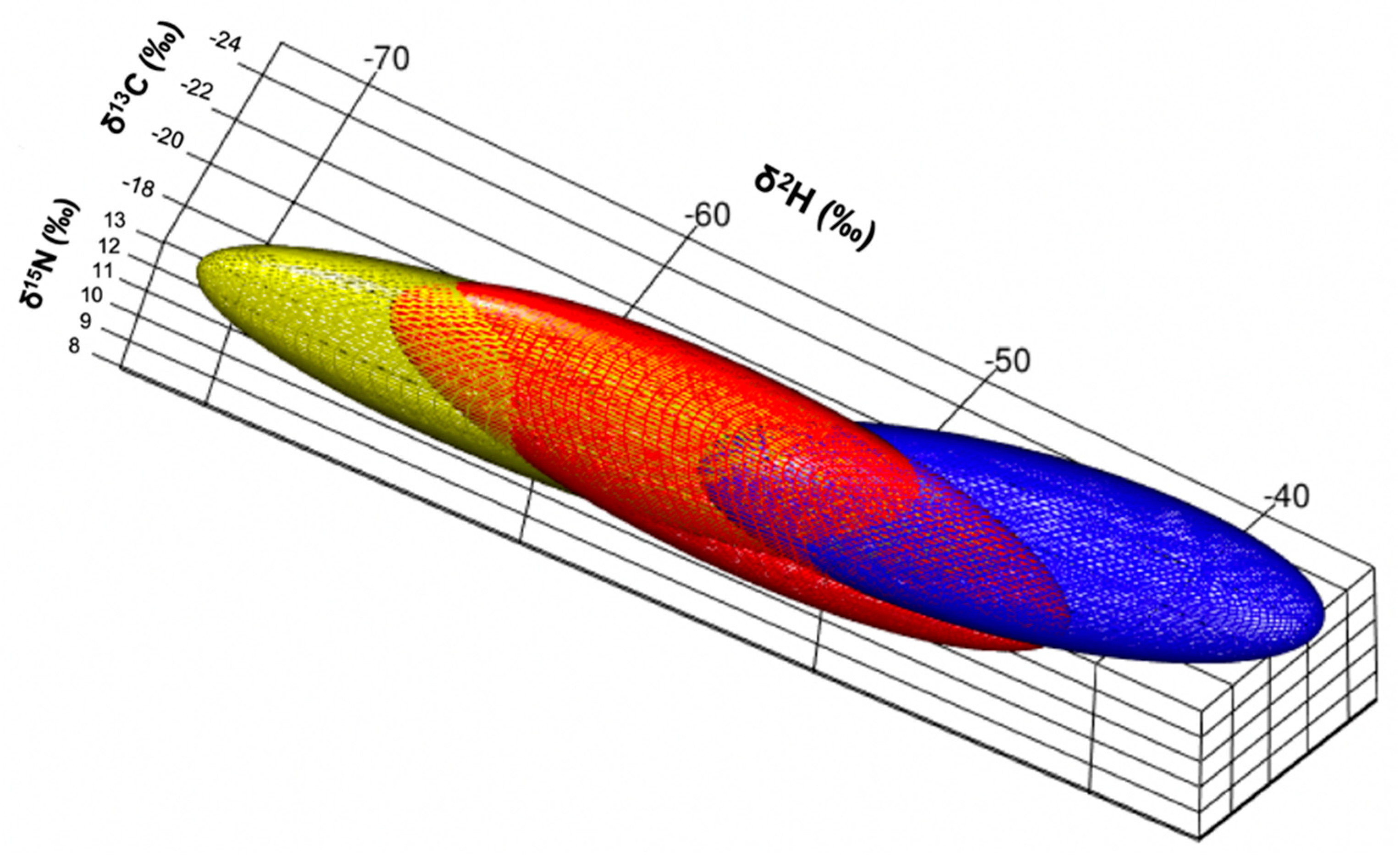
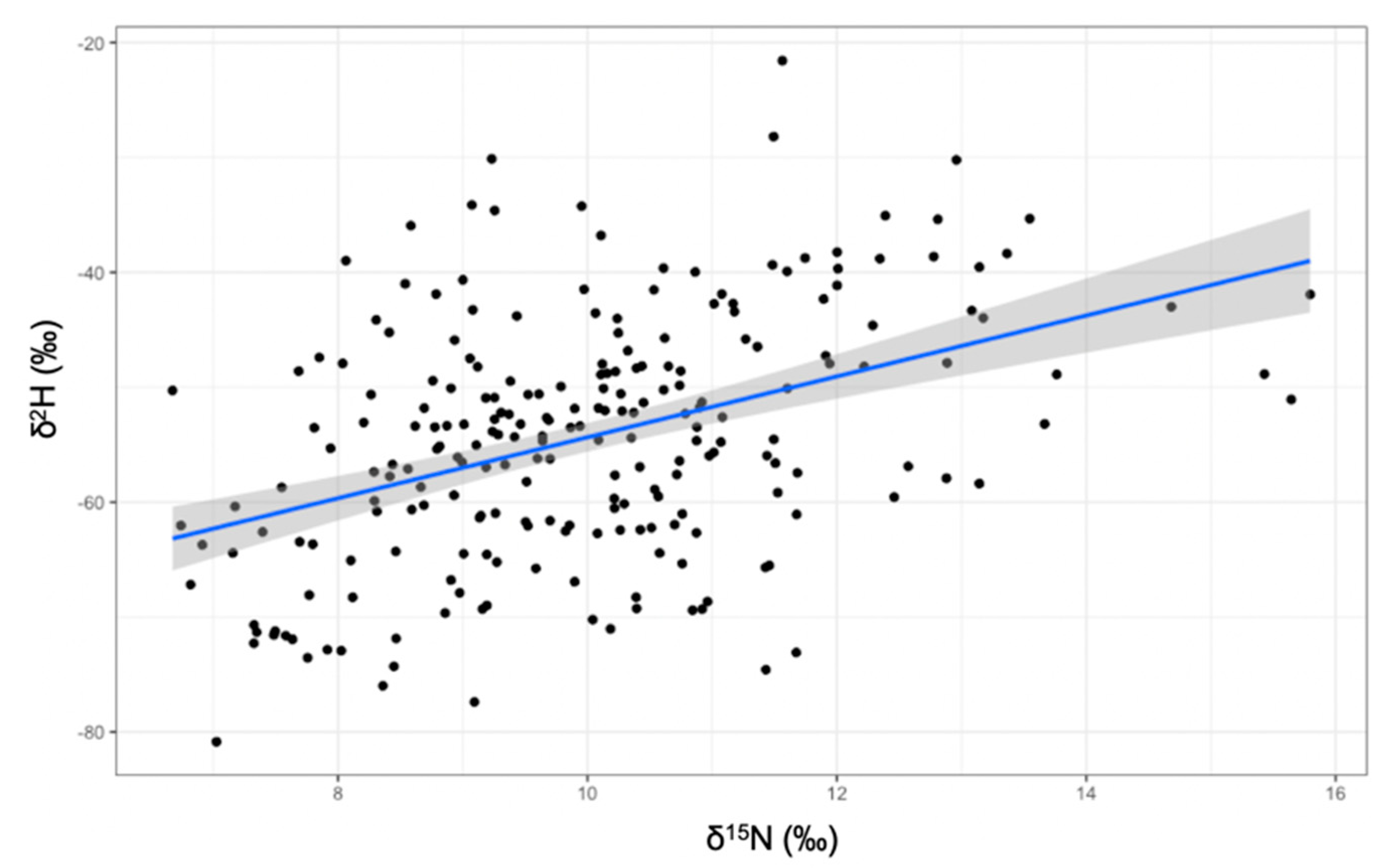
When incorporating δ2H values and examining isotopic niches for each guild as volumes (Figure 3), the herbivorous guild had the largest standard ellipse volume at a 97.5 credible interval (216‰3) followed by the granivorous guild (158‰3) and lastly, the insectivorous guild (138‰3). Distance between centroids was largest between herbivorous and insectivorous (0.92‰), then between insectivorous and granivorous (0.89‰), and lastly between granivorous and herbivorous finches (0.62‰). The only significant relationship between isotopes, was that between δ15N and δ2H values (Figure 4).
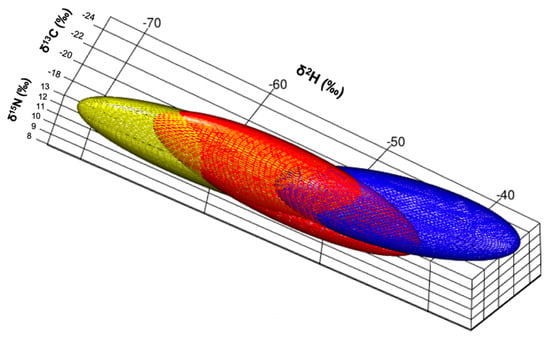
Figure 3.
Estimated isotopic niches for the three foraging guilds: granivorous (red; SGF and MGF), herbivorous (yellow; LGF, CF and VF) and insectivorous finches (blue; STF, GWF and WF) graphed in three dimensions (following the code proposed by Rossman et al. [33]).
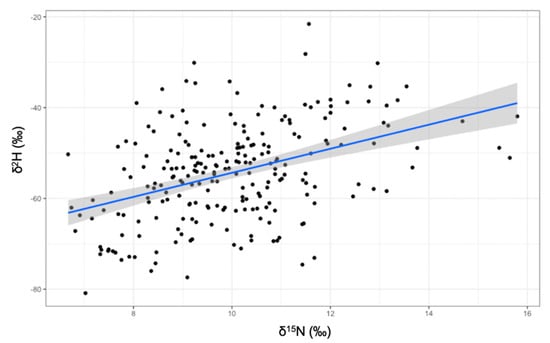
Figure 4.
Significant relationship between δ15N and δ2H values (F1,229 = 49.14, p < 0.001).
4. Discussion
Darwin’s Finches are considered a classic example of adaptive radiation and niche segregation [2,4,5] and so provided a compelling test of the stable isotope approach as a means of confirming or elucidating the degree to which expected biological/ecological niche segregation is mirrored in isotopic niche segregation [13,45,46]. Isotopic similarity among species and guilds by no means implies true biological similarity as we are at the mercy of how environmental drivers may differ isotopically. Indeed, we expect much overlap among diets that are trophically similar (δ15N, δ2H) or based on similar photosynthetic pathway (δ13C, δ2H) or microhabitat (δ15N, δ13C, δ2H). Nonetheless, stable isotopes are well suited to revealing biological segregation when they differ and so can illuminate factors resulting in true biological segregation that would otherwise remain cryptic [46]. Our multi-isotope approach suggested considerable isotopic overlap among species and guilds of Darwin’s finches on Santa Cruz Island at least during the period of feather molt [47].
Ours is the first characterization, as far as we are aware, of isotopic niches of Darwin’s finches. Michel et al. [48] examined isotopic composition of feathers of some species of finches; however, their study was focused on the gut microbiome of the Vampire Finch (Geospiza septentrionalis), for which a complementary stable isotope analysis revealed that its feathers had exceptionally high δ15N values, resembling top marine predators. Our investigation confirms that Darwin’s finches tend to be “imperfect generalists”, as suggested by De León et al. [6]. We provide evidence confirming that Darwin’s finches are opportunistic feeders and hence demonstrate large isotopic niche overlap across a number of sites and habitats (Table 1 and Table 3, Supplementary Figure S3). Darwin’s finches on Santa Cruz Island are composed primarily of granivorous and herbivorous foraging guilds, both with large isotopic niches (Figure 2 and Figure 3). Specifically, the granivorous foraging guild (formed by the small and medium ground finches, SGF and MGF), is the most abundant [30], and previous evidence shows that SGF consumes the highest proportion of plants and is the most important seed disperser on the island [40,49]. These ground finches (i.e., SGF and MGF) occur in several habitat types and so can consume resources that span multiple trophic levels [50] and can depend on a variety of food webs [37]. Similarly, a study examining the degree of niche overlap among three sympatric species of thrush (i.e., Turdus albicollis, T. amaurochalinus, T. rufiventris) in Brazil confirmed they were generalist species, partitioning some resources but coexisting at the same trophic positions [38]. Buelow et al. [51] also demonstrated that birds in a mangrove forest in Australia had more generalist and opportunistic foraging strategies than previously assumed, and they suggest that the environmental heterogeneity in resource availability results in such strategies. Environmental heterogeneity [37], often linked to variation in precipitation on the Galápagos [2,8] may also be an important factor driving niche overlap of Darwin’s finches on Santa Cruz Island. Feathers of live birds analyzed in this research were likely formed after the breeding season in 2018 and 2019, around April-June [47]. This period constitutes the transition from the wet to the dry season (Supplementary Figure S3 shows precipitation in Santa Cruz Island from Dec 2017 to June 2019 [7,52]), at which time food availability decreases and this in turn, tends to increase diet overlap in Darwin’s finches [6,7]. Additionally, an El Niño event occurred from 2018–2019 [53], which presumably affected our feather isotope data. During El Niño years, the surface ocean around Galápagos warms, and this considerably increases rainfall in the archipelago [54]. This combination of factors, along with the variable precipitation patterns in Galápagos in general, together may have contributed to the strong isotopic niche overlap we found and further studies during different phases of ENSO (El Niño Southern Oscillation) are encouraged.
While tissue δ2H values have been largely used for tracking migratory animals due to the close correspondence with hydrogen precipitation isoscapes [25,55], recent studies have shown that microhabitat differences (e.g., forest interior versus coffee plantation) were good predictors of avian δ2H values [56] or for identifying aquatic and terrestrial food webs [57]. Here we found that feather δ2H values differentiated among foraging guilds and trophic positions (Figure 2 and Figure 3, Supplementary Figures S1 and S2 [58]). Similarly, previous studies in Central America with birds [56] and bats [59] have shown that secondary consumers had higher tissue δ2H values than primary consumers. The lack of an increase in δ15N values from granivorous (or herbivorous) to insectivorous finches was surprising but we note that this may be linked to the inclusion of agricultural sites in our study whereby nitrogen from a variety of inputs to the foodweb was possible (see below). Conversely, we did find a significant increase in δ2H values (58–46‰) across this trophic gradient (but see [60]). These results highlight the potential application of δ2H values for studying habitat use and dietary specialization in animal communities, especially in areas where there is high sympatry and niche overlap [38]. However, a challenging characteristic of using δ2H values in this way is that the isotopic composition of consumer tissues reflect a blend of dietary and body water isotopic compositions that can vary considerably due to abiotic factors affecting environmental waters [26,61]. Here, we confirmed, for example, that both feather δ13C and δ2H values significantly decreased with altitude [62]. Local hydrological patterns must be considered and accounted for, then, in studies that use δ2H values as a proxy of trophic position.
Of considerable interest in community ecology is understanding the degree of individual dietary variation within species. Isotopic groups with high dietary diversity is consistent with individual dietary specialization [51]. Individual dietary variation is driven by several factors (e.g., sex, species, location, etc.) and stable isotope data can be definitive or ambiguous. Individuals that have different tissue stable isotope values will differ in location or type of diet but those with similar isotope values may also differ in these factors [63]. Anthropogenic factors on Santa Cruz Island clearly affect diet of ground finches and this, in turn, has been implicated in decreasing niche segregation among species [64]. During environmentally benign periods, finches tend to consume foods that are easily accessible and under such conditions, high dietary overlap is expected [64]. In the case of the MGF, human impacts such as habitat alteration, introduction of non-native plants and provisioning of foods are changing finch diets and consequently the adaptive landscape for beak size [65].
We only found difference among habitats for feather δ13C values (Table 1 and Table 3). However, farms had higher δ15N values than other sites, and the two farms differed in this isotope. Farms grew crops of coffee and citrus fruits. For some species of Darwin’s finches, feather samples at Farm B had higher δ13C and δ15N values than Farm A. The farm A is organic and does not use fertilizers or herbicides, whereas at Farm B, inorganic fertilizer is spread on crops, which is known to result in higher foodweb δ15N values [19,20,66,67]. As noted above, this could have influenced our results of δ15N values and their failure to discriminate among foraging guilds. However, when excluding Farm B from the analysis, results on δ15N values among foraging guilds were the same (results not included). The strong relationship between δ15N and δ2H values (Figure 4) further supports our use of δ2H values as an alternative and additional means of determining isotopic niches of foraging guilds. When examining difference in feather δ13C, δ15N and δ2H values among guilds within each habitat, we found that the insectivorous guild differed from the other guilds, especially for the humid zone (Supplementary Table S4). This supports our findings that insectivorous finches are more differentiated from the other guilds, and also that granivorous and herbivorous finches are mainly composed of generalist species which tend to dominate the avian community on Santa Cruz Island. It is also likely that the humid zone provides different resources for Darwin’s finches that are not available in other habitats, given the presence of native vegetation patches and its higher altitude and precipitation [29,31].
For our retrospective study involving museum specimens, we did find that feathers of museum specimens had significantly higher δ13C and δ15N values than feathers from live birds. This could suggest a possible trophic shift over time [39,67], however, due to our small sample size we cannot say if there has been climatic variation over time or some other human factor affecting isotope values, hence further sampling of museum specimens of multiple Darwin’s finch species is recommended.
We conclude that Darwin’s finches are mainly generalist and opportunistic species, a finding largely consistent with more recent dietary considerations of these species [6,64]. We clearly demonstrate that stable isotope analysis constitutes a useful tool for studying variation in diet and ecological segregation among sympatric bird species because it provides information on dimensions rarely explored with just observational purposes. Future studies in the Galápagos should compare feather samples from an island with high human disturbance, such as Santa Cruz, with feathers from an island with no or with very little human disturbance, such as Fernandina or Santiago. Such future studies should also examine isotopic values of local foodwebs as well as birds, such analyses will provide a more powerful analysis of ecological segregation among species.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/d13040147/s1, Table S1: Total numbers of individuals of Darwin’s finches sampled in four different habitats in Santa Cruz Island and from museum specimens, Table S2: Museum collections at the Vertebrate Collection of the Charles Darwin Research Station (VCCDRS) in Puerto Ayora, Santa Cruz Island, Table S3: Best generalized linear models (GLM) that explain variation in feather δ13C, δ15N and δ2H values in each dataset analyzed: (A) Live birds (N = 231), and (B) Samples with live and museum data (N = 85). Significant parameters are in bold, Table S4: Differences among foraging guilds within each habitat. Significant guilds are in bold, Figure S1: Standard ellipse areas (SEA) based on δ13C~δ15N (A) and δ13C~δ2H (B), Figure S2: Biplots that show the trophic position of each Darwin’s finch species, with the Y axis given by δ15N values (upper graph) or δ2H values (lower graph). Figure S3: Monthly precipitation (mean ± SD) from December 2017 to June 2019, based on data from the two climatological stations on Santa Cruz Island (Puerto Ayora at 2 masl, and Bella Vista at 223 masl; [52]). This time frame covers breeding seasons and molt periods of feathers of live birds analyzed in this research. Breeding season in Darwin’s finches starts around December when precipitation is higher, and molt after the breeding season, around April to June [47].
Author Contributions
Conceptualization, K.A.H., M.V. and C.S.; methodology, M.V. and K.A.H.; field work, M.V., C.S. and G.J.-U.; laboratory analyses, S.M.; resources, K.A.H., C.S. and G.J.-U.; data curation and analysis, M.V.; writing—original draft preparation, M.V. and K.A.H.; writing—review and editing, M.V., C.S., G.J.-U., S.M. and K.A.H.; supervision, K.A.H.; project funding, K.A.H. and C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by K.A. Hobson, Stable Isotope fund #108402, University of Saskatchewan, and by Environment and Climate Change Canada. No additional funding was received.
Institutional Review Board Statement
Bird sampling was conducted in accordance with the IACUC approved by the Animal Research Ethics Board (AREB) at the University of Saskatchewan (Animal Use Protocol #20190050). All procedures followed the Guidelines for the Use of Animals in Research (Charles Darwin Research Station, Government of Ecuador, and Galápagos National Park).
Data Availability Statement
The data presented in this study are stored in Dryad at https://doi.org/10.5061/dryad.nzs7h44r2, accessed on 1 March 2021.
Acknowledgments
We thank Joel Alava and Tatiana Torres for their much appreciated assistance in field work. We thank Jamille Macleod for her administrative and laboratory help, and Blanca X. Mora-Alvarez for assistance with stable isotope analyses. We are very grateful to the staff at the Charles Darwin Research Station for their logistical support and the opportunity to work in Galápagos National Park. We also thank farm owners, Maria Elena Guerra and Lorenzo Freire, for their time and for permission to work at their farms. This investigation was conducted thanks to the research permit DPNG PC-03-19, and thanks to the valuable assistance of park guards and staff from the Dirección del Parque Nacional Galápagos (DPNG). For their crucial help in one way or another, we thank M. Romoleroux, M. Cruz, U. Ellecosta, F. Echeverría, I. Peña, D. Nuñez, C. Sevilla, F. Gaona, and C. Cevallos. Finally, we thank the Agencia de Regulación y Control de la Bioseguridad y Cuarentena para Galápagos (ABG) and Canadian Food Inspection Agency (CFIA) for providing permits. This publication is contribution number 2388 of the Charles Darwin Foundation for the Galápagos Islands.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grant, P.R. Ecology and Evolution of Darwin’s Finches; Princeton University Press: Princeton, NJ, USA, 1999. [Google Scholar]
- Grant, P.R.; Grant, B.R. Adaptive radiation of Darwin’s finches: Recent data help explain how this famous group of Galapagos birds evolved, although gaps in our understanding remain. Am. Sci. 2002, 130, 1–7. [Google Scholar]
- Tebbich, S.; Stereln, K.; Teschke, I. The tale of the finch: Adaptive radiation and behavioural flexibility. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Farrington, H.L.; Lawson, L.P.; Clark, C.M.; Petren, K. The evolutionary history of Darwin’s Finches: Speciation, gene flow, and introgression in a fragmented landscape. Evolution 2014, 68, 2932–2944. [Google Scholar] [CrossRef] [PubMed]
- Lamichhaney, S.; Berglund, J.; Almén, M.S.; Maqbool, K.; Grabherr, M.; Martínez-Barrio, A.; Promerová, M.; Rubin, C.-J.; Wang, C.; Zamani, N.; et al. Evolution of Darwin’s finches and their beaks revealed by genome sequencing. Nature 2015, 518, 371–377. [Google Scholar] [CrossRef] [PubMed]
- De León, L.F.; Podos, J.; Gardezi, T.; Herrel, A.; Hendry, A.P. Darwin’s finches and their diet niches: The sympatric coexistence of imperfect generalists. J. Evol. Biol. 2014, 27, 1093–1104. [Google Scholar] [CrossRef]
- Smith, J.N.M.; Grant, P.R.; Grant, B.R.; Abbott, I.J.; Abbott, L.K. Seasonal variation in feeding habits of Darwin’s ground finches. Ecology 1978, 59, 1137–1150. [Google Scholar] [CrossRef]
- Boag, P.T.; Grant, P.R. Darwin’s finches (Geospiza) on Isla Daphne Major, Galapagos: Breeding and feeding ecology in a climatically variable environment. Ecol. Monogr. 1984, 54, 463–489. [Google Scholar] [CrossRef]
- Christensen, R.; Kleindorfer, S. Jack-of-all-trades or master of one? Variation in foraging specialisation across years in Darwin’s Tree Finches (Camarhynchus spp.). J. Ornithol. 2009, 150, 383–391. [Google Scholar] [CrossRef]
- Grant, B.R.; Grant, P.R. What Darwin’s Finches can teach us about the evolutionary origin and regulation of biodiversity. Bioscience 2003, 53, 965–975. [Google Scholar] [CrossRef]
- Bearhop, S.; Adams, C.E.; Waldron, S.; Fuller, R.A.; Macleod, H. Determining trophic niche width: A novel approach using stable isotope analysis. J. Anim. Ecol. 2004, 73, 1007–1012. [Google Scholar] [CrossRef]
- West, J.B.; Bowen, G.J.; Cerling, T.E.; Ehleringer, J.R. Stable isotopes as one of nature’s ecological recorders. Trends Ecol. Evol. 2006, 21, 408–414. [Google Scholar] [CrossRef]
- Newsome, S.D.; Martinez del Rio, C.; Bearhop, S.; Phillips, D.L. A Niche for Isotope Ecology. Front. Ecol. Environ. 2007, 5, 429–436. [Google Scholar] [CrossRef]
- Sulzman, E.W. Stable Isotope Chemistry and Measurement: A primer. In Stable Isotopes in Ecology and Environmental Science; Michener, R.H., Lajtha, K., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 1–18. [Google Scholar]
- McLoughlin, P.D.; Lysak, K.; Debeffe, L.; Perry, T.; Hobson, K.A. Density-dependent resource selection by a terrestrial herbivore in response to sea-to-land nutrient transfer by seals. Ecology 2016, 97, 1929–1937. [Google Scholar] [CrossRef] [PubMed]
- Wiley, A.E.; James, H.F.; Ostrom, P.H. Emerging Techniques for Isotope Studies of Avian Ecology. In The Extended Specimen: Emerging Frontiers in Collections-Based Ornithological Research; Webster, M.S., Ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 89–109. [Google Scholar]
- Kelly, J.F. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. Can. J. Zool. 2000, 78, 1–27. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Kendall, C.; Elliott, E.M.; Wankel, S.D. Tracing anthropogenic inputs of nitrogen to ecosystems. In Stable Isotopes in Ecology and Environmental Science, 2nd ed.; Michener, R., Lajtha, K., Eds.; Blackwell Publishing: Oxford, UK, 2007; pp. 375–449. [Google Scholar]
- Fairhurst, G.D.; Vögeli, M.; Serrano, D.; Delgado, A.; Tella, J.L.; Bortolotti, G.R. Can synchronizing feather-based measures of corticosterone and stable isotopes help us better understand habitat-physiology relationships? Oecologia 2013, 173, 731–743. [Google Scholar] [CrossRef]
- Bowen, G.J. Isoscapes: Spatial pattern in isotopic biogeochemistry. Annu. Rev. Earth Planet. Sci. 2010, 38, 161–187. [Google Scholar] [CrossRef]
- Bowen, G.J.; West, J.B. Isotope landscapes for terrestrial migration research. In Tracking Animal Migration with Stable Isotopes; Hobson, K.A., Wassenaar, L.I., Eds.; Elsevier Inc.: Saskatoon, SK, Canada, 2008; pp. 89–105. [Google Scholar]
- Hong, P.; Wiley, D.N.; Powers, K.D.; Michener, R.H.; Kaufman, L.; Hatch, K.A. Stable Isotope analyses of multiple tissues of Great Shearwaters (Ardenna gravis) reveals long-term dietary stability, short-term changes in diet, and can be used as a tool to monitor food webs. Diversity 2019, 11, 163. [Google Scholar] [CrossRef]
- Rubenstein, D.R.; Hobson, K.A. From birds to butterflies: Animal movement patterns and stable isotopes. Trends Ecol. Evol. 2004, 19, 256–263. [Google Scholar] [CrossRef]
- Wassenaar, L.I.; Hobson, K.A. Stable-carbon and hydrogen isotope ratios reveal breeding origins of red-winged blackbirds. Ecol. Appl. 2000, 10, 911–916. [Google Scholar] [CrossRef]
- Vander Zanden, H.B.; Soto, D.X.; Bowen, G.J.; Hobson, K.A. Expanding the isotopic toolbox: Applications of hydrogen and oxygen stable isotope ratios to food web studies. Front. Ecol. Evol. 2016, 4, 1–19. [Google Scholar] [CrossRef]
- Newsome, S.D.; Sabat, P.; Wolf, N.; Rader, J.A.; Martinez del Rio, C. Multi-tissue δ2H analysis reveals altitudinal migration and tissue-specific discrimination patterns in Cinclodes. Ecosphere 2015, 6, 1–18. [Google Scholar] [CrossRef]
- Hobson, K.A.; Wassenaar, L.I.; Milá, B.; Lovette, I.; Dingle, C.; Smith, T.B. Stable isotopes as indicators of altitudinal distributions and movements in an Ecuadorean hummingbird community. Oecologia 2003, 136, 302–308. [Google Scholar] [CrossRef]
- Snell, H.M.; Stone, P.A.; Snell, H.L. A summary of geographical characteristics of the Galápagos Islands. J. Biogeog. 1996, 23, 619–624. [Google Scholar] [CrossRef]
- Dvorak, M.; Fessl, B.; Nemeth, E.; Kleindorfer, S.; Tebbich, S. Distribution and abundance of Darwin’s finches and other land birds on Santa Cruz Island, Galápagos: Evidence for declining populations. Oryx 2011, 46, 78–86. [Google Scholar] [CrossRef]
- Rivas-Torres, G.F.; Benítez, F.L.; Rueda, D.; Sevilla, C.; Mena, C.F. A methodology for mapping native and invasive vegetation coverage in archipelagos: An example from the Galápagos Islands. Profress Phys. Geogr. 2018, 42, 83–111. [Google Scholar] [CrossRef]
- Herrera, L.G.; Hobson, K.A. Trophic partitioning in tropical rain forest birds: Insights from stable isotope analysis. Oecologia 2003, 136, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Rossman, S.; Ostrom, P.H.; Gordon, F.; Zipkin, E.F. Beyond carbon and nitrogen: Guidelines for estimating three-dimensional isotopic niche space. Ecol. Evol. 2016, 6, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Uzcátegui, G.; Vaca, L.; Cotín, J.; García, C.; Costales, A.; Sevilla, C.; Páez-Rosas, D. Using referential values of δ13C and δ15N to infer the foraging ecology of Galápagos seabirds. Marine Ornithol. 2019, 47, 5–10. [Google Scholar]
- Hette-Tronquart, N. Isotopic niche is not equal to trophic niche. Ecol. Lett. 2019. [Google Scholar] [CrossRef] [PubMed]
- Yeakel, J.D.; Bhat, U.; Elliott Smith, E.A.; Newsome, S.D. Exploring the isotopic niche: Isotopic variance, physiological incorporation, and the temporal dynamics of foraging. Front. Ecol. Evol. 2016, 4. [Google Scholar] [CrossRef]
- Rader, J.A.; Newsome, S.D.; Sabat, P.; Chesser, R.T.; Dillon, M.E.; Martinez del Río, C. Isotopic niches support the niche breadth hypothesis. J. Anim. Ecol. 2017, 86, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Bosenbecker, C.; Bugoni, L. Trophic niche similarities of sympatric Turdus thrushes determined by fecal contents, stable isotopes and bipartite network approaches. Ecol. Evol. 2020, 10, 9073–9084. [Google Scholar] [CrossRef]
- English, P.A.; Green, D.J.; Nocera, J.J. Stable isotopes from museum specimens may provide evidence of long-term change in the trophic ecology of a migratory aerial insectivore. Front. Ecol. Environ. 2018, 6, 1–13. [Google Scholar] [CrossRef]
- Guerrero, A.M.; Tye, A. Darwin’s Finches as Seed Predators and Dispersers. Wilson J. Ornithol. 2009, 121, 752–764. [Google Scholar] [CrossRef]
- Kleindorfer, S.; Fessl, B.; Peters, K.; Achundia, D. Guía de Campo: Aves Terrestres Residentes de Galápagos; Fundación Charles Darwin para las Islas Galápagos: Puerto Ayora, Ecuador, 2019. [Google Scholar]
- Wassenaar, L.I.; Hobson, K.A. Comparative equilibration and online technique for determination of non-exchangeable hydrogen of keratins for use in animal migration studies. Isotopes Environ. Health Stud. 2003, 39, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER—Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Dammhahn, M.; Goodman, S. Trophic niche differentiation and microhabitat utilization revealed by stable isotope analyses in a dry forest bat assemblage at Ankarana, northern Madagascar. J. Trop. Ecol. 2014, 30, 97–109. [Google Scholar] [CrossRef]
- Shipley, O.N.; Matich, P. Studying animal niches using bulk stable isotope ratios: An updated synthesis. Oecologia 2020. [Google Scholar] [CrossRef]
- Snow, D.W. Moult and the breeding cycle in Darwin’s finches. J. Ornithol. 1966, 107, 283–291. [Google Scholar] [CrossRef]
- Michel, A.J.; Ward, L.M.; Goffredi, S.K.; Dawson, K.S.; Baldassarre, D.T.; Brenner, K.; Gotanda, K.A.; McCormack, J.E.; Mullin, S.W.; O’Neill, A.; et al. The gut of the finch: Uniqueness of the gut microbiome of the Galápagos vampire finch. Microbiome 2018, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Heleno, R.H.; Olesen, J.M.; Nogales, M.; Vargas, P.; Traveset, A. Seed dispersal networks in the Galápagos and the consequences of alien plant invasions. Proc. R. Soc. B Biol. Sci. 2013, 280, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, K.; Bozinovic, F.; Newsome, S.D.; Sabat, P. Testing the niche variation hypothesis in a community of passerine birds. Ecology 2017, 98, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Buelow, C.A.; Reside, A.E.; Baker, R.; Sheaves, M. Stable isotopes reveal opportunistic foraging in a spatiotemporally heterogenous environment: Bird assemblages in mangrove forests. PLoS ONE 2018, 13, e0206145. [Google Scholar] [CrossRef]
- Charles Darwin Foundation. Available online: https://www.darwinfoundation.org/en/datazone/climate (accessed on 13 January 2021).
- National Geographic. 2019 May Be the Hottest Year Yet—Here’s Why. Available online: https://www.nationalgeographic.com/environment/article/2019-may-be-hottest-year-yet-el-nino-climate-change (accessed on 15 March 2021).
- Sachs, J.P.; Ladd, S.M. Climate and oceanography of the Galapagos in the 21st century: Expected changes and research needs. Galapagos Res. 2010, 67, 50–54. [Google Scholar]
- Hobson, K.A. Tracing origins and migration of wildlife using stable isotopes: A review. Oecologia 1999, 120, 314–326. [Google Scholar] [CrossRef]
- Fraser, K.C.; McKinnon, E.A.; Diamond, A.W.; Chavarría, L. The influence of microhabitat, moisture and diet on stable-hydrogen isotope variation in a Neotropical avian food web. J. Trop. Ecol. 2011, 27, 563–572. [Google Scholar] [CrossRef]
- Voigt, C.C.; Lehmann, D.; Greif, S. Stable isotope ratios of hydrogen separate mammals of aquatic and terrestrial food webs. Methods Ecol. Evol. 2015, 6, 1332–1340. [Google Scholar] [CrossRef]
- Quezada-Romegialli, C.; Jackson, A.L.; Hayden, B.; Kahilainen, K.K.; Lopes, C.; Harrod, C. tRophicPosition, an r package for the Bayesian estimation of trophic position from consumer stable isotope ratios. Methods Ecol. Evol. 2018, 9, 1592–1599. [Google Scholar] [CrossRef]
- Voigt, C.C.; Schneeberger, K.; Luckner, A. Ecological and dietary correlates of stable hydrogen isotope ratios in fur an body water of syntopic tropical bats. Ecology 2013, 94, 346–355. [Google Scholar] [CrossRef]
- Peters, J.M.; Wolf, N.; Stricker, C.A.; Collier, T.R.; Martínez del Rio, C. Effects of trophic level and metamorphosis on discrimination of hydrogen isotopes in a plant-herbivore system. PLoS ONE 2012, 7, e32744. [Google Scholar] [CrossRef]
- Magozzi, S.; Vander Zanden, H.B.; Wunder, M.B.; Bowen, G.J. Mechanistic model predicts tissue–environment relationships and trophic shifts in animal hydrogen and oxygen isotope ratios. Oecologia 2019, 191, 777–789. [Google Scholar] [CrossRef]
- Poage, M.A.; Chamberlain, P. Empirical relationships between elevation and the stable isotope composition of precipitation and surface waters: Considerations for studies of paleoelevation change. Am. J. Sci. 2001, 301, 1–15. [Google Scholar] [CrossRef]
- Bond, A.L.; Jardine, T.D.; Hobson, K.A. Multi-tissue stable-isotope analyses can identify dietary specialization. Methods Ecol. Evol. 2016, 7, 1428–1437. [Google Scholar] [CrossRef]
- De León, L.F.; Sharpe, D.M.T.; Gotanda, K.M. Urbanization erodes niche segregation in Darwin’s finches. Evol. Appl. 2018. [Google Scholar] [CrossRef] [PubMed]
- Hendry, A.P.; Grant, P.R.; Grant, B.R.; Ford, H.A.; Brewer, M.J.; Podos, J. Possible human impacts on adaptive radiation: Beak size bimodality in Darwin’s finches. Proc. R. Soc. B 2006, 273, 1887–1894. [Google Scholar] [CrossRef]
- Hobson, K.A.; Slater, G.L.; Lank, D.B.; Milner, R.L.; Gardiner, R. Agricultural lands subsidize winter diet of the dunlin at two major estuaries. Condor 2013, 115, 515–524. [Google Scholar] [CrossRef]
- Mason, N.A.; Unitt, P.; Sparks, J.P. Agriculture induces isotopic shifts and niche contraction in Horned Larks (Eremophila alpestris) of the Colorado Desert. J. Ornithol. 2020. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).