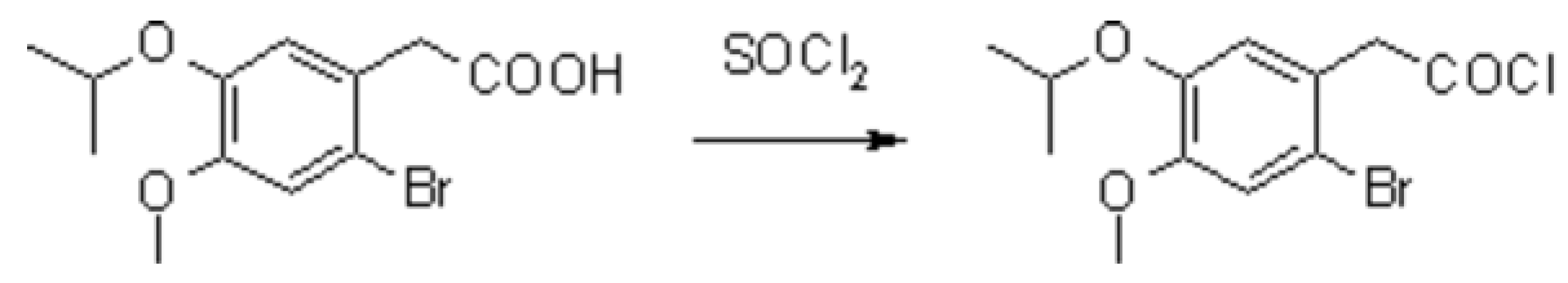
2-Bromo-4-methoxy-5-(1-methylethoxy)benzeneacetic acid [1] (4.00 g, 13.2 mmol) and DMF (100 µL) were stirred in thionyl chloride (40 mL) for 3 h at ambient temperature. The mixture was concentrated in vacuo, and the residue was purified by kugelrohr distillation (100 °C/0.01 mbar) and triturated with dry petroleum ether (3 x 20 mL). Yield: yellow crystals (4.24 g, 99%); mp. 55 - 57 °C.
TLC: petroleum ether : EtOAc = 80 : 20, Rf = 0.6 [2].
Anal. Calcd for C12H14BrClO3: C, 44.82; H, 4.39. Found: C, 44.57; H, 4.67.
1H NMR (CDCl3): δ 7.03 (s, 1H), 6.80 (s, 1H), 4.48 (septet, J = 6.7 Hz, 1H), 4.21 (s, 2H), 3.81 (s, 3H), 1.32 (d, J = 6.7 Hz, 6H).
13C NMR (CDCl3): δ 170.9 (s), 151.0 (s), 146.7 (s), 123.5 (s), 118.5 (d), 116.0 (d), 115.4 (s), 71.9 (d), 56.0 (q), 52.6 (t), 21.8 (q).
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Treu, M.; Jordis, U. Molbank 2002, M294.
- Converted into the ethyl ester for TLC analysis by quenching with dry EtOH.
- Samples Availability: Available from the authors.
© 2003 MDPI. All rights reserved.