Abstract
Allergen sensitization profiles are increasingly affected by environmental and climate changes. This study exemplifies fundamental differences in molecular IgE sensitization profiles in two nearby regions in Argentina with different climatic conditions (La Plata and Bahía Blanca). A cross-sectional study was conducted involving 155 patients with allergic symptoms from La Plata and Bahía Blanca (34.0 ± 11.2 years, female/male: 83/72). Serum samples were analyzed for IgE reactivity using a chip containing 101 micro-arrayed allergen molecules. Statistical analyses were performed to compare allergen-specific IgE levels, sensitization prevalences and reported symptoms. Patients from La Plata—with subtropical weather—showed a higher prevalence of IgE reactivity to house dust mite (HDM) allergens (Der p 23: 74%; Der p 1: 53% and Der p 2: 56%) and more frequently reported asthma (AS) symptoms (40% vs. 24%) than patients from Bahía Blanca. In contrast, patients from Bahía Blanca, with dry and windy weather, exhibited higher sensitization rates to pollen allergens, particularly Phl p 1 (49%) and Ole e 1 (22%) as well as to Alternaria alternata (Alt a 1, 35%) and reported a significantly higher prevalence of skin manifestations (54% vs. 31%) than those from La Plata. Cat allergen Fel d 1 was an equally important sensitizer in both regions (La Plata 30% and Bahía Blanca 37%). Sensitization to class 1 food allergens was rare in both groups (1–8%), including non-specific lipid transfer proteins (peanut Ara h 9 and peach Pru p 3) but IgE sensitizations to genuine peanut allergens were almost absent. Important regional differences in allergen sensitization profiles were observed between two geographically close regions with different climatic conditions. Our findings underscore the relevance of region-specific allergen profiling and highlight the clinical utility of molecular allergy diagnosis for a more precise allergen identification and improved management of allergic diseases.
1. Introduction
The global increase in allergic diseases is a major public health concern, with growing evidence that environmental changes play a key role. The rise in atmospheric CO2 levels has led to higher pollen yields, extended pollen seasons, and the expansion of fungi and HDMs due to increased surface humidity [1,2,3,4,5]. These changes disproportionally affect regions in the tropics and subtropics, including Argentina, which exhibits considerable climatic diversity. According to the Köppen–Geiger system classification, Argentina comprises eleven different climate types and six different climatic regions [6]. This study focuses on two major cities, La Plata and Bahía Blanca, which lie only 570 km apart, but in different climatic zones, in close proximity to the South Atlantic Ocean. La Plata is located in the humid pampas characterized by temperate weather and the absence of a dry season and hot summers (Cfa), whereas Bahía Blanca lies in dry pampas, with arid steppe conditions, windy, dry weather and colder average temperatures (BSk) (Figure 1).
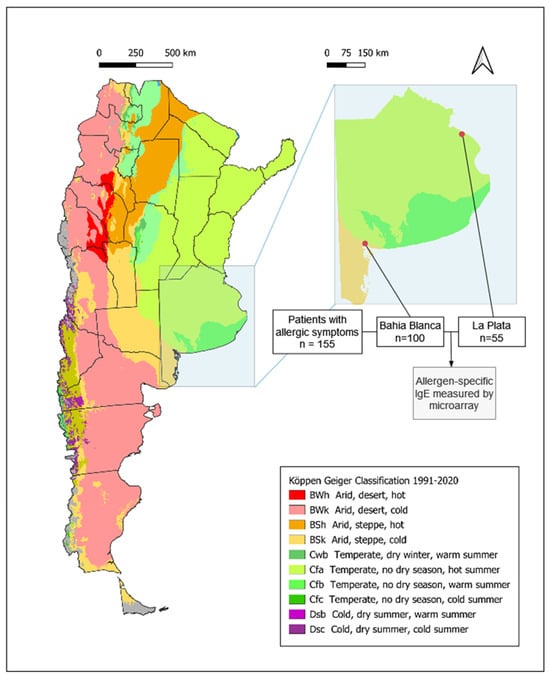
Figure 1.
Flow chart illustrating the process of patient selection and involvement in the analyses for our study. Köpper–Geiger climate classification map for Argentina (1991–2020) modified according to Beck HE et al. [6] Demographic and clinical characterization of patients. Number and percentage of patients reporting symptoms of rhinoconjunctivitis, cutaneous manifestations and asthma within the specific regions of Bahía Blanca, La Plata, and the total study population. Symptoms were assessed using the ISAAC questionnaire.
Despite the known burden of allergic diseases, such as asthma and rhinoconjunctivitis in Argentina, no studies to date have used molecular allergy diagnosis [7] to analyze allergen-specific IgE sensitization in this region or even in Latin America [8,9,10]. Previous studies on allergic sensitization in Argentina and Latin America have relied primarily on allergen extract-based assays [11,12]. However, accurate identification of disease-eliciting allergen molecules is necessary for allergen-specific management of allergies, such as allergen-specific immunotherapy (AIT) or allergen avoidance [13].
This study is the first to apply an in-house-developed allergen microarray to assess molecular IgE sensitization patterns in adult allergic patients from two cities in Argentina, linking allergen-specific IgE profiles to clinical symptoms. We provide detailed insights into the regional variations in molecular sensitization to respiratory and food allergens, which are critical for improving the diagnosis and management of allergic diseases in Argentina. Importantly, our study reveals profound differences in two nearby areas due to regional climatic differences.
2. Results
2.1. Demographic and Clinical Characterization of the Study Population
The study included adolescents and adults with allergic symptoms, aged 15–62 years (mean 34.0 years). Serum samples were collected from patients attending allergy centers in La Plata (n = 55) and Bahía Blanca (n = 100) (Figure 1, Table S1). The gender distribution was comparable between regions, with a slight predominance of females (82 females and 73 males overall). Bahía Blanca is situated at a latitude of −38.71° in an arid steppe (Bsk) climatic zone, whereas La Plata, located further north at a latitude of −34.92°, belongs to the humid subtropical (Cfa) climatic region (Figure 1).
Participants completed the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire, which provided data on allergic symptoms. The most frequently reported symptom was rhinoconjunctivitis (RC), with similar frequencies in both regions (93.0% in Bahía Blanca and 90.9% in La Plata). However, skin manifestations (D) were more frequent in Bahía Blanca (54.0%) compared to La Plata (30.9%). Conversely, asthma (AS) was more prevalent in La Plata (40.0%) than in Bahía Blanca (24.0%) (Figure 1).
Sera from all participants were analyzed for allergen-specific IgE reactivity using an in-house developed allergen microarray (Table S2). Overall, 78.0% of participants (121/155) exhibited detectable IgE levels (cut-off ISU ≥ 0.1) to one or more allergens on the microarray (Figure 1). The number of allergens recognized per patient varied between regions, though differences were not statistically significant. In Bahía Blanca, 55.1% of sensitized patients recognized up to five respiratory allergens, 33.3% between six and ten, and 11.5% more than ten allergens. In La Plata, 51.2% of sensitized patients reacted to up to five respiratory allergens, 23.3% between six and ten, and 25.6% to more than ten allergens. These findings indicate a broader respiratory allergen sensitization profile in patients from La Plata (Figure S1).
2.2. Bahía Blanca and La Plata Show Different Molecular IgE Sensitization Patterns Associated with Different Climatic and Environmental Factors
IgE sensitization patterns of Bahía Blanca and La Plata revealed substantial differences, reflecting the distinct climatic and environmental conditions of the two regions. In La Plata, the most common IgE-reactive respiratory allergens were derived from HDM, with Der p 23 being the most frequently recognized allergen (74%), followed by Der p 2 (56%), Der p 1 (53%), Der p 7 (40%), Der p 21 (37%), Der p 5 (35%) and Blo t 21 (28%). Sensitization to pollen allergens, such as Phl p 1, was less common, namely 19% (Table S3). The most frequently recognized animal-derived allergen was cat major allergen Fel d 1, with a recognition frequency of 30%, whereas IgE sensitization to dog allergen molecules was quite rare in both regions (Figure 2 and Table S3).
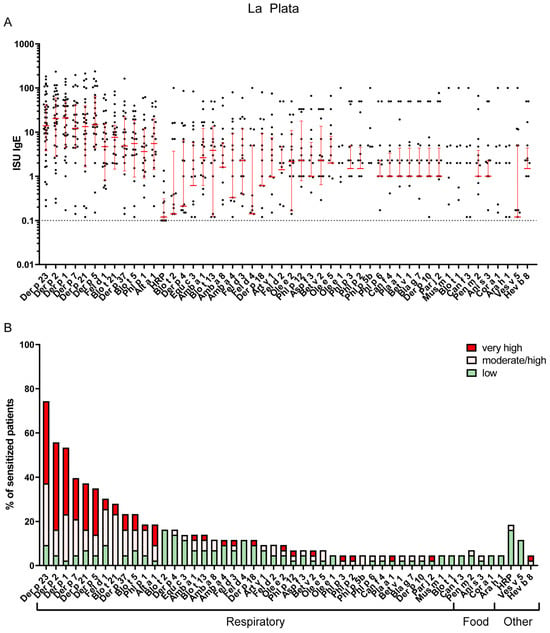
Figure 2.
(A) IgE levels of the most frequent sensitizers in La Plata. Error bars (red) indicate medians ± interquartile ranges of positive ISU values. Cutoff for positive results (>=0.1) is indicated by a dashed horizontal line. Values below the cutoff are not shown. (B) Most frequently recognized respiratory, food and other allergens in La Plata (prevalence of IgE sensitizations of at least 5% is shown). The percentage of sensitized patients is divided into three different ISU gradings reflecting allergen-specific IgE levels: 0.1 < ISU < 1 low; 1 < ISU < 15 moderate/high; ISU ≥ 15 very high.
In contrast, Bahía Blanca exhibited a higher prevalence of sensitization to pollen allergens, with timothy grass allergen Phl p 1 being the most frequent sensitizer (49%), followed by the olive pollen allergen Ole e 1 (28%), other grass pollen allergens (Phl p 2, 3 and 5) and the major mugwort allergen, Art v 1 (15%) (Figure 3 and Table S4). The sensitization rate to the major cat allergen, Fel d 1 (37%), in Bahía Blanca was comparable to that in La Plata (Figure 2 and Figure 3 and Tables S3 and S4). Notably, IgE sensitization of the major Alternaria allergen Alt a 1 (35%) was more than twice as frequent in Bahía Blanca as in La Plata (Figure 2 and Figure 3 and Tables S3 and S4). Conversely, IgE sensitization to HDM allergens, including Der p 23 (33%) and Der p 2 (33%), was less than half that in La Plata, with Blo t 21 showing minimal prevalence (1%). Sensitization to Ole e 1 (28%) was substantially higher in Bahía Blanca than in La Plata (5%) (Figure 2 and Figure 3 and Tables S3 and S4).
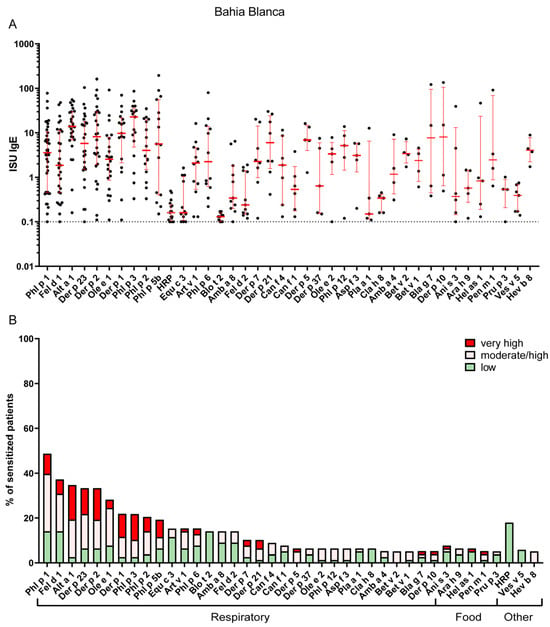
Figure 3.
(A) IgE levels of the most frequent sensitizers in La Bahía Blanca. Error bars (red) indicate medians ± interquartile ranges of positive ISU values. Cutoff for positive results (>=0.1) is indicated by a dashed horizontal line. Values below the cutoff are not shown. (B) Most frequently recognized respiratory, food and other allergens in Bahía Blanca (prevalence of IgE sensitizations of at least 5% is shown). The percentage of sensitized patients is divided into three different ISU gradings reflecting allergen-specific IgE levels: 0.1 < ISU < 1 low; 1 < ISU < 15 moderate/high; ISU ≥ 15 very high.
These patterns correlate with regional climatic and land cover data obtained in the region (Table S5). La Plata, with higher annual relative humidity (78.9%) and precipitation (1072.7 mm), provides favorable conditions for HDM proliferation, consistent with dominant HDM sensitization. Conversely, Bahía Blanca, characterized by lower annual precipitation (639.1 mm), lower relative humidity (64.6%), and higher wind flow speed (Bahía Blanca 18.8 km/h vs. La Plata 8.2 km/h), favors grass pollen and mold spore dispersal and persistence [14] consistent with the predominance of Phl p 1 and Alt a 1 sensitization. Furthermore, the larger proportion of pastureland in Bahía Blanca (64.3% of total area versus 25.4% in La Plata) likely increases the grass pollen exposure in Bahía Blanca, contributing to the observed sensitization differences. The higher frequency of Ole e 1 sensitization in Bahía Blanca is consistent with the presence of olive trees both in agricultural production areas and in urban spaces as ornamental trees.
2.3. Cross-Reactive Arginine Kinase and Tropomyosin Represent the Most Frequently Recognized Potential Food Allergens in Both Cities
Sensitization rates to food allergens were similar in both cities, ranging from 1% to 8%. In Bahía Blanca, the most frequently recognized potential food allergens were cross-reactive tropomyosins (TMs). Sensitization rates and IgE levels (median and maximum ISU) for food-derived TM were Pen m 1 from shrimp, 5% (2.4 and 91.0 ISU), Hel as 1 from snail, 6% (0.8 and 46.4 ISU); and Ani s 3 from Anisakis worm, 8% (0.3 and 39.1 ISU). For respiratory TM, the corresponding figures were: Der p 10, 5% (8.0 and 135.7 ISU), Bla g 7, 5% (7.6 and 122.1 ISU); and Blo t 10, 4% (3.7 and 53.6 ISU). Notably, all patients sensitized to Pen m 1 were co-sensitized to Der p 10. These findings suggest that the frequency and levels of specific IgE to food-derived TM were similar to those for the highly cross–reactive HDM tropomyosin, Der p 10, making it difficult to determine whether sensitization was primarily driven by food allergens, Der p 10, or other respiratory TM allergens (Figure 2 and Figure 3 and Tables S3 and S4).
IgE sensitization to plant-derived non-specific lipid transfer proteins (nsLTP), Ara h 9 (peanut, 2–6%) and Pru p 3 (peach, 2–5%) was found in both cities. For class 1 food allergens, sensitization to egg (Gal d 1) was 2–3%, slightly higher than milk sensitization (Bos d LF, 1–2%), consistent with previous evidence that egg allergy can persist into adulthood [15,16]. Sensitization to genuine peanut storage proteins (Ara h 1, Ara h 2 and Ara h 6) was rare (≤2%). No IgE sensitization to fish or wheat allergens was detected and milk sensitization was very rare in both cities.
IgE sensitization to pollen-related, cross-reactive class 2 food allergens was detected at low frequency. Bet v 1 homologues, including Gly m 4 (soy), Ara h 8 (peanut), Cor a 1 (hazelnut), and Pru p 1 (peach), were identified in a few patients (≤2–3%), consistent with secondary food allergy mediated by cross-reactivity to the major birch pollen allergen Bet v 1. Profilin-related food sensitizations were not directly assessed; however, based on 5–14% sensitized to the pollen profilins (Amb a 8, Bet v 2, Ole e 2, and Phl p 12), a similar rate of food profilin sensitization can be expected (Figure 2 and Figure 3 and Tables S3 and S4).
2.4. Peculiarities of IgE Sensitizations to Other Antigens
IgE sensitization to Ves v 5 from wasp venom was more frequent than to bee venom allergens and was more prevalent in La Plata (12%) compared with Bahía Blanca (9%). This finding is in line with previous reports showing that wasp sensitization is generally more common than bee sensitization in many populations [17,18,19]. Latex allergen Hev b 8 was detected in both regions, though at low prevalence (5% in La Plata and 1% in Bahía Blanca), and was mainly attributed to cross-reactive pollen profilins rather than genuine latex sensitization (Figure 2 and Figure 3 and Tables S3 and S4).
IgE sensitization prevalence to cross-reactive carbohydrate determinants (CCDs)- represented by HRP on the microarray-was comparable in patients of both cities (18.6% La Plata, median ISU 0.26; 17.9% Bahía Blanca, median ISU 0.17), but specific IgE levels were low (Figure 4, Tables S3 and S4).
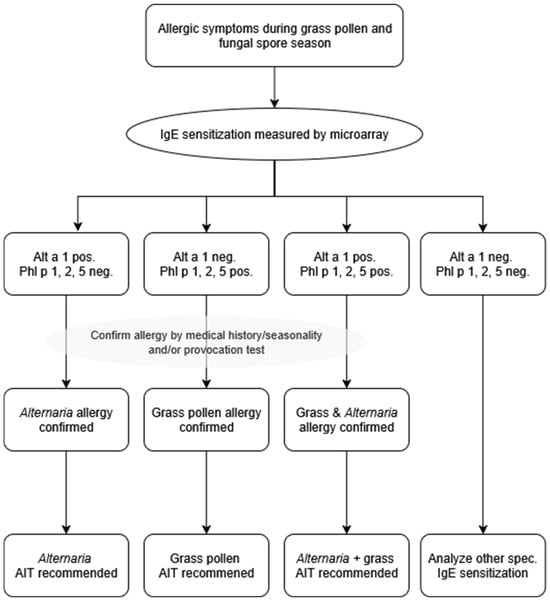
Figure 4.
Numbers and percentages of patients sensitized to Alternaria alternata major allergen, Alt a 1 only (“Alt a 1 only”); or to any of the genuine grass pollen allergens, Phl p 1, 2 or 5 (“Grass only”) or to both (“Co-sensitization”), and to HRP, as a marker for cross-reactive carbohydrate determinants (CCD) recognition. Molecular allergy diagnosis to facilitate the correct prescription of allergen-specific immunotherapy for Alternaria and/or grass pollen allergy.
2.5. Regional Variation in Allergic Symptoms in La Plata and Bahía Blanca
The prevalence of allergic symptoms differed significantly between La Plata and Bahía Blanca, reflecting regional variations in clinical presentations among study participants. Rhinoconjunctivitis was the most frequently reported symptom in both cities, affecting 91% of participants in La Plata and 93% in Bahía Blanca, with no significant difference observed between the regions (χ2 = 0.64, p = n.s.). La Plata, characterized by a humid subtropical climate (Cfa), had significantly higher asthma prevalence (40%), compared to 24% in Bahía Blanca (χ2 = 3.70, p = 0.04). In contrast, in Bahía Blanca, characterized by an arid steppe climate (Bsk), with a colder and windier winter, skin manifestations were more prevalent (54%) compared to La Plata (31%) (χ2 = 7.74, p < 0.01) (Figure 1 and Figure 5). No symptoms of food allergy were reported in the patients investigated in both cities.
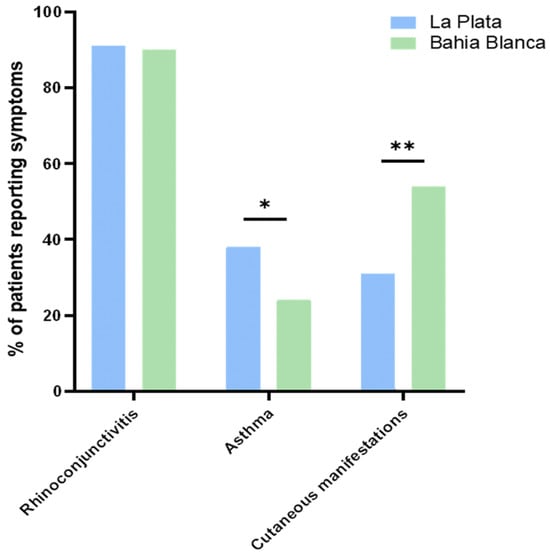
Figure 5.
Percentage of patients reporting symptoms compared between Bahía Blanca and La Plata. Asterisks denote statistical significance at * p ≤ 0.05, and ** p ≤ 0.01.
2.6. High Sensitization Rates to Grass Pollen Allergen Phl p 1 and Fungal Spore Allergen from Alternaria alternata Alt a 1, with Evidence of Seasonal Overlap
Sensitization to Alternaria alternata (Alt a 1) and genuine grass pollen marker allergens (Phl p 1, 2 and 5) was highly frequent in both La Plata and Bahía Blanca, underscoring their clinical relevance in these regions. Of note, the combined use of Phl p 1, Phl p 2, and Phl p 5 identified the same proportion of grass pollen allergic patients as the broader panel including Phl p 1, 2, 3, 5, and 6. This indicates that these three genuine marker allergens are sufficient to identify all grass pollen allergic patients in our cohorts. In La Plata, 16.2% of participants were sensitized to Alt a 1 but not to grass pollen marker allergens, while 16.2% were sensitized to grass pollen marker allergens but not to Alt a 1 (Figure 4). Only 2.3% of participants showed co-sensitization to grass pollen and Alternaria. In Bahía Blanca, the prevalence of exclusive sensitization was higher for grass pollen allergens (34.6%) compared to Alternaria (16.6%), with a co-sensitization rate of 17.9%.
In 2005, the grass pollen season for Poaceae species extended from August to April, with peak concentrations observed in November (1706 grains/m3) (Table S5). By comparison, Alternaria spores were present throughout the year, with peak concentrations during summer and autumn. Monthly spore counts ranged from 9000 spores/m3 in May to 14,500 spores/m3 in February, Ref. [20] indicating a consistent exposure burden across seasons (Table S5). Together, these findings point to periods of overlapping exposure to grass pollen and Alternaria spores.
3. Discussion
Our study provides the first detailed insight into molecular IgE-sensitization profiles in Latin America, specifically in Argentina, using micro-arrayed allergen molecules in a cross-sectional study, revealing significant regional differences between La Plata and Bahía Blanca. Our findings underscore the influence of climatic conditions on allergic sensitization patterns, highlighting the different importance of house dust mite and grass pollen allergens in two cities of Argentina, La Plata and Bahía Blanca, respectively.
Interestingly, differences were observed in the IgE recognition profile and in the reported symptoms of the two regions analyzed. In La Plata, we identified major HDM allergens (Der p 23, 2, and 1) and cat allergen Fel d 1 as the most important indoor sensitizers, and patients from La Plata more often reported symptoms of asthma than patients from Bahía Blanca. This is in line with the observations that high frequencies of IgE sensitizations to HDM allergens significantly increase bronchial responsiveness in sensitized individuals [3,21,22]. In contrast, in Bahía Blanca, characterized by a drier and windier climate, compared to La Plata, patients with asthma were frequently sensitized to the fungal allergen Alt a 1, in line with findings by Peat JK et al. linking Alternaria exposure to asthma in dry rural regions [23,24,25]. In the study by Kiewet MBG et al., similar climate-related regional differences have been reported in Southern Europe (Spain), where Der p 23 prevalence was higher in subtropical areas [19]. However, it was found that Der p 2 typically dominates HDM sensitization [19]. This aligns with studies indicating Der p 23’s superior prevalence in subtropical regions, suggesting a climatic influence on allergen distribution and sensitization patterns [26,27].
Moreover, La Plata exhibited a high sensitization frequency to major allergens Blo t 5 (23%) and Blo t 21 (33%) from Blomia tropicalis, the mite prevalent in the tropics and subtropics [28,29], while patients in Bahía Blanca showed very low IgE recognition prevalence to these allergens. Sensitization to Blomia allergens might be explained by cross-reactivity to Der p allergens from the same allergen group; however, it is tempting to speculate that Blomia tropicalis represents an individual allergen source and must be distinguished from Dermatophagoides pteronyssinus in certain regions [28].
Conversely, Bahía Blanca exhibited higher sensitization rates to pollen allergens, particularly to the timothy grass allergen, Phl p 1, and to the fungal spore allergen Alt a 1.
Sensitization to Fel d 1, the major cat allergen, was very common in both regions (La Plata 30%, Bahía Blanca 37%), which is not surprising given Argentina’s high rate of pet ownership and its high cat population of approximately 3 million cats [30,31].
Interestingly, IgE sensitization to dog allergens, Can f 1, Can f 2, and Can f 4 was much less prevalent. This may be due to the fact that dogs are kept more often outside than cats but one must also consider that we have not included the major dog allergen Can f 5 in the panel of micro-arrayed allergens tested and thus did not identify all dog-sensitized subjects. Among tree pollen allergens, Ole e 1 was the most prominent, with a prevalence of 5% in La Plata and 28% in Bahía Blanca. Sensitization to Ole e 1 may be attributable to the presence of olive trees in both rural and urban areas; however, cross-reactivity of IgE antibodies specific to ash pollen allergen Fra e 1 [32], recognized as the second most abundant pollen-producing tree in Bahía Blanca [33], should also be considered. Nevertheless, given Argentina’s status as a leading producer and exporter of olive oil in the Americas, with an estimated 90,000 hectares of olive cultivation, including significant areas in Buenos Aires province, genuine sensitization to Ole e 1 remains highly plausible, as Olea pollen grains have been detected in Bahía Blanca in the air (Table S5). It is a limitation of our study that certain tree pollen allergens are underrepresented on our microarray, potentially missing other species relevant to Argentina’s diverse tree population. Studies indicate that Cupressaceae pollen is an important pollen allergen source in Bahía Blanca [33,34] and cypress is a major contributor to respiratory allergies in Mediterranean climates, which share similarities with parts of Argentina [35]. However, the major cypress allergens are glycosylated proteins and the expression of functional IgE-reactive major allergens devoid of IgE-reactive CCDs has not been solved yet.
In contrast to regions like Northern Europe, where Bet v 1 (birch pollen allergen) dominates the hierarchy of aeroallergen sensitization [19,36,37], our study revealed very low recognition frequencies for this allergen in Argentina. Although birch trees have been introduced to Argentina, they are not native to the area, and the proportion of birch trees compared to native species is minimal, which explains the low prevalence of PR-10 sensitization in the population. Bet v 1 was recognized only by 5% of the study population, and the IgE recognition of cross-reactive PR10 class 2 allergens such as Cor a 1.041, Gly m 4, Ara h 8, Mal d 1 and Pru p 1 was even lower.
Studies from other subtropical regions like South Africa show frequent sensitization to the egg allergen Gal d 1 among food allergens in children [38]. Gal d 1 was also one of the most frequently detected class 1 allergens besides nsLTPs from peanut (Ara h 9) and peach (Pru p 3) in patients from La Plata and Bahía Blanca. IgE sensitization to shellfish allergens, tropomyosin and arginine kinase was frequent, which aligns with numerous publications that identify shellfish allergy as the most prevalent food allergy among adults in the United States [39,40], Asia [41,42] and other regions [43,44,45]. Moreover, shellfish are a leading cause of food-induced anaphylaxis worldwide [46,47,48,49]. However, it is quite possible that IgE reactivity to the latter allergens was due to IgE cross-reactivity with the homologous allergens from HDM. Of note, IgE sensitization to Ves v 5 was very common, affecting between 9 and 12% of the patients, whereas IgE sensitization to bee venom allergens was rare. Like in Europe, Ves v 5 is therefore the most frequently recognized venom allergen in our population.
Out of 155 patients reporting allergic symptoms, 78% showed IgE sensitization to allergens included on our microarray. This may be due to the fact that certain allergens, such as Can f 5 and cypress allergens, were not included in our micro-array, but it is even more likely that the ISAAC questionnaire may frequently yield positive results for subjects without IgE sensitizations. In fact, the presence of symptoms such as rhinitis, asthma or dermatitis does not necessarily indicate IgE-mediated sensitization. Respiratory virus infections, in particular rhinovirus, are well-established triggers of wheeze, asthma, and rhinitis, and can exacerbate allergic symptoms or mimic them in non-sensitized individuals. Recent studies have highlighted that virus-driven airway inflammation can account for acute exacerbations both in IgE-sensitized and non-sensitized patients, supporting the notion that symptomatic but IgE-negative cases may represent virus-induced disease rather than allergy [50,51].
Our study also highlights another important clinical problem that can be addressed by molecular allergy diagnosis. Allergen extract-based assays, which are still widely utilized by practitioners, can yield false-negative and positive results due to the absence of key allergens or cross-reactivity caused by allergen glycosylation and IgE-reactive CCDs, leading to potential misdiagnoses and suboptimal treatment strategies. We found considerable sensitization rates to CCDs, major grass pollen and Alternaria alternata allergens, which are present in the air simultaneously (Table S5). This underlines the critical need for accurate differential diagnosis to identify the symptom-eliciting allergen and to guide targeted therapy such as allergen-specific immunotherapy. Indeed, grass pollen extracts often contain cross-reactive carbohydrate determinants [52,53] leading to false-positive results, while Alternaria extracts may lack Alt a 1 [54,55], the major allergen essential for diagnosing Alternaria allergy. Molecular allergy diagnosis, as demonstrated by our allergen microarray, provides a precise method for identifying the relevant allergen sources and overcomes the limitations of extract-based assays. We propose a clinically applicable diagnostic decision tree to translate molecular IgE results into therapeutic recommendations (Figure 4). Patients with allergy symptoms during the grass pollen and mold season should undergo IgE testing by molecular allergy diagnosis. Sensitization to the genuine grass pollen markers Phl p 1, 2, and 5 [56,57] confirms grass pollen allergy, while sensitization to the major Alternaria marker Alt a 1 [58] establishes Alternaria allergy. Patients positive for both sets of markers are classified as co-sensitized. In contrast, patients showing IgE reactivity restricted to minor grass allergens or only CCDs are classified as cross-reactive without clinical relevance. Based on this stratification, tailored allergen immunotherapy can be recommended: grass AIT for grass-sensitized patients, Alternaria AIT for Alt a 1-positive patients, combined AIT for co-sensitized patients, and no AIT in cases of cross-reactivity or negative results. This approach ensures that AIT is only offered when genuine sensitization is demonstrated, minimizing unnecessary treatment while addressing the true drivers of allergic disease.
The main limitation of this study is the small sample size in both cities. Another important limitation, considering Argentina’s diverse climatic conditions, is the exclusion of populations from the northern (BSh) and western (BWk) regions (Figure 1). Further research is warranted to assess sensitization patterns in these areas.
Argentina’s diverse climatic regions and geographical variations (e.g., Andes Mountains) might influence allergen profiles; therefore, future studies collecting samples from a broader area and larger cohorts will be necessary to comprehensively map allergen sensitization across the entire country. The ongoing assessment of IgE patterns and vegetation shifts due to climate change is critical, as it may predict emerging allergenic threats as climate zones shift towards temperate regions. Research studies based on tailored research microarrays will be crucial to precisely target symptom-eliciting allergens and improve immunotherapy for patients suffering from allergic diseases.
4. Materials and Methods
4.1. Cohorts and Design of the Study
The IgE sensitization profiles in two different regions in Argentina, Bahía Blanca and La Plata, were examined. A total of 155 serum samples (patients aged 15–62, mean age 34.0, 83 females and 72 males) were collected from adolescents and adults with allergic symptoms in allergy centers of La Plata (n = 55; 33.9 ± 12.1 years, female/male 40/15) and Bahía Blanca (n = 100; 34.1 ± 10.8 years, female/male 43/57) (Figure 1 and Table S1). Patients were recruited from a public hospital in La Plata, Argentina (Hospital San Juan de Dios de La Plata) and two centers in Bahía Blanca, Argentina (Instituto de Alergia e Inmunología del Sur and Hospital Italiano Regional del Sur). Participants were randomly selected, regardless of symptoms or disease severity. No selection bias was introduced, and the study population is considered representative of the general adult population of both cities.
Our study aimed to compare the allergen sensitization patterns between these geographically and environmentally distinct areas (Figure 1). Participants completed an ISAAC questionnaire modified for adults to record symptoms indicative of allergy and disease severity [59]. Specific questions from the questionnaire addressing symptoms related to possible allergic rhinitis, skin manifestations, and asthma were summarized and selected for further analysis (Table S6). Ethics approval was acquired and informed consent was obtained from all subjects involved in the study. The protocol and informed consent forms were analyzed and approved by the Ethics Committee of the Hospital San Juan de Dios de La Plata (LP3680/022021). Anonymized sera were analyzed in the Medical University of Vienna, Department of Pathophysiology and Allergy Research, with the permission of the ethics committee of the Medical University of Vienna, EK1641/2014. Participants aged 15–17 years were included with written parental consent, in accordance with the ethics approval.
4.2. Immunoglobulin E Measurements
For the evaluation of IgE sensitization, serum samples were analyzed using an allergen microarray chip produced by the Allergochip working group (see authors), which includes 101 different recombinant and natural allergens, including a cross-reactive carbohydrate determinant (CCD) marker (Table S2). The protein printing procedure was performed as previously described [60]. Allergens were printed onto glass slides comprising six microarrays within an epoxy frame (Paul Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany). Slides were pre-coated with an amino-active polymer, MCP-2 (Lucidant Polymers, Sunnyvale, CA, USA), to facilitate protein immobilization. Allergens were spotted in triplicate in 75 mM Na2HPO4/NaH2PO4 buffer at pH 8.4 in a concentration ranging from 0.5 to 1 mg/mL using a SciFlexArrayer S12 (Scienion AG, Berlin, Germany). For allergen-specific IgE measurements, slides were washed with phosphate-buffered saline containing 0.5% Tween 20 (PBST) and dried by centrifugation. To each array, 30 µL of human serum was added along with controls, including sample diluent (SD) (Thermo Fisher, Waltham, MA, USA) and an in-house IgE calibration serum containing a mix of sensitized individuals’ sera. The slides were incubated for 2 h at room temperature. After washing, arrays were incubated with mouse anti-human IgE (Roche, Basel, Switzerland) conjugated with DyLight 550-2xPEG NHS Ester amine-reactive fluorescent dye (Pierce, Rockford, IL, USA) for 30 min in a final concentration of 1 μg/mL. Following a washing and drying step, slides were scanned using a confocal laser scanner (Tecan, Männedorf, Switzerland).
4.3. Data Analysis
Microarray image analysis was processed using MAPIX microarray image acquisition and analysis software Version 8.5.0 (Innopsys, Carbonne, France). The fluorescent intensities (FI) of IgE values were normalized against the sample diluent, and an IgE calibration serum was used to establish a linear conversion equation to ISAC standardized units (ISU). A percentage of patients with IgE sensitization were grouped according to their ISU class (low ≥ 0.1–1 ISU, moderate = 1–15 ISU, high > 15) (Figure 2 and Figure 3). Allergen molecules were categorized by their source (e.g., animal dander and food allergens) and sensitization route. Sensitization rates of patients from two different regions, La Plata (Table S3) and Bahía Blanca (Table S4), were calculated for each allergen, and allergens were ranked by their IgE recognition frequencies. Statistical analyses were performed to identify any significant regional differences in reported allergic symptoms and to evaluate differences in allergen-specific IgE levels and their correlation with reported symptoms. Chi-square (χ2) test was used to assess the statistical significance. All statistical analyses were conducted using GraphPad Prism 8 software.
4.4. Climatic and Geospatial Data Acquisition and Map Generation
Maps were generated using QGIS software (version 3.38.2; open-source Geographic Information System software) to visualize the spatial distribution of climate zones and calculate land cover in Argentina. Monthly climatological statistics, including average temperature, maximum and minimum temperatures, relative humidity, wind speed and precipitation for the period 1991–2020, were obtained from the official meteorological website of Argentina, Servicio Meteorológico Nacional (SMN). The Köppen–Geiger climate classification data by Beck et al. [6,61] covering the same period (1991–2020) were obtained in GeoTIFF format from https://www.gloh2o.org/koppen/, with a resolution of 1 km (accessed on 6 May 2025). Land cover information was provided by the MapBiomas Argentina Project, from “Collection 1” of the Annual Land Use and Land Cover Maps of Argentina, accessed in 2022. In QGIS, a base map of Argentina, displaying provincial boundaries and climate regions, was created by importing the vector and raster datasets. To refine the focus on this region, the raster climate data were clipped using the provincial boundary as a mask. An inset map was generated, magnifying the selected province while maintaining the context of the national map.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms262412101/s1.
Author Contributions
Conceptualization, G.D. and R.V.; methodology, E.S., M.T., S.V. and R.V.; investigation, E.S. and M.C., (expression, purification, and characterization of allergens); resources, S.V. and R.V. (supervision of allergen production teams); visualization, E.S., G.D., S.V. and R.V.; validation, E.S., H.-J.H., M.T. and T.S.; formal analysis, E.S., P.S., M.C., M.A., P.B., A.R., M.E.B.L., G.R. (Gonzalo Ramón), G.R. (Germán Ramón), T.S., M.T., H.-J.H., S.V., G.D. and R.V.; data curation, E.S.; writing—original draft preparation, E.S., G.D. and R.V.; writing—review and editing, E.S., G.D., R.V. and S.V.; supervision, S.V., R.V. and G.D.; project administration, S.V. and R.V.; funding acquisition, R.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Danube Allergy Research Cluster of the country of Lower Austria and by grant no. 23-75-30016 from the Russian Science Foundation, for its part related to allergen characterization, by the INSPIRED (Innovative Strategies for Prevention, diagnosis and therapy of ragweed pollen Induced REspiratoryDiseases) project, COP 2014–2020 92/09.09.2016, P_37_747 from the European Regional Development Fund (ERDF) and the Romanian national budget.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Hospital San Juan de Dios de La Plata on 18 February 2021 (LP3680/022021). Anonymized sera were analyzed at the Medical University of Vienna, Department of Pathophysiology and Allergy Research with the permission of the ethics committee of the Medical University of Vienna EK1641/2014 (last renewal on 3 September 2025). Informed consent was obtained from all subjects involved in the study.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
Investigation, A.O.A., M.-R.B., K.-W.C., R.C., M.D.C., Y.D., N.D., A.D., M.F.-T., P.G., A.K., E.K., A.K., B.L., C.P., D.T., M.W., and H.G. (expression, purification, and characterization of allergens); resources, A.K., C.P. (supervision of allergen production teams); A.K., C.P. (supervision of allergen production teams); supervision, A.K., C.P.
Conflicts of Interest
Rudolf Valenta serves as a consultant for HVD Biotech, Vienna, Austria.
Abbreviations
| AIT | Allergen-specific immunotherapy |
| AS | Asthma |
| CCD | Cross-reactive carbohydrate determinant |
| CRD | Component-resolved diagnostics |
| D | Dermatitis/skin manifestations |
| FI | Fluorescence intensity |
| HDM | House dust mite |
| IgE | Immunoglobulin E |
| ISAAC | International Study of Asthma and Allergies in Childhood |
| ISUs | IgE standardized units |
| RC | Rhinoconjunctivitis |
| TM | Tropomyosin |
Appendix A
- Allergochip Working Group: Adebanke Oluwatoyin Akinfenwa1 Maria-Roxana Buzan2,3, Kuan-Wei Chen2, Raffaela Campana1, Monica Daniela Cotarcă3, Mirela Curin1, Yulia Dorofeeva1, Nishelle Dsouza1, Alexandra Dubovets4,5, Margarete Focke-Tejkl1, Pia Gattinger1, Heinrich Grausgruber6, Alexander Karaulov4,5, Evgenii Kozlov4,5, Andrea Krisai6, Birgit Linhart1, Carmen Panaitescu2,3, Daria Trifonova1,4,5, Milena Weber1
- 1Division of Immunopathology, Department of Pathophysiology and Allergy Research, Center for Pathophysiology, Infectiology and Immunology, Medical University of Vienna, Vienna, Austria 2OncoGen Center, Pius Brinzeu County Clinical Emergency Hospital, Timisoara, Romania; 3Center of Immuno-Physiology and Biotechnologies, Department of Functional Sciences, Victor Babes University of Medicine and Pharmacy, Timisoara, Romania; 4Laboratory of Immunopathology, Department of Clinical Immunology and Allergy, Sechenov First Moscow State Medical University, Moscow, Russia; 5Life Improvement by Future Technologies (LIFT) Center, Moscow, Russia; 6BOKU University, Department of Agricultural Sciences, Institute of Crop Breeding and Genomics; Tulln an der Donau, Austria.
References
- Katelaris, C.H.; Beggs, P.J. Climate change: Allergens and allergic diseases. Intern. Med. J. 2018, 48, 129–134. [Google Scholar] [CrossRef]
- Basilico, M.d.l.L.Z.; Chiericatti, C.; Aringoli, E.E.; Althaus, R.L.; Basilico, J.C. Influence of environmental factors on airborne fungi in houses of Santa Fe City, Argentina. Sci. Total Environ. 2007, 376, 143–150. [Google Scholar] [CrossRef]
- Acevedo, N.; Zakzuk, J.; Caraballo, L. House dust mite allergy under changing environments. Allergy Asthma Immunol. Res. 2019, 11, 450–469. [Google Scholar] [CrossRef]
- D’Amato, G.; Chong-Neto, H.J.; Monge Ortega, O.P.; Vitale, C.; Ansotegui, I.; Rosario, N.; Haahtela, T.; Galan, C.; Pawankar, R.; Murrieta-Aguttes, M.; et al. The effects of climate change on respiratory allergy and asthma induced by pollen and mold allergens. Allergy 2020, 75, 2219–2228. [Google Scholar] [CrossRef]
- Price, D.; Hughes, K.M.; Thien, F.; Suphioglu, C. Epidemic thunderstorm asthma: Lessons learned from the storm Down-Under. J. Allergy Clin. Immunol. Pract. 2021, 9, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Lutsko, N.J.; Dufour, A.; Zeng, Z.; Jiang, X.; van Dijk, A.I.J.M.; Miralles, D.G. High-resolution (1 km) Köppen–Geiger maps for 1901–2099 based on constrained CMIP6 projections. Sci. Data 2023, 10, 724. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.M.; van Hage, M.; Custovic, A.; Korosec, P.; Santos, A.F.; Valenta, R. Molecular allergy diagnosis enabling personalized medicine. J. Allergy Clin. Immunol. 2025, 156, 485–502. [Google Scholar] [CrossRef]
- Andiappan, A.K.; Puan, K.J.; Lee, B.; Nardin, A.; Poidinger, M.; Connolly, J.; Chew, F.T.; Wang, D.Y.; Rotzschke, O. Allergic airway diseases in a tropical urban environment are driven by dominant mono-specific sensitization against house dust mites. Allergy 2014, 69, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Neffen, H.E.; Maillo, M. Letter from Argentina. Respirology 2024, 29, 82–83. [Google Scholar] [CrossRef]
- Vázquez, D.; Medina, I.; Logusso, G.; Arias, S.; Gattolin, G.; Parisi, C. Cross-sectional survey about the prevalence of allergic rhinitis in Argentina: Study PARA. Rev. Alerg. Mex. 2019, 66, 55–64. [Google Scholar] [CrossRef]
- Pendino, P.; Agüero, C.; Cavagnero, P.; Lopez, K.; Kriunis, I.; Molinas, J. Aeroallergen sensitization in wheezing children from Rosario, Argentina. World Allergy Organ. J. 2011, 4, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Ramon, G.D.; Barrionuevo, L.B.; Viego, V.; Vanegas, E.; Felix, M.; Cherrez-Ojeda, I. Sensitization to subtropical grass pollens in patients with seasonal allergic rhinitis from Bahía Blanca, Argentina. World Allergy Organ. J. 2019, 12, 100062. [Google Scholar] [CrossRef]
- Dorofeeva, Y.; Shilovskiy, I.; Tulaeva, I.; Focke-Tejkl, M.; Flicker, S.; Kudlay, D.; Khaitov, M.; Karsonova, A.; Riabova, K.; Karaulov, A.; et al. Past, present, and future of allergen immu-notherapy vaccines. Allergy 2021, 76, 131–149. [Google Scholar] [CrossRef]
- Emmerson, K.M.; Silver, J.D.; Thatcher, M.; Wain, A.; Jones, P.J.; Dowdy, A.; Newbigin, E.J.; Picking, B.W.; Choi, J.; Ebert, E.; et al. Atmospheric modelling of grass pollen rupturing mechanisms for thunderstorm asthma prediction. PLoS ONE 2021, 16, e0249488. [Google Scholar] [CrossRef]
- Dona, D.W.; Suphioglu, C. Egg allergy: Diagnosis and immunotherapy. Int. J. Mol. Sci. 2020, 21, 5010. [Google Scholar] [CrossRef]
- Dhanapala, P.; De Silva, C.; Doran, T.; Suphioglu, C. Cracking the egg: An insight into egg hypersensitivity. Mol. Immunol. 2015, 66, 375–383. [Google Scholar] [CrossRef]
- Mittermann, I.; Zidarn, M.; Silar, M.; Markovic-Housley, Z.; Aberer, W.; Korosec, P.; Kosnik, M.; Valenta, R. Recombinant allergen-based IgE testing to distinguish bee and wasp allergy. J. Allergy Clin. Immunol. 2010, 125, 1097–1103. [Google Scholar] [CrossRef]
- Gattinger, P.; Lupinek, C.; Kalogiros, L.; Silar, M.; Zidarn, M.; Korosec, P.; Koessler, C.; Novak, N.; Valenta, R.; Mittermann, I. The culprit insect—But not severity—Of allergic reactions to bee and wasp venom can be determined by molecular diagnosis. PLoS ONE 2018, 13, e0199250. [Google Scholar] [CrossRef]
- Kiewiet, M.B.G.; Lupinek, C.; Vrtala, S.; Wieser, S.; Baar, A.; Kiss, R.; Kull, I.; Melén, E.; Wickman, M.; Porta, D.; et al. A molecular sensitization map of European children reveals exposome- and climate-dependent sensitization profiles. Allergy 2023, 78, 2007–2018. [Google Scholar] [CrossRef] [PubMed]
- Mallo, A.C.; Nitiu, D.S.; Gardella Sambeth, M.C. Airborne fungal spore content in the atmosphere of the city of La Plata, Argentina. Aerobiologia 2011, 27, 77–84. [Google Scholar] [CrossRef]
- Huang, H.J.; Breyer-Kohansal, R.; Niespodziana, K.; Lim, C.J.M.; Breyer, M.K.; Valenta, R.; Hartl, S.; Buzan, M.R.; Chen, K.W.; Cotarcă, M.D.; et al. Molecular IgE sensitization profiling with micro-arrayed allergen molecules in adult patients with asthma from the LEAD cohort: A precision medicine approach. Allergy 2025, in press. [Google Scholar] [CrossRef]
- Portnoy, J.; Miller, J.D.; Williams, P.B.; Chew, G.L.; Miller, J.D.; Zaitoun, F.; Phipatanakul, W.; Kennedy, K.; Barnes, C.; Grimes, C.; et al. Environmental assessment and exposure control of dust mites: A practice parameter. Allergy Asthma Immunol. 2013, 111, 465–507. [Google Scholar] [CrossRef]
- Peat, J.K.; Tovey, E.; Mellis, C.M.; Leeder, S.R.; Woolcock, A.J. Importance of house dust mite and Alternaria allergens in childhood asthma: An epidemiological study in two climatic regions of Australia. Clin. Exp. Allergy 1993, 23, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.M.; Price, D.; Torriero, A.A.J.; Symonds, M.R.E.; Suphioglu, C. Impact of fungal spores on asthma prevalence and hospitalization. Int. J. Mol. Sci. 2022, 23, 4313. [Google Scholar] [CrossRef] [PubMed]
- Twaroch, T.E.; Curin, M.M.; Valenta, R.; Swoboda, I. Mold allergens in respiratory allergy: From structure to therapy. Allergy Asthma Immunol. Res. 2015, 7, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Calzada, D.; Bartra, J.; Serrano, C.; Riggioni, S.; Moran, E.; Maselli, J.; Silva, D.; Ramirez, L.; Pascal, M.; Carnés, J.; et al. Differences in molecular sensitization profiles between Spanish and Latin American mite-allergic patients. J. Investig. Allergol. Clin. Immunol. 2024, 35, 114–121. [Google Scholar] [CrossRef]
- Muddaluru, V.; Valenta, R.; Vrtala, S.; Schlederer, T.; Hindley, J.; Hickey, P.; Larché, M.; Tonti, E. Comparison of house dust mite sensitization profiles in allergic adults from Canada, Europe, South Africa and USA. Allergy 2021, 76, 2177–2188. [Google Scholar] [CrossRef]
- Pauli, G.; Wurmser, C.; Roos, A.; Kokou, C.; Huang, H.J.; D’souza, N.; Lupinek, C.; Zakzuk, J.; Regino, R.; Acevedo, N.; et al. Frequent IgE recognition of Blomia tropicalis allergen molecules in asthmatic children and young adults in equatorial Africa. Front. Immunol. 2023, 14, 1133935. [Google Scholar] [CrossRef]
- D’souza, N.; Weber, M.; Sarzsinszky, E.; Vrtala, S.; Curin, M.; Schaar, M.; Garib, V.; Focke-Tejkl, M.; Li, Y.; Jones, R.; et al. The molecular allergen recognition profile in China as basis for allergen-specific immunotherapy. Front. Immunol. 2021, 12, 719573. [Google Scholar] [CrossRef]
- Cat Population by Country 2025. World Population Review. 2025. Available online: https://worldpopulationreview.com/country-rankings/cat-population-by-country (accessed on 17 May 2024).
- Mattsson, L.; Lundgren, T.; Everberg, H.; Larsson, H.; Lidholm, J. Prostatic kallikrein: A new major dog allergen. J. Allergy Clin. Immunol. 2009, 123, 362–368. [Google Scholar] [CrossRef]
- Palomares, O.; Swoboda, I.; Villalba, M.; Balic, N.; Spitzauer, S.; Rodríguez, R.; Valenta, R. The major allergen of olive pollen Ole e 1 is a diagnostic marker for sensitization to Oleaceae. Int. Arch. Allergy Immunol. 2006, 141, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.G.; Galán, C.; Villamil, C.B. Airborne pollen in Bahía Blanca, Argentina: Seasonal distribution of pollen types. Aerobiologia 2010, 26, 195–207. [Google Scholar] [CrossRef]
- Ramon, G.D.; Felix, M.; Barrionuevo, L.B.; Benedetti, G.M.; Duval, V.S.; Vanegas, E.; Cherrez-Ojeda, I. Persistence of airborne tree pollen from the Cupressaceae family during the last decade in the city of Bahía Blanca. Allergol. Immunopathol. 2022, 50, 75–77. [Google Scholar] [CrossRef]
- De Linares, C.; Plaza, M.P.; Valle, A.M.; Alcázar, P.; de la Guardia, C.D.; Galán, C. Airborne Cupressaceae pollen and its major allergen, Cup a 1, in urban green areas of southern Iberian Peninsula. Forests 2021, 12, 254. [Google Scholar] [CrossRef]
- Siroux, V.; Lupinek, C.; Resch, Y.; Curin, M.; Just, J.; Keil, T.; Kiss, R.; Lødrup Carlsen, K.; Melén, E.; Nadif, R.; et al. Specific IgE and IgG measured by the MeDALL allergen-chip depend on allergen and route of exposure: The EGEA study. J. Allergy Clin. Immunol. 2017, 139, 643–654.e6. [Google Scholar] [CrossRef]
- Elisyutina, O.; Lupinek, C.; Fedenko, E.; Litovkina, A.; Smolnikov, E.; Ilina, N.; Kudlay, D.; Shilovskiy, I.; Valenta, R.; Khaitov, M. IgE-reactivity profiles to allergen molecules in Russian children with and without symptoms of allergy revealed by micro-array analysis. Pediatr. Allergy Immunol. 2021, 32, 251–263. [Google Scholar] [CrossRef]
- Mittermann, I.; Dzoro, S.; Gattinger, P.; Botha, M.; Basera, W.; Facey-Thomas, H.E.; Gaunt, B.; Genuneit, J.; Gray, C.L.; Hlela, C.; et al. Molecular IgE sensitization profiles of urban and rural children in South Africa. Pediatr. Allergy Immunol. 2021, 32, 234–241. [Google Scholar] [CrossRef]
- Sicherer, S.H.; Muñoz-Furlong, A.; Sampson, H.A. Prevalence of seafood allergy in the United States determined by a random telephone survey. J. Allergy Clin. Immunol. 2004, 114, 159–165. [Google Scholar] [CrossRef]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.Y.Y.; Leung, N.Y.H.; Chu, K.H.; Leung, P.S.C.; Leung, A.S.Y.; Wong, G.W.K.; Leung, T.F. Overcoming shellfish allergy: How far have we come? Int. J. Mol. Sci. 2020, 21, 2234. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.Y.Y.; Leung, N.Y.H.; Leung, A.S.Y.; Wong, G.W.K.; Leung, T.F. Seafood Allergy in Asia: Geographical Specificity and Beyond. Front. Allergy 2021, 2, 676903. [Google Scholar] [CrossRef]
- Ben-Shoshan, M.; Harrington, D.W.; Soller, L.; Fragapane, J.; Joseph, L.; St Pierre, Y.; Godefroy, S.B.; Elliot, S.J.; Clarke, A.E. A population-based study on peanut, tree nut, fish, shellfish, and sesame allergy prevalence in Canada. J. Allergy Clin. Immunol. 2010, 125, 1327–1335, Correction in J. Allergy Clin. Immunol. 2011, 127, 840.. [Google Scholar] [CrossRef]
- Moonesinghe, H.; Mackenzie, H.; Venter, C.; Kilburn, S.; Turner, P.; Weir, K.; Dean, T. Prevalence of fish and shellfish allergy: A systematic review. Ann. Allergy Asthma Immunol. 2016, 117, 264–272.e4. [Google Scholar] [CrossRef]
- Kline, A.A.; Vysochyn, M.; Dushenko, N.; Stewart, K. Revisiting Shellfish as the Leading Allergen in Adult-Onset Food Allergy. Cureus 2025, 17, e94357. [Google Scholar] [CrossRef]
- Mullins, R.J.; Wainstein, B.K.; Barnes, E.H.; Liew, W.K.; Campbell, D.E. Increases in anaphylaxis fatalities in Australia from 1997 to 2013. Clin. Exp. Allergy 2016, 46, 1099–1110. [Google Scholar] [CrossRef]
- Nantanee, R.; Suratannon, N.; Chatchatee, P. Characteristics and laboratory findings of food-induced anaphylaxis in children: Study in an Asian developing country. Int. Arch. Allergy Immunol. 2022, 183, 59–67. [Google Scholar] [CrossRef]
- Conrado, A.B.; Patel, N.; Turner, P.J. Global patterns in anaphylaxis due to specific foods: A systematic review. J. Allergy Clin. Immunol. 2021, 148, 1515–1525.e3. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Garcia, R.; Lopez-de-Andres, A.; Hernandez-Barrera, V.; Zamorano-Leon, J.J.; Cuadrado-Corrales, N.; de Miguel-Diez, J.; Del-Barrio, J.L.; Jimenez-Sierra, A.; Carabantes-Alarcon, D. Hospitalizations for Food-Induced Anaphylaxis Between 2016 and 2021: Population-Based Epidemiologic Study. JMIR Public Health Surveill. 2024, 10, e57340. [Google Scholar] [CrossRef] [PubMed]
- Niespodziana, K.; Stenberg-Hammar, K.; Papadopoulos, N.G.; Focke-Tejkl, M.; Errhalt, P.; Konradsen, J.R.; Söderhäll, C.; van Hage, M.; Hedlin, G.; Valenta, R. Microarray technology may reveal the contribution of allergen exposure and rhinovirus infections as possible triggers for acute wheezing attacks in preschool children. Viruses 2021, 13, 915. [Google Scholar] [CrossRef]
- Borochova, K.; Niespodziana, K.; Focke-Tejkl, M.; Hofer, G.; Keller, W.; Valenta, R. Dissociation of the respiratory syncytial virus F protein-specific human IgG, IgA and IgM response. Sci. Rep. 2021, 11, 3551. [Google Scholar] [CrossRef] [PubMed]
- Gangl, K.; Niederberger, V.; Valenta, R. Multiple grass mixes as opposed to single grasses for allergen immunotherapy in allergic rhinitis. Clin. Exp. Allergy 2013, 43, 1202–1216. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, W.; Altmann, F.; Holzweber, F.; Gruber, C.; Wantke, F.; Wöhrl, S. ImmunoCAP cellulose displays cross-reactive carbohydrate determinant (CCD) epitopes and can cause false-positive test results in patients with high anti-CCD IgE antibody levels. J. Allergy Clin. Immunol. 2018, 141, 372–381.e3. [Google Scholar] [CrossRef]
- Bush, R.K.; Prochnau, J.J. Alternaria-induced asthma. J. Allergy Clin. Immunol. 2004, 113, 227–234. [Google Scholar] [CrossRef]
- Vailes, L.; Sridhara, S.; Cromwell, O.; Weber, B.; Breitenbach, M.; Chapman, M. Quantitation of the major fungal allergens, Alt a 1 and Asp f 1, in commercial allergenic products. J. Allergy Clin. Immunol. 2001, 107, 641–646. [Google Scholar] [CrossRef]
- Kazemi-Shirazi, L.; Niederberger, V.; Linhart, B.; Lidholm, J.; Kraft, D.; Valenta, R. Recombinant marker allergens: Diagnostic gatekeepers for the treatment of allergy. Int. Arch. Allergy Immunol. 2002, 127, 259–268. [Google Scholar] [CrossRef]
- Westritschnig, K.; Horak, F.; Swoboda, I.; Balic, N.; Spitzauer, S.; Kundi, M.; Fiebig, H.; Suck, R.; Cromwell, O.; Valenta, R. Different allergenic activity of grass pollen allergens revealed by skin testing. Eur. J. Clin. Investig. 2008, 38, 260–267. [Google Scholar] [CrossRef]
- Twaroch, T.E.; Arcalís, E.; Sterflinger, K.; Stöger, E.; Swoboda, I.; Valenta, R. Predominant localization of the major Alternaria allergen Alt a 1 in the cell wall of airborne spores. J. Allergy Clin. Immunol. 2012, 129, 1148–1149. [Google Scholar] [CrossRef]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef]
- Gattinger, P.; Niespodziana, K.; Stiasny, K.; Sahanic, S.; Tulaeva, I.; Borochova, K.; Dorofeeva, Y.; Schlederer, T.; Sonnweber, T.; Hofer, G.; et al. Neutralization of SARS-CoV-2 requires antibodies against conformational receptor-binding domain epitopes. Allergy 2022, 77, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen–Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).