Network Toxicology and Molecular Docking Reveal the Toxicological Mechanisms of DEHP in Bone Diseases
Abstract
1. Introduction
2. Results
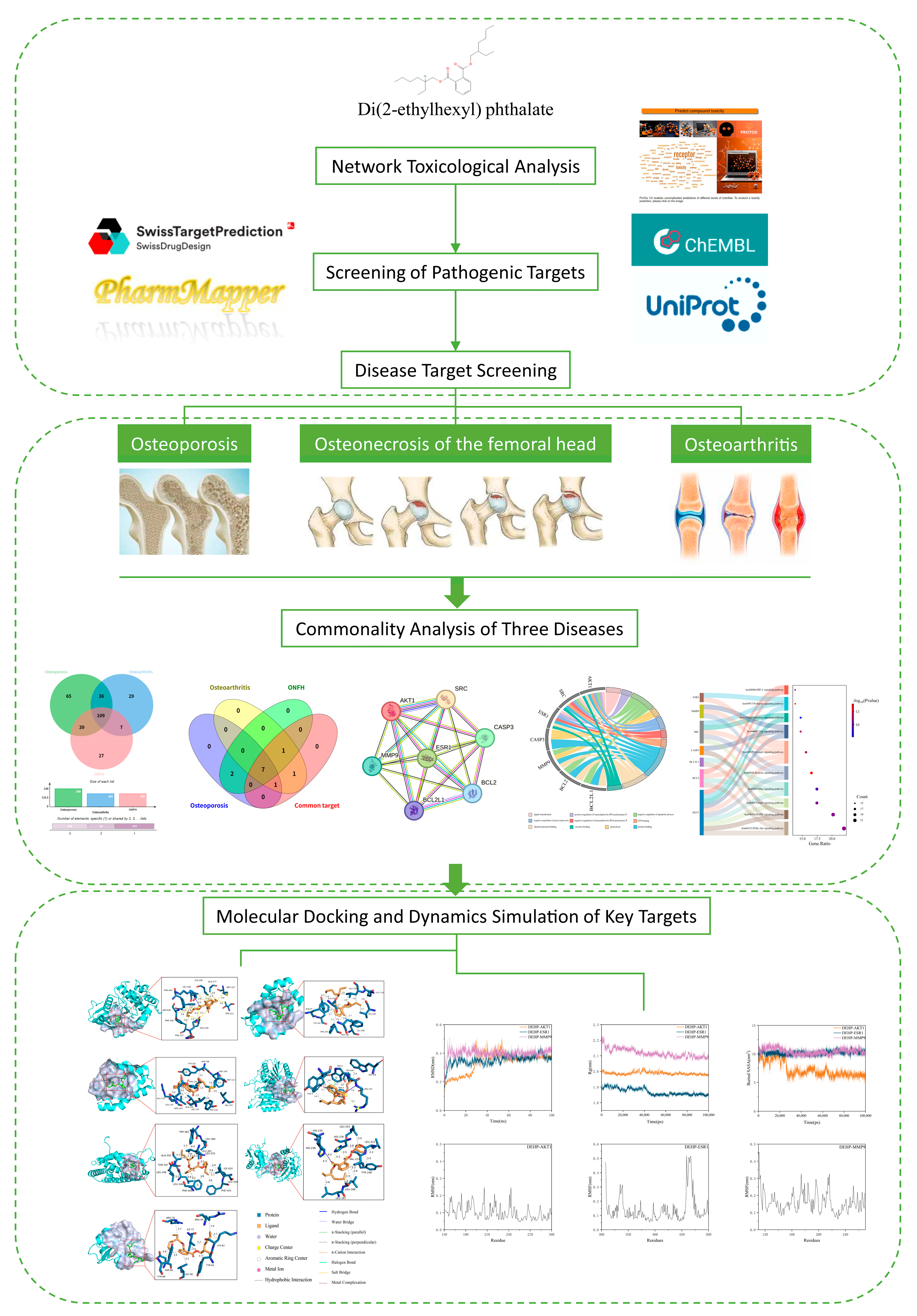
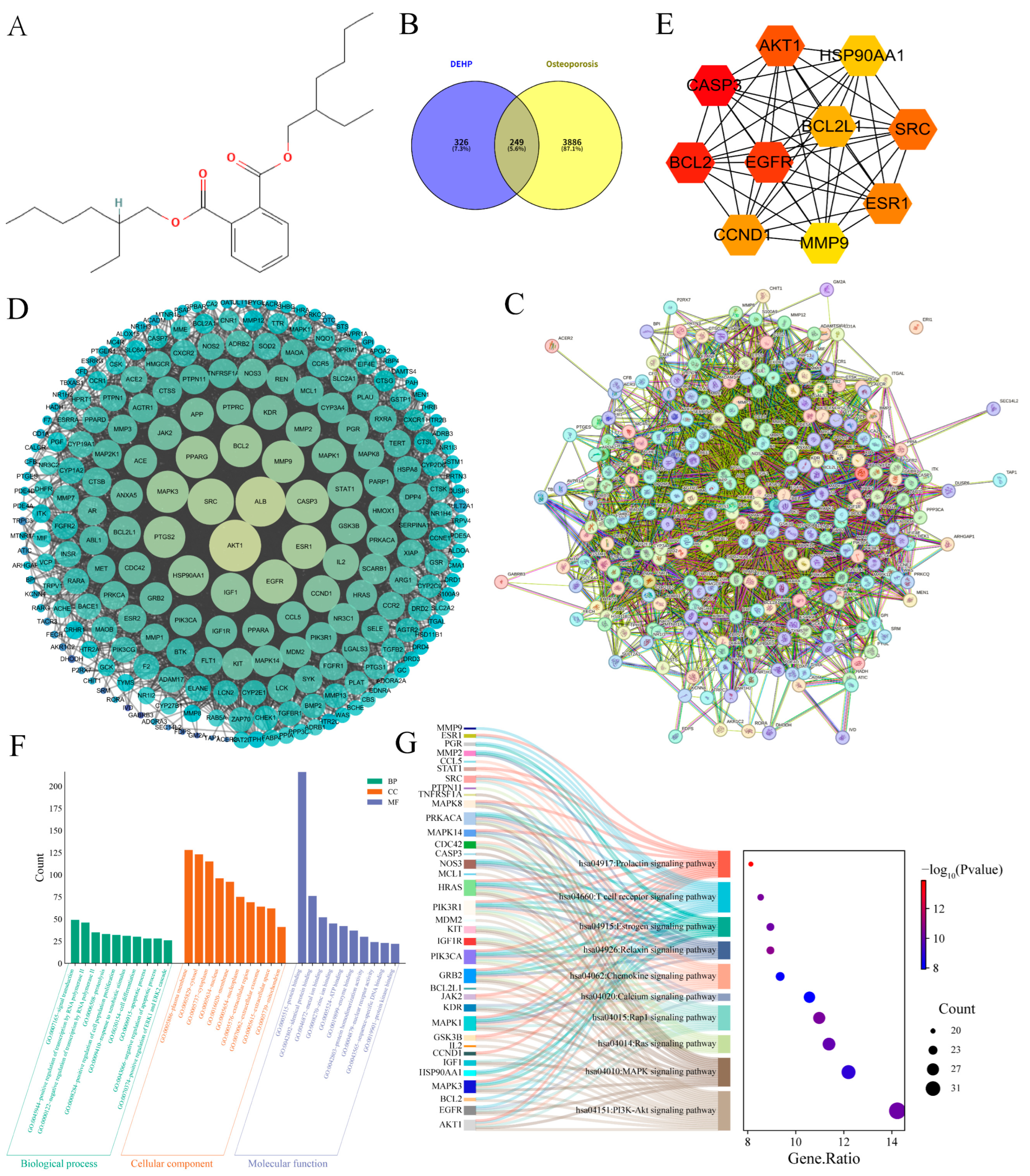
2.1. Preliminary Toxicological Network Evaluation of DEHP
2.2. Impact of DEHP on Osteoporosis
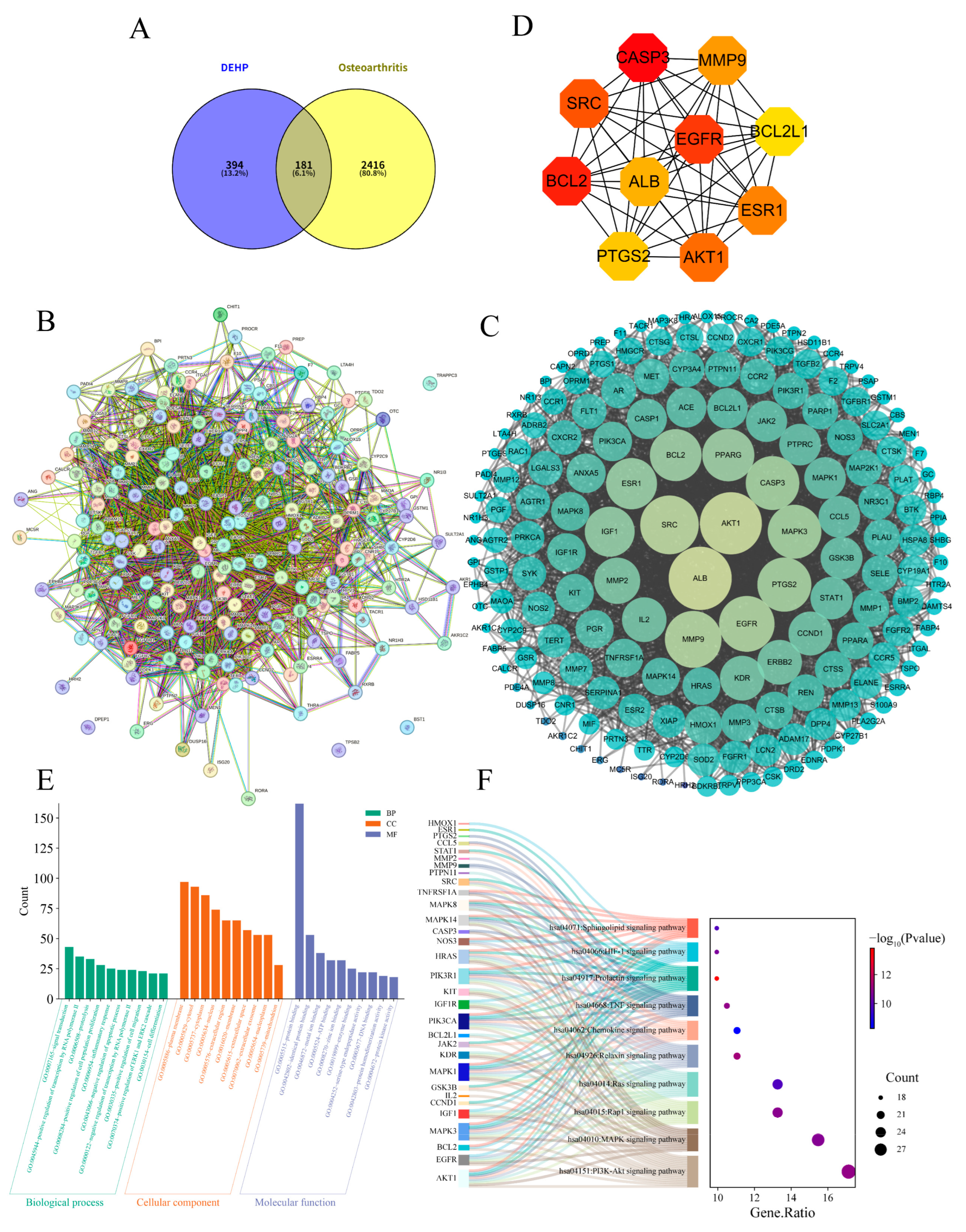
2.3. Impact of DEHP on Osteoarthritis
2.4. Impact of DEHP on Osteonecrosis of the Femoral Head
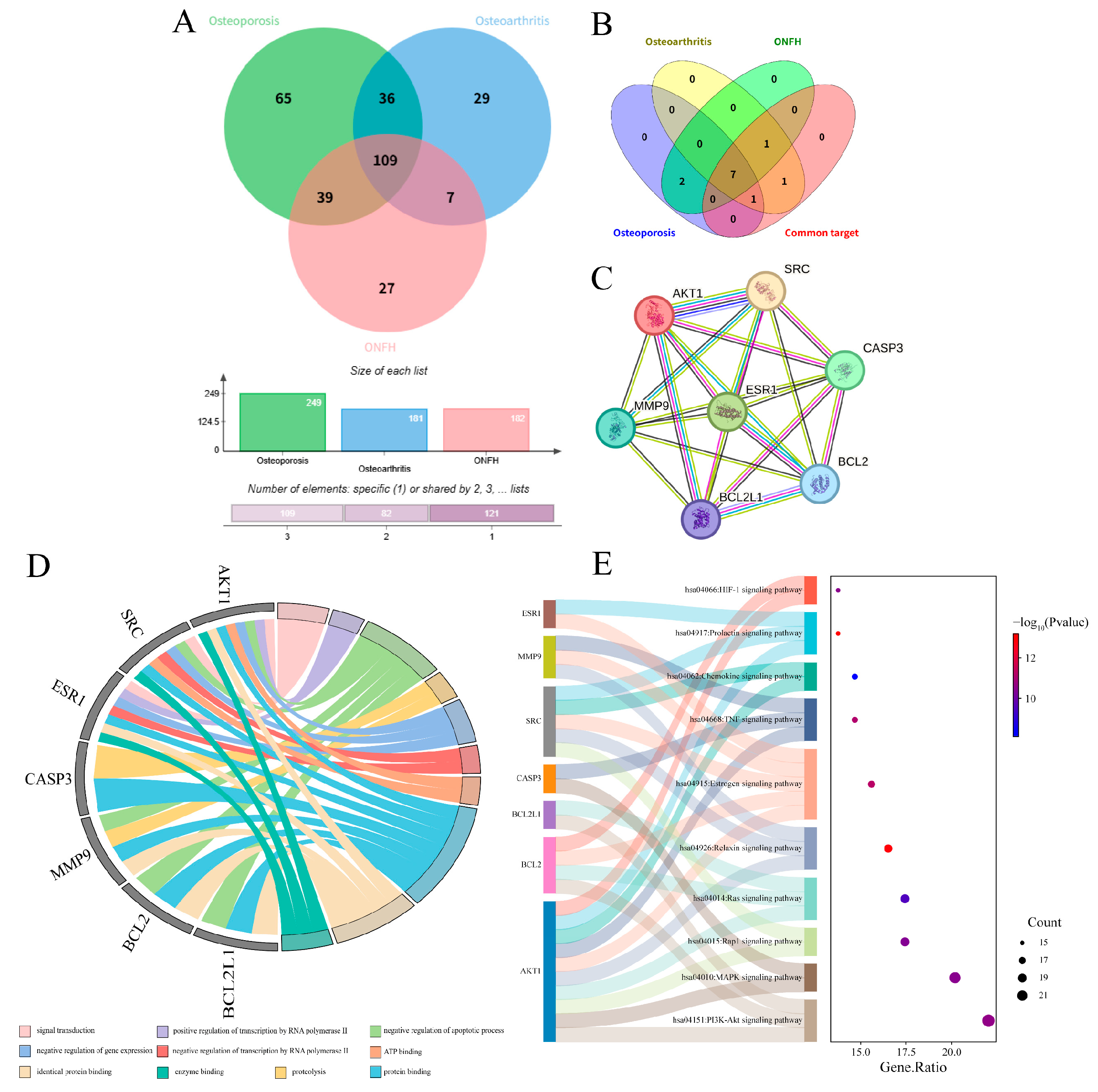
2.5. Synergistic Analysis of Core Targets Across Three Bone Diseases
2.6. Molecular Docking of DEHP with Core Targets Associated with Bone Diseases
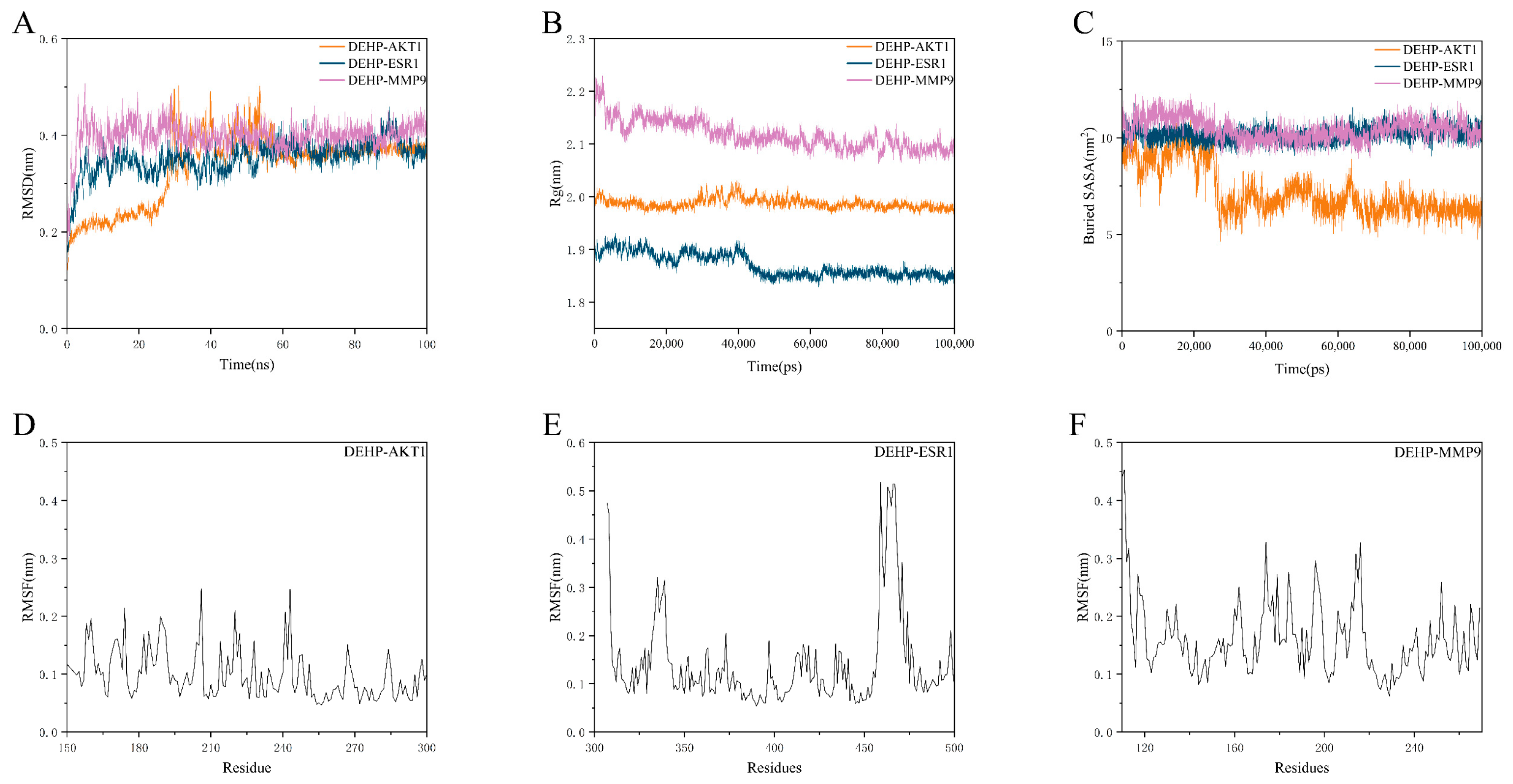
2.7. Molecular Dynamics Simulation Validation of Key Toxicity Targets
3. Discussion
4. Materials and Methods
4.1. Network Toxicological Analysis of DEHP
4.2. Collection of DEHP Targets
4.3. Construction of Disease Target Library
4.4. Intersection of DEHP Targets and Disease Targets
4.5. Construction of Protein–Protein Interaction (PPI) Network
4.6. GO and KEGG Pathway Enrichment Analysis
4.7. Molecular Docking
4.8. Molecular Dynamics (MD) Simulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DEHP | Di(2-ethylhexyl) phthalate |
| OP | Osteoporosis |
| OA | Osteoarthritis |
| ONFH | Osteonecrosis of the femoral head |
| PPI | Protein–protein interaction |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MD | Molecular Dynamics |
| PVC | Polyvinyl Chloride |
| EDC | Endocrine-Disrupting Chemical |
| MEHP | Mono(2-ethylhexyl) phthalate |
| AR | Androgen receptor |
| ER | Estrogen receptor |
| PPARα/γ | Peroxisome proliferator-activated receptors |
| NF-κB | Nuclear factor-kappa B |
| MAPK | Mitogen-activated protein kinase |
| ROS | reactive oxygen species |
| SEA | Similarity Ensemble Approach |
| IUPAC | International Union of Pure and Applied Chemistry |
| MCC | Maximal Clique Centrality |
| RMSD | Root Mean Square Deviation |
| Rg | Radius of Gyration |
| SASA | Solvent Accessible Surface Area |
| RMSF | Root Mean Square Fluctuation |
| PME | Particle-Mesh Ewald |
References
- Zeng, F.; Zhang, L.; Deng, F.; Lou, S. Early-Life Exposure to Di (2-Ethyl-Hexyl) Phthalate: Role in Children with Endocrine Disorders. Front. Cell Dev. Biol. 2023, 11, 1115229. [Google Scholar] [CrossRef]
- Chapon, V.; Brignon, J.-M.; Gasperi, J. Non-Persistent Chemicals in Polymer and Non-Polymer Products Can Cause Persistent Environmental Contamination: Evidence with DEHP in Europe. Environ. Sci. Pollut. Res. Int. 2023, 30, 44952–44962. [Google Scholar] [CrossRef]
- Bider, R.-C.; Lluka, T.; Himbert, S.; Khondker, A.; Qadri, S.M.; Sheffield, W.P.; Rheinstädter, M.C. Stabilization of Lipid Membranes through Partitioning of the Blood Bag Plasticizer Di-2-Ethylhexyl Phthalate (DEHP). Langmuir 2020, 36, 11899–11907. [Google Scholar] [CrossRef] [PubMed]
- Safarpour, S.; Ghasemi-Kasman, M.; Safarpour, S.; Darban, Y.M. Effects of Di-2-Ethylhexyl Phthalate on Central Nervous System Functions: A Narrative Review. Curr. Neuropharmacol. 2022, 20, 766–776. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, J.; Wang, Z.; Wang, J.; Allen, D.T. Coupled Dynamic Material Flow, Multimedia Environmental Model, and Ecological Risk Analysis for Chemical Management: A Di(2-Ethylhexhyl) Phthalate Case in China. Environ. Sci. Technol. 2022, 56, 11006–11016. [Google Scholar] [CrossRef] [PubMed]
- Carli, F.; Tait, S.; Busani, L.; Ciociaro, D.; Della Latta, V.; Pala, A.P.; Deodati, A.; Raffaelli, A.; Pratesi, F.; Conte, R.; et al. Exposure to Endocrine Disruptors (Di(2-Ethylhexyl)Phthalate (DEHP) and Bisphenol A (BPA)) in Women from Different Residing Areas in Italy: Data from the LIFE PERSUADED Project. Int. J. Mol. Sci. 2022, 23, 16012. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yan, J.; Yin, T.; Pan, Y.; Chen, M.; Wang, X.; Wu, L.; Ding, H. Di (2-Ethylhexyl) Phthalate Decrease Pregnancy Rate via Disrupting the Microbe-Gut-Hypothalamic-Pituitary-Ovarian Axis in Mice. npj Biofilms Microbiomes 2025, 11, 107. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y. Mechanism Exploration of Di(2-Ethylhexyl) Phthalate (DEHP)-Induced Breast Cancer via Network Toxicology and Molecular Docking Analysis. Sci. Rep. 2025, 15, 27257. [Google Scholar] [CrossRef]
- Xie, Q.; Cao, H.; Liu, H.; Xia, K.; Gao, Y.; Deng, C. Prenatal DEHP Exposure Induces Lifelong Testicular Toxicity by Continuously Interfering with Steroidogenic Gene Expression. Transl. Androl. Urol. 2024, 13, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, Y.; Wang, X.; Zhang, Q.; Wang, L.; Zhang, X.; Cui, W.; Han, X.; Ma, N.; Li, H.; et al. Role of Hepatocyte- and Macrophage-Specific PPARγ in Hepatotoxicity Induced by Diethylhexyl Phthalate in Mice. Environ. Health Perspect. 2022, 130, 17005. [Google Scholar] [CrossRef]
- Kratochvil, I.; Hofmann, T.; Rother, S.; Schlichting, R.; Moretti, R.; Scharnweber, D.; Hintze, V.; Escher, B.I.; Meiler, J.; Kalkhof, S.; et al. Mono(2-Ethylhexyl) Phthalate (MEHP) and Mono(2-Ethyl-5-Oxohexyl) Phthalate (MEOHP) but Not Di(2-Ethylhexyl) Phthalate (DEHP) Bind Productively to the Peroxisome Proliferator-Activated Receptor γ. Rapid Commun. Mass Spectrom. 2019, 33 (Suppl. 1), 75–85. [Google Scholar] [CrossRef]
- Wei, X.; Yang, D.; Zhang, B.; Fan, X.; Du, H.; Zhu, R.; Sun, X.; Zhao, M.; Gu, N. Di-(2-Ethylhexyl) Phthalate Increases Plasma Glucose and Induces Lipid Metabolic Disorders via FoxO1 in Adult Mice. Sci. Total Environ. 2022, 842, 156815. [Google Scholar] [CrossRef]
- Ding, W.-J.; Huang, S.-L.; Huang, S.; Xu, W.-P.; Wei, W. Di(2-Ethylhexyl) Phthalate Mediates Oxidative Stress and Activates p38MAPK/NF-kB to Exacerbate Diabetes-Induced Kidney Injury In Vitro and In Vivo Models. Toxicol. Res. 2023, 12, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in Biology and Targeted Therapy: New Insights and Translational Implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Cheng, D.; Lei, C.; Li, H.; Zhou, J.; Zhang, C.; Song, F.; Zeng, T.; Zhao, X. Chronic Di(2-Ethylhexyl) Phthalate Exposure at Environmental-Relevant Doses Induces Osteoporosis by Disturbing the Differentiation of Bone Marrow Mesenchymal Stem Cells. Sci. Total Environ. 2024, 914, 169918. [Google Scholar] [CrossRef]
- Heilmann, N.Z.; Reeves, K.W.; Hankinson, S.E. Phthalates and Bone Mineral Density: A Systematic Review. Environ. Health 2022, 21, 108. [Google Scholar] [CrossRef]
- Lai, C.-C.; Liu, F.-L.; Tsai, C.-Y.; Wang, S.-L.; Chang, D.-M. Di-(2-Ethylhexyl) Phthalate Exposure Links to Inflammation and Low Bone Mass in Premenopausal and Postmenopausal Females: Evidence from Ovariectomized Mice and Humans. Int. J. Rheum. Dis. 2022, 25, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Giannattasio, R.; Lisco, G.; Giagulli, V.A.; Settembrini, S.; De Pergola, G.; Guastamacchia, E.; Lombardi, G.; Triggiani, V. Bone Disruption and Environmental Pollutants. Endocr. Metab. Immune Disord.-Drug Targets 2022, 22, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Q. Novel Insights into Osteocyte and Inter-Organ/Tissue Crosstalk. Front. Endocrinol. 2023, 14, 1308408. [Google Scholar] [CrossRef]
- Furuya, M.; Kikuta, J.; Fujimori, S.; Seno, S.; Maeda, H.; Shirazaki, M.; Uenaka, M.; Mizuno, H.; Iwamoto, Y.; Morimoto, A.; et al. Direct Cell-Cell Contact between Mature Osteoblasts and Osteoclasts Dynamically Controls Their Functions In Vivo. Nat. Commun. 2018, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-H.; Chen, L.-R.; Chen, K.-H. Primary Osteoporosis Induced by Androgen and Estrogen Deficiency: The Molecular and Cellular Perspective on Pathophysiological Mechanisms and Treatments. Int. J. Mol. Sci. 2024, 25, 12139. [Google Scholar] [CrossRef]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP Signaling in Osteoblast, Skeletal Development, and Bone Formation, Homeostasis and Disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Lecka-Czernik, B.; Rosen, C.J.; Napoli, N. The Role of Bone in Whole-Body Energy Metabolism. Nat. Rev. Endocrinol. 2025. [Google Scholar] [CrossRef]
- Huang, K.; Cai, H. The Interplay between Osteoarthritis and Osteoporosis: Mechanisms, Implications, and Treatment Considerations–A Narrative Review. Exp. Gerontol. 2024, 197, 112614. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, M.; Namane, M.; Cicuttini, F. Osteoarthritis. Lancet 2025, 405, 71–85, Erratum in Lancet 2025, 405, 2278. https://doi.org/10.1016/S0140-6736(25)01292-9. [Google Scholar] [CrossRef]
- Ma, M.; Tan, Z.; Li, W.; Zhang, H.; Liu, Y.; Yue, C. Osteoimmunology and Osteonecrosis of the Femoral Head. Bone Joint Res. 2022, 11, 26–28. [Google Scholar] [CrossRef]
- Kim, E.H.; Jeon, S.; Park, J.; Ryu, J.H.; Mobasheri, A.; Matta, C.; Jin, E.-J. Progressing Future Osteoarthritis Treatment toward Precision Medicine: Integrating Regenerative Medicine, Gene Therapy and Circadian Biology. Exp. Mol. Med. 2025, 57, 1133–1142. [Google Scholar] [CrossRef]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Haqqi, T.M. A Wogonin-Rich-Fraction of Scutellaria baicalensis Root Extract Exerts Chondroprotective Effects by Suppressing IL-1β-Induced Activation of AP-1 in Human OA Chondrocytes. Sci. Rep. 2017, 7, 43789. [Google Scholar] [CrossRef]
- Hines, J.T.; Jo, W.L.; Cui, Q.; Mont, M.A.; Koo, K.H.; Cheng, E.Y.; Goodman, S.B.; Ha, Y.C.; Hernigou, P.; Jones, L.C.; et al. Osteonecrosis of the Femoral Head: An Updated Review of ARCO on Pathogenesis, Staging and Treatment. J. Korean Med. Sci. 2021, 36, e177. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, J.; Liu, Y.; Zhao, H. Advances in the Pathogenesis of Steroid-Associated Osteonecrosis of the Femoral Head. Biomolecules 2024, 14, 667. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, S.; Leng, Z.; Chen, S.; Shi, Y.; Shi, L.; Li, J.; Mao, K.; Tang, H.; Meng, B.; et al. Pathological Mechanisms and Related Markers of Steroid-Induced Osteonecrosis of the Femoral Head. Ann. Med. 2024, 56, 2416070. [Google Scholar] [CrossRef]
- Zhivodernikov, I.V.; Kirichenko, T.V.; Markina, Y.V.; Postnov, A.Y.; Markin, A.M. Molecular and Cellular Mechanisms of Osteoporosis. Int. J. Mol. Sci. 2023, 24, 15772. [Google Scholar] [CrossRef]
- Gopinath, V. Osteoporosis. Med. Clin. N. Am. 2023, 107, 213–225. [Google Scholar] [CrossRef]
- Liu, C.; Zuo, M.; Zhao, J.; Niu, T.; Hu, A.; Wang, H.; Zeng, X. DPHB Inhibits Osteoclastogenesis by Suppressing NF-κB and MAPK Signaling and Alleviates Inflammatory Bone Destruction. Int. Immunopharmacol. 2025, 152, 114377. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ibarra, A.; Cerbón, M.; Martínez-Razo, L.D.; Morales-Pacheco, M.; Torre-Villalvazo, I.; Kawa, S.; Rodríguez-Dorantes, M. Impact of DEHP Exposure on Female Reproductive Health: Insights into Uterine Effects. Environ. Toxicol. Pharmacol. 2024, 107, 104391. [Google Scholar] [CrossRef] [PubMed]
- Valls-Margarit, J.; Piñero, J.; Füzi, B.; Cerisier, N.; Taboureau, O.; Furlong, L.I. Assessing Network-Based Methods in the Context of System Toxicology. Front. Pharmacol. 2023, 14, 1225697. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, L.; Pang, L.; Pu, K.; Fu, J.; Shen, Y.; Zhang, W.; Xu, H.; Niu, Y. Application and Development Trends of Network Toxicology in the Safety Assessment of Traditional Chinese Medicine. J. Ethnopharmacol. 2025, 343, 119480. [Google Scholar] [CrossRef]
- Shah, A.B.; Baiseitova, A.; Amzeyeva, U.; Shang, X.; Jenis, J. α-Glucosidase Inhibition-Guided Network Pharmacology and Molecular Docking Reveal the Antidiabetic Potential of Cichorium intybus as a Functional Food. Int. J. Mol. Sci. 2025, 26, 9497. [Google Scholar] [CrossRef]
- Huang, C.; Xiao, Y.; Qing, L.; Tang, J.; Wu, P. Exosomal Non-Coding RNAs in the Regulation of Bone Metabolism Homeostasis: Molecular Mechanism and Therapeutic Potential. Heliyon 2025, 11, e41632. [Google Scholar] [CrossRef]
- Győri, D.S. Research on Bone Cells in Health and Disease. Int. J. Mol. Sci. 2024, 25, 8758. [Google Scholar] [CrossRef]
- Choi, J.I.; Cho, H.H. Effects of Di(2-Ethylhexyl)Phthalate on Bone Metabolism in Ovariectomized Mice. J. Bone Metab. 2019, 26, 169–177. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, J.; Ye, Z.; Luo, J.; Peng, B.; Wang, Z. Research on the Role and Mechanism of the PI3K/Akt/mTOR Signalling Pathway in Osteoporosis. Front. Endocrinol. 2025, 16, 1541714. [Google Scholar] [CrossRef]
- Matsubara, T.; Yasuda, K.; Mizuta, K.; Kawaue, H.; Kokabu, S. Tyrosine Kinase Src Is a Regulatory Factor of Bone Homeostasis. Int. J. Mol. Sci. 2022, 23, 5508. [Google Scholar] [CrossRef]
- Pelusi, L.; Mandatori, D.; Di Pietrantonio, N.; Del Pizzo, F.; Di Tomo, P.; Di Pietro, N.; Buda, R.; Genovese, S.; Epifano, F.; Pandolfi, A.; et al. Estrogen Receptor 1 (ESR1) and the Wnt/β-Catenin Pathway Mediate the Effect of the Coumarin Derivative Umbelliferon on Bone Mineralization. Nutrients 2022, 14, 3209. [Google Scholar] [CrossRef]
- Bai, X.-H.; Su, J.; Mu, Y.-Y.; Zhang, X.-Q.; Li, H.-Z.; He, X.-F.; He, X.-F. Association between the ESR1 and ESR2 Polymorphisms and Osteoporosis Risk: An Updated Meta-Analysis. Medicine 2023, 102, e35461. [Google Scholar] [CrossRef]
- Toumba, M.; Kythreotis, A.; Panayiotou, K.; Skordis, N. Estrogen Receptor Signaling and Targets: Bones, Breasts and Brain (Review). Mol. Med. Rep. 2024, 30, 144. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Yu, Y.; Li, C.; Han, J.; Xu, J. Phosphorylation of BCL2 at the Ser70 Site Mediates RANKL-Induced Osteoclast Precursor Autophagy and Osteoclastogenesis. Mol. Med. 2022, 28, 22. [Google Scholar] [CrossRef] [PubMed]
- Vogler, M.; Braun, Y.; Smith, V.M.; Westhoff, M.-A.; Pereira, R.S.; Pieper, N.M.; Anders, M.; Callens, M.; Vervliet, T.; Abbas, M.; et al. The BCL2 Family: From Apoptosis Mechanisms to New Advances in Targeted Therapy. Signal Transduct. Target. Ther. 2025, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Garcia-Saez, A.J. Mechanisms of BCL-2 Family Proteins in Mitochondrial Apoptosis. Nat. Rev. Mol. Cell Biol. 2023, 24, 732–748. [Google Scholar] [CrossRef]
- Eskandari, E.; Negri, G.L.; Tan, S.; MacAldaz, M.E.; Ding, S.; Long, J.; Nielsen, K.; Spencer, S.E.; Morin, G.B.; Eaves, C.J. Dependence of Human Cell Survival and Proliferation on the CASP3 Prodomain. Cell Death Discov. 2024, 10, 63. [Google Scholar] [CrossRef]
- You, L.; Gu, W.; Chen, L.; Pan, L.; Chen, J.; Peng, Y. MiR-378 Overexpression Attenuates High Glucose-Suppressed Osteogenic Differentiation through Targeting CASP3 and Activating PI3K/Akt Signaling Pathway. Int. J. Clin. Exp. Pathol. 2014, 7, 7249–7261. [Google Scholar]
- Miura, M.; Chen, X.-D.; Allen, M.R.; Bi, Y.; Gronthos, S.; Seo, B.-M.; Lakhani, S.; Flavell, R.A.; Feng, X.-H.; Robey, P.G.; et al. A Crucial Role of Caspase-3 in Osteogenic Differentiation of Bone Marrow Stromal Stem Cells. J. Clin. Investig. 2004, 114, 1704–1713. [Google Scholar] [CrossRef] [PubMed]
- Iantomasi, T.; Romagnoli, C.; Palmini, G.; Donati, S.; Falsetti, I.; Miglietta, F.; Aurilia, C.; Marini, F.; Giusti, F.; Brandi, M.L. Oxidative Stress and Inflammation in Osteoporosis: Molecular Mechanisms Involved and the Relationship with microRNAs. Int. J. Mol. Sci. 2023, 24, 3772. [Google Scholar] [CrossRef]
- Ru, J.-Y.; Wang, Y.-F. Osteocyte Apoptosis: The Roles and Key Molecular Mechanisms in Resorption-Related Bone Diseases. Cell Death Dis. 2020, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zou, J.; Niimi, M.; Qiu, X.; Zhang, S.; Yang, H.; Zhu, M.; Fan, J. Matrix Metalloproteinase-9 Enhances Osteoclastogenesis: Insights from Transgenic Rabbit Bone Marrow Models and In Vitro Studies. Int. J. Mol. Sci. 2025, 26, 3194. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.E.-Z.A.; Abd-Elhafeez, H.H.; Taha, R.S.; Osman, A.; Almaki, M.M.; Baker, Z.M.; Alghamdi, A.A.A.; Massoud, D.; Elmasry, M.; Soliman, S.A.; et al. MMP-9 Expression in Bone Cells in the Developing Femur. Tissue Cell 2025, 95, 102862. [Google Scholar] [CrossRef]
- Qadeer, A.; Anis, M.; Warner, G.R.; Potts, C.; Giovanoulis, G.; Nasr, S.; Archundia, D.; Zhang, Q.; Ajmal, Z.; Tweedale, A.C.; et al. Global Environmental and Toxicological Data of Emerging Plasticizers: Current Knowledge, Regrettable Substitution Dilemma, Green Solution and Future Perspectives. Green Chem. 2024, 26, 5635–5683. [Google Scholar] [CrossRef]
- Fu, L.; Shi, S.; Yi, J.; Wang, N.; He, Y.; Wu, Z.; Peng, J.; Deng, Y.; Wang, W.; Wu, C.; et al. ADMETlab 3.0: An Updated Comprehensive Online ADMET Prediction Platform Enhanced with Broader Coverage, Improved Performance, API Functionality and Decision Support. Nucleic Acids Res. 2024, 52, W422–W431. [Google Scholar] [CrossRef]
- Banerjee, P.; Kemmler, E.; Dunkel, M.; Preissner, R. ProTox 3.0: A Webserver for the Prediction of Toxicity of Chemicals. Nucleic Acids Res. 2024, 52, W513–W520. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Keiser, M.J.; Roth, B.L.; Armbruster, B.N.; Ernsberger, P.; Irwin, J.J.; Shoichet, B.K. Relating Protein Pharmacology by Ligand Chemistry. Nat. Biotechnol. 2007, 25, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Zdrazil, B.; Felix, E.; Hunter, F.; Manners, E.J.; Blackshaw, J.; Corbett, S.; de Veij, M.; Ioannidis, H.; Lopez, D.M.; Mosquera, J.F.; et al. The ChEMBL Database in 2023: A Drug Discovery Platform Spanning Multiple Bioactivity Data Types and Time Periods. Nucleic Acids Res. 2024, 52, D1180–D1192. [Google Scholar] [CrossRef]
- Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. PharmMapper Server: A Web Server for Potential Drug Target Identification Using Pharmacophore Mapping Approach. Nucleic Acids Res. 2010, 38, W609–W614. [Google Scholar] [CrossRef] [PubMed]
- UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2025, 53, D609–D617. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Bader, G.D.; Hogue, C.W.V. An Automated Method for Finding Molecular Complexes in Large Protein Interaction Networks. BMC Bioinform. 2003, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A Web Server for Functional Enrichment Analysis and Functional Annotation of Gene Lists (2021 Update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate Structure Prediction of Biomolecular Interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Schake, P.; Bolz, S.N.; Linnemann, K.; Schroeder, M. PLIP 2025: Introducing Protein-Protein Interactions to the Protein-Ligand Interaction Profiler. Nucleic Acids Res. 2025, 53, W463–W465. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, A.; Wenzel, C.; Hackenstrass, K.; Schwarzl, R.; Rüttiger, C.; Hugel, T.; Gallei, M.; Netz, R.R.; Balzer, B.N. Opposing Temperature Dependence of the Stretching Response of Single PEG and PNiPAM Polymers. J. Am. Chem. Soc. 2019, 141, 11603–11613. [Google Scholar] [CrossRef] [PubMed]

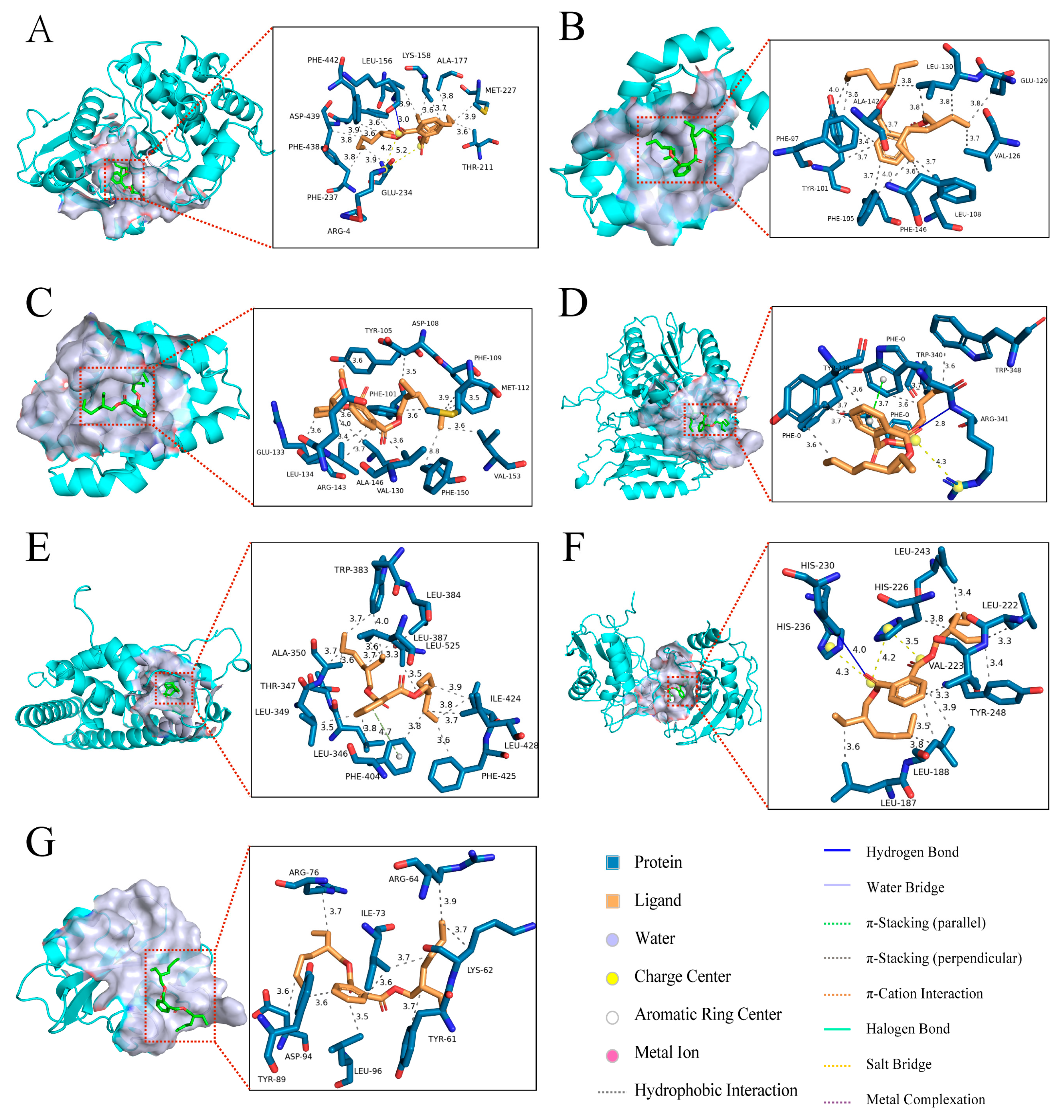
| Target Name | PDB ID | Binding Energy (kcal/mol) | Three-Dimensional Coordinates | Key Interacting Residues | RMSD |
|---|---|---|---|---|---|
| AKT1 | 3MVH | −8.2 | center_x = 18.326 center_y = −2.446 center_z = 28.06 | LYS158, ALA177, MET227, THR211, GLU234, PHE237, PHE438, ASP439, PHE442, LEU156 | 2.479 |
| BCL2L1 | 4EHR | −6.3 | center_x = 13.921 center_y = 30.454 center_z = 7.391 | LEU130, GLU129, VAL126, LEU108, PHE146, PHE105, TYR101, PHE97, ALA142, | 3.301 |
| BCL2 | 4LXD | −6.8 | center_x = 23.766 center_y = 32.657 center_z = 10.164 | TYR105, ASP108, PHE109, MET112, VAL153, PHE150, VAL130, ALA146, ARG143, LEU134, GLU133, PHE101 | 4.390 |
| CASP3 | 1RHJ | −6.5 | center_x = −96.163 center_y = 25.222 center_z = 45.774 | PHE0, TRP340, TRP348, ARG341, TYR338 | 4.004 |
| ESR1 | 1SJ0 | −7.7 | center_x = 31.406 center_y = −1.491 center_z = 25.297 | TRP383, LEU384, LEU387, LEU525, ILE424, LEU428, PHE425, PHE404, LEU346, LEU349, THR347, ALA350 | 2.494 |
| MMP9 | 4H1Q | −7.6 | center_x = 29.306 center_y = −5.638 center_z = 25.733 | LEU243, LEU222, TYR248, LEU188, LEU187, HIS236, HIS230, HIS226, VAL223 | 5.571 |
| SRC | 1O43 | −5.0 | center_x = 19.624 center_y = 22.011 center_z = 21.789 | ARG76, ARG64, ILE73, LYS62, TYR61, LEU96, ASP94, TYR89 | 1.509 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Z.; Du, H.; Zhou, X.; Wang, C.; Zhang, M.; Sun, T.; Wang, Y.; Wang, P. Network Toxicology and Molecular Docking Reveal the Toxicological Mechanisms of DEHP in Bone Diseases. Int. J. Mol. Sci. 2025, 26, 10895. https://doi.org/10.3390/ijms262210895
Fan Z, Du H, Zhou X, Wang C, Zhang M, Sun T, Wang Y, Wang P. Network Toxicology and Molecular Docking Reveal the Toxicological Mechanisms of DEHP in Bone Diseases. International Journal of Molecular Sciences. 2025; 26(22):10895. https://doi.org/10.3390/ijms262210895
Chicago/Turabian StyleFan, Zhonghao, Haitao Du, Xinyi Zhou, Cheng Wang, Mengru Zhang, Tiefeng Sun, Yi Wang, and Ping Wang. 2025. "Network Toxicology and Molecular Docking Reveal the Toxicological Mechanisms of DEHP in Bone Diseases" International Journal of Molecular Sciences 26, no. 22: 10895. https://doi.org/10.3390/ijms262210895
APA StyleFan, Z., Du, H., Zhou, X., Wang, C., Zhang, M., Sun, T., Wang, Y., & Wang, P. (2025). Network Toxicology and Molecular Docking Reveal the Toxicological Mechanisms of DEHP in Bone Diseases. International Journal of Molecular Sciences, 26(22), 10895. https://doi.org/10.3390/ijms262210895







