Abstract
Wheat (Triticum aestivum L.) is the most widely cultivated staple food crop globally. As a primary food source for 35–40% of the world’s population, the stability of its yield is directly linked to global food security. However, extreme weather events triggered by climate change have led to reductions in wheat yield, resulting in an urgent need to enhance the stress tolerance of wheat against drought and high temperatures. In this study, we successfully isolated and cloned a myo-inositol oxygenase gene from wheat. Further research revealed that high temperatures and drought stress significantly increased the expression level of the TaMIOXA gene in wheat leaves. A batch of overexpressing lines was obtained via Agrobacterium-mediated transformation. Compared to the control group, wheat plants with molecularly modified TaMIOXA overexpression exhibited stronger resistance to high temperatures and drought. This significantly increased their survival rates by 10% to 40%. The cumulative amount of hydrogen peroxide decreased from 7.86 × 10−4 to 1.54 × 10−2 mmol/g, and that of malondialdehyde decreased from 8.42 × 10−7 to 2.21 × 10−6 mmol/g. This confirms that overexpression of myo-inositol oxygenase significantly enhances wheat’s tolerance to drought and high temperatures. This study offers valuable genetic resources for wheat stress tolerance.
1. Introduction
Wheat (Triticum aestivum L.) is the most widely cultivated staple crop worldwide [], spanning a broad latitudinal range from 60° N to 40° S and occupying 30.7% of the global grain-cropping area []. It serves as a primary food source for 35–40% of the world’s population [], supplying approximately 20% of humanity’s caloric and protein requirements []. Consequently, the stability of wheat yields is directly linked to global food security []. However, extreme weather events driven by climate change are threatening wheat production systems at an unprecedented rate []. Current studies indicate that for every 1 °C rise in global temperature, the environmental adaptability of existing wheat varieties declines by 8.7% []. High temperatures directly damage the photosynthetic apparatus of wheat leaves, disrupting their chloroplast structure [] and reducing Rubisco activity by 55–70%, which severely weakens carbon assimilation []. Moreover, stress-induced bursts of reactive oxygen species (ROS) compromise the cellular membrane system, trigger oxidative stress [], and accelerate plant senescence []. In the water dimension, drought stress leads to abnormal stomatal opening and closing, markedly decreasing water use efficiency and thereby inhibiting nutrient uptake and material transport []. In addition, drought stress damages the cellular membrane system of crops [], elevates the production of reactive oxygen species (ROS), and disrupts the balance between ROS generation and scavenging []. This leads to membrane lipid peroxidation and oxidative stress in plant cells, resulting in increased malondialdehyde (MDA) content and heightened cell-membrane permeability []. Moreover, drought stress inactivates membrane-associated proteins, enzymes, and other biomolecules, impairing the structure and function of the biofilm and, in severe cases, causing crop death [].
Currently, research on plant resistance to abiotic stresses such as drought and high temperature has evolved into a multidimensional, multilevel systematic framework. Significant progress has been made in screening and functional analysis of related genes. For instance, TaSnRK2.10 enhances drought tolerance by phosphorylating TaERD15 and TaENO1 [], whereas TaSHN1 improves drought resistance by modulating leaf architecture without compromising yield []. In terms of hormonal and signaling regulation, progesterone contributes to the stability of photosystem II under heat stress []. Moreover, endoplasmic reticulum stress-related miRNAs are widely implicated in stress responses []. In rye and maize, exogenous application of 2,4-epibrassinolide and introduction of C4-type PEPC genes have been shown to markedly enhance photosynthetic performance and improve tolerance to high temperatures [,]. In addition, members of transcription-factor families such as MYB and SPL (e.g., TaMYB31 and TaSPL6) exert opposite regulatory effects on drought-response processes, with MYB acting as a positive regulator and SPL as a negative regulator [,]. Moreover, other crop genes—including maize ZmSCE1e, ZmERF21, and tobacco NtabDOG1L—enhance drought and heat tolerance through sumoylation modification and the regulation of hormone-signaling pathways and antioxidant mechanisms, respectively [,,]. The down-regulation of the cytokinin receptor SlHK2 enhances tomato resistance to combined stresses [], while the heat shock factor TaHsfA2d also participates in the response to phosphorus deficiency []. These studies systematically elucidate the complex regulatory network involving multiple species and genes in abiotic stress responses, and they provide a wealth of candidate genes and a solid theoretical foundation for the genetic improvement of wheat stress resistance.
Myo-inositol oxygenase (MIOX) is a pivotal metabolic enzyme that plays a crucial role in plant growth, development, and metabolism, particularly in response to abiotic stress []. As a key enzyme in the inositol metabolic pathway, MIOX regulates the cellular inositol pool, thereby influencing the synthesis of L-ascorbic acid (L-ASA) and the biosynthesis of cell-wall polysaccharides []. Moreover, MIOX significantly contributes to plant tolerance against various abiotic stresses, including drought, salinity, and low temperature. For example, overexpression of the MIOX gene in cotton enhances the plant’s drought and salt resistance [], while its overexpression in Arabidopsis improves tolerance to a range of abiotic stresses []. Current studies have demonstrated that MIOX is associated with plant resistance across various species. Elevating the expression level of myo-inositol oxygenase enhances plant tolerance to abiotic stresses. Moreover, the expression pattern of the MIOX gene varies among different tissues and is induced by drought, salinity, and cold stress.
In view of the increasingly severe drought and high-temperature conditions that have affected Henan in recent years [], we aimed to investigate the role of TaMIOX in wheat tolerance to these stresses. We selected the main cultivated wheat varieties from different latitudes in the Huang-Huai-Hai Wheat Region of China for this study. The resulting transgenic lines were then subjected to RT-qPCR and leaf protein extraction, confirming that the pCAMBIA1302-TaMIOXA construct functions effectively in wheat. The transgenic lines overexpressing myo-inositol oxygenase and the corresponding control plants were subjected to simulated drought and high-temperature stress. The overexpression group displayed a markedly higher survival rate than the control group, demonstrating that elevated TaMIOX expression significantly enhances wheat tolerance to combined drought and heat stress. In response to the increasingly severe meteorological stress issues in the Huang-Huai-Hai wheat region, particularly in Henan Province, this study aims to provide precise genetic targets for the stress resistance genetic improvement of the main cultivated wheat varieties in this area. To furnish new genetic resources for the in-depth elucidation of the molecular mechanisms underlying wheat’s response to combined drought and high-temperature stress, and to consolidate the theoretical and experimental foundation for stress-tolerant molecular breeding in wheat.
2. Results
2.1. Gene Sequence Information of Myo-Inositol Oxygenase
The coding sequence of TaMIOXA obtained from wheat was aligned with the reference sequence deposited in the public database (Figure S1). One nucleotide substitution was identified across all four wheat varieties. The phylogenetic tree was constructed from retrieved coding sequences (CDSs) encoding TaMIOXA-homologous proteins to elucidate evolutionary relationships among these homologs (Figure 1).
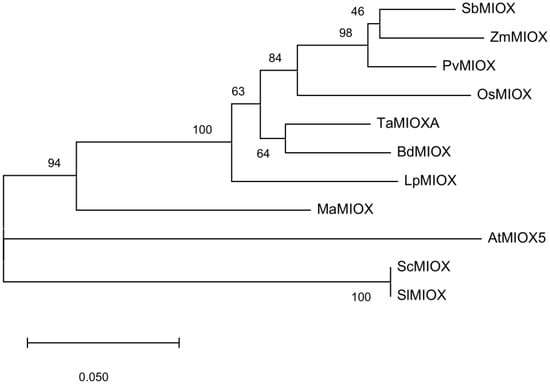
Figure 1.
A phylogenetic tree was generated using the wheat-derived TaMIOXA sequence. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The abbreviations and corresponding species names are as follows: TaMIOXA (Triticum aestivum L.), OsMIOX (Oryza sativa L.), BdMIOX (Brachypodium distachyon (L.) P. Beauv.), LpMIOX (Lolium perenne L.), PvMIOX (Panicum virgatum L.), SbMIOX (Sorghum bicolor (L.) Moench), ZmMIOX (Zea mays L.), AtMIOX5 (Arabidopsis thaliana (L.) Heynh.), MaMIOX (Musa acuminata Colla), ScMIOX (Solanum chilense), SlMIOX (Solanum lycopersicum L.).
2.2. Changes in Myo-Inositol Oxygenase Transcription Level Under Drought and High-Temperature Stress
We first subjected the cultivar Zhengmai 7698 to the drought regimen and subsequently extracted total RNA from its leaves. Quantitative reverse transcription PCR (RT qPCR) analysis revealed that drought stress significantly upregulated the transcription of the myo-inositol oxygenase gene in Zhengmai 7698. Subsequently, two additional wheat cultivars—Yangmai 13 and Bainong 207—were subjected to 40 °C heat treatment for 24 h. Quantitative reverse transcription PCR (RT-qPCR) analysis revealed that the abundant transcript of TaMIOX was significantly upregulated in all three wheat varieties. This demonstrates that both drought and high-temperature stress markedly upregulated TaMIOX transcription in wheat leaves (Figure 2).
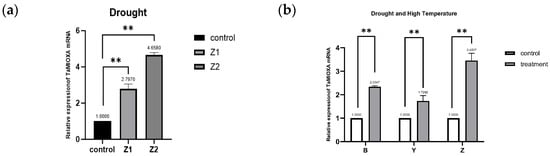
Figure 2.
(a) Expression of TaMIOXA in Zhengmai 7698 after 5-day drought treatment. (b) Expression levels of TaMIOXA in Bainong 207, Yangmai 13, and Zhengmai 7698 following 24-h combined drought and high-temperature treatment for MIOXA overexpression lines. Statistical analyses were performed using one-way analysis of variance (ANOVA). ** p < 0.01, Data are presented as mean ± SD; all experiments included n = 3 biological replicates.
2.3. Construction of Wheat MIOXA Overexpression Lines
For the construction of the pCAMBIA1302-TaMIOXA vector plasmid map, RNA was isolated from wheat leaves, and the TaMIOXA coding sequence was amplified from reverse-transcribed cDNA. The pCAMBIA1302-TaMIOXA overexpression plasmid (Figure 3d) was first assembled and subsequently introduced into Agrobacterium tumefaciens. Agrobacterium-mediated infection then yielded a batch of wheat plants transiently overexpressing TaMIOXA.
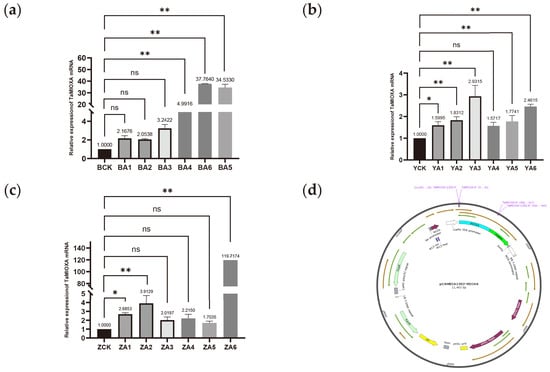
Figure 3.
(a) The expression level of TaMIOXA in the Bainong 207 overexpression strain. (b) The expression level of TaMIOXA in the Yangmai 13 overexpression strain. (c) The expression level of TaMIOXA in the Zhengmai 7698 overexpression strain. Statistical analyses were performed using one-way analysis of variance (ANOVA). ** p < 0.01, * p < 0.05; ns, not significant. Data are presented as mean ± SD; all experiments included n = 3 biological replicates. (d) pCAMBIA1302-TaMIOXA plasmid map.
Next, total RNA was extracted from the leaves of three independent TaMIOXA overexpression wheat lines and subjected to RT-qPCR analysis. As shown in Figure 3, the expression level of TaMIOXA was significantly upregulated in transgenic lines compared to the wild-type control group.
Subsequently, leaf proteins from the three wheat genotypes were extracted and purified using a His tag affinity protocol. The purified proteins were then resolved using SDS-PAGE (Figure 4). A distinct band corresponding to the expected molecular weight (~61.7 kDa) was observed in the supernatant of TaMIOX overexpression lines, whereas no detectable band appeared in the purified fractions from the control plants. The HIS-tag purified proteins from the overexpression lines and the corresponding Western blot (WB) results are shown in Figure S2. These results confirm that the pCAMBIA1302-TaMIOXA construct is functionally expressed in wheat leaves.
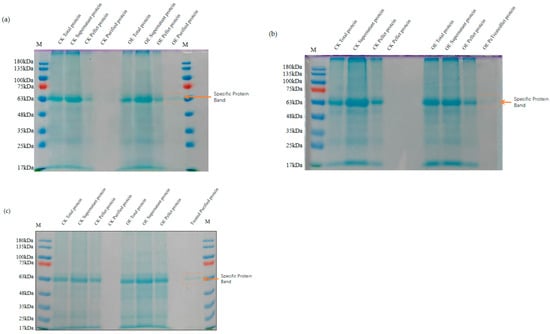
Figure 4.
SDS-PAGE electrophoresis results for the extraction of wheat leaf protein. OE: TaMIOXA overexpression. (a) Bainong 207. (b) Yangmai 13. (c) Zhengmai 7698. The specific protein band for the His-tagged fusion protein was detected at 63 kDa.
2.4. Detection of Various Indicators of Overexpressing Strains
2.4.1. Phenotype of Drought and High-Temperature Treatment
After the initial characterization, the three wheat cultivars were grown under identical conditions for 73 days and then subjected to combined drought and high-temperature stress treatment for 7 days, followed by a 10-day recovery period with adequate irrigation (Figure 5). Survival rates were recorded and are summarized in Table 1. The myo-inositol oxygenase overexpression lines displayed markedly higher survival rates than their respective wild-type controls. Notably, the TaMIOXA overexpression line of Yangmai 13 achieved the highest survival rate (53.33%), representing a 40% increase relative to the control. Among the three cultivars, the TaMIOXA overexpression line of Zhengmai 7698 exhibited the most pronounced improvement in survival compared to its control group. The quantitative PCR analysis of the three wheat cultivars revealed that lines exhibiting high TaMIOXA expression displayed markedly enhanced tolerance to both drought and high-temperature stress. Among the tested varieties, the Zhengmai 7698 cultivar showed the most pronounced improvement in stress resistance.
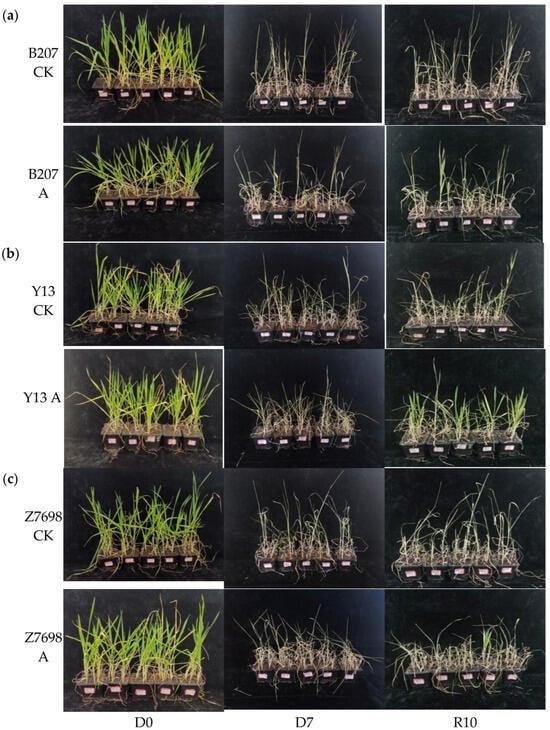
Figure 5.
Images of control and overexpression groups subjected to dry-heat treatment for 0 days and 7 days, followed by 10 days of recovery from rehydration. (a) Bainong 207. (b) Yangmai 13. (c) Zhengmai 7698. D0 (Day 0); D7 (Day 7); R10 (Recovery Day 10).

Table 1.
The recovery of wheat under stress conditions.
2.4.2. Detection of Physiological Indexes
To further elucidate the role of TaMIOXA in the tolerance of wheat to high temperatures and drought, five plants were randomly selected from each treatment group after exposure to combined heat–drought stress. Through DAB staining observations [], we found that the content of H2O2 in wheat leaves from the overexpression group was significantly lower than that of the control group after high-temperature and drought treatment (Figure 6). Among the tested wheat cultivars, Zhengmai 7698 displayed the greatest variation in staining, followed by Bainong 207, whereas the variation in Yangmai 13 was relatively unremarkable.
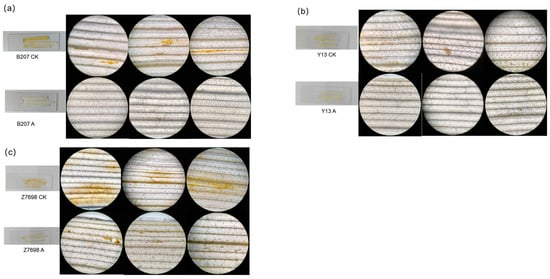
Figure 6.
The control and overexpression groups were subjected to 24 h dry-heat treatment, after which the leaves were examined using DAB staining. (a) Bainong 207. (b) Yangmai 13. (c) Zhengmai 7698.
The quantitative determination of H2O2 content before and after stress treatment corroborated the DAB-staining results. As shown in Figure 7, the H2O2 levels in all TaMIOXA-overexpression lines were significantly lower than the corresponding control plants. Specifically, the control plants Bainong 207, Yangmai 13, and Zhengmai 7698 exhibited H2O2 concentrations 1.04-, 1.19-, and 1.27-fold higher, respectively, than those of overexpression lines. Moreover, the relative accumulation of H2O2 (post-treatment versus pre-treatment) in the control groups was 1.42-fold, 1.02-fold, and 1.47-fold greater than that of the overexpression groups (Figure 7).
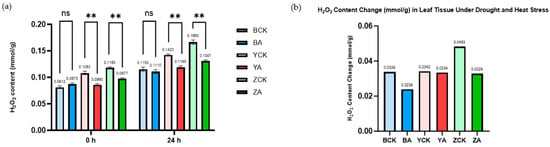
Figure 7.
The control and TaMIOXA-overexpression groups were subjected to 24 h dry-heat treatment, after which the above-ground tissues of the wheat plants were harvested for hydrogen peroxide (H2O2) quantification. (a) H2O2 content in the three wheat cultivars (Bainong 207, Yangmai 13, and Zhengmai 7698) for both control and overexpression lines, measured before and after the 24 h dry-heat stress test. Statistical analyses were performed using two-way analysis of variance (ANOVA). ** p < 0.01; ns, not significant. Data are presented as mean ± SD; all experiments included n = 3 biological replicates. (b) A cumulative change in H2O2 levels (post-treatment minus pre-treatment) for the same three cultivars, comparing control and overexpression groups after the 24 h dry-heat exposure test.
We also measured the catalase (CAT) activity of wheat before and after a 1-day drought and high-temperature treatment (Figure 8). As shown in Figure 8, the CAT activities of all TaMIOXA-overexpression lines were lower than those of the corresponding control plants after the stress. Specifically, the CAT activities in the control groups of Bainong 207, Yangmai 13, and Zhengmai 7698 were 1.13-, 1.08-, and 1.72-fold higher, respectively, than in the overexpression lines. Moreover, the increase in CAT activity from pre- to post-treatment was markedly greater in the control plants. The fold increase in the control groups of Bainong 207, Yangmai 13, and Zhengmai 7698 was 1.58-, 1.11-, and 4.00-fold higher.
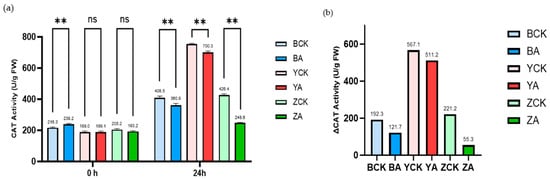
Figure 8.
The control and TaMIOXA-overexpression groups were subjected to a 24 h dry-heat treatment, after which the above-ground tissues of the wheat plants were harvested for catalase (CAT) activity measurement. (a) CAT activity in the three wheat cultivars (Bainong 207, Yangmai 13, and Zhengmai 7698) for both control and overexpression lines, measured before and after the 24 h dry-heat stress. (b) Cumulative changes in CAT activity (post-treatment minus pre-treatment) for the same three cultivars, comparing control and overexpression. Statistical analyses were performed using two-way analysis of variance (ANOVA). ** p < 0.01; ns, not significant. Data are presented as mean ± SD; all experiments included n = 3 biological replicates.
As illustrated in Figure 9, panel (a) displays the malondialdehyde (MDA) content in both control and TaMIOXA-overexpressing groups across three wheat varieties before and after combined drought and heat stress treatment. Panel (b) presents the cumulative MDA content values before and after treatment. Here, we found an interesting phenomenon, normal in the face of abiotic stress in plants: MDA content will gradually increase, accelerating cell death. However, we performed a one-day dry-heat treatment on three wheat varieties. It was found that compared with the control group, the MDA accumulation in the Yangmai 13 overexpression group decreased more. The MDA reduction in the control plants of Bainong 207 and Zhengmai 7698 was 1.67- and 1.83-fold greater, respectively, than that observed in the overexpression lines. This shows that the increase in MIOXA expression will reduce the content of MDA in wheat.
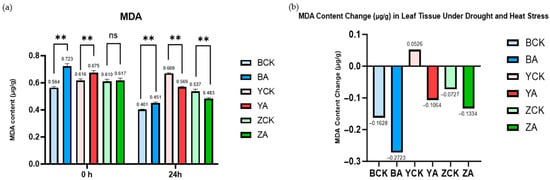
Figure 9.
The control and TaMIOXA-overexpression groups were subjected to 24 h dry-heat treatment, after which the above-ground tissues of the wheat plants were harvested for malondialdehyde (MDA) measurement. (a) MDA content in the three wheat cultivars (Bainong 207, Yangmai 13, and Zhengmai 7698) was measured for both control and overexpression lines, before and after the 24 h dry-heat stress test. (b) Net change in MDA levels (post-treatment minus pre-treatment) for the same three cultivars, comparing control and overexpression groups after 24 h of dry-heat exposure. Statistical analyses were performed using two-way analysis of variance (ANOVA). ** p < 0.01; ns, not significant. Data are presented as mean ± SD; all experiments included n = 3 biological replicates.
The detection results of glucuronic acid (GlcA) content in the control and TaMIOXA-overexpression groups (Figure 10) demonstrated that GlcA levels in all overexpression lines were lower than those in the corresponding controls. The reduction in GlcA in the control plants of Bainong 207, Yangmai 13, and Zhengmai 7698 was 1.09-, 1.05-, and 1.51-fold greater, respectively, than in the overexpression lines—an outcome that contradicts our initial hypothesis. Consequently, overexpression of TaMIOXA leads to a decrease in GlcA content.
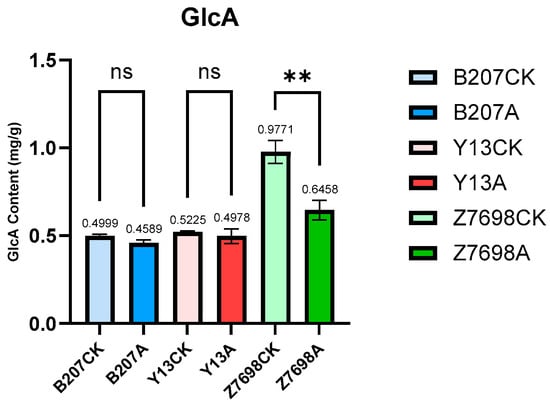
Figure 10.
The glucuronic acid (GlcA) content was measured in the above-ground tissues of wheat from both the control and TaMIOXA-overexpression groups. Statistical analyses were performed using one-way analysis of variance (ANOVA). ** p < 0.01; ns, not significant. Data are presented as mean ± SD; all experiments included n = 3 biological replicates.
In addition, the detection results of POD (peroxidase) and SOD (superoxide dismutase) showed that (Figure 11) the enzyme activity of POD is not significantly affected by TaMIOXA overexpression. The results of SOD enzyme activity were similar to those of CAT enzyme activity, and the increase in enzyme activity in the overexpression group was significantly lower than that in the control group. This indicates that the reaction substrate of TaMIOXA may partially overlap with the substrate of SOD, but the specific type of superoxide consumed by oxygenase requires further investigation.
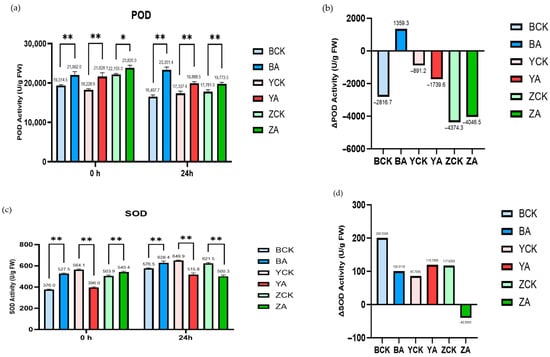
Figure 11.
The control and TaMIOXA-overexpression groups were subjected to 24 h dry-heat treatment, after which the above-ground tissues of the wheat plants were harvested for peroxidase (POD) and superoxide dismutase (SOD) activity assays. (a) POD activity in the three wheat cultivars (Bainong 207, Yangmai 13, and Zhengmai 7698) for both control and overexpression lines, measured before and after the 24 h dry-heat stress test. (b) The net change in POD activity (post-treatment minus pre-treatment) for the same three cultivars, comparing control and overexpression groups, after 24 h dry-heat exposure. (c) SOD activity in the three wheat cultivars for control and overexpression lines, measured before and after the 24 h dry-heat treatment test. (d) The net change in SOD activity (post-treatment minus pre-treatment) for the three cultivars, comparing control and overexpression groups, after the 24 h dry-heat exposure test. Statistical analyses were performed using two-way analysis of variance (ANOVA). ** p < 0.01, * p < 0.05. Data are presented as mean ± SD; n = 3.
3. Discussion
We isolated the TaMIOXA gene from four wheat cultivars—Chinese Spring, Zhengmai 7698, Yangmai 13, and Bainong 207. Compared with the reference sequence, all four isolates shared an identical nucleotide substitution that resulted in a single amino-acid change, converting tyrosine to cysteine at position 185 (Y185C). This mutation likely represents a novel natural variant that arose through regional or environmental selection. In addition, we designed distinct primers (Table S1) for the PCR amplification of TaMIOXB (Triticum aestivum myo-inositol oxygenase B) and TaMIOXD (Triticum aestivum myo-inositol oxygenase D) based on the CDS of TaMIOXA, TaMIOXB, and TaMIOXD. Despite numerous attempts, neither allele could be amplified from any developmental stage of the four wheat varieties.
In this study, we generated transient TaMIOXA-overexpressing lines in three wheat cultivars and subjected them to heat and drought stress. Our results demonstrate that TaMIOXA overexpression markedly enhances wheat tolerance to both drought and high temperatures; this effect is especially pronounced in Zhengmai 7698, a principal wheat variety cultivated in Henan Province. This demonstrates that up-regulating TaMIOXA expression offers a novel strategy for mitigating wheat yield losses caused by recent dry and hot wind events under increasingly extreme high-temperature and drought conditions. TaMIOXA can be used as a screening marker for new drought- and high-temperature-resistant wheat varieties. However, for the same overexpression lines, the RT-qPCR results obtained the lowest overexpression for Yangmai 13, which may be related to the breeding screening. The primary cultivation region of Yangmai 13 lies south of the Bainong 207 and Zhengmai 7698 zones, where temperatures are comparatively higher. Targeted breeding has endowed Yangmai 13 with enhanced tolerance to elevated temperatures, and its baseline expression of myo-inositol oxygenase is relatively high. This elevated expression may also explain why the control group of Yangmai 13 exhibited the greatest survival rate after 7 days of combined drought and heat stress treatment. As such, myo-inositol oxygenase can serve as a novel screening marker for wheat varieties exhibiting high temperature and drought tolerance.
In addition, studies investigating which oxidant is consumed by plant Myo-inositol oxygenase during its catalytic activity are scarce. The prevailing view is that plant Myo-inositol oxygenase functions as an oxidase that utilizes molecular oxygen. Other radioisotope-labeling studies have demonstrated that, after 18O2 labeling, a portion of the oxygen atoms incorporated into the reaction product of glucuronic acid originates from molecular oxygen [].
In this study, simultaneous monitoring of multiple wheat parameters during treatment allowed us to propose novel hypotheses concerning the oxidative substrates and stress-resistance mechanisms involved in the TaMIOXA reaction. After 1 day of treatment, the H2O2 levels in all overexpression groups were significantly lower than those in the control group. The cumulative amount of H2O2 in the overexpression groups was also comparatively reduced. On the contrary, the CAT enzyme activity of all overexpression wheat groups after drought and high-temperature treatment was lower than that of the control group. The level of CAT enzyme activity before and after treatment in the control group was significantly higher than that in the overexpression group. A comparison of cumulative H2O2 levels before and after wheat treatment indicates that the reduction in H2O2 accumulation observed in the overexpression lines is not attributable to catalase activity but is directly associated with the overexpression of TaMIOXA. TaMIOXA likely consumes H2O2 directly during the oxidation of myo-inositol.
We employed the AlphaFold3 online service to generate an ab initio model of TaMIOXA and subsequently performed molecular docking with AutoDock 4.26 to systematically screen and investigate the oxidation substrates and their corresponding binding sites on TaMIOXA. The results are displayed in a PyMOL map (Figure 12), and the corresponding hydrogen bond interaction network is marked. The mechanism of action and binding sites of myo-inositol oxygenase, inositol, and hydrogen peroxide were further predicted, as shown in Figure 12. The amino acids that have direct hydrogen bond interaction with inositol are serine (S103), aspartic acid oxygenase (D104), histidine (H139), aspartic acid (D140), histidine (H212), histidine (H238), serine (S239), aspartic acid (D271), and lysine (K275). Arginine (R76), methionine (M208), aspartic acid (D214), and arginine (R236) all experience direct hydrogen bonding interactions with hydrogen peroxide.
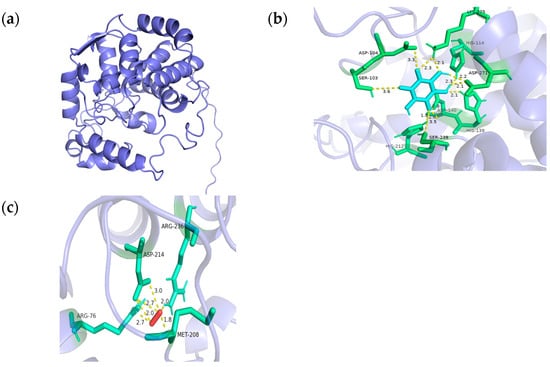
Figure 12.
Molecular docking results of the TaMIOXA protein. (a) Protein modeling results of myo-inositol oxygenase generated by AlphaFold3. (b) The hydrogen bonding network from the docking of myo-inositol oxygenase with inositol. (c) The hydrogen bonding network from the docking of myo-inositol oxygenase with hydrogen peroxide.
We performed a one-day dry-heat treatment test on three wheat cultivars. Apart from the control group of Yangmai 13, which showed an increase in MDA accumulation, the MDA content decreased in all other wheat groups. The increase in TaMIOXA expression during the early phase of treatment could have enhanced ASA synthesis while consuming a portion of MDA. Yangmai 13, the principal cultivar grown in southern regions, exhibits enhanced tolerance to high-temperature stress. Consequently, the expression level of TaMIOXA in the control group of Yangmai 13 was low, and the consumption (depletion) of malondialdehyde (MDA) was reduced. Therefore, we infer that TaMIOXA enhances wheat tolerance to high temperatures and drought, both due to the scavenging of reactive oxygen species and the promotion of ASA synthesis and other related pathways.
Finally, we measured the glucuronic acid content in both the control and overexpression groups of the three wheat varieties. The results demonstrated that the overexpression lines had a significantly lower glucuronic acid concentration than the controls, with the most pronounced decrease observed in the Zhengmai 7698 cultivar. Studies have demonstrated that overexpression of the myo-inositol oxygenase (MIOX) gene in Arabidopsis thaliana [], tomato (Solanum lycopersicum) [], and cucumber (Cucumis sativus) [] markedly enhances the content of ascorbic acid (ASA). Combining the phenotypic observations and indices of three wheat cultivars after dry-heat treatment, we hypothesize that, during the early stages of abiotic stress, the expression of myo-inositol oxygenase is upregulated. This enzyme scavenges accumulated reactive oxygen species (ROS) and produces glucuronic acid. Subsequently, a portion of glucuronic acid may be converted into ascorbic acid (ASA) via the ASA pathway, thereby reducing malondialdehyde (MDA) levels and enhancing the stress tolerance of wheat (Figure 13).
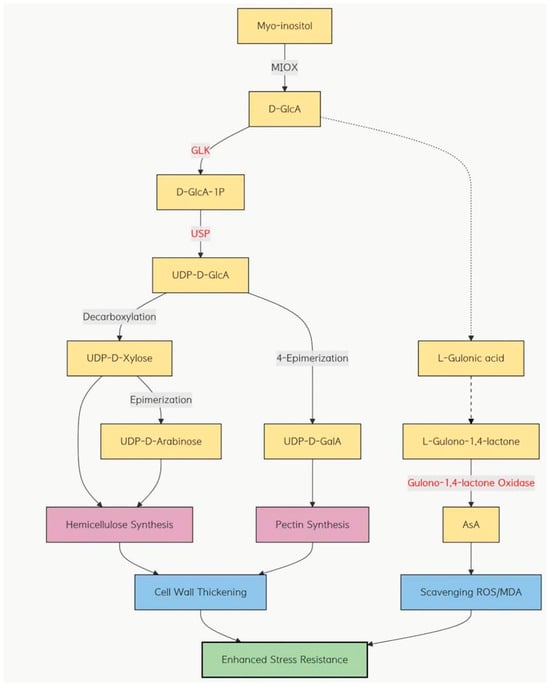
Figure 13.
The myo-inositol oxygenase (MIOX) pathway and its hypothesized role in related pathways under combined drought and heat stress.
4. Materials and Methods
4.1. Isolation and Cloning of TaMIOXA
Total RNA was extracted from wheat leaves using the total RNA extraction kit TRIpure Reagent (Heruibio, Fuzhou, China) [], and then, RNA was reverse-transcribed into cDNA using HisScript II Q RT superMix for qPCR (Vazyme, Nanjing, China) []. The primers were designed based on the nucleotide sequence of TaMIOXA. The cDNA was amplified by polymerase chain reaction (PCR) using 2 × Es Taq MasterMix (Dye). The PCR product was then recovered using agarose gel electrophoresis and purified with the HiPure Gel Pure DNA Mini Kit (Magen, Guangzhou, China). The recovered products were submitted for sequencing; the resulting reads were aligned using SnapeGene® 6.0.2 and subsequently translated into amino acid sequences [].
4.2. Bioinformatics Analysis of TaMIOXA
The nucleotide sequence of TaMIOXA was translated into a protein sequence using SnapeGene® 6.0.2 software. The protein structure of TaMIOXA was predicted using the AlphaFold Server (https://alphafoldserver.com/fold/5c40373bf5717a2e, accessed on 22 September 2025) provided by Google DeepMind. The platform is based on the AlphaFold3 model and can process various biomolecular inputs such as proteins and nucleic acids. The default parameters are used for prediction, and the custom template is not enabled. In order to perform molecular docking, this study obtained the three-dimensional structure files (format PDB) of hydrogen peroxide (H2O2) and superoxide anion (O2−) from the NCBI PubChem database (https://pubchem.ncbi.nlm.nih.gov/). The PubChem CIDs of the compounds were 784 and 5359597, respectively, and the structure of inositol was derived from ChemSpider (Inositol|C6H12O6) (https://www.chemspider.com/Chemical-Structure.10239179.html, accessed on 22 September 2025).
The translated amino acid sequences were analyzed using BLAST on NCBI platform, and the sequences with significant homology were selected for download []. Subsequently, phylogenetic tree analysis was performed using MEGA 7.0 []. After that, we used AutoDock 4.26 to perform molecular docking on the protein prediction structure of TaMIOXA [], and the results were displayed using PyMOLWin 2.5.5 [].
4.3. Expression Analysis of the TaMIOXA Gene
According to the method described by Wang et al. [], the expression of TaMIOXA was evaluated via RT-qPCR. Customized RT-qPCR primers (Table S1) were produced by targeting the conserved sequences of 26S Ribosomal RNA gene []. For the RT-qPCR process, we used the SYBR Green Realtime PCR premix from TaKaRa in Beijing, China [] to ensure accurate and reliable results. The relative expression of TaMIOXA was determined using the 2−ΔΔCt method. The experiment was repeated using three techniques [].
4.4. Preparation of Vector and Transformation of Wheat Plants
In this study, based on the coding-region sequence of the TaMIOXA gene and the sequence features of the pCAMBIA1302-GFP vector (Abcam, Hong Kong, China), NcoI and BglII were selected as restriction sites, and specific primers incorporating these sites were designed for homologous recombination experiments (Table S1). Subsequently, the constructed vector plasmid was transferred into Agrobacterium tumefaciens and transformed into wheat via Agrobacterium tumefaciens to obtain positive plants [].
4.5. Plant Materials and Treatments
According to the planting conditions at different latitudes in the Huang-Huai wheat region, three wheat varieties were selected: Zhengmai 7698 (34°16′ N–36°22′ N), Yangmai 13 (29°–33° N), and Bainong 207 (31°23′ N–36°22′ N). From each variety, 150 seeds were chosen, primarily ovoid or elliptical, with intact morphology, a balanced seed-coat to endosperm ratio, and fine surface striations or hairs. After sterilization with hydrogen peroxide [], the seeds were divided into two groups: a control group and a group subjected to Agrobacterium-mediated transformation. Following 2–3 days of germination, 50 healthy seeds from each treatment were transferred to cultivation pots, with 6–8 seeds per pot and 0.14 kg of dry soil per pot, which was then fully saturated with water. All treatment groups were maintained at 22 °C and 80 ± 5% relative humidity under a photoperiod of 16 h light/8 h dark and cultivated for 60–70 days. In each group, 30 healthy plants with complete roots, stems, and leaves were selected for stress exposure. After sampling, the samples were stored at −80 °C for further analysis.
4.6. Analysis of Physiological Indicators After Stress Overexpression of TaMIOXA in Wheat
Wheat plants in the overexpression groups (B207 A, Y13 A, Z7698 A) and the control groups (B207 CK, Y13 CK, Z7698 CK) were cultured in a light incubator for 10 weeks at 22 °C and 85% relative humidity, under a 16 h light/8 h dark photoperiod. The overexpression and control groups were simultaneously subjected to drought and high-temperature stress for 7 days, with conditions of 40 °C and 50% relative humidity for 14 h during the day, and 25 °C and 50% relative humidity for 8 h at night. After drought and high-temperature stress, the plants were re-watered and allowed to recover for 10 days. The above-ground parts of wheat were harvested, rapidly frozen in liquid nitrogen, and then stored at −80 °C for subsequent experiments. We quantified malondialdehyde (MDA) using the thiobarbituric acid (TBA) assay []. Hydrogen peroxide content was measured using the ammonium molybdate colorimetric method []; glucuronic acid was determined with high-performance liquid chromatography (HPLC); catalase (CAT) activity was assessed via UV spectrophotometry []; peroxidase (POD) activity was assessed using the guaiacol oxidation assay []; and superoxide dismutase (SOD) activity was assessed using the nitroblue tetrazolium (NBT) reduction assay [], respectively.
5. Conclusions
In summary, wheat plants were subjected to combined drought and heat stress, which induced a significant upregulation of myo-inositol oxygenase transcripts. To investigate the role of myo-inositol oxygenase in wheat under abiotic stress, we generated three TaMIOXA-overexpressing wheat lines. Following exposure to high-temperature and drought treatments, the results demonstrated that TaMIOXA overexpression markedly enhanced the tolerance of wheat to both heat and drought stresses. Analysis of various physiological indices indicated that myo-inositol oxygenase enhances the plant’s stress resistance via the coordinated regulation of multiple pathways.
Under abiotic stress conditions, wheat plants accumulate reactive oxygen species (ROS), which upregulate the expression of myo-inositol oxygenase. This enzyme subsequently utilizes ROS (such as hydrogen peroxide) and myo-inositol to produce glucuronic acid [], a process that may activate the ASA biosynthetic pathway, ultimately leading to ASA accumulation. ASA, in conjunction with superoxide dismutase (SOD), functions synergistically to scavenge malondialdehyde (MDA) in plants []. This cascade of reactions contributes to the maintenance of physiological homeostasis and improves abiotic stress tolerance. Thus, myo-inositol oxygenase represents a promising genetic target for enhancing heat and drought resistance in wheat, offering a novel strategy to mitigate the impact of extreme high-temperature events associated with global warming.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms262210894/s1.
Author Contributions
Conceptualization, K.C. and W.Z. (Wenming Zheng); methodology, K.C.; software, S.Z. and S.H.; validation, W.Z. (Wenmei Zhou), W.Q., Y.L. (Yimeng Liu) and Z.Z.; formal analysis, K.C. and K.Z.; investigation, P.S.; resources, L.L., P.S. and Y.L. (Yuhang Liu); data curation, L.L., S.Z. and S.H.; writing—original draft preparation, S.Z.; writing—review and editing, K.C. and W.Z. (Wenming Zheng); project administration, W.Z. (Wenming Zheng); funding acquisition, K.C. and D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Sub-project of the National Key R&D Program of China, grant number 2021YFF1000203-12.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Statement: The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We want to express our gratitude to the National Key R&D Program of China for funding this project.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| TaMIOXA | myo-inositol oxygenase gene in Triticum aestivum A-genome (Triticum urartu) |
| TaMIOXB | myo-inositol oxygenase gene in Triticum aestivum B-genome (Aegilops speltoides) |
| TaMIOXD | myo-inositol oxygenase gene in Triticum aestivum D-genome (Aegilops tauschii) |
| B207 A | TaMIOXA overexpressing lines of wheat cultivar Bainong 207 |
| Y13 A | TaMIOXA overexpressing lines of wheat cultivar Yangmai 13 |
| Z7698 A | TaMIOXA overexpressing lines of wheat cultivar Zhengmai 7698 |
| B207 CK | control line of wheat cultivar Bainong 207 |
| Y13 CK | control line of wheat cultivar Yangmai 13 |
| Z7698 CK | control line of wheat cultivar Zhengmai 7698 |
| CAT | catalase |
| SOD | superoxide dismutase |
| POD | peroxidase |
| GlcA | glucuronic acid |
| ASA | ascorbic acid |
| MDA | malondialdehyde |
References
- Wang, Y.; Yang, M.; Wei, S.; Qin, F.; Zhao, H.; Suo, B. Identification of Circular RNAs and Their Targets in Leaves of Triticum aestivum L. under Dehydration Stress. Front. Plant Sci. 2016, 7, 2024. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Li, Y.; Zhang, Z.; Yang, L.; Wang, M.; Zhang, Y.; Zhang, J.; Li, C.; Li, L.; et al. Wheat TaSnRK2.10 phosphorylates TaERD15 and TaENO1 and confers drought tolerance when overexpressed in rice. Plant Physiol. 2023, 191, 1344–1364. [Google Scholar] [CrossRef]
- Li, H.; Yu, M.; Zhu, X.; Nai, F.; Yang, R.; Wang, L.; Liu, Y.; Wei, Y.; Ma, X.; Yu, H.; et al. TaGSr contributes to low-nitrogen tolerance by optimizing nitrogen uptake and assimilation in Arabidopsis. Environ. Exp. Bot. 2024, 219, 105657. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Lewis, J.M.; Ammar, K.; Basnet, B.R.; Crespo-Herrera, L.; Crossa, J.; Dhugga, K.S.; Dreisigacker, S.; Juliana, P.; Karwat, H.; et al. Harnessing translational research in wheat for climate resilience. J. Exp. Bot. 2021, 72, 5134–5157. [Google Scholar] [CrossRef] [PubMed]
- Rathor, P.; Rouleau, V.; Gorim, L.Y.; Chen, G.; Thilakarathna, M.S. Humalite enhances the growth, grain yield, and protein content of wheat by improving soil nitrogen availability and nutrient uptake. J. Plant Nutr. Soil Sci. 2024, 187, 247–259. [Google Scholar] [CrossRef]
- Zhao, G.; Xu, H.; Zhang, P.; Su, X.; Zhao, H. Effects of 2,4-epibrassinolide on photosynthesis and Rubisco activase gene expression in Triticum aestivum L. seedlings under a combination of drought and heat stress. Plant Growth Regul. 2017, 81, 377–384. [Google Scholar] [CrossRef]
- Xiong, W.; Reynolds, M.P. Wheat breeding strategies for increased climate resilience. Nat. Clim. Change 2024, 14, 791–792. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Zhang, P.; Gao, M.; Wang, D.; Zhao, H. High temperature effects on D1 protein turnover in three wheat varieties with different heat susceptibility. Plant Growth Regul. 2017, 81, 1–9. [Google Scholar] [CrossRef]
- Perdomo, J.A.; Capó-Bauçà, S.; Carmo-Silva, E.; Galmés, J. Rubisco and Rubisco Activase Play an Important Role in the Biochemical Limitations of Photosynthesis in Rice, Wheat, and Maize under High Temperature and Water Deficit. Front. Plant Sci. 2017, 8, 490. [Google Scholar] [CrossRef]
- Liu, J.; Hasanuzzaman, M.; Wen, H.; Zhang, J.; Peng, T.; Sun, H.; Zhao, Q. High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma 2019, 256, 1217–1227. [Google Scholar] [CrossRef]
- Ding, H.; Ma, D.; Huang, X.; Hou, J.; Wang, C.; Xie, Y.; Wang, Y.; Qin, H.; Guo, T. Exogenous hydrogen sulfide alleviates salt stress by improving antioxidant defenses and the salt overly sensitive pathway in wheat seedlings. Acta Physiol. Plant. 2019, 41, 123. [Google Scholar] [CrossRef]
- Guo, X.; Xin, Z.; Yang, T.; Ma, X.; Zhang, Y.; Wang, Z.; Ren, Y.; Lin, T. Metabolomics Response for Drought Stress Tolerance in Chinese Wheat Genotypes (Triticum aestivum). Plants 2020, 9, 520. [Google Scholar] [CrossRef] [PubMed]
- Maseyk, K.; Lin, T.; Cochavi, A.; Schwartz, A.; Yakir, D. Quantification of leaf-scale light energy allocation and photoprotection processes in a Mediterranean pine forest under extensive seasonal drought. Tree Physiol. 2019, 39, 1767–1782. [Google Scholar] [CrossRef] [PubMed]
- Bouremani, N.; Cherif-Silini, H.; Silini, A.; Bouket, A.C.; Luptakova, L.; Alenezi, F.N.; Baranov, O.; Belbahri, L. Plant Growth-Promoting Rhizobacteria (PGPR): A Rampart against the Adverse Effects of Drought Stress. Water 2023, 15, 418. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, S.; Zhang, S.; Yan, P.; Wang, J.; Zhang, H. Glutathione, carbohydrate and other metabolites of Larix olgensis A. Henry reponse to polyethylene glycol-simulated drought stress. PLoS ONE 2021, 16, e0253780. [Google Scholar] [CrossRef]
- Liu, F.; Zhao, Y.; Wang, X.; Wang, B.; Xiao, F.; He, K. Physiological response and drought resistance evaluation of Gleditsia sinensis seedlings under drought-rehydration state. Sci. Rep. 2023, 13, 19963. [Google Scholar] [CrossRef]
- Bi, H.; Shi, J.; Kovalchuk, N.; Luang, S.; Bazanova, N.; Chirkova, L.; Zhang, D.; Shavrukov, Y.; Stepanenko, A.; Tricker, P.; et al. Overexpression of the TaSHN1 transcription factor in bread wheat leads to leaf surface modifications, improved drought tolerance, and no yield penalty under controlled growth conditions. Plant Cell Environ. 2018, 41, 2549–2566. [Google Scholar] [CrossRef]
- Xue, R.L.; Wang, S.Q.; Xu, H.L.; Zhang, P.J.; Li, H.; Zhao, H.J. Progesterone increases photochemical efficiency of photosystem II in wheat under heat stress by facilitating D1 protein phosphorylation. Photosynthetica 2017, 55, 664–670. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, X. Endoplasmic reticulum stress-responsive microRNAs are involved in the regulation of abiotic stresses in wheat. Plant Cell Rep. 2023, 42, 1433–1452. [Google Scholar] [CrossRef]
- Qi, X.; Xu, W.; Zhang, J.; Guo, R.; Zhao, M.; Hu, L.; Wang, H.; Dong, H.; Li, Y. Physiological characteristics and metabolomics of transgenic wheat containing the maize C4 phosphoenolpyruvate carboxylase (PEPC) gene under high temperature stress. Protoplasma 2017, 254, 1017–1030. [Google Scholar] [CrossRef]
- Zhao, Y.; Cheng, X.; Liu, X.; Wu, H.; Bi, H.; Xu, H. The Wheat MYB Transcription Factor TaMYB31 Is Involved in Drought Stress Responses in Arabidopsis. Front. Plant Sci. 2018, 9, 1426. [Google Scholar] [CrossRef]
- Zhao, Y.; He, J.; Liu, M.; Miao, J.; Ma, C.; Feng, Y.; Qian, J.; Li, H.; Bi, H.; Liu, W. The SPL transcription factor TaSPL6 negatively regulates drought stress response in wheat. Plant Physiol. Biochem. 2024, 206, 108264. [Google Scholar] [CrossRef]
- Dai, X.; Wang, Y.; Chen, Y.; Li, H.; Xu, S.; Yang, T.; Zhang, X.; Su, X.; Xia, Z. Overexpression of NtDOG1L-T Improves Heat Stress Tolerance by Modulation of Antioxidant Capability and Defense-, Heat-, and ABA-Related Gene Expression in Tobacco. Front. Plant Sci. 2020, 11, 568489. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Xia, Z. Overexpression of a maize SUMO conjugating enzyme gene (ZmSCE1e) increases Sumoylation levels and enhances salt and drought tolerance in transgenic tobacco. Plant Sci. 2019, 281, 113–121. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Ren, Z.; Abou-Elwafa, S.F.; Pu, X.; Zhu, Y.; Dou, D.; Su, H.; Cheng, H.; Liu, Z.; et al. ZmERF21 directly regulates hormone signaling and stress-responsive gene expression to influence drought tolerance in maize seedlings. Plant Cell Environ. 2022, 45, 312–328. [Google Scholar] [CrossRef]
- Mushtaq, N.; Wang, Y.; Fan, J.; Li, Y.; Ding, J. Down-Regulation of Cytokinin Receptor Gene SlHK2 Improves Plant Tolerance to Drought, Heat, and Combined Stresses in Tomato. Plants 2022, 11, 154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Miao, J.; He, J.; Tian, X.; Gao, K.; Ma, C.; Tian, X.; Men, W.; Li, H.; Bi, H.; et al. Wheat heat shock factor TaHsfA2d contributes to plant responses to phosphate deficiency. Plant Physiol. Biochem. 2022, 185, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Alok, A.; Singh, S.; Kumar, P.; Bhati, K.K. Potential of engineering the myo-inositol oxidation pathway to increase stress resilience in plants. Mol. Biol. Rep. 2022, 49, 8025–8035. [Google Scholar] [CrossRef] [PubMed]
- Ivanov Kavkova, E.; Blöchl, C.; Tenhaken, R. The Myo-inositol pathway does not contribute to ascorbic acid synthesis. Plant Biol. 2019, 21 (Suppl. 1), 95–102. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Z.; Wei, Y.; Liu, Y.; Xing, L.; Liu, M.; Li, P.; Lu, Q.; Peng, R. Genome-wide identification of the MIOX gene family and their expression profile in cotton development and response to abiotic stress. PLoS ONE 2021, 16, e0254111. [Google Scholar] [CrossRef]
- Nepal, N.; Yactayo-Chang, J.P.; Medina-Jiménez, K.; Acosta-Gamboa, L.M.; González-Romero, M.E.; Arteaga-Vázquez, M.A.; Lorence, A. Mechanisms underlying the enhanced biomass and abiotic stress tolerance phenotype of an Arabidopsis MIOX over-expresser. Plant Direct 2019, 3, e00165. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.F.; Guo, Z.P.; Su, H.; Wang, D.; Guo, R.R. Henan Province: Precise measures to fully ensure the water supply demand for drought resistance, planting and seedling protection. China Flood Drought Manag. 2024, 34, 81–82. [Google Scholar]
- Carril, P.; da Silva, A.B.; Tenreiro, R.; Cruz, C. An Optimized in situ Quantification Method of Leaf H2O2 Unveils Interaction Dynamics of Pathogenic and Beneficial Bacteria in Wheat. Front. Plant Sci. 2020, 11, 889. [Google Scholar] [CrossRef] [PubMed]
- Moskala, R.; Reddy, C.C.; Minard, R.D.; Hamilton, G.A. An oxygen-18 tracer investigation of the mechanism of myo-inositol oxygenase. Biochem. Biophys. Res. Commun. 1981, 99, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Lorence, A.; Chevone, B.I.; Mendes, P.; Nessler, C.L. myo-inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol. 2004, 134, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Mumtaz, M.A.; Ahiakpa, J.K.; Liu, G.; Chen, W.; Zhou, G.; Zheng, W.; Ye, Z.; Zhang, Y. Genome-wide analysis of Myo-inositol oxygenase gene family in tomato reveals their involvement in ascorbic acid accumulation. BMC Genom. 2020, 21, 284. [Google Scholar] [CrossRef]
- Xue, Z. Main Pathway and Key Gene Contribution to the Increasing AsA Concentration in Cucumber Leaves under Nitrogen Deficiency; Chinese Academy of Agricultural Sciences: Beijing, China, 2016. [Google Scholar]
- Tatineni, S.; Alexander, J.; Qu, F. Differential Synergistic Interactions Among Four Different Wheat-Infecting Viruses. Front. Microbiol. 2021, 12, 800318. [Google Scholar] [CrossRef]
- Wang, C.; Gan, J.; Mi, X. On four species of the genus Argiope Audouin, 1826 (Araneae, Araneidae) from China. Zookeys 2021, 1019, 15–34. [Google Scholar] [CrossRef]
- Wang, X.; Qin, Z.; Zhang, M.; Shang, B.; Li, Z.; Zhao, M.; Tang, Q.; Tang, Q.; Luo, J. Immunogenicity and protection of recombinant self-assembling ferritin-hemagglutinin nanoparticle influenza vaccine in mice. Clin. Exp. Vaccine Res. 2025, 14, 23–34. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef]
- Tan, B.; Yan, L.; Li, H.; Lian, X.; Cheng, J.; Wang, W.; Zheng, X.; Wang, X.; Li, J.; Ye, X.; et al. Genome-wide identification of HSF family in peach and functional analysis of PpHSF5 involvement in root and aerial organ development. PeerJ 2021, 9, e10961. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Du, Y.; Li, Z.; Guo, R.; Li, Y.; Wei, J.; Yin, X.; Su, L. Soldier Caste-Specific Protein 1 Is Involved in Soldier Differentiation in Termite Reticulitermes aculabialis. Insects 2022, 13, 502. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. AMDock: A versatile graphical tool for assisting molecular docking with AutoDock Vina and AutoDock4. Biol. Direct 2020, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, H.; Zhao, C.; Li, S.; Kong, L.; Wu, W.; Kong, W.; Liu, Y.; Wei, Y.; Zhu, J.K.; et al. The inhibition of protein translation mediated by AtGCN1 is essential for cold tolerance in Arabidopsis thaliana. Plant Cell Environ. 2017, 40, 56–68. [Google Scholar] [CrossRef]
- Galbiati, M.; Matus, J.T.; Francia, P.; Rusconi, F.; Cañón, P.; Medina, C.; Conti, L.; Cominelli, E.; Tonelli, C.; Arce-Johnson, P. The grapevine guard cell-related VvMYB60 transcription factor is involved in the regulation of stomatal activity and is differentially expressed in response to ABA and osmotic stress. BMC Plant Biol. 2011, 11, 142. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, K.; Bai, C.; Yin, D.; Li, G.; Qi, K.; Wang, G.; Li, Y. Development of a SYBR Green I real-time PCR for the detection of the orf virus. AMB Express 2017, 7, 21. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, X.; Yang, L.; Raziq, A.; Hao, S.; Zeng, W.; Huang, J.; Cao, Y.; Duan, Q. Nontransformation methods for studying signaling pathways and genes involved in Brassica rapa pollen-stigma interactions. Plant Physiol. 2024, 196, 1802–1812. [Google Scholar] [CrossRef]
- Gao, Y.; Yang, J.; Duan, W.; Ma, X.; Qu, L.; Xu, Z.; Yang, Y.; Xu, J. NtRAV4 negatively regulates drought tolerance in Nicotiana tabacum by enhancing antioxidant capacity and defence system. Plant Cell Rep. 2022, 41, 1775–1788. [Google Scholar] [CrossRef]
- Sirohi, R.; Tarafdar, A.; Kumar Gaur, V.; Singh, S.; Sindhu, R.; Rajasekharan, R.; Madhavan, A.; Binod, P.; Kumar, S.; Pandey, A. Technologies for disinfection of food grains: Advances and way forward. Food Res. Int. 2021, 145, 110396. [Google Scholar] [CrossRef]
- Ji, X.; Hou, H.; Wang, X.; Qiu, Y.; Ma, Y.; Wang, S.; Guo, S.; Huang, S.; Zhang, C. Effect of dietary Glycyrrhiza polysaccharides on growth performance, hepatic antioxidant capacity and anti-inflammatory capacity of broiler chickens. Res. Vet. Sci. 2024, 167, 105114. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Liu, N.; Wei, S.; Wang, J.; Qin, F.; Suo, B. The iTRAQ-based chloroplast proteomic analysis of Triticum aestivum L. leaves subjected to drought stress and 5-aminolevulinic acid alleviation reveals several proteins involved in the protection of photosynthesis. BMC Plant Biol. 2020, 20, 96. [Google Scholar] [CrossRef]
- Su, X.; Fan, X.; Shao, R.; Guo, J.; Wang, Y.; Yang, J.; Yang, Q.; Guo, L. Physiological and iTRAQ-based proteomic analyses reveal that melatonin alleviates oxidative damage in maize leaves exposed to drought stress. Plant Physiol. Biochem. 2019, 142, 263–274. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Ali, B.; Kumar, S.; Sui, X.; Niu, J.; Yang, J.; Zheng, M.; Tang, Y.; Li, H. Exogenous acetylsalicylic acid mitigates cold stress in common bean seedlings by enhancing antioxidant defense and photosynthetic efficiency. Front. Plant Sci. 2025, 16, 1589706. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).