Functional Analysis of Hyaluronidase-like Genes in Ovarian Development of Macrobrachium nipponense and Comparative Evaluation with Other Key Regulatory Genes
Abstract
1. Introduction
2. Results
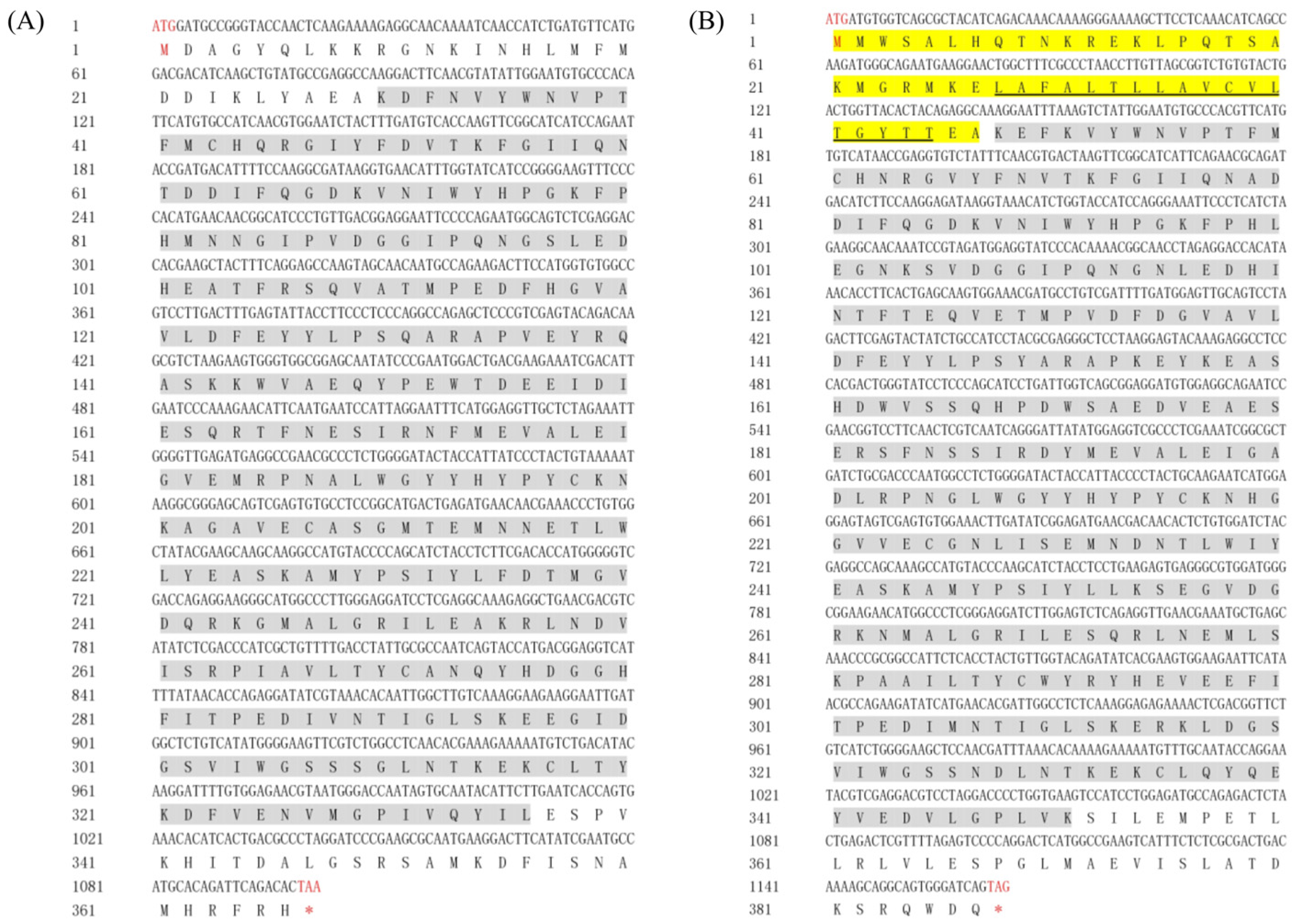
2.1. Characterization of the Full-Length Sequences of Mn-HyaL1 and Mn-HyaL2
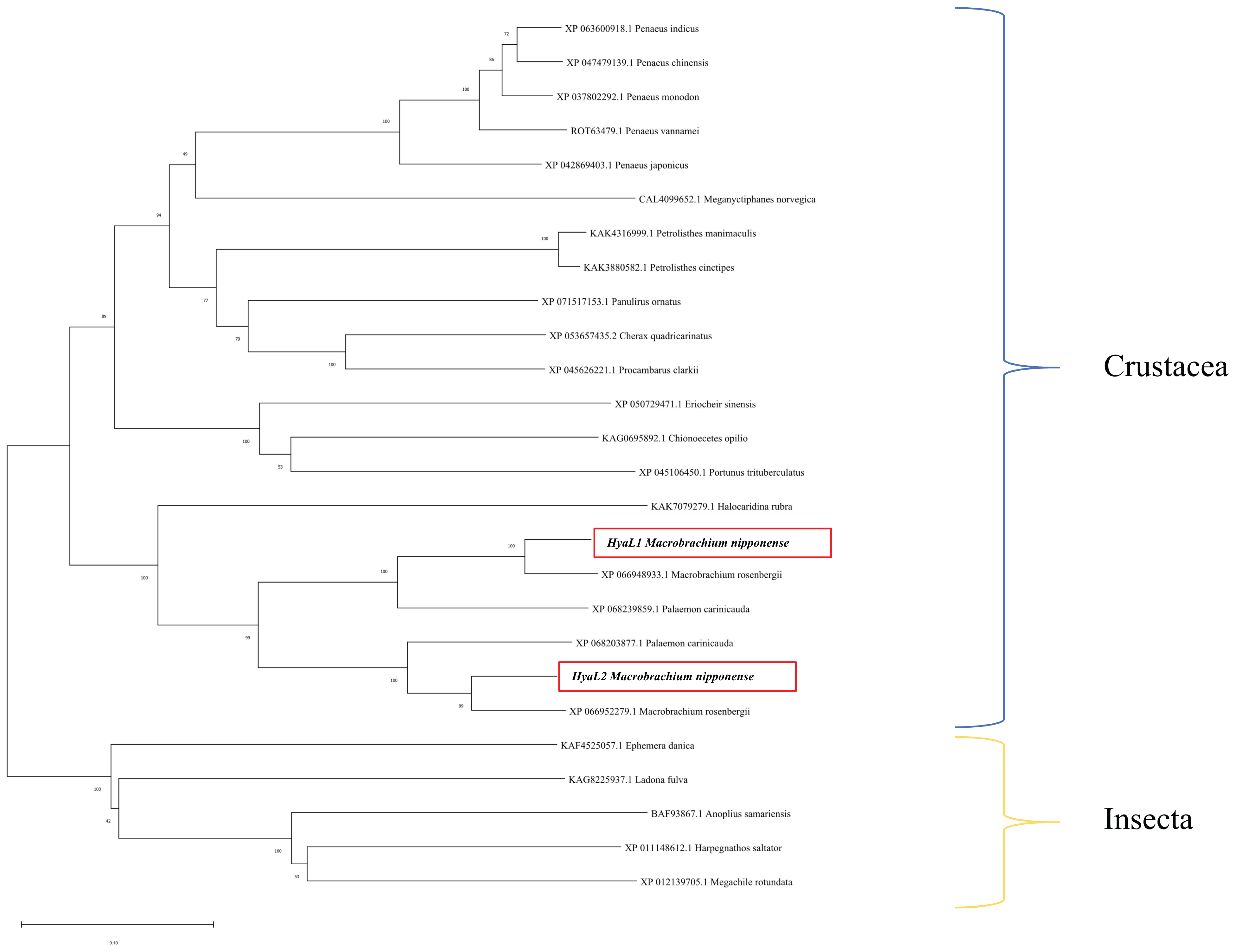
2.2. Analysis of Species Similarity and Phylogeny of Mn-HyaL1 and Mn-HyaL2
2.3. Analysis of Spatiotemporal Expression Patterns of Mn-HyaL1 and Mn-HyaL2
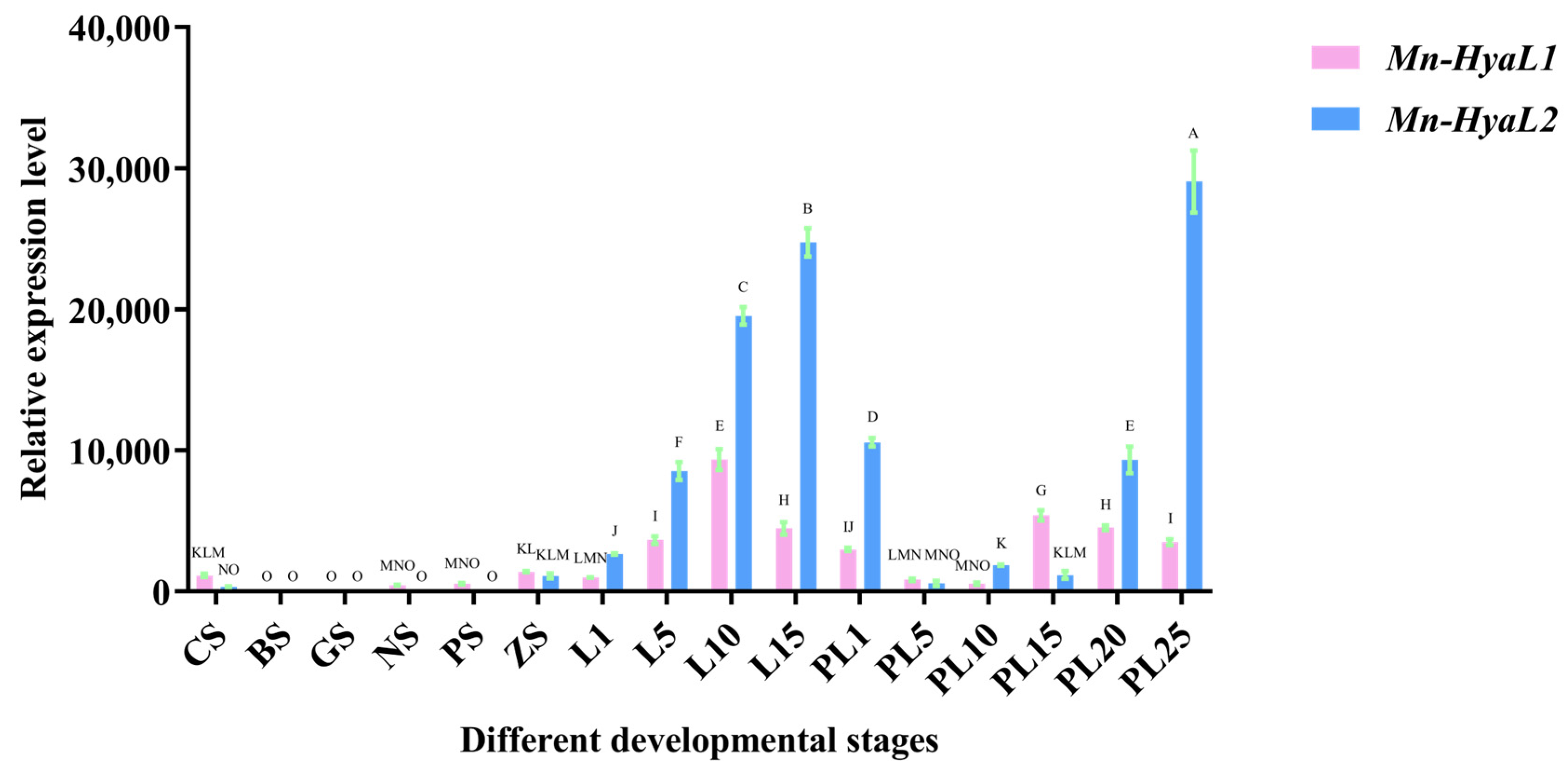
2.3.1. Analysis of Stage-Specific Expression Patterns of Mn-HyaL1 and Mn-HyaL2
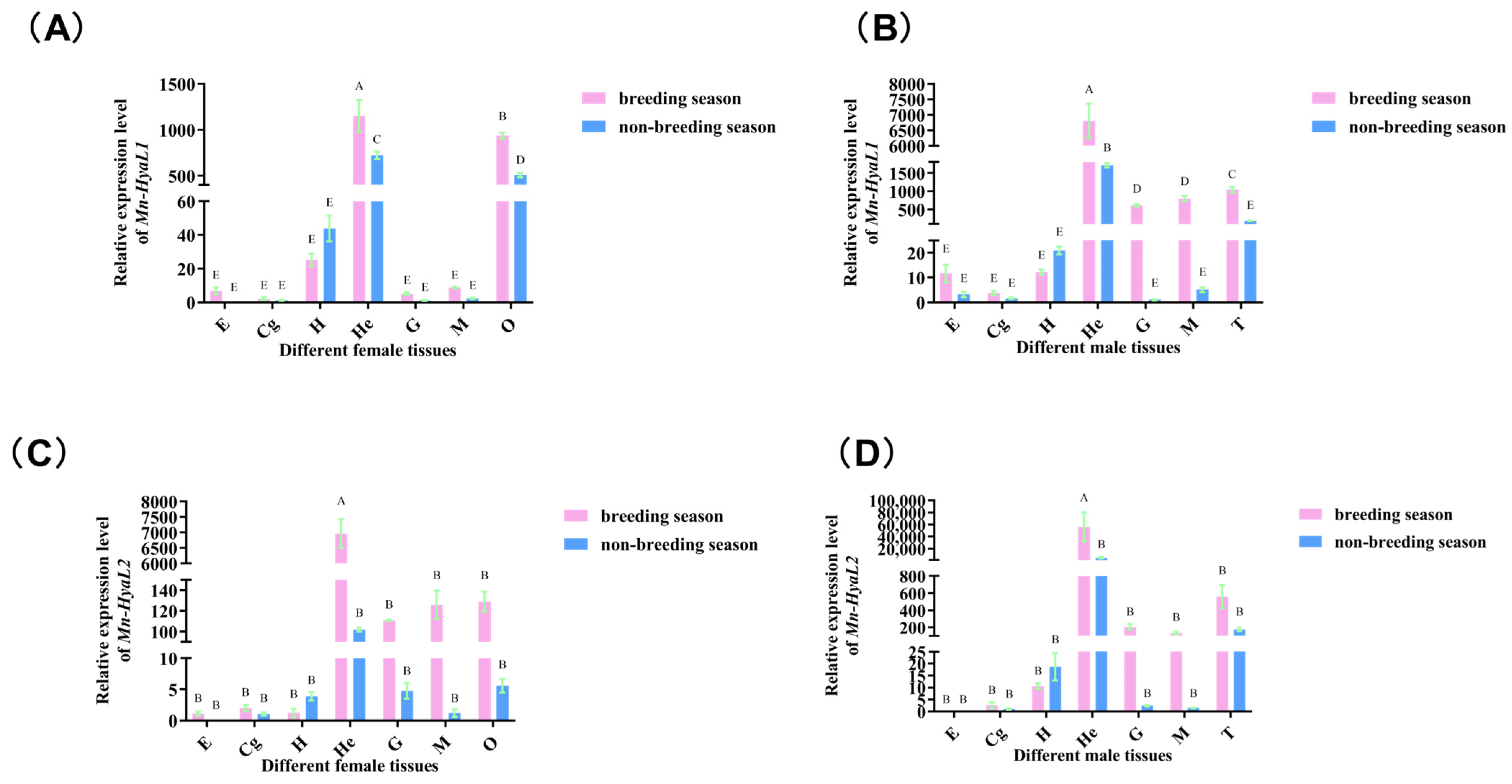
2.3.2. Analysis of Tissue-Specific Expression Patterns of Mn-HyaL1 and Mn-HyaL2
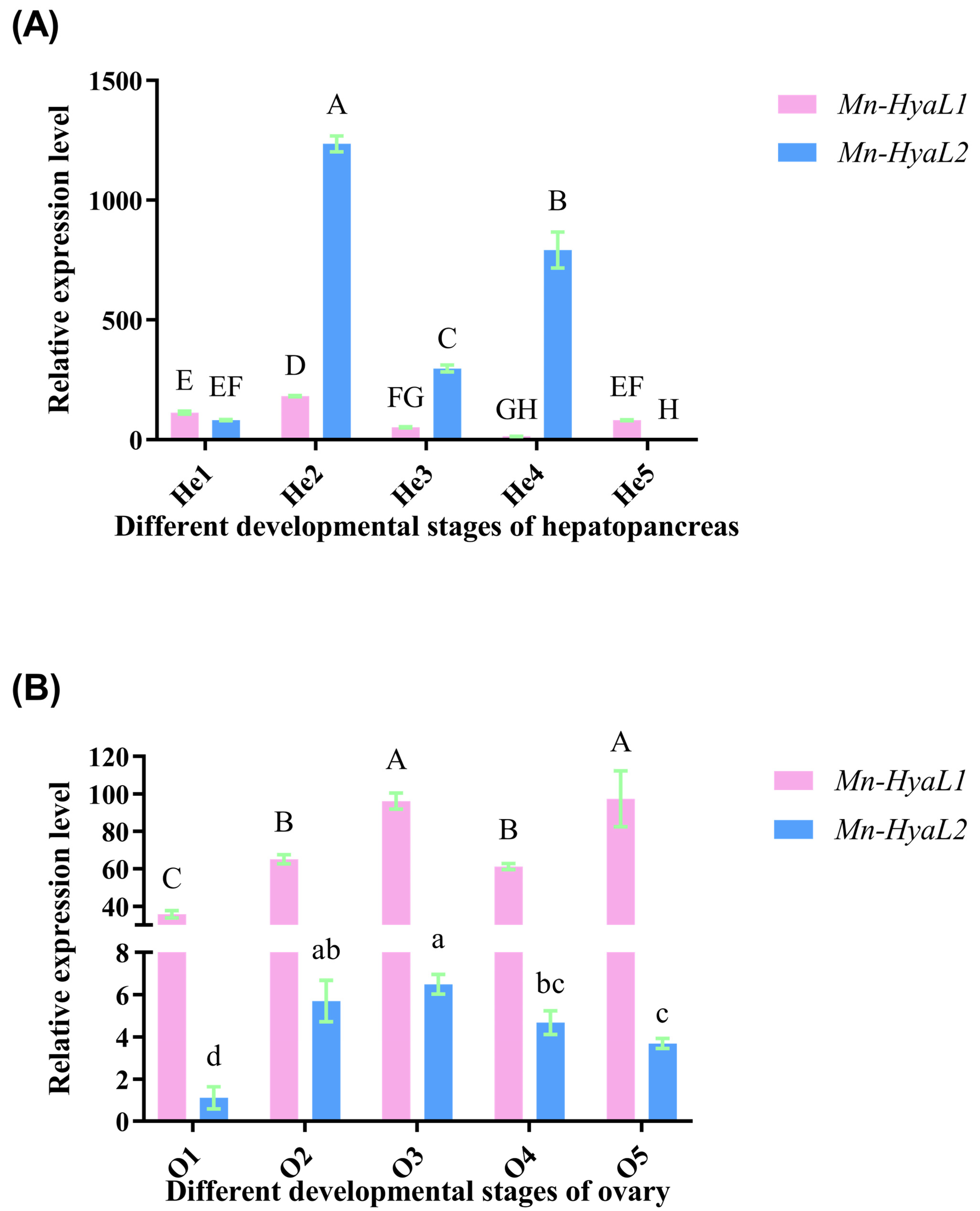
2.3.3. Analysis of Stage-Specific Expression Patterns of Mn-HyaL1 and Mn-HyaL2 in Hepatopancreatic and Ovarian Tissues
2.4. Functional Characterization and Comparative Analysis of Genes
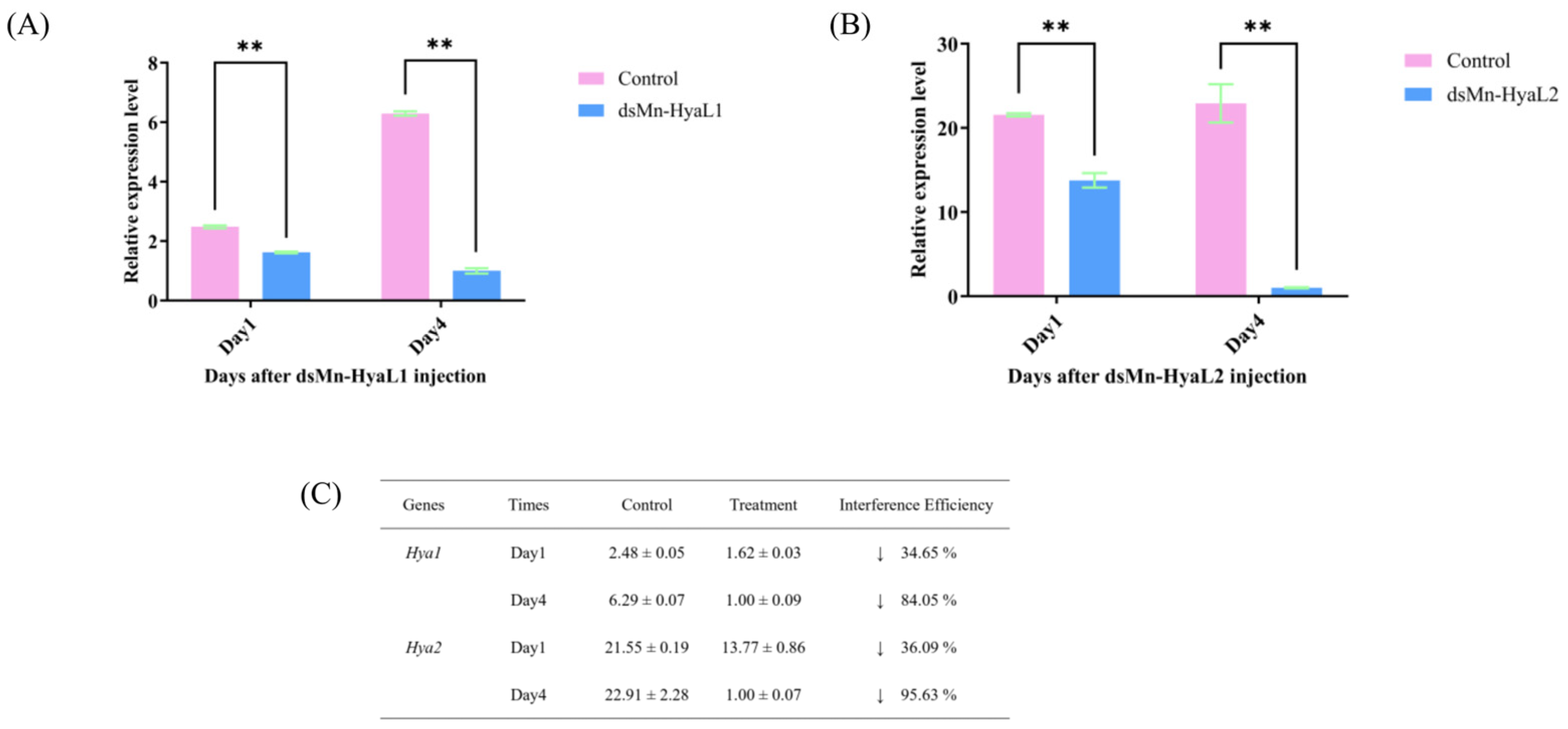
2.4.1. Evaluation of RNA Interference Efficiency
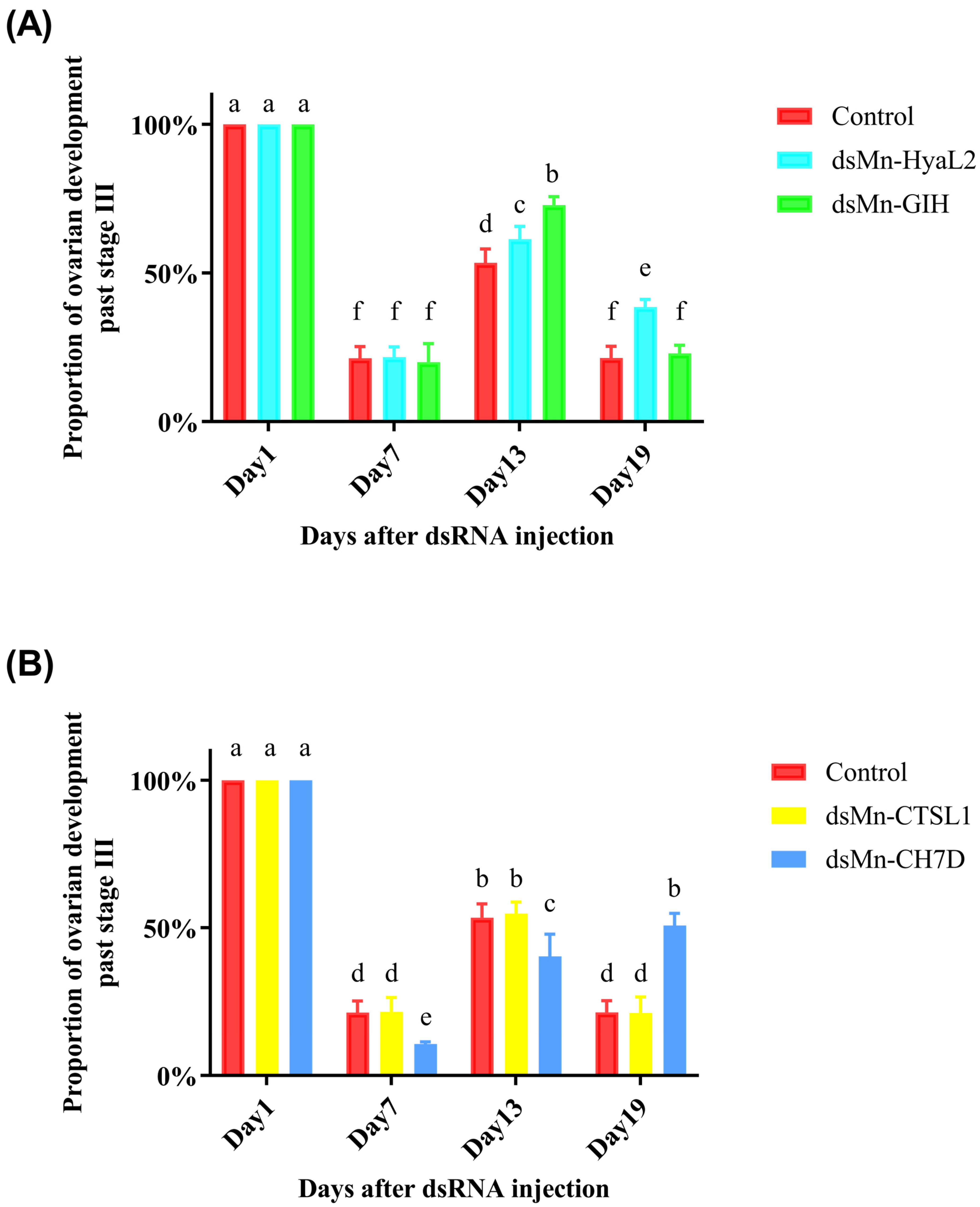
2.4.2. Comparative Functional Analysis of Key Genes in Ovarian Development of M. nipponense
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Breeding Conditions
4.2. Tissue Sample Collection
4.3. Molecular Biology Methods
4.4. Bioinformatics Analysis
4.5. RNA Interference Experiment
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, D.; Zhang, W.; Jiang, S.; Xiong, Y.; Jin, S.; Pan, F.; Zhu, J.; Gong, Y.; Wu, Y.; Qiao, H.; et al. Cathepsin D Plays a Vital Role in Macrobrachium nipponense of Ovary Maturation: Identification, Characterization, and Function Analysis. Genes 2022, 13, 1495. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, S.; Zhang, W.; Xiong, Y.; Jin, S.; Cheng, D.; Zheng, Y.; Qiao, H.; Fu, H. Function Analysis of Cholesterol 7-Desaturase in Ovarian Maturation and Molting in Macrobrachium nipponense: Providing Evidence for Reproductive Molting Progress. Int. J. Mol. Sci. 2023, 24, 6940. [Google Scholar] [CrossRef]
- Li, X.; Han, T.; Zheng, S.; Wu, G. Nutrition and Functions of Amino Acids in Aquatic Crustaceans. In Amino Acids in Nutrition and Health: Amino Acids in the Nutrition of Companion, Zoo and Farm Animals; Wu, G., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 169–198. ISBN 978-3-030-54462-1. [Google Scholar]
- Izquierdo, M.S.; Fernández-Palacios, H.; Tacon, A.G.J. Effect of Broodstock Nutrition on Reproductive Performance of Fish. Aquaculture 2001, 197, 25–42. [Google Scholar] [CrossRef]
- Fatima, H.; Ayub, Z.; Ali, S.; Siddiqui, G. Biochemical Composition of the Hemolymph, Hepatopancreas, Ovary, and Muscle during Ovarian Maturation in the Penaeid Shrimps Fenneropenaeus merguiensis and F. penicillatus (Crustacea: Decapoda). Turk. J. Zool. 2013, 37, 334–347. [Google Scholar] [CrossRef]
- Feng, W.; Zhao, Z.; Wang, J.; Han, T. Nutrient Composition of Ovary, Hepatopancreas and Muscle Tissues in Relation to Ovarian Development Stage of Female Swimming Crab, Portunus trituberculatus. Animals 2023, 13, 3220. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, W.; Xiong, Y.; Cheng, D.; Wang, J.; Jin, S.; Gong, Y.; Wu, Y.; Qiao, H.; Fu, H. Hepatopancreas Transcriptome Analyses Provide New Insights into the Molecular Regulatory Mechanism of Fast Ovary Maturation in Macrobrachium nipponense. BMC Genom. 2022, 23, 625. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Qiao, H.; Fu, H.; Gu, Z. Hepatopancreas Proteomic Analysis Reveals Key Proteins and Pathways in Regulatory of Ovary Maturation of Macrobrachium nipponense. Animals 2023, 13, 977. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, S.; Zhang, W.; Jin, S.; Xiong, Y.; Xu, M.; Gao, Z.; Xu, M.; Qiao, H.; Fu, H. Functional Characterization of Two β-Hexosaminidase A Isoforms During Ovarian Development in Macrobrachium nipponense. Int. J. Mol. Sci. 2025, 26, 5459. [Google Scholar] [CrossRef]
- Zhu, J.; Fu, H.; Qiao, H.; Jin, S.; Zhang, W.; Jiang, S.; Gong, Y.; Xiong, Y. Expression and Functional Analysis of Cathepsin L1 in Ovarian Development of the Oriental River Prawn, Macrobrachium nipponense. Aquac. Rep. 2021, 20, 100724, Correction in Int. J. Appl. Earth Obs. Geoinf. 2022, 114, 102880. https://doi.org/10.1016/j.jag.2022.102880. [Google Scholar] [CrossRef]
- Jiang, S.; Xiong, Y.; Zhang, W.; Zhu, J.; Cheng, D.; Gong, Y.; Wu, Y.; Qiao, H.; Fu, H. Molecular Characterization of a Novel Cathepsin L in Macrobrachium nipponense and Its Function in Ovary Maturation. Front. Endocrinol. 2022, 12, 816813. [Google Scholar] [CrossRef]
- Qiao, H.; Xiong, Y.; Zhang, W.; Fu, H.; Jiang, S.; Sun, S.; Bai, H.; Jin, S.; Gong, Y. Characterization, Expression, and Function Analysis of Gonad-Inhibiting Hormone in Oriental River Prawn, Macrobrachium nipponense and Its Induced Expression by Temperature. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 185, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Fu, H.; Zhang, W.; Xiong, Y.; Jin, S.; Qiao, H.; Jiang, S. Regulation Roles of Juvenile Hormone Epoxide Hydrolase Gene 2 in the Female River Prawn Macrobrachium Nipponense Reproductive Process. Curr. Issues Mol. Biol. 2024, 46, 13456–13470. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.; Xiong, Y.; Wang, J.; Jin, S.; Qiao, H.; Jiang, S.; Fu, H. Dual Roles of CYP302A1 in Regulating Ovarian Maturation and Molting in Macrobrachium nipponense. J. Steroid Biochem. Mol. Biol. 2023, 232, 106336. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, W.; Xiong, Y.; Zhang, M.; Yuan, H.; Niu, Y.; Qiao, H.; Fu, H. NPC Intracellular Cholesterol Transporter 1 Regulates Ovarian Maturation and Molting in Female Macrobrachium Nipponense. Int. J. Mol. Sci. 2024, 25, 6049. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, W.; Jiang, J.; Zhang, M.; Wang, J.; Xiong, Y.; Qiao, H.; Fu, H. Molecular Characterization and Functional Analysis of CYP315 A1 in Ovarian Development in Macrobrachium nipponense. Aquac. Rep. 2025, 41, 102700. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, S.; Zhang, W.; Xiong, Y.; Jin, S.; Wang, J.; Qiao, H.; Fu, H. Functional Study of the Role of the Methyl Farnesoate Epoxidase Gene in the Ovarian Development of Macrobrachium Nipponense. Int. J. Mol. Sci. 2024, 25, 7318. [Google Scholar] [CrossRef]
- Bai, H.; Qiao, H.; Li, F.; Fu, H.; Sun, S.; Zhang, W.; Jin, S.; Gong, Y.; Jiang, S.; Xiong, Y. Molecular Characterization and Developmental Expression of Vitellogenin in the Oriental River Prawn Macrobrachium Nipponense and the Effects of RNA Interference and Eyestalk Ablation on Ovarian Maturation. Gene 2015, 562, 22–31. [Google Scholar] [CrossRef]
- Zhou, Z.; Fu, H.; Jin, S.; Qiao, H.; Zhang, W.; Jiang, S.; Xiong, Y.; Gong, Y.; Hu, Y.; Gu, X.; et al. Function Analysis and Molecular Characterization of Cyclin A in Ovary Development of Oriental River Prawn, Macrobrachium nipponense. Gene 2021, 788, 145583. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Qiao, H.; Li, F.; Fu, H.; Jiang, S.; Zhang, W.; Yan, Y.; Xiong, Y.; Sun, S.; Jin, S.; et al. Molecular and Functional Characterization of the Vitellogenin Receptor in Oriental River Prawn, Macrobrachium nipponense. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 194, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yuan, H.; Qiao, H.; Jiang, S.; Zhang, W.; Xiong, Y.; Fu, H.; Jin, S. Mn-XRN1 Has an Inhibitory Effect on Ovarian Reproduction in Macrobrachium Nipponense. Genes 2023, 14, 1454. [Google Scholar] [CrossRef]
- Cui, W.; Liu, X.; Zhang, S.; Li, X.; Wang, Z.; Li, Y.; Zhang, M.; Wang, L.; Yu, M.; Qiao, Z.; et al. Identification and Ovarian Developmental Regulation of Ribosomal Protein S6 Kinase in Macrobrachium Nipponense. Int. J. Biol. Macromol. 2025, 328, 147666. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zhang, S.; Li, H.; Zhang, R.; Li, X.; Zhang, M.; Wang, L.; Yu, M.; Qiao, Z.; et al. cDNA Characterization of the Ribosomal Protein L10a Gene and Its Functional Analysis in Ovarian Development of Macrobrachium nipponense. Aquac. Rep. 2024, 34, 101899. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Li, Y.; Liu, X.; Zhang, S.; Li, H.; Zhang, M.; Wang, L.; Yu, M.; Qiao, Z. Molecular and Functional Characterization of Ribosome Protein S24 in Ovarian Development of Macrobrachium nipponense. Int. J. Biol. Macromol. 2024, 254, 127934. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Y.; Zhang, S.; Li, H.; Liu, X.; Zhang, R.; Zhang, M.; Wang, L.; Yu, M.; Qiao, Z.; et al. Identification of the Cyclooxygenase (COX) Gene and Its Role in Ovarian Development and Ovulation of the Oriental River Prawn Macrobrachium nipponense. Aquac. Rep. 2023, 29, 101488. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, X.; Li, Y.; Zhang, R.; Liu, H.; Ma, X.; Wu, L.; Qiao, Z.; Li, X. Identification of Ribosomal Protein L24 (RPL24) from the Oriental River Prawn, Macrobrachium Nipponense, and Its Roles in Ovarian Development. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2022, 266, 111154. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Sun, Y.; Hou, F.; Zhang, Y.; Li, F.; Guo, J.; Wang, Y.; Gu, Z.; Liu, X. Molecular and Functional Characterization of Nucleoside Diphosphate Kinase (Nm23) Gene in Oriental River Prawn Macrobrachium Nipponense during Ovarian Development. Aquac. Res. 2018, 49, 1219–1231. [Google Scholar] [CrossRef]
- Orimoto, R.; Adachi, E.; Gau, M.; Saito, Y.; Yamano, H.; Nakatani, H.; Kirino, S.; Moriyama, K.; Yamaguchi, Y.; Mizuno, T.; et al. Hyaluronidase 2 Deficiency Due to Novel Compound Heterozygous Variants in HYAL2: A Case Report of Siblings with HYAL2 Deficiency Showing Different Clinical Severity and Literature Review. J. Hum. Genet. 2025, 70, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Marei, W.F.A.; Raheem, K.A.; Salavati, M.; Tremaine, T.; Khalid, M.; Fouladi-Nashta, A.A. Hyaluronan and Hyaluronidase, Which Is Better for Embryo Development? Theriogenology 2016, 86, 940–948. [Google Scholar] [CrossRef]
- Sliadovskii, D.; Ponomareva, T.; Molchanov, M.; Pozdnyakova-Filatova, I.; Timchenko, M.; Marchenkov, V.; Gusev, O.; Sogorin, E. β-Elimination of Hyaluronate by Red King Crab Hyaluronidase. Sci. Rep. 2021, 11, 22600. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K. 11 Hyaluronidases. In The Enzymes; Boyer, P.D., Ed.; Academic Press: Cambridge, MA, USA, 1971; Volume 5, pp. 307–320. [Google Scholar]
- Cramer, J.A.; Bailey, L.C.; Bailey, C.A.; Miller, R.T. Kinetic and Mechanistic Studies with Bovine Testicular Hyaluronidase. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1994, 1200, 315–321. [Google Scholar] [CrossRef]
- Linker, A.; Hoffman, P.; Meyer, K. The Hyaluronidase of the Leech: An Endoglucuronidase. Nature 1957, 180, 810–811. [Google Scholar] [CrossRef]
- Jin, P.; Kang, Z.; Zhang, N.; Du, G.; Chen, J. High-Yield Novel Leech Hyaluronidase to Expedite the Preparation of Specific Hyaluronan Oligomers. Sci. Rep. 2014, 4, 4471. [Google Scholar] [CrossRef] [PubMed]
- Rigden, D.J.; Littlejohn, J.E.; Joshi, H.V.; De Groot, B.L.; Jedrzejas, M.J. Alternate Structural Conformations of Streptococcus Pneumoniae Hyaluronan Lyase: Insights into Enzyme Flexibility and Underlying Molecular Mechanism of Action. J. Mol. Biol. 2006, 358, 1165–1178. [Google Scholar] [CrossRef]
- Gmachl, M.; Kreil, G. Bee Venom Hyaluronidase Is Homologous to a Membrane Protein of Mammalian Sperm. Proc. Natl. Acad. Sci. USA 1993, 90, 3569–3573. [Google Scholar] [CrossRef]
- Modarressi, S.M.; koolivand, Z.; Akbari, M. Enhancing Hyaluronidase Enzyme Activity: Insights from Advancement in Bovine and Ovine Testicular Hyaluronidase Purification. J. Chromatogr. B 2024, 1234, 124031. [Google Scholar] [CrossRef] [PubMed]
- El-Safory, N.S.; Fazary, A.E.; Lee, C.K. Hyaluronidases, a Group of Glycosidases: Current and Future Perspectives. Carbohydr. Polym. 2010, 81, 165–181. [Google Scholar] [CrossRef]
- Kim, E.; Yamashita, M.; Kimura, M.; Honda, A.; Kashiwabara, S.; Baba, T. Sperm Penetration through Cumulus Mass and Zona Pellucida. Int. J. Dev. Biol. 2008, 52, 677–682. [Google Scholar] [CrossRef]
- Reitinger, S.; Laschober, G.T.; Fehrer, C.; Greiderer, B.; Lepperdinger, G. Mouse Testicular Hyaluronidase-like Proteins SPAM1 and HYAL5 but Not HYALP1 Degrade Hyaluronan. Biochem. J. 2007, 401, 79–85. [Google Scholar] [CrossRef]
- Orimoto, A.M.; Karine, D.D.; Jin-Yi, J.; Nongnuj, T.; Tsang, B.K.; Euridice, C. Mammalian Hyaluronidase Induces Ovarian Granulosa Cell Apoptosis and Is Involved in Follicular Atresia. Endocrinology 2008, 149, 5835–5847. [Google Scholar] [CrossRef]
- Yoffou, P.H.; Edjekouane, L.; Meunier, L.; Tremblay, A.; Provencher, D.M.; Mes-Masson, A.-M.; Carmona, E. Subtype Specific Elevated Expression of Hyaluronidase-1 (HYAL-1) in Epithelial Ovarian Cancer. PLoS ONE 2011, 6, e20705. [Google Scholar] [CrossRef]
- Nykopp, T.K.; Rilla, K.; Tammi, M.I.; Tammi, R.H.; Sironen, R.; Hämäläinen, K.; Kosma, V.-M.; Heinonen, S.; Anttila, M. Hyaluronan Synthases (HAS1-3) and Hyaluronidases (HYAL1-2) in the Accumulation of Hyaluronan in Endometrioid Endometrial Carcinoma. BMC Cancer 2010, 10, 512. [Google Scholar] [CrossRef]
- Nykopp, T.K.; Rilla, K.; Sironen, R.; Tammi, M.I.; Tammi, R.H.; Hämäläinen, K.; Heikkinen, A.-M.; Komulainen, M.; Kosma, V.-M.; Anttila, M. Expression of Hyaluronan Synthases (HAS1–3) and Hyaluronidases (HYAL1–2) in Serous Ovarian Carcinomas: Inverse Correlation between HYAL1 and Hyaluronan Content. BMC Cancer 2009, 9, 143. [Google Scholar] [CrossRef]
- Amargant, F.; Manuel, S.L.; Tu, Q.; Parkes, W.S.; Rivas, F.; Zhou, L.T.; Rowley, J.E.; Villanueva, C.E.; Hornick, J.E.; Shekhawat, G.S.; et al. Ovarian Stiffness Increases with Age in the Mammalian Ovary and Depends on Collagen and Hyaluronan Matrices. Aging Cell 2020, 19, e13259. [Google Scholar] [CrossRef] [PubMed]
- Ponomareva, T.; Timchenko, M.; Filippov, M.; Lapaev, S.; Sogorin, E. Prospects of Red King Crab Hepatopancreas Processing: Fundamental and Applied Biochemistry. Recycling 2021, 6, 3. [Google Scholar] [CrossRef]
- Ponomareva, T.; Sliadovskii, D.; Timchenko, M.; Molchanov, M.; Timchenko, A.; Sogorin, E. The Effect of Hepatopancreas Homogenate of the Red King Crab on HA-Based Filler. PeerJ 2020, 8, e8579. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rios, L.; Díaz-Peña, L.F.; Lazcano-Pérez, F.; Arreguín-Espinosa, R.; Rojas-Molina, A.; García-Arredondo, A. Hyaluronidase-like Enzymes Are a Frequent Component of Venoms from Theraphosid Spiders. Toxicon 2017, 136, 34–43. [Google Scholar] [CrossRef]
- Biner, O.; Trachsel, C.; Moser, A.; Kopp, L.; Langenegger, N.; Kämpfer, U.; von Ballmoos, C.; Nentwig, W.; Schürch, S.; Schaller, J.; et al. Isolation, N-Glycosylations and Function of a Hyaluronidase-Like Enzyme from the Venom of the Spider Cupiennius Salei. PLoS ONE 2015, 10, e0143963. [Google Scholar] [CrossRef]
- Bala, E.; Hazarika, R.; Singh, P.; Yasir, M.; Shrivastava, R. A Biological Overview of Hyaluronidase: A Venom Enzyme and Its Inhibition with Plants Materials. Mater. Today Proc. 2018, 5, 6406–6412. [Google Scholar] [CrossRef]
- Ferrer, V.P.; de Mari, T.L.; Gremski, L.H.; Silva, D.T.; da Silveira, R.B.; Gremski, W.; Chaim, O.M.; Senff-Ribeiro, A.; Nader, H.B.; Veiga, S.S. A Novel Hyaluronidase from Brown Spider (Loxosceles Intermedia) Venom (Dietrich’s Hyaluronidase): From Cloning to Functional Characterization. PLoS Neglected Trop. Dis. 2013, 7, e2206. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Andersen, C.L.; Ye, X. Functions of Lysosomes in Mammalian Female Reproductive System. Reprod. Dev. Med. 2020, 4, 109–122. [Google Scholar] [CrossRef]
- Esmaeilian, Y.; Hela, F.; Bildik, G.; İltumur, E.; Yusufoglu, S.; Yakin, K.; Oktem, O. Discovery of Autophagy as a Universal Mechanism for Sex Steroid Synthesis in Human Ovary and Testis. Autophagy Rep. 2023, 2, 2251804. [Google Scholar] [CrossRef]
- Niswender, G.D. Response of the Corpus Luteum to Luteinizing Hormone. Environ. Health Perspect. 1981, 38, 47–50. [Google Scholar] [CrossRef]
- Krishnamurthy, H.; Kishi, H.; Shi, M.; Galet, C.; Bhaskaran, R.S.; Hirakawa, T.; Ascoli, M. Postendocytotic Trafficking of the Follicle-Stimulating Hormone (FSH)-FSH Receptor Complex. Mol. Endocrinol. 2003, 17, 2162–2176. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Rao, C.V. Receptors for Gonadotropins and Prostaglandins in Lysosomes of Bovine Corpora Lutea. Arch. Biochem. Biophys. 1978, 185, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Carmona, E.; Dumaresq-Doiron, K.; Orimoto, A.M.; Yoffou, P.H.; Edjekouane, L.; Triggs-Raine, B. Impact of Hyaluronidase-1 and Hyaluronidase-3 Deficiency on Mouse Female Reproduction. Biol. Reprod. 2009, 81, 196. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, M.; Xiong, J.; Zhang, M.; Wu, X. Sequence Characteristics, Evolutionary History and Expression Pattern of BCO2 in Chinese Mitten Crab Eriocheir sinensis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2025, 56, 101524. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, L.; Wu, P.; Zhao, W.; Li, E.; Qin, J. cDNA Cloning and Expression of Ubc9 in the Developing Embryo and Ovary of Oriental River Prawn, Macrobrachium nipponense. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2010, 155, 288–293. [Google Scholar] [CrossRef]
- Hu, Y.; Fu, Y.; Jin, S.; Fu, H.; Qiao, H.; Zhang, W.; Jiang, S.; Gong, Y.; Xiong, Y.; Wu, Y.; et al. Comparative Transcriptome Analysis of Lethality in Response to RNA Interference of the Oriental River Prawn (Macrobrachium nipponense). Comp. Biochem. Physiol. Part D Genom. Proteom. 2021, 38, 100802. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| First Author | Genes | Methodological Approach | Year | Reference |
|---|---|---|---|---|
| Jisheng Wang | Cholesterol 7-desaturase | The proportion of ovarian development over the third stage and gonadosomatic index (GSI) | 2023 | [2] |
| Jisheng Wang | Juvenile hormone epoxide hydrolase | The proportion of ovarian development over the third stage and GSI | 2024 | [13] |
| Yalu Zheng | CYP302A1 | The proportion of ovarian development over the third stage and GSI | 2023 | [14] |
| Sufei Jiang | NPC intracellular cholesterol transporter 1 | The proportion of ovarian development over the third stage and GSI | 2024 | [15] |
| Sufei Jiang | CYP315A1 | The proportion of ovarian development over the third stage and GSI | 2025 | [16] |
| Mengying Zhang | Methyl farnesoate epoxidase | The proportion of ovarian development over the third stage and GSI | 2024 | [17] |
| Hongkun Bai | Vitellogenin (Vg) | Ovarian developmental stages, relative expression levels of Vg and GSI | 2015 | [18] |
| Junpeng Zhu | Cathepsin L1 | Ovarian developmental stages, relative expression levels of Vg and GSI | 2021 | [10] |
| Zhenyu Zhou | Cyclin A | Ovarian developmental stages and GSI | 2021 | [19] |
| Dan Cheng | Cathepsin D | Ovarian developmental stages and GSI | 2022 | [1] |
| Hui Qiao | Gonad-inhibiting hormone | Ovarian developmental stages and GSI | 2015 | [12] |
| Hongkun Bai | Vitellogenin receptor | Relative expression levels of Vg and GSI | 2016 | [20] |
| Sufei Jiang | Cathepsin L2 | Relative expression levels of Vg and GSI | 2022 | [11] |
| Zhiming Wang | β-Hexosaminidase A | The proportion of ovarian development over the third stage | 2025 | [9] |
| Tianyong Chen | XRN1 | The proportion of ovarian development over the third stage | 2023 | [21] |
| Wenshan Cui | Ribosomal protein S6 kinase | Ovarian developmental stages and relative expression levels of Vg | 2025 | [22] |
| Xuewei Liu | ribosomal protein L10a | Relative expression levels of Vg | 2024 | [23] |
| Hongxia Jiang | ribosome protein S24 | Ovarian developmental stages, relative expression levels of Vg and GSI | 2024 | [24] |
| Hongxia Jiang | Cyclooxygenase | Ovarian developmental stages, relative expression levels of Vg and GSI | 2023 | [25] |
| Hongxia Jiang | ribosomal protein L24 | Ovarian developmental stages, relative expression levels of Vg and GSI | 2022 | [26] |
| Hongxia Jiang | nm23 | Ovarian developmental stages, relative expression levels of Vg and GSI | 2018 | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Dong, H.; Qiao, H.; Zhang, W.; Jin, S.; Xiong, Y.; Ye, Z.; Gong, Y.; Jiang, S.; Fu, H. Functional Analysis of Hyaluronidase-like Genes in Ovarian Development of Macrobrachium nipponense and Comparative Evaluation with Other Key Regulatory Genes. Int. J. Mol. Sci. 2025, 26, 10748. https://doi.org/10.3390/ijms262110748
Wang Z, Dong H, Qiao H, Zhang W, Jin S, Xiong Y, Ye Z, Gong Y, Jiang S, Fu H. Functional Analysis of Hyaluronidase-like Genes in Ovarian Development of Macrobrachium nipponense and Comparative Evaluation with Other Key Regulatory Genes. International Journal of Molecular Sciences. 2025; 26(21):10748. https://doi.org/10.3390/ijms262110748
Chicago/Turabian StyleWang, Zhiming, Hao Dong, Hui Qiao, Wenyi Zhang, Shubo Jin, Yiwei Xiong, Zhenghao Ye, Yan Gong, Sufei Jiang, and Hongtuo Fu. 2025. "Functional Analysis of Hyaluronidase-like Genes in Ovarian Development of Macrobrachium nipponense and Comparative Evaluation with Other Key Regulatory Genes" International Journal of Molecular Sciences 26, no. 21: 10748. https://doi.org/10.3390/ijms262110748
APA StyleWang, Z., Dong, H., Qiao, H., Zhang, W., Jin, S., Xiong, Y., Ye, Z., Gong, Y., Jiang, S., & Fu, H. (2025). Functional Analysis of Hyaluronidase-like Genes in Ovarian Development of Macrobrachium nipponense and Comparative Evaluation with Other Key Regulatory Genes. International Journal of Molecular Sciences, 26(21), 10748. https://doi.org/10.3390/ijms262110748








