Placenta-Derived Secretions Promote Liver Dysfunction, and Hepatic Serum Amyloid A Mediates Kidney Inflammatory Response in a Preeclampsia-like Mouse Model
Abstract
1. Introduction
2. Results
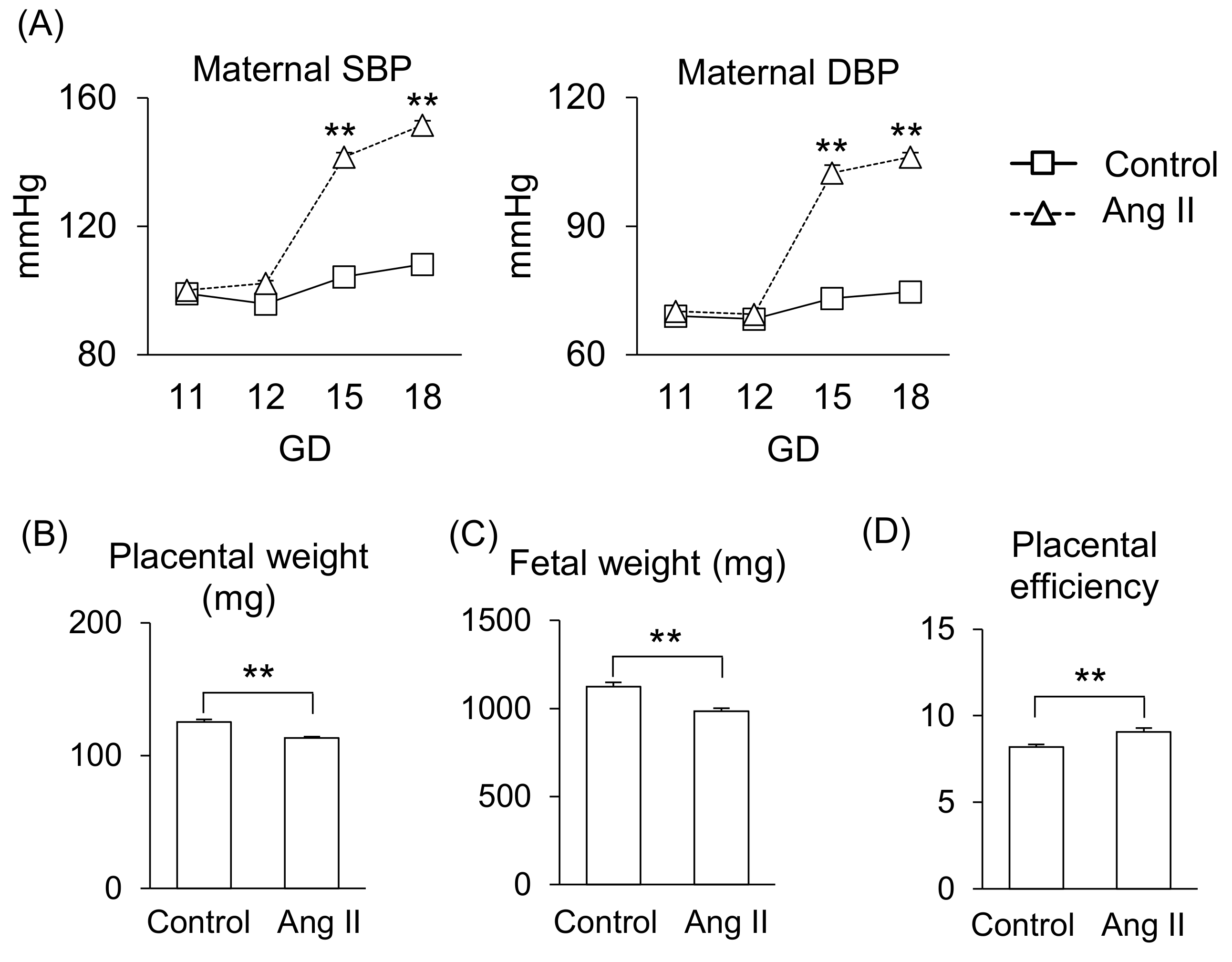
2.1. Effect of Angiotensin II (Ang II) on Blood Pressure and Fetal Development in Pregnant Mice
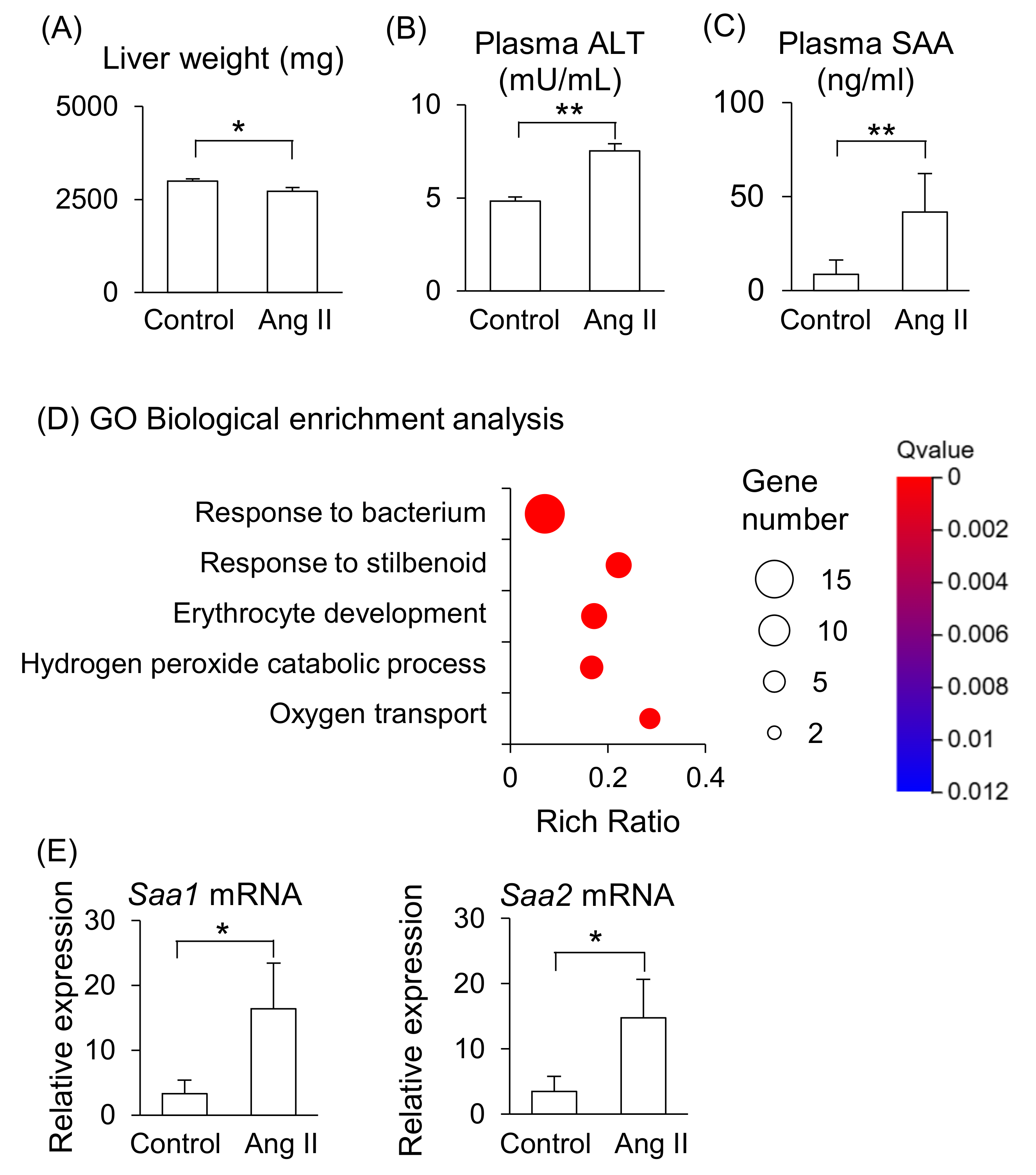
2.2. Effect of Ang II on Maternal Liver in Pregnant Mice
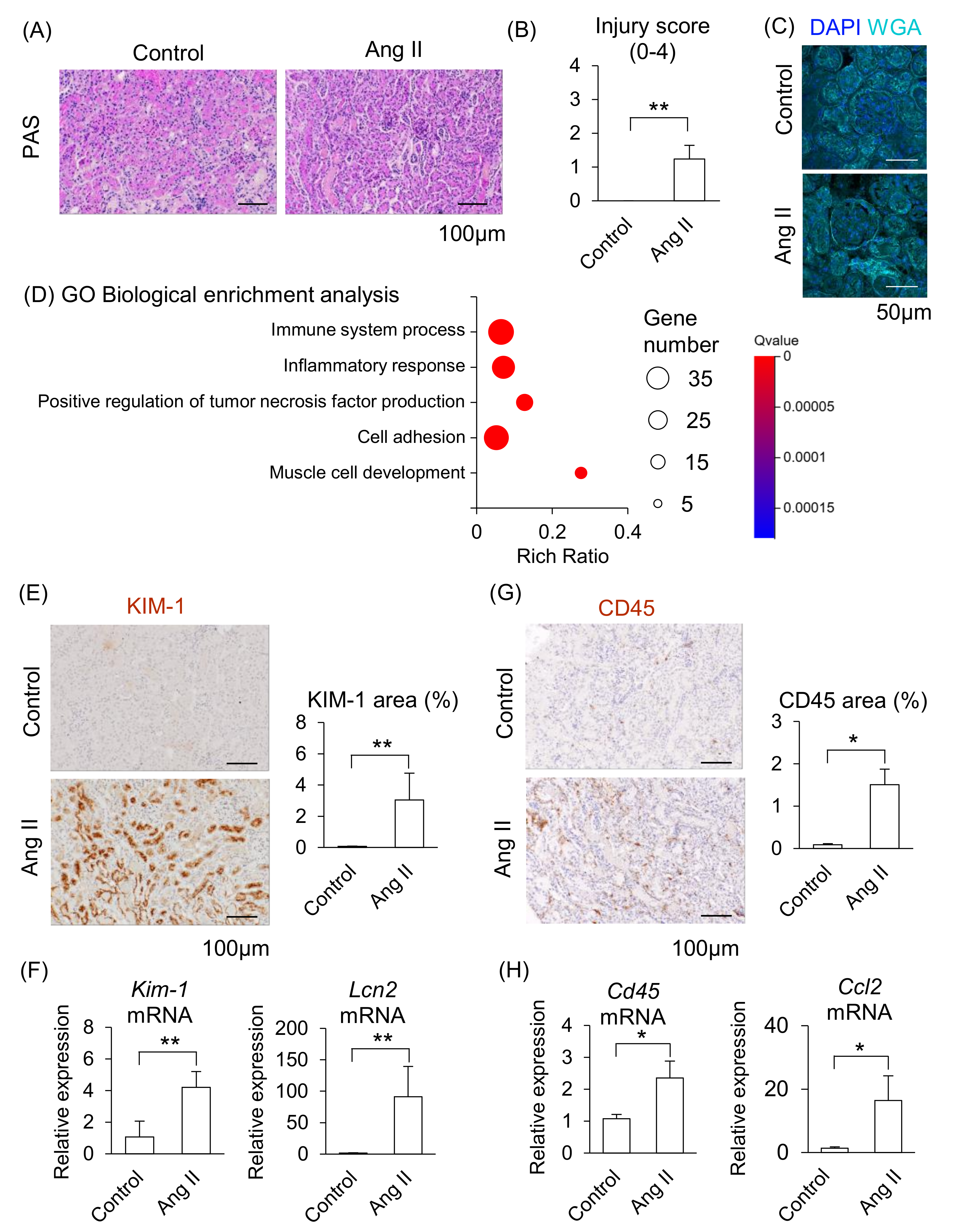
2.3. Effect of Ang II on Maternal Kidneys in Pregnant Mice
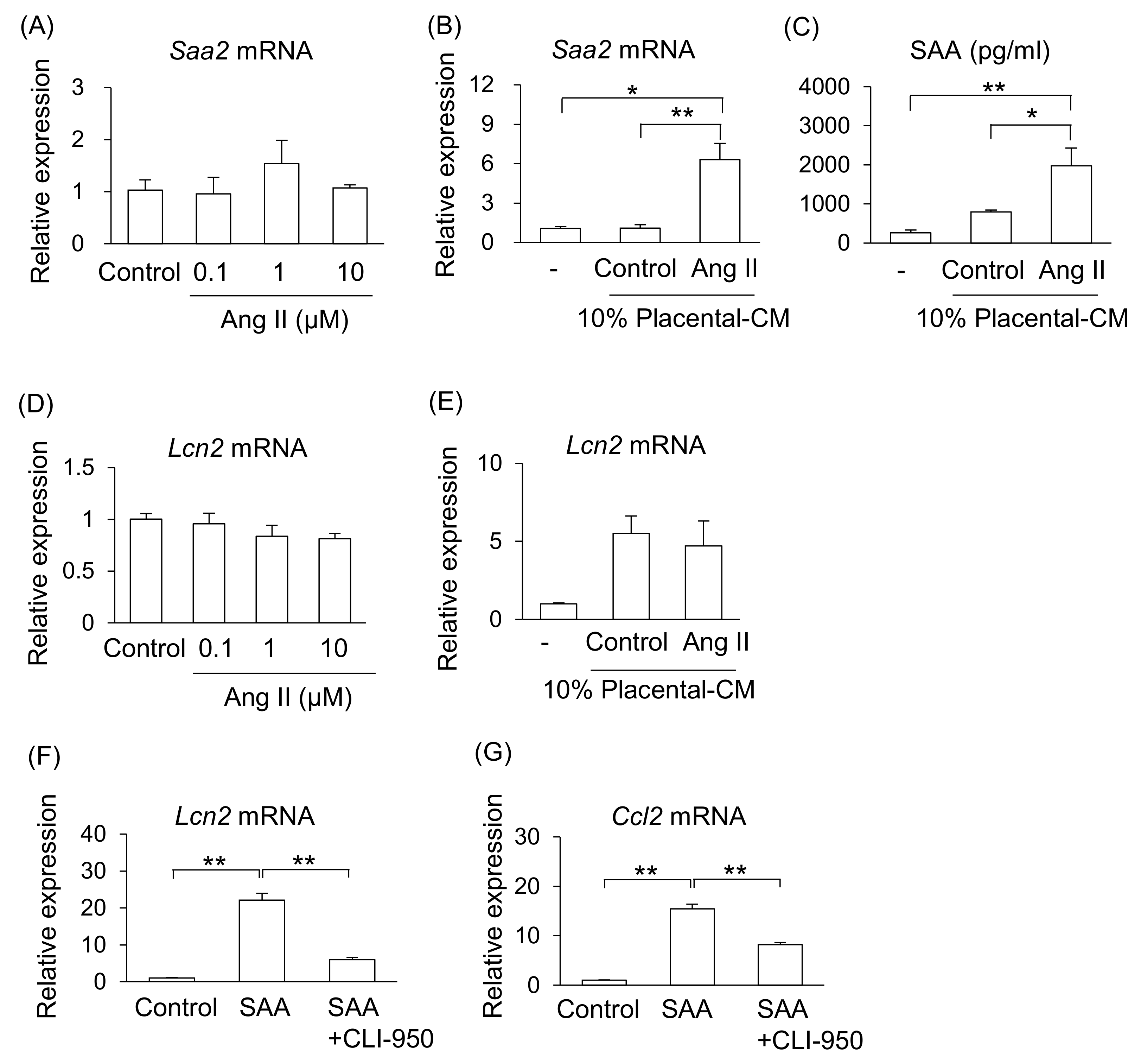
2.4. Effect of Placenta-Derived Conditioned Medium (CM) on Liver and Kidney Cells
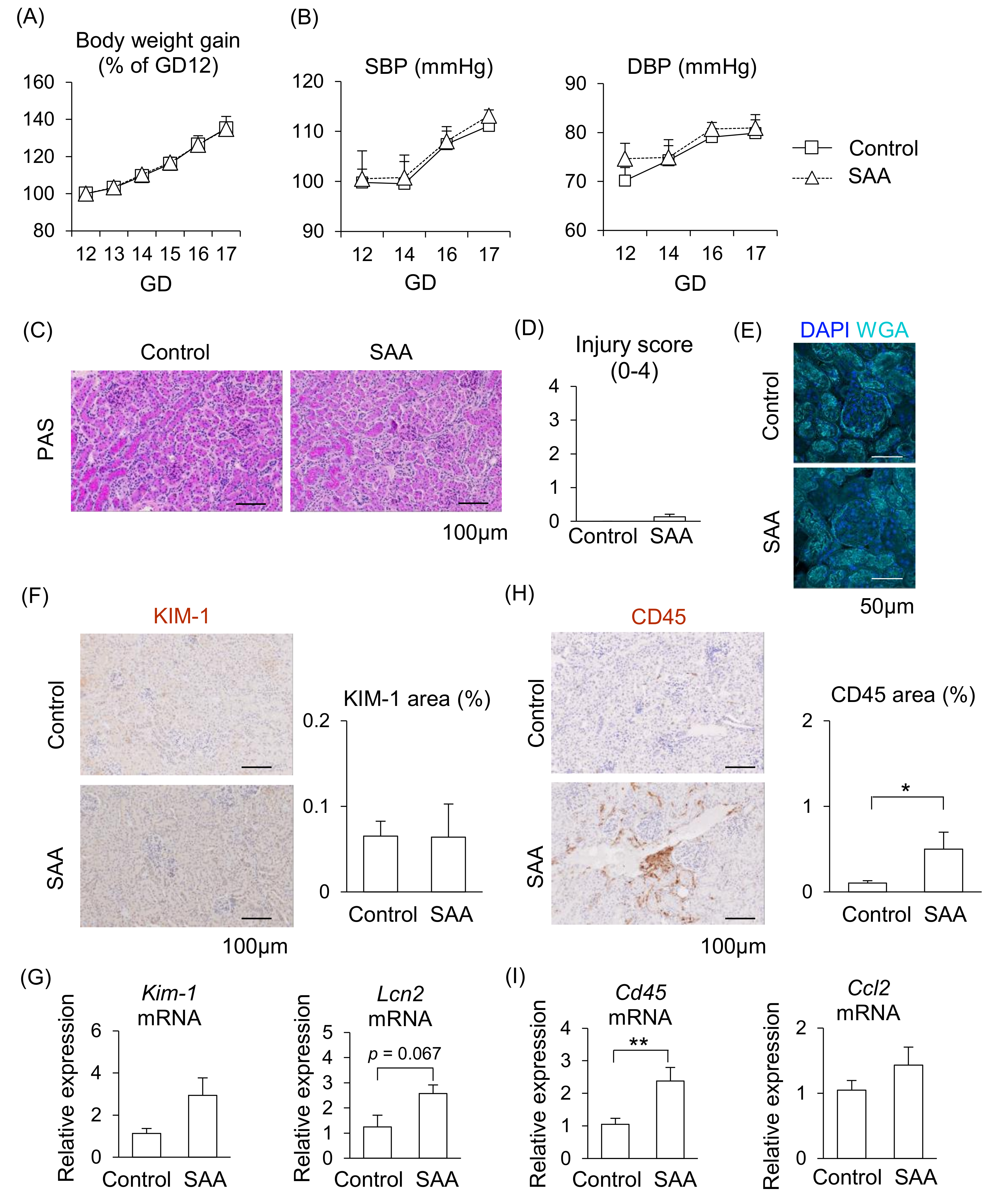
2.5. Effect of SAA on Kidneys in Pregnant Mice
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Cell Culture and Experiments
4.3. Measurements of SAA and ALT
4.4. RNA Sequencing (RNA-Seq)
4.5. Real-Time Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
4.6. Histology
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PE | Preeclampsia |
| Ang II | Angiotensin II |
| GD | Gestational day |
| SAA | Serum amyloid A |
| CM | Conditioned medium |
| ALT | Alanine aminotransferase |
| sFlt-1 | Soluble fms-like tyrosine kinase-1 |
| SBP | Systolic blood pressure |
| DBP | Diastolic blood pressure |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1β |
References
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef]
- Engin-Üstün, Y.; Üstün, Y.; Karabulut, A.B.; Özkaplan, E.; Meydanlı, M.M.; Kafkaslı, A. Serum Amyloid A Levels Are Increased in Pre-Eclampsia. Gynecol. Obstet. Investig. 2006, 64, 117–120. [Google Scholar] [CrossRef]
- Bartal, M.F.; Lindheimer, M.D.; Sibai, B.M. Proteinuria during pregnancy: Definition, pathophysiology, methodology, and clinical significance. Am. J. Obstet. Gynecol. 2022, 226, S819–S834. [Google Scholar] [CrossRef]
- Vigil-De Gracia, P.; Vargas, C.; Sánchez, J.; Collantes-Cubas, J. Preeclampsia: Narrative review for clinical use. Heliyon 2023, 9, e14187. [Google Scholar] [CrossRef] [PubMed]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Deer, E.; Herrock, O.; Campbell, N.; Cornelius, D.; Fitzgerald, S.; Amaral, L.M.; LaMarca, B. The role of immune cells and mediators in preeclampsia. Nat. Rev. Nephrol. 2023, 19, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Schuster, J.; Cheng, S.B.; Padbury, J.; Sharma, S. Placental extracellular vesicles and pre-eclampsia. Am. J. Reprod. Immunol. 2021, 85, e13297. [Google Scholar] [CrossRef]
- Di Marco, G.S.; Reuter, S.; Hillebrand, U.; Amler, S.; König, M.; Larger, E.; Oberleithner, H.; Brand, E.; Pavenstädt, H.; Brand, M. The soluble VEGF receptor sFlt1 contributes to endothelial dysfunction in CKD. J. Am. Soc. Nephrol. 2009, 20, 2235–2245. [Google Scholar] [CrossRef]
- Wallace, K.; Morris, R.; Kyle, P.B.; Cornelius, D.; Darby, M.; Scott, J.; Moseley, J.; Chatman, K.; Lamarca, B. Hypertension, inflammation and T lymphocytes are increased in a rat model of HELLP syndrome. Hypertens. Pregnancy 2014, 33, 41–54. [Google Scholar] [CrossRef]
- Moghaddas Sani, H.; Zununi Vahed, S.; Ardalan, M. Preeclampsia: A close look at renal dysfunction. Biomed. Pharmacother. 2019, 109, 408–416. [Google Scholar] [CrossRef]
- Sack, G.H., Jr. Serum amyloid A—A review. Mol. Med. 2018, 24, 46. [Google Scholar] [CrossRef]
- Fosheim, I.K.; Jacobsen, D.P.; Sugulle, M.; Alnaes-Katjavivi, P.; Fjeldstad, H.E.S.; Ueland, T.; Lekva, T.; Staff, A.C. Serum amyloid A1 and pregnancy zone protein in pregnancy complications and correlation with markers of placental dysfunction. Am. J. Obstet. Gynecol. MFM 2023, 5, 100794. [Google Scholar] [CrossRef]
- Ozawa, R.; Iwata, H.; Kuwayama, T.; Shirasuna, K. Maternal hypertensive condition alters adipose tissue function and blood pressure sensitivity in offspring. Biochem. Biophys. Res. Commun. 2024, 707, 149617. [Google Scholar] [CrossRef]
- Liu, W.; Baker, S.S.; Baker, R.D.; Nowak, N.J.; Zhu, L. Upregulation of hemoglobin expression by oxidative stress in hepatocytes and its implication in nonalcoholic steatohepatitis. PLoS ONE 2011, 6, e24363. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Gómez, R.; Lois, A.; Pino, J.; Gómez-Reino, J.J.; Lago, F.; Mobasheri, A.; Gualillo, O. The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers 2015, 20, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Asimakopoulou, A.; Weiskirchen, S.; Weiskirchen, R. Lipocalin 2 (LCN2) expression in hepatic malfunction and therapy. Front. Physiol. 2016, 7, 430. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.A.; Gomułkiewicz, A.; Serafińska, S.; Simon, K.A.; Dzięgiel, P. Metallothioneins in the Pathogenesis of Liver Diseases: A Review. Int. J. Hepatol. 2025, 2025, 8880889. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Reid, K.B. DMBT1, a regulator of mucosal homeostasis through the linking of mucosal defense and regeneration? FEBS Lett. 2003, 540, 21–25. [Google Scholar] [CrossRef]
- Bisgaard, H.C.; Holmskov, U.; Santoni-Rugiu, E.; Nagy, P.; Nielsen, O.; Ott, P.; Hage, E.; Dalhoff, K.; Rasmussen, L.J.; Tygstrup, N. Heterogeneity of ductular reactions in adult rat and human liver revealed by novel expression of deleted in malignant brain tumor 1. Am. J. Pathol. 2002, 161, 1187–1198. [Google Scholar] [CrossRef]
- Sintes, J.; Romero, X.; de Salort, J.; Terhorst, C.; Engel, P. Mouse CD84 is a pan-leukocyte cell-surface molecule that modulates LPS-induced cytokine secretion by macrophages. J. Leukoc. Biol. 2010, 88, 687–697. [Google Scholar] [CrossRef]
- Ichii, O.; Otsuka, S.; Sasaki, N.; Yabuki, A.; Ohta, H.; Takiguchi, M.; Hashimoto, Y.; Endoh, D.; Kon, Y. Local overexpression of interleukin-1 family, member 6 relates to the development of tubulointerstitial lesions. Lab. Investig. 2010, 90, 459–475. [Google Scholar] [CrossRef]
- Malamud, M.; Brown, G.D. The Dectin-1 and Dectin-2 clusters: C-type lectin receptors with fundamental roles in immunity. EMBO Rep. 2024, 25, 5239–5264. [Google Scholar] [CrossRef]
- Deng, M.; Gui, X.; Kim, J.; Xie, L.; Chen, W.; Li, Z.; He, L.; Chen, Y.; Chen, H.; Luo, W. LILRB4 signalling in leukaemia cells mediates T cell suppression and tumour infiltration. Nature 2018, 562, 605–609. [Google Scholar] [CrossRef]
- Leśniak, W.; Filipek, A. S100A6 protein—Expression and function in norm and pathology. Int. J. Mol. Sci. 2023, 24, 1341. [Google Scholar] [CrossRef]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef]
- Ichimura, T.; Asseldonk, E.J.P.; Humphreys, B.D.; Gunaratnam, L.; Duffield, J.S.; Bonventre, J.V. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J. Clin. Investig. 2008, 118, 1657–1668. [Google Scholar] [CrossRef]
- Webb, N.R.; De Beer, M.C.; Wroblewski, J.M.; Ji, A.; Bailey, W.; Shridas, P.; Charnigo, R.J.; Noffsinger, V.P.; Witta, J.; Howatt, D.A. Deficiency of Endogenous Acute-Phase Serum Amyloid A Protects apoE−/− Mice From Angiotensin II–Induced Abdominal Aortic Aneurysm Formation. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Villapol, S.; Kryndushkin, D.; Balarezo, M.G.; Campbell, A.M.; Saavedra, J.M.; Shewmaker, F.P.; Symes, A.J. Hepatic expression of serum amyloid A1 is induced by traumatic brain injury and modulated by telmisartan. Am. J. Pathol. 2015, 185, 2641–2652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, J.; Hu, Z.; Han, G.; Delafontaine, P.; Garcia, G.; Mitch, W.E. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J. Am. Soc. Nephrol. 2009, 20, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Hagihara, K.; Nishikawa, T.; Isobe, T.; Song, J.; Sugamata, Y.; Yoshizaki, K. IL-6 plays a critical role in the synergistic induction of human serum amyloid A (SAA) gene when stimulated with proinflammatory cytokines as analyzed with an SAA isoform real-time quantitative RT-PCR assay system. Biochem. Biophys. Res. Commun. 2004, 314, 363–369. [Google Scholar] [CrossRef]
- Hagihara, K.; Nishikawa, T.; Sugamata, Y.; Song, J.; Isobe, T.; Taga, T.; Yoshizaki, K. Essential role of STAT3 in cytokine-driven NF-κB-mediated serum amyloid A gene expression. Genes Cells 2005, 10, 1051–1063. [Google Scholar] [CrossRef]
- Shirasuna, K.; Karasawa, T.; Usui, F.; Kobayashi, M.; Komada, T.; Kimura, H.; Kawashima, A.; Ohkuchi, A.; Taniguchi, S.; Takahashi, M. NLRP3 Deficiency Improves Angiotensin II-Induced Hypertension But Not Fetal Growth Restriction During Pregnancy. Endocrinology 2015, 156, 4281–4292. [Google Scholar] [CrossRef] [PubMed]
- Strand, S.; Strand, D.; Seufert, R.; Mann, A.; Lotz, J.; Blessing, M.; Lahn, M.; Wunsch, A.; Broering, D.C.; Hahn, U. Placenta-derived CD95 ligand causes liver damage in hemolysis, elevated liver enzymes, and low platelet count syndrome. Gastroenterology 2004, 126, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Oe, Y.; Ko, M.; Fushima, T.; Sato, E.; Karumanchi, S.A.; Sato, H.; Sugawara, J.; Ito, S.; Takahashi, N. Hepatic dysfunction and thrombocytopenia induced by excess sFlt1 in mice lacking endothelial nitric oxide synthase. Sci. Rep. 2018, 8, 102. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Y.; Loyd, S.; Jia, X.; Groome, L.J. Increased urinary levels of podocyte glycoproteins, matrix metallopeptidases, inflammatory cytokines, and kidney injury biomarkers in women with preeclampsia. Am. J. Physiol.-Ren. Physiol. 2015, 309, F1009–F1017. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, Y.; Gu, X.; Cooper, D.B.; Lewis, D.F. Evidence of kidney injury in preeclampsia: Increased maternal and urinary levels of NGAL and KIM-1 and their enhanced expression in proximal tubule epithelial cells. Front. Med. 2023, 10, 1130112. [Google Scholar] [CrossRef]
- Ren, J.; Crowley, S.D. Role of T-cell activation in salt-sensitive hypertension. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H1345–H1353. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.K.; Umene, R.; Wu, C.H.; Nakamura, Y.; Washimine, N.; Yamamoto, R.; Ngugi, C.; Linge, K.; Kweri, J.K.; Inoue, T. Renal macrophages induce hypertension and kidney fibrosis in Angiotensin II salt mice model. Biochem. Biophys. Res. Commun. 2024, 715, 149997. [Google Scholar] [CrossRef]
- Krishnan, J.; Hennen, E.M.; Ao, M.; Kirabo, A.; Ahmad, T.; de la Visitación, N.; Patrick, D.M. NETosis Drives Blood Pressure Elevation and Vascular Dysfunction in Hypertension. Circ. Res. 2024, 134, 1483–1494. [Google Scholar] [CrossRef]
- Cabarcas-Barbosa, O.; Capalbo, O.; Ferrero-Fernández, A.; Musso, C.G. Kidney–placenta crosstalk in health and disease. Clin. Kidney J. 2022, 15, 1284–1289. [Google Scholar] [CrossRef]
- Fukazawa, K.; Lee, H.T. Updates on hepato-renal syndrome. J. Anesth. Clin. Res. 2013, 4, 352. [Google Scholar]
- De Buck, M.; Gouwy, M.; Wang, J.M.; Van Snick, J.; Proost, P.; Struyf, S.; Van Damme, J. The cytokine-serum amyloid A-chemokine network. Cytokine Growth Factor Rev. 2016, 30, 55–69. [Google Scholar] [CrossRef]
- Gaiser, A.-K.; Bauer, S.; Ruez, S.; Holzmann, K.; Fändrich, M.; Syrovets, T.; Simmet, T. Serum amyloid A1 induces classically activated macrophages: A role for enhanced fibril formation. Front. Immunol. 2021, 12, 691155. [Google Scholar] [CrossRef]
- Gathiram, P.; Moodley, J. The role of the renin-angiotensin-aldosterone system in preeclampsia: A review. Curr. Hypertens. Rep. 2020, 22, 89. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, E.; Ishida, J.; Sugiyama, F.; Horiguchi, H.; Murakami, K.; Fukamizu, A. Hypertension induced in pregnant mice by placental renin and maternal angiotensinogen. Science 1996, 274, 995–998. [Google Scholar] [CrossRef]
- Burke, S.D.; Zsengellér, Z.K.; Khankin, E.V.; Lo, A.S.; Rajakumar, A.; DuPont, J.J.; McCurley, A.; Moss, M.E.; Zhang, D.; Clark, C.D.; et al. Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia. J. Clin. Investig. 2016, 126, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- Yanai, N.; Satoh, T.; Kyo, S.; Abe, K.; Suzuki, M.; Obinata, M. A tubule cell line established from transgenic mice harboring temperature-sensitive simian virus 40 large T-antigen gene. Jpn. J. Cancer Res. 1991, 82, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, G.L.; Sanford, K.K.; Evans, V.J.; Earle, W.R. Establishment of a clone of mouse liver cells from a single isolated cell. J. Natl. Cancer Inst. 1957, 18, 701–707. [Google Scholar]
- Taru, M.; Katoh, T.; Koshimizu, K.; Kuribayashi, S.; Miura, R.; Hamano, S.; Shirasuna, K. Inflammatory uterine microenvironment in long-term infertility repeat breeder cows compared with normal fertile cows. Vet. Anim. Sci. 2024, 25, 100369. [Google Scholar] [CrossRef]
- Komada, T.; Usui, F.; Kawashima, A.; Kimura, H.; Karasawa, T.; Inoue, Y.; Kobayashi, M.; Mizushina, Y.; Kasahara, T.; Taniguchi, S.; et al. Role of NLRP3 Inflammasomes for Rhabdomyolysis-induced Acute Kidney Injury. Sci. Rep. 2015, 5, 10901. [Google Scholar] [CrossRef]
- Baatarjav, C.; Komada, T.; Karasawa, T.; Yamada, N.; Sampilvanjil, A.; Matsumura, T.; Takahashi, M. dsDNA-induced AIM2 pyroptosis halts aberrant inflammation during rhabdomyolysis-induced acute kidney injury. Cell Death Differ. 2022, 29, 2487–2502. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozawa, R.; Suzuki, S.; Shirota, A.; Nomura, S.; Komada, T.; Takahashi, M.; Iwata, H.; Shirasuna, K. Placenta-Derived Secretions Promote Liver Dysfunction, and Hepatic Serum Amyloid A Mediates Kidney Inflammatory Response in a Preeclampsia-like Mouse Model. Int. J. Mol. Sci. 2025, 26, 10737. https://doi.org/10.3390/ijms262110737
Ozawa R, Suzuki S, Shirota A, Nomura S, Komada T, Takahashi M, Iwata H, Shirasuna K. Placenta-Derived Secretions Promote Liver Dysfunction, and Hepatic Serum Amyloid A Mediates Kidney Inflammatory Response in a Preeclampsia-like Mouse Model. International Journal of Molecular Sciences. 2025; 26(21):10737. https://doi.org/10.3390/ijms262110737
Chicago/Turabian StyleOzawa, Ren, Sae Suzuki, Ayaka Shirota, Shota Nomura, Takanori Komada, Masafumi Takahashi, Hisataka Iwata, and Koumei Shirasuna. 2025. "Placenta-Derived Secretions Promote Liver Dysfunction, and Hepatic Serum Amyloid A Mediates Kidney Inflammatory Response in a Preeclampsia-like Mouse Model" International Journal of Molecular Sciences 26, no. 21: 10737. https://doi.org/10.3390/ijms262110737
APA StyleOzawa, R., Suzuki, S., Shirota, A., Nomura, S., Komada, T., Takahashi, M., Iwata, H., & Shirasuna, K. (2025). Placenta-Derived Secretions Promote Liver Dysfunction, and Hepatic Serum Amyloid A Mediates Kidney Inflammatory Response in a Preeclampsia-like Mouse Model. International Journal of Molecular Sciences, 26(21), 10737. https://doi.org/10.3390/ijms262110737





