A Novel Procedure for Preparing Mango Jellies with Higher Antioxidant Capacity and Reduced Sugar Content
Abstract
1. Introduction
2. Results and Discussion
2.1. Biological Properties of Jellies
2.1.1. Phenolic Profile and Antioxidant Activity
2.1.2. Antidiabetic Activity
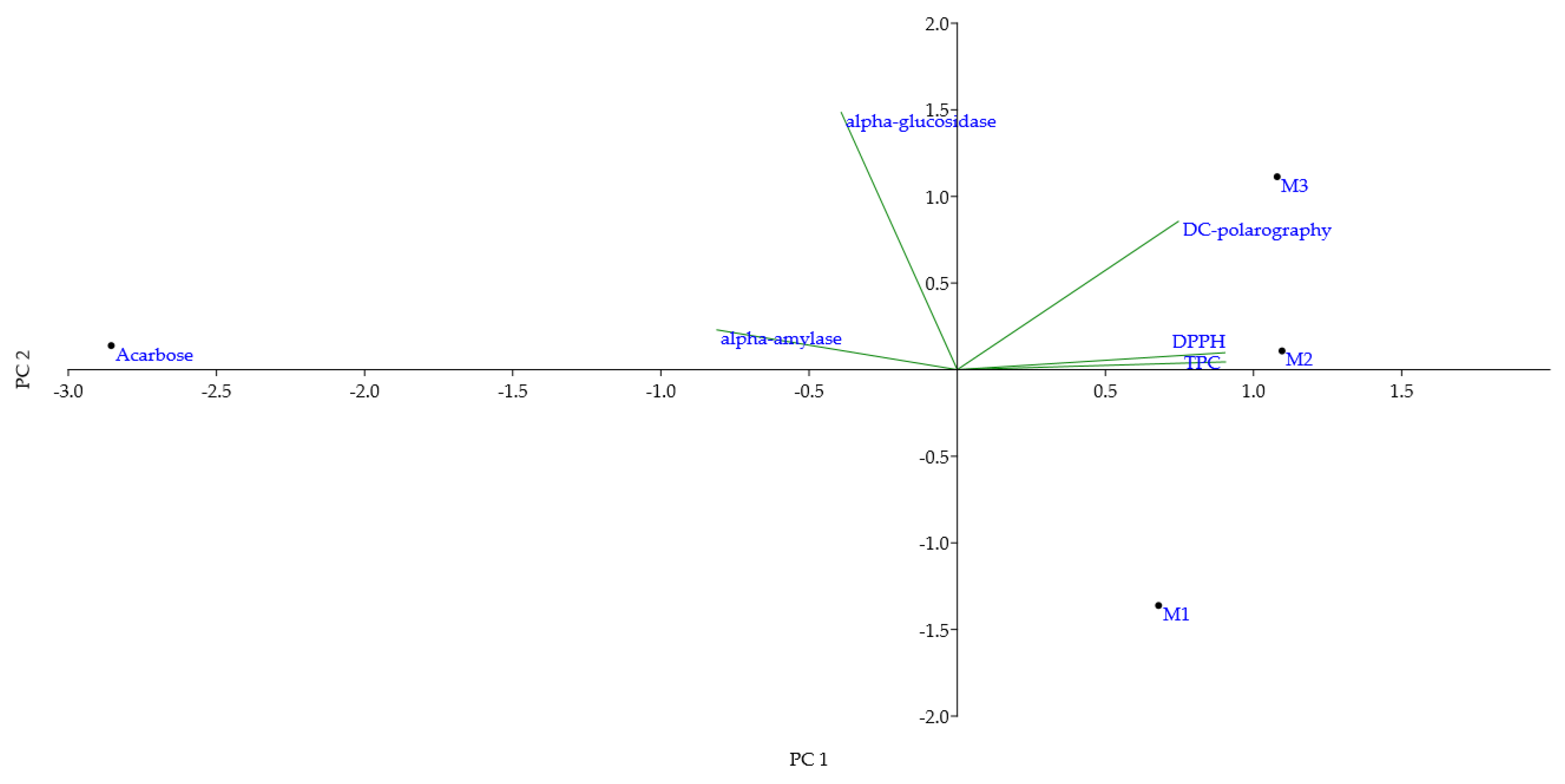
2.1.3. Principal Component and Pearson Correlation Analyses
2.2. Sensory Evaluation of Jellies
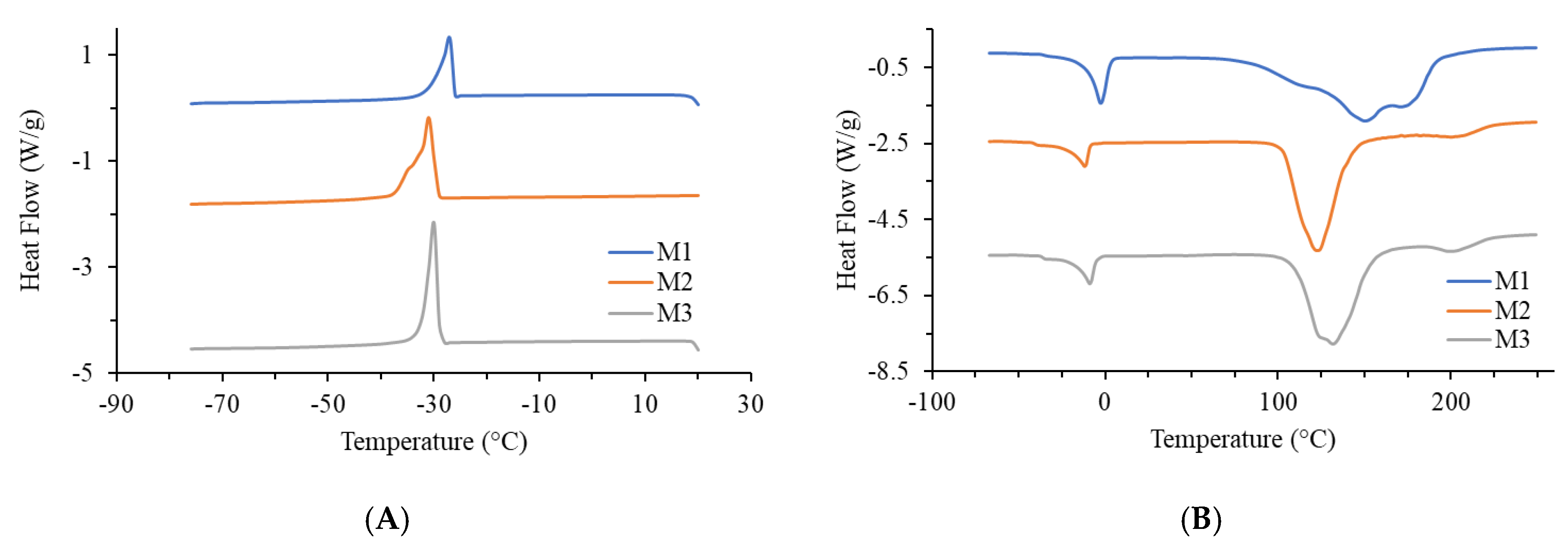
2.3. Thermal Analysis of Jellies
2.4. Limitations of the Study
3. Materials and Methods
3.1. Chemicals
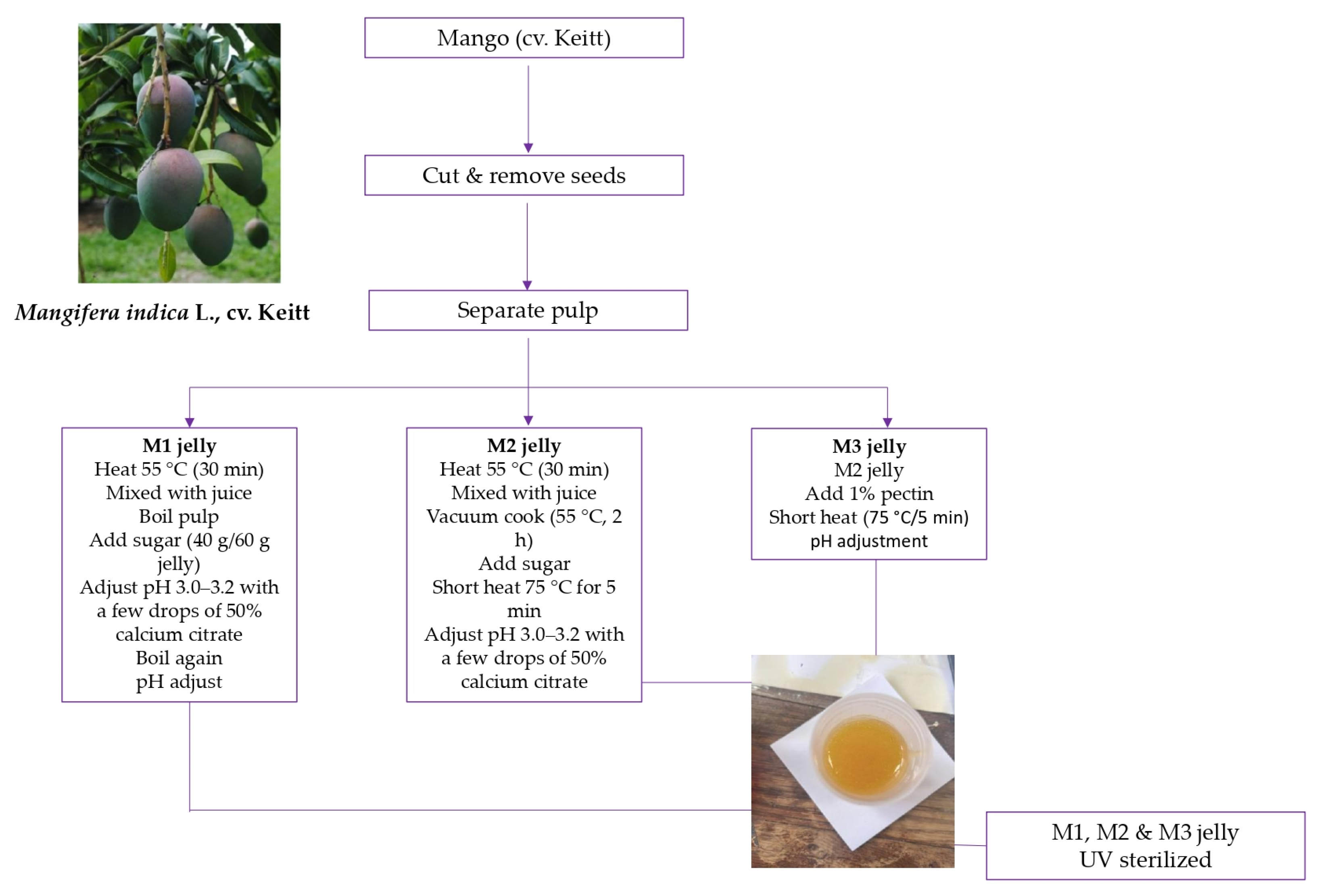
3.2. Fruit Samples and Preparation of Mango Jellies
3.3. Determination of Jelly Moisture and Total Soluble Contents
3.4. Preparation of Samples for Analysis
3.5. Determination of Total Phenolic Content
3.6. Determination of Antioxidant Activity by the DPPH Spectrophotometric Method
3.7. Direct Current (DC)-Polarographic Method for the Determination of Antioxidant Activity
3.8. In Vitro Antidiabetic Potential
3.8.1. α-Amylase Inhibitory Activity
3.8.2. α-Glucosidase Inhibitory Activity
3.9. Sensory Evaluation of the Mango Jellies
3.10. Thermal Analysis
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brecht, J.K.; Yahia, E.M. Postharvest physiology. In The Mango: Botany, Production and Uses, 2nd ed.; Litz, R.E., Ed.; CABI Publishing: Wallingford, UK, 2009; pp. 484–528. [Google Scholar] [CrossRef]
- Yahia, E.M. Postharvest Biology and Technology of Tropical and Subtropical Fruits. In Postharvest Biology and Technology of Tropical and Subtropical Fruits, 1st ed.; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2011. [Google Scholar] [CrossRef]
- Yahia, E.M.; de Jesús Ornelas-Paz, J.; Brecht, J.K.; García-Solís, P.; Maldonado Celis, M.E. The contribution of mango fruit (Mangifera indica L.) to human nutrition and health. Arab. J. Chem. 2023, 16, 104860. [Google Scholar] [CrossRef]
- Matheyambath, A.C.; Subramanian, J.; Paliyath, G. Mangoes. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Elsevier: Cambridge, MA, USA, 2016; pp. 641–645. [Google Scholar] [CrossRef]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Mangos and their bioactive components: Adding variety to the fruit plate for health. Food Funct. 2017, 8, 3010–3032. [Google Scholar] [CrossRef]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef]
- Wall-Medrano, A.; Olivas-Aguirre, F.J.; Velderrain-Rodríguez, G.R.; González-Aguilar, A.; de la Rosa, L.A.; López-Díaz, J.A.; Álvarez-Parrilla, E. El mango: Aspectos agroindustriales, valor nutricional/funcional y efectos en la salud. Nutr. Hosp. 2015, 31, 67–75. [Google Scholar] [CrossRef]
- Zapata-Londoño, M.B.; Ramos Polo, A.; Alzate-Arbelaez, A.F.; Restrepo-Betancur, L.F.; Rojano, B.A.; Maldonado-Celis, M.E. Effect of mango (Mangifera indica) cv. azucar juice consumption on plasma antioxidant capacity and oxidative stress biomarkers. Vitae 2020, 27, 1–10. [Google Scholar] [CrossRef]
- Işık, M.; Dikici, E.; Altın, S.; Alp, C.; Kırboğa, K.K.; Köksal, E.; Beydemir, Ş. Phenolic content, antioxidant capacity, and therapeutic potential of mango (Mangifera indica L.) leaves. Food Sci. Nutr. 2025, 13, e70263. [Google Scholar] [CrossRef]
- Shah, K.A.; Patel, M.B.; Patel, R.J.; Parmar, P.K. Mangifera indica (mango). Pharmacogn. Rev. 2010, 4, 42–48. [Google Scholar] [CrossRef]
- Karigidi, M.E.; Fakunle, O.E.; Karigidi, K.O. Antioxidant, antidiabetic and antifungal activities of leaf and bark ethanol extracts of Mangifera indica and their antagonistic biochemical effects. Int. J. Funct. Nutr. 2025, 6, 5. [Google Scholar] [CrossRef]
- Gafforov, Y.; Bekić, S.; Yarasheva, M.; Mišković, J.; Živanović, N.; Chen, J.J.; Petri, E.; Abdullaev, B.; Rapior, S.; Lim, Y.W.; et al. Bioactivity profiling of Sanghuangporus lonicerinus: Antioxidant, hypoglycaemic, and anticancer potential via in-vitro and in-silico approaches. J. Enzym. Inhib. Med. Chem. 2025, 40, 2461185. [Google Scholar] [CrossRef]
- Rašeta, M.; Mišković, J.; Kebert, M.; Berežni, S.; Krstić, S.; Gojgić-Cvijović, G.; Pirker, T.; Bauer, R.; Karaman, M. Mycochemical profiles and bioactivities of Fistulina hepatica and Volvopluteus gloiocephalus from Serbia: Antioxidant, enzyme inhibition, and cytotoxic potentials. Food Biosci. 2025, 66, 106221. [Google Scholar] [CrossRef]
- Masibo, M.; Qian, H. Major mango polyphenols and their potential significance to human health. Compr. Rev. Food Sci. Food Saf. 2008, 7, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.; Faizi, S.; Naqvi, S.; Roome, T.; Zikr-Ur-Rehman, S.; Ali, M.; Firdous, S.; Moin, S.T. Analgesic and antioxidant activity of mangiferin and its derivatives: The structure activity relationship. Biol. Pharm. Bull. 2005, 28, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Andreu, G.L.; Delgado, R.; Núñez-Sellés, A.J.; Vercesi, A.E. Dual mechanism of mangiferin protection against iron-induced damage to 2-deoxyribose and ascorbate oxidation. Pharmacol. Res. 2006, 53, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Andreu, G.L.; Sánchez-Baldoquín, C.; Ávila-González, R.; Yamamoto, E.T.S.; Revilla, A.; Uyemura, S.A.; Naal, Z.; Delgado, R.; Curti, C. Interaction of Vimang (Mangifera indica L. extract) with Fe(III) improves its antioxidant and cytoprotecting activity. Pharmacol. Res. 2006, 54, 389–395. [Google Scholar] [CrossRef]
- Asuncion, P.; Liu, C.; Castro, R.; Yon, V.; Rosas, M.; Hooshmand, S.; Kern, M.; Hong, M.Y. The effects of fresh mango consumption on gut health and microbiome–randomized controlled trial. Food Sci. Nutr. 2023, 11, 2069–2078. [Google Scholar] [CrossRef]
- Corrales-Bernal, A.; Jaramillo, G.; Rodríguez, B.; Yahia Kazuz, E.; Maldonado-Celis, M.E. Mango (Mangifera indica cv. Azúcar) antiinflammatory and chemopreventive role during colorectal carcinogenesis. Emir. J. Food Agric. 2016, 28, 704–712. [Google Scholar] [CrossRef]
- Ge, X.X.; Xing, M.Y.; Yu, L.F.; Shen, P. Carotenoid intake and esophageal cancer risk: A meta-analysis. Asian Pac. J. Cancer Prev. 2013, 14, 1911–1918. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. A review on ethnopharmacological applications, pharmacological activities, and bioactive compounds of Mangifera indica (Mango). Evid. Based Complement. Alternat. Med. 2017, 2017, 6949835. [Google Scholar] [CrossRef]
- Gondi, M.; Basha, S.A.; Bhaskar, J.J.; Salimath, P.V.; Prasada Rao, U.J.S. Anti-diabetic effect of dietary mango (Mangifera indica L.) peel in streptozotocin-induced diabetic rats. J. Sci. Food Agric. 2015, 95, 991–999. [Google Scholar] [CrossRef]
- Lucas, E.A.; Li, W.; Peterson, S.K.; Brown, A.; Kuvibidila, S.; Perkins-Veazie, P.; Clarke, S.L.; Smith, B.J. Mango modulates body fat and plasma glucose and lipids in mice fed a high-fat diet. Br. J. Nutr. 2011, 106, 1495–1505. [Google Scholar] [CrossRef]
- Xu, X.; Yu, E.; Liu, L.; Zhang, W.; Wei, X.; Gao, X.; Song, N.; Fu, C. Dietary intake of vitamins A, C, and E and the risk of colorectal adenoma: A meta-analysis of observational studies. Eur. J. Cancer Prev. 2013, 22, 529–539. [Google Scholar] [CrossRef]
- de Almeida Costa, N.; de Almeida Paula, D.; Brêda, J.D.; Rufino Vieira, É.N.; Furtado Martins, E.M.; Ramos, A.M. A symbiotic dessert composed of yam (Dioscorea sp.) and Ubá mango pulp (Mangifera indica L.). LWT 2020, 133, 110074. [Google Scholar] [CrossRef]
- Ekpong, A.; Ngarmsak, T.; Winger, R.J. Comparing sensory methods for the optimisation of mango gel snacks. Food Qual. Prefer. 2006, 17, 622–628. [Google Scholar] [CrossRef]
- Low, Y.L.; Pui, L.P. Optimization of mango-pineapple jelly sphere production by frozen reverse spherification using a full factorial design. Acta Sci. Pol. Technol. Aliment. 2020, 19, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. Changes in carotenoids during processing and storage of foods. Arch. Latinoam. Nutr. 1999, 49 (Suppl. S1), 38S–47S. [Google Scholar] [PubMed]
- Bekele, M.; Satheesh, N.; Awol, S.J. Screening of Ethiopian mango cultivars for suitability for preparing jam and determination of pectin, sugar, and acid effects on physico-chemical and sensory properties of mango jam. Sci. Afr. 2020, 7, e00277. [Google Scholar] [CrossRef]
- Market Data Forecast. 2024. Available online: https://www.marketdataforecast.com/market-reports/jelly-market (accessed on 24 August 2025).
- Future Market Insights. 2025. Available online: https://www.futuremarketinsights.com/reports/low-calorie-jelly-market (accessed on 24 August 2025).
- Verified Market Reports. 2021. Available online: https://www.verifiedmarketreports.com/product/sugar-free-jelly-market/ (accessed on 24 August 2025).
- Innova Market Insights. 2024. Available online: https://www.innovamarketinsights.com/ (accessed on 24 August 2025).
- Narayan, M.; Valente, A.J.M.; Mikhailov, O.V. Gelatin as it is: History and modernity. Int. J. Mol. Sci. 2023, 24, 3583. [Google Scholar] [CrossRef]
- Muralidhara, B.M.; Veena, G.L.; Bhattacherjee, A.K.; Rajan, S. Antioxidants in ripe peel and pulp of twelve mango (Mangifera indica) cultivars. Indian J. Agric. Sci. 2019, 89, 1580–1584. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Chen, J.; Xie, J.; Wu, H.; Zhan, R.; Li, W. Major antioxidants and in vitro antioxidant capacity of eleven mango (Mangifera indica L.) cultivars. Int. J. Food Prop. 2014, 17, 1872–1887. [Google Scholar] [CrossRef][Green Version]
- Amakura, Y.; Umino, Y.; Tsuji, S.; Tonogai, Y. Influence of jam processing on the radical scavenging activity and phenolic content in berries. J. Agric. Food Chem. 2000, 48, 6292–6297. [Google Scholar] [CrossRef]
- Heinonen, I.M.; Meyer, A.S.; Frankel, E.N. Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation. J. Agric. Food Chem. 1998, 46, 4107–4112. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Yahia, E.M.; González-Aguilar, G.A. Identification and quantification of major phenolic compounds from mango (Mangifera indica, cv. Ataulfo) fruit by HPLC–DAD–MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012, 135, 105–111. [Google Scholar] [CrossRef]
- Lebaka, V.R.; Wee, Y.J.; Ye, W.; Korivi, M. Nutritional composition and bioactive compounds in three different parts of mango fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Tian, S.; Jiang, J.; Han, D.; Yu, X.; Wang, K.; Li, D.; Lu, D.; Yu, A.; Zhang, Z. Determination of antioxidant capacity of diverse fruits by electron spin resonance (ESR) and UV-vis spectrometries. Food Chem. 2017, 15, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Ahmed, I.A.M.; Doğu, S.; Uslu, N.; Fadimu, G.J.; Al Juhaimi, F.; Babiker, E.E.; Özcan, M.M. The effect of heating temperature on total phenolic content, antioxidant activity, and phenolic compounds of plum and mahaleb fruits. Int. J. Food Eng. 2019, 15, 20170302. [Google Scholar] [CrossRef]
- Barron-García, O.Y.; Gaytan-Martínez, M.; Ramírez-Jimenez, A.K.; Luzardo-Ocampo, I.; Velazquez, G.; Morales-Sanchez, E. Physicochemical characterization and polyphenol oxidase inactivation of Ataulfo mango pulp pasteurized by conventional and ohmic heating processes. LWT-Food Sci. Technol. 2021, 143, 111113. [Google Scholar] [CrossRef]
- Rawson, A.; Patras, A.; Tiwari, B.K.; Noci, F.; Koutchma, T.; Brunton, N. Effect of thermal and non thermal processing technologies on the bioactive content of exotic fruits and their products: Review of recent advances. Food Res. Int. 2011, 44, 1875–1887. [Google Scholar] [CrossRef]
- Barron-García, O.Y.; Morales-Sanchez, E.; Ramírez Jimenez, A.K.; Antunes-Ricardo, M.; Luzardo-Ocampo, I.; Gonzalez-Jasso, E.; Gaytan-Martínez, M. Phenolic compounds profile and antioxidant capacity of ‘Ataulfo’ mango pulp processed by ohmic heating at moderate electric field strength. Food Res. Int. 2022, 154, 111032. [Google Scholar] [CrossRef]
- Okino-Delgado, C.H.; Prado, D.Z.; Pereira, M.S.; Camargo, D.A.; Koike, M.A.; Fleuri, L.F. Mango. In Valorization of Fruit Processing By-Products; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 2016. [Google Scholar] [CrossRef]
- Herrera-Cazares, L.A.; Hernández-Navarro, F.; Ramírez-Jiménez, A.K.; Campos-Vega, R.; Reyes-Vega, M.L.; Loarca-Piña, G.; Morales-Sánchez, E.; Wall-Medrano, A.; Gaytán-Martínez, M. Mango-bagasse functional-confectionery: Vehicle for enhancing bioaccessibility and permeability of phenolic compounds. Food Funct. 2017, 8, 3906–3916. [Google Scholar] [CrossRef]
- Palafox-Carlos, H.; Gil-Chavez, J.; Sotelo-Mundo, R.R.; Namiesnik, J.; Gorinstein, S.; Gonzalez-Aguilar, G.A. Antioxidant interactions between major phenolic compounds found in ‘Ataulfo’ mango pulp: Chlorogenic, gallic, protocatechuic and vanillic acids. Molecules 2012, 17, 12657–12664. [Google Scholar] [CrossRef] [PubMed]
- Wall-Medrano, A.; Olivas-Aguirre, F.J.; Ayala-Zavala, J.F.; Domínguez-Avila, J.A.; Gonzalez-Aguilar, G.A.; Herrera-Cazares, L.A.; Gaytan-Martinez, M. Health benefits of mango by-products. In Food Wastes and By-Products, 1st ed.; Campos-Vega, R., Oomah, B.D., Vergara-Castaneda, H.A., Eds.; John Wiley & Sons Ltd.: West Sussex, UK, 2020; pp. 159–191. [Google Scholar] [CrossRef]
- Sha, H.; Zeng, H.; Zhao, J.; Jin, H. Mangiferin ameliorates gestational diabetes mellitus-induced placental oxidative stress, inflammation and endoplasmic reticulum stress and improves fetal outcomes in mice. Eur. J. Pharmacol. 2019, 859, 172522. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.M.; Kwon, E.B.; Lee, B.; Kim, C.Y. Recent trends in controlling the enzymatic browning of fruit and vegetable products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef]
- Zawawi, N.A.F.; Hazmi, N.A.M.; How, M.S.; Kantono, K.; Silva, F.V.M.; Sulaiman, A. Thermal, high pressure, and ultrasound inactivation of various fruit cultivars’ polyphenol oxidase: Kinetic inactivation models and estimation of treatment energy requirement. Appl. Sci. 2022, 12, 1864. [Google Scholar] [CrossRef]
- Smith, D. Fruit Jellies. Food Processing for Entrepreneurs Series. 2006. Available online: https://ucanr.edu/sites/cottagefoods/files/199766.pdf (accessed on 15 August 2025).
- Bai, Y.; Atluri, S.; Zhang, Z.; Gidley, M.; Li, E.; Gilbert, R. Structural reasons for inhibitory effects of pectin on α-amylase enzyme activity and in vitro digestibility of starch. Food Hydrocoll. 2021, 114, 106581. [Google Scholar] [CrossRef]
- Pluschke, A.; Williams, B.; Zhang, D.; Gidley, M. Dietary pectin and mango pulp effects on small intestinal enzyme activity levels and macronutrient digestion in grower pigs. Food Funct. 2018, 9, 991–999. [Google Scholar] [CrossRef]
- Lasano, N.F.; Hamid, A.H.; Karim, R.; Pak Dek, M.S.; Shukri, R.; Ramli, N.S. Nutritional composition, anti-diabetic properties and identification of active compounds using UHPLC-ESI-Orbitrap-MS/MS in Mangifera odorata L. peel and seed kernel. Molecules 2019, 24, 320. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, N.; Wang, Y.; Li, N.; Qi, X.; Ouyang, X.; Wang, Q.; Liu, M. Exploring the competitive inhibition of α-glucosidase by citrus pectin enzymatic hydrolysate and its mechanism: An integrated experimental and simulation approach. Food Chem. 2025, 464, 141819. [Google Scholar] [CrossRef]
- Bai, Y.; Gilbert, R.G. Mechanistic understanding of the effects of pectin on in vivo starch digestion: A review. Nutrients 2022, 14, 5107. [Google Scholar] [CrossRef]
- Gawkowska, D.; Cybulska, J.; Zdunek, A. Structure-related gelling of pectins and linking with other natural compounds: A review. Polymers 2018, 10, 762. [Google Scholar] [CrossRef]
- Liu, Y.; Weng, P.; Liu, Y.; Wu, Z.; Wang, L.; Liu, L. Citrus pectin research advances: Derived as a biomaterial in the construction and applications of micro/nano-delivery systems. Food Hydrocoll. 2022, 133, 107910. [Google Scholar] [CrossRef]
- Ganogpichayagrai, A.; Palanuvej, C.; Ruangrungsi, N. Antidiabetic and anticancer activities of Mangifera indica cv. Okrong leaves. J. Adv. Pharm. Technol. Res. 2017, 8, 19–24. [Google Scholar] [CrossRef]
- Sferrazzo, G.; Palmeri, R.; Restuccia, C.; Parafati, L.; Siracusa, L.; Spampinato, M.; Carota, G.; Distefano, A.; Di Rosa, M.; Tomasello, B.; et al. Mangifera indica L. leaves as a potential food source of phenolic compounds with biological activity. Antioxidants 2022, 11, 1313. [Google Scholar] [CrossRef]
- Selim, D.A.; Eldin, S.M.S.; González, F.P.; Ghareeb, D.A.; Shawky, E. Metabolomics and chemometrics-driven valorisation of mango leaves: Unveiling putative α-amylase and α-glycosidase inhibitory metabolites. Food Biosci. 2024, 61, 104652. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, M.; Zhuang, Y.; Li, Y.; Fei, P. Amidation pectin with high viscosity and enhanced gelation properties: Preparation, characterization and viscoelastic behaviors. Int. J. Biol. Macromol. 2025, 318, 145108. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, G. Improving method, properties and application of polysaccharide as emulsifier. Food Chem. 2022, 376, 131937. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Chen, Z.; Han, D. Development of multi-cultivar models for predicting the soluble solid content and firmness of European pear (Pyrus communis L.) using portable vis–NIR spectroscopy. Postharvest Biol. Technol. 2017, 129, 143–151. [Google Scholar] [CrossRef]
- Liu, Y.; Yamaguchi, S.; Ishigaki, Y.; Chen, J.; Liu, X.; Li, J.; Zhou, Z. Protease-lipase co-mediated cheese microstructural changes enhanced proteolysis and lipolysis kinetics during in vitro dynamic digestion for the elderly. Food Chem. 2025, 493, 146045. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgos, V. Hydrocolloids as Texture Modifiers. In Food Texturology: Measurement and Perception of Food Textural Properties, 1st ed.; Rosenthal, A., Chen, J., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Benthin, A.; Zimathies, A.; Gorke, O.; Drusch, S. Pectin-water interactions: Comparison of different analytical methods and influence of storage. Food Hydrocoll. 2015, 43, 577–583. [Google Scholar] [CrossRef]
- Hishida, M.; Kaneko, A.; Yamamura, Y.; Saito, K. Contrasting changes in strongly and weakly bound hydration water of a protein upon denaturation. J. Phys. Chem. B 2023, 127, 6296–6305. [Google Scholar] [CrossRef]
- Liu, B.; Xin, Q.; Zhang, M.; Chen, J.; Lu, Q.; Zhou, X.; Li, X.; Zhang, W.; Feng, W.; Pei, H.; et al. Research progress on mango post-harvest ripening physiology and the regulatory technologies. Foods 2022, 12, 173. [Google Scholar] [CrossRef]
- Simonovic, M.; Ostojic, S.; Micic, D.; Pejin, B. Low sugar jellies of berry fruits: The impact of low vs. high temperature regime on their chemical composition and antioxidativity. Nat. Prod. Res. 2021, 35, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Downing, D.L. Preserves (jams), jellies, and related products. In A Complete Course in Canning and Related Processes; Downing, D.L., Ed.; CTI Publications, Inc.: Lutherville-Timonium, MD, USA, 1996; Volume III, pp. 385–462. [Google Scholar]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific: Oxford, UK, 1994. [Google Scholar]
- Liyana-Pathirana, C.M.; Shahidi, F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J. Agric. Food Chem. 2005, 53, 2433–2440. [Google Scholar] [CrossRef]
- Suznjevic, D.; Pastor, F.; Gorjanovic, S. DC polarographic examination of Hg2+ reduction applicability to antioxidant activity determination. Electrochim. Acta 2015, 168, 240–245. [Google Scholar] [CrossRef]
- Yang, X.W.; Huang, M.Z.; Jin, Y.S.; Sun, L.N.; Song, Y.; Chen, H.S. Phenolics from Bidens bipinnata and their amylase inhibitory properties. Fitoterapia 2012, 83, 1169–1175. [Google Scholar] [CrossRef]
- Palanisamy, U.D.; Ling, L.T.; Manaharan, T.; Appleton, D. Rapid isolation of geraniin from Nephelium lappaceum rind waste and its anti-hyperglycemic activity. Food Chem. 2011, 127, 21–27. [Google Scholar] [CrossRef]

| Samples | Soluble Solids (%) | TPC (mg GAE/kg Jelly) | DPPH (mg AAE/100 g Jelly) |
|---|---|---|---|
| M1 | 60.1 ± 0.1 a | 70.0 ± 3.7 b | 53.0 ± 3.4 b |
| M2 | 60.5 ± 0.4 a | 82.0 ± 2.0 a | 65.0 ± 2.2 a |
| M3 | 60.9 ± 0.4 a | 80.4 ± 1.9 a | 63.3 ± 3.1 a |
| Samples | Mol Reduced Hg(II)/kg of Jelly | R2 |
|---|---|---|
| M1 | 3.74 × 10−3 ± 2.6 × 10−8 b | 0.973 |
| M2 | 12.40 × 10−3 ± 3.0 × 10−8 a | 0.984 |
| M3 | 11.84 × 10−3 ± 3.0 × 10−8 a | 0.982 |
| Samples | α-Amylase | α-Glucosidase |
|---|---|---|
| M1 | n.a. | n.a. |
| M2 | 37.50 ± 2.11 b | 32.63 ± 7.18 c |
| M3 | 1.10 ± 0.15 c | 92.88 ± 2.06 b |
| Acarbose | 95.52 ± 4.62 *,a | 86.26 ± 1.30 *,a |
| Samples | Taste | Spreadability | Gelation |
|---|---|---|---|
| M1 | 4.4 ± 0.4 c | 3.9 ± 0.3 a | 4.1 ± 0.4 b |
| M2 | 4.8 ± 0.4 a | 4.0 ± 0.3 a | 4.0 ± 0.4 b |
| M3 | 4.7 ± 0.5 b | 2.7 ± 0.3 b | 4.7 ± 0.4 a |
| DSC | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Cooling | Heating | ||||||||||||
| Ton (°C) | Tp (°C) | Tend (°C) | ΔH (J/g) | Ton (°C) | Tp (°C) | Tend (°C) | ΔH (J/g) | Ton (°C) | Tp1 (°C) | Tp2 (°C) | Tp3 (°C) | Tend (°C) | ΔH (J/g) | |
| M1 | −26.1 ± 1.0 | −22.5 ± 1.1 | −30.6 ± 1.2 | 59.3 ± 1.6 | −10.4 ± 0.1 | −2.6 ± 0.1 | 3.0 ± 0.1 | 162.3 ± 4.8 | 111.9 ± 5.6 | 115.2 ± 4.9 | 150.1 ± 3.8 | 171.5 ± 3.8 | 191.9 ± 2.6 | 1365 ± 9.8 |
| M2 | −29.7 ± 1.0 | −30.7 ± 1.2 | −37.6 ± 1.8 | 78.7 ± 2.0 | −21.8 ± 1.2 | −11.7 ± 1.0 | −8.8 ± 0.8 | 87.6 ± 2.7 | 102.4 ± 3.8 | 122.8 ± 4.2 | - | 202.3 ± 7.2 | 222.3 ± 6.0 | 1057 ± 9.9 |
| M3 | −29.2 ± 1.0 | −29.3 ± 1.3 | −32.8 ± 1.0 | 88.5 ± 1.9 | −17.6 ± 1.1 | −9 ± 1.0 | −4.9 ± 0.2 | 98.0 ± 2.5 | 109.8 ± 4.1 | 131.7 ± 2.5 | - | 200.6 ± 7.1 | 221.8 ± 6.9 | 1018 ± 5.6 |
| TGA | ||||||||||||||
| Sample | WL1 (%) | Ts (°C) | Tp1 (°C) | Tp2 (°C) | Te (°C) | WL2 (%) | Ts (°C) | Tp (°C) | Te (°C) | Res at 700 °C (%) | ||||
| M1 | 64.0 ± 1.8 | 25.6 ± 1.8 | 60.8 ± 2.8 | - | 160.2 ± 4.2 | 22.1 ± 1.8 | 160.4 ± 3.5 | 206.3 ± 4.8 | 401.3 ± 5.8 | 10.1 ± 1.8 | ||||
| M2 | 46.3 ± 1.5 | 25.1 ± 0.7 | 25.3 ± 1.6 | 110.7 ± 2.8 | 169.3 ± 4.5 | 34.7 ± 2.5 | 169.3 ± 3.8 | 213.4 ± 3.7 | 400.7 ± 6.1 | 13.3 ± 1.2 | ||||
| M3 | 47.5 ± 1.6 | 25.9 ± 0.9 | 50.2 ± 2.0 | 115.4 ± 1.9 | 173.3 ± 5.1 | 33.4 ± 2.1 | 173.3 ± 3.9 | 213.2 ± 2.8 | 400.4 ± 5.9 | 12.7 ± 0.8 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonović, M.; Rašeta, M.; Lekic, S.; Micic, D.; Savic, D.; Nale, D.; Vukovic, I.; Karaman, M.; Fischer, A.; Adrar, N.; et al. A Novel Procedure for Preparing Mango Jellies with Higher Antioxidant Capacity and Reduced Sugar Content. Int. J. Mol. Sci. 2025, 26, 10637. https://doi.org/10.3390/ijms262110637
Simonović M, Rašeta M, Lekic S, Micic D, Savic D, Nale D, Vukovic I, Karaman M, Fischer A, Adrar N, et al. A Novel Procedure for Preparing Mango Jellies with Higher Antioxidant Capacity and Reduced Sugar Content. International Journal of Molecular Sciences. 2025; 26(21):10637. https://doi.org/10.3390/ijms262110637
Chicago/Turabian StyleSimonović, Mladen, Milena Rašeta, Stefan Lekic, Darko Micic, Danica Savic, Djordje Nale, Ivan Vukovic, Maja Karaman, Annik Fischer, Nabil Adrar, and et al. 2025. "A Novel Procedure for Preparing Mango Jellies with Higher Antioxidant Capacity and Reduced Sugar Content" International Journal of Molecular Sciences 26, no. 21: 10637. https://doi.org/10.3390/ijms262110637
APA StyleSimonović, M., Rašeta, M., Lekic, S., Micic, D., Savic, D., Nale, D., Vukovic, I., Karaman, M., Fischer, A., Adrar, N., & Esatbeyoglu, T. (2025). A Novel Procedure for Preparing Mango Jellies with Higher Antioxidant Capacity and Reduced Sugar Content. International Journal of Molecular Sciences, 26(21), 10637. https://doi.org/10.3390/ijms262110637









