Biological Effect of Green Synthesis of Silver Nanoparticles Derived from Malva parviflora Fruits
Abstract
1. Introduction
2. Results
2.1. Green Synthesis of Silver Nanoparticles
2.2. Characterization of AgNPs Biosynthesized from M. parviflora Fruits
2.2.1. UV-Visible Spectral Analysis
2.2.2. Technical Specifications of EDX
2.2.3. Zeta Potential Determination and Particle Size Distribution
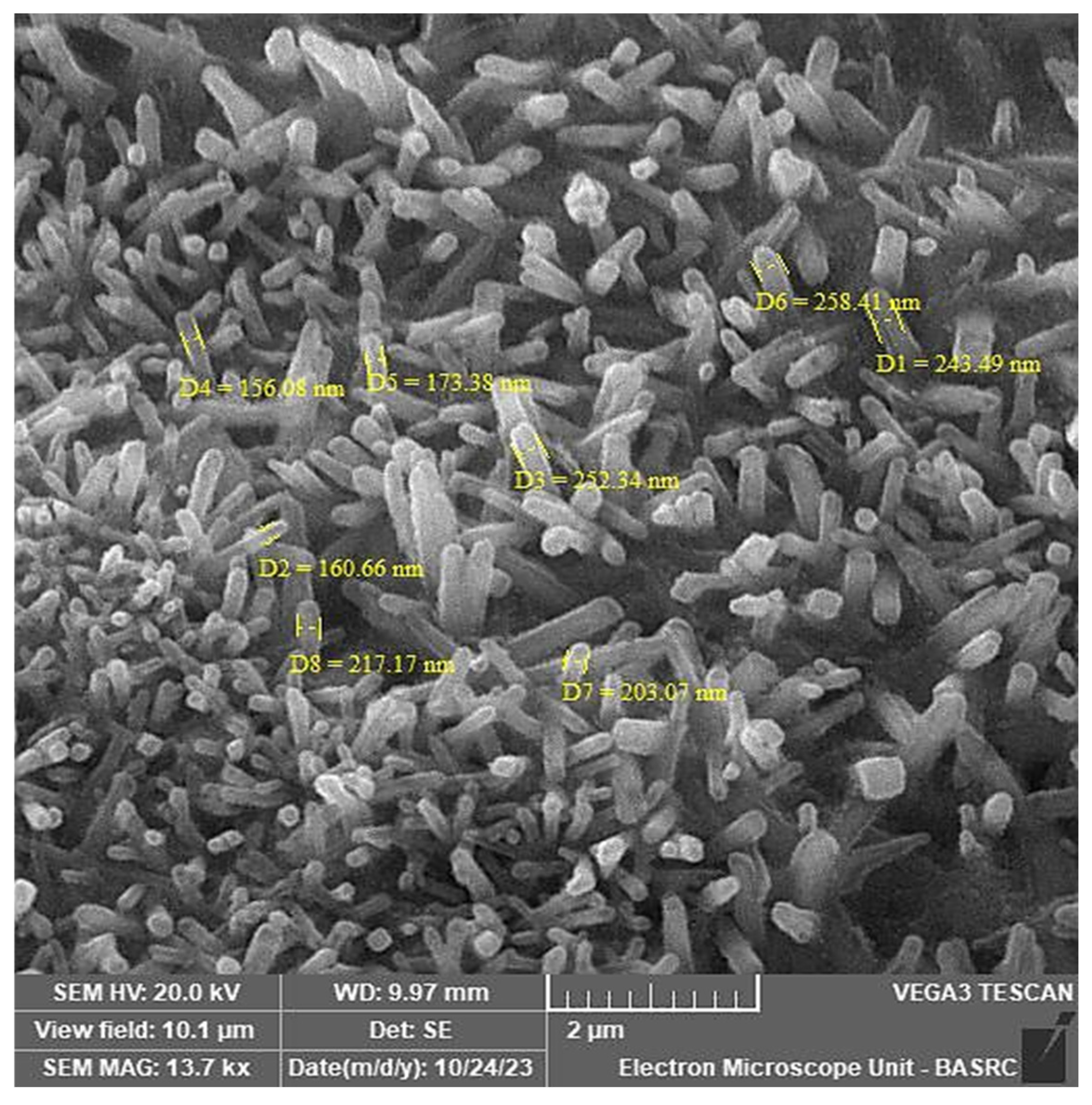
2.2.4. Scanning Electron Microscopy (SEM)
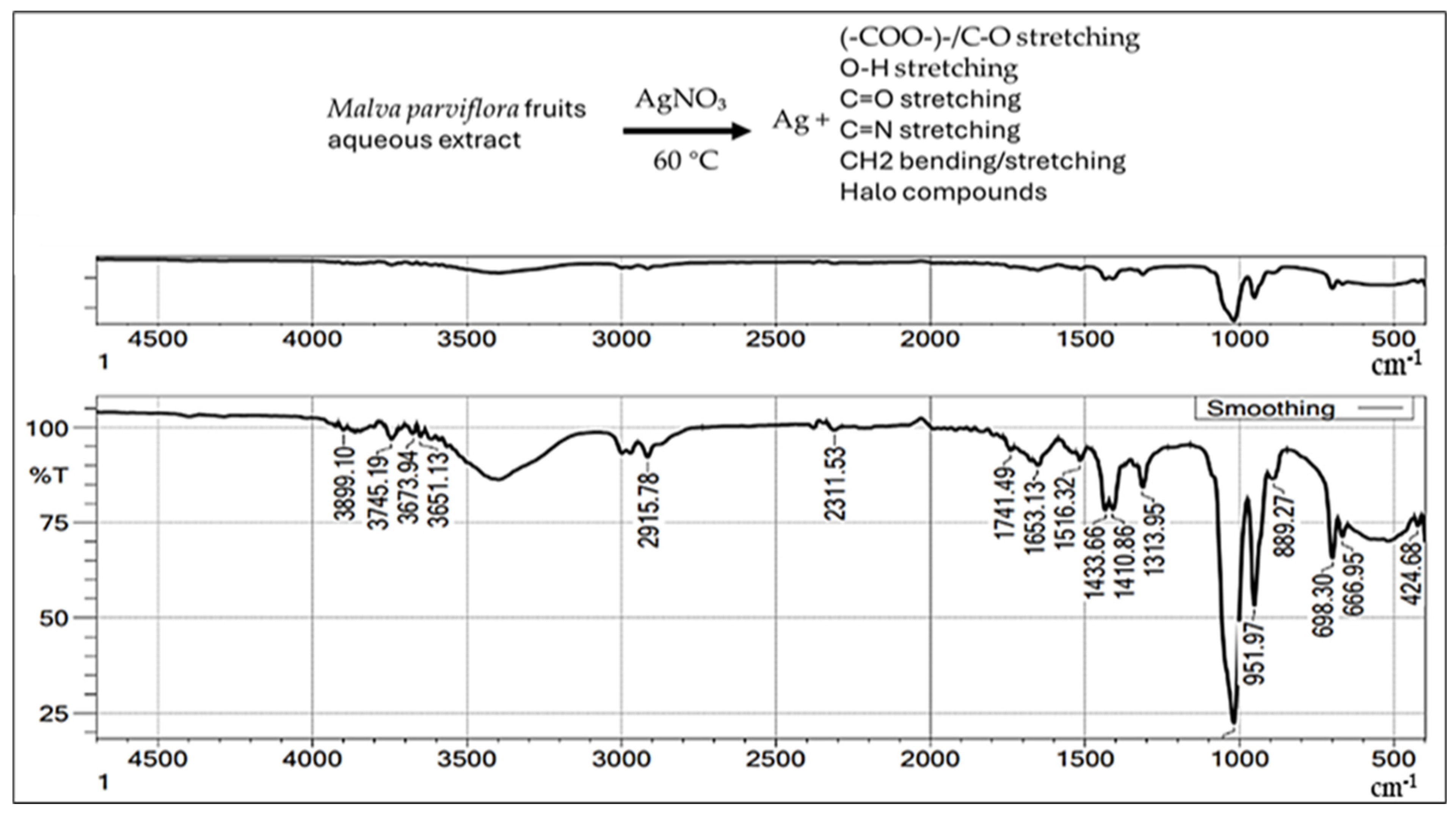
2.2.5. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3. Evaluation of the Biological Activity of Biosynthesized AgNPs from M. parviflora Fruits
2.3.1. Antibacterial Assay Against Multidrug-Resistant Bacteria
Diffusion Technique Assay
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
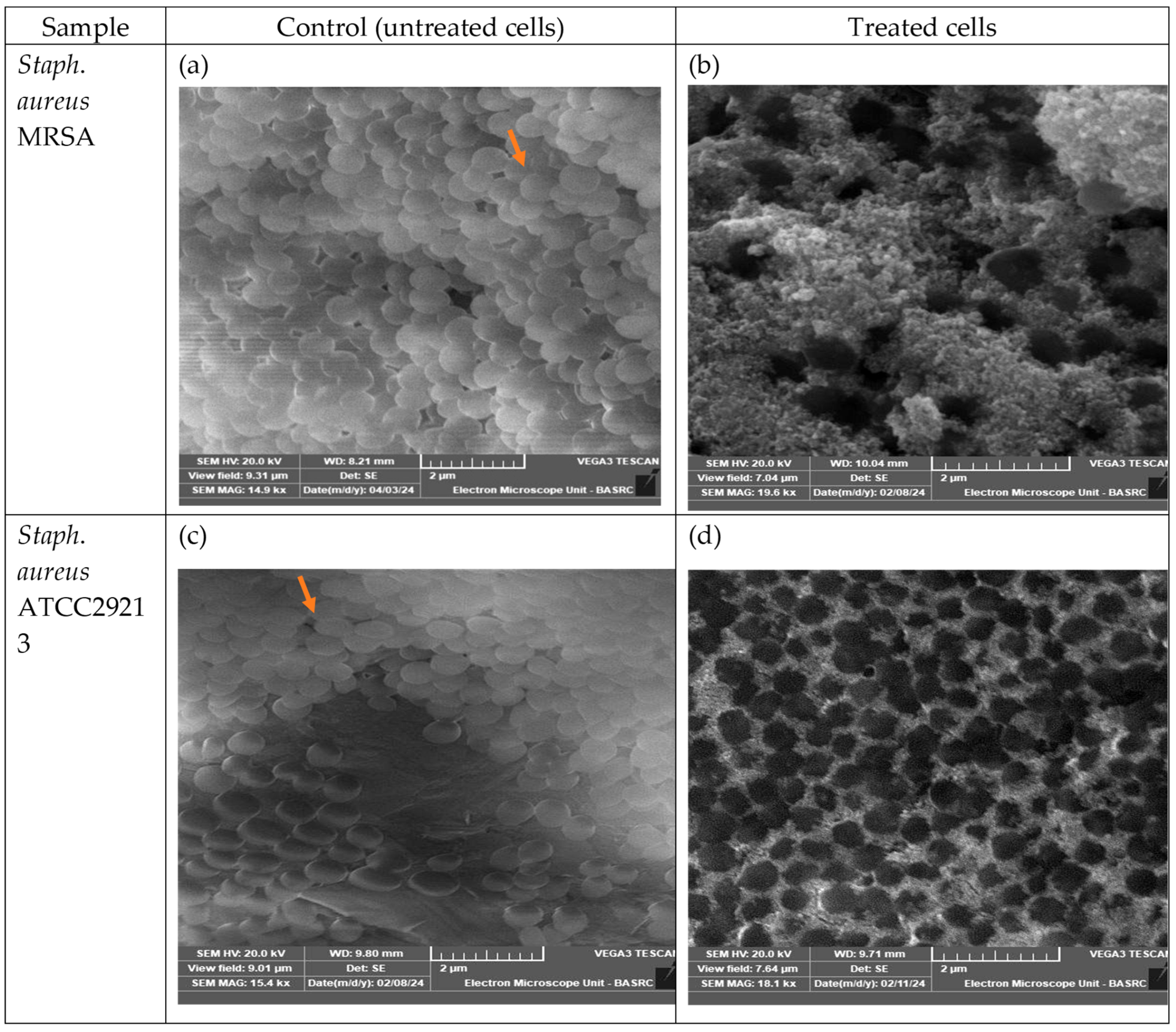
Scanning Electron Microscope Analysis of the Antibacterial Effects of M. parviflora AgNPs on Bacterial Cells
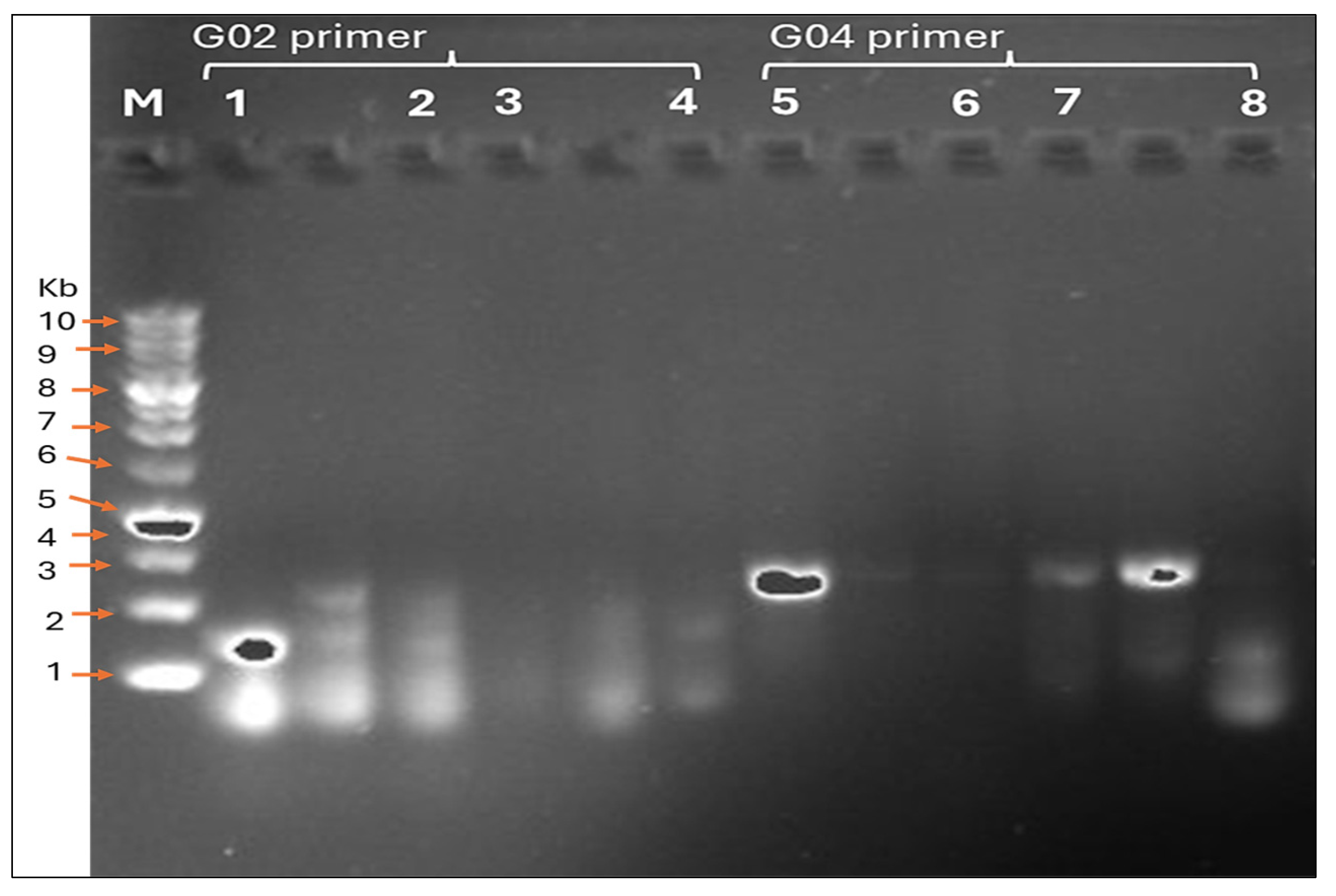
The Antibacterial Screening Effect of Biosynthesized M. parviflora AgNPs on Genomic DNA
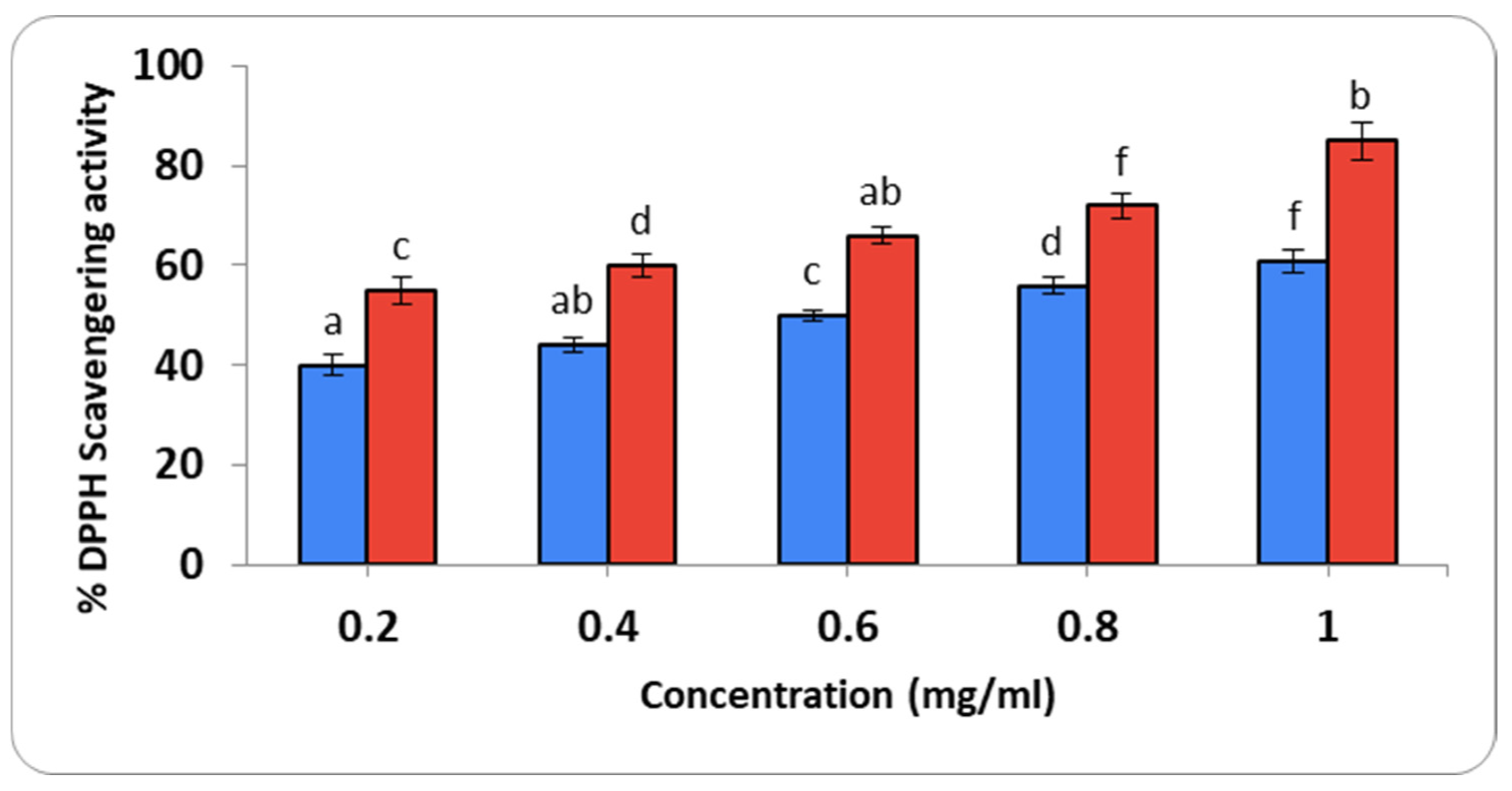
2.3.2. Determination of Antioxidant Activity
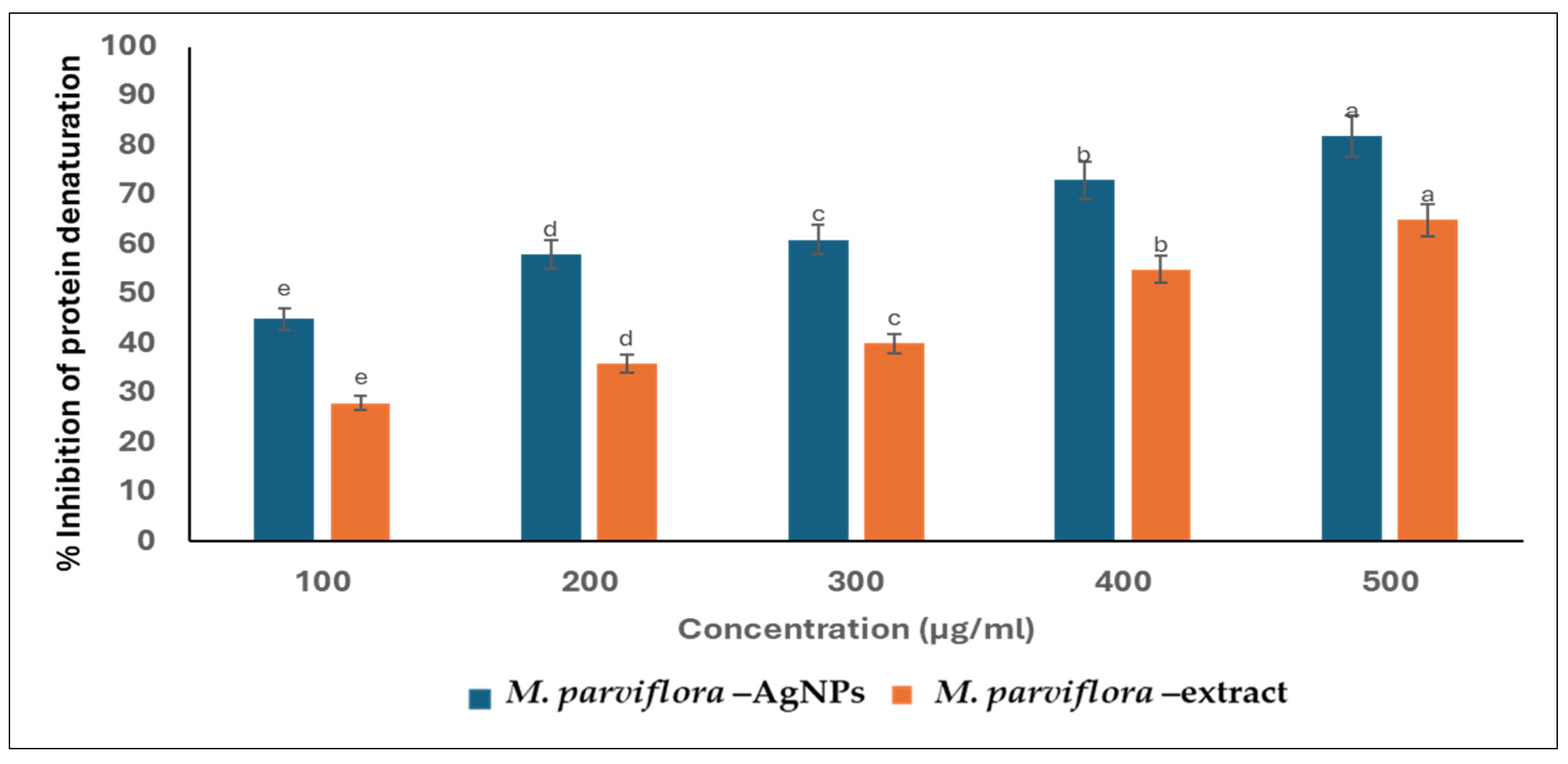
2.3.3. Anti-Inflammatory Action
Suppression of the Protein Denaturation
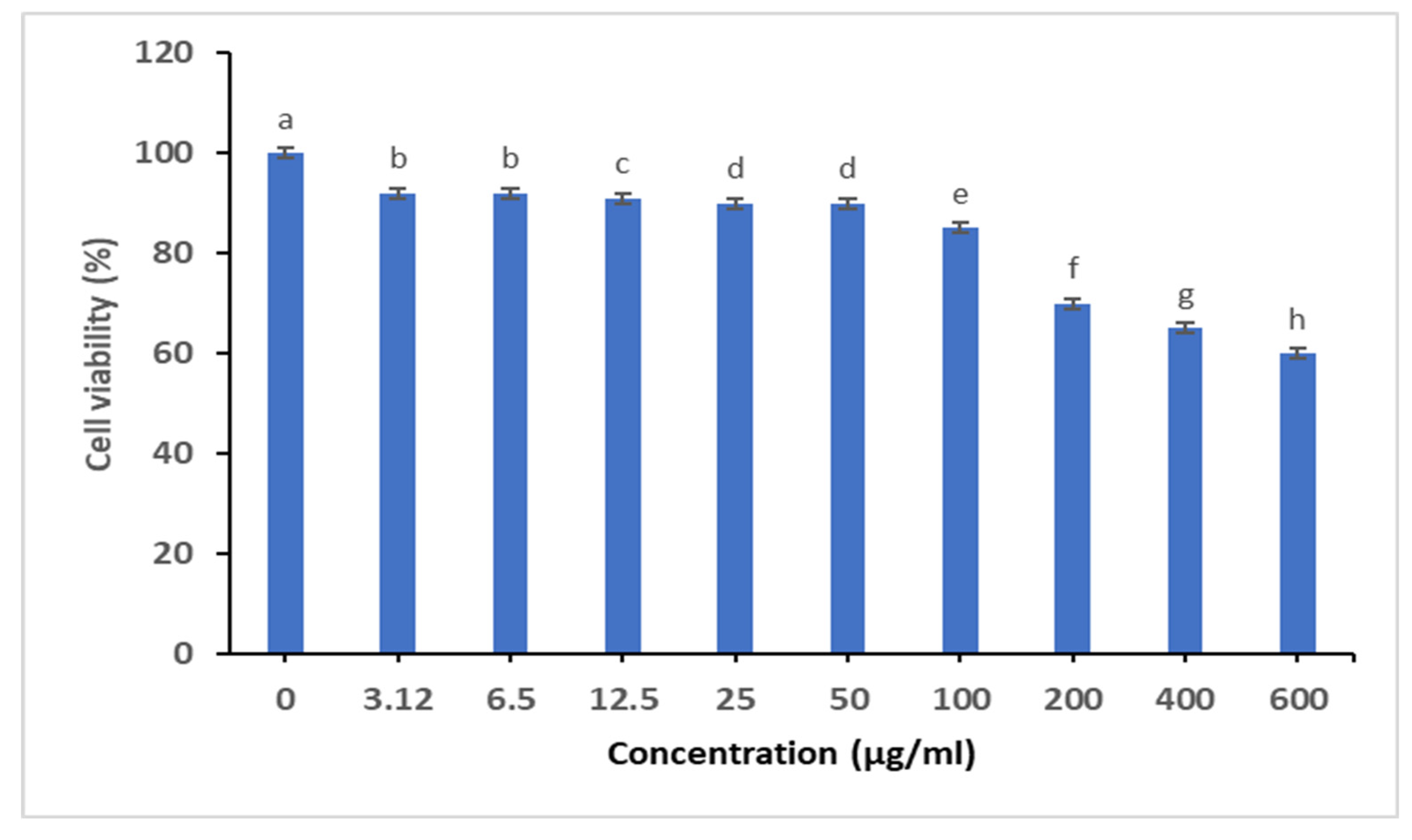
2.3.4. Cytotoxicity Studies
3. Discussion
4. Materials and Methods
4.1. Source of the Plant Sample
4.2. Green Synthesis of Silver Nanoparticles (AgNPs)
4.3. Silver Nanoparticle Characterization
4.3.1. UV Spectral Analysis
4.3.2. Technical Energy-Dispersive X-Ray Spectroscopy (EDX) Specifications
4.3.3. Zeta Potential Determination and Particle Size Distribution
4.3.4. Scanning Electron Microscopy (SEM)
4.3.5. Fourier-Transform Infrared Spectroscopy (FTIR)
4.4. Evaluation of the Biological Activity of Biosynthesized AgNPs from M. parviflora Fruits
4.4.1. Antibacterial Assay Against Multidrug-Resistant Bacteria
Bacterial Isolates Source
Diffusion Technique Assay
Determination of Minimum Inhibitory Concentration (MIC)
Determination of Minimum Bactericidal Concentration (MBC)
Scanning Electron Microscope Analysis of the Antibacterial Effects of M. parviflora AgNPs on Bacterial Cells
Screening Experiment on Biosynthesized AgNPs’ Antibacterial Effect on Genomic DNA
4.4.2. Determination of Antioxidant Activity
4.4.3. Anti-Inflammatory Activity of the Biosynthesized AgNPs
Suppression of the Protein Denaturation
4.4.4. Cytotoxicity Study on Breast Cancer Cell Line
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AgNPs | Silver nanoparticles |
| SEM | Scanning electron microscopy |
| EDX | Energy-dispersive X-ray spectroscopy |
| FTIR | Fourier-transform infrared spectroscopy |
| MIC | Minimum inhibitory concentration |
| MBC | Minimum bactericidal concentration |
| RAPD-PCR | Random amplification of polymorphic DNA–polymerase chain reaction |
References
- World Health Organization World. Health Statistics 2023: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2023; Available online: https://iris.who.int/handle/10665/367912 (accessed on 30 May 2025).
- Meshref, E.M.; Omar, A.A.D.; Moussa, S.H.; Alabdalall, A.H.; Al-Saggaf, M.S.; Alalawy, A.I.; Almutairi, F.M.; Gad, H.A.; Tayel, A.A. Antimicrobial Nanocomposites from Chitosan and Squash Synthesized Nano-Selenium Eradicate Skin Pathogens. ChemistrySelect 2024, 9, e202400881. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Pimentel, B.; Andrade, V.; Zverev, V.; Gimaev, R.R.; Pomorov, A.S.; Pyatakov, A.; Alekhina, Y.; Komlev, A.; Makarova, L.; et al. Understanding the Dependence of Nanoparticles Magnetothermal Properties on Their Size for Hyperthermia Applications: A Case Study for La-Sr Manganites. Nanomaterials 2021, 11, 1826. [Google Scholar] [CrossRef] [PubMed]
- Di Foggia, M.; Tugnoli, V.; Ottani, S.; Dettin, M.; Zamuner, A.; Sanchez-Cortes, S.; Cesini, D.; Torreggiani, A. SERS Investigation on Oligopeptides Used as Biomimetic Coatings for Medical Devices. Biomolecules 2021, 11, 959. [Google Scholar] [CrossRef]
- Bila, D.; Radwan, Y.; Dobrovolskaia, M.A.; Panigaj, M.; Afonin, K.A. The Recognition of and Reactions to Nucleic Acid Nanoparticles by Human Immune Cells. Molecules 2021, 26, 4231. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics 2020, 10, 8996. [Google Scholar] [CrossRef]
- Shehzad, A.; Qureshi, M.; Jabeen, S.; Ahmad, R.; Alabdalall, A.H.; Aljafary, M.A.; Al-Suhaimi, E. Synthesis, characterization and antibacterial activity of silver nanoparticles using Rhazya stricta. PeerJ 2018, 6, e6086. [Google Scholar] [CrossRef] [PubMed]
- Erenler, R.; Dag, B. Biosynthesis of silver nanoparticles using Origanum majorana L. and evaluation of their antioxidant activity. Inorg. Nano Met. Chem. 2021, 52, 485–492. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; HASAN, M.; Salih, A.M.; Roushdy, S.S.; Al-Shammari, A.S.; Alsanie, S.I.; El-Zaidy, M. Silver nanoparticles improve growth and protect against oxidative damage in eggplant fruitslings under drought stress. Plant Soil Environ. 2021, 67, 617–624. [Google Scholar] [CrossRef]
- AL-Moaikal, R.M.; Alabdallah, N.M.; Alsanie, S.I. Interaction of nanoparticles with photosynthetic machinery. In Molecular Impacts of Nanoparticles on Plants and Algae; Academic Press: New York, NY, USA, 2024; pp. 159–194. [Google Scholar]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Musino, D.; Devcic, J.; Lelong, C.; Luche, S.; Rivard, C.; Dalzon, B.; Landrot, G.; Rabilloud, T.; Capron, I. Impact of Physico-Chemical Properties of Cellulose Nanocrystal/Silver Nanoparticle Hybrid Suspensions on Their Biocidal and Toxicological Effects. Nanomaterials 2021, 11, 1862. [Google Scholar] [CrossRef]
- Flieger, J.; Franus, W.; Panek, R.; Szymańska-Chargot, M.; Flieger, W.; Flieger, M.; Kołodziej, P. Green synthesis of silver nanoparticles using natural extracts with proven antioxidant activity. Molecules 2021, 26, 4986. [Google Scholar] [CrossRef]
- Majithia, M.; Barretto, D.A. Biocompatible green-synthesized nanomaterials for therapeutic applications. In Advances in Nano and Biochemistry; Academic Press: New York, NY, USA, 2023; pp. 285–367. [Google Scholar]
- Patil Shriniwas, P.; Kumbhar Subhash, T. Antioxidant, antibacterial and cytotoxic potential of silver nanoparticles synthesized using terpenes rich extract of Lantana camara L. leaves. Biochem. Biophys. Rep. 2017, 10, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Khandel, P.; Shahi, S.K.; Soni, D.K.; Yadaw, R.K.; Kanwar, L. Alpinia calcarata: Potential source for the fabrication of bioactive silver nanoparticles. Nano Converg. 2018, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Alzubaidi, A.K.; Al-Kaabi, W.J.; Ali, A.A.; Albukhaty, S.; Al-Karagoly, H.; Sulaiman, G.M.; Asiri, M.; Khane, Y. Green synthesis and characterization of silver nanoparticles using Flaxseed extract and evaluation of their antibacterial and antioxidant activities. Appl. Sci. 2023, 13, 2182. [Google Scholar] [CrossRef]
- Al Baloushi, K.S.Y.; Senthilkumar, A.; Kandhan, K.; Subramanian, R.; Kizhakkayil, J.; Ramachandran, T.; Shehab, S.; Kurup, S.S.; Alyafei, M.A.M.; Al Dhaheri, A.S.; et al. Green synthesis and characterization of silver nanoparticles using Moringa peregrina and their toxicity on MCF-7 and Caco-2 human cancer cells. Int. J. Nanomed. 2024, 19, 3891–3905. [Google Scholar] [CrossRef]
- Kini, R.; Trivedi, R.; Mujahid, M.H.; Patra, P.; Alharbi, S.A.; Alshammari, F.D.; Bealy, M.B.; Elkhalifa, A.E.O.; Siddiqui, S.; Upadhyay, T.K. Green synthesis and characterization of silver nanoparticles from Nerium indicum and investigation of antioxidant and anti-cancerous potential against cervical cancer cell line (HeLa). Indian J. Pharm. Educ. Res. 2024, 58, 565–578. [Google Scholar] [CrossRef]
- Lekkala, V.D.V.; Muktinutalapati, A.V.; Lebaka, V.R.; Lomada, D.; Korivi, M.; Li, W.; Reddy, M.C. Green Synthesis and Characterization of Silver Nanoparticles from Tinospora cordifolia Leaf Extract: Evaluation of Their Antioxidant, Anti-Inflammatory, Antibacterial, and Antibiofilm Efficacies. Nanomaterials 2025, 15, 381. [Google Scholar] [CrossRef]
- Naser, Z.A.; Al-Tamimi, J.Z.; Alotaibi, B.S.; Alenzi, M.M.; Alshammari, G.M.; Alshammari, F.R. Malva parviflora: A review on its phytochemistry, pharmacological activities, and traditional uses. Saudi Pharm. J. 2022, 30, 1325–1333. [Google Scholar]
- Shadid, K.A.; Shakya, A.K.; Naik, R.R.; Jaradat, N.; Farah, H.S.; Shalan, N.; Khalaf, N.A.; Oriquat, G.A. Phenolic content and antioxidant and antimicrobial activities of Malva sylvestris L., Malva oxyloba Boiss., Malva parviflora L., and Malva aegyptia L. leaves extract. J. Chem. 2021, 2021, 8867400. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Taheri, Y.; Shaheen, S.; Kregiel, D.; Antolak, H.; Pawlikowska, E.; Brdar-Jokanović, M.; Rajkovic, J.; et al. Malva species: Insights on its chemical composition towards pharmacological applications. Phytother. Res. 2020, 34, 546–567. [Google Scholar] [CrossRef]
- Mahmoodi Esfanddarani, H.; Abbasi Kajani, A.; Bordbar, A.K. Green synthesis of silver nanoparticles using flower extract of Malva sylvestris and investigation of their antibacterial activity. IET Nanobiotechnol. 2018, 12, 412–416. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Perveen, K.; Al-Saif, N.A.; Alharbi, R.I.; Bokhari, N.A.; Albasher, G.; Al-Otaibi, R.M.; Al-Mosa, M.A. Biosynthesis of silver nanoparticles using Malva parviflora and their antifungal activity. Saudi J. Biol. Sci. 2021, 28, 2229–2235. [Google Scholar] [CrossRef]
- He, X.; Zhang, C.; Huang, F. Physico-Chemical Characterization and Antilaryngeal Cancer Effects of the Silver Nanoparticles Green-Mediated by Malva parviflora L. Extract. Appl. Organomet. Chem. 2025, 39, e70225. [Google Scholar] [CrossRef]
- Balan, K.; Qing, W.; Wang, Y.; Liu, X.; Palvannan, T.; Wang, Y.; Ma, F.; Zhang, Y. Antidiabetic activity of silver nanoparticles from green synthesis using Lonicera japonica leaf extract. RSC Adv. 2016, 6, 40162–40168. [Google Scholar] [CrossRef]
- Kajani, A.A.; Bordbar, A.K.; Zarkesh Esfahani, S.H.; Khosropour, A.R.; Razmjou, A. Green synthesis of anisotropic silver nanoparticles with potent anticancer activity using Taxus baccata extract. RSC Adv. 2014, 4, 61394–61403. [Google Scholar] [CrossRef]
- Vanlalveni, C.; Lallianrawna, S.; Biswas, A.; Selvaraj, M.; Changmai, B.; Rokhum, S.L. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: A review of recent literature. RSC Adv. 2021, 11, 2804–2837. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, S.; Sood, H.; Saxena, S.; Chaurasia, O.P. Green synthesis of silver nanoparticles using Rhodiola imbricata and Withania somnifera root extract and their potential catalytic, antioxidant, cytotoxic and growth-promoting activities. Bioprocess Biosyst. Eng. 2022, 45, 365–380. [Google Scholar] [CrossRef]
- Farooqi, M.A.; Bae, S.; Kim, S.; Bae, S.; Kausar, F.; Farooqi, H.M.U.; Hyun, C.G.; Kang, C.U. Eco-friendly synthesis of bioactive silver nanoparticles from black roasted gram (Cicer arietinum) for biomedical applications. Sci. Rep. 2024, 14, 22922. [Google Scholar] [CrossRef] [PubMed]
- Younes, K.M.; Romeilah, R.M.; El-Beltagi, H.S.; Moll, H.E.; Rajendrasozhan, S.; El-Shemy, H.A.; Shalaby, E.A. In-vitro evaluation of antioxidant and antiradical potential of successive extracts, semi-purified fractions and biosynthesized silver nanoparticles of Rumex vesicarius. Not. Bot. Horti Agrobot. Cluj Napoca 2021, 49, 12293. [Google Scholar] [CrossRef]
- Banu, A.; Gousuddin, M.; Yahya, E.B. Green synthesized monodispersed silver nanoparticles’ characterization and their efficacy against cancer cells. Biomed. Res. Ther. 2021, 8, 4476–4482. [Google Scholar] [CrossRef]
- Yusuf-Salihu, B.O.; Abdulmumini, S.A.; Bajepade, T.T.; Durosinmi, H.A.; Kazeem, M.O.; Ajayi, V.A.; Lateef, A. Novel green synthesis of silver nanoparticles from empty fruit bunch waste: Biomedical applications and mechanistic insights. Next Nanotechnol. 2025, 7, 100136. [Google Scholar] [CrossRef]
- Flores-Lopez, N.S.; Cervantes-Chávez, J.A.; Téllez de Jesús, D.G.; Cortez-Valadez, M.; Estévez-González, M.; Esparza, R. Bactericidal and fungicidal capacity of Ag2O/Ag nanoparticles synthesized with Aloe vera extract. J. Environ. Sci. Health Part A 2021, 56, 762–768. [Google Scholar] [CrossRef]
- Osman, E.E.; Shemis, M.A.; Abdel-Hameed, E.-S.S.; Gouda, A.E.; Hassan, H.; Atef, N.; Mamdouh, S. Phytoconstituent analysis, anti-inflammatory, antimicrobial and anticancer effects of nanoencapsulated Convolvulus arvensis L. extracts. BMC Complement. Med. Ther. 2024, 24, 122. [Google Scholar] [CrossRef]
- Hassan, W.A.; Mohammed, A.E.; AlShaye, N.A.; Sonbol, H.; Alghamdi, S.A.; Iamonico, D.; Korany, S.M. Characterization of Amaranthus species: Ability in nanoparticles fabrication and the antimicrobial activity against human pathogenic bacteria. PeerJ 2024, 11, e16708. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Mishra, P. Antimicrobial and antibiofilm activity of curcumin-silver nanoparticles with improved stability and selective toxicity to bacteria over mammalian cells. Med. Microbiol. Immunol. 2018, 207, 39–53. [Google Scholar] [CrossRef]
- Li, W.R.; Xie, X.B.; Shi, Q.S.; Zeng, H.Y.; Ou-Yang, Y.S.; Chen, Y.B. Antibacterial activity and mechanism of silver nano-particles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef]
- Madisha, J.K. Preliminary Phytochemical analysis and antibacterial activity of Malva parviflora against respiratory and dermatological pathogens. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Dabhi, M.; Patel, R.; Shah, V.; Soni, R.; Saraf, M.; Rawal, R.; Goswami, D. Penicillin-binding proteins: The master builders and breakers of bacterial cell walls and its interaction with β-lactam antibiotics. J. Proteins Proteom. 2024, 15, 215–232. [Google Scholar] [CrossRef]
- Singh, P.; Mijakovic, I. Strong Antimicrobial Activity of Silver Nanoparticles Obtained by the Green Synthesis in Viridibacillus sp. Extracts. Front. Microbiol. 2022, 13, 820048. [Google Scholar] [CrossRef] [PubMed]
- Fady, M.; Rizwana, H.; Alarjani, K.M.; Alghamdi, M.A.; Ibrahim, S.S.; Geyer, J.; Abbas, A. Evaluation of antibiofilm and cytotoxicity effect of Rumex vesicarius methanol extract. Open Chem. 2023, 21, 20220286. [Google Scholar] [CrossRef]
- Yadav, P.; Singhal, M.; Chatterjee, S.; Nimesh, S.; Gupta, N. Grewia tenax-Mediated Silver Nanoparticles as Efficient Antibacterial and Antifungal Agents. Nanomater. Nanotechnol. 2024, 2024, 9912599. [Google Scholar] [CrossRef]
- Khasapane, N.G.; Koos, M.; Nkhebenyane, S.J.; Khumalo, Z.T.; Ramatla, T.; Thekisoe, O. Detection of Staphylococcus isolates and their antimicrobial resistance profiles and virulence genes from subclinical mastitis cattle milk using MALDI-TOF MS, PCR and sequencing in free state province, South Africa. Animals 2024, 14, 154. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.A.; Al-Faifi, Z.E.; Masood, M.F.; El-Amier, Y.A. Investigation and biological assessment of Rumex vesicarius L. extract: Characterization of the chemical components and antioxidant, antimicrobial, cytotoxic, and anti-dengue vector activity. Molecules 2022, 27, 3177. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Abu-Ghazaleh, A.; Lightfoot, D.A. Evaluation of the antimicrobial activities of ultrasonicated spinach leaf extracts using rapid markers and electron microscopy. Arch. Microbiol. 2017, 199, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Alshareef, A.; Laird, K.; Cross, R.B.M. Shape-dependent antibacterial activity of silver nanoparticles on Escherichia coli and Enterococcus faecium bacterium. Appl. Surf. Sci. 2017, 424, 310–315. [Google Scholar] [CrossRef]
- Joshi, A.S.; Singh, P.; Mijakovic, I. Interactions of gold and silver nanoparticles with bacterial biofilms: Molecular interactions behind inhibition and resistance. Int. J. Mol. Sci. 2020, 21, 7658. [Google Scholar] [CrossRef]
- Hossain, A.; Hong, X.; Ibrahim, E.; Li, B.; Sun, G.; Meng, Y.; Wang, Y.; An, Q. Green synthesis of silver nanoparticles with culture supernatant of a bacterium Pseudomonas rhodesiae and their antibacterial activity against soft rot pathogen Dickeya dadantii. Molecules 2019, 24, 2303. [Google Scholar] [CrossRef] [PubMed]
- Girma, A.; Alamnie, G.; Bekele, T.; Mebratie, G.; Mekuye, B.; Abera, B.; Workineh, D.; Tabor, A.; Jufar, D. Green-synthesised silver nanoparticles: Antibacterial activity and alternative mechanisms of action to combat multidrug-resistant bacterial pathogens: A systematic literature review. Green Chem. Lett. Rev. 2024, 17, 2412601. [Google Scholar] [CrossRef]
- Monedeiro, F.; Pomastowski, P.; Milanowski, M.; Ligor, T.; Buszewski, B. Monitoring of Bactericidal Effects of Silver Nanoparticles Based on Protein Signatures and VOC Emissions from Escherichia coli and Selected Salivary Bacteria. J. Clin. Med. 2019, 8, 2024. [Google Scholar] [CrossRef]
- Priya, R.S.; Geetha, D.; Ramesh, P.S. Antioxidant activity of chemically synthesized AgNPs and biosynthesized Pongamia pinnata leaf extract mediated AgNPs—A comparative study. Ecotoxicol. Environ. Saf. 2016, 134, 308–318. [Google Scholar] [CrossRef]
- Kharat, S.N.; Mendhulkar, V.D. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater. Sci. Eng. C 2016, 62, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Tyavambiza, C.; Elbagory, A.M.; Madiehe, A.M.; Meyer, M.; Meyer, S. The Antimicrobial and Anti-Inflammatory Effects of Silver Nanoparticles Synthesised from Cotyledon orbiculata Aqueous Extract. Nanomaterials 2021, 11, 1343. [Google Scholar] [CrossRef]
- Rhimi, F.; Rejili, M.; Benabderrahim, M.A.; Hannachi, H. Diversity of Phytochemical Content, Antioxidant Activity, and Fruit Morphometry of Three Mallow, Malva Species (Malvaceae). Plants 2025, 14, 930. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Ali, M. Comparative phyto and physico-chemical standardization of fresh and different market samples with the anti-inflammatory studies of fruit parts of Malva sylvestris L. Res. J. Pharmacol. Pharmacodyn. 2022, 14, 219–224. [Google Scholar] [CrossRef]
- Kayel, I.; Essghaier, B.; Benabderrahim, M.A.; Rodríguez-Arcos, R.; Jiménez-Araujo, A.; Guillén-Bejarano, R.; Hannachi, H. Three Mediterranean species from natural plant communities (Ceratonia siliqua, Pistacia lentiscus, and Olea europaea var sylvestris): Phenolic acids, flavonoids, and biological activities. S. Afr. J. Bot. 2024, 175, 620–627. [Google Scholar] [CrossRef]
- Miladi, M.; Abdellaoui, K.; Hamouda, A.B.; Boughattas, I.; Mhafdhi, M.; Acheuk, F.; Halima-Kamel, M.B. Physiological, histopathological and cellular immune effects of Pergularia tomentosa extract on Locusta migratoria nymphs. J. Integr. Agric. 2019, 18, 2823–2834. [Google Scholar] [CrossRef]
- Kubinova, R.; Spackova, V.; Svajdlenka, E.; Lucivjanska, K. Antioxidant activity of extracts and HPLC analysis of flavonoids from Capsella bursa-pastoris (L.) Medik. Ceska Slov. Farm. 2013, 62, 174–176. [Google Scholar]
- Huq, M.A.; Ashrafudoulla, M.; Rahman, M.M.; Balusamy, S.R.; Akter, S. Green synthesis and potential antibacterial applications of bioactive silver nanoparticles: A review. Polymers 2022, 14, 742. [Google Scholar] [CrossRef]
- Huq, M.A.; Akter, S. Bacterial mediated rapid and facile synthesis of silver nanoparticles and their antimicrobial efficacy against pathogenic microorganisms. Materials 2021, 14, 2615. [Google Scholar] [CrossRef]
- Faber, T.; McConville, J.T.; Lamprecht, A. Focused ion beam-scanning electron microscopy provides novel insights of drug delivery phenomena. J. Control. Release 2024, 366, 312–327. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, N.E.; Shiha, A.M.; Mahrous, H.; Mohammed, A.B.A. Green synthesis of chitosan nanoparticles, optimization, characterization and antibacterial efficacy against multi drug resistant biofilm-forming Acinetobacter baumannii. Sci. Reports. 2022, 18, 19869. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret FTIR spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Ferraro, M.J. Performance Standards for Antimicrobial Susceptibility Testing: Ninth Informational Supplement: NCCLS Document M100-S9; National Committee for Clinical Laboratory Standards: Villanova, PA, USA, 1999; ISBN 978-1-56238-358-9. [Google Scholar]
- Ababutain, I.M.; Alghamdi, A.I. In Vitro Anticandidal Activity and Gas Chromatography-Mass Spectrometry (GC-MS) Screening of Vitex Agnus-Castus Leaf Extracts. PeerJ 2021, 9, e10561. [Google Scholar] [CrossRef]
- Nalezinková, M.; Loskot, J.; Myslivcová Fučíková, A. The use of scanning electron microscopy and fixation methods to evaluate the interaction of blood with the surfaces of medical devices. Sci. Rep. 2024, 14, 4622. [Google Scholar] [CrossRef]
- Reinoso, E.; Bettera, S.; Frigerio, C.; DiRenzo, M.; Calzolari, A.; Bogni, C. RAPD-PCR Analysis of Staphylococcus aureus Strains Isolated from Bovine and Human Hosts. Microbiol. Res. 2004, 159, 245–255. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Souza, C.D.A.; Rao, V.S.N.; Souza, T.M.A. Evaluation of anti-inflammatory activity of some plant extracts using albumin denaturation assay. Braz. J. Pharm. Sci. 2006, 42, 239–245. [Google Scholar]
- Aldayel, M. Biofabrication of Silver Nanoparticles Using Pergularia tomentosa Extract and Evaluation of Their Antibacterial, Antioxidant, and Cytotoxic Properties. Life 2024, 40, 945–948. [Google Scholar] [CrossRef]





| Bacteria | Gram-Positive Bacteria | Gram-Negative Bacteria | ||
|---|---|---|---|---|
| S. aureus MRSA | S. aureus ATCC29213 | E. coli ESBL | E. coli ATCC25922 | |
| Zone of Inhibition (mm) ± Standard Deviation | ||||
| M. parviflora AgNPs | 20.33 ± 0.88 | 16.67 ± 0.33 | 13.33 ± 0.33 | 16.67 ± 1.33 |
| Gentamycin | 14 ± 0 | 15 ± 0 | 14 ± 0 | 15.33 ± 0.33 |
| Significance (p ≤ 0.01) | 0.12 | 0.020 | 0.001 | 0.010 |
| Test Bacteria | MIC µg/mL | MBC µg/mL |
|---|---|---|
| S. aureus MRSA | 0.8 | 1.56 |
| S. aureus ATCC29213 | 3.125 | 6.25 |
| E. coli ESBL | 0.8 | 1.56 |
| E. coli ATCC25922 | 0.8 | 1.56 |
| Strain | S. aureus MRSA | S. aureus ATCC29213 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Primer Name | Total Bands * | Number of Bands | Percentage of Bands | Total Bands | Number of Bands | Percentage of Bands | ||||
| Monomorphic | Polymorphic | Monomorphic | Polymorphic | Monomorphic | Polymorphic | Monomorphic | Polymorphic | |||
| Primer G02 | 6 | 4 | 2 | 67% | 33% | 7 | 2 | 5 | 29% | 71% |
| Primer G04 | 3 | 0 | 3 | 0% | 100% | 10 | 4 | 6 | 40% | 60% |
| Total | 9 | 4 | 5 | - | - | 17 | 6 | 11 | - | - |
| RAPD Primer | Primer Sequence 5′-3′ |
|---|---|
| G02 | GGCACTGAGG |
| G04 | AGCGTGTCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Audah, S.A.; Alghamdi, A.I.; Alsanie, S.I.; Ababutain, I.M.; Kotb, E.; Alabdalall, A.H.; Aldosary, S.K.; AlAhmady, N.F.; Alhamad, S.; Alaudah, A.A.; et al. Biological Effect of Green Synthesis of Silver Nanoparticles Derived from Malva parviflora Fruits. Int. J. Mol. Sci. 2025, 26, 8135. https://doi.org/10.3390/ijms26178135
Al-Audah SA, Alghamdi AI, Alsanie SI, Ababutain IM, Kotb E, Alabdalall AH, Aldosary SK, AlAhmady NF, Alhamad S, Alaudah AA, et al. Biological Effect of Green Synthesis of Silver Nanoparticles Derived from Malva parviflora Fruits. International Journal of Molecular Sciences. 2025; 26(17):8135. https://doi.org/10.3390/ijms26178135
Chicago/Turabian StyleAl-Audah, Suzan Abdullah, Azzah I. Alghamdi, Sumayah I. Alsanie, Ibtisam M. Ababutain, Essam Kotb, Amira H. Alabdalall, Sahar K. Aldosary, Nada F. AlAhmady, Salwa Alhamad, Amnah A. Alaudah, and et al. 2025. "Biological Effect of Green Synthesis of Silver Nanoparticles Derived from Malva parviflora Fruits" International Journal of Molecular Sciences 26, no. 17: 8135. https://doi.org/10.3390/ijms26178135
APA StyleAl-Audah, S. A., Alghamdi, A. I., Alsanie, S. I., Ababutain, I. M., Kotb, E., Alabdalall, A. H., Aldosary, S. K., AlAhmady, N. F., Alhamad, S., Alaudah, A. A., Aldayel, M. F., & Aldakheel, A. A. (2025). Biological Effect of Green Synthesis of Silver Nanoparticles Derived from Malva parviflora Fruits. International Journal of Molecular Sciences, 26(17), 8135. https://doi.org/10.3390/ijms26178135








