Improving Poly(3-Hydroxybutyrate) Properties Using Nanocellulose in Biomedical Applications: Thermal, Mechanical and Biological Studies
Abstract
1. Introduction
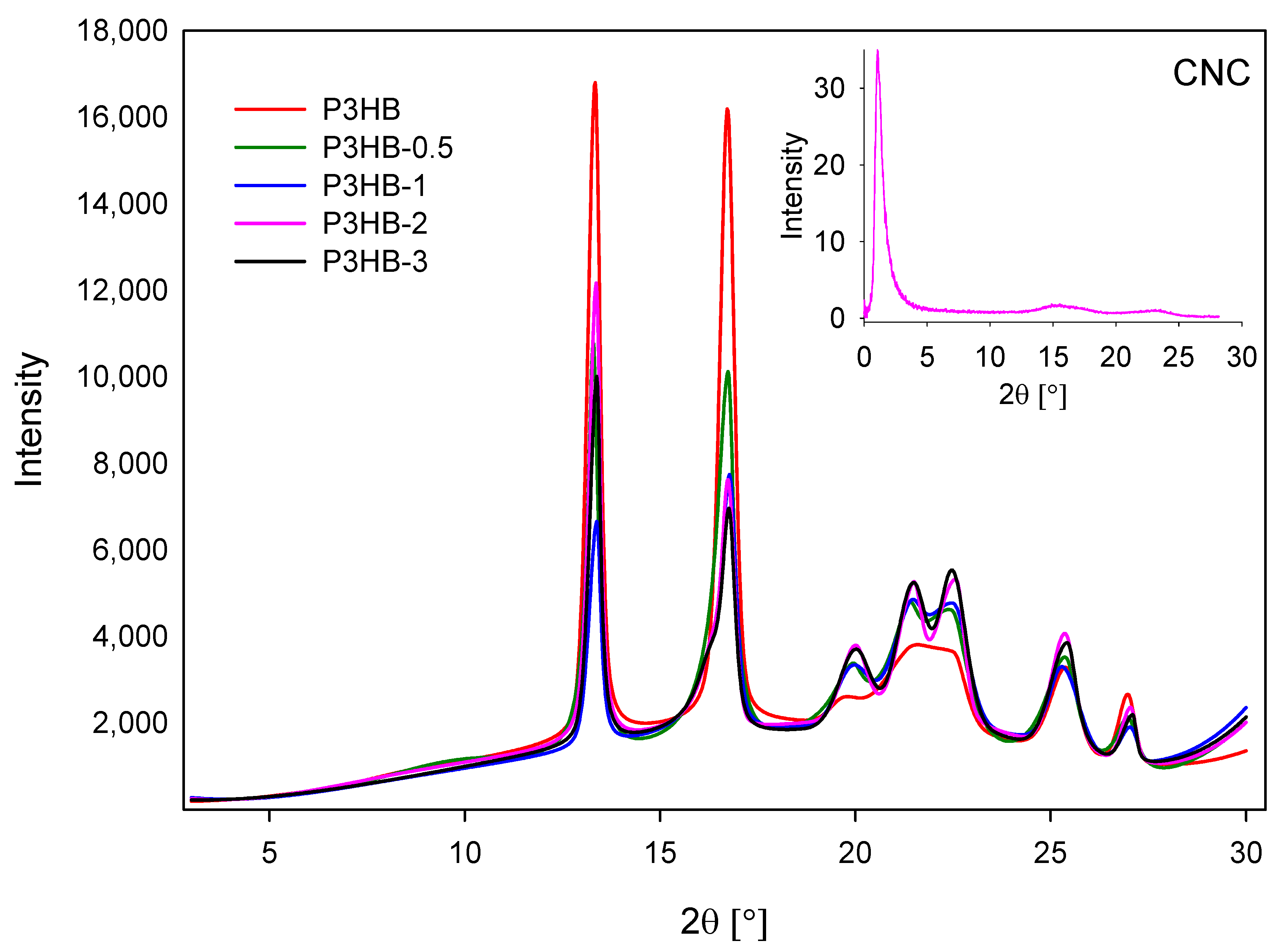
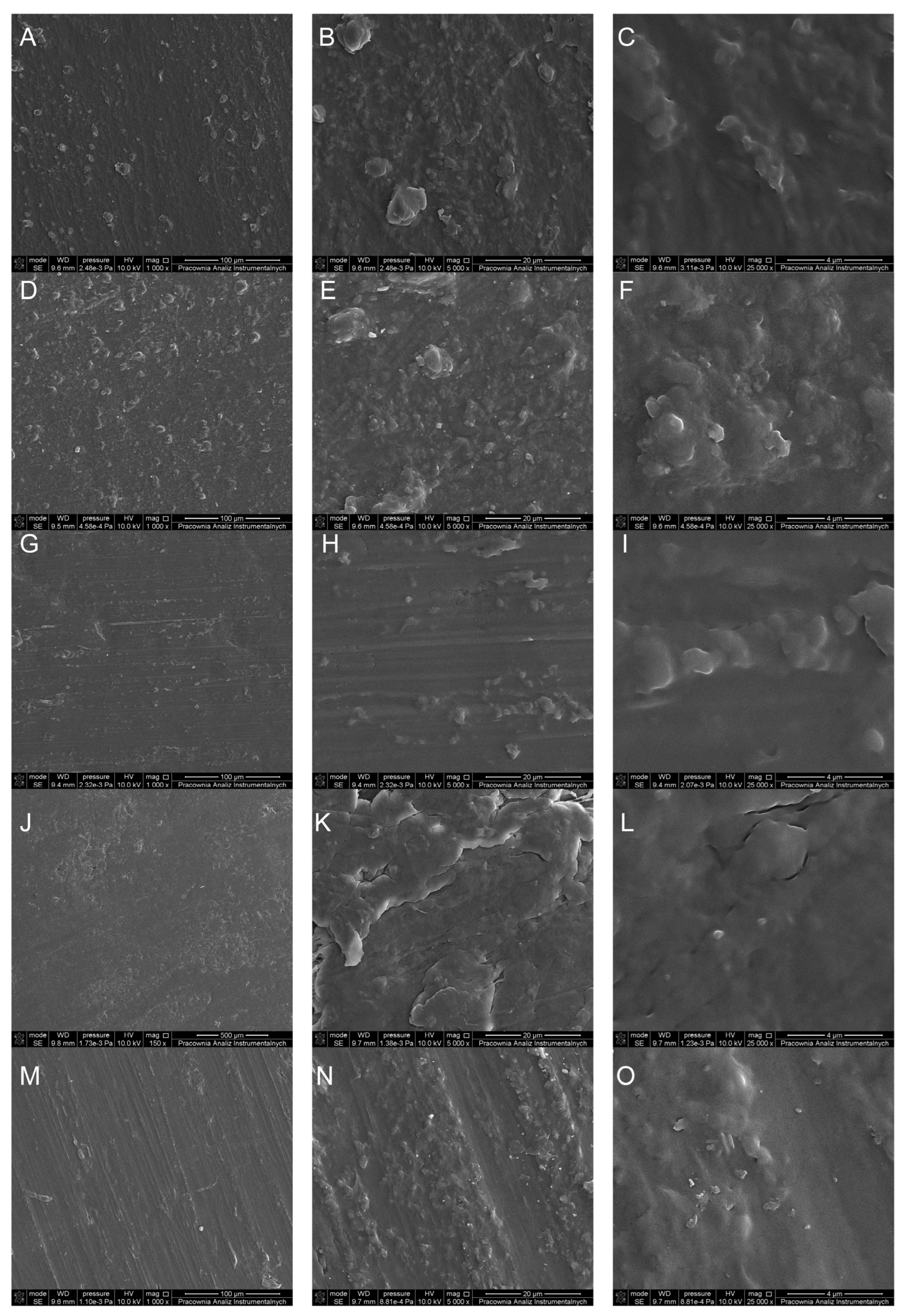
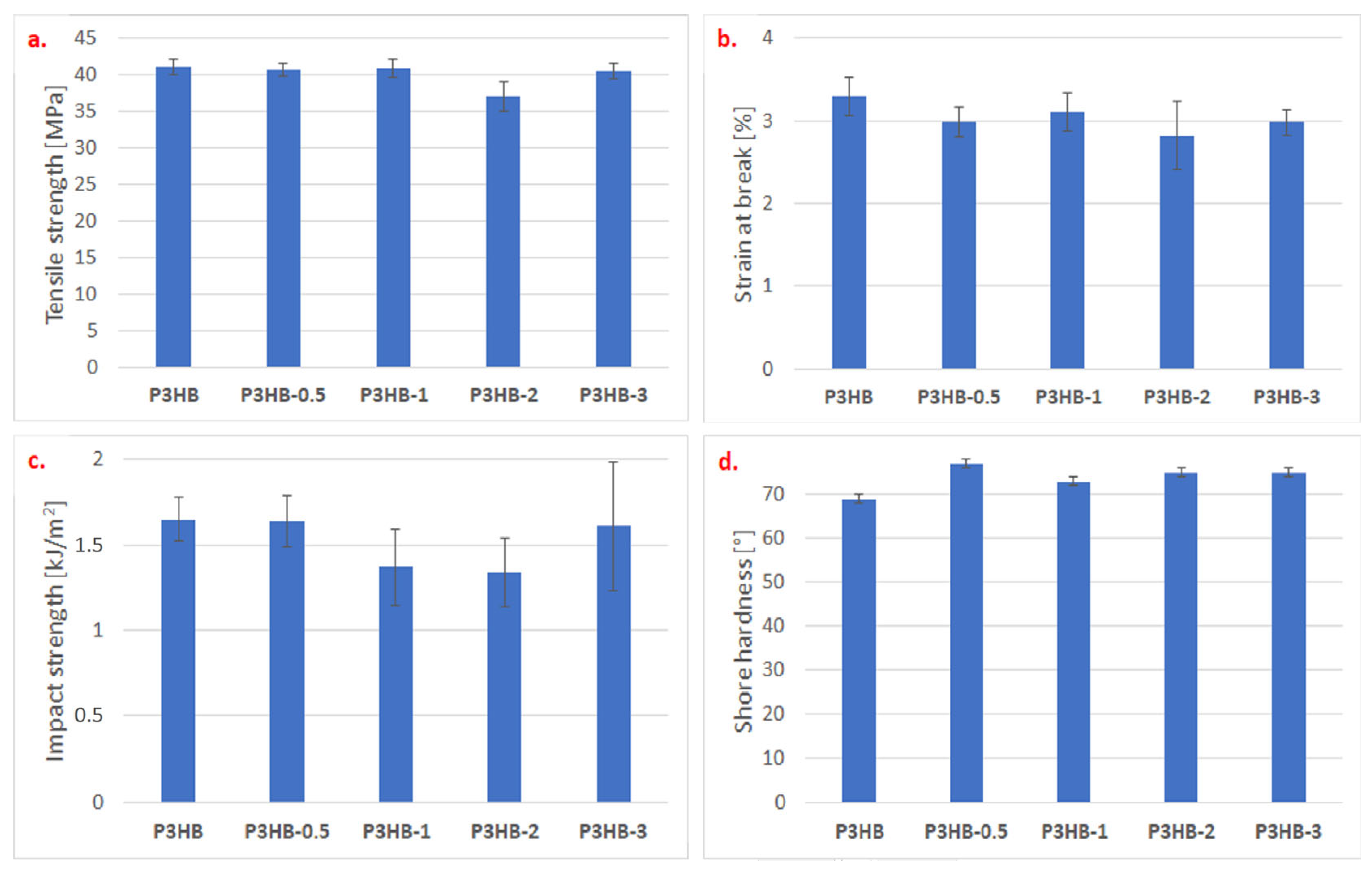
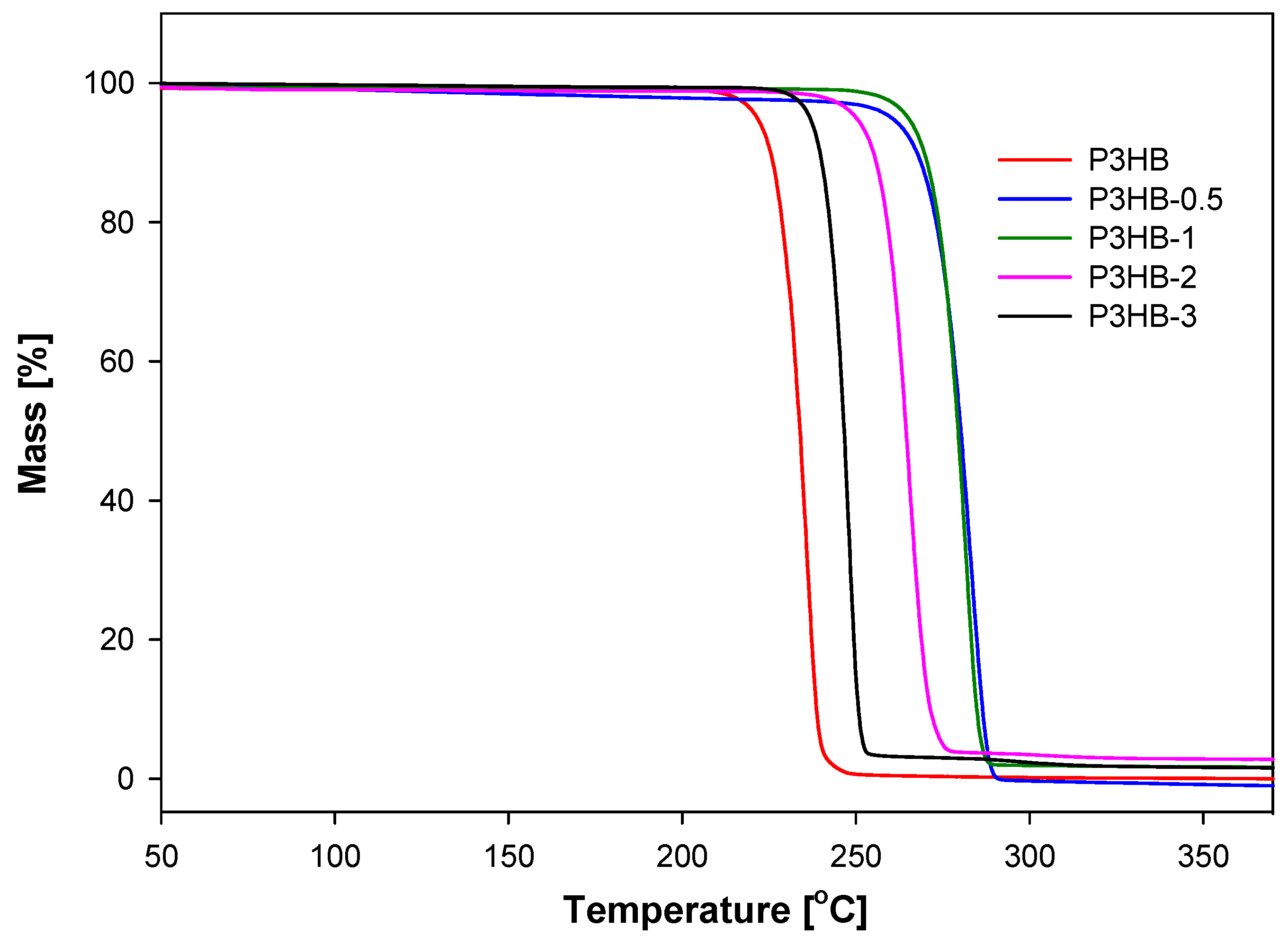
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods and Instrumentation
3.2.1. Nanobiocomposites Preparation
3.2.2. Thermogravimetry
3.2.3. Differential Scanning Calorimetry
3.2.4. X-Rays
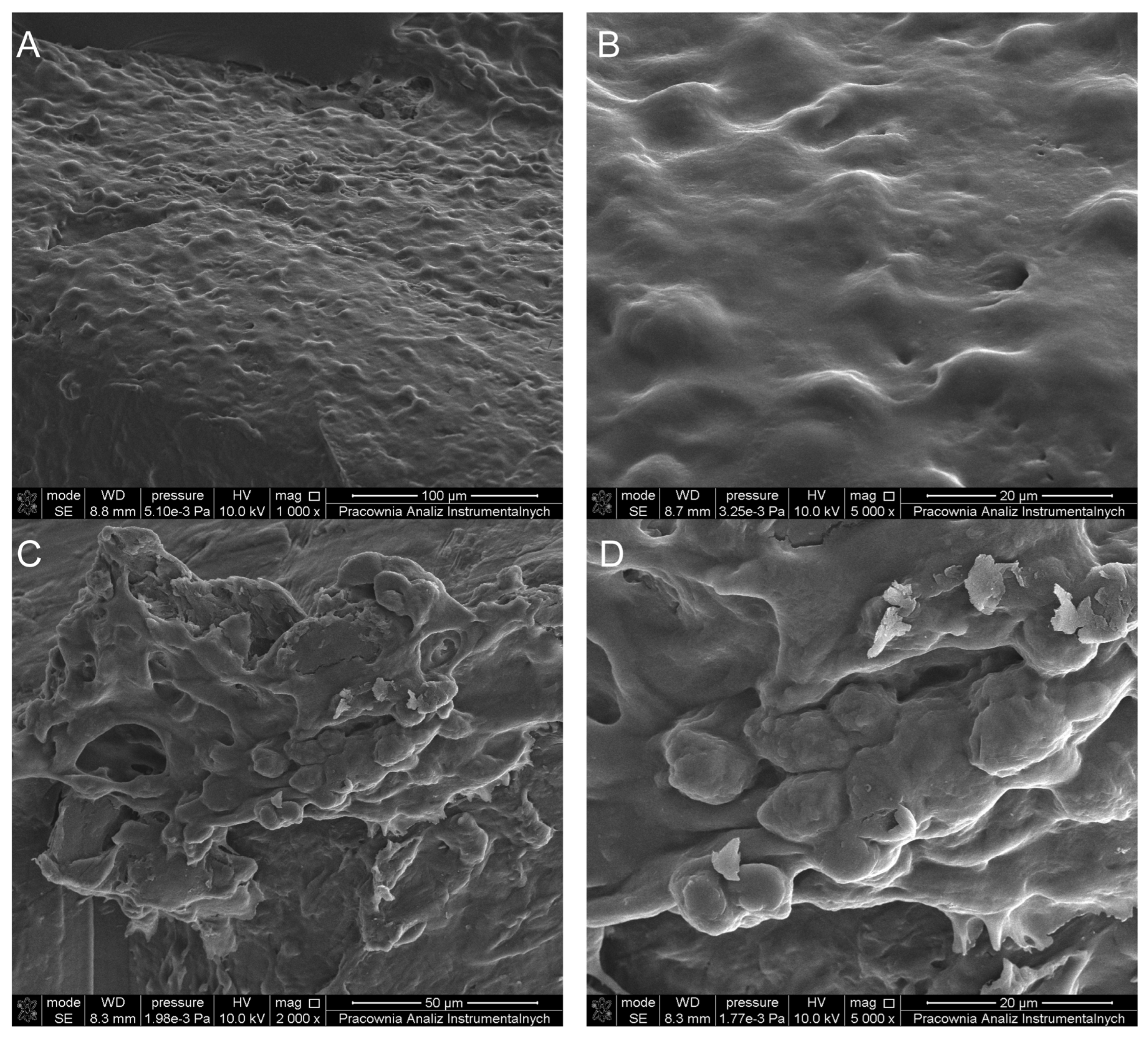
3.2.5. Scanning Electron Microscopy
3.2.6. Mechanical Tests
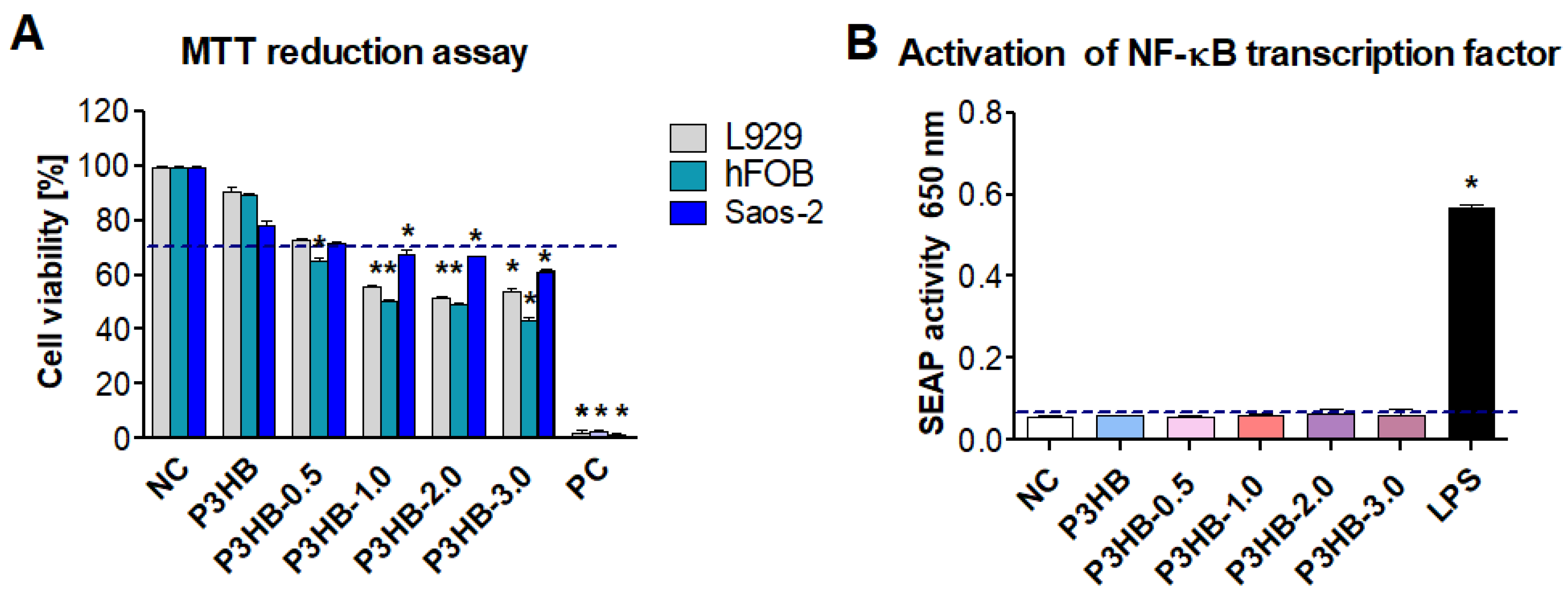
3.2.7. Cell Culture and In Vitro Biocompatibility Assessment
3.2.8. Monocyte Activation Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García, A.; Aguirre, C.; Pérez, A.; Bahamonde, S.S.; Urtuvia, V.; Díaz-Barrera, A.; Peña, C. Recent Trends in the Production and Recovery of Bioplastics Using Polyhydroxyalkanoates Copolymers. Microorganisms 2024, 12, 2135. [Google Scholar] [CrossRef] [PubMed]
- Guzik, M.; Witko, T.; Steinbüchel, A.; Wojnarowska, M.; Sołtysik, M.; Wawak, S. What Has Been Trending in the Research of Polyhydroxyalkanoates? A Systematic Review. Front. Bioeng. Biotechnol. 2020, 8, 959. [Google Scholar] [CrossRef] [PubMed]
- Fradinho, J.C.; Oehmen, A.; Reis, M.A.M. Improving polyhydroxyalkanoates production in phototrophic mixed cultures by optimizing accumulator reactor operating conditions. Int. J. Biol. Macromol. 2019, 126, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The Chemomechanical Properties of Polyhydroxyalkanoates (PHAs) and Their Blends. Prog. Polym. Sci. 2014, 39, 397–442. [Google Scholar] [CrossRef]
- Rodríguez-Cendal, A.I.; Gómez-Seoane, I.; de Toro-Santos, F.J.; Fuentes-Boquete, I.M.; Señarís-Rodríguez, J.; Díaz-Prado, S.M. Biomedical Applications of the Biopolymer Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Drug Encapsulation and Scaffold Fabrication. Int. J. Mol. Sci. 2023, 24, 11674. [Google Scholar] [CrossRef]
- Safaeian, P.; Yazdian, F.; Khosravi-Darani, K.; Rashedi, M.; Lackner, M. P3HB from CH4 Using Methanotrophs: Aspects of Bioreactor, Fermentation Process and Modelling for Cost-Effective Biopolymer Production. Front. Bioeng. Biotechnol. 2023, 11, 1137749. [Google Scholar] [CrossRef]
- García-Cerna, S.; Sánchez-Pacheco, U.; Meneses-Acosta, A.; Rojas-García, J.; Campillo-Illanes, B.; Segura-González, D.; Peña-Malacara, C. Evaluation of Poly-3-Hydroxybutyrate (P3HB) Scaffolds Used for Epidermal Cells Growth as Potential Biomatrix. Polymers 2022, 14, 4021. [Google Scholar] [CrossRef]
- Chen, X.; Yang, X.; Pan, Y.; Wang, L.; Xu, K. Degradation Behaviors of Bioabsorbable P3/4HB Monofilament Suture In Vitro and In Vivo. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 356–365. [Google Scholar] [CrossRef]
- Pena, C.; Castillo, T.; Garcia, A.; Millan, M.; Segura, D. Biotechnological strategies to improve production of microbial poly-(3-hydroxybutyrate): A review of recent research work. Microb. Biotechnol. 2014, 7, 278–293. [Google Scholar] [CrossRef]
- Verlinden, R.A.J.; Hill, D.J.; Kenward, M.; Williams, C.D.; Radecka, I. Bacterial synthesis of biodegradable polyhydroxyalkanoates. J. Appl. Microbiol. 2007, 102, 1437–1449. [Google Scholar] [CrossRef]
- Peña, C.; López, S.; García, A.; Espín, G.; Romo-Uribe, A.; Segura, D. Biosynthesis of poly-β-hydroxybutyrate (PHB) with a high molecular mass by a mutant strain of Azotobacter vinelandii (OPN). Ann. Microbiol. 2013, 64, 39–47. [Google Scholar] [CrossRef]
- Anderson, A.J.; Dawes, E.A. Occurrence, metabolism, metabolic role, and industrial uses of bacterial Poly (βhydroxyalkanoate). Microbiol. Rev. 1990, 54, 450–472. [Google Scholar] [CrossRef]
- Moradi, M.; Rashedi, M.; Khosravi-Darani, A.; Yazdian, C. Polyhydroxybutyrate production from natural gas in a bubble column bioreactor: Simulation using COMSOL. Bioengineering 2019, 6, 84. [Google Scholar] [CrossRef]
- Vadlja, D.; Koller, M.; Novak, M.; Braunegg, G.; Horvat, P. Footprint area analysis of binary imaged Cupriavidus necator cells to study PHB production at balanced, transient, and limited growth conditions in a cascade process. Appl. Microbiol. Biotechnol. 2016, 100, 10065–10080. [Google Scholar] [CrossRef] [PubMed]
- Delayey, P.; Dubruel, P.; Van Vlierberghe, S. Shape-Memory Polymers for Biomedical Applications. Adv. Funct. Mater. 2023, 33, 2301234. [Google Scholar] [CrossRef]
- Brigham, C.J.; Sinskey, A.J. Applications of Polyhydroxyalkanoates in the Medical Industry. Int. J. Biotech. Well. 2012, 1, 52–60. [Google Scholar] [CrossRef]
- Sastri, V.R. Chapter 9—Other Polymers: Styrenics, Silicones, Thermoplastic Elastomers, Biopolymers, and Thermosets. In Plastics in Medical Devices. Properties, Requirements, and Applications, 2nd ed.; Plastics Design Library: Norwich, NY, USA, 2010; pp. 221–253. [Google Scholar]
- European Bioplastics. Market Data—European Bioplastics. Available online: https://www.european-bioplastics.org/market/ (accessed on 26 September 2025).
- Zhang, W.-L.; Dai, Z.-W.; Chen, S.-Y.; Guo, W.-X.; Wang, Z.-W.; Wei, J.-S. A novel poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)-PEG-melatonin composite scaffold for inhibiting bone tumor recurrence and enhancing bone regeneration. Front. Pharmacol. 2023, 14, 1246783. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Huang, S.; Dai, Z.; Liang, J.; Qiu, Q.; Chen, S.; Guo, W.; Wang, Z.; Wei, J. Poly-3-hydroxybutyrate-co-3-hydroxyvalerate(PHBV)-Polyethylene glycol 20k(PEG20k) as a promising delivery system for PT2399 in the treatment of disc degeneration. J. Biol. Eng. 2024, 18, 1660. [Google Scholar] [CrossRef]
- Craveiro, H.D.; Rahnavard, R.; Henriques, J.; Simos, R.A. Structural Fire Performance of Concrete-Filled Built-Up Cold-Formed Steel Columns. Materials 2022, 15, 2159. [Google Scholar] [CrossRef]
- Schoolz, C. Poly(β-hydroxyalkanoates) as Potential Biomedical Materials: An Overview. In Polymers from Resources; ACS Symposium Series; ASC: Washington, DC, USA, 2001; Volume 21, pp. 328–334. [Google Scholar]
- Skibiński, S.; Czechowska, J.P.; Cichoń, E.; Seta, M.; Gondek, A.; Cudnoch-Jędrzejewska, A.; Ślósarczyk, A.; Guzik, M.; Zima, A. Study on βTCP/P(3HB) Scaffolds-Physicochemical Properties and Biological Performance in Low Oxygen Concentration. Int. J. Biol. Macromol. 2022, 23, 11587. [Google Scholar] [CrossRef]
- Baptista-Perianes, A.; Simbara, M.M.O.; Malmonge, S.M.; da Cunha, M.R.; Buchaim, D.V.; Miglino, M.A.; Kassis, E.N.; Buchaim, R.L.; Santos, A.R., Jr. Innovative Biocompatible Blend Scaffold of Poly(hydroxybutyrate-co-hydroxyvalerate) and Poly(ε-caprolactone) for Bone Tissue Engineering: In Vitro and In Vivo Evaluation. Polymers 2024, 16, 3054. [Google Scholar] [CrossRef]
- Ghafari, F.; Karbasi, S.; Baghaban Eslaminejad, M.; Sayahpour, F.A.; Kalantari, N. Biological evaluation and osteogenic potential of polyhydroxybutyrate-keratin/Al2O3 electrospun nanocomposite scaffold: A novel bone regeneration construct. Int. J. Biol. Macromol. 2023, 242, 124602. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.E.A.; Huaman, N.R.C.; Folly, M.M.; Gomez, J.G.C.; Sánchez Rodríguez, R.J. A potential hybrid nanocomposite of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and fullerene for bone tissue regeneration and sustained drug release against bone infections. Int. J. Biol. Macromol. 2023, 251, 126531. [Google Scholar] [CrossRef] [PubMed]
- Salekh, K.M.; Muraev, A.A.; Dolgalev, A.A.; Dymnikov, A.B.; Bonartseva, G.A.; Makhina, T.K.; Chesnokova, D.V.; Voinova, V.V.; Bonartsev, A.P.; Mokrenko, M.E.; et al. Efficacy of Poly-3-Hydroxybutyrate Enriched with Simvastatin in Bone Regeneration after Tooth Extraction (Experimental Study). Sovremennye. Tekhnol. Med. 2024, 16, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Tyubaeva, P.M.; Gasparyan, K.G.; Fedotov, A.Y.; Lobzhanidze, P.V.; Baranov, O.V.; Egorov, A.A.; Sirotinkin, V.P.; Komlev, V.S.; Olkhov, A.A. Development of Nonwoven Fibrous Materials Based on Poly-3-Hydroxybutyrate with a High Content of α-Tricalcium Phosphate. Polymers 2023, 15, 3167. [Google Scholar] [CrossRef]
- Krzykowska, B.; Fajdek-Bieda, A.; Jakubus, A.; Kostrzewa, J.; Białkowska, A.; Kisiel, M.; Dvořáčková, Š.; Frącz, W.; Zarzyka, I. Bio-Based Poly(3-hydroxybutyrate) and Polyurethane Blends: Preparation, Properties Evaluation and Structure Analysis. Materials 2025, 18, 1914. [Google Scholar] [CrossRef]
- Zharkova, I.I.; Volkov, A.V.; Muraev, A.A.; Makhina, T.K.; Voinova, V.V.; Ryabova, V.M.; Gazhva, Y.V.; Kashirina, A.S.; Kashina, A.V.; Bonartseva, G.A.; et al. Poly(3-hydroxybutyrate) 3D-Scaffold-Conduit for Guided Tissue Sprouting. Int. J. Mol. Sci. 2023, 24, 6965. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Y.; Li, C. Advances in 3D printing technology for preparing bone tissue engineering scaffolds from biodegradable materials. Front. Bioeng. Biotechnol. 2024, 12, 1483547. [Google Scholar] [CrossRef]
- Motiee, E.S.; Karbasi, S.; Bidram, E.; Sheikholeslam, M. Investigation of physical, mechanical and biological properties of polyhydroxybutyrate-chitosan/graphene oxide nanocomposite scaffolds for bone tissue engineering applications. Int. J. Biol. Macromol. 2023, 247, 125593. [Google Scholar] [CrossRef]
- Petousis, M.; David, C.; Sagris, D.; Nasikas, N.K.; Papadakis, V.; Argyros, A.; Stratiotou Efstratiadis, V.; Gaganatsiou, A.; Michailidis, N.; Vidakis, N. Reinforced PHA/CNC Biocomposites in Extrusion-Based Additive Manufacturing. ACS Omega 2025, 10, 36613–36630. [Google Scholar] [CrossRef]
- Liu, Y.; Ahmed, S.; Sameen, D.E.; Wang, Y.; Lu, R.; Dai, J.; Li, S.; Qin, W. A review of cellulose and its derivatives in biopolymer-based for food packaging application. Trends Food Sci. Technol. 2021, 112, 532–546. [Google Scholar] [CrossRef]
- Khairnar, Y.; Hansora, D.; Hazra, C.; Kundu, D.; Tayde, S.; Tonde, S.; Naik, J.; Chatterjee, A. Cellulose bionanocomposites for sustainable planet and people: A global snapshot of preparation, properties, and applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100065. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Teodoro, K.B.R.; Sanfelice, R.C.; Migliorini, F.L.; Pavinatto, A.; Facure, M.H.M.; Correa, D.S. A review on the role and performance of cellulose nanomaterials in sensors. ACS Sens. 2021, 6, 2473–2496. [Google Scholar] [CrossRef]
- Vatansever, E.; Arslan, D.; Nofar, M. Polylactide cellulose-based nanocomposites. Int. J. Biol. Macromol. 2019, 137, 912–938. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Nicolae, C.A.; Gabor, A.R.; Trusca, R. Thermal and mechanical properties of poly(3-hydroxybutyrate) reinforced with cellulose fibers from wood waste. Ind. Crops Prod. 2020, 145, 112071. [Google Scholar] [CrossRef]
- Rajendran, M.; Oscar, V.; Tizazu, H.M. Recent developments in short- and medium-chain- length Polyhydroxyalkanoates: Production, properties, and applications. Int. J. Biol. Macromol. 2021, 187, 11674. [Google Scholar] [CrossRef]
- Mohammadalipour, M.; Karbasi, S.; Behzad, T.; Mohammadalipour, Z.; Zamani, M. Effect of cellulose nanofibers on polyhydroxybutyrate electrospun scaffold for bone tissue engineering applications. Int. J. Biol. Macromol. 2022, 220, 1402–1414. [Google Scholar] [CrossRef]
- Choi, J.; Kang, J.; Yun, S.I. Nanofibrous foams of poly(3-hydroxybutyrate)/cellulose nanocrystal composite fabricated using nonsolvent-induced phase separation. Langmuir 2021, 37, 1173–1182. [Google Scholar] [CrossRef]
- Seoane, I.T.; Luzi, F.; Puglia, D.; Cyras, V.P.; Manfredi, L.B. Enhancement of paperboard performance as packaging material by layering with plasticized polyhydroxybutyrate/nanocellulose coatings. J. Appl. Polym. Sci. 2018, 135, 46872. [Google Scholar] [CrossRef]
- Czerniecka-Kubicka, A.; Zarzyka, I.; Pyda, M. Advanced analysis of poly(3-hydroxybutyrate) phases based on vibrational heat capacity. J. Therm. Anal. Calorim. 2017, 127, 905–914. [Google Scholar] [CrossRef][Green Version]
- Czerniecka-Kubicka, A.; Skotnicki, M.; Gonciarz, W.; Zarzyka, I.; Jadach, B.; Lovecka, L.; Maternia-Dudzik, K.; Kovarova, M.; Pyda, M.; Tutka, P.; et al. The cytisine-enriched poly(3-hydroxybutyrate) fibers for sustained-release dosage form. Int. J. Biol. Macromol. 2023, 245, 125544. [Google Scholar] [CrossRef]
- Czerniecka-Kubicka, A.; Zielecki, W.; Frącz, W.; Janus-Kubiak, M.; Kubisz, L.; Pyda, M. Vibrational heat capacity of the linear 6,4-polyurethane. Thermochim. Acta 2020, 683, 178433. [Google Scholar] [CrossRef]
- Zarzyka, I.; Czerniecka-Kubicka, A.; Hęclik, K.; Dobrowolski, L.; Pyda, M.; Leś, K.; Walczak, M.; Białkowska, A.; Bakar, M. Thermally stable biopolymer composites based on poly(3-hydroxybutyrate) modified with linear aliphatic polyurethanes—Preparation and properties. Acta Bioeng. Biomech. 2021, 23, 2. [Google Scholar] [CrossRef]
- Mantovani, A.; Biswas, S.K.; Galdiero, M.R.; Sica, A.; Locati, M. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 2013, 229, 176–185. [Google Scholar] [CrossRef]
- PN-EN ISO 527-1; Plastics—Determination of Mechanical Properties Under Static Stretching—Part 1: General Principles. Polish Committee for Standardization: Warsaw, Poland, 2020.
- PN-EN ISO 179-1; Plastics—Determination of Charpy Impact Properties, Part 1: Non-Instrumented Impact Test. Polish Committee for Standardization: Warsaw, Poland, 2023.
- PN-ISO 868; Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (Shore Hardness). Polish Committee for Standardization: Warsaw, Poland, 2003.
- ISO 10993-5; Biological Evaluation of Medical Devices, Part 5: Tests for In Vitro Cytotoxicity, Edition 3. ISO: Geneva, Switzerland, 2009.
- D’Arienzo, L.; Acierno, S.; Patti, A.; Di Maio, L. Cellulose/Polyhydroxybutyrate (PHB) Composites as a Sustainable Bio-Based Feedstock to 3D-Printing Applications. Materials 2024, 17, 916. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Ionita, E.R.; Nicolae, C.-A.; Gabor, A.R.; Ionita, M.D.; Trusca, R.; Lixandru, B.-E.; Codita, I.; Dinescu, G. Poly(3-hydroxybutyrate) Modified by Nanocellulose and Plasma Treatment for Packaging Applications. Polymers 2018, 10, 1249. [Google Scholar] [CrossRef]
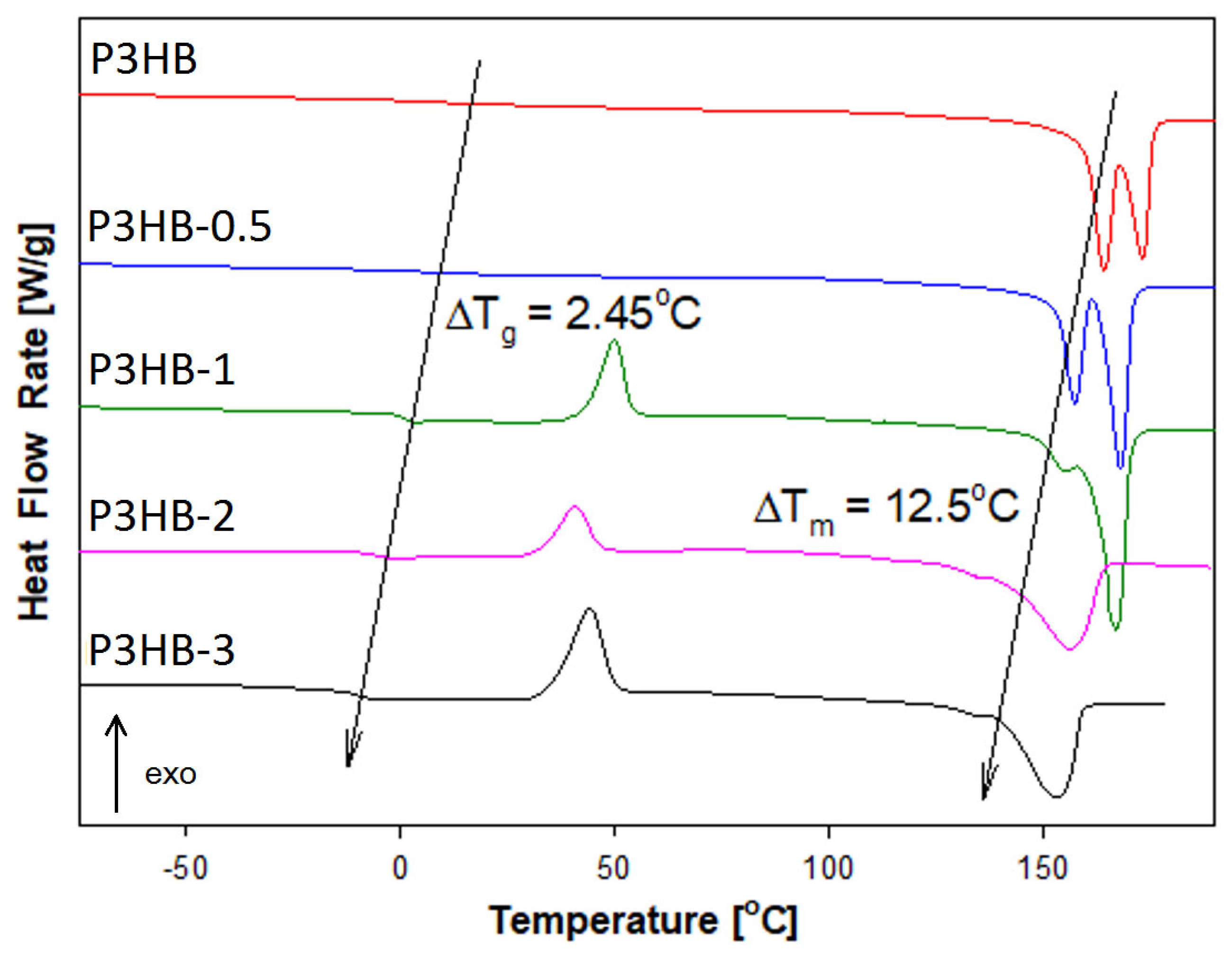
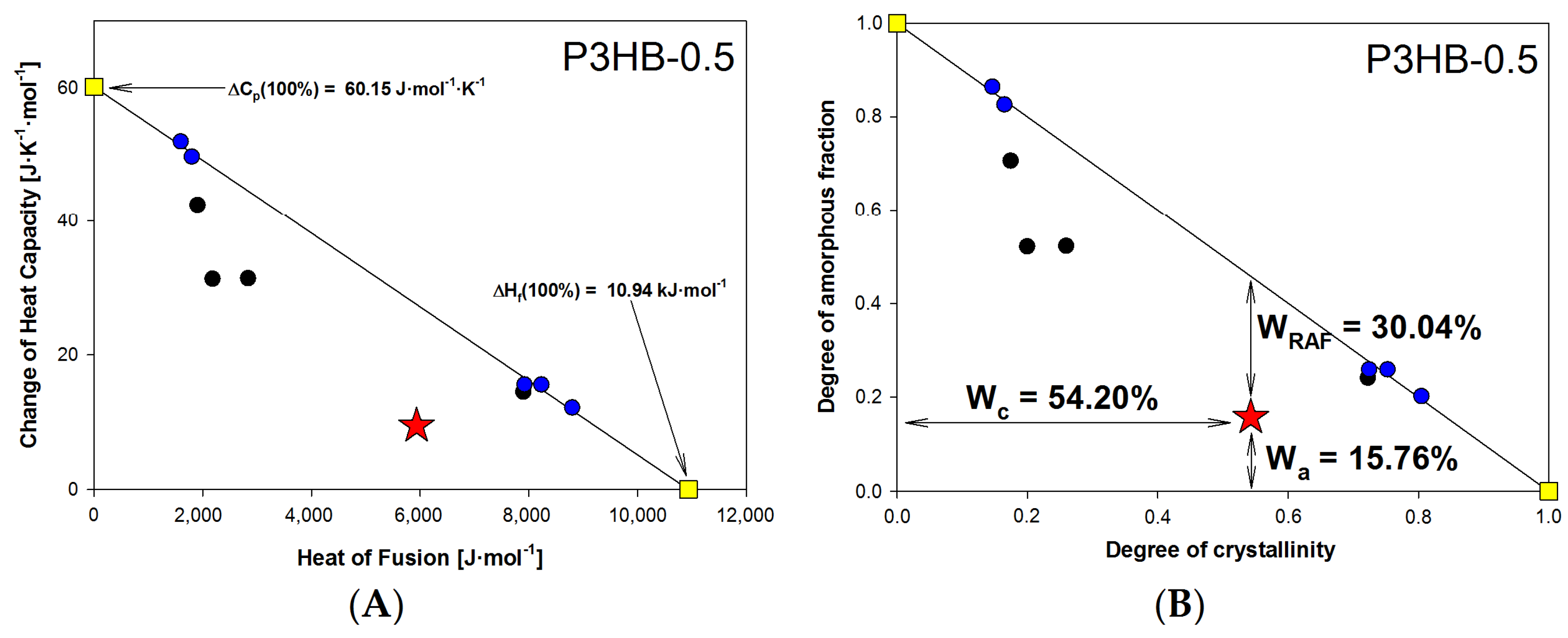
| Name of Sample | Tg [°C] | ΔCp [J·mol−1·°C−1] | Tm1 [°C] | Tm2 [°C] | ΔHf [kJ·mol−1] |
|---|---|---|---|---|---|
| P3HB | 9.8 | 8.3 | - | 165.9 | 9.5 |
| P3HB-0.5 | 7.4 | 9.48 | 157.3 | 167.9 | 5.93 |
| P3HB-1 | 0.6 | 34.51 | 155.5 | 166.6 | 2.71 |
| P3HB-2 | −5.7 | 34.98 | 134.4 | 156.3 | 2.04 |
| P3HB-3 | −10.2 | 12.22 | 134.2 | 153.3 | 7.44 |
| Name of Sample | Wa [%] | ΔCp (100%) [J·mol−1·°C−1] | Wc [%] | ΔHf (100%) [kJ·mol−1] | WRAF [%] |
|---|---|---|---|---|---|
| P3HB | 20.24 | 41.00 | 76.00 | 12.5 | 3.76 |
| P3HB-0.5 | 15.76 | 60.15 | 54.20 | 10.94 | 30.04 |
| P3HB-1 | 64.05 | 53.88 | 23.14 | 11.71 | 12.81 |
| P3HB-2 | 62.59 | 55.89 | 17.88 | 11.41 | 19.53 |
| P3HB-3 | 24.95 | 48.97 | 71.40 | 10.42 | 3.65 |
| Sample Designation | Content of Nanocrystalline Cellulose | Temperature of Extrusion | Speed of Rotation |
|---|---|---|---|
| P3HB | 0 | Zone 1: 125 °C | 350 rpm |
| P3HB-0.5 | 0.5 | Zone 2: 135 °C | |
| P3HB-1 | 1 | Zone 3: 150 °C | |
| P3HB-2 | 2 | Zone 4: 160 °C | |
| P3HB-3 | 3 | Head: 166 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maternia-Dudzik, K.; Ożóg, Ł.; Bober, Z.; Oliwa, R.; Oleksy, M.; Kamizela, A.; Szyszkowska, A.; Rafińska, K.; Gonciarz, W.; Gancarczyk, K.; et al. Improving Poly(3-Hydroxybutyrate) Properties Using Nanocellulose in Biomedical Applications: Thermal, Mechanical and Biological Studies. Int. J. Mol. Sci. 2025, 26, 9795. https://doi.org/10.3390/ijms26199795
Maternia-Dudzik K, Ożóg Ł, Bober Z, Oliwa R, Oleksy M, Kamizela A, Szyszkowska A, Rafińska K, Gonciarz W, Gancarczyk K, et al. Improving Poly(3-Hydroxybutyrate) Properties Using Nanocellulose in Biomedical Applications: Thermal, Mechanical and Biological Studies. International Journal of Molecular Sciences. 2025; 26(19):9795. https://doi.org/10.3390/ijms26199795
Chicago/Turabian StyleMaternia-Dudzik, Karolina, Łukasz Ożóg, Zuzanna Bober, Rafał Oliwa, Mariusz Oleksy, Angelika Kamizela, Agnieszka Szyszkowska, Katarzyna Rafińska, Weronika Gonciarz, Kamil Gancarczyk, and et al. 2025. "Improving Poly(3-Hydroxybutyrate) Properties Using Nanocellulose in Biomedical Applications: Thermal, Mechanical and Biological Studies" International Journal of Molecular Sciences 26, no. 19: 9795. https://doi.org/10.3390/ijms26199795
APA StyleMaternia-Dudzik, K., Ożóg, Ł., Bober, Z., Oliwa, R., Oleksy, M., Kamizela, A., Szyszkowska, A., Rafińska, K., Gonciarz, W., Gancarczyk, K., & Czerniecka-Kubicka, A. (2025). Improving Poly(3-Hydroxybutyrate) Properties Using Nanocellulose in Biomedical Applications: Thermal, Mechanical and Biological Studies. International Journal of Molecular Sciences, 26(19), 9795. https://doi.org/10.3390/ijms26199795










