Lidocaine-Based Derivatives for the Treatment of Staphylococcal Enterotoxin B-Induced Chronic Rhinosinusitis
Abstract
1. Introduction
2. Results
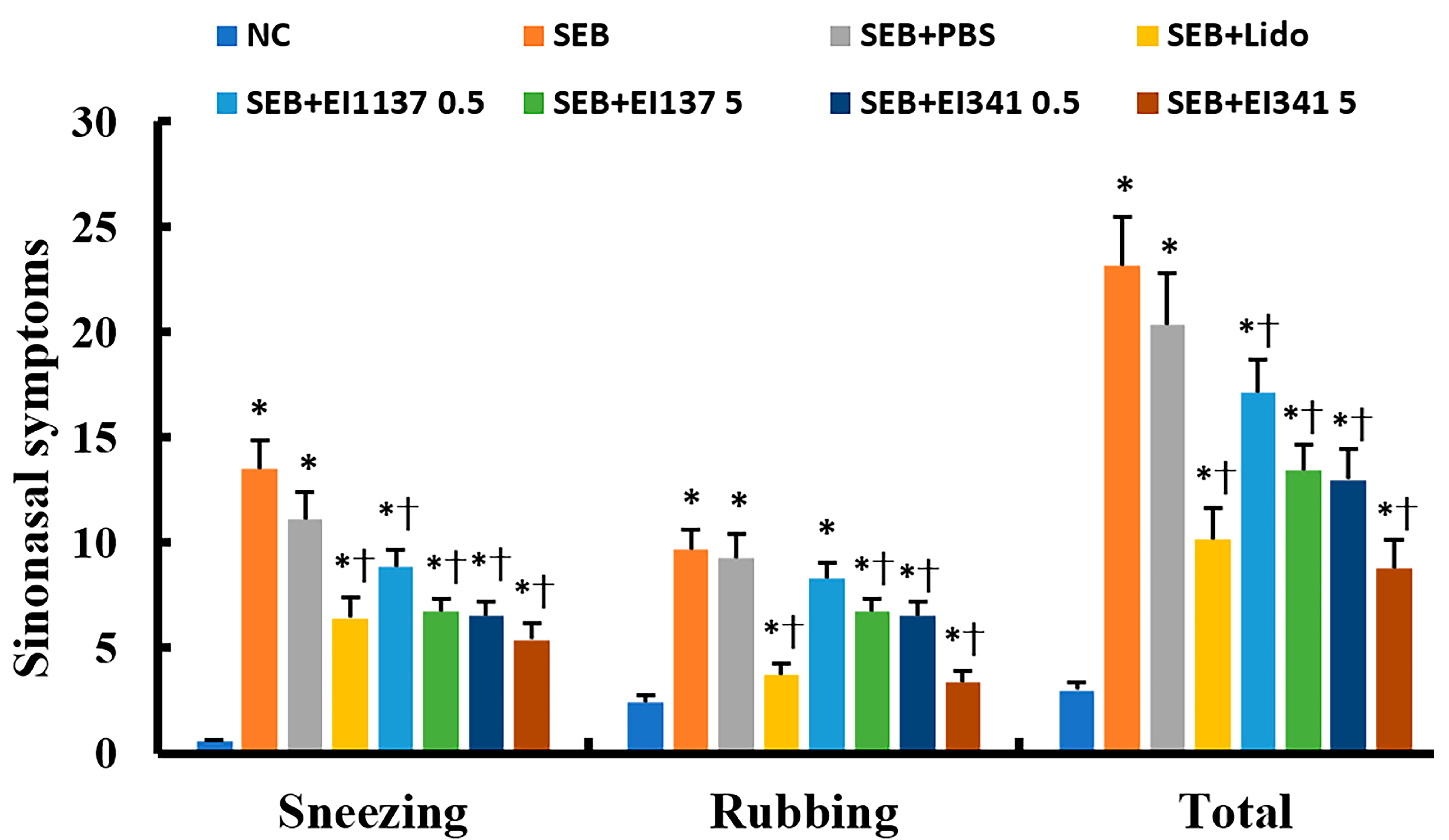
2.1. Effect of Lidocaine-Derived Compounds on Sinonasal Symptoms
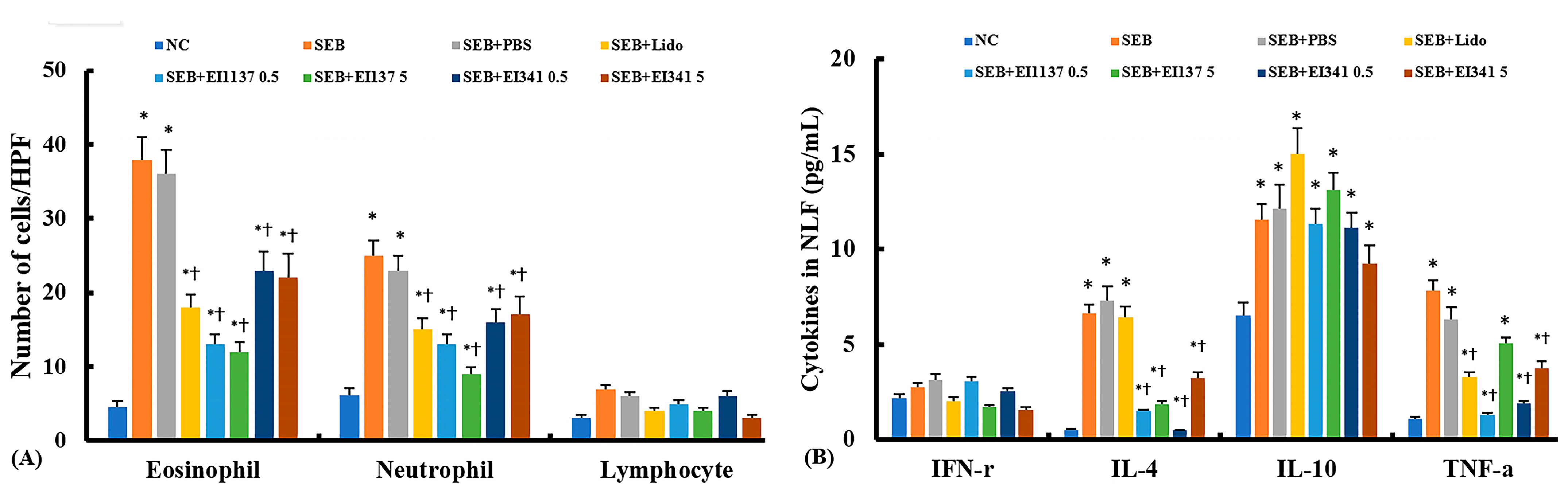
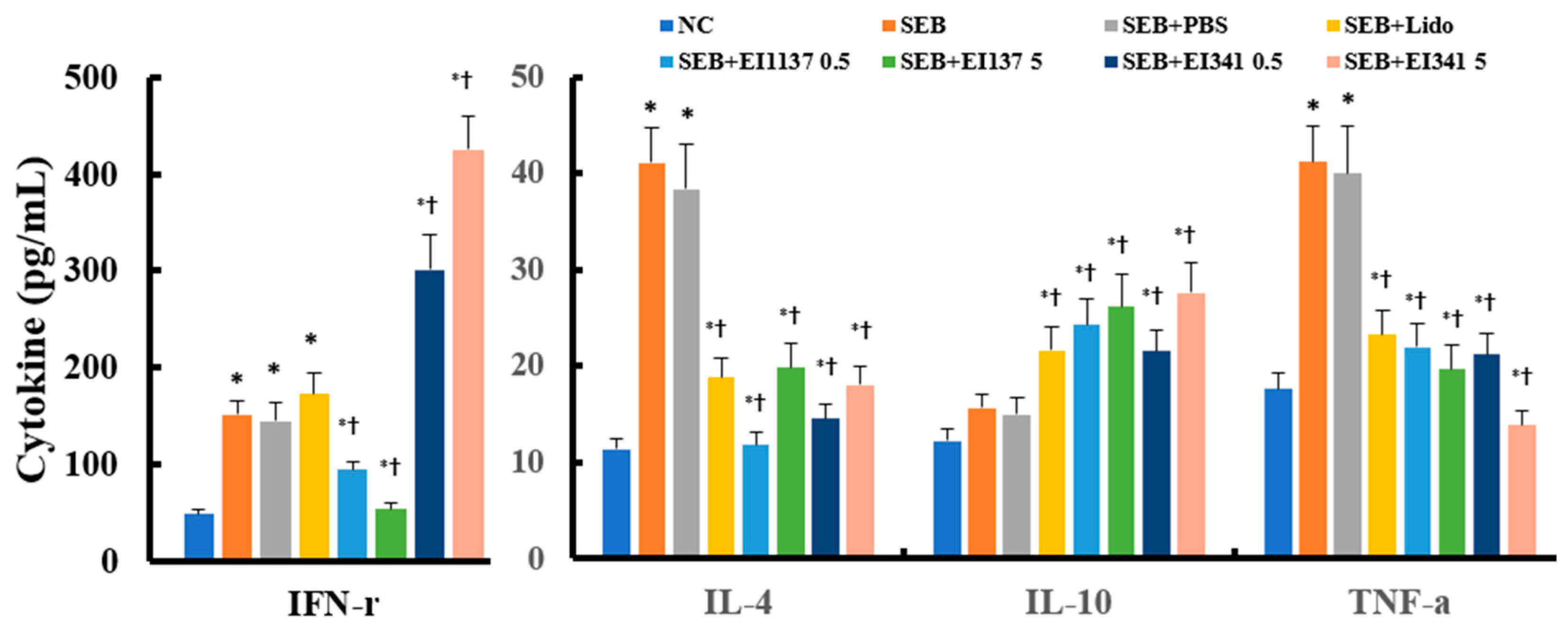
2.2. Effect of Lidocaine-Derived Compounds on Inflammatory Cell Profiles and Cytokine Levels in the Nasal Lavage Fluid (NLF)
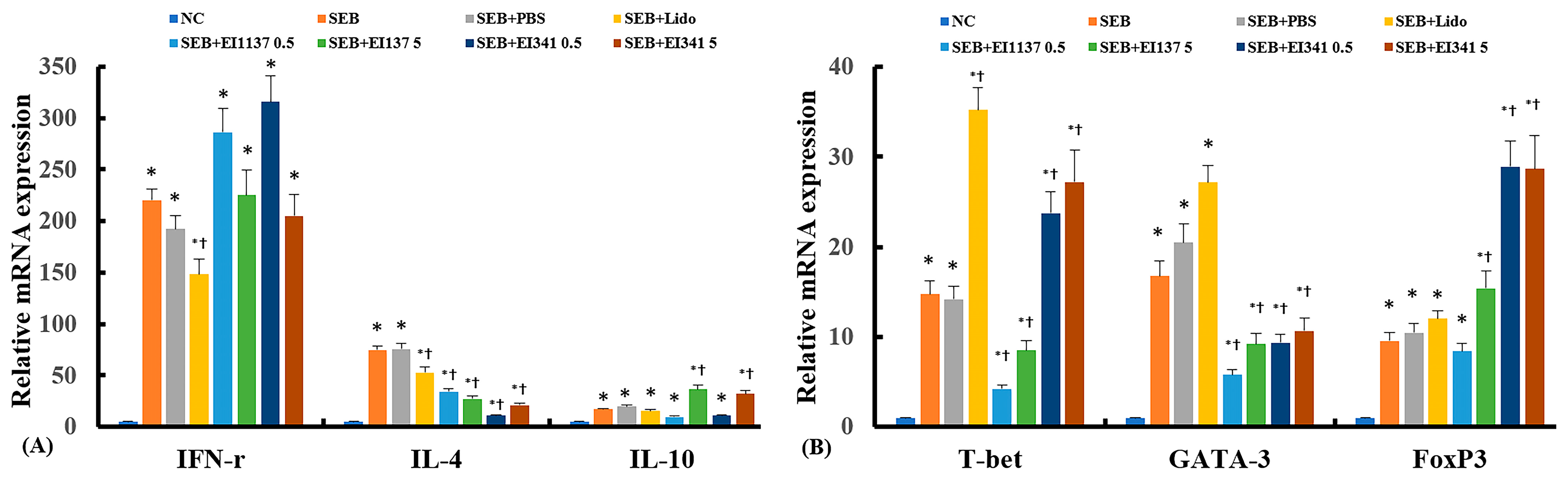
2.3. Effect of Lidocaine-Derived Compounds on Cytokine and Their Transcription Factor mRNA Expression in the Sinonasal Mucosa
2.4. Effect of Lidocaine-Derived Compounds on SEB-Induced Splenocyte Activation
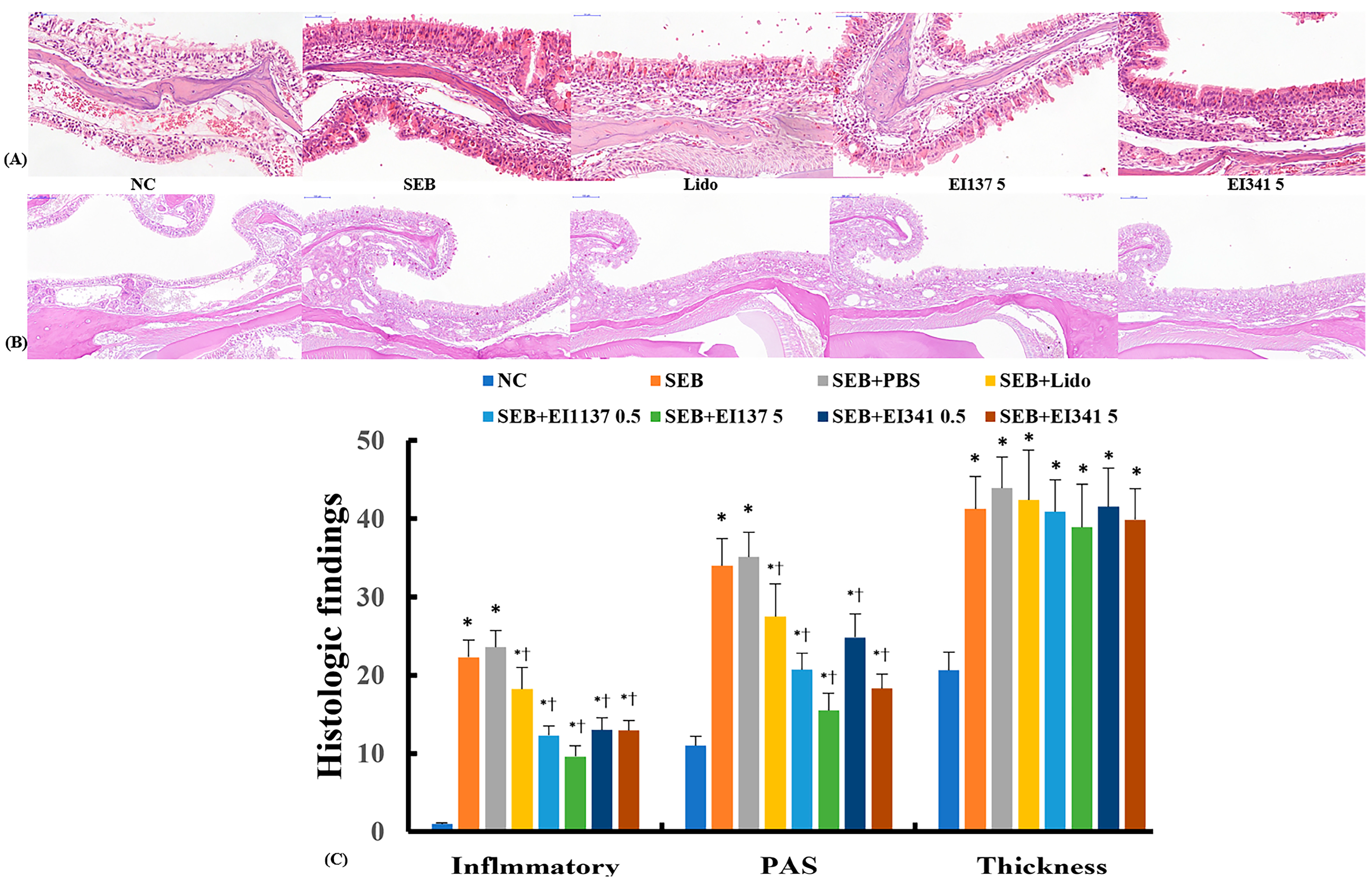
2.5. Effect of Lidocaine-Derived Compounds on Histological Changes in the Sinonasal Mucosa
3. Discussion
4. Materials and Methods
4.1. Lidocaine-Derived Organic Compound Preparation
4.2. Development of SEB-Induced Eosinophilic CRS and the Experimental Protocol
4.3. Evaluation of the Sinonasal Symptoms
4.4. Assessment of Inflammatory Cells and Cytokines in NLF
4.5. Measurement of Cytokine and Transcription Factor mRNA in the Sinonasal Mucosa
4.6. Measurement of Cytokines from SEB-Stimulated Splenocytes
4.7. Histological Evaluation of the Sinonasal Mucosa
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CRS | Chronic rhinosinusitis |
| ELISA | Enzyme-linked immunosorbent assay |
| IFN | Interferon |
| IL | Interleukin |
| NLF | Nasal lavage fluid |
| OVA | Ovalbumin |
| PAS | Periodic acid–Schiff |
| PBS | Phosphate buffered saline |
| RBC | Red blood cell |
| RT-PCR | Reverse-transcription polymerase chain reaction |
| SEB | Staphylococcal enterotoxin B |
| Th | T-helper |
| TNF | Tumor necrosis factor |
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef]
- Bochner, B.S.; Stevens, W.W. Biology and Function of Eosinophils in Chronic Rhinosinusitis with or Without Nasal Polyps. Allergy Asthma Immunol. Res. 2021, 13, 8–22. [Google Scholar] [CrossRef]
- Tai, J.; Han, M.; Kim, T.H. Therapeutic Strategies of Biologics in Chronic Rhinosinusitis: Current Options and Future Targets. Int. J. Mol. Sci. 2022, 23, 5523. [Google Scholar] [CrossRef]
- Caracas, H.C.; Maciel, J.V.; Martins, P.M.; de Souza, M.M.; Maia, L.C. The use of lidocaine as an anti-inflammatory substance: A systematic review. J. Dent. 2009, 37, 93–97. [Google Scholar] [CrossRef]
- Xiang, J.; Yang, Z.; Zhou, Q. Lidocaine relieves murine allergic rhinitis by regulating the NF-kappaB and p38 MAPK pathways. Exp. Ther. Med. 2022, 23, 193. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, M.; Li, S.; Wu, H.; Shen, Q.; Zhang, S.; Fang, L.; Liu, R. Nebulized lidocaine ameliorates allergic airway inflammation via downregulation of TLR2. Mol. Immunol. 2018, 97, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Huang, G.; Chen, G. Synthesis and biological activities of local anesthetics. RSC Adv. 2019, 13, 41173–41191. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.F.; Neves, J.S.; Couto, G.C.; Cotias, A.C.; Pao, C.R.; Olsen, P.C.; de Carvalho, K.I.; Anjos-Valotta, E.A.; Faria, R.X.; Costa, J.C.; et al. JM25-1, a Lidocaine Analog Combining Airway Relaxant and Antiinflammatory Properties: Implications for New Bronchospasm Therapy. Anesthesiology 2016, 124, 109–120. [Google Scholar] [CrossRef]
- Shin, S.H.; Ye, M.K.; Chae, M.H.; Geum, S.Y.; Aboraia, A.S.; Abdel-Aal, A.M.; Qayed, W.S.; El-Wahab, H.; Abou-Ghadir, O.F.; Aboul-Fadl, T. Anti-Allergic and Anti-Inflammatory Effects of Lidocaine-Derived Organic Compounds in a House Dust Mite-Induced Allergic Rhinitis Mouse Model. Biomedicines 2024, 12, 1965. [Google Scholar] [CrossRef]
- Kim, D.W.; Khalmuratova, R.; Hur, D.G.; Jeon, S.Y.; Kim, S.W.; Shin, H.W.; Lee, C.H.; Rhee, C.S. Staphylococcus aureus enterotoxin B contributes to induction of nasal polypoid lesions in an allergic rhinosinusitis murine model. Am. J. Rhinol. Allergy 2011, 25, e255–e261. [Google Scholar] [CrossRef]
- Shin, S.H.; Ye, M.K.; Chae, M.H.; Geum, S.Y.; Aboraia, A.S.; Abdel-Aal, A.M.; Qayed, W.S.; Abd El-Wahab, H.A.A.; Abou-Ghadir, O.F.; Aboul-Fadl, T. Effects of Lidocaine-Derived Organic Compounds on Eosinophil Activation and Survival. Molecules 2023, 28, 5696. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.I.; Bae, J.S.; Kim, E.H.; Kim, J.H.; Lyu, L.; Chung, Y.J.; Mo, J.H. Strain-Specific Differences in House Dust Mite (Dermatophagoides farinae)-Induced Mouse Models of Allergic Rhinitis. Clin. Exp. Otorhinolaryngol. 2020, 13, 396–406. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, W.; Zou, Z.; Yu, W.; Gao, P.; Wang, Y.; Chen, J. A Preliminary Study in Immune Response of BALB/c and C57BL/6 Mice with a Locally Allergic Rhinitis Model. Am. J. Rhinol. Allergy 2023, 37, 410–418. [Google Scholar] [CrossRef]
- Chung, J.; Im, S.Y.; Park, S.K.; Heo, D.B.; Sung, H.W.J.; Ohm, D.; Chung, E.H.; Park, J.T.; Kim, Y.M. Inotodiol attenuates mucosal inflammation in a mouse model of eosinophilic chronic rhinosinusitis. Allergy Asthma Immunol. Res. 2025, 17, 77–93. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Z.; Li, X.; Chen, X.; Li, Y.; Huang, W.; Chang, L.; Zhang, G. IL-17A facilitates type 2 inflammation in a modified eosinophilic chronic rhinosinusitis mouse model. Int. Forum Allergy Rhinol. 2023, 13, 1726–1737. [Google Scholar] [CrossRef]
- Lee, E.; Lee, S.Y.; Park, M.J.; Hong, S.J. TNF-alpha (rs1800629) polymorphism modifies the effect of sensitization to house dust mite on asthma and bronchial hyperresponsiveness in children. Exp. Mol. Pathol. 2020, 115, 104467. [Google Scholar] [CrossRef]
- Delemarre, T.; Holtappels, G.; De Ruyck, N.; Zhang, N.; Nauwynck, H.; Bachert, C.; Gevaert, E. A substantial neutrophilic inflammation as regular part of severe type 2 chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2021, 147, 179–188.e172. [Google Scholar] [CrossRef] [PubMed]
- Delemarre, T.; Bochner, B.S.; Simon, H.U.; Bachert, C. Rethinking neutrophils and eosinophils in chronic rhinosinusitis. J. Allergy Clin. Immunol. 2021, 148, 327–335. [Google Scholar] [CrossRef]
- Kempe-Dustin, J.J.; Aboul-Fadl, T.; Christensen, C.; Palais, R.; Parsawar, K.; Gleich, G.J.; Wagner, L.A. Cell screening assay for identifying inhibitors of eosinophil proliferation. Drug Dev. Res. 2011, 72, 353–360. [Google Scholar] [CrossRef]
- Fu, M.; Fu, S.; Ni, S.; Zou, L.; Liu, Y.; Hong, T. Anti-inflammatory effect of epigallocatechin gallate in a mouse model of ovalbumin-induced allergic rhinitis. Int. Immunopharmacol. 2017, 49, 102–108. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.-H.; Ye, M.-K.; Chae, M.-H.; Lee, D.-W.; Aboraia, A.S.; Abdel-Aal, A.-B.M.; Qayed, W.S.; El-wahab, H.A.A.A.; Abou-Ghadir, O.F.; Aboul-Fadl, T. Lidocaine-Based Derivatives for the Treatment of Staphylococcal Enterotoxin B-Induced Chronic Rhinosinusitis. Int. J. Mol. Sci. 2025, 26, 8137. https://doi.org/10.3390/ijms26178137
Shin S-H, Ye M-K, Chae M-H, Lee D-W, Aboraia AS, Abdel-Aal A-BM, Qayed WS, El-wahab HAAA, Abou-Ghadir OF, Aboul-Fadl T. Lidocaine-Based Derivatives for the Treatment of Staphylococcal Enterotoxin B-Induced Chronic Rhinosinusitis. International Journal of Molecular Sciences. 2025; 26(17):8137. https://doi.org/10.3390/ijms26178137
Chicago/Turabian StyleShin, Seung-Heon, Mi-Kyung Ye, Mi-Hyun Chae, Dong-Won Lee, Ahmed S. Aboraia, Abu-Baker M. Abdel-Aal, Wesam S. Qayed, Hend A. A. Abd El-wahab, Ola F. Abou-Ghadir, and Tarek Aboul-Fadl. 2025. "Lidocaine-Based Derivatives for the Treatment of Staphylococcal Enterotoxin B-Induced Chronic Rhinosinusitis" International Journal of Molecular Sciences 26, no. 17: 8137. https://doi.org/10.3390/ijms26178137
APA StyleShin, S.-H., Ye, M.-K., Chae, M.-H., Lee, D.-W., Aboraia, A. S., Abdel-Aal, A.-B. M., Qayed, W. S., El-wahab, H. A. A. A., Abou-Ghadir, O. F., & Aboul-Fadl, T. (2025). Lidocaine-Based Derivatives for the Treatment of Staphylococcal Enterotoxin B-Induced Chronic Rhinosinusitis. International Journal of Molecular Sciences, 26(17), 8137. https://doi.org/10.3390/ijms26178137








