Antioxidant Enzymes and Their Potential Use in Breast Cancer Treatment
Abstract
1. Introduction
2. Oxidative Stress
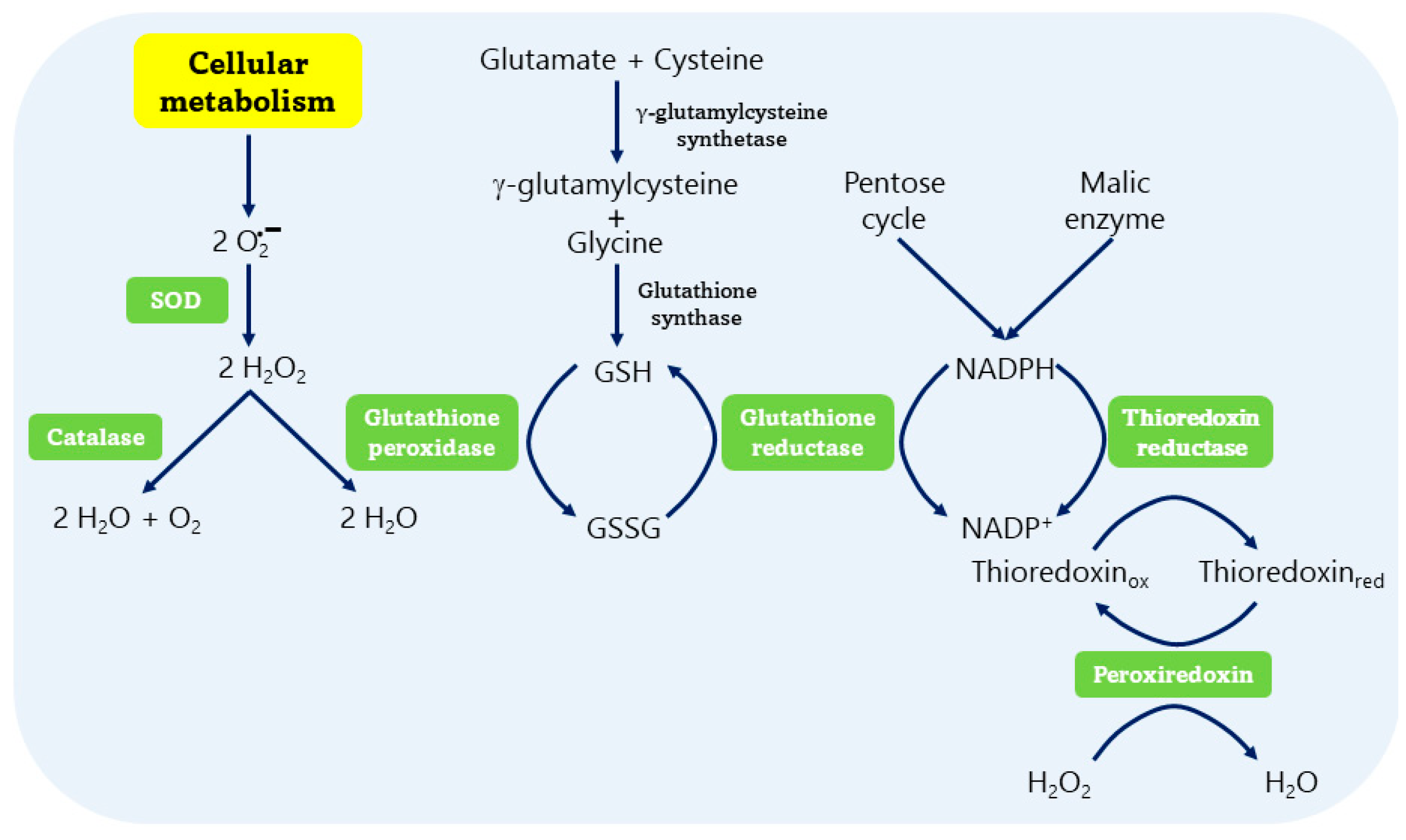
3. Antioxidant Systems
3.1. Non-Enzymatic Antioxidant Systems
3.2. Enzymatic Antioxidant Systems
3.2.1. Superoxide Dismutase (SOD)
3.2.2. Catalase (CAT)
3.2.3. Glutathione Peroxidase (GPx)
3.2.4. Glutathione Reductase (GR)
3.2.5. Thioredoxin Reductase (TrxR)
3.2.6. Peroxiredoxin (Prx)
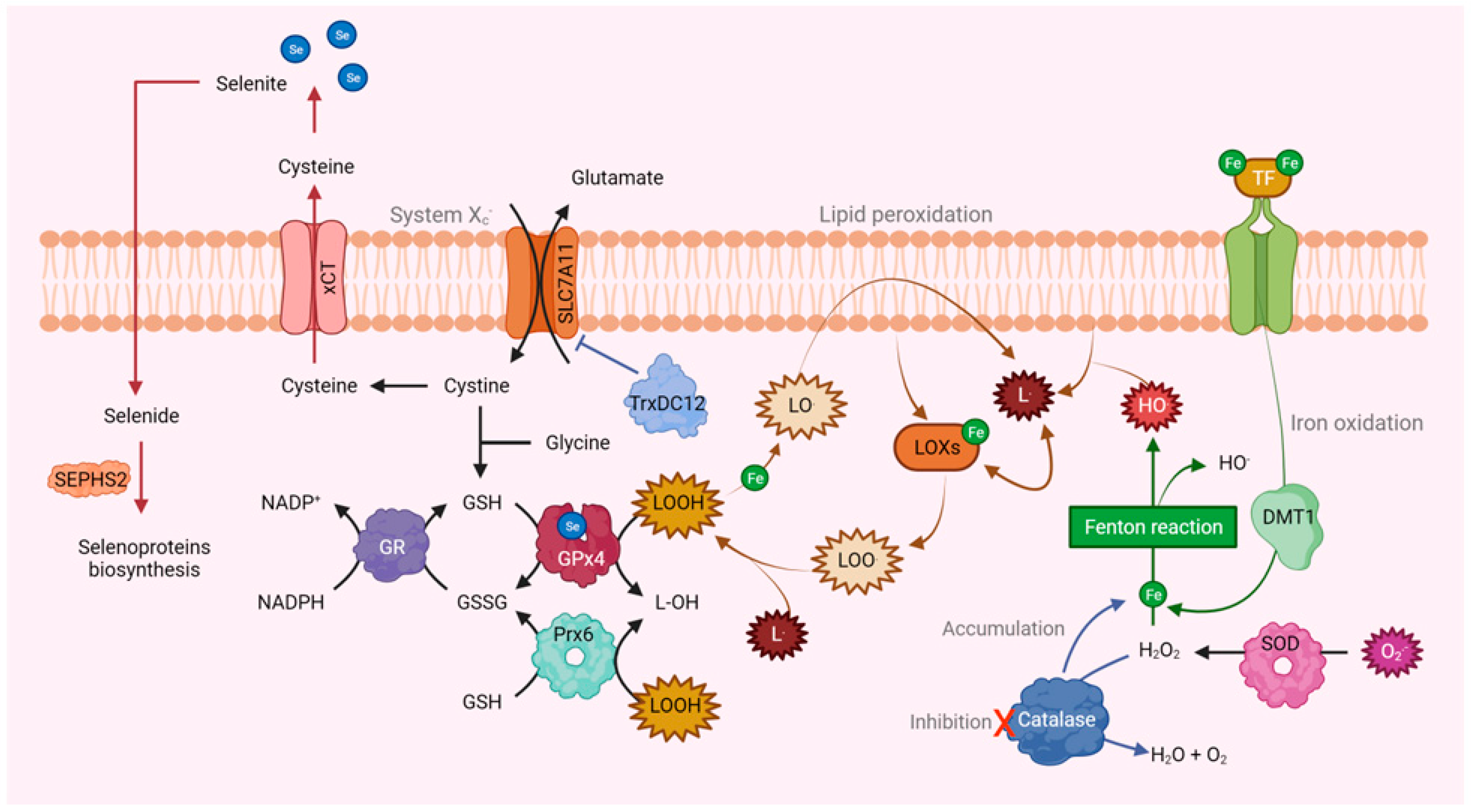
4. Dichotomy of Some Antioxidant Enzymes
5. Ferroptosis
6. Nanotechnology Applied to the Clinic
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Breast Cancer; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- International Agency for Research on Cancer. Available online: https://gco.iarc.fr/ (accessed on 10 May 2024).
- Pinto, J.A.; Pinillos, L.; Villarreal-Garza, C.; Morante, Z.; Villaran, M.V.; Mejia, G.; Caglevic, C.; Aguilar, A.; Fajardo, W.; Usuga, F.; et al. Barriers in Latin America for the management of locally advanced breast cancer. Ecancermedicalscience 2019, 13, 897. [Google Scholar] [CrossRef] [PubMed]
- INEGI. Estadisticas a Proposito del día Mundial de la Lucha Contra el Cáncer de Mama. Comunicado de Prensa; INEGI: Aguascalientes, Mexico, 2021; p. 5. [Google Scholar]
- Nourazarian, A.R.; Kangari, P.; Salmaninejad, A. Roles of oxidative stress in the development and progression of breast cancer. Asian Pac. J. Cancer Prev. 2014, 15, 4745–4751. [Google Scholar] [CrossRef] [PubMed]
- Rusolo, F.; Capone, F.; Pasquale, R.; Angiolillo, A.; Colonna, G.; Castello, G.; Costantini, M.; Costantini, S. Comparison of the seleno-transcriptome expression between human non-cancerous mammary epithelial cells and two human breast cancer cell lines. Oncol. Lett. 2017, 13, 2411–2417. [Google Scholar] [CrossRef] [PubMed]
- Majumder, D.; Nath, P.; Debnath, R.; Maiti, D. Understanding the complicated relationship between antioxidants and carcinogenesis. J. Biochem. Mol. Toxicol. 2021, 35, e22643. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive Oxygen Species: A Key Hallmark of Cardiovascular Disease. Adv. Med. 2016, 2016, 9152732. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Hawk, M.A.; McCallister, C.; Schafer, Z.T. Antioxidant Activity during Tumor Progression: A Necessity for the Survival of Cancer Cells? Cancers 2016, 8, 92. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Tochhawng, L.; Deng, S.; Pervaiz, S.; Yap, C.T. Redox regulation of cancer cell migration and invasion. Mitochondrion 2013, 13, 246–253. [Google Scholar] [CrossRef]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Klaunig, J.E.; Zemin, W. Oxidative stress in carcinogenesis. Curr. Opin. Toxicol. 2018, 7, 116–121. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Hewala, T.I.; Elsoud, M.R.A. The clinical significance of serum oxidative stress biomarkers in breast cancer females. Med. Res. J. 2019, 4, 1–7. [Google Scholar] [CrossRef]
- Bjelland, S.; Seeberg, E. Mutagenicity, toxicity and repair of DNA base damage induced by oxidation. Mutat. Res. 2003, 531, 37–80. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hosokawa, K.; Tamura, T.; Kanno, H.; Urabe, M.; Honjo, H. Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels in women with or without gynecologic cancer. J. Obstet. Gynaecol. Res. 1996, 22, 359–363. [Google Scholar] [CrossRef]
- Murrell, T.G. Epidemiological and biochemical support for a theory on the cause and prevention of breast cancer. Med. Hypotheses 1991, 36, 389–396. [Google Scholar] [CrossRef]
- Miao, L.; St Clair, D.K. Regulation of superoxide dismutase genes: Implications in disease. Free Radic. Biol. Med. 2009, 47, 344–356. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Schafer, Z.T.; Grassian, A.R.; Song, L.; Jiang, Z.; Gerhart-Hines, Z.; Irie, H.Y.; Gao, S.; Puigserver, P.; Brugge, J.S. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature 2009, 461, 109–113. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Dec, K.; Kaldunska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lek. 2020, 48, 124–127. [Google Scholar]
- Jakubczyk, K.; Kaldunska, J.; Dec, K.; Kawczuga, D.; Janda, K. Antioxidant properties of small-molecule non-enzymatic compounds. Pol. Merkur. Lek. 2020, 48, 128–132. [Google Scholar]
- Sharma, S.K.; Singh, D.; Pandey, H.; Jatav, R.B.; Singh, V.; Pandey, D. An Overview of Roles of Enzymatic and Nonenzymatic Antioxidants in Plant. In Antioxidant Defense in Plants; Aftab, T., Hakeem, K.R., Eds.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Shariati, M.A.; Rebezov, M.; Rengasamy, K.R.R.; Mubarak, M.S. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2022, 62, 7282–7300. [Google Scholar] [CrossRef] [PubMed]
- Bafana, A.; Dutt, S.; Kumar, S.; Ahuja, P.S. Superoxide dismutase: An industrial perspective. Crit. Rev. Biotechnol. 2011, 31, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Oberley, L.W. Mechanism of the tumor suppressive effect of MnSOD overexpression. BioMed. Pharmacother. 2005, 59, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Bohm, B.; Heinzelmann, S.; Motz, M.; Bauer, G. Extracellular localization of catalase is associated with the transformed state of malignant cells. Biol. Chem. 2015, 396, 1339–1356. [Google Scholar] [CrossRef]
- Chance, B.; Sies, H.; Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 1979, 59, 527–605. [Google Scholar] [CrossRef]
- Galasso, M.; Gambino, S.; Romanelli, M.G.; Donadelli, M.; Scupoli, M.T. Browsing the oldest antioxidant enzyme: Catalase and its multiple regulation in cancer. Free Radic. Biol. Med. 2021, 172, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, C.; Sandoval, J.M.; Dejeans, N.; Nonckreman, S.; Bahloula, K.; Poirel, H.A.; Calderon, P.B. Evaluation of Potential Mechanisms Controlling the Catalase Expression in Breast Cancer Cells. Oxidative Med. Cell. Longev. 2018, 2018, 5351967. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Keykhaee, M.; Khorramdelazad, H.; Karimi, M.Y.; Nejatbakhsh Samimi, L.; Aghamohamadi, N.; Karimi, M.; Falak, R.; Khoobi, M. Catalase application in cancer therapy: Simultaneous focusing on hypoxia attenuation and macrophage reprogramming. BioMed. Pharmacother. 2022, 153, 113483. [Google Scholar] [CrossRef] [PubMed]
- Negahdar, M.; Jalali, M.; Abtahi, H.; Sadeghi, M.R.; Javadi, E.; Aghvami, T.; Layegh, H. Blood superoxide dismutase and catalase activities in women affected with breast cancer. Iran J. Public Health 2005, 34, 39–43. [Google Scholar]
- Sahu, A.; Varma, M.; Kachhawa, K. A prognostic study of MDA, SOD and catalase in breast Cancer patients. Int. J. Sci. Res. 2015, 4, 157–159. [Google Scholar]
- Finley, L.W.; Carracedo, A.; Lee, J.; Souza, A.; Egia, A.; Zhang, J.; Teruya-Feldstein, J.; Moreira, P.I.; Cardoso, S.M.; Clish, C.B.; et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1alpha destabilization. Cancer Cell 2011, 19, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Papa, L.; Hahn, M.; Marsh, E.L.; Evans, B.S.; Germain, D. SOD2 to SOD1 switch in breast cancer. J. Biol. Chem. 2014, 289, 5412–5416. [Google Scholar] [CrossRef]
- Ambrosone, C.B.; Freudenheim, J.L.; Thompson, P.A.; Bowman, E.; Vena, J.E.; Marshall, J.R.; Graham, S.; Laughlin, R.; Nemoto, T.; Shields, P.G. Manganese superoxide dismutase (MnSOD) genetic polymorphisms, dietary antioxidants, and risk of breast cancer. Cancer Res. 1999, 59, 602–606. [Google Scholar]
- Griess, B.; Tom, E.; Domann, F.; Teoh-Fitzgerald, M. Extracellular superoxide dismutase and its role in cancer. Free Radic. Biol. Med. 2017, 112, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.L. Extracellular superoxide dismutase in human tissues and human cell lines. J. Clin. Investig. 1984, 74, 1398–1403. [Google Scholar] [CrossRef]
- Teoh, M.L.; Fitzgerald, M.P.; Oberley, L.W.; Domann, F.E. Overexpression of extracellular superoxide dismutase attenuates heparanase expression and inhibits breast carcinoma cell growth and invasion. Cancer Res. 2009, 69, 6355–6363. [Google Scholar] [CrossRef]
- Teoh-Fitzgerald, M.L.; Fitzgerald, M.P.; Zhong, W.; Askeland, R.W.; Domann, F.E. Epigenetic reprogramming governs EcSOD expression during human mammary epithelial cell differentiation, tumorigenesis and metastasis. Oncogene 2014, 33, 358–368. [Google Scholar] [CrossRef]
- Brigelius-Flohe, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Jin, L.; Li, D.; Alesi, G.N.; Fan, J.; Kang, H.B.; Lu, Z.; Boggon, T.J.; Jin, P.; Yi, H.; Wright, E.R.; et al. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell 2015, 27, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Wu, H.T.; Chen, W.J.; Xu, Y.; Ye, Q.Q.; Shen, J.X.; Liu, J. Involvement of glutathione peroxidases in the occurrence and development of breast cancers. J. Transl. Med. 2020, 18, 247. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.; Zhou, J.; Shao, Q. Glutathione Peroxidase GPX1 and Its Dichotomous Roles in Cancer. Cancers 2022, 14, 2560. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Liang, H.; Galbo, P.M., Jr.; Dharmaratne, M.; Kulkarni, A.S.; Fard, A.T.; Aoun, M.L.; Martinez-Lopez, N.; Suyama, K.; Benard, O.; et al. Redox signaling by glutathione peroxidase 2 links vascular modulation to metabolic plasticity of breast cancer. Proc. Natl. Acad. Sci. USA 2022, 119, e2107266119. [Google Scholar] [CrossRef] [PubMed]
- Saelee, P.; Pongtheerat, T.; Sophonnithiprasert, T. Reduced Expression of GPX3 in Breast Cancer Patients in Correlation with Clinical Significance. Glob. Med. Genet. 2020, 7, 87–91. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, X.; Liu, C.; Ge, W.; Wang, Q.; Hao, X.; Wang, M.; Chen, Y.; Zhang, Q. Identification of a small molecule as inducer of ferroptosis and apoptosis through ubiquitination of GPX4 in triple negative breast cancer cells. J. Hematol. Oncol. 2021, 14, 19. [Google Scholar] [CrossRef]
- Lee, J.; Roh, J.L. Targeting GPX4 in human cancer: Implications of ferroptosis induction for tackling cancer resilience. Cancer Lett. 2023, 559, 216119. [Google Scholar] [CrossRef]
- Khatib, A.; Solaimuthu, B.; Ben Yosef, M.; Abu Rmaileh, A.; Tanna, M.; Oren, G.; Schlesinger Frisch, M.; Axelrod, J.H.; Lichtenstein, M.; Shaul, Y.D. The glutathione peroxidase 8 (GPX8)/IL-6/STAT3 axis is essential in maintaining an aggressive breast cancer phenotype. Proc. Natl. Acad. Sci. USA 2020, 117, 21420–21431. [Google Scholar] [CrossRef] [PubMed]
- Abboud, M.M.; Al Awaida, W.; Alkhateeb, H.H.; Abu-Ayyad, A.N. Antitumor Action of Amygdalin on Human Breast Cancer Cells by Selective Sensitization to Oxidative Stress. Nutr. Cancer 2019, 71, 483–490. [Google Scholar] [CrossRef]
- di Ilio, C.; Sacchetta, P.; del Boccio, G.; la Rovere, G.; Federici, G. Glutathione peroxidase, glutathione S-transferase and glutathione reductase activities in normal and neoplastic human breast tissue. Cancer Lett. 1985, 29, 37–42. [Google Scholar] [CrossRef]
- el-Sharabasy, M.M.; el-Dosoky, I.; Horria, H.; Khalaf, A.H. Elevation of glutathione, glutathione-reductase and nucleic acids in both normal tissues and tumour of breast cancer patients. Cancer Lett. 1993, 72, 11–15. [Google Scholar] [CrossRef]
- Raninga, P.V.; Lee, A.C.; Sinha, D.; Shih, Y.Y.; Mittal, D.; Makhale, A.; Bain, A.L.; Nanayakarra, D.; Tonissen, K.F.; Kalimutho, M.; et al. Therapeutic cooperation between auranofin, a thioredoxin reductase inhibitor and anti-PD-L1 antibody for treatment of triple-negative breast cancer. Int. J. Cancer 2020, 146, 123–136. [Google Scholar] [CrossRef]
- Dahou, H.; Minati, M.A.; Jacquemin, P.; Assi, M. Genetic Inactivation of Peroxiredoxin-I Impairs the Growth of Human Pancreatic Cancer Cells. Antioxidants 2021, 10, 570. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.W.; Chae, H.Z.; Seo, M.S.; Kim, K.; Baines, I.C.; Rhee, S.G. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J. Biol. Chem. 1998, 273, 6297–6302. [Google Scholar] [CrossRef]
- Noh, D.Y.; Ahn, S.J.; Lee, R.A.; Kim, S.W.; Park, I.A.; Chae, H.Z. Overexpression of peroxiredoxin in human breast cancer. Anticancer Res. 2001, 21, 2085–2090. [Google Scholar] [PubMed]
- Park, J.H.; Kim, Y.S.; Lee, H.L.; Shim, J.Y.; Lee, K.S.; Oh, Y.J.; Shin, S.S.; Choi, Y.H.; Park, K.J.; Park, R.W.; et al. Expression of peroxiredoxin and thioredoxin in human lung cancer and paired normal lung. Respirology 2006, 11, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Song, I.S.; Jeong, Y.J.; Jung, Y.; Park, Y.H.; Shim, S.; Kim, S.J.; Eom, D.W.; Hong, S.M.; Lee, P.C.W.; Kim, S.U.; et al. The sulfiredoxin-peroxiredoxin redox system regulates the stemness and survival of colon cancer stem cells. Redox Biol. 2021, 48, 102190. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Diaz, A.J.; Yen, Y. The role of peroxiredoxin II in chemoresistance of breast cancer cells. Breast Cancer Targets Ther. 2014, 6, 73–80. [Google Scholar] [CrossRef]
- Chandimali, N.; Jeong, D.K.; Kwon, T. Peroxiredoxin II Regulates Cancer Stem Cells and Stemness-Associated Properties of Cancers. Cancers 2018, 10, 305. [Google Scholar] [CrossRef]
- Chua, P.J.; Lee, E.H.; Yu, Y.; Yip, G.W.; Tan, P.H.; Bay, B.H. Silencing the Peroxiredoxin III gene inhibits cell proliferation in breast cancer. Int. J. Oncol. 2010, 36, 359–364. [Google Scholar]
- Karihtala, P.; Mantyniemi, A.; Kang, S.W.; Kinnula, V.L.; Soini, Y. Peroxiredoxins in breast carcinoma. Clin. Cancer Res. 2003, 9, 3418–3424. [Google Scholar]
- Jin, D.Y.; Chae, H.Z.; Rhee, S.G.; Jeang, K.T. Regulatory role for a novel human thioredoxin peroxidase in NF-kappaB activation. J. Biol. Chem. 1997, 272, 30952–30961. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, Y.J.; Park, J.; Kim, T.H.; Hong, S.C.; Jung, E.J.; Ju, Y.T.; Jeong, C.Y.; Park, H.J.; Ko, G.H.; et al. PRDX4 overexpression is associated with poor prognosis in gastric cancer. Oncol. Lett. 2020, 19, 3522–3530. [Google Scholar] [CrossRef]
- Thapa, P.; Ding, N.; Hao, Y.; Alshahrani, A.; Jiang, H.; Wei, Q. Essential Roles of Peroxiredoxin IV in Inflammation and Cancer. Molecules 2022, 27, 6513. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Kim, Y.; Lee, H.; Lee, D.S.; Lee, H.S. Interplay Between Mitochondrial Peroxiredoxins and ROS in Cancer Development and Progression. Int. J. Mol. Sci. 2019, 20, 4407. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, Y.S.; Ahn, H.M.; Lee, H.J.; Jung, M.K.; Jeong, H.Y.; Choi, D.K.; Lee, J.H.; Lee, S.R.; Kim, J.M.; et al. Peroxiredoxin 5 overexpression enhances tumorigenicity and correlates with poor prognosis in gastric cancer. Int. J. Oncol. 2017, 51, 298–306. [Google Scholar] [CrossRef]
- Seong, J.B.; Kim, B.; Kim, S.; Kim, M.H.; Park, Y.H.; Lee, Y.; Lee, H.J.; Hong, C.W.; Lee, D.S. Macrophage peroxiredoxin 5 deficiency promotes lung cancer progression via ROS-dependent M2-like polarization. Free Radic. Biol. Med. 2021, 176, 322–334. [Google Scholar] [CrossRef]
- Sjoblom, T.; Jones, S.; Wood, L.D.; Parsons, D.W.; Lin, J.; Barber, T.D.; Mandelker, D.; Leary, R.J.; Ptak, J.; Silliman, N.; et al. The consensus coding sequences of human breast and colorectal cancers. Science 2006, 314, 268–274. [Google Scholar] [CrossRef]
- Chang, X.Z.; Li, D.Q.; Hou, Y.F.; Wu, J.; Lu, J.S.; Di, G.H.; Jin, W.; Ou, Z.L.; Shen, Z.Z.; Shao, Z.M. Identification of the functional role of peroxiredoxin 6 in the progression of breast cancer. Breast Cancer Res. 2007, 9, R76. [Google Scholar] [CrossRef] [PubMed]
- Forshaw, T.E.; Holmila, R.; Nelson, K.J.; Lewis, J.E.; Kemp, M.L.; Tsang, A.W.; Poole, L.B.; Lowther, W.T.; Furdui, C.M. Peroxiredoxins in Cancer and Response to Radiation Therapies. Antioxidants 2019, 8, 11. [Google Scholar] [CrossRef]
- Nguyen, P.; Awwad, R.T.; Smart, D.D.; Spitz, D.R.; Gius, D. Thioredoxin reductase as a novel molecular target for cancer therapy. Cancer Lett. 2006, 236, 164–174. [Google Scholar] [CrossRef]
- Rackham, O.; Shearwood, A.M.; Thyer, R.; McNamara, E.; Davies, S.M.; Callus, B.A.; Miranda-Vizuete, A.; Berners-Price, S.J.; Cheng, Q.; Arner, E.S.; et al. Substrate and inhibitor specificities differ between human cytosolic and mitochondrial thioredoxin reductases: Implications for development of specific inhibitors. Free Radic. Biol. Med. 2011, 50, 689–699. [Google Scholar] [CrossRef]
- Rodriguez-Fanjul, V.; Lopez-Torres, E.; Mendiola, M.A.; Pizarro, A.M. Gold(III) bis(thiosemicarbazonate) compounds in breast cancer cells: Cytotoxicity and thioredoxin reductase targeting. Eur. J. Med. Chem. 2018, 148, 372–383. [Google Scholar] [CrossRef]
- Seo, M.J.; Kim, I.Y.; Lee, D.M.; Park, Y.J.; Cho, M.Y.; Jin, H.J.; Choi, K.S. Dual inhibition of thioredoxin reductase and proteasome is required for auranofin-induced paraptosis in breast cancer cells. Cell Death Dis. 2023, 14, 42. [Google Scholar] [CrossRef]
- Galassi, R.; Burini, A.; Ricci, S.; Pellei, M.; Rigobello, M.P.; Citta, A.; Dolmella, A.; Gandin, V.; Marzano, C. Synthesis and characterization of azolate gold(I) phosphane complexes as thioredoxin reductase inhibiting antitumor agents. Dalton Trans. 2012, 41, 5307–5318. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Bandyopadhyay, J.; Hwaang, H.S.; Park, B.J.; Cho, J.H.; Lee, J.I.; Ahnn, J.; Lee, S.K. Two thioredoxin reductases, trxr-1 and trxr-2, have differential physiological roles in Caenorhabditis elegans. Mol. Cells 2012, 34, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A.; Lu, J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Fessel, J.P.; Sherrill, T.; Kocurek, E.G.; Yull, F.E.; Blackwell, T.S. Enhanced Expression of Catalase in Mitochondria Modulates NF-kappaB-Dependent Lung Inflammation through Alteration of Metabolic Activity in Macrophages. J. Immunol. 2020, 205, 1125–1134. [Google Scholar] [CrossRef] [PubMed]
- Zamocky, M.; Koller, F. Understanding the structure and function of catalases: Clues from molecular evolution and in vitro mutagenesis. Prog. Biophys. Mol. Biol. 1999, 72, 19–66. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.C.; Tang, B.K.; Rao, V.; Agarwal, S.; Martin, L.; Tritchler, D.; Yaffe, M.; Boyd, N.F. Cytochrome P450 1A2 (CYP1A2) activity, mammographic density, and oxidative stress: A cross-sectional study. Breast Cancer Res. 2004, 6, R338–R351. [Google Scholar] [CrossRef]
- Radenkovic, S.; Milosevic, Z.; Konjevic, G.; Karadzic, K.; Rovcanin, B.; Buta, M.; Gopcevic, K.; Jurisic, V. Lactate dehydrogenase, catalase, and superoxide dismutase in tumor tissue of breast cancer patients in respect to mammographic findings. Cell Biochem. Biophys. 2013, 66, 287–295. [Google Scholar] [CrossRef]
- Kattan, Z.; Minig, V.; Leroy, P.; Dauca, M.; Becuwe, P. Role of manganese superoxide dismutase on growth and invasive properties of human estrogen-independent breast cancer cells. Breast Cancer Res. Treat. 2008, 108, 203–215. [Google Scholar] [CrossRef]
- Handschuh, L.; Kazmierczak, M.; Milewski, M.C.; Goralski, M.; Luczak, M.; Wojtaszewska, M.; Uszczynska-Ratajczak, B.; Lewandowski, K.; Komarnicki, M.; Figlerowicz, M. Gene expression profiling of acute myeloid leukemia samples from adult patients with AML-M1 and -M2 through boutique microarrays, real-time PCR and droplet digital PCR. Int. J. Oncol. 2018, 52, 656–678. [Google Scholar] [CrossRef]
- Glorieux, C.; Dejeans, N.; Sid, B.; Beck, R.; Calderon, P.B.; Verrax, J. Catalase overexpression in mammary cancer cells leads to a less aggressive phenotype and an altered response to chemotherapy. Biochem. Pharmacol. 2011, 82, 1384–1390. [Google Scholar] [CrossRef]
- Ruqayah Ali, O.F.A.-R. Decreased catalase activity and glutathione concentration levels in women patients with breast cancer. Ann. Trop. Med. Public Health 2020, 23, SP231371. [Google Scholar] [CrossRef]
- Zinczuk, J.; Maciejczyk, M.; Zareba, K.; Romaniuk, W.; Markowski, A.; Kedra, B.; Zalewska, A.; Pryczynicz, A.; Matowicka-Karna, J.; Guzinska-Ustymowicz, K. Antioxidant Barrier, Redox Status, and Oxidative Damage to Biomolecules in Patients with Colorectal Cancer. Can Malondialdehyde and Catalase Be Markers of Colorectal Cancer Advancement? Biomolecules 2019, 9, 637. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Adamiec, M.; Skonieczna, M. UV radiation in HCT 116 cells influences intracellular H2O2 and glutathione levels, antioxidant expression, and protein glutathionylation. Acta Biochim. Pol. 2019, 66, 605–610. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sanchez-Perez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef] [PubMed]
- OA, I.O.a.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in theentire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–290. [Google Scholar] [CrossRef]
- Koeberle, S.C.; Gollowitzer, A.; Laoukili, J.; Kranenburg, O.; Werz, O.; Koeberle, A.; Kipp, A.P. Distinct and overlapping functions of glutathione peroxidases 1 and 2 in limiting NF-kappaB-driven inflammation through redox-active mechanisms. Redox Biol. 2020, 28, 101388. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman Khan, M.; Tania, M.; Zhang, D. Antioxidant enzymes and cancer. Cancer Res. 2010, 22, 87–92. [Google Scholar] [CrossRef]
- Cecerska-Heryc, E.; Surowska, O.; Heryc, R.; Serwin, N.; Napiontek-Balinska, S.; Dolegowska, B. Are antioxidant enzymes essential markers in the diagnosis and monitoring of cancer patients—A review. Clin. Biochem. 2021, 93, 1–8. [Google Scholar] [CrossRef]
- Lorestani, S.; Hashemy, S.I.; Mojarad, M.; Keyvanloo Shahrestanaki, M.; Bahari, A.; Asadi, M.; Zahedi Avval, F. Increased Glutathione Reductase Expression and Activity in Colorectal Cancer Tissue Samples: An Investigational Study in Mashhad, Iran. Middle East J. Cancer 2018, 9, 99–104. [Google Scholar] [CrossRef]
- Zhao, Y.; Seefeldt, T.; Chen, W.; Carlson, L.; Stoebner, A.; Hanson, S.; Foll, R.; Matthees, D.P.; Palakurthi, S.; Guan, X. Increase in thiol oxidative stress via glutathione reductase inhibition as a novel approach to enhance cancer sensitivity to X-ray irradiation. Free Radic. Biol. Med. 2009, 47, 176–183. [Google Scholar] [CrossRef]
- Niu, B.; Liao, K.; Zhou, Y.; Wen, T.; Quan, G.; Pan, X.; Wu, C. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials 2021, 277, 121110. [Google Scholar] [CrossRef]
- Weydert, C.J.; Zhang, Y.; Sun, W.; Waugh, T.A.; Teoh, M.L.; Andringa, K.K.; Aykin-Burns, N.; Spitz, D.R.; Smith, B.J.; Oberley, L.W. Increased oxidative stress created by adenoviral MnSOD or CuZnSOD plus BCNU (1,3-bis(2-chloroethyl)-1-nitrosourea) inhibits breast cancer cell growth. Free Radic. Biol. Med. 2008, 44, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Bouchmaa, N.; Ben Mrid, R.; Boukharsa, Y.; Nhiri, M.; Ait Mouse, H.; Taoufik, J.; Ansar, M.; Zyad, A. Cytotoxicity of new pyridazin-3(2H)-one derivatives orchestrating oxidative stress in human triple-negative breast cancer (MDA-MB-468). Arch. Pharm. 2018, 351, e1800128. [Google Scholar] [CrossRef]
- Bouchmaa, N.; Ben Mrid, R.; Bouargalne, Y.; Ajouaoi, S.; Cacciola, F.; El Fatimy, R.; Nhiri, M.; Zyad, A. In vitro evaluation of dioscin and protodioscin against ER-positive and triple-negative breast cancer. PLoS ONE 2023, 18, e0272781. [Google Scholar] [CrossRef]
- Arner, E.S.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef]
- Mustacich, D.; Powis, G. Thioredoxin reductase. Biochem. J. 2000, 346 Pt 1, 1–8. [Google Scholar] [CrossRef]
- Arner, E.S.; Holmgren, A. The thioredoxin system in cancer. Semin. Cancer Biol. 2006, 16, 420–426. [Google Scholar] [CrossRef]
- Gandin, V.; Fernandes, A.P. Metal- and Semimetal-Containing Inhibitors of Thioredoxin Reductase as Anticancer Agents. Molecules 2015, 20, 12732–12756. [Google Scholar] [CrossRef]
- Gencheva, R.; Cheng, Q.; Arner, E.S.J. Thioredoxin reductase selenoproteins from different organisms as potential drug targets for treatment of human diseases. Free Radic. Biol. Med. 2022, 190, 320–338. [Google Scholar] [CrossRef]
- Kalin, S.N.; Altay, A.; Budak, H. Inhibition of thioredoxin reductase 1 by vulpinic acid suppresses the proliferation and migration of human breast carcinoma. Life Sci. 2022, 310, 121093. [Google Scholar] [CrossRef]
- Penney, R.B.; Roy, D. Thioredoxin-mediated redox regulation of resistance to endocrine therapy in breast cancer. Biochim. Biophys. Acta 2013, 1836, 60–79. [Google Scholar] [CrossRef]
- Hampton, M.B.; O’Connor, K.M. Peroxiredoxins and the Regulation of Cell Death. Mol. Cells 2016, 39, 72–76. [Google Scholar] [CrossRef]
- Hall, A.; Nelson, K.; Poole, L.B.; Karplus, P.A. Structure-based insights into the catalytic power and conformational dexterity of peroxiredoxins. Antioxid. Redox Signal. 2011, 15, 795–815. [Google Scholar] [CrossRef]
- Morais, M.A.; Giuseppe, P.O.; Souza, T.A.; Alegria, T.G.; Oliveira, M.A.; Netto, L.E.; Murakami, M.T. How pH modulates the dimer-decamer interconversion of 2-Cys peroxiredoxins from the Prx1 subfamily. J. Biol. Chem. 2015, 290, 8582–8590. [Google Scholar] [CrossRef]
- Stocker, S.; Van Laer, K.; Mijuskovic, A.; Dick, T.P. The Conundrum of Hydrogen Peroxide Signaling and the Emerging Role of Peroxiredoxins as Redox Relay Hubs. Antioxid. Redox Signal. 2018, 28, 558–573. [Google Scholar] [CrossRef]
- Hampton, M.B.; Vick, K.A.; Skoko, J.J.; Neumann, C.A. Peroxiredoxin Involvement in the Initiation and Progression of Human Cancer. Antioxid. Redox Signal. 2018, 28, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Gupta Vallur, P.; Phaeton, R.; Mythreye, K.; Hempel, N. Insights into the Dichotomous Regulation of SOD2 in Cancer. Antioxidants 2017, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Zahra, K.F.; Lefter, R.; Ali, A.; Abdellah, E.C.; Trus, C.; Ciobica, A.; Timofte, D. The Involvement of the Oxidative Stress Status in Cancer Pathology: A Double View on the Role of the Antioxidants. Oxidative Med. Cell. Longev. 2021, 2021, 9965916. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.K.; Tangpong, J.; Chaiswing, L.; Oberley, T.D.; St Clair, D.K. Manganese superoxide dismutase is a p53-regulated gene that switches cancers between early and advanced stages. Cancer Res. 2011, 71, 6684–6695. [Google Scholar] [CrossRef] [PubMed]
- Gaya-Bover, A.; Hernandez-Lopez, R.; Alorda-Clara, M.; Ibarra de la Rosa, J.M.; Falco, E.; Fernandez, T.; Company, M.M.; Torrens-Mas, M.; Roca, P.; Oliver, J.; et al. Antioxidant enzymes change in different non-metastatic stages in tumoral and peritumoral tissues of colorectal cancer. Int. J. Biochem. Cell Biol. 2020, 120, 105698. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.R.; He, C.; Danes, J.M.; Paviani, V.; Coelho, D.R.; Gantner, B.N.; Bonini, M.G. Mitochondrial superoxide dismutase: What the established, the intriguing, and the novel reveal about a key cellular redox switch. Antioxid. Redox Signal. 2020, 32, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gawlinski, E.T. The glycolytic phenotype in carcinogenesis and tumor invasion: Insights through mathematical models. Cancer Res. 2003, 63, 3847–3854. [Google Scholar] [PubMed]
- Kurono, S.; Kaneko, Y.; Matsuura, N.; Oishi, H.; Noguchi, S.; Kim, S.J.; Tamaki, Y.; Aikawa, T.; Kotsuma, Y.; Inaji, H.; et al. Identification of potential breast cancer markers in nipple discharge by protein profile analysis using two-dimensional nano-liquid chromatography/nanoelectrospray ionization-mass spectrometry. Proteom. Clin. Appl. 2016, 10, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.J.; Wang, X.B.; Cao, A.G. Screening and functional analysis of a differential protein profile of human breast cancer. Oncol. Lett. 2014, 7, 1851–1856. [Google Scholar] [CrossRef]
- O’Leary, P.C.; Terrile, M.; Bajor, M.; Gaj, P.; Hennessy, B.T.; Mills, G.B.; Zagozdzon, A.; O’Connor, D.P.; Brennan, D.J.; Connor, K.; et al. Peroxiredoxin-1 protects estrogen receptor alpha from oxidative stress-induced suppression and is a protein biomarker of favorable prognosis in breast cancer. Breast Cancer Res. 2014, 16, R79. [Google Scholar] [CrossRef]
- Liu, J.; Du, J.; Zhang, Y.; Sun, W.; Smith, B.J.; Oberley, L.W.; Cullen, J.J. Suppression of the malignant phenotype in pancreatic cancer by overexpression of phospholipid hydroperoxide glutathione peroxidase. Hum. Gene Ther. 2006, 17, 105–116. [Google Scholar] [CrossRef]
- Krol, M.B.; Galicki, M.; Gresner, P.; Wieczorek, E.; Jablonska, E.; Reszka, E.; Morawiec, Z.; Wasowicz, W.; Gromadzinska, J. The ESR1 and GPX1 gene expression level in human malignant and non-malignant breast tissues. Acta. Biochim. Pol. 2018, 65, 51–57. [Google Scholar] [CrossRef]
- Al-Taie, O.H.; Uceyler, N.; Eubner, U.; Jakob, F.; Mork, H.; Scheurlen, M.; Brigelius-Flohe, R.; Schottker, K.; Abel, J.; Thalheimer, A.; et al. Expression profiling and genetic alterations of the selenoproteins GI-GPx and SePP in colorectal carcinogenesis. Nutr. Cancer 2004, 48, 6–14. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, X.; Shen, Y.; He, Q.; Qin, Y.; Shao, Y.; Yuan, L.; Ye, H. GPX2 predicts recurrence-free survival and triggers the Wnt/beta-catenin/EMT pathway in prostate cancer. PeerJ 2022, 10, e14263. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, Y.; Guo, S.; Wang, G. Glutathione peroxidases as oncotargets. Oncotarget 2017, 8, 80093–80102. [Google Scholar] [CrossRef]
- Lee, O.J.; Schneider-Stock, R.; McChesney, P.A.; Kuester, D.; Roessner, A.; Vieth, M.; Moskaluk, C.A.; El-Rifai, W. Hypermethylation and loss of expression of glutathione peroxidase-3 in Barrett’s tumorigenesis. Neoplasia 2005, 7, 854–861. [Google Scholar] [CrossRef]
- Falck, E.; Karlsson, S.; Carlsson, J.; Helenius, G.; Karlsson, M.; Klinga-Levan, K. Loss of glutathione peroxidase 3 expression is correlated with epigenetic mechanisms in endometrial adenocarcinoma. Cancer Cell Int. 2010, 10, 46. [Google Scholar] [CrossRef]
- Yu, Y.P.; Yu, G.; Tseng, G.; Cieply, K.; Nelson, J.; Defrances, M.; Zarnegar, R.; Michalopoulos, G.; Luo, J.H. Glutathione peroxidase 3, deleted or methylated in prostate cancer, suppresses prostate cancer growth and metastasis. Cancer Res. 2007, 67, 8043–8050. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, T.; Zhu, S.; Mukaisho, K.; El-Rifai, W.; Peng, D.F. Glutathione peroxidase 7 suppresses cancer cell growth and is hypermethylated in gastric cancer. Oncotarget 2017, 8, 54345–54356. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, S.C.; Kipp, A.P.; Stuppner, H.; Koeberle, A. Ferroptosis-modulating small molecules for targeting drug-resistant cancer: Challenges and opportunities in manipulating redox signaling. Med. Res. Rev. 2023, 43, 614–682. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.; Xu, S.; Pang, D. Emerging role of ferroptosis in breast cancer: New dawn for overcoming tumor progression. Pharmacol. Ther. 2022, 232, 107992. [Google Scholar] [CrossRef]
- Liu, H.; Schreiber, S.L.; Stockwell, B.R. Targeting Dependency on the GPX4 Lipid Peroxide Repair Pathway for Cancer Therapy. Biochemistry 2018, 57, 2059–2060. [Google Scholar] [CrossRef]
- Chen, M.; Shi, Z.; Sun, Y.; Ning, H.; Gu, X.; Zhang, L. Prospects for Anti-Tumor Mechanism and Potential Clinical Application Based on Glutathione Peroxidase 4 Mediated Ferroptosis. Int. J. Mol. Sci. 2023, 24, 1607. [Google Scholar] [CrossRef]
- Liu, H.; Forouhar, F.; Lin, A.J.; Wang, Q.; Polychronidou, V.; Soni, R.K.; Xia, X.; Stockwell, B.R. Small-molecule allosteric inhibitors of GPX4. Cell Chem. Biol. 2022, 29, 1680–1693.e9. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Jiang, X. The Chemistry and Biology of Ferroptosis. Cell Chem. Biol. 2020, 27, 365–375. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, H.; Tang, J.; Wang, R. Ferulic Acid Mitigates Growth and Invasion of Esophageal Squamous Cell Carcinoma through Inducing Ferroptotic Cell Death. Dis. Markers 2022, 2022, 4607966. [Google Scholar] [CrossRef]
- Xu, F.L.; Wu, X.H.; Chen, C.; Wang, K.; Huang, L.Y.; Xia, J.; Liu, Y.; Shan, X.F.; Tang, N. SLC27A5 promotes sorafenib-induced ferroptosis in hepatocellular carcinoma by downregulating glutathione reductase. Cell Death Dis. 2023, 14, 22. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, X.S.; Zhou, X.M.; Gao, Y.Y.; Chen, C.L.; Liu, J.P.; Ye, Z.N.; Zhang, Z.H.; Wu, L.Y.; Li, W.; et al. Peroxiredoxin 1/2 protects brain against H(2)O(2)-induced apoptosis after subarachnoid hemorrhage. FASEB J. 2019, 33, 3051–3062. [Google Scholar] [CrossRef]
- Vabulas, R.M. Ferroptosis-Related Flavoproteins: Their Function and Stability. Int. J. Mol. Sci. 2021, 22, 430. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, S.; Liu, Y.; Chang, X.; Liang, Y.; Li, X.; Xu, Z.; Wang, S.; Lu, Y.; Liu, Y.; et al. Biotin-Targeted Au(I) Radiosensitizer for Cancer Synergistic Therapy by Intervening with Redox Homeostasis and Inducing Ferroptosis. J. Med. Chem. 2022, 65, 8401–8415. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef]
- Tang, X.; Loc, W.S.; Dong, C.; Matters, G.L.; Butler, P.J.; Kester, M.; Meyers, C.; Jiang, Y.; Adair, J.H. The use of nanoparticulates to treat breast cancer. Nanomedicine 2017, 12, 2367–2388. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, Y.; Yuan, Q.; Lu, C.; Zhang, X.; Yuan, J.; Hou, K.; Zhang, C.; Du, Z.; Gao, X.; et al. Gold clusters prevent breast cancer bone metastasis by suppressing tumor-induced osteoclastogenesis. Theranostics 2020, 10, 4042–4055. [Google Scholar] [CrossRef]
- Zhou, J.; Li, K.; Zang, X.; Xie, Y.; Song, J.; Chen, X. ROS-responsive Galactosylated-nanoparticles with Doxorubicin Entrapment for Triple Negative Breast Cancer Therapy. Int. J. Nanomed. 2023, 18, 1381–1397. [Google Scholar] [CrossRef]
- Hong, S.; Choi, D.W.; Kim, H.N.; Park, C.G.; Lee, W.; Park, H.H. Protein-Based Nanoparticles as Drug Delivery Systems. Pharmaceutics 2020, 12, 604. [Google Scholar] [CrossRef]
- Homayouni Tabrizi, M. Fabrication of folic acid-conjugated chitosan-coated PLGA nanoparticles for targeted delivery ofPeganum harmalasmoke extract to breast cancer cells. Nanotechnology 2022, 33, 495101. [Google Scholar] [CrossRef]
- Solak, K.; Mavi, A.; Yilmaz, B. Disulfiram-loaded functionalized magnetic nanoparticles combined with copper and sodium nitroprusside in breast cancer cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 119, 111452. [Google Scholar] [CrossRef] [PubMed]
- Feuser, P.E.; Cordeiro, A.P.; de Bem Silveira, G.; Borges Correa, M.E.A.; Lock Silveira, P.C.; Sayer, C.; de Araujo, P.H.H.; Machado-de-Avila, R.A.; Dal Bo, A.G. Co-encapsulation of sodium diethyldithiocarbamate (DETC) and zinc phthalocyanine (ZnPc) in liposomes promotes increases phototoxic activity against (MDA-MB 231) human breast cancer cells. Colloids Surf. B Biointerfaces 2021, 197, 111434. [Google Scholar] [CrossRef]
- Tian, W.; Wang, S.; Tian, Y.; Su, X.; Sun, H.; Tang, Y.; Lu, G.; Liu, S.; Shi, H. Periodic mesoporous organosilica coupled with chlorin e6 and catalase for enhanced photodynamic therapy to treat triple-negative breast cancer. J. Colloid Interface Sci. 2022, 610, 634–642. [Google Scholar] [CrossRef]
- Hei, Y.; Teng, B.; Zeng, Z.; Zhang, S.; Li, Q.; Pan, J.; Luo, Z.; Xiong, C.; Wei, S. Multifunctional Immunoliposomes Combining Catalase and PD-L1 Antibodies Overcome Tumor Hypoxia and Enhance Immunotherapeutic Effects Against Melanoma. Int. J. Nanomed. 2020, 15, 1677–1691. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Li, M.; Zhang, Z.; Yao, Q.; Shao, K.; Xu, F.; Xu, N.; Li, H.; Fan, J.; Sun, W.; et al. Catalase-based liposomal for reversing immunosuppressive tumor microenvironment and enhanced cancer chemo-photodynamic therapy. Biomaterials 2020, 233, 119755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Song, X.; Liang, C.; Yi, X.; Song, G.; Chao, Y.; Yang, Y.; Yang, K.; Feng, L.; Liu, Z. Catalase-loaded cisplatin-prodrug-constructed liposomes to overcome tumor hypoxia for enhanced chemo-radiotherapy of cancer. Biomaterials 2017, 138, 13–21. [Google Scholar] [CrossRef]
- Li, G.; Wang, S.; Deng, D.; Xiao, Z.; Dong, Z.; Wang, Z.; Lei, Q.; Gao, S.; Huang, G.; Zhang, E.; et al. Fluorinated Chitosan To Enhance Transmucosal Delivery of Sonosensitizer-Conjugated Catalase for Sonodynamic Bladder Cancer Treatment Post-intravesical Instillation. ACS Nano 2020, 14, 1586–1599. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Chen, Z.; Sheng, Z.; Gao, D.; Yan, F.; Ma, T.; Zheng, H.; Hong, M. A catalase-loaded hierarchical zeolite as an implantable nanocapsule for ultrasound-guided oxygen self-sufficient photodynamic therapy against pancreatic cancer. Nanoscale 2018, 10, 17283–17292. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, J.; Yang, Z.; Xu, J.; Xu, L.; Liang, C.; Han, X.; Liu, Z. Nanoparticle-Enhanced Radiotherapy to Trigger Robust Cancer Immunotherapy. Adv. Mater. 2019, 31, e1802228. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zhang, L.; Peng, C.; Zhang, S.; Chen, S.; Qian, X.; Luo, W.; Dan, Q.; Ren, Y.; Li, Y.; et al. Tumor microenvironments self-activated nanoscale metal-organic frameworks for ferroptosis based cancer chemodynamic/photothermal/chemo therapy. Acta Pharm. Sin. B 2021, 11, 3231–3243. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, M.M.; Luo, Q.W.; Zhang, Y.C.; Liu, T.T.; Yang, Z.; Liao, M.; Tu, P.; Zeng, K.W. Carbon Quantum Dots-Based Nanozyme from Coffee Induces Cancer Cell Ferroptosis to Activate Antitumor Immunity. ACS Nano 2022, 16, 9228–9239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, J.; Li, R.; Wei, L.; Xiong, H.; Wang, C.; Chai, K.; Chen, M.; Zhu, Z.; Yao, T.; et al. Metal-Polyphenol-Network Coated Prussian Blue Nanoparticles for Synergistic Ferroptosis and Apoptosis via Triggered GPX4 Inhibition and Concurrent In Situ Bleomycin Toxification. Small 2021, 17, e2103919. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, M.; Liu, L.; Xue, C.; Fei, Y.; Wang, X.; Zhang, Y.; Cai, K.; Zhao, Y.; Luo, Z. Cell-Specific Metabolic Reprogramming of Tumors for Bioactivatable Ferroptosis Therapy. ACS Nano 2022, 16, 3965–3984. [Google Scholar] [CrossRef]
- Li, K.; Lin, C.; Li, M.; Xu, K.; He, Y.; Mao, Y.; Lu, L.; Geng, W.; Li, X.; Luo, Z.; et al. Multienzyme-like Reactivity Cooperatively Impairs Glutathione Peroxidase 4 and Ferroptosis Suppressor Protein 1 Pathways in Triple-Negative Breast Cancer for Sensitized Ferroptosis Therapy. ACS Nano 2022, 16, 2381–2398. [Google Scholar] [CrossRef]
- Zhou, L.L.; Guan, Q.; Li, W.Y.; Zhang, Z.; Li, Y.A.; Dong, Y.B. A Ferrocene-Functionalized Covalent Organic Framework for Enhancing Chemodynamic Therapy via Redox Dyshomeostasis. Small 2021, 17, e2101368. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, M.; Zhai, T.; Zhou, H.; Zhou, Z.; Liu, X.; Yang, S.; Yang, H. Glutathione-Responsive Chemodynamic Therapy of Manganese(III/IV) Cluster Nanoparticles Enhanced by Electrochemical Stimulation via Oxidative Stress Pathway. Bioconjugate Chem. 2022, 33, 152–163. [Google Scholar] [CrossRef]
- He, H.; Du, L.; Guo, H.; An, Y.; Lu, L.; Chen, Y.; Wang, Y.; Zhong, H.; Shen, J.; Wu, J.; et al. Redox Responsive Metal Organic Framework Nanoparticles Induces Ferroptosis for Cancer Therapy. Small 2020, 16, e2001251. [Google Scholar] [CrossRef]
- Fereidoonnezhad, M.; Ahmadi, M.; Abedanzadeh, S.; Yazdani, A.; Alamdarlou, A.; Babaghasabha, M.; Almansaf, Z.; Faghih, Z.; McConnell, Z.; Shahsavari, H.R.; et al. Synthesis and biological evaluation of thiolate gold(I) complexes as thioredoxin reductases (TrxRs) and glutathione reductase (GR) inhibitors. New J. Chem. 2019, 43, 13173–13182. [Google Scholar] [CrossRef]
- Bajor, M.; Graczyk-Jarzynka, A.; Marhelava, K.; Kurkowiak, M.; Rahman, A.; Aura, C.; Russell, N.; Zych, A.O.; Firczuk, M.; Winiarska, M.; et al. Triple Combination of Ascorbate, Menadione and the Inhibition of Peroxiredoxin-1 Produces Synergistic Cytotoxic Effects in Triple-Negative Breast Cancer Cells. Antioxidants 2020, 9, 320. [Google Scholar] [CrossRef]
- Kato, I.; Kasukabe, T.; Kumakura, S. Menin-MLL inhibitors induce ferroptosis and enhance the anti-proliferative activity of auranofin in several types of cancer cells. Int. J. Oncol. 2020, 57, 1057–1071. [Google Scholar] [CrossRef]
- Hatem, E.; Azzi, S.; El Banna, N.; He, T.; Heneman-Masurel, A.; Vernis, L.; Baille, D.; Masson, V.; Dingli, F.; Loew, D.; et al. Auranofin/Vitamin C: A Novel Drug Combination Targeting Triple-Negative Breast Cancer. J. Natl. Cancer Inst. 2018, 111, 597–608. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, X.; Zhao, Y. Nanozymes: Versatile Platforms for Cancer Diagnosis and Therapy. Nanomicro Lett. 2022, 14, 95. [Google Scholar] [CrossRef]
- Pei, P.; Shen, W.; Zhang, Y.; Zhang, Y.; Qi, Z.; Zhou, H.; Liu, T.; Sun, L.; Yang, K. Radioactive nano-oxygen generator enhance anti-tumor radio-immunotherapy by regulating tumor microenvironment and reducing proliferation. Biomaterials 2022, 280, 121326. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Han, X.; Zhang, T.; Xie, D.; Zhang, H.; Hu, Y. An Oxygen Self-Evolving, Multistage Delivery System for Deeply Located Hypoxic Tumor Treatment. Adv. Health Mater. 2020, 9, e1901303. [Google Scholar] [CrossRef] [PubMed]
- Kheshtchin, N.; Hadjati, J. Targeting hypoxia and hypoxia-inducible factor-1 in the tumor microenvironment for optimal cancer immunotherapy. J. Cell. Physiol. 2022, 237, 1285–1298. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Wang, Z.; Dong, C.; Zhou, R.; Chen, L.; Huang, H.; Feng, W.; Wang, Z.; Wang, Y.; Chen, Y. Ultrasound-Amplified Enzyodynamic Tumor Therapy by Perovskite Nanoenzyme-Enabled Cell Pyroptosis and Cascade Catalysis. Adv. Mater. 2023, 35, e2208817. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Zhao, X.; Wang, X.; Huang, T.; Ding, Y.; Zhang, J.; Zhang, Z.; Wang, Z.L.; Li, L. Bioinspired Electron Polarization of Nanozymes with a Human Self-Generated Electric Field for Cancer Catalytic Therapy. Adv. Mater. 2022, 34, e2109568. [Google Scholar] [CrossRef] [PubMed]
- Adair, J.H.; Parette, M.P.; Altinoglu, E.I.; Kester, M. Nanoparticulate alternatives for drug delivery. ACS Nano 2010, 4, 4967–4970. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; He, Q.; Dai, X.; Zhang, X.; Song, D. The potential role of nanomedicine in the treatment of breast cancer to overcome the obstacles of current therapies. Front. Pharmacol. 2023, 14, 1143102. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; Andre, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Khan, J.A.; Murtaza, S. Nanotechnology: An Emerging Therapeutic Option for Breast Cancer. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Chen, C.; Xi, Z.; Chen, J.; Zhang, Q.; Cao, S.; Jiang, X. In vivo behavior and safety of lapatinib-incorporated lipid nanoparticles. Curr. Pharm. Biotechnol. 2014, 14, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, B.; Wu, Y.; Lee, R.J.; Lee, L.J. Efficient down-regulation of CDK4 by novel lipid nanoparticle-mediated siRNA delivery. Anticancer Res. 2011, 31, 1619–1626. [Google Scholar]
- American Type Culture Collection. 2024. The Global Bioresource Center. Available online: https://www.atcc.org/ (accessed on 8 May 2024).
- Briem, E.; Ingthorsson, S.; Traustadottir, G.A.; Hilmarsdottir, B.; Gudjonsson, T. Application of the D492 Cell Lines to Explore Breast Morphogenesis, EMT and Cancer Progression in 3D Culture. J. Mammary Gland Biol. Neoplasia 2019, 24, 139–147. [Google Scholar] [CrossRef] [PubMed]

| Enzyme | Type | Tissue Expression | Cellular Localization | Pathological Function | Therapeutical Use | Sample Type | References |
|---|---|---|---|---|---|---|---|
| Catalase | Typical | Highest enzyme activity in liver and erythrocytes, high activity in kidney and adipose tissue, intermediate in lung and pancreas, and very low in heart and brain | Peroxisomes | Dichotomous role: Protection from tumor formation and progression; however, it is also necessary for tumor progression and metastasis. It is frequently decreased in breast tumors and blood of patients with BC and BC cell cultures | Increasing CAT levels in breast tumors decreases hypoxia and attenuates the tumoral microenvironment immunosuppression condition through tumor-associated macrophages reprogramming from M2 (pro-tumoral) to M1 (anti-tumoral) due to increased O2 in the tumor, reversing hypoxia-induced chemotherapy resistance | Serum and tissue samples from BC patients and human BC MCF-7 cell line | [38,39,40,41,42,43,44] |
| SOD1 | CuZn-SOD | Pons, substantia nigra pars compacta, dorsal root ganglion, lateral nuclear group of the thalamus | Cytosol, nucleus, and mitochondria | Reduced expression and activity, generating an increase in oxidative stress within the cell | In BC cells, the decrease in NAD-dependent deacetylase sirtuin-3 (SIRT3) expression can be counteracted by an upregulation of SOD1. As a result, the total level of ROS in the mitochondria is maintained within a window compatible with cell survival. In addition, it was reported that, using a panel of mammary cell lines, SOD1 is overexpressed and SIRT3 is decreased | MCF-10A, MCF-7, MDA-MB-231, and MDA-MB-157 | [45,46] |
| SOD2 | Mn-SOD | Lungs, placenta, kidney, pancreas and uterus, cartilage, skeletal muscle, brain, and eye | Mitochondrial matrix | Reduced expression and activity, generating an increase in oxidative stress within the cell and mitochondria | Almost all tumors have reduced Mn-SOD activity. Extensive epidemiological studies have mainly focused on the Ala16Val dimorphism of Mn-SOD as a risk factor for BC. Ambrosone and his colleagues were the first to report Ala16-MnSOD as a risk factor for BC | Data were collected in a case–control study of diet and BC in western New York from 1986 to 1991. Caucasian women with incident, primary, histologically confirmed BC were frequency-matched by age and county of residence to community controls. Blood specimens were collected and processed from a subset of participants in the study (266 cases and 295 controls) | [37,47,48] |
| SOD3 | Ec-SOD | Cardiovascular endothelium, lungs, and placenta. Displays moderate activity within the kidney, pancreas, uterus, cartilage, skeletal muscle, adipose tissue, brain, and eye | Extracellular | Reduced activity, OH· levels are increased through the Fenton and Harber–Weiss reactions. In addition, the oxidation of NO• mediated by superoxide is increased, generating high concentrations of peroxynitrite (ONOO−) | Ec-SOD overexpression inhibited in vitro proliferation, clonogenic survival, and invasion of a triple-negative breast cancer cell (TNCB) line, in part by suppressing heparanase-mediated cleavage of cell surface proteoglycans and by reducing the bioavailability of VEGFA (vascular endothelial growth factor A). Ec-SOD overexpression also significantly inhibited tumor metastasis in both an experimental lung and a mouse model of spontaneous metastasis | Non-malignant, post-stasis human mammary epithelial cells extracted from reduction mammoplasty, human mammary epithelial cells (HMEC) immortalized, non-malignant breast epithelial MCF-10A cells, human mammary adenocarcinoma cell lines, MCF-7 cells, MDA-MB-231 cells, and MDA-MB-435 cells | [48,49,50,51] |
| GPx1 | Selenium-dependent | Red blood cells, liver, lung, and kidney | Cytosol, nucleus, and mitochondria | Acts as a tumor promoter by regulating the proliferation, invasion, migration, apoptosis, immune response, and drug sensitivity of tumor cells | Enzyme glutamate dehydrogenase 1 (GDH1) controlling the intracellular levels of alpha-ketoglutarate (α-KG) and subsequent metabolite fumarate. Fumarate binds to and activates GPx1, leading to attenuated cancer, cell proliferation, and tumor growth (cell culture) (decreased GPx1 expression in tumorous breast tissue) (in human BC cell lines, GPx1 is downregulated) | BC cell line MDA-MB-231. Tissue samples from human patients aged 44–82 years with BC and MCF-7 human carcinoma cells. Human BC MCF-7 and MDA-MB-231 cell lines compared with healthy breast MCF-10A cells | [52,53,54,55] |
| GPx2 | Selenium-dependent | Gastrointestinal tract, breast | Cytosol and nucleus | It is upregulated in a variety of tumor cells and is associated with tumor cell proliferation and a poor prognosis of patients. It causes vascular malfunction and hypoxia | Once a cell has been programmed to proliferate in an uncontrolled way, GPx2 supports the growth of cells by inhibiting apoptosis. GPx2 loss stimulates malignant progression due to reactive oxygen species/hypoxia inducible factor-α (HIF1α)/VEGFA signaling, causing poor perfusion and hypoxia (in human BC cell lines, GPx2 is upregulated) | MCF-7 and MDA-MB-231 cell lines compared with healthy breast MCF-10A cells | [7,52,54,56] |
| GPx3 | Selenium-dependent | Kidney, lung, epididymis, breast, heart, and muscle | Plasma and mitochondria | Reduced expression can promote the proliferation, motility, and invasion of melanoma cells | GPx3 directly targets the ERα gene in white adipose tissue, for which it was proposed as an important mediator of the estrogen effects in association with fat mass. Considering the link between visceral fat and BC initiation and progression, it is reasonable to observe an overexpression of GPx3 in BC cells (in human BC cell lines, GPx2 is upregulated) | MCF-7 and MDA-MB-231 cell lines compared with healthy breast MCF-10A cells | [7,57] |
| GPx4 | Selenium-dependent | Thyroid gland, bronchus, duodenum, lung, breast, heart, and muscle | Nucleus, cytosol, and mitochondria | Increased expression may promote the malignant progression of BC | It is an inducer of ferroptosis and apoptosis through ubiquitination of GPx4 (GPx4 is downregulated, with reduced expression in several cell lines, including human BC) | MCF-7 and MDA-MB-231 cell lines compared with healthy breast MCF-10A cells | [7,54,58,59] |
| GPx5 | Non-Selenium-dependent | Epididymis | Extracellular | Downregulated | In human BC cell lines, GPx5 is downregulated | MCF-7 and MDA-MB-231 cell lines compared with healthy breast MCF-10A cells | [7] |
| GPx6 | Selenium-dependent | Olfactory epithelium | Epithelium | No data | No data | No data | [52] |
| GPx7 | Non-Selenium-dependent | Preadipocytes | Lumen of the endoplasmic reticulum. | Downregulated | In human breast cancer cell lines, GPx7 is downregulated | MCF-7 and MDA-MB2-31 cell lines compared with healthy breast MCF-10A cells | [7,52] |
| GPx8 | Non-Selenium-dependent | Lung | Transmembrane of the endoplasmic reticulum | Expression is upregulated | In human BC cell lines, GPx8 is upregulated. If GPx8 is suppressed in these cells, they express a non-functional IL-6 receptor, which does not interact with IL-6. This altered binding hinders the activation of the JAK/STAT3 signaling pathway, thus inhibiting the transition of cancer cells to an aggressive phenotype | Human breast cancer cell line MDA-MB-231 | [54,60] |
| GR | Selenoprotein | Pylorus, islet of Langerhans, epithelium of nasopharynx | Mitochondria, nucleus, and cytoplasm | Protects cancer cells against increased oxidative stress and provides a survival advantage | Increased GR activity in tumor cells and in the blood of BC patients. Therefore, inhibition of glutathione reductase in BC cells causes increased oxidative stress in the cell, which stops the growth of the cancer cell | The studies have been carried out on tissue samples from human patients with BC, aged 20 to 65 years; some were taken from ductal carcinoma; in the case of human BC cell lines, T-47D and MCF-7, D492 have been used | [61,62,63,64] |
| PrxI | 2-Cysteine peroxidase | Thyroid gland, nasal cavity epithelium, olfactory segment of nasal mucosa, palpebral conjunctiva | Cytoplasm, melanosome, nucleus | Overexpressed in BC tissue. Correlated with shortened patient survival | Inhibition of PrxI gene upregulation may cause disadvantage to the survival and proliferation of tumor cells | Tissue from BC (type I to IV stage) patients | [65,66,67,68,69] |
| PrxII | 2-Cysteine peroxidase | Thalamus, trabecular bone tissue, substantia nigra pars compacta, substantia nigra pars reticulata | Cytoplasm, nucleus | Overexpressed in BC tissue. Induces carcinogenic changes, maintains cancer stem cells phenotype and stemness properties | Inhibition of PrxII with siRNA partially reverses the radioresistant phenotype in radiation-resistant BC cells | Tissue from BC (type I to IV stage) patients | [67,69,70] |
| PrxIII | 2-Cysteine peroxidase | Adrenal tissue, adrenal gland cortex, heart right ventricle, biceps brachii | Mitochondrion, cytoplasm, early endosome | Overexpressed in BC tissue. Related to tumorigenesis | Potential proliferation marker. Related to a better prognosis | Human BC MCF-7 and MDA-MB-231 cell lines. Tissue from BC patients | [68,69,71,72,73] |
| PrxIV | 2-Cysteine peroxidase | Pancreas, tibia, adrenal tissue | Cytoplasm, endoplasmic reticulum | Overexpressed in progesterone receptor positive cases. Promoted migration and invasion of cancer cells | Promising therapeutic target for inflammatory diseases and cancer. Related to a better prognosis | Tissue from BC patients | [73,74,75,76] |
| PrxV | 2-Cysteine peroxidase | Bronchial epithelial cell, epithelium of nasopharynx, palpebral conjunctiva, fallopian tube (uterine tube) | Mitochondria, cytoplasm, peroxisome matrix | Overexpression of PrxV gene is correlated with a larger tumor size, positive lymph node status, and shorter survival. Deficiency induced M2 macrophage polarization | PrxV is a putative therapeutic target and clinical strategy in breast, bladder, lung, cervical, ovarian, prostate, esophageal, and hepatocellular tumors | Human BC MCF-7 cell line | [68,73,77,78,79,80] |
| PrxVI | 1-Cysteine peroxidase (GSHs reductant) | Corpus epididymis, gastrocnemius, mucosa of stomach, amniotic fluid | Cytoplasm, lysosome, lamellar bodies, nucleus | Upregulated in progesterone receptor positive cases. Overexpression of PrxVI leads to a more invasive phenotype and metastatic potential in BC. Increased in most metastatic cell lines | Prx6 stable knockdown xenografts exhibited decreased tumor growth and metastasis | BC cell lines, xenograft tumor model in athymic mice | [81,82] |
| TrxR1 | Selenocysteine-containing protein | Ovary, spleen, heart, liver, kidney, and pancreas | Cytoplasm | TrxR overexpression has been correlated with aggressive tumor growth, worse prognosis, and decreased patient survival. | Inhibition of TrxR causes malignant cells to become more susceptible to cytotoxicity, cytostasis, and cell death. | Human BC MDA-MB-435 S, MDA-MB-231, BT-549, and MCF-10A cell lines | [83,84,85,86] |
| TrxR2 | Selenocysteine-containing protein | Pharyngeal and body wall muscles | Mitochondria | Overexpressed in cancer cells, conferring apoptosis resistance. | Increases the mitochondrial concentration of reactive oxygen species and shifts the thiol redox state toward a more oxidized condition | Human BC MCF-7 cell line | [87,88] |
| TrxR3 | Selenocysteine-containing protein | Testis | No data | No data | No data | No data | [89] |
| Target Antioxidant Enzyme | Compound | Action Mode | References |
|---|---|---|---|
| SOD | PSE-PCF-NPs: Shell poly (lactic acid-co-glycolic) (PLGA)-NPs coated with folic acid (FA)-chitosan (PCF-NPs) loaded with Peganum harmala smoke extract (PSE) | The combination of chitosan and PGLA increases the bioavailability, toxicity, and release of the drug. In addition, the use of folic acid on the surface of the NPs is one of the most effective strategies to internalize into cancer cells through receptor-mediated endocytosis and the administration of anticancer agents. As a result, an increase in ROS and a decrease in the SOD enzyme was obtained in MCF-7 cells treated with PSE-PCF-NP. | [160] |
| Fe3O4@mSiO2-DSF@PEI-FA, mMDPF: Disulfiram (DSF) loading, encapsulated folic acid (FA) conjugated polyethyleneimine magnetic mesoporous silica (Fe3O4@mSiO2) NPs | Disulfiram is a SOD inhibitor due to its affinity for sulfhydryl groups and the ability to bind to the copper and zinc of SOD. Inhibition of SOD can cause superoxide accumulation in cells inducing oxidative stress, apoptosis, and cell cycle arrest; it also reduces cancer cell proliferation, angiogenesis, tumor metastasis, and multidrug resistance. Fe3O4@mSiO2 is a drug carrier system based on magnetic mesoporous silica NPs and folic acid, and it is used to increase both its solubility in water and its specificity for cancer cells. Finally, the addition of Cu2+ increases the therapeutic effect of DSF in different types of cancer cells. | [161] | |
| PC + C22PEG900GlcNAc: Diethyldithiocarbamate (DETC), zinc phthalocyanine (ZnPc) loaded in liposomes | The encapsulation of photosensitizers with liposomes improves their therapeutic activity while preserving their photophysical properties, in addition to reducing their toxic effect. The principle of photodynamic therapy (PDT) involves the production of high levels of ROS photosensitizing molecules, which when exposed to visible light can kill nearby cells. ZnPc is a second-generation photosensitizer used in PDT to produce singlet oxygen, while DETC is a hydrophilic metal chelating agent and a known SOD inhibitor. Thus, the inhibition of SOD increases the ROS generated by PDT, causing an increase in the death of tumor cells. | [162] | |
| CAT | PMO-Ce6@Catalase: Mesoporous organosilica (PMO) coupled with chlorine e6 (Ce6) and CAT | PMO-Ce6@Catalase is selectively absorbed and stored by tumor tissue. Then, after local irradiation with light of appropriate wavelength, the photosensitizer (Ce6@) is activated to produce a photosensitizing effect to generate high levels of ROS. CAT increases the concentration of oxygen around the cells and solves the problem of hypoxia in the tumor, in addition to enhancing the effects of Ce6@. | [163] |
| CAT@PDL1-SSLs: CAT-loaded and PDL1 (programmed death-ligand 1) monoclonal antibody modified immunoliposomes | PDL1 monoclonal antibodies are used as immune checkpoint blockers (ICBs) to significantly improve the efficacy of tumor immunotherapy by blocking the PD-1/PD-L1 inhibitory pathway. CAT helps the system overcome hypoxia, which is a limitation for PDL1. The results of this nanoparticle are activating and increasing the infiltration of CD8+ T cells at the tumor site and inhibiting tumor growth with low systemic toxicity. | [164] | |
| FA-L@MBDP@CAT: Lyso-targeted NIR photosensitizer (MBDP), CAT and doxorubicin (Dox) encapsulated within folic acid (FA) modified liposomes | Increased M1-MQ polarization; increased recruitment of CD8+ T cells. Photosensitizer (MBDP) has deep tissue penetration and high phototoxicity toward cancer cells. Doxorubicin has a good therapeutic effect on BC and metastatic tumors by inducing DNA damage and inhibiting the progression of topoisomerase II enzyme. For these liposomes, folate increases active targeting and prevents them from being recognized and phagocytosed by the reticuloendothelial system (RES) due to the existence of the PEG framework. The released CAT catalyzes overexpressed hydrogen peroxide to increase tumor oxygenation, providing sufficient oxygen for PDT and reversing the immunosuppressive TME by modulating immune cytokines to favor antitumor immunities, enhancing tumor inhibition in vivo. | [165] | |
| CAT@Pt(IV)-liposome: CAT-loaded cisplatin constructed liposome | CAT is encapsulated together with cisplatin (IV), forming a CAT@Pt(IV) liposome to improve cancer chemoradiotherapy. After loading into the liposomes, the CAT within the CAT@Pt(IV) liposome shows retained and well-protected enzymatic activity and is capable of triggering the breakdown of H2O2 produced by tumor cells, to produce additional oxygen for relieve hypoxia. As a result, CAT@Pt(IV) liposome treatment induces the highest level of DNA damage in cancer cells after X-ray irradiation. | [166] | |
| CAT-TCPP/FCS: Assembled FCS (fluorinated chitosan) with meso-tetra(4-carboxyphenyl) porphyrin (TCPP) conjugated CAT | These NPs exhibit greatly improved transmucosal adsorption and intratumoral penetration, due to their abilities to reversibly modulate transepithelial electrical resistance (TEER) and open tight junctions of the bladder epithelium. Such actions, together with the in situ O2 generation triggered by the CAT-catalyzed decomposition of the endogenous H2O2 of the tumor, would contribute to drastically improve the efficacy of sonodynamic therapy to destroy orthotopic bladder tumors. | [167] | |
| ZCM nanocapsule: CAT and methylene blue co-loaded into mesoporous of zeolite nanocarriers | Free CAT efficiently modulates tumor hypoxia and enhances intratumoral contrast through sustained decomposition of endogenous H2O2 and in situ production of O2 gas bubbles. Meanwhile, loading methylene blue into zeolite matrices prevents rapid leaching of photosensitizer in tumor tissue, achieving well-sustained release effect of photosensitizer. According to synchronous mechanisms, after near-infrared laser irradiation, local pancreatic cancer cells die completely, and no therapy-induced toxicity or recurrence is observed. | [168] | |
| PLGA-R837@CAT: CAT and R837 co-loaded in core/shell poli (lactic acid-co-glycolic) (PLGA) NPs platform | Reduced tumor metastasis; increased M1-MQ polarization; enhanced immunological cell death. The formed PLGA-R837@CAT nanoparticles can greatly improve the efficacy of radiotherapy by alleviating tumor hypoxia and modulating the immunosuppressive tumor microenvironment. Antigens with R837 will induce strong antitumor immune responses, which together with the blockade of the cytotoxic T lymphocyte-associated protein 4 (CTLA-4) checkpoint will be able to effectively inhibit tumor metastases through a strong abscopal effect (the reduction or disappearance of tumors in parts of the body that were not the direct target of local therapy, such as radiotherapy. | [169] | |
| GPx4 | FCSP@DOX MOF: Fe and Cu ions bridged by disulfide bonds with PEGylation (FCSP MOFs) loaded with doxorubicin (Dox) | FCSP@DOX MOFs are structures activated by the redox environment of the tumor, which causes the release of Fe2+/Cu+ ions to produce ROS through the Fenton reaction, triggering the depletion of GSH levels and the inhibition of GPx4, which causes an elevation of lipid peroxidation and the onset of ferroptosis. Additionally, better tumor therapeutic efficiency is achieved by loading DOX, since it can not only cause apoptosis, but also indirectly produces H2O2 to amplify the Fenton reaction, which allows the notable antitumor effect of FCSP@DOX MOFs. | [170] |
| ChA CQDs: Carbon quantum dots (CQDs) prepared into nanozymes from chlorogenic acid (ChA) | CQDs have GSH oxidase-like activity by catalyzing the conversion of GSH to GSSG. Due to this, ChACQDs induce ferroptosis by promoting an unbalanced redox reaction due to the depletion of GSH and the inactivation of GPx4, with the consequent accumulation of ROS and lipid peroxidation. | [171] | |
| HMTBF: Metal-polyphenol network coated Prussian blue NPs | The HMTBF nanocomplex promotes ferroptosis/apoptosis synergism. During the intracellular degradation of this nanocomplex, the Fe3+/Fe2+ conversion mediated by tannic acid (TA) is favored, initiating the Fenton reaction and increasing the level of ROS, subsequent lipid peroxidation and, therefore, causing ferroptotic cell death. Furthermore, the degradation of HMTBF allows the release of the compound ML210, which inhibits the activity of GPx4 to activate the ferroptosis pathway. | [172] | |
| DBCO-RSL3-DHA: Dibenzocyclooctyne-modified disulfide-bridged nanoassemblies loaded with RSL3 and dihydroartemisinin | DBCO-RSL3-DHA nanoassemblies are loaded with the ferroptosis inducer RSL3 and the ferritinophagy initiator dihydroartemisinin (DHA). DHA induces ferritinophagy to release iron in the form of Fe2+. The cellular abundance of Fe2+ is the driving force of lipid peroxidation, together with the inhibition of GPx4 caused by RSL3, which triggers iron-dependent cell death (ferroptosis). | [173] | |
| Cu-TCPP(Fe): Metal-organic framework (MOF) incorporated with gold NPs and RSL3 | Cu2+ ions immobilized on Cu-TCPP(Fe) nanosheets rapidly oxidize GSH to GSSG, potentially depleting GPx4 cofactors to inactivate its antiferroptotic functions. Furthermore, the nanosheet system can release the attached RSL3, which binds to the catalytic selenocysteine residue of GPx4. Overall, the multienzyme reactivity can simultaneously inhibit the GPx4 and ferroptosis suppressor protein 1 (FSP1) pathways that catalyze the recycling of coenzyme Q10 to ubiquinol, both ferroptosis-suppressing mechanisms. | [174] | |
| (RSL3@COF-Fc): Ferrocene-functionalized covalent organic framework loaded with RSL3 | The RSL3@COF-Fc nanodrug carries a GPx4 inhibitor and RSL3. This nanodrug promotes in situ reactions similar to the Fenton reaction, triggering the production of hydroxyl radicals (·OH) by increasing the level of ROS in cells and irreversible covalent inhibition of GPx4, resulting in massive lipid peroxide accumulation, cellular damage, and ultimately, ferroptosis. | [175] | |
| Mn12-heparin: Manganese cluster NPs (Mn12) encapsulated with heparin | Manganese (Mn12) NPs encapsulated with heparin (Mn12-heparin) are a chemodynamic therapeutic agent that mediates the increase in ROS levels since manganese reacts with H2O2 to generate ·OH through a pathway similar to the reaction of Fenton. Increased ROS and depletion of endogenous GSH indirectly leads to GPx4 inhibition, consequently increasing the level of lipid peroxidation to cause ferroptosis. | [176] | |
| PFP@Fe/Cu-SS MOF: Phloroglucinol, iron (Fe3+), and copper (Cu2+) are the corresponding coordination metals. Perfluoropentane (PFP) was loaded into MOF | The high concentration of GSH present in tumor cells will accelerate the breakdown of the PFP@Fe/Cu-SS MOF nanocarrier structure, producing the release of Fe2+/Cu2+ ions that react with H2O2, producing ·OH through the Fenton reaction, causing the depletion of GSH levels and inhibition of GPx4. In turn, this causes the accumulation of intracellular lipid peroxides to eventually induce ferroptosis. | [177] | |
| GR | HSpyN: pyrimidine-2 thiol | Phosphine-gold(I) thiolate complexes are promising anticancer agents for antiproliferative activities in vitro and in vivo. The ability of HSpyN to inhibit the proliferation of human BC cells, MCF-7, was evaluated by measuring cell death through the induction of apoptosis. In addition, this compound is a potent inhibitor of GR. | [178] |
| Prx1 | Auf-Asc-Men: Auronofin (Auf) treatment in combination with ascorbate (Asc) and menadione (Men) | Prx1 can protect TNBC cells from the effects of prooxidant compounds, while Asc and Men treatment increases H2O2 levels. When Auf is added, Prx1 is inhibited, so the effects of H2O2 cause rapid toxicity, irreversible cell damage, and as a consequence, cell death instead of adaptation or survival. | [179] |
| TrxR | Auf-MI-463: Auronofin plus MI-463 | Menin-MLL inhibitors have been shown to be effective against BC. MI-463 unexpectedly induced ferroptotic cell death. In addition, heme oxygenase-1 (HO-1) was inhibited, which increased the effect of MI-463 plus Auf. | [180] |
| Auf-anti-PD-L1: Auronofin plus anti-PD-L1 antibody | Auf treatment acts as a TrxR inhibitor, causing specific cell death and affects cell growth. Auf increased tumor infiltration of CD8 + Ve T cells in vivo and amplified the expression of the immune checkpoint PD-L1 in an ERK1/2-MYC-dependent manner. Furthermore, the combination of Auf with anti-PD-L1 antibody synergistically impaired the growth of 4T1.2 TNBC primary tumors. | [64] | |
| Auf-Vitamin C: Auronofin plus vitamin C | Auf simultaneously targeted the antioxidant systems thioredoxin and glutathione, causing cell death. AUF/VC combinations exerted synergistic H2O2-mediated cytotoxicity on BC cell lines. | [181] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilchis-Landeros, M.M.; Vázquez-Meza, H.; Vázquez-Carrada, M.; Uribe-Ramírez, D.; Matuz-Mares, D. Antioxidant Enzymes and Their Potential Use in Breast Cancer Treatment. Int. J. Mol. Sci. 2024, 25, 5675. https://doi.org/10.3390/ijms25115675
Vilchis-Landeros MM, Vázquez-Meza H, Vázquez-Carrada M, Uribe-Ramírez D, Matuz-Mares D. Antioxidant Enzymes and Their Potential Use in Breast Cancer Treatment. International Journal of Molecular Sciences. 2024; 25(11):5675. https://doi.org/10.3390/ijms25115675
Chicago/Turabian StyleVilchis-Landeros, María Magdalena, Héctor Vázquez-Meza, Melissa Vázquez-Carrada, Daniel Uribe-Ramírez, and Deyamira Matuz-Mares. 2024. "Antioxidant Enzymes and Their Potential Use in Breast Cancer Treatment" International Journal of Molecular Sciences 25, no. 11: 5675. https://doi.org/10.3390/ijms25115675
APA StyleVilchis-Landeros, M. M., Vázquez-Meza, H., Vázquez-Carrada, M., Uribe-Ramírez, D., & Matuz-Mares, D. (2024). Antioxidant Enzymes and Their Potential Use in Breast Cancer Treatment. International Journal of Molecular Sciences, 25(11), 5675. https://doi.org/10.3390/ijms25115675








