Royal Jelly: Biological Action and Health Benefits
Abstract
1. Introduction
Royal Jelly as Functional Food
2. Chemical Composition of Royal Jelly
3. Royal Jelly as Nutrient for Queen and Larvae
4. RJ Values in Human Nutrition and as a Nutraceutical
5. Biological Function of Royal Jelly
5.1. Antioxidant and Anti-Inflammatory Activity of RJ
5.2. Royal Jelly, Beauty, and Postponement of Ageing
5.3. Royal Jelly and Its Effect on Brain Cells
5.4. Royal Jelly and Diabetes
5.5. Positive Effect of RJ on Overweight and Obesity
5.6. Effectiveness of RJ in Reducing Blood Pressure and Protection of Vascular System and Heart
5.7. Estrogen Effect of Royal Jelly
5.8. Effect of Royal Jelly on Spematogenesis
5.9. Royal Jelly and Osteoporosis
5.10. Anticancer Effectiveness of Royal Jelly and Hematopoiesis Stimulation
5.11. RJ as Protective Agent against Pro-Oxidants’ Toxicity and Chemo- and Radiotherapy Side Effects
5.12. Antimicrobial Activity of Royal Jelly
5.13. Wound-Healing Activity of Royal Jelly
5.14. Antiallergic Effect of Royal Jelly
5.15. Allergic Reactions as Side Effects of RJ Application
6. Scientific Claims about Effectiveness of RJ to Human Health According to PASSCLAIM Classification
- (1)
- Diet and cardiovascular diseases,
- (2)
- Bone health and osteoporosis,
- (3)
- Physical strength and fitness,
- (4)
- The regulation of body mass, insulin sensitivity, and risk of diabetes,
- (5)
- Diet and cancer,
- (6)
- Mental health and effectiveness,
- (7)
- Bowel health, digestion, and immunity.
7. Need for Standardization of Important Biologically Active Compounds and Determination of Validity and Quality of Products with RJ
8. Closing Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Tsopmo, A.; Hosseinian, F. Antioxidants in functional foods. J. Food. Biochem. 2022, 46, e14167. [Google Scholar] [CrossRef]
- Popescu, O.; Mǎrghitaş, L.A.; Dezmirean, D.S. A study about physicochemical composition of fresh and lyophilized royal jelly. Zooteh. Si Biotehnol. 2008, 41, 328–332. [Google Scholar]
- Collazo, N.; Carpena, M.; Nuñez-Estevez, B.; Otero, P.; Simal-Gandara, J.; Prieto, M.A. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients 2021, 13, 543. [Google Scholar] [CrossRef]
- Oršolić, N.; Jazvinšćak Jembrek, M. Molecular and Cellular Mechanisms of Propolis and Its Polyphenolic Compounds against Cancer. Int. J. Mol. Sci. 2022, 23, 10479. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Campos, M.G.; Fratini, F.; Altaye, S.Z.; Li, J. New Insights into the Biological and Pharmaceutical Properties of Royal Jelly. Int. J. Mol. Sci. 2020, 21, 382. [Google Scholar] [CrossRef]
- Strant, M.; Yücel, B.; Topal, E.; Puscasu, A.M.; Margaoan, R.; Varadi, A. Use of Royal Jelly as Functional Food on Human and Animal Health. Hayvansal Üretim 2019, 60, 131–144. [Google Scholar] [CrossRef]
- Botezan, S.; Baci, G.-M.; Bagameri, L.; Pașca, C.; Dezmirean, D.S. Current Status of the Bioactive Properties of Royal Jelly: A Comprehensive Review with a Focus on Its Anticancer, Anti-Inflammatory, and Antioxidant Effects. Molecules 2023, 28, 1510. [Google Scholar] [CrossRef]
- Xue, X.; Wu, L.; Wang, K. Chemical composition of royal jelly. In Bee Products-Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 181–190. [Google Scholar]
- Buttstedt, A. The role of 10-hydroxy-Δ2-decenoic acid in the formation of fibrils of the major royal jelly protein 1/apisimin/24-methylenecholesterol complex isolated from honey bee (Apis mellifera) royal jelly. Eur. J. Entomol. 2022, 119, 448–453. [Google Scholar] [CrossRef]
- Furusawa, T.; Arai, Y.; Kato, K.; Ichihara, K. Quantitative Analysis of Apisin, a Major Protein Unique to Royal Jelly. Evid. Based Complement. Altern. Med. 2016, 2016, 5040528. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Feng, M.; Ma, C.; Rueppell, O.; Li, J. Major royal jelly proteins influence the neurobiological regulation of the division of labor among honey bee workers. Int. J. Biol. Macromol. 2023, 225, 848–860. [Google Scholar] [CrossRef]
- Ramanathan, A.N.K.G.; Nair, A.J.; Sugunan, V.S. A review on Royal Jelly proteins and peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Bagameri, L.; Botezan, S.; Bobis, O.; Bonta, V.; Dezmirean, D.S. Molecular Insights into Royal Jelly Anti-Inflammatory Properties and Related Diseases. Life 2023, 13, 1573. [Google Scholar] [CrossRef] [PubMed]
- Mureşan, C.I.; Dezmirean, D.S.; Marc, B.D.; Suharoschi, R.; Pop, O.L.; Buttstedt, A. Biological properties and activities of major royal jelly proteins and their derived peptides. J. Funct. Foods 2022, 98, 105286. [Google Scholar] [CrossRef]
- Wang, X.; Dong, J.; Qiao, J.; Zhang, G.; Zhang, H. Purification and characteristics of individual major royal jelly protein 1–3. J. Apic. Res. 2020, 59, 1049–1060. [Google Scholar] [CrossRef]
- Tian, W.; Li, M.; Guo, H.; Peng, W.; Xue, X.; Hu, Y.; Liu, Y.; Zhao, Y.; Fang, X.; Wang, K.; et al. Architecture of the native major royal jelly protein 1 oligomer. Nat. Commun. 2018, 9, 3373. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tao, L.; Yu, X.; Zheng, H.; Wu, J.; Hu, F. Royal Jelly Proteins and Their Derived Peptides: Preparation, Properties, and Biological Activities. J. Agric. Food Chem. 2021, 69, 14415–14427. [Google Scholar] [CrossRef] [PubMed]
- Albert, S.; Klaudiny, J. The MRJP/YELLOW protein family of Apis mellifera: Identification of new members in the EST library. J. Insect Physiol. 2004, 50, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, M.D.; Albert, S.; Kucharski, R.; Prusko, C.; Maleszka, R. Evolution of the Yellow/Major Royal Jelly Protein family and the emergence of social behavior in honey bees. Genome Res. 2006, 16, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.; Coffey, M.; Kavanagh, K. Proteomic analysis of summer and winter Apis mellifera workers shows reduced protein abundance in winter samples. J. Insect Physiol. 2022, 139, 104397. [Google Scholar] [CrossRef]
- Kimura, M.; Kimura, Y.; Tsumura, K.; Okihara, K.; Sugimoto, H.; Yamada, H.; Yonekura, M. 350-kDa royal jelly glycoprotein (apisin), which stimulates proliferation of human monocytes, bears the beta1-3galactosylated N-glycan: Analysis of the N-glycosylation site. Biosci. Biotechnol. Biochem. 2003, 67, 2055–2058. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Zhang, Z.; Pan, Y. Proteomic analysis of royal jelly from three strains of western honeybees (Apis mellifera). J. Agric. Food Chem. 2007, 55, 8411–8422. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F.; Al-Ghamdi, A. Bioactive compounds and health-promoting properties of royal jelly: A review. J. Funct. Foods 2012, 4, 39–52. [Google Scholar] [CrossRef]
- Nazemi-Rafie, J.; Fatehi, F.; Hasrak, S. A comparative transcriptome analysis of the head of 1 and 9 days old worker honeybees (Apis mellifera). Bull. Entomol. Res. 2023, 113, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Andyshe, R.; Nazemi-Rafie, J.; Maleki, M.; Fatehi, F. Comparative proteomics analysis of the head proteins of worker honey bees (Apis mellifera) in production stage of royal jelly. J. Apic. Res. 2022, 1–15. [Google Scholar] [CrossRef]
- Lin, Y.; Shao, Q.; Zhang, M.; Lu, C.; Fleming, J.; Su, S. Royal jelly-derived proteins enhance proliferation and migration of human epidermal keratinocytes in an in vitro scratch wound model. BMC Complement. Altern. Med. 2019, 19, 175. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, M.; Moriyama, T.; Sakaki, T. Changes in hepatic gene expression associated with the hypocholesterolaemic activity of royal jelly. J. Pharm. Pharmacol. 2006, 58, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Mishima, S.; Suzuki, K.M.; Isohama, Y.; Kuratsu, N.; Araki, Y.; Inoue, M.; Miyata, T. Royal jelly has estrogenic effects in vitro and in vivo. J. Ethnopharmacol. 2005, 101, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Kim, B.Y.; Deng, Y.; Park, H.G.; Choi, Y.S.; Lee, K.S.; Jin, B.R. Antioxidant capacity of major royal jelly proteins of honeybee (Apis mellifera) royal jelly. J. Asia. Pac. Entomol. 2020, 23, 445–448. [Google Scholar] [CrossRef]
- Wan, D.C.; Morgan, S.L.; Spencley, A.L.; Mariano, N.; Chang, E.Y.; Shankar, G.; Luo, Y.; Li, T.H.; Huh, D.; Huynh, S.K.; et al. Honey bee Royalactin unlocks conserved pluripotency pathway in mammals. Nat. Commun. 2018, 9, 5078. [Google Scholar] [CrossRef]
- Minegaki, N.; Koshizuka, T.; Nishina, S.; Kondo, H.; Takahashi, K.; Sugiyama, T.; Inoue, N. The carboxyl-terminal penta-peptide repeats of major royal jelly protein 3 enhance cell proliferation. Biol. Pharm. Bull. 2020, 43, 1911–1916. [Google Scholar] [CrossRef]
- Narita, Y.; Ohta, S.; Suzuki, K.M.; Nemoto, T.; Abe, K.; Mishima, S. Effects of long-term administration of royal jelly on pituitary weight and gene expression in middle-aged female rats. Biosci. Biotechnol. Biochem. 2009, 73, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Shinmoto, H.; Kobori, M.; Tsushida, T.; Shinohara, K.; Kanaeda, J.; Yonekura, M. Stimulation of cell growth in the U-937 human myeloid cell line by honey royal jelly protein. Cytotechnology 1998, 26, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, M.; Onda, H.; Sasaki, K.; Yukiyoshi, A.; Tachibana, H.; Yamada, K. Effect of royal jelly on bisphenol A-induced proliferation of human breast cancer cells. BioSci. Biotechnol. Biochem. 2007, 71, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Simúth, J.; Bíliková, K.; Kovácová, E.; Kuzmová, Z.; Schroder, W. Immunochemical approach to detection of adulteration in honey: Physiologically active royal jelly protein stimulating TNF-alpha release is a regular component of honey. J. Agric. Food Chem. 2004, 52, 2154–2158. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal jelly inhibits the production of proinflammatory cytokines by activated macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, I.; Taniguchi, Y.; Kunikata, T.; Kohno, K.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Major royal jelly protein 3 modulates immune responses in vitro and in vivo. Life Sci. 2003, 73, 2029–2045. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Jin, B.R. Antimicrobial activity of the C-terminal of the major royal jelly protein 4 in a honeybee (Apis cerana). J. Asia Pac. Entomol. 2019, 22, 561–564. [Google Scholar] [CrossRef]
- Ferioli, F.; Marcazzan, G.L.; Caboni, M.F. Determination of (E)-10-hydroxy-2-decenoic acid content in pure royal jelly: A comparison between a new CZE method and HPLC. J. Sep. Sci. 2007, 30, 1061–1069. [Google Scholar] [CrossRef]
- Uversky, V.N.; Albar, A.H.; Khan, R.H.; Redwan, E.M. Multifunctionality and intrinsic disorder of royal jelly proteome. Proteomics 2021, 21, e2000237. [Google Scholar] [CrossRef]
- Buttstedt, A.; Mureşan, C.I.; Lilie, H.; Hause, G.; Ihling, C.H.; Schulze, S.H.; Pietzsch, M.; Moritz, R.F.A. How Honeybees Defy Gravity with Royal Jelly to Raise Queens. Curr. Biol. 2018, 28, 1095–1100.e3. [Google Scholar] [CrossRef]
- Kanelis, D.; Tananaki, C.; Liolios, V.; Dimou, M.; Goras, G.; Rodopoulou, M.A.; Karazafiris, E.; Thrasyvoulou, A. A suggestion for royal jelly specifications. Arh. Hig. Rada Toksikol. 2015, 66, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, M. Royalactin induces queen differentiation in honeybees. Nature 2011, 473, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Renard, T.; Gueydan, C.; Aron, S. DNA methylation and expression of the egfr gene are associated with worker size in monomorphic ants. Sci. Rep. 2022, 12, 21228. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Nagao, T.; Sasaki, K. Consumption of tyrosine in royal jelly increases brain levels of dopamine and tyramine and promotes transition from normal to reproductive workers in queenless honey bee colonies. Gen. Comp. Endocrinol. 2015, 211, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pyrzanowska, J.; Piechal, A.; Blecharz-Klin, K.; Joniec-Maciejak, I.; Graikou, K.; Chinou, I.; Widy-Tyszkiewicz, E. Long-term administration of Greek royal jelly improves spatial memory and influences the concentration of brain neurotransmitters in naturally aged Wistar male rats. J. Ethnopharmacol. 2014, 155, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Alhosin, M. Epigenetics Mechanisms of Honeybees: Secrets of Royal Jelly. Epigenet. Insights. 2023, 16, 25168657231213717. [Google Scholar] [CrossRef] [PubMed]
- Bagameri, L.; Baci, G.-M.; Dezmirean, D.S. Royal Jelly as a Nutraceutical Natural Product with a Focus on Its Antibacterial Activity. Pharmaceutics 2022, 14, 1142. [Google Scholar] [CrossRef] [PubMed]
- Mumoki, F.N.; Crewe, R.M. Pheromone communication in honey bees (Apis mellifera). In Insect Pheromone Biochemistry and Molecular Biology, 2nd ed.; Blomquist, G.J., Vogt, R.G., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Cambridge, MA, USA, 2021; pp. 183–204. [Google Scholar] [CrossRef]
- Polsinelli, G.A.; Yu, H.D. Regulation of histone deacetylase 3 by metal cations and 10-hydroxy-2E-decenoic acid: Possible epigenetic mechanisms of queen-worker bee differentiation. PLoS ONE 2018, 13, e0204538. [Google Scholar] [CrossRef] [PubMed]
- Buttstedt, A.; Ihling, C.H.; Pietzsch, M.; Moritz, R.F.A. Royalactin is not a royal making of a queen. Nature 2016, 537, E10–E12. [Google Scholar] [CrossRef]
- Moraru, D.; Alexa, E.; Cocan, I.; Obistioiu, D.; Radulov, I.; Simiz, E.; Berbecea, A.; Grozea, A.; Dragomirescu, M.; Vintila, T.; et al. Chemical Characterization and Antioxidant Activity of Apilarnil, Royal Jelly, and Propolis Collected in Banat Region, Romania. Appl. Sci. 2024, 14, 1242. [Google Scholar] [CrossRef]
- Kunugi, H.; Mohammed Ali, A. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. [Google Scholar] [CrossRef] [PubMed]
- Terker, A.S.; Zhang, C.; McCormick, J.A.; Lazelle, R.A.; Zhang, C.; Meermeier, N.P.; Siler, D.A.; Park, H.J.; Fu, Y.; Cohen, D.M.; et al. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015, 21, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef] [PubMed]
- Cormick, G.; Betran, A.P.; Romero, I.B.; Cormick, M.S.; Belizán, J.M.; Bardach, A.; Ciapponi, A. Effect of Calcium Fortified Foods on Health Outcomes: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 316. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Yu, L.; Gou, W.; Wang, L.; Sun, J.; Li, D.; Lu, Y.; Cai, X.; Yu, H.; Yuan, C.; et al. Health effects of high serum calcium levels: Updated phenome-wide Mendelian randomisation investigation and review of Mendelian randomisation studies. eBioMedicine 2022, 76, 103865. [Google Scholar] [CrossRef] [PubMed]
- Mureşan, C.I.; Schierhorn, A.; Buttstedt, A. The Fate of Major Royal Jelly Proteins during Proteolytic Digestion in the Human Gastrointestinal Tract. J. Agric. Food Chem. 2018, 66, 4164–4170. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Royal Jelly as an Intelligent Anti-Aging Agent—A Focus on Cognitive Aging and Alzheimer’s Disease: A Review. Antioxidants 2020, 9, 937. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Ansarian, A.; Ghanbari, E. New Findings on Biological Actions and Clinical Applications of Royal Jelly: A Review. J. Diet Suppl. 2018, 15, 757–775. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.A.M.; Elenany, Y.E.; Nassrallah, A.; Cheng, W.; Abd El-Maksoud, A.A. Royal Jelly Improves the Physicochemical Properties and Biological Activities of Fermented Milk with Enhanced Probiotic Viability. Lebensm. Wiss. Technol. 2022, 155, 112912. [Google Scholar] [CrossRef]
- Nabas, Z.; Haddadin, M.S.Y.; Haddadin, J.; Nazer, I.K. Chemical composition of royal jelly and effects of synbiotic with two different locally isolated probiotic strains on antioxidant activities. Pol. J. Food Nutr. Sci. 2014, 64, 171–180. [Google Scholar] [CrossRef]
- Kocot, J.; Kiełczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxidative Med. Cell. Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, W.; Zhou, E.; Tao, Y.; Wang, M.; Qi, S.; Zhao, L.; Tan, Y.; Wu, L. Integrated microbiomic and metabolomic analyses reveal the mechanisms by which bee pollen and royal jelly lipid extracts ameliorate colitis in mice. Food Res. Int. 2023, 171, 113069. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Martinello, M.; Mutinelli, F. Antioxidant Activity in Bee Products: A Review. Antioxidants 2021, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Chi, X.; Liu, Z.; Wang, H.; Wang, Y.; Wei, W.; Xu, B. Royal jelly enhanced the antioxidant activities and modulated the gut microbiota in healthy mice. J. Food Biochem. 2021, 45, e13701. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Chou, W.M.; Widowati, D.A.; Lin, I.P.; Peng, C.C. 10-hydroxy-2-decenoic acid of royal jelly exhibits bactericide and anti-inflammatory activity in human colon cancer cells. BMC Complement. Med. Ther. 2018, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Eid, N.; Abd El-Wahed, A.A.; Rateb, M.E.; Afifi, H.S.; Algethami, A.F.; Zhao, C.; Al Naggar, Y.; Alsharif, S.M.; Tahir, H.E.; et al. Honey Bee Products: Preclinical and Clinical Studies of Their Anti-inflammatory and Immunomodulatory Properties. Front. Nutr. 2022, 8, 761267. [Google Scholar] [CrossRef]
- Aslan, Z.; Aksoy, L. Anti-inflammatory effects of royal jelly on ethylene glycol induced renal inflammation in rats. Int. Braz. J. Urol. 2015, 41, 1008–1013. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Jiang, J.; Sun, L.; Lai, C.; Sun, L.; Wu, X. Proteomic characterization of royal jelly proteins in Chinese (Apis cerana) and European (Apis mellifera) honeybees. Biochemistry 2008, 73, 676–680. [Google Scholar] [CrossRef]
- Guo, J.; Ma, B.; Wang, Z.; Chen, Y.; Tian, W.; Dong, Y. Royal Jelly Protected against Dextran-Sulfate-Sodium-Induced Colitis by Improving the Colonic Mucosal Barrier and Gut Microbiota. Nutrients 2022, 14, 2069. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, S.; Ning, W.; Wu, X.; Xu, X.; Liu, Z.; Liu, W.; Liu, K.; Shen, L.; Wang, J. Major royal-jelly proteins intake modulates immune functions and gut microbiota in mice. Food Sci. Hum. Wellness 2024, 13, 444–453. [Google Scholar] [CrossRef]
- Miyauchi-Wakuda, S.; Kagota, S.; Maruyama-Fumoto, K.; Wakuda, H.; Yamada, S.; Shinozuka, K. Effect of Royal Jelly on Mouse Isolated Ileum and Gastrointestinal Motility. J. Med. Food 2019, 22, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Kleerebezem, M.; Brummer, R.-J.; Cani, P.D.; Mercenier, A.; MacDonald, T.T.; Garcia-Rodenas, C.L.; Wells, J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 2017, 117, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, X.; Li, D.; Pan, F.; Fang, X.; Peng, W.; Tian, W. Effects of Major Royal Jelly Proteins on the Immune Response and Gut Microbiota Composition in Cyclophosphamide-Treated Mice. Nutrients 2023, 15, 974. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Curto, E.; Milligan, G. Metabolism Meets Immunity: The Role of Free Fatty Acid Receptors in the Immune System. Biochem. Pharmacol. 2016, 114, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.D.; Pittet, M.J.; Weissleder, R. The Chemical Biology of IL-12 Production: Via the Non-Canonical NFkB Pathway. RSC Chem. Biol. 2020, 1, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-KB) Signaling in Cancer Development and Immune Diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, G.; Okamura, T.; Majima, S.; Senmaru, T.; Okada, H.; Ushigome, E.; Nakanishi, N.; Nishimoto, Y.; Yamada, T.; Okamoto, H.; et al. Effects of Royal Jelly on Gut Dysbiosis and NAFLD in db/db Mice. Nutrients 2023, 15, 2580. [Google Scholar] [CrossRef] [PubMed]
- Mazzoccoli, G.; Vinciguerra, M.; Oben, J.; Tarquini, R.; De Cosmo, S. Non-alcoholic fatty liver disease: The role of nuclear receptors and circadian rhythmicity. Liver Int. 2014, 34, 1133–1152. [Google Scholar] [CrossRef]
- You, M.M.; Liu, Y.C.; Chen, Y.F.; Pan, Y.M.; Miao, Z.N.; Shi, Y.Z.; Si, J.J.; Chen, M.L.; Hu, F.L. Royal jelly attenuates nonalcoholic fatty liver disease by inhibiting oxidative stress and regulating the expression of circadian genes in ovariectomized rats. J. Food Biochem. 2020, 44, e13138. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Meng, X.C.; Zhou, Y.J.; Zhu, J.X.; Chang, Y.N. Major royal jelly proteins alleviate non-alcoholic fatty liver disease in mice model by regulating disordered metabolic pathways. J. Food Biochem. 2022, 46, e14214. [Google Scholar] [CrossRef] [PubMed]
- Felemban, A.H.; Alshammari, G.M.; Yagoub, A.E.A.; Al-Harbi, L.N.; Alhussain, M.H.; Yahya, M.A. Activation of AMPK Entails the Protective Effect of Royal Jelly against High-Fat-Diet-Induced Hyperglycemia, Hyperlipidemia, and Non-Alcoholic Fatty Liver Disease in Rats. Nutrients 2023, 15, 1471. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Yang, D.S.; Wang, J.M.; Li, C.Y.; Lei, K.F.; Chen, X.F.; Shen, N.H.; Jin, L.Q.; Wang, J.G. 10-Hydroxy-2-decenoic acid from Royal jelly: A potential medicine for RA. J. Ethnopharmacol. 2010, 128, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Matsumaru, D.; Shimizu, S.; Hiromori, Y.; Nagase, H.; Nakanishi, T. Evaluation of the Estrogenic Action Potential of Royal Jelly by Genomic Signaling Pathway In Vitro and In Vivo. Biol. Pharm. Bull. 2022, 45, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Hanai, R.; Matsushita, H.; Minami, A.; Abe, Y.; Tachibana, R.; Watanabe, K.; Takeuchi, H.; Wakatsuki, A. Effects of 10-Hydroxy-2-decenoic Acid and 10-Hydroxydecanoic Acid in Royal Jelly on Bone Metabolism in Ovariectomized Rats: A Pilot Study. J. Clin. Med. 2023, 12, 5309. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, W.; Zou, H.; Lin, Y.; Lin, K.; Zhou, Z.; Qiang, J.; Lin, J.; Chuka, C.M.; Ge, R.; et al. 10-Hydroxy-2-decenoic acid inhibiting the proliferation of fibroblast-like synoviocytes by PI3K-AKT pathway. Int. Immunopharmacol. 2015, 28, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Oshvandi, K.; Aghamohammadi, M.; Kazemi, F.; Masoumi, S.Z.; Mazdeh, M.; Molavi Vardanjani, M. Effect of royal jelly capsule on quality of life of patients with multiple sclerosis: A double-blind randomized controlled clinical trial. Iran. Red. Crescent. Med. J. 2020, 22, e74. [Google Scholar] [CrossRef]
- Seyyedi, F.; Kopaei, M.R.; Miraj, S. Comparison between vaginal royal jelly and vaginal estrogen effects on quality of life and vaginal atrophy in postmenopausal women: A clinical trial study. Electron Phys. 2016, 8, 3184–3192. [Google Scholar] [CrossRef] [PubMed]
- Vahid, M.; Fatemeh, D. Royal jelly for genitourinary syndrome of menopause: A randomized controlled trial. Gyn. Obst. Clin. Med. 2021, 1, 211–215. [Google Scholar] [CrossRef]
- Pourmobini, H.; Kazemi Arababadi, M.; Salahshoor, M.R.; Roshankhah, S.; Taghavi, M.M.; Taghipour, Z.; Shabanizadeh, A. The effect of royal jelly and silver nanoparticles on liver and kidney inflammation. Avicenna J. Phytomed. 2021, 11, 218–223. [Google Scholar]
- Salahshoor, M.R.; Jalili, C.; Roshankhah, S. Can royal jelly protect against renal ischemia/reperfusion injury in rats? Chin. J. Physiol. 2019, 62, 131–137. [Google Scholar] [CrossRef]
- Elmallah, M.I.Y.; Elkhadragy, M.F.; Al-Olayan, E.M.; Abdel Moneim, A.E. Protective Effect of Fragaria ananassa Crude Extract on Cadmium-Induced Lipid Peroxidation, Antioxidant Enzymes Suppression, and Apoptosis in Rat Testes. Int. J. Mol. Sci. 2017, 18, 957. [Google Scholar] [CrossRef] [PubMed]
- Almeer, R.S.; Soliman, D.; Kassab, R.B.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Metwally, D.; Abdel Moneim, A.E. Royal Jelly Abrogates Cadmium-Induced Oxidative Challenge in Mouse Testes: Involvement of the Nrf2 Pathway. Int. J. Mol. Sci. 2018, 19, 3979. [Google Scholar] [CrossRef] [PubMed]
- Jenkhetkana, W.; Thitiorulb, S.; Jansomc, C.; Ratanavalachai, T. Genoprotective effects of that royal jelly against doxorubicin in human lymphocytes in vitro. Nat. Prod. Commun. 2018, 13, 79–84. [Google Scholar] [CrossRef]
- Inoue, Y.; Hara, H.; Mitsugi, Y.; Yamaguchi, E.; Kamiya, T.; Itoh, A.; Adachi, T. 4-Hydroperoxy-2-decenoic acid ethyl ester protects against 6-hydroxydopamine-induced cell death via activation of Nrf2-ARE and eIF2α-ATF4 pathways. Neurochem. Int. 2018, 112, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2018, 217, 65–77. [Google Scholar] [CrossRef]
- Pedroza-Diaz, J.; Arroyave-Ospina, J.C.; Serna Salas, S.; Moshage, H. Modulation of Oxidative Stress-Induced Senescence during Non-Alcoholic Fatty Liver Disease. Antioxidants 2022, 11, 975. [Google Scholar] [CrossRef]
- Calder, P.C.; Carr, A.C.; Gombart, A.F.; Eggersdorfer, M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients 2020, 12, 1181. [Google Scholar] [CrossRef]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Bee honey protects astrocytes against oxidative stress: A preliminary in vitro investigation. Neuropsychopharmacol. Rep. 2019, 39, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.X.; Chen, Y.; Chen, D.; Xiao, F.; Parnell, L.D.; Zhao, J.; Liu, L.; Ordovas, J.M.; Lai, C.Q.; Shen, L.R. Supplementation with Major Royal-Jelly Proteins Increases Lifespan, Feeding, and Fecundity in Drosophila. J. Agric. Food Chem. 2016, 64, 5803–5812. [Google Scholar] [CrossRef] [PubMed]
- Detienne, G.; De Haes, W.; Ernst, U.R.; Schoofs, L.; Temmerman, L. Royalactin extends lifespan of Caenorhabditis elegans through epidermal growth factor signaling. Exp. Gerontol. 2014, 60, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cook, L.F.; Grasso, L.M.; Cao, M.; Dong, Y. Royal Jelly-Mediated Prolongevity and Stress Resistance in Caenorhabditis elegans Is Possibly Modulated by the Interplays of DAF-16, SIR-2.1, HCF-1, and 14-3-3 Proteins. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Araki, Y.; Hata, T.; Ichihara, K.; Ito, M.; Tanaka, M.; Honda, S. 10-Hydroxy-2-decenoic Acid, the Major Lipid Component of Royal Jelly, Extends the Lifespan of Caenorhabditis elegans through Dietary Restriction and Target of Rapamycin Signaling. J. Aging Res. 2015, 2015, 425261. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.M.; Kunugi, H. Apitherapy for Age-Related Skeletal Muscle Dysfunction (Sarcopenia): A Review on the Effects of Royal Jelly, Propolis, and Bee Pollen. Foods 2020, 9, 1362. [Google Scholar] [CrossRef] [PubMed]
- Sahin, H. Royal jelly: Healthy aging and longevity. In Bee Products and Their Applications in the Food and Pharmaceutical Industries; Boyacioglu, D., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 245–260. [Google Scholar] [CrossRef]
- Ji, W.Z.; Zhang, C.P.; Wei, W.T.; Hu, F.L. The in vivo antiaging effect of enzymatic hydrolysate from royal jelly in d-galactose induced aging mouse. J. Chin. Inst. Food Sci. Technol. 2016, 16, 18–25. [Google Scholar] [CrossRef]
- Jiang, C.M.; Liu, X.; Li, C.X.; Qian, H.C.; Chen, D.; Lai, C.Q.; Shen, L.R. Anti-senescence effect and molecular mechanism of the major royal jelly proteins on human embryonic lung fibroblast (HFL-I) cell line. J. Zhejiang Univ. Sci. B. 2018, 19, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Olivo, L.A.; Paz-Gonzalez, V. Screening of biological activities present in honeybee (Apis mellifera) royal jelly. Toxicol. Vitr. 2005, 19, 645–651. [Google Scholar] [CrossRef]
- Kamakura, M.; Suenobu, N.; Fukushima, M. Fifty-seven-kDa protein in royal jelly enhances proliferation of primary cultured rat hepatocytes and increases albumin production in the absence of serum. Biochem. Biophys Res. Commun. 2001, 282, 865–874. [Google Scholar] [CrossRef]
- Kawano, Y.; Makino, K.; Jinnin, M.; Sawamura, S.; Shimada, S.; Fukushima, S.; Ihn, H. Royal jelly regulates the proliferation of human dermal microvascular endothelial cells through the down-regulation of a photoaging-related microRNA. Drug Discov. Ther. 2019, 13, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N. Allergic Inflammation: Effect of Propolis and Its Flavonoids. Molecules 2022, 27, 6694. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Hwang, E.; Lee, K.G.; Han, S.M.; Cho, Y.; Kim, S.Y. Royal jelly protects against ultraviolet B-induced photoaging in human skin fibroblasts via enhancing collagen production. J. Med. Food 2011, 14, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Chen, X.; Tian, Y.; Wu, D.; Du, M.; Wang, S. Protection against oxidative stress and anti-aging effect in Drosophila of royal jelly-collagen peptide. Food Chem. Toxicol. 2020, 135, 110881. [Google Scholar] [CrossRef] [PubMed]
- Al-U’datt, D.G.F.; Alu’datt, M.H.; Tranchant, C.C.; Al-Dwairi, A.; Al-Shboul, O.; Almajwal, A.; Elsalem, L.; Jaradat, S.; Alzoubi, K.H.; Faleh, B.G.; et al. Royal jelly mediates fibrotic signaling, collagen cross-linking and cell proliferation in cardiac fibroblasts. Biomed. Pharmacother. 2023, 164, 114922. [Google Scholar] [CrossRef] [PubMed]
- Koya-Miyata, S.; Okamoto, I.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Identification of a collagen production-promoting factor from an extract of royal jelly and its possible mechanism. Biosci. Biotechnol. Biochem. 2004, 68, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Bălan, A.; Moga, M.A.; Dima, L.; Toma, S.; Elena Neculau, A.; Anastasiu, C.V. Royal Jelly—A Traditional and Natural Remedy for Postmenopausal Symptoms and Aging-Related Pathologies. Molecules 2020, 25, 3291. [Google Scholar] [CrossRef] [PubMed]
- Khani-Eshratabadi, M.; Talebpour, A.; Bagherzadeh, A.; Mehranfar, P.; Motallebzadeh Khanmiri, J.; Ghorbani, M.; Abtahi-Eivary, S.H. Potential Anti-Apoptotic Impacts and Telomerase Activity of Royal Jelly on Different Tissues of Rats. Arch. Med. Lab. Sci. 2022, 8, 1–10.e3. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, Q.; Ren, Y.; Guo, C.; Ge, X.; Wang, L.; Cheng, Q.; Luo, P.; Zhang, Y.; Han, X. Immunosenescence: Molecular mechanisms and diseases. Sig. Transduct. Target. Ther. 2023, 8, 200. [Google Scholar] [CrossRef]
- Bouamama, S.; Merzouk, H.; Latrech, H.; Charif, N.; Bouamama, A. Royal jelly alleviates the detrimental effects of aging on immune functions by enhancing the in vitro cellular proliferation, cytokines, and nitric oxide release in aged human PBMCS. J. Food Biochem. 2021, 45, e13619. [Google Scholar] [CrossRef]
- Natarajan, O.; Angeloni, J.T.; Bilodeau, M.F.; Russi, K.E.; Dong, Y.; Cao, M. The Immunomodulatory Effects of Royal Jelly on Defending against Bacterial Infections in the Caenorhabditis elegans Model. J. Med. Food 2021, 24, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Rojasawasthien, T.; Takeuchi, S.Y.; Okamoto, H.; Okumura, N.; Shirakawa, T.; Matsubara, T.; Kawamoto, T.; Kokabu, S. Royal Jelly Enhances the Ability of Myoblast C2C12 Cells to Differentiate into Multilineage Cells. Molecules 2024, 29, 1449. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Koya-Miyata, S.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal Jelly prolongs the life span of C3H/HeJ mice: Correlation with reduced DNA damage. Exp. Gerontol. 2003, 38, 965–969. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Nemrava, J.; Jeleč, Ž.; Kukolj, M.; Odeh, D.; Jakopović, B.; Jazvinšćak Jembrek, M.; Bagatin, T.; Fureš, R.; Bagatin, D. Antioxidative and Anti-Inflammatory Activities of Chrysin and Naringenin in a Drug-Induced Bone Loss Model in Rats. Int. J. Mol. Sci. 2022, 23, 2872. [Google Scholar] [CrossRef] [PubMed]
- Jazvinšćak Jembrek, M.; Oršolić, N.; Karlović, D.; Peitl, V. Flavonols in Action: Targeting Oxidative Stress and Neuroinflammation in Major Depressive Disorder. Int. J. Mol. Sci. 2023, 24, 6888. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Romanatto, T.; Gomes de Assis, C.; Buis, A.; Kowaltowski, A.J.; Aguilaniu, H.; Marques da Cunha, F. Lifespan-extending interventions enhance lipid-supported mitochondrial respiration in Caenorhabditis elegans. FASEB J. 2020, 34, 9972–9981. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, G.; Öz Bağcı, F. Effects of royal jelly on the antisenescence, mitochondrial viability and osteogenic differentiation capacity of umbilical cord-derived mesenchymal stem cells. Histochem. Cell Biol. 2023, 161, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee Products in Dermatology and Skin Care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef] [PubMed]
- Haston, S.; Gonzalez-Gualda, E.; Morsli, S.; Ge, J.; Reen, V.; Calderwood, A.; Moutsopoulos, I.; Panousopoulos, L.; Deletic, P.; Carreno, G.; et al. Clearance of senescent macrophages ameliorates tumorigenesis in KRAS-driven lung cancer. Cancer Cell 2023, 41, 1242–1260.e6. [Google Scholar] [CrossRef]
- Zhou, L.; Ruscetti, M. Senescent macrophages: A new “old” player in lung cancer development. Cancer Cell 2023, 41, 1201–1203. [Google Scholar] [CrossRef]
- Reynolds, L.E.; Maallin, S.; Haston, S.; Martinez-Barbera, J.P.; Hodivala-Dilke, K.M.; Pedrosa, A.R. Effects of senescence on the tumour microenvironment and response to therapy. FEBS J. 2023; ahead of print. [Google Scholar] [CrossRef]
- Li, J.; Sun, M.; Cui, X.; Li, C. Protective Effects of Flavonoids against Alzheimer’s Disease: Pathological Hypothesis, Potential Targets, and Structure-Activity Relationship. Int. J. Mol. Sci. 2022, 23, 10020. [Google Scholar] [CrossRef]
- Zubčić, K.; Hof, P.R.; Šimić, G.; Jazvinšćak Jembrek, M. The Role of Copper in Tau-Related Pathology in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 572308. [Google Scholar] [CrossRef] [PubMed]
- Charlton, T.; Prowse, N.; McFee, A.; Heiratifar, N.; Fortin, T.; Paquette, C.; Hayley, S. Brain-derived neurotrophic factor (BDNF) has direct anti-inflammatory effects on microglia. Front. Cell. Neurosci. 2023, 7, 1188672. [Google Scholar] [CrossRef]
- Raoufi, S.; Salavati, Z.; Komaki, A.; Shahidi, S.; Zarei, M. Royal jelly improves learning and memory deficits in an amyloid β-induced model of Alzheimer’s disease in male rats: Involvement of oxidative stress. Metab. Brain Dis. 2023, 38, 1239–1248. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Sun, P.; Fan, Z.; Zhang, W.; Feng, C. Royal jelly peptides: Potential inhibitors of β-secretase in N2a/APP695swe cells. Sci. Rep. 2019, 9, 168. [Google Scholar] [CrossRef] [PubMed]
- Nisa, N.; Rasmita, B.; Arati, C.; Uditraj, C.; Siddhartha, R.; Dinata, R.; Bhanushree, B.; Bidanchi, R.M.; Manikandan, B.; Laskar, S.A.; et al. Repurposing of phyto-ligand molecules from the honey bee products for Alzheimer’s disease as novel inhibitors of BACE-1: Small molecule bioinformatics strategies as amyloid-based therapy. Environ. Sci. Pollut. Res. Int. 2023, 30, 51143–51169. [Google Scholar] [CrossRef]
- Gong, Y.; Luo, H.; Li, Z.; Feng, Y.; Liu, Z.; Chang, J. Metabolic Profile of Alzheimer’s Disease: Is 10-Hydroxy-2-decenoic Acid a Pertinent Metabolic Adjuster? Metabolites 2023, 13, 954. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Liu, J.-C.; Sun, J.-A.; Mao, X.-Z. Tyrosinase Inhibitory Peptides from Enzyme Hydrolyzed Royal Jelly: Production, Separation, Identification and Docking Analysis. Foods 2023, 12, 2240. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, J.; Jin, P.; Yang, Q.; Zhu, K.; You, M.; Hu, F.; Chen, M. Royal Jelly Ameliorates Behavioral Deficits, Cholinergic System Deficiency, and Autonomic Nervous Dysfunction in Ovariectomized Cholesterol-Fed Rabbits. Molecules 2019, 24, 1149. [Google Scholar] [CrossRef]
- Sirinupong, N.; Chansuwan, W.; Kaewkaen, P. Hydrolase-Treated Royal Jelly Attenuates H2O2- and Glutamate-Induced SH-SY5Y Cell Damage and Promotes Cognitive Enhancement in a Rat Model of Vascular Dementia. Int. J. Food Sci. 2021, 2021, 2213814. [Google Scholar] [CrossRef]
- You, M.; Pan, Y.; Liu, Y.; Chen, Y.; Wu, Y.; Si, J.; Wang, K.; Hu, F. Royal Jelly Alleviates Cognitive Deficits and β-Amyloid Accumulation in APP/PS1 Mouse Model Via Activation of the cAMP/PKA/CREB/BDNF Pathway and Inhibition of Neuronal Apoptosis. Front. Aging Neurosci. 2019, 10, 428. [Google Scholar] [CrossRef]
- You, M.; Miao, Z.; Sienkiewicz, O.; Jiang, X.; Zhao, X.; Hu, F. 10-Hydroxydecanoic acid inhibits LPS-induced inflammation by targeting p53 in microglial cells. Int. Immunopharmacol. 2020, 84, 106501. [Google Scholar] [CrossRef]
- Ali, A.M.; Hendawy, A.O. Royal jelly acid, 10-hydroxy-trans-2-decenoic acid, for psychiatric and neurological disorders: How helpful could it be?! Edelweiss Food Sci. Technol. 2019, 1, 1–4. [Google Scholar] [CrossRef]
- Wang, X.; Cao, M.; Dong, Y. Royal jelly promotes DAF-16-mediated proteostasis to tolerate β-amyloid toxicity in C. elegans model of Alzheimer’s disease. Oncotarget 2016, 7, 54183–54193. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Wang, K.; Pan, Y.; Tao, L.; Ma, Q.; Zhang, G.; Hu, F. Combined royal jelly 10-hydroxydecanoic acid and aspirin has a synergistic effect against memory deficit and neuroinflammation. Food Funct. 2022, 13, 2336–2353. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xu, J.; Chen, C.; Chen, F.; Jin, P.; Zhu, K.; Hu, C.W.; You, M.; Chen, M.; Hu, F. Royal Jelly Reduces Cholesterol Levels, Ameliorates Aβ Pathology and Enhances Neuronal Metabolic Activities in a Rabbit Model of Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 50. [Google Scholar] [CrossRef]
- de Souza E Silva, T.G.; do Val de Paulo, M.E.F.; da Silva, J.R.M.; da Silva Alves, A.; Britto, L.R.G.; Xavier, G.F.; Lopes Sandoval, M.R. Oral treatment with royal jelly improves memory and presents neuroprotective effects on icv-STZ rat model of sporadic Alzheimer’s disease. Heliyon 2020, 6, e03281. [Google Scholar] [CrossRef]
- Karimi, E.; Arab, A.; Sepidarkish, M.; Khorvash, F.; Saadatnia, M.; Amani, R. Effects of the royal jelly consumption on post-stroke complications in patients with ischemic stroke: Results of a randomized controlled trial. Front. Nutr. 2024, 10, 1227414. [Google Scholar] [CrossRef]
- Hashimoto, M.; Kanda, M.; Ikeno, K.; Hayashi, Y.; Nakamura, T.; Ogawa, Y.; Fukumitsu, H.; Nomoto, H.; Furukawa, S. Oral administration of royal jelly facilitates mRNA expression of glial cell line-derived neurotrophic factor and neurofilament H in the hippocampus of the adult mouse brain. Biosci. Biotechnol. Biochem. 2005, 69, 800–805. [Google Scholar] [CrossRef]
- Hattori, N.; Ohta, S.; Sakamoto, T.; Mishima, S.; Furukawa, S. Royal jelly facilitates restoration of the cognitive ability in trimethyltin-intoxicated mice. Evid. Based Complement. Alternat. Med. 2011, 2011, 165968. [Google Scholar] [CrossRef]
- You, M.M.; Chen, Y.F.; Pan, Y.M.; Liu, Y.C.; Tu, J.; Wang, K.; Hu, F.L. Royal jelly attenuates LPS-induced inflammation in BV-2 microglial cells through modulating NF-kappaB and p38/JNK signaling pathways. Mediat. Inflamm. 2018, 2018, 7834381. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Nomoto, H.; Fukumitsu, H.; Mishima, S.; Furukawa, S. Royal jelly and its unique fatty acid, 10-hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed. Res. 2007, 28, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Nomoto, H.; Fukumitsu, H.; Mishima, S.; Furukawa, S. AMP N(1)-oxide, a unique compound of royal jelly, induces neurite outgrowth from PC12 cells via signaling by protein kinase A independent of that by mitogen-activated protein kinase. Evid. Based Complement. Alternat. Med. 2011, 7, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Aslan, A.; Beyaz, S.; Gok, O.; Parlak, G.; Can, M.I.; Agca, C.A.; Ozercan, I.H.; Parlak, A.E. Royal jelly protects brain tissue against fluoride-induced damage by activating Bcl-2/NF-κB/caspase-3/caspase-6/Bax and Erk signaling pathways in rats. Environ. Sci. Pollut. Res. Int. 2023, 30, 49014–49025. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Liu, F.; Wan, J.-B.; Lai, C.Q.; Shen, L. Effect of Major Royal Jelly Proteins on Spatial Memory in Aged Rats: Metabolomics Analysis in Urine. J. Agric. Food Chem. 2017, 65, 3151–3159. [Google Scholar] [CrossRef] [PubMed]
- Pourfard, H.; Ahmadi, A.; Habibi, Z.; Asadi-Samani, M.; Shahinfard, N.; Soleimani, A. The Effect of Tang Forte (Royal Jelly) Capsule on Hypoglycemia and Clinical Course in COVID-19 Patients Under Corticosteroid Therapy. J. Evid. Based Integr. Med. 2023, 28, 2515690X231165333. [Google Scholar] [CrossRef] [PubMed]
- Al Nohair, S.F.; Ahmed, S.S.; Ismail, M.S.; El Maadawy, A.A.; Albatanony, M.A.; Rasheed, Z. Potential of honey against the onset of autoimmune diabetes and its associated nephropathy, pancreatitis, and retinopathy in type 1 diabetic animal model. Open Life Sci. 2022, 17, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Maleki, V.; Jafari-Vayghan, H.; Saleh-Ghadimi, S.; Adibian, M.; Kheirouri, S.; Alizadeh, M. Effects of Royal jelly on metabolic variables in diabetes mellitus: A systematic review. Complement. Ther. Med. 2019, 43, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Horvat, A.; Vlašić, I.; Štefulj, J.; Oršolić, N.; Jazvinšćak Jembrek, M. Flavonols as a Potential Pharmacological Intervention for Alleviating Cognitive Decline in Diabetes: Evidence from Preclinical Studies. Life 2023, 13, 2291. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Salama, S.; El-Wahed, A.A.A.; Guo, Z.; Di Minno, A.; Daglia, M.; Li, C.; Guan, X.; Buccato, D.G.; Khalifa, S.A.M.; et al. Exploring the Therapeutic Potential of Royal Jelly in Metabolic Disorders and Gastrointestinal Diseases. Nutrients 2024, 16, 393. [Google Scholar] [CrossRef]
- Ghanbari, E.; Nejati, V.; Khazaei, M. Antioxidant and protective effects of Royal jelly on histopathological changes in testis of diabetic rats. Int. J. Reprod. Biomed. 2016, 14, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Maruo, N.; Zamami, Y.; Takatori, S.; Doi, S.; Kawasaki, H. Effect of long-term treatment with royal jelly on insulin resistance in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. Yakugaku Zasshi 2007, 127, 1877–1882. [Google Scholar] [CrossRef] [PubMed]
- Zamami, Y.; Takatori, S.; Goda, M.; Koyama, T.; Iwatani, Y.; Jin, X.; Takai-Doi, S.; Kawasaki, H. Royal jelly ameliorates insulin resistance in fructose-drinking rats. Biol. Pharm. Bull. 2008, 31, 2103–2107. [Google Scholar] [CrossRef] [PubMed]
- Omer, K.; Gelkopf, M.J.; Newton, G. Effectiveness of royal jelly supplementation in glycemic regulation: A systematic review. World J. Diabetes 2019, 10, 96–113. [Google Scholar] [CrossRef] [PubMed]
- Gohari, I.; Khajehlandi, A.; Mohammadi, A. Effect of High-intensity Interval Training with Royal Jelly Consumption on Serum Levels of Glucose, Insulin, and Insulin Resistance Index of Overweight and Obese Middle-aged Men: A Quasi-experimental Study. Jundishapur J. Chronic Dis. Care 2022, 11, e123363. [Google Scholar] [CrossRef]
- Münstedt, K.; Bargello, M.; Hauenschild, A. Royal jelly reduces the serum glucose levels in healthy subjects. J. Med. Food 2009, 12, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Khoshpey, B.; Djazayeri, S.; Amiri, F.; Malek, M.; Hosseini, A.F.; Hosseini, S.; Shidfar, S.; Shidfar, F. Effect of Royal Jelly Intake on Serum Glucose, Apolipoprotein A-I (ApoA-I), Apolipoprotein B (ApoB) and ApoB/ApoA-I Ratios in Patients with Type 2 Diabetes: A Randomized, Double-Blind Clinical Trial Study. Can. J. Diabetes 2016, 40, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Nohair, S.F.A. Antidiabetic efficacy of a honey-royal jelly mixture: Biochemical study in rats. Int. J. Health Sci. 2021, 15, 4–9. [Google Scholar]
- Pourmoradian, S.; Mahdavi, R.; Mobasseri, M.; Faramarzi, E.; Mobasseri, M. Effects of royal jelly supplementation on glycemic control and oxidative stress factors in type 2 diabetic female: A randomized clinical trial. Chin. J. Integr. Med. 2014, 20, 347–352. [Google Scholar] [CrossRef]
- Shidfar, F.; Jazayeri, S.; Mousavi, S.N.; Malek, M.; Hosseini, A.F.; Khoshpey, B. Does Supplementation with Royal Jelly Improve Oxidative Stress and Insulin Resistance in Type 2 Diabetic Patients? Iran. J. Public Health 2015, 44, 797–803. [Google Scholar]
- Siavash, M.; Shokri, S.; Haghighi, S.; Shahtalebi, M.A.; Farajzadehgan, Z. The efficacy of topical royal jelly on healing of diabetic foot ulcers: A double-blind placebo-controlled clinical trial. Int. Wound J. 2015, 12, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Yakoot, M.; Abdelatif, M.; Helmy, S. Efficacy of a new local limb salvage treatment for limb-threatening diabetic foot wounds—A randomized controlled study. Diabetes Metab. Syndr. Obes. 2019, 12, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Kagota, S.; Maruyama, K.; Oonishi, Y.; Miyauchi-Wakuda, S.; Ito, Y.; Yamada, S.; Shinozuka, K. Royal jelly increases peripheral circulation by inducing vasorelaxation through nitric oxide production under healthy conditions. Biomed. Pharmacother. 2018, 106, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Sirovina, D.; Odeh, D.; Gajski, G.; Balta, V.; Šver, L.; Jazvinšćak Jembrek, M. Efficacy of Caffeic Acid on Diabetes and Its Complications in the Mouse. Molecules 2021, 26, 3262. [Google Scholar] [CrossRef] [PubMed]
- Ilić, I.; Oršolić, N.; Rođak, E.; Odeh, D.; Lovrić, M.; Mujkić, R.; Delaš Aždajić, M.; Grgić, A.; Tolušić Levak, M.; Vargek, M.; et al. The effect of high-fat diet and 13-cis retinoic acid application on lipid profile, glycemic response and oxidative stress in female Lewis rats. PLoS ONE 2020, 15, e0238600. [Google Scholar] [CrossRef] [PubMed]
- Vajdi, M.; Musazadeh, V.; Khajeh, M.; Safaei, E.; Darzi, M.; Noshadi, N.; Bazyar, H.; Askari, G. The effects of royal jelly supplementation on anthropometric indices: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Front. Nutr. 2023, 10, 1196258. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Kaede, R.; Nagaya, K.; Aoyama, J.; Saito, M.; Okamatsu-Ogura, Y.; Kimura, K.; Terao, A. Royal jelly ameliorates diet-induced obesity and glucose intolerance by promoting brown adipose tissue thermogenesis in mice. Obes. Res. Clin. Pract. 2016, 12 (Suppl. S2), 127–137. [Google Scholar] [CrossRef] [PubMed]
- Mesri Alamdari, N.; Irandoost, P.; Roshanravan, N.; Vafa, M.; Asghari Jafarabadi, M.; Alipour, S.; Roshangar, L.; Alivand, M.; Farsi, F.; Shidfar, F. Effects of Royal Jelly and Tocotrienol Rich Fraction in obesity treatment of calorie-restricted obese rats: A focus on white fat browning properties and thermogenic capacity. Nutr. Metab. 2020, 17, 42. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Hayashi, K.; Watadani, R.; Okano, Y.; Tanimura, K.; Kotoh, J.; Sasaki, D.; Matsumoto, K.; Maeda, A. Royal jelly improves hyperglycemia in obese/diabetic KK-Ay mice. J. Vet. Med. Sci. 2017, 79, 299–307. [Google Scholar] [CrossRef]
- Watadani, R.; Kotoh, J.; Sasaki, D.; Someya, A.; Matsumoto, K.; Maeda, A. Hydroxy-2-decenoic acid, a natural product, improves hyperglycemia and insulin resistance in obese/diabetic KK-Ay mice, but does not prevent obesity. J. Vet. Med. Sci. 2017, 79, 1596–1602. [Google Scholar] [CrossRef]
- Çakir, S. The Effect of Royal Jelly on Irisin in Experimentally Diabetic Rats. SAUJS 2023, 27, 912–919. [Google Scholar] [CrossRef]
- Irandoost, P.; Mesri Alamdari, N.; Saidpour, A.; Shidfar, F.; Farsi, F.; Asghari Jafarabadi, M.; Alivand, M.R.; Vafa, M. The effect of royal jelly and tocotrienol-rich fraction along with calorie restriction on hypothalamic endoplasmic reticulum stress and adipose tissue inflammation in diet-induced obese rats. BMC Res. Notes 2020, 13, 409. [Google Scholar] [CrossRef] [PubMed]
- Kashima, Y.; Kanematsu, S.; Asai, S.; Kusada, M.; Watanabe, S.; Kawashima, T.; Nakamura, T.; Shimada, M.; Goto, T.; Nagaoka, S. Identification of a novel hypocholesterolemic protein, major royal jelly protein 1, derived from royal jelly. PLoS ONE 2014, 9, e105073. [Google Scholar] [CrossRef] [PubMed]
- Petelin, A.; Kenig, S.; Kopinč, R.; Deželak, M.; Černelič Bizjak, M.; Jenko Pražnikar, Z. Effects of Royal Jelly Administration on Lipid Profile, Satiety, Inflammation, and Antioxidant Capacity in Asymptomatic Overweight Adults. Evid. Based Complement. Alternat. Med. 2019, 2019, 4969720. [Google Scholar] [CrossRef] [PubMed]
- Pandeya, P.R.; Lamichhane, R.; Lee, K.H.; Kim, S.G.; Lee, D.H.; Lee, H.K.; Jung, H.J. Bioassay-guided isolation of active anti-adipogenic compound from royal jelly and the study of possible mechanisms. BMC Complement. Altern. Med. 2019, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Tahir, R.A.; Bashir, A.; Yousaf, M.N.; Ahmed, A.; Dali, Y.; Khan, S.; Sehgal, S.A. In Silico identification of angiotensin-converting enzyme inhibitory peptides from MRJP1. PLoS ONE 2020, 15, e0228265. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Yukiyoshi, A.; Doi, S.; Sugimoto, H.; Yamada, H.; Matsumoto, K. Gastrointestinal enzyme production of bioactive peptides from royal jelly protein and their antihypertensive ability in SHR. J. Nutr. Biochem. 2002, 13, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, K.H.; Yoshida, C.; Suzuki, K.M.; Maruyama, H.; Futamura, Y.; Araki, Y.; Mishima, S. Antihypertensive effect of peptides from royal jelly in spontaneously hypertensive rats. Biol. Pharm. Bull. 2004, 27, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Ikeda, T.; Kajita, K.; Fujioka, K.; Mori, I.; Okada, H.; Uno, Y.; Ishizuka, T. Effect of royal jelly ingestion for six months on healthy volunteers. Nutr. J. 2012, 11, 77. [Google Scholar] [CrossRef]
- Guo, H.; Saiga, A.; Sato, M.; Miyazawa, I.; Shibata, M.; Takahata, Y.; Morimatsu, F. Royal jelly supplementation improves lipoprotein metabolism in humans. J. Nutr. Sci. Vitaminol. 2007, 53, 345–348. [Google Scholar] [CrossRef]
- Vittek, J. Effect of royal jelly on serum lipids in experimental animals and humans with atherosclerosis. Experientia 1995, 51, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Takaki-Doi, S.; Hashimoto, K.; Yamamura, M.; Kamei, C. Antihypertensive activities of royal jelly protein hydrolysate and its fractions in spontaneously hypertensive rats. Acta Med. Okayama 2009, 63, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Rong, Y.; You, M.; Ma, Q.; Chen, M.; Hu, F. Royal jelly causes hypotension and vasodilation induced by increasing nitric oxide production. Food Sci. Nutr. 2019, 7, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Akhmedov, A.; Chen, C.H. Spotlight on very-low-density lipoprotein as a driver of cardiometabolic disorders: Implications for disease progression and mechanistic insights. Front. Cardiovasc. Med. 2022, 9, 993633. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Unuma, H.; Ebina, K. Royal Jelly Proteins Inhibit Macrophage Proliferation: Interactions with Native- and Oxidized-Low Density Lipoprotein. Protein J. 2021, 40, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.-H.; Yang, K.-M.; Sheu, S.-C.; Chen, C.-W. The Bioactive Compound Contents and Potential Protective Effects of Royal Jelly Protein Hydrolysates against DNA Oxidative Damage and LDL Oxidation. Antioxidants 2021, 10, 580. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, Z.; Lu, Y.; Chi, X.; Han, K.; Wang, H.; Wang, Y.; Ma, L.; Xu, B. Glucose metabolism enhancement by 10-hydroxy-2-decenoic acid via the PI3K/AKT signaling pathway in high-fat-diet/streptozotocin induced type 2 diabetic mice. Food Funct. 2022, 13, 9931–9946. [Google Scholar] [CrossRef] [PubMed]
- Lambrinoudaki, I.; Augoulea, A.; Rizos, D.; Politi, M.; Tsoltos, N.; Moros, M.; Chinou, I.; Graikou, K.; Kouskouni, E.; Kambani, S.; et al. Greek-origin royal jelly improves the lipid profile of postmenopausal women. Gynecol. Endocrinol. 2016, 32, 835–839. [Google Scholar] [CrossRef]
- Moutsatsou, P.; Papoutsi, Z.; Kassi, E.; Heldring, N.; Zhao, C.; Tsiapara, A.; Melliou, E.; Chrousos, G.P.; Chinou, I.; Karshikoff, A.; et al. Fatty acids derived from royal jelly are modulators of estrogen receptor functions. PLoS ONE 2010, 5, e15594. [Google Scholar] [CrossRef]
- Oršolić, N.; Nemrava, J.; Jeleč, Ž.; Kukolj, M.; Odeh, D.; Terzić, S.; Fureš, R.; Bagatin, T.; Bagatin, D. The Beneficial Effect of Proanthocyanidins and Icariin on Biochemical Markers of Bone Turnover in Rats. Int. J. Mol. Sci. 2018, 19, 2746. [Google Scholar] [CrossRef]
- Siddiqui, S.; Mahdi, A.A.; Arshad, M. Genistein contributes to cell cycle progression and regulates oxidative stress in primary culture of osteoblasts along with osteoclasts attenuation. BMC Complement. Med. Ther. 2020, 20, 277. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Hayashi, M.; Nagamatsu, K.; Ono, T.; Kamakura, M.; Iwata, T.; Nakashima, T. The key royal jelly component 10-hydroxy-2-decenoic acid protects against bone loss by inhibiting NF-κB signaling downstream of FFAR4. J. Biol. Chem. 2020, 295, 12224–12232. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.M.; Isohama, Y.; Maruyama, H.; Yamada, Y.; Narita, Y.; Ohta, S.; Araki, Y.; Miyata, T.; Mishima, S. Estrogenic activities of fatty acids and a sterol isolated from royal jelly. Evid. Complement. Alternat. Med. 2008, 5, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Husein, M.Q.; Kridli, R.T. Reproductive responses following royal jelly treatment administered orally or intramuscularly into progesterone-treated Awassi ewes. Anim. Reprod. Sci. 2002, 74, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Husein, M.Q.; Haddad, S.G. A new approach to enhance reproductive performance in sheep using royal jelly in comparison with equine chorionic gonadotropin. Anim. Reprod. Sci. 2006, 93, 24–33. [Google Scholar] [CrossRef] [PubMed]
- El-Tarabany, M.S.; Nassan, M.A.; Salah, A.S. Royal Jelly Improves the Morphology of the Reproductive Tract, Internal Egg Quality, and Blood Biochemical Parameters in Laying Hens at the Late Stage of Production. Animals 2021, 11, 1861. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.N.; Darsareh, F. Effect of royal jelly on menopausal symptoms: A randomized placebo-controlled clinical trial. Complement. Ther. Clin. Pract. 2019, 37, 47–50. [Google Scholar] [CrossRef]
- Ab Hamid, N.; Abu Bakar, A.B.; Mat Zain, A.A.; Nik Hussain, N.H.; Othman, Z.A.; Zakaria, Z.; Mohamed, M. Composition of Royal Jelly (RJ) and Its Anti-Androgenic Effect on Reproductive Parameters in a Polycystic Ovarian Syndrome (PCOS) Animal Model. Antioxidants 2020, 9, 499. [Google Scholar] [CrossRef]
- Ghanbari, E.; Khazaei, M.R.; Khazaei, M.; Nejati, V. Royal jelly promotes ovarian follicles growth and increases steroid hormones in immature rats. Int. J. Fertil. Steril. 2018, 11, 263–269. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, C.; Chen, Y.; Shi, F.; Lai, C.; Shen, L. Major royal jelly proteins accelerate onset of puberty and promote ovarian follicular development in immature female mice. Food Sci. Hum. Wellness. 2020, 9, 338–345. [Google Scholar] [CrossRef]
- Shahzad, Q.; Mehmood, M.U.; Khan, H.; ul Husna, A.; Qadeer, S.; Azam, A.; Naseer, Z.; Ahmad, E.; Safdar, M.; Ahmad, M. Royal jelly supplementation in semen extender enhances post-thaw quality and fertility of Nili-Ravi buffalo bull sperm. Anim. Reprod. Sci. 2016, 167, 83–88. [Google Scholar] [CrossRef] [PubMed]
- El-Hanoun, A.M.; Elkomy, A.E.; Fares, W.A.; Shahien, E.H. Impact of royal jelly to improve reproductive performance of male rabbits under hot summer conditions. World Rabbit Sci. 2014, 22, 241–248. [Google Scholar] [CrossRef]
- Al-Nahari, H.; Al Eisa, R. Effect of turmeric (Curcuma longa) on some pituitary, thyroid and testosterone hormone against aluminum chloride (AlCl3) induced toxicity in rat. Adv. Environ. Biol. 2016, 10, 250–261. [Google Scholar]
- El Helew, E.A.; Hamed, W.S.; Moustafa, A.M.; Sakkara, Z.A. Structural changes in testes of Streptozotocin induced diabetic rats and possible protective effect of royal jelly: Light and electron microscopic study. Ultrastruct. Pathol. 2024, 48, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Enayatullah, H.; Lv, Z.; Dai, H.; Wei, Q.; Shen, L.; Karwand, B.; Shi, F. Freeze-Dried Royal Jelly Proteins Enhanced the Testicular Development and Spermatogenesis in Pubescent Male Mice. Animals 2019, 9, 977. [Google Scholar] [CrossRef] [PubMed]
- Kafadar, I.H.; Güney, A.; Türk, C.Y.; Oner, M.; Silici, S. Royal jelly and bee pollen decrease bone loss due to osteoporosis in an oophorectomized rat model. Eklem Hastalik Cerrahisi 2012, 23, 100–105. [Google Scholar] [PubMed]
- Hattori, S.; Omi, N. The effects of royal jelly protein on bone mineral density and strength in ovariectomized female rats. Phys. Act. Nutr. 2021, 25, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Matsushita, H.; Minami, A.; Kanazawa, H.; Suzuki, T.; Watanabe, K.; Wakatsuki, A. Royal jelly does not prevent bone loss but improves bone strength in ovariectomized rats. Climacteric 2018, 21, 601–606. [Google Scholar] [CrossRef]
- Matsushita, H.; Shimizu, S.; Morita, N.; Watanabe, K.; Wakatsuki, A. Effects of royal jelly on bone metabolism in postmenopausal women: A randomized, controlled study. Climacteric 2021, 24, 164–170. [Google Scholar] [CrossRef]
- Münstedt, K.; Männle, H. Apitherapy for menopausal problems. Arch. Gynecol. Obstet. 2020, 302, 1495–1502. [Google Scholar] [CrossRef]
- Aydin, B.; Müge Atar, M.; Pirgon, Ö. The effect of royal jelly supplementation for three months on bone markers in postmenopausal osteoporotic women. Mellifera 2021, 21, 59–68. [Google Scholar]
- Attia, W.; Gabry, M.; Othman, G. Immunostimulatory effect of royal jelly and its relation with host resistance against tumor in mice. Egypt. J. Exp. Biol. 2007, 3, 241–250. [Google Scholar]
- Orsolić, N.; Knezević, A.; Sver, L.; Terzić, S.; Hackenberger, B.K.; Basić, I. Influence of honey bee products on transplantable murine tumors. Vet. Comp. Oncol. 2003, 1, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Sacases, F.; Percie Du Sert, P.; Bašić, I. Antimetastatic ability of honey bee products. Period. Biol. 2007, 109, 173–180. [Google Scholar]
- Oršolić, N.; Terzić, S.; Šver, L.; Bašić, I. Honey-bee products in preventive and/or terapy of murine transplantable tumours. J. Sci. Food Agr. 2005, 85, 363–370. [Google Scholar] [CrossRef]
- Sver, L.; Orsolić, N.; Tadić, Z.; Njari, B.; Valpotić, I.; Basić, I. A royal jelly a new potential immunomodulator in rats and mice. Comp. Immun. Microbiol. Infect. Dis. 1996, 19, 31–38. [Google Scholar] [CrossRef]
- Kimura, Y. Antitumor and antimetastatic actions of various natural products. Stud. Nat. Prod. Chem. 2008, 34, 35–76. [Google Scholar] [CrossRef]
- Vucevic, D.; Melliou, E.; Vasilijic, S.; Gasic, S.; Ivanovski, P.; Chinou, I.; Colic, M. Fatty acids isolated from royal jelly modulate dendritic cell-mediated immune response in vitro. Int. Immunopharmacol. 2007, 7, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.C.; Sun, H.T.; Lin, I.P.; Kuo, P.C.; Li, J.C. The functional property of royal jelly 10-hydroxy-2-decenoic acid as a melanogenesis inhibitor. BMC Complement. Altern. Med. 2017, 17, 392. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.M.; Liu, S.B.; Luo, Y.H.; Xu, W.T.; Zhang, Y.; Zhang, T.; Xue, H.; Zuo, W.B.; Li, Y.N.; Lu, B.X.; et al. 10-HDA Induces ROS-Mediated Apoptosis in A549 Human Lung Cancer Cells by Regulating the MAPK, STAT3, NF-κB, and TGF-β1 Signaling Pathways. Biomed. Res. Int. 2020, 2020, 3042636. [Google Scholar] [CrossRef]
- Kamiya, T.; Watanabe, M.; Hara, H.; Mitsugi, Y.; Yamaguchi, E.; Itoh, A.; Adachi, T. Induction of Human-Lung-Cancer-A549-Cell Apoptosis by 4-Hydroperoxy-2-decenoic Acid Ethyl Ester through Intracellular ROS Accumulation and the Induction of Proapoptotic CHOP Expression. J. Agric. Food Chem. 2018, 66, 10741–10747. [Google Scholar] [CrossRef] [PubMed]
- Shakib Khoob, M.; Hosseini, S.M.; Kazemi, S. In Vitro and In Vivo Antioxidant and Anticancer Potentials of Royal Jelly for Dimethylhydrazine-Induced Colorectal Cancer in Wistar Rats. Oxid. Med. Cell. Longev. 2022, 2022, 9506026. [Google Scholar] [CrossRef] [PubMed]
- Gismondi, A.; Trionfera, E.; Canuti, L.; Di Marco, G.; Canini, A. Royal jelly lipophilic fraction induces antiproliferative effects on SH-SY5Y human neuroblastoma cells. Oncol. Rep. 2017, 38, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Simsek Ozek, N. Exploring the in vitro potential of royal jelly against glioblastoma and neuroblastoma: Impact on cell proliferation, apoptosis, cell cycle, and the biomolecular content. Analyst 2024, 149, 1872–1884. [Google Scholar] [CrossRef] [PubMed]
- Makino, J.; Ogasawara, R.; Kamiya, T.; Hara, H.; Mitsugi, Y.; Yamaguchi, E.; Itoh, A.; Adachi, T. Royal Jelly Constituents Increase the Expression of Extracellular Superoxide Dismutase through Histone Acetylation in Monocytic THP-1 Cells. J. Nat. Prod. 2016, 79, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.S.; Yeh, F.T.; Yu, F.S.; Cheng, K.C.; Chung, J.G. Royal jelly inhibited N-acetylation and metabolism of 2-aminofluorene in human liver tumor cells (J5). Toxicol. Environ. Chem. 2005, 87, 83–90. [Google Scholar] [CrossRef]
- Abu-Serie, M.M.; Habashy, N.H. Two purified proteins from royal jelly with in vitro dual anti-hepatic damage potency: Major royal jelly protein 2 and its novel isoform X1. Int. J. Biol. Macromol. 2019, 128, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Albalawi, A.E.; Althobaiti, N.A.; Alrdahe, S.S.; Alhasani, R.H.; Alaryani, F.S.; BinMowyna, M.N. Antitumor Activity of Royal Jelly and Its Cellular Mechanisms against Ehrlich Solid Tumor in Mice. Biomed. Res. Int. 2022, 2022, 7233997. [Google Scholar] [CrossRef] [PubMed]
- Bincoletto, C.; Eberlin, S.; Figueiredo, C.A.; Luengo, M.B.; Queiroz, M.L. Effects produced by Royal Jelly on haematopoiesis: Relation with host resistance against Ehrlich ascites tumour challenge. Int. Immunopharmacol. 2005, 5, 679–688. [Google Scholar] [CrossRef]
- Zang, S.; Ma, X.; Wu, Y.; Liu, W.; Cheng, H.; Li, J.; Liu, J.; Huang, A. PGE2 synthesis and signaling in malignant transformation and progression of human hepatocellular carcinoma. Hum. Pathol. 2017, 63, 120–127. [Google Scholar] [CrossRef]
- Xie, C.; Xu, X.; Wang, X.; Wei, S.; Shao, L.; Chen, J.; Cai, J.; Jia, L. Cyclooxygenase-2 induces angiogenesis in pancreatic cancer mediated by prostaglandin E2. Oncol. Lett. 2018, 16, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Chen, C.W. Effects of royal jelly extracts on growth inhibition, differentiation human leukemic U937 cells and its immunomodulatory activity. Biocell 2019, 43, 29–41. [Google Scholar]
- Cihan, Y.; Deniz, K. The effects of royal jelly against radiation-induced acute oral mucositis. Int. J. Hematol. Oncol. 2014, 24, 45–53. [Google Scholar] [CrossRef]
- Rafat, N.; Monfared, A.S.; Shahidi, M.; Pourfallah, T.A. The modulating effect of royal jelly consumption against radiation-induced apoptosis in human peripheral blood leukocytes. J. Med. Phys. 2016, 41, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Cihan, Y.; Ozturk, A.; Gokalp, S.S. Protective role of royal jelly against radiation-induced oxidative stress in rats. Int. J. Hematol. Oncol. 2013, 23, 79–87. [Google Scholar] [CrossRef]
- Azab, K.S.; Bashandy, M.; Salem, M.; Ahmed, O.; Tawfik, Z.; Helal, H. Royal jelly modulates oxidative stress and tissue injury in gamma irradiated male Wister Albino rats. N. Am. J. Med. Sci. 2011, 3, 268–276. [Google Scholar] [CrossRef]
- Kwon, Y. Potential Pro-Tumorigenic Effect of Bisphenol A in Breast Cancer via Altering the Tumor Microenvironment. Cancers 2022, 14, 3021. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Sakai, H. Anti-Cancer and Protective Effects of Royal Jelly for Therapy-Induced Toxicities in Malignancies. Int. J. Mol. Sci. 2018, 19, 3270. [Google Scholar] [CrossRef] [PubMed]
- Salama, S.; Shou, Q.; Abd El-Wahed, A.A.; Elias, N.; Xiao, J.; Swillam, A.; Umair, M.; Guo, Z.; Daglia, M.; Wang, K.; et al. Royal Jelly: Beneficial Properties and Synergistic Effects with Chemotherapeutic Drugs with Particular Emphasis in Anticancer Strategies. Nutrients 2022, 14, 4166. [Google Scholar] [CrossRef]
- Roohi, M.A.; Langroudi, L.; Nosratabadi, R.; Ranjbar, M.; Khajepour, F.; Zangouyee, M.R. Royal Jelly Nanoparticle Alleviates Experimental Model of Breast Cancer Through Suppressing Regulatory T Cells and Upregulating TH1 Cells. Clin. Lab. 2023, 69, 1–5. [Google Scholar] [CrossRef]
- Xu, H.; Li, L.; Wang, S.; Wang, Z.; Qu, L.; Wang, C.; Xu, K. Royal jelly acid suppresses hepatocellular carcinoma tumorigenicity by inhibiting H3 histone lactylation at H3K9la and H3K14la sites. Phytomedicine 2023, 118, 154940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shao, Q.; Geng, H.; Su, S. The effect of royal jelly on the growth of breast cancer in mice. Oncol. Lett. 2017, 14, 7615–7621. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N.; Horvat-Knežević, A.; Benković, V.; Bašić, I. Benefits of use of propolis and related flavonoids against the toxicity of chemotherapeutic agents. In Scientific Evidence of the Use of Propolis in Ehtnomedicine; Ethnopharmacology—Review Book; Oršolić, N., Bašić, I., Eds.; Transworld Research Network: Trivandrum, India, 2008; pp. 195–222. [Google Scholar]
- Oršolić, N.; Bevanda, M.; Bendelja, K.; Horvat-Knežević, A.; Benković, V.; Bašić, I. Propolis and related polyphenolic compounds; their relevance on host resistance and interaction with chemotherapy. In Scientific Evidence of the Use of Propolis in Ethnomedicine; Ethnopharmacology—Review Book; Oršolić, N., Bašić, I., Eds.; Transworld Research Network: Trivandrum, India, 2008; pp. 223–250. [Google Scholar]
- Kučan, D.; Oršolić, N.; Odeh, D.; Ramić, S.; Jakopović, B.; Knežević, J.; Jazvinšćak Jembrek, M. The Role of Hyperthermia in Potentiation of Anti-Angiogenic Effect of Cisplatin and Resveratrol in Mice Bearing Solid Form of Ehrlich Ascites Tumour. Int. J. Mol. Sci. 2023, 24, 11073. [Google Scholar] [CrossRef] [PubMed]
- Abandansari, R.M.; Parsian, H.; Kazerouni, F.; Porbagher, R.; Zabihi, E.; Rahimipour, A. Effect of Simultaneous Treatment with Royal Jelly and Doxorubicin on the Survival of the Prostate Cancer Cell Line (PC3): An In Vitro Study. Int. J. Cancer Manag. 2018, 11, e13780. [Google Scholar] [CrossRef]
- Safaei Pourzamani, M.; Oryan, S.; Yaghmaei, P.; Jalili, C.; Ghanbari, A. Royal jelly alleviates side effects of Doxorubicin on male reproductive system: A mouse model simulated human chemotherapy cycles. Res. J. Pharmacogn. 2022, 9, 77–87. [Google Scholar] [CrossRef]
- Mohamed, H.K.; Mobasher, M.A.; Ebiya, R.A.; Hassen, M.T.; Hagag, H.M.; El-Sayed, R.; Abdel-Ghany, S.; Said, M.M.; Awad, N.S. Anti-Inflammatory, Anti-Apoptotic, and Antioxidant Roles of Honey, Royal Jelly, and Propolis in Suppressing Nephrotoxicity Induced by Doxorubicin in Male Albino Rats. Antioxidants 2022, 11, 1029. [Google Scholar] [CrossRef] [PubMed]
- Bhalchandra, W.; Alqadhi, Y.A. Administration of Honey and Royal Jelly Ameliorate Cisplatin Induced Changes in Liver and Kidney Function in Rat. Biomed. Pharmacol. J. 2018, 11, 2191–2199. [Google Scholar] [CrossRef]
- Osama, H.; Abdullah, A.; Gamal, B.; Emad, D.; Sayed, D.; Hussein, E.; Mahfouz, E.; Tharwat, J.; Sayed, S.; Medhat, S.; et al. Effect of Honey and Royal Jelly against Cisplatin-Induced Nephrotoxicity in Patients with Cancer. J. Am. Coll. Nutr. 2017, 36, 342–346. [Google Scholar] [CrossRef]
- Abdel-Hafez, S.M.N.; Rifaai, R.A.; Abdelzaher, W.Y. Possible protective effect of royal jelly against cyclophosphamide induced prostatic damage in male albino rats; a biochemical, histological and immuno-histo-chemical study. Biomed. Pharmacother. 2017, 90, 15–23. [Google Scholar] [CrossRef]
- Albalawi, A.E.; Althobaiti, N.A.; Alrdahe, S.S.; Alhasani, R.H.; Alaryani, F.S.; BinMowyna, M.N. Anti-Tumor Effects of Queen Bee Acid (10-Hydroxy-2-Decenoic Acid) Alone and in Combination with Cyclophosphamide and Its Cellular Mechanisms against Ehrlich Solid Tumor in Mice. Molecules 2021, 26, 7021. [Google Scholar] [CrossRef]
- Khazaei, F.; Ghanbari, E.; Khazaei, M. Protective Effect of Royal Jelly against Cyclophosphamide-Induced Thrombocytopenia and Spleen and Bone Marrow Damages in Rats. Cell J. 2020, 22, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Han, B.; Hu, H.; Wei, Q.; Zhang, X.; Meng, L.; Nie, J.; Tang, X.; Tian, X.; Zhang, L.; et al. Proteome of thymus and spleen reveals that 10-hydroxydec-2-enoic acid could enhance immunity in mice. Expert Opin. Ther. Targets 2020, 24, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, A.; Simsek, N.; Karakus, E.; Yildirim, S.; Kara, A.; Can, I.; Kisa, F.; Emre, H.; Turkeli, M. Royal jelly modulates oxidative stress and apoptosis in liver and kidneys of rats treated with cisplatin. Oxid. Med. Cell. Longev. 2011, 2011, 981793. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.Z.; Al-Eisa, R.A.; El-Shenawy, N.S. Possible Ameliorative Effects of the Royal Jelly on Hepatotoxicity and Oxidative Stress Induced by Molybdenum Nanoparticles and/or Cadmium Chloride in Male Rats. Biology 2022, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Tohamy, H.G.; El-Neweshy, M.S.; Soliman, M.M.; Sayed, S.; Shukry, M.; Ghamry, H.I.; Abd-Ellatieff, H. Protective potential of royal jelly against hydroxyurea -induced hepatic injury in rats via antioxidant, anti-inflammatory, and anti-apoptosis properties. PLoS ONE 2022, 17, e0265261. [Google Scholar] [CrossRef] [PubMed]
- Zargar, H.R.; Hemmati, A.A.; Ghafourian, M.; Arzi, A.; Rezaie, A.; Javad-Moosavi, S.A. Long-term treatment with royal jelly improves bleomycin-induced pulmonary fibrosis in rats. Can. J. Physiol. Pharmacol. 2017, 95, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Moskwa, J.; Naliwajko, S.K.; Dobiecka, D.; Socha, K. Bee Products and Colorectal Cancer—Active Components and Mechanism of Action. Nutrients 2023, 15, 1614. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, L.; Alhusayni, F. Cytotoxicity effect of 5-fluorouracil and bee products on the HTC-116 human colon cancer cell line. Life Sci. J. 2019, 16, 56–61. [Google Scholar] [CrossRef]
- Moubarak, M.M.; Chanouha, N.; Abou Ibrahim, N.; Khalife, H.; Gali-Muhtasib, H. Thymoquinone anticancer activity is enhanced when combined with royal jelly in human breast cancer. World J. Clin. Oncol. 2021, 12, 342–354. [Google Scholar] [CrossRef]
- Filipič, B.; Gradišnik, L.; Rihar, K.; Šooš, E.; Pereyra, A.; Potokar, J. The influence of royal jelly and human interferon-alpha (HuIFN-αN3) on proliferation, glutathione level and lipid peroxidation in human colorectal adenocarcinoma cells in vitro. Arh. Hig. Rada Toxicol. 2015, 66, 269–274. [Google Scholar] [CrossRef]
- Okic-Djordjevic, I.; Trivanovic, D.; Krstic, J.; Jaukovic, A.; Mojsilovic, S.; Santibanez, J.F.; Terzic, M.; Vesovic, D.; Bugarski, D. GE132+Natural: Novel promising dietetic supplement with antiproliferative influence on prostate, colon, and breast cancer cells. J. BUON. 2013, 18, 504–510. [Google Scholar] [PubMed]
- Damiani, A.P.; Magenis, M.L.; Dagostin, L.S.; Beretta, Â.C.D.L.; Sarter, R.J.; Longaretti, L.M.; Monteiro, I.O.; Andrade, V.M. Royal jelly reduce DNA damage induced by alkylating agent in mice. Mutat Res. 2022, 825, 111796. [Google Scholar] [CrossRef]
- Sarhan, H.; Naoum, L. Protective Role of Royal Jelly Against Gamma Radiation Induced Oxidative Stress, Cardio-Toxicity and Organ Dysfunctions in Male Rats. Egypt J. Hosp. Med. 2020, 78, 62–67. [Google Scholar] [CrossRef]
- Erdem, O.; Güngörmüş, Z. The effect of royal jelly on oral mucositis in patients undergoing radiotherapy and chemotherapy. Holist. Nurs. Pract. 2014, 28, 242–246. [Google Scholar] [CrossRef]
- Campos, M.G.; Anjos, O.; Ahmad, S. Prevention of side effects from chemoradiotherapy and antitumor potential of royal jelly and its components: A systematic review. In Bee Products and Their Applications in the Food and Pharmaceutical Industries; Boyacioglu, D., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 221–244. [Google Scholar] [CrossRef]
- El-Fiky, S.A.; Othman, O.E.; Balabel, E.A.; Abd-Elbaset, S.A. The protective role of royal jelly against mutagenic effect of adriamycin and gamma radiation separately and in combination. Trends Appl. Sci. Res. 2008, 3, 303–318. [Google Scholar]
- Fatmawati, F.; Erizka, E.; Hidayat, R. Royal Jelly (Bee Product) Decreases Inflammatory Response in Wistar Rats Induced with Ultraviolet Radiation. Open Access Maced. J. Med. Sci. 2019, 7, 2723–2727. [Google Scholar] [CrossRef]
- Okumura, N.; Ito, T.; Degawa, T.; Moriyama, M.; Moriyama, H. Royal Jelly Protects against Epidermal Stress through Upregulation of the NQO1 Expression. Int. J. Mol. Sci. 2021, 22, 12973. [Google Scholar] [CrossRef] [PubMed]
- Almeer, R.S.; Alarifi, S.; Alkahtani, S.; Ibrahim, S.R.; Ali, D.; Moneim, A. The potential hepatoprotective effect of royal jelly against cadmium chloride-induced hepatotoxicity in mice is mediated by suppression of oxidative stress and upregulation of Nrf2 expression. Biomed. Pharmacother. 2018, 106, 1490–1498. [Google Scholar] [CrossRef]
- Almeer, R.S.; AlBasher, G.I.; Alarifi, S.; Alkahtani, S.; Ali, D.; Abdel Moneim, A.E. Royal jelly attenuates cadmium-induced nephrotoxicity in male mice. Sci. Rep. 2019, 9, 5825. [Google Scholar] [CrossRef]
- Focak, M.; Suljevic, D. Ameliorative Effects of Propolis and Royal Jelly against CCl4 -Induced Hepatotoxicity and Nephrotoxicity in Wistar Rats. Chem. Biodivers. 2023, 20, e202200948. [Google Scholar] [CrossRef]
- Khalifa, H.A.M.I.; Eleiwa, N.Z.H.; Nazim, H.A. Royal Jelly, A Super Food, Protects Against Celecoxib-Induced Renal Toxicity in Adult Male Albino Rats. Can. J. Kidney Health Dis. 2024, 11, 20543581241235526. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Abdel Moneim, A.E.; Hafez, T.A.; Mubaraki, M.A.; Mohamed, W.F.; Thagfan, F.A.; Al-Quraishy, S. Myristica fragrans Kernels Prevent Paracetamol-Induced Hepatotoxicity by Inducing Anti-Apoptotic Genes and Nrf2/HO-1 Pathway. Int. J. Mol. Sci. 2019, 20, 993. [Google Scholar] [CrossRef]
- Nejati, V.; Zahmatkesh, E.; Babaei, M. Protective Effects of Royal Jelly on Oxymetholone-Induced Liver Injury in Mice. Iran. Biomed. J. 2016, 20, 229–234. [Google Scholar] [CrossRef]
- Qu, L.; Wang, L.; Ji, H.; Fang, Y.; Lei, P.; Zhang, X.; Jin, L.; Sun, D.; Dong, H. Toxic Mechanism and Biological Detoxification of Fumonisins. Toxins 2022, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Brito, J.C.M.; Verly, R.M.; Lima, M.E. Jelleine, a Family of Peptides Isolated from the Royal Jelly of the Honey Bees (Apis mellifera), as a Promising Prototype for New Medicines: A Narrative Review. Toxins 2024, 16, 24. [Google Scholar] [CrossRef]
- Wu, Q.; Patočka, J.; Kuča, K. Insect Antimicrobial Peptides, a Mini Review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, S.; Imai, J.; Fujiwara, M.; Yaeshima, T.; Kawashima, T.; Kobayashi, K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J. Biol. Chem. 1990, 265, 11333–11337. [Google Scholar] [CrossRef]
- Bílikova, K.; Huang, S.C.; Lin, I.P.; Šimuth, J.; Peng, C.C. Structure and antimicrobial activity relationship of royalisin, an antimicrobial peptide from royal jelly of Apis mellifera. Peptides 2015, 68, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Fontana, R.; Mendes, M.A.; de Souza, B.M.; Konno, K.; César, L.M.; Malaspina, O.; Palma, M.S. Jelleines: A family of antimicrobial peptides from the Royal Jelly of honeybees (Apis mellifera). Peptides 2004, 25, 919–928. [Google Scholar] [CrossRef]
- Han, B.; Fang, Y.; Feng, M.; Lu, X.; Huo, X.; Meng, L.; Wu, B.; Li, J. In-depth phosphoproteomic analysis of royal jelly derived from western and eastern honeybee species. J. Proteome Res. 2014, 13, 5928–5943. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; He, Y.; Liu, K.; Zhang, F.; Zhang, H.; Lu, Y.; Yang, C.; Wang, Z.; Fareed, M.S.; et al. An optimized analog of antimicrobial peptide Jelleine-1 shows enhanced antimicrobial activity against multidrug resistant P. aeruginosa and negligible toxicity in vitro and in vivo. Eur. J. Med. Chem. 2021, 219, 113433. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Yu, Q.; Wang, R.; Zhao, L.; Yuan, F.; Guo, H.; Shen, Y.; He, F. Optimized Antimicrobial Peptide Jelleine-I Derivative Br-J-I Inhibits Fusobacterium Nucleatum to Suppress Colorectal Cancer Progression. Int. J. Mol. Sci. 2023, 24, 1469. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Brudzynski, K.; Sjaarda, C.; Lannigan, R. MRJP1-containing glycoproteins isolated from honey, a novel antibacterial drug candidate with broad spectrum activity against multi-drug resistant clinical isolates. Front. Microbiol. 2015, 6, 711. [Google Scholar] [CrossRef] [PubMed]
- Leiva-Sabadini, C.; Alvarez, S.; Barrera, N.P.; Schuh, C.M.A.P.; Aguayo, S. Antibacterial Effect of Honey-Derived Exosomes Containing Antimicrobial Peptides against Oral Streptococci. Int. J. Nanomed. 2021, 16, 4891–4900. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, S.; Contreras-Kallens, P.; Aguayo, S.; Ramírez, O.; Vallejos, C.; Ruiz, J.; Carrasco-Gallardo, E.; Troncoso-Vera, S.; Morales, B.; Schuh, C.M.A.P. Royal jelly extracellular vesicles promote wound healing by modulating underlying cellular responses. Mol. Ther. Nucleic Acids 2023, 31, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Uthaibutra, V.; Kaewkod, T.; Prapawilai, P.; Pandith, H.; Tragoolpua, Y. Inhibition of Skin Pathogenic Bacteria, Antioxidant and Anti-Inflammatory Activity of Royal Jelly from Northern Thailand. Molecules 2023, 28, 996. [Google Scholar] [CrossRef]
- Naghib, M.; Kariminik, A.; Arababadi, M.K.; Rokhbakhsh-Zamin, F.; Mollaei, H.R. Effect of Royal Jelly on Gene Expression of Toll-like Receptors 1–9 in Patients with Hepatitis, B. Clin Lab. 2023, 69, 77. [Google Scholar] [CrossRef] [PubMed]
- Alkhaibari, A.M.; Alanazi, A.D. Insecticidal, Antimalarial, and Antileishmanial Effects of Royal Jelly and Its Three Main Fatty Acids, trans-10-Hydroxy-2-decenoic Acid, 10-Hydroxydecanoic Acid, and Sebacic Acid. Evid. Based. Complement. Alternat. Med. 2022, 2022, 7425322. [Google Scholar] [CrossRef]
- Mahdavi, M.; Cheragh, M.; Bagheri, K.P.; Adibzadeh, M.M.; Tajik, A.H.; Memariani, H.; Savoji, M.A.; MahdiGhahari, S.M.; Shahbazzadeh, D. Adjuvant Effect of Royal Jelly on HIV-1 Multi-Epitope Vaccine Candidate: Induction of Th1 Cytokine Pattern. MOJ Immunol. 2017, 5, 43–48. [Google Scholar] [CrossRef]
- Habashy, N.H.; Abu-Serie, M.M. The potential antiviral effect of major royal jelly protein2 and its isoform X1 against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Insight on their sialidase activity and molecular docking. J. Funct. Foods 2020, 75, 104282. [Google Scholar] [CrossRef] [PubMed]
- Habashy, N.H.; Hamouda, A.F.; Abu Serie, M.M. Identification of effective anti-HCV and anti-HIV royal jelly constituents and their acute toxicity evaluation in Albino rats. Food Chem Toxicol. 2023, 182, 114170. [Google Scholar] [CrossRef] [PubMed]
- Abu-Serie, M.M.; Habashy, N.H. Major royal jelly proteins elicited suppression of SARS-CoV-2 entry and replication with halting lung injury. Int. J. Biol. Macromol. 2023, 228, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Elfiky, A.A. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci. 2020, 253, 117592. [Google Scholar] [CrossRef] [PubMed]
- Ozgok Kangal, M.K.; Regan, J.P. Wound Healing. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Majd, S.A.; Rabbani Khorasgani, M.; Zaker, S.R.; Khezri, M.; Aliasghari Veshareh, A. Comparative Efficacy Study of N-Chromosome Royal Jelly Versus Semelil (ANGIPARS) on Wound Healing of Diabetic Rats. Int. J. Diabetes Obes. 2022, 14, 223–230. [Google Scholar] [CrossRef]
- El-Gayar, M.H.; Ishak, R.A.H.; Esmat, A.; Aboulwafa, M.M.; Aboshanab, K.M. Evaluation of lyophilized royal jelly and garlic extract emulgels using a murine model infected with methicillin-resistant Staphylococcus aureus. AMB Express 2022, 12, 37. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, M.; Wang, L.; Lin, T.; Wang, G.; Peng, J.; Su, S. The in vitro and in vivo wound-healing effects of royal jelly derived from Apis mellifera L. during blossom seasons of Castanea mollissima Bl. and Brassica napus L. in South China exhibited distinct patterns. BMC Complement. Med. Ther. 2020, 20, 357. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.M.; Šeklić, D.S.; Rakobradović, J.D.; Planojević, N.S.; Vuković, N.L.; Vukić, M.D.; Marković, S.D. Royal Jelly and trans-10-Hydroxy-2-Decenoic Acid Inhibit Migration and Invasion of Colorectal Carcinoma Cells. Food Technol. Biotechnol. 2022, 60, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, N.; Ali, A.; Yasmeen, A.; Khalid, H.; Safi, S.Z.; Sharif, F. Fabrication of green composite hand knitted silk mesh reinforced with silk hydrogel. Int. J. Biol. Macromol. 2023, 253, 127284. [Google Scholar] [CrossRef]
- Parhizkari, N.; Eidi, M.; Mahdavi-Ortakand, M.; Ebrahimi-Kia, Y.; Zarei, S.; Pazoki, Z. The effect of oral treatment of royal jelly on the expression of the PDGF-β gene in the skin wound of male mice. J. Tissue Viab. 2023, 32, 536–540. [Google Scholar] [CrossRef]
- Ashrafi, B.; Chehelcheraghi, F.; Rashidipour, M.; Hadavand, S.; Beiranvand, B.; Taherikalani, M.; Soroush, S. Electrospun Nanofibrous Biocomposite of Royal Jelly/Chitosan/Polyvinyl Alcohol (RJ/CS/PVA) Gel as a Biological Dressing for P. aeruginosa-Infected Burn Wound. Appl. Biochem. Biotechnol. 2023; ahead of print. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Tanga, B.M.; Bang, S.; Maigoro, A.Y.; Kang, H.; Cha, D.; Lee, S.; Lee, S.; Cho, J. MicroRNA profiling of royal jelly extracellular vesicles and their potential role in cell viability and reversing cell apoptosis. Funct. Integr. Genomics 2023, 23, 200. [Google Scholar] [CrossRef] [PubMed]
- Kudłacik-Kramarczyk, S.; Krzan, M.; Jamroży, M.; Przybyłowicz, A.; Drabczyk, A. Exploring the Potential of Royal-Jelly-Incorporated Hydrogel Dressings as Innovative Wound Care Materials. Int. J. Mol. Sci. 2023, 24, 8738. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Zhu, W.; Liu, L.; Pan, Y.; Xu, Y.; Huang, Q.; Li, L.; Rao, L. In situ formed scaffold with royal jelly-derived extracellular vesicles for wound healing. Theranostics 2023, 13, 2811–2824. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Pan, C.; Xu, P.; Liu, K. Hydrogel-mediated extracellular vesicles for enhanced wound healing: The latest progress, and their prospects for 3D bioprinting. J. Nanobiotechnol. 2024, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, G.; Rosicka-Kaczmarek, J.; Miśkiewicz, K.; Zakłos-Szyda, M.; Rohn, S.; Kanzler, C.; Wiktorska, M.; Niewiarowska, J. Arabinoxylan-Based Microcapsules Being Loaded with Bee Products as Bioactive Food Components Are Able to Modulate the Cell Migration and Inflammatory Response—In Vitro Study. Nutrients 2022, 14, 2529. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, O.J.; Alvarez, S.; Contreras-Kallens, P.; Barrera, N.P.; Aguayo, S.; Schuh, C.M.A.P. Type I collagen hydrogels as a delivery matrix for royal jelly derived extracellular vesicles. Drug Deliv. 2020, 27, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, K.; Kogashiwa, Y.; Moro, Y.; Kohno, N. The effect of topical application of royal jelly on chemoradiotherapy-induced mucositis in head and neck cancer: A preliminary study. Int. J. Otolaryngol. 2014, 2014, 974967. [Google Scholar] [CrossRef] [PubMed]
- Suemaru, K.; Cui, R.; Li, B.; Watanabe, S.; Okihara, K.; Hashimoto, K.; Yamada, H.; Araki, H. Topical application of royal jelly has a healing effect for 5-fluorouracil-induced experimental oral mucositis in hamsters. Methods Find. Exp. Clin. Pharmacol. 2008, 30, 103–106. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, L.; Feng, X.; Zhao, N.; Feng, N.; Du, X. Did you choose appropriate mouthwash for managing chemoradiotherapy-induced oral mucositis? The therapeutic effect compared by a Bayesian network meta-analysis. Front Oral Health 2023, 3, 977830. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Severo, M.L.B.; Thieme, S.; Silveira, F.M.; Tavares, R.P.M.; Gonzaga, A.K.G.; Zucolotto, S.M.; de Araújo, A.A.; Martins, M.A.T.; Martins, M.D.; da Silveira, É.J.D. Comparative study of royal jelly, propolis, and photobiomodulation therapies in 5-fluorouracil-related oral mucositis in rats. Support. Care Cancer 2022, 30, 2723–2734. [Google Scholar] [CrossRef]
- Watanabe, S.; Suemaru, K.; Takechi, K.; Kaji, H.; Imai, K.; Araki, H. Oral mucosal adhesive films containing royal jelly accelerate recovery from 5-fluorouracil-induced oral mucositis. J. Pharmacol. Sci. 2013, 121, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Daugėlaitė, G.; Užkuraitytė, K.; Jagelavičienė, E.; Filipauskas, A. Prevention and Treatment of Chemotherapy and Radiotherapy Induced Oral Mucositis. Medicina 2019, 55, 25. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xin, X.X.; Qian, H.C.; Yu, Z.Y.; Shen, L.R. Evaluation of the major royal jelly proteins as an alternative to fetal bovine serum in culturing human cell lines. J. Zhejiang Univ. Sci. B 2016, 17, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Oka, H.; Emori, Y.; Kobayashi, N.; Hayashi, Y.; Nomoto, K. Suppression of allergic reactions by royal jelly in association with the restoration of macrophage function and the improvement of Th1/Th2 cell responses. Int. Immunopharmacol. 2001, 1, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Kohno, K.; Inoue, S.; Koya-Miyata, S.; Okamoto, I.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Oral administration of royal jelly inhibits the development of atopic dermatitis-like skin lesions in NC/Nga mice. Int. Immunopharmacol. 2003, 3, 1313–1324. [Google Scholar] [CrossRef]
- Mannoor, M.K.; Shimabukuro, I.; Tsukamotoa, M.; Watanabe, H.; Yamaguchi, K.; Sato, Y. Honeybee royal jelly inhibits autoimmunity in SLE-prone NZB × NZW F1 mice. Lupus 2009, 18, 44–52. [Google Scholar] [CrossRef]
- Zahran, A.M.; Elsayh, K.I.; Saad, K.; Eloseily, E.M.; Osman, N.S.; Alblihed, M.A.; Badr, G.; Mahmoud, M.H. Effects of royal jelly supplementation on regulatory T cells in children with SLE. Food Nutr. Res. 2016, 60, 32963. [Google Scholar] [CrossRef]
- Erem, C.; Deger, O.; Ovali, E.; Barlak, Y. The effects of royal jelly on autoimmunity in Graves’ disease. Endocrine 2006, 30, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Guendouz, M.; Haddi, A.; Grar, H.; Kheroua, O.; Saidi, D.; Kaddouri, H. Preventive effects of royal jelly against anaphylactic response in a murine model of cow’s milk allergy. Pharm. Biol. 2017, 55, 2145–2152. [Google Scholar] [CrossRef]
- Kai, H.; Motomura, Y.; Saito, S.; Hashimoto, K.; Tatefuji, T.; Takamune, N.; Misumi, S. Royal jelly enhances antigen-specific mucosal IgA response. Food Sci. Nutr. 2013, 1, 222–227. [Google Scholar] [CrossRef]
- Hata, T.; Furusawa-Horie, T.; Arai, Y.; Takahashi, T.; Seishima, M.; Ichihara, K. Studies of royal jelly and associated cross-reactive allergens in atopic dermatitis patients. PLoS ONE 2020, 15, e0233707. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, Y.; Shibuya, Y.; Takahashi, T.; Tsunoda, T.; Moriyama, T.; Seishima, M. Major royal jelly protein 3 as a possible allergen in royal jelly-induced anaphylaxis. J. Dermatol. 2011, 38, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, T.; Rakwal, R.; Nam, H.W.; Shibato, J.; Agrawal, G.K.; Kim, Y.S.; Ogawa, Y.; Yoshida, Y.; Kouzuma, Y.; Masuo, Y.; et al. Comprehensive royal jelly (RJ) proteomics using one- and two-dimensional proteomics platforms reveals novel RJ proteins and potential phospho/glycoproteins. J. Proteome Res. 2008, 7, 3194–3229. [Google Scholar] [CrossRef] [PubMed]
- Rosmilah, M.; Shahnaz, M.; Patel, G.; Lock, J.; Rahman, D.; Masita, A.; Noormalin, A. Characterization of major allergens of royal jelly Apis mellifera. Trop. Biomed. 2008, 25, 243–251. [Google Scholar] [PubMed]
- Li, J.D.; Cui, L.; Xu, Y.Y.; Guan, K. A Case of Anaphylaxis Caused by Major Royal Jelly Protein 3 of Royal Jelly and Its Cross-Reactivity with Honeycomb. J. Asthma Allergy 2021, 14, 1555–1557. [Google Scholar] [CrossRef] [PubMed]
- El-Nekeety, A.A.; El-Kholy, W.; Abbas, N.F.; Ebaid, A.; Amra, H.A.; Abdel-Wahhab, M.A. Efficacy of royal jelly against the oxidative stress of fumonisin in rats. Toxicon 2007, 50, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Han, B.; Feng, M.; Fang, Y.; Zhang, L.; Hu, H.; Hao, Y.; Qi, Y.; Zhang, X.; Li, J. Functional and Proteomic Investigations Reveal Major Royal Jelly Protein 1 Associated with Anti-Hypertension Activity in Mouse Vascular Smooth Muscle Cells. Sci. Rep. 2016, 6, 30230. [Google Scholar] [CrossRef] [PubMed]
- Escamilla, K.I.A.; Ordóñez, Y.B.M.; Sandoval-Peraza, V.M.; Fernández, J.J.A.; Ancona, D.A.B. Anti-Hypertensive Activity In Vitro and In Vivo on Royal Jelly Produced by Different Diets. Emir. J. Food Agric. 2022, 34, 9–15. [Google Scholar] [CrossRef]
- Mazzei, P.; Piccolo, A.; Brescia, M.; Caprio, E. Assessment of geographical origin and production period of royal jelly by NMR metabolomics. Chem. Biol. Technol. Agric. 2020, 7, 24. [Google Scholar] [CrossRef]
- Emir, M. Effect of harvesting period on chemical and bioactive properties of royal jelly from Turkey. Eur. Food Sci. Eng. 2020, 1, 9–12. [Google Scholar]
- Ghosh, S.; Jung, C. Chemical Composition and Nutritional Value of Royal Jelly Samples Obtained from Honey Bee (Apis mellifera) Hives Fed on Oak and Rapeseed Pollen Patties. Insects 2024, 15, 141. [Google Scholar] [CrossRef] [PubMed]
- Milone, J.P.; Chakrabarti, P.; Sagili, R.R.; Tarpy, D.R. Colony-level pesticide exposure affects honey bee (Apis mellifera L.) royal jelly production and nutritional composition. Chemosphere 2021, 263, 128183. [Google Scholar] [CrossRef] [PubMed]
- Sagona, S.; Coppola, F.; Giannaccini, G.; Betti, L.; Palego, L.; Tafi, E.; Casini, L.; Piana, L.; Dall’Olio, R.; Felicioli, A. Impact of Different Storage Temperature on the Enzymatic Activity of Apis mellifera Royal Jelly. Foods 2022, 11, 3165. [Google Scholar] [CrossRef] [PubMed]
- Arfa, A.; Reyad, Y.M.; Nikeety, M.E. Quality Parameters of Royal Jelly in national and international standards: Specifications, differences and suggestions. Ann. Rom. Soc. Cell Biol. 2021, 25, 7977–7997. [Google Scholar]
- Chen, D.; Guo, C.; Lu, W.; Zhang, C.; Xiao, C. Rapid quantification of royal jelly quality by mid-infrared spectroscopy coupled with backpropagation neural network. Food Chem. 2023, 418, 135996. [Google Scholar] [CrossRef] [PubMed]
- Oršolić, N. Učinkovitost biološki aktivnih sastavnica matične mliječi: Analiza i standardizacija. Arh. Hig. Rada Toksikol. 2013, 64, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Spanidi, E.; Athanasopoulou, S.; Liakopoulou, A.; Chaidou, A.; Hatziantoniou, S.; Gardikis, K. Royal Jelly Components Encapsulation in a Controlled Release System-Skin Functionality, and Biochemical Activity for Skin Applications. Pharmaceuticals 2022, 15, 907. [Google Scholar] [CrossRef] [PubMed]
- Mureşan, C.I.; Buttstedt, A. pH-dependent stability of honey bee (Apis mellifera) major royal jelly proteins. Sci. Rep. 2019, 9, 9014. [Google Scholar] [CrossRef] [PubMed]
- Daniele, G.; Casabianca, H. Sugar composition of French Royal Jelly for comparison with commercial and artificial sugar samples. Food Chem. 2012, 134, 1025–1029. [Google Scholar] [CrossRef]
- Virgiliou, C.; Kanelis, D.; Pina, A.; Gika, H.; Tananaki, C.; Zotou, A.; Theodoridis, G. A targeted approach for studying the effect of sugar bee feeding on the metabolic profile of Royal Jelly. J. Chromatogr. A. 2020, 1616, 460783. [Google Scholar] [CrossRef]
- Fuente-Ballesteros, A.; Jano, A.; Bernal, J.; Ares, A.M. Development and validation of an analytical methodology based on solvent extraction and gas chromatography for determining pesticides in royal jelly and propolis. Food Chem. 2024, 437, 137911. [Google Scholar] [CrossRef] [PubMed]
- Stocker, A. Isolation and Characterisation of Substances from Royal Jelly. Ph.D. Thesis, Universite´ d’Orle´ans, Orle´ans, France, 2003. [Google Scholar]
- Wang, Z.; Ren, P.; Wu, Y.; He, Q. Recent advances in analytical techniques for the detection of adulteration and authenticity of bee products—A review. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk. Assess. 2021, 38, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Minegaki, N.; Koshizuka, T.; Hatasa, K.; Kondo, H.; Kato, H.; Tannaka, M.; Takahashi, K.; Tsuji, M.; Inoue, N. The C-Terminal Penta-Peptide Repeats of Major Royal Jelly Protein 3 Ameliorate the Progression of Inflammation In Vivo and In Vitro. Biol Pharm Bull. 2022, 45, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Tahereh, F.N.; Nayereh, K.; Narjes, B.; Mojtaba, M.; Shirin, S.; Sareh, D. The Fertility Outcome of Royal Jelly versus Intra Uterine Insemination: A Pilot Randomized Controlled Trial Study, Jundishapur. J. Nat. Prod. 2021, 16, e107420. [Google Scholar]
- Basmeh, K.; Farzad, S.; Shima, J.; Mojtaba, M.A.; Fatemeh, H. Effect of royal jelly intake on serum glucose, HbA1c, and total antioxidant capacity (TAC) in type 2 diabetic patients: A randomized, double blind clinical trial study. Red Jacket Middle Sch. 2013, 19, 33–40. [Google Scholar]
- Ohba, K.; Miyata, Y.; Shinzato, T.; Funakoshi, S.; Maeda, K.; Matsuo, T.; Mitsunari, K.; Mochizuki, Y.; Nishino, T.; Sakai, H. Effect of oral intake of royal jelly on endothelium function in hemodialysis patients: Study protocol for multicenter, double-blind, randomized control trial. Trials 2022, 22, 950. [Google Scholar] [CrossRef] [PubMed]
- Yumi, M.; Fumihiko, T.; Yuji, K.; Hiroyuki, H. The Effects of The Royal Jelly on Dry Mouth Sensation with Normal Saliva Function: A Double-Blind, Placebo-Controlled, Cross-Over Trial Clinical Study. JCDR 2023, 17, KC01–KC04. [Google Scholar] [CrossRef]
- Shafiee, Z.; Hanifi, N.; Rashtchi, V. The effect ofroyal jelly on the level of consciousness in patients with traumatic brain injury: A double-blind randomized clinical trial. Nurs. Midwifery Stud. 2022, 11, 96–102. [Google Scholar] [CrossRef]
- Peivandi, S.; Khalili, S.S.; Abbasi, Z.; Zamaniyan, M.; Gordani, N.; Moradi, S. Effect of Royal Jelly on Sperm Parameters and Testosterone Levels in Infertile Men. J. Maz. Univ. Med. Sci. 2022, 31, 43–52. [Google Scholar]
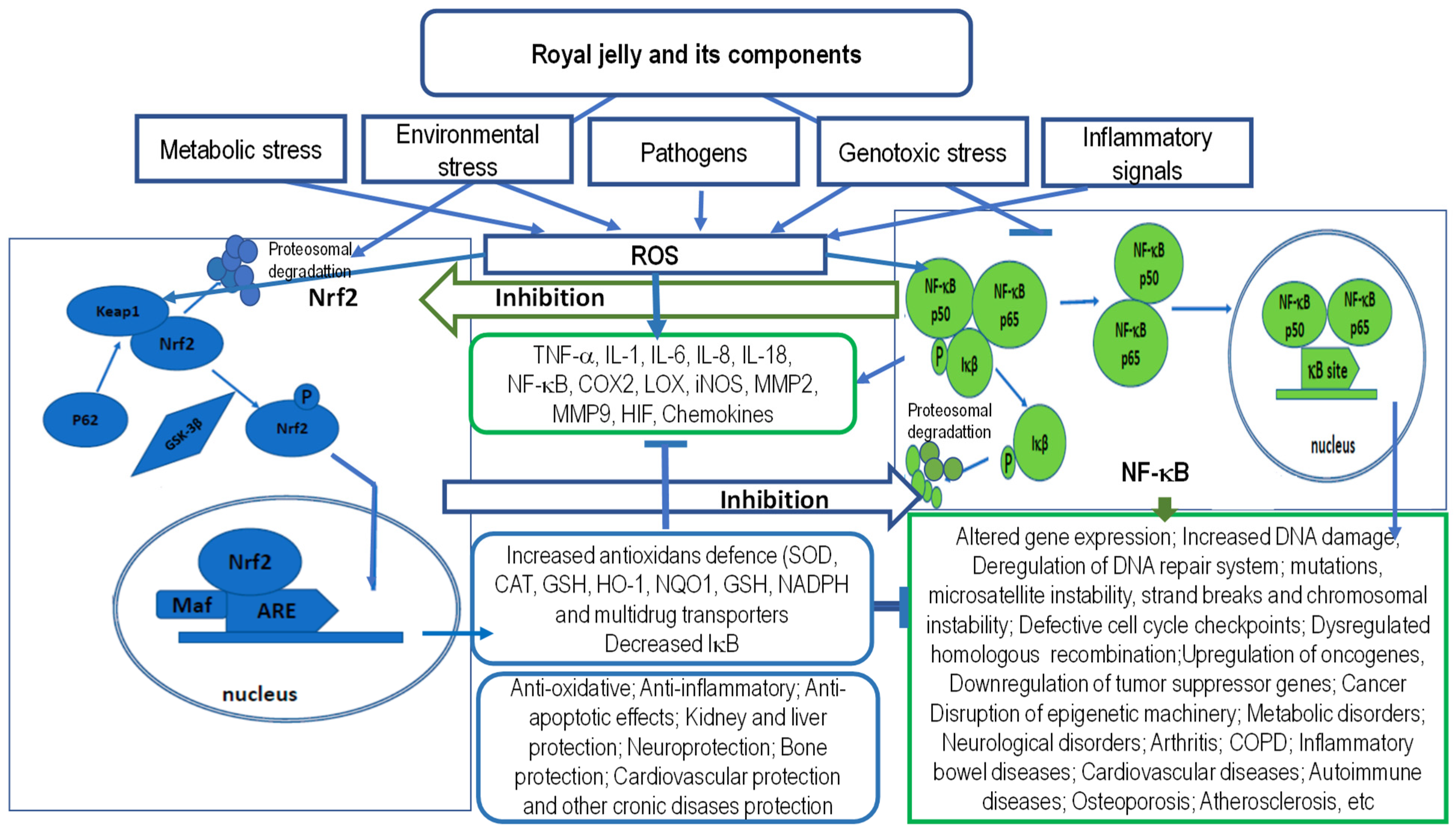

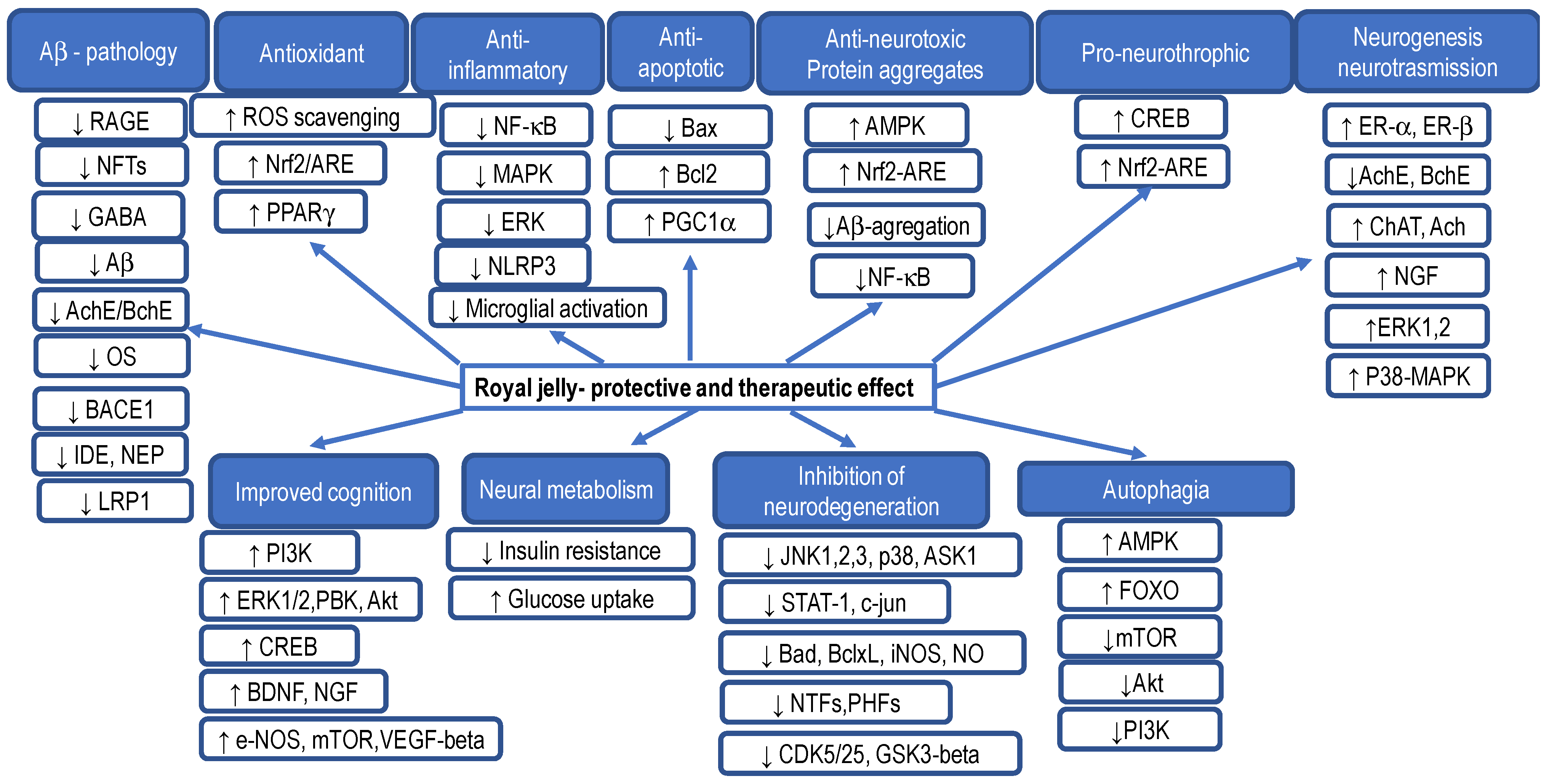
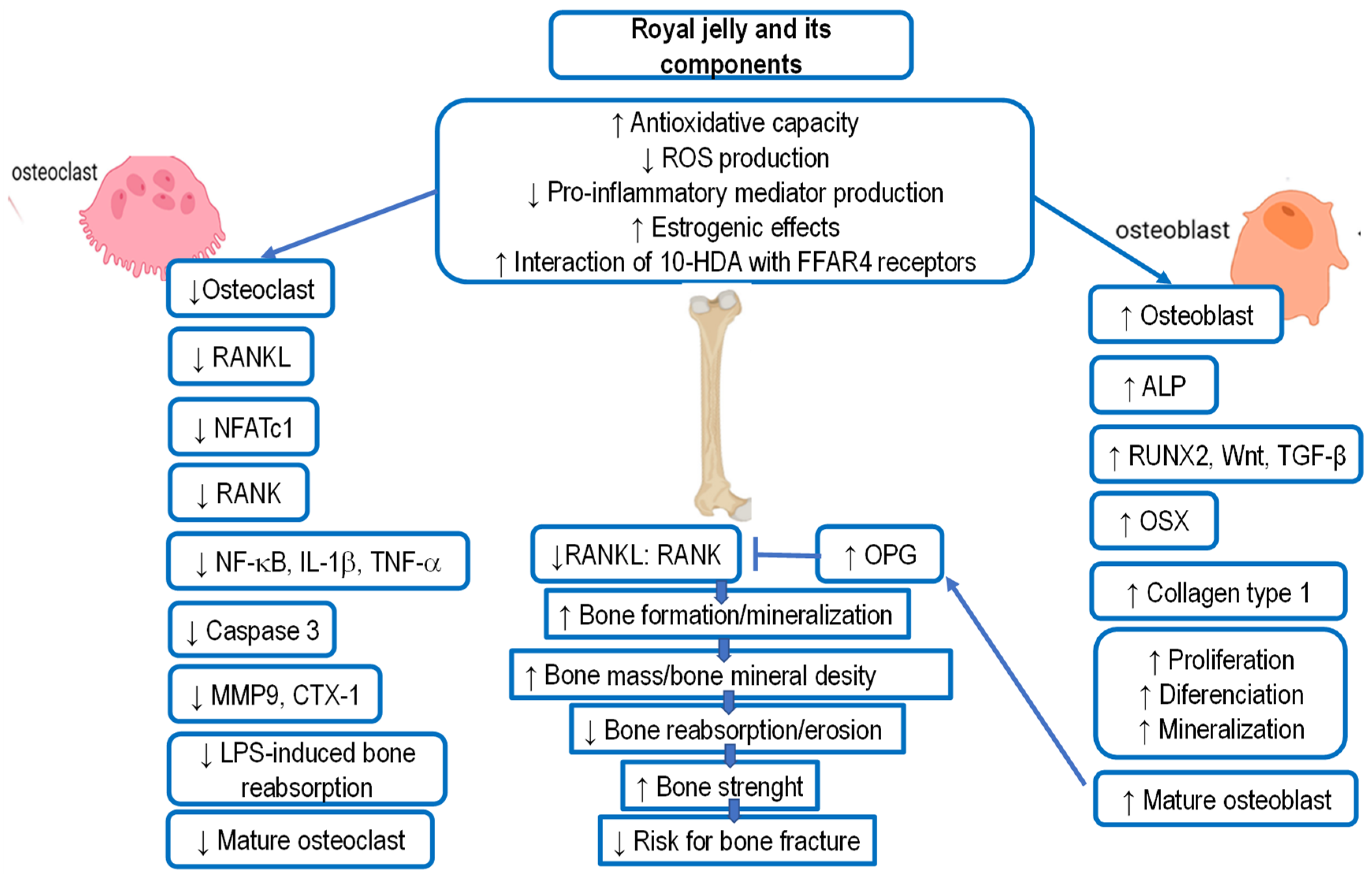
| Contents | Fresh Royal Jelly (%) | Lyophilized Royal Jelly (%) | References |
|---|---|---|---|
| Water | 60–70 | <5 | |
| Lipids | 3–8 | 8–19 | |
| 10-hydroxy-2-decenoic acid (10-HDA) | >1.4 | >3.5 | |
| Proteins | 9–18 | 27–41 | |
| Fructose + glucose + sucrose | 7–18 | - | |
| Fructose | 3–13 | - | |
| Glucose | 4–8 | - | [2,3,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23] |
| Sucrose | 0.5–2.0 | - | |
| Minerals | 0.7–1.5 | ||
| Ash (dry weight) | 0.8–3.0 | 2–5 | |
| pH | 3.4–4.5 | 3.4–4.5 | |
| Acidity (mL 0.1N NaOH/g) | 3.0–6.0 | - | |
| Furasine (mg/100 g protein) | <50 | - |
| Bioactive Component | Fresh Royal Jelly (%) | Lyophilized Royal Jelly (%) | Functional Activities | References |
|---|---|---|---|---|
| Proteins | ||||
| MRPJ1 (Alternative name: Royalactin, apalbumin 1, D III) | 5.89% | Antimicrobial, antibacterial, antifungal, | [2,3,12,14,18,48,63] | |
| wound healing, | [3,7,13,26,31,48,57] | |||
| antiproliferative, antioxidant, anti-inflammatory, antitumoral, | [7,13,29,58,68,69,70,71,72,73,74,75,76] | |||
| immunomodulatory, | [13,14,15,17,19,69] | |||
| hypocholesterolemic, anti-hypertensive, | [27,28] | |||
| proliferation of intestinal epithelial cells (IEC-6), | [31] | |||
| increase in lifespan in invertebrates, | [11,26,53] | |||
| proliferation rat hepatocytes, | [26] | |||
| larvae differentiation into queen via epidermal growth factor signaling, | [11,30,43,44,47,48] | |||
| self-renewal of stem cells, | [11,26,30] | |||
| proliferation of human monocytes and Jurkat lymphoid cell | [33,35,44] | |||
| MRPJ2 and isoform (Alternative name: Apalbumin 2) | 1.41% | Antimicrobial, antibacterial, antifungal, antiviral, | [2,3,12,14,18,48,63] | |
| wound healing, | [26,31] | |||
| antioxidant, antitumoral, | [29,62,63,64,65,66,67,68] | |||
| anti-allergic, | [31,37] | |||
| hepato-renal protective, | ||||
| promotione of caspase-dependent apoptosis, inhibition of bcl-2 and p53 expression in HepG2 cells | [60,61] | |||
| MRPJ3 | 1.66% | Wound healing, | [26,31] | |
| anti-allergic, | [31,37] | |||
| anti-aging, | [53] | |||
| anti-inflammatory, antitumoral, | [34,36,69,70,71] | |||
| immunoregulatory effect, modulation of immune responses of T cells, suppression of proinflammatory cytokine secretion, decrease of IL-4, IL-2, and IFN-γ in vitro and anti-OVA IgE and IgG1 in vivo | [13,37] | |||
| MRPJ4 | 0.89% | Antimicrobial | [2,3,12,14,18] | |
| MRPJ7 | 0.51% | Wound healing | [14,17,19,26] | |
| Enzymes | ||||
| Glucose oxidase | 0.08% | Carbohydrate metabolism, antibacterial | [3,5,15] | |
| Gluco-cerebrosidase | Hydrolysis glucosylceramide | [22,53] | ||
| Alpha-glucosidase | Hydrolysis of polysaccharides and oligosaccharides into monomers | [22,53] | ||
| Antimicrobial peptides and proteins | ||||
| Royalisin | 0.83% | Antimicrobial, antibacterial, antifungal, inhibition of Gram-positive bacteria through damage to cell walls and cell membranes | [12,13,14,15,16,48,63,68] | |
| Apisimin | 0.13% | Antibacterial, | [9,10,12,17,48] | |
| increases the proliferation and stimulation of human monocytes | [21,33] | |||
| Yelleines I-III | 0.37% | Antimicrobial, antibacterial, inhibition of yeast, Gram-positive and Gram-negative bacteria, cell degranulation, hemolysis, increase immune response | [13,14,15,16,48,63,68] | |
| Yelleine IV | - | Antimicrobial | [48] | |
| Venom protein 2 | - | Protection of larvae from diseases infection | [48,63] | |
| Apolipophorin-III-like protein | 0.08% | Antimicrobial, immunoregulatory effect, stimulation of immune response | [48,63] | |
| Lipids and fatty acids | ||||
| 10-hydroxy-2-decenoic (10-HDA), | 0.75–3.39% | Immunomodulatory, | [13,67,69,76,77] | |
| antioxidant, | [7,52,53,62,68] | |||
| antiaging, | [53,67] | |||
| neurotrophic and neurogenesis inductor, | [4,58,68] | |||
| anti-inflammatory functions, | [7,13,58,62,63,68,69] | |||
| antimicrobial, antibacterial, | [48,67,68] | |||
| estrogenic, | [28,58] | |||
| antialergic, | [7] | |||
| antiosteoporetic, | [35,58] | |||
| antitumor activity, | [7,34,60,61] | |||
| activation of TRPA1 and TRPV1 receptors and increased longevity in C. Elegans, | [52,53,59] | |||
| anti-ultraviolet B properties and skin protection, | [59] | |||
| decrease of IL-6 production by reducing expression of IκBζ in RAW264 cells, | [48,63,68] | |||
| inhibition of NO production through the inhibition of NF-κB activation | [13,58,69] | |||
| 10-hydroxydecanoic acid (10-HDAA) | 0.78–1.05% | Anti-inflammatory, | [67,68] | |
| estrogenic, | [28,60] | |||
| activation of TRPA1 and TRPV1 receptors | [52,53,59] | |||
| 8-hydroxy octanoic acid | 0.18–0.39% | Varroa-repellent | [3,35,67,68] | |
| 3-hydroxydecanoic acid | 0.05–0.09% | Antifungal | [3,5,35,67,68] | |
| 3,10-dihydroxydecanoic acid | 0.26–0.46% | Immunomodulatory, stimulation of dendritic cell, differentiation, inhibition of the proliferation of allogeneic T cells and dendritic cell-dependent production of IL-2 | [22,23,35] | |
| 9-hydroxy-2-decenoic acid | 0.07–0.15% | Signal components (pheromone) of honeybee queen | [35,49,50] | |
| 1,10-decanedioic acid (sebacic) | 0.15–0.24% | Estrogenic, | [28,53] | |
| anti-inflammatory, | [36,68,69] | |||
| hypotensive | [8,23] | |||
| 2-Decenedioic | 0.18–0.33% | <0.1–3.6% | Anti-inflammatory | [36,68,69] |
| Phenols | 0.24–0.6% | 4–10% | Antioxidant | [8,9,10,11,62,63,64,65,66,67] |
| Phenolic Acids | ||||
| Ferulic acid | 68.42 mg/100 g | Antioxidant, anti-inflammatory, and antiviral properties | [62,63] | |
| Chlorogenic Acid | 37.61 mg/100 g | |||
| Caffeic Acid | 5.14 mg/100 g | |||
| Flavonoids | ||||
| Quercetin | 16.13 mg/100 g | Anticancer, antioxidant, anti-inflammatory, and antiviral properties, neuroprotective and cardio-protective | [62,63,64,65,66,67] | |
| Naringin | 0.47 mg/100 g | |||
| Hesperetin | 0.85 mg/100 g | |||
| Galangin | 0.51 mg/100 g | |||
| Waxes | 0.3–0.36% | 5–6% | - | |
| Steroids | 0.18–0.24% | 3–4% | Effects on collagen synthesis | |
| 24-methylene cholesterol | 6.06 mg/lipid | Estrogenic | [2,3,28] | |
| Phospholipids | 0.02–0.04% | (0.4–0.8%) | - | |
| Carbohydrates | ||||
| Fructose + glucose + sucrose Fructose glucose | 7–18% 3–13% 4–8% | 90% of the total sugar 2.3–7.6% 2.9–8.1% | Act as an energy source, help in control of blood glucose and insulin metabolism, participate in cholesterol and triglyceride metabolism, and help with fermentation | [2,3,5,6,7,52] |
| Sucrose | 0.5–2.0% | <0.1–3.6% | Increases mental alertness, memory, reaction readiness, attention and the ability to solve mathematical problems, as well as reducing the feeling of fatigue | [2,3,5,6,7,52] |
| Trehalose, maltose, erlose, melibiose, ribose gentiobiose, isomaltose, raffinose, and melezitose | Modulate glucose homeostasis, reduce bone resorption and inflammation, induce autophagy and alleviate Huntington’s disease, neurodegenerative and cardiometabolic diseases, enhance resistance to oxidative stress | [2,3,5,6,7,52] | ||
| Vitamins | ||||
| Vitamin A | 1.10 mg/100 g | Helps form and maintain healthy teeth, skeletal and soft tissue, mucus membranes, and skin, reproduction, immunity, maintenance of the visual system, and epithelial cellular integrity | [2,3,5,6,7] | |
| Vitamin B1 | 2.06 mg/100 g | Transketolation, metabolism of fats, proteins, and nucleic acids | [2,3,5,6,7] | |
| Vitamin B2 | 2.77 mg/100 g | Precursor of FMN and FAD | [2,3,5,6,7] | |
| Niacin (B3) | 42.42 mg/100 g | Increase HDL | [2,3,5,6,7] | |
| Vitamin B5 (Pantothenic acid) | 52.80 mg/100 g | Constituent of coenzyme A, plays a role in breaking down fats and carbohydrates for energy, crucial for red blood cell production, production of sex and stress-related hormones from the adrenal glands, reduces cholesterol levels | [2,3,5,6,7] | |
| Vitamin B6 | 11.90 mg/100 g | Plays a role in cognitive development through the biosynthesis of neurotransmitters and in maintaining normal levels of homocysteine, an amino acid in the blood, gluconeogenesis and glycogenolysis, immune function, and hemoglobin formation, transamination, and decarboxylation of amino acids | [2,3,5,6,7] | |
| Vitamin B9 (Folic acid) | 0.40 mg/100 g | Helps to form DNA, RNA, and protein metabolism, rapid growth during pregnancy and fetal development, supports healthy blood cells, plays a key role in breaking down homocysteine, DNA biosynthesis, and methylation | [2,3,5,6,7] | |
| Vitamin B12 | 0.15 mg/100 g | Plays an essential role in red blood cell formation, cell metabolism, nerve function, and the production of DNA, the molecules inside cells that carry genetic information | [2,3,5,6,7] | |
| Vitamin C (Ascorbic acid) | 2.00 mg/100 g | Antioxidant activity, form blood vessels, cartilage, muscle, and collagen in bones | [2,3,5,6,7] | |
| Vitamin D | 0.2 mg/100 g | Calcium and phosphorus absorption, essential for the bones and teeth, the immune system, brain health, and for regulating inflammation | [2,3,5,6,7] | |
| Vitamin E | 5.00 mg/100 g | Antioxidant activity | [2,3,5,6,7] | |
| Free amino acid | ||||
| Lysine | 62.43 mg/100 g | Prevent chronic fatigue, help maintain skin, hair, nails, bones, joints, hormonal regulation, sexual vitality, weight regulation, body vitality, recovery from illness, stimulation of the immune system, health of the cardiovascular system | [3,57,64,65] | |
| Proline | 58.76 mg/100 g | |||
| Cysteine | 21.76 mg/100 g | |||
| aspartic acid | 17.33 mg/100 g | |||
| valine, glutamic acid, serine, glycine, cysteine, threonine, alanine, tyrosine, phenylalanine, hydroxyproline, leucine-isoleucine, and glutamine | less than 5 mg/100 g | |||
| Minerals | ||||
| Macroelements | 321.1–357.4 mg/100 g | |||
| Na | 0.30–13.8 mg/100 g | Helps maintain normal blood pressure, supports the work of your nerves and muscles, and regulates your body’s fluid balance | [54,55] | |
| K | 321.1–357.4 mg/100 g | Regulates fluid balance, decreases blood pressure, regulates electrical activity of muscle cells and heart, and improves bone mineral density | [53,54] | |
| Ca | 22.8–24.0 mg/100 | 14.5–113 | Effect on bone mineral density and bone mineral content, prevent fat accumulation, regulate blood pressure and premenstrual syndrome, and reduce the risk of colon cancer | [57,58] |
| Mg | 44.0–50.4 mg/100 | Helps with high blood pressure, cardiovascular diseases, osteoporosis and diabetes, reduces the inflammatory processes, improves glucose and insulin metabolism, and normalizes the lipid profile | [52,53,54,55,56] | |
| P | 338.4–412.1 mg/100 | Plays key roles in regulation of gene transcription, activation of enzymes, maintenance of normal pH in extracellular fluid, and intracellular energy storage, helps in the formation of bones and teeth | [52,53,54,55,56] | |
| S | 153.2–169.3 mg/100 | Plays role in formation of collagen and keratin proteins, responsible for wound healing and for the health of the skin, hair, nails, and connective tissue, is important for proper development of cartilage and tendons, participates in detoxification processes, blood coagulation, and the production of some bile of acids | [52,53,54,55,56] | |
| Microelements | ||||
| Fe | n.d. | Oxygen transport, DNA synthesis, and electron transport, metabolic processes and energy, regulates macrophage polarization, activity and function of neutrophils, NK, T, and B cells | [3,11,50,52] | |
| Mn | 0.01–0.08 mg/100 | Oxidative phosphorylation, fatty acids, and cholesterol metabolism, mucopolysaccharide metabolism, and urea cycle | [3,11,52] | |
| Zn | 2.07–2.58 mg/100 | Supports growth, development, and immune functions | [3,11,52] | |
| Cr | 0.03–0.15 mg/100 | Helps control whole body metabolism, utilizes energy, controls body sugar, and body function. | [3,11,52] | |
| Cu | 0.31–0.39 mg/100 | Supports energy production, iron metabolism, neuropeptide activation, connective tissue synthesis, and neurotransmitter synthesis, angiogenesis, neurohormone homeostasis, and regulation of gene expression, brain development, pigmentation, and immune system functioning, defense against oxidative damage | [3,11,50,52] |
| Effect | References |
|---|---|
| Antibacterial, antiviral, antiparasitic effect (model—microrganisms) | |
| Antibacterial | [48,294,300,301,302] |
| Antifungal (fungicidal) | [3,7,48,57,295,304] |
| Antiviral | [293,305,306,307,308,309,310,311] |
| Activity against different parasites of family Trypanosomidae | [306] |
| Bio-stimulatory effect and anti-ageing effect (model cell culture and laboratory animals) | |
| Estrogenic and gonadotropic effects confirmed on cells and in rats | [28,86,207] |
| Increase in growth and animal weight | [52,108,109,110,291,345] |
| Anticancer effects, increases immune cells’ activity and stress resistance | [36,37,226,227,228,229,230,232,243,246,254] |
| Increases reproduction capacity in rats and sheep | [121,209,211,212,213,214] |
| In vitro increases oxygen consumption in tissues, antihypoxic effect | [180,191,250] |
| Against male rabbits’ infertility, increases sexual effectiveness in rats and hamsters | [55,59,212] |
| Prolongs lifespan in rats and hamsters | [39,104,105,106,107,129] |
| Immunomodulating effects: anticancer, antiallergic, and anti-inflammatory (model—laboratory animals and humans) | |
| Immunostimulant activity, increase in number of leukocytes | [68,77,125,230,246,268] |
| Anticancer effects | [34,35,36,37,38,39,40,42,44,49,50] |
| Prevents autoimmunity in mice | [335,336,337] |
| Anti-inflammatory effects | [7,13,36,68,69,70,127,137,262,271,304,334] |
| Heart and circulatory system effects (model—laboratory animals and humans) | |
| Decreases increased blood pressure, hypotensive effect, vasodilatation | [53,54,55,56,58,59,177,187,190,191,193,194,195,196,197] |
| Antiatherosclerotic effect: decreases cholesterol and triglyceride concentration in blood, increases HDL, decreases fibrinogen in plasma and thrombosis | [27,28,127,143,150,172,173,174,187,203,308] |
| Cardio-protective effect, prevents myocarditis | [58,77,78,79,80,92,93,100,101,102,103,172,173,178,202,345] |
| Increases thyroxin levels, albumin/globulin ratio in blood, and decreases serum proteins after oral application in rats | [75,76,113] |
| Effects on central and vegetative nervous system (model—laboratory animals) | |
| Effects on central nervous activity—protection and activation | [28,29] |
| Increases brain cell differentiation | [154,156,157] |
| Improves memory and learning through upregulation of neurotrophic factor synthesis such as that of GDNF and other neurotrophins | [137,139,156,157,176] |
| Acetyl-choline-like effects on bowels and smooth muscles nerves | [137,154,156,176,177,197] |
| Calming effects (rat) | [112,157] |
| Antioxidative, hepatoprotective and radio protective effects (model—laboratory animals) | |
| Antioxidative effect | [52,58,62,64,67,83,103,108,127,147] |
| Liver protection | [81,82,83,92,100,240,263,269,290] |
| Reducing stress and teratogen effects, lung edema, and liver or kidney damage after mycotoxine intake in rats | [67,98,99,100,101,102,107] |
| Stimulates DNA synthesis in hepatocytes and protects cells from apoptosis and mitogen effect, prolongs cell proliferation, and increases albumin production | [26,113] |
| Radiation protective effect in animal experiments | [247,249,250,278,279,280,281,282,283] |
| Other effects (model—laboratory animals and humans) | |
| Prevents osteoporosis and promotes bone formation | [87,127,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220] |
| Skin protection: promotes collagen build-up | [114,117,118,119,120,131,304,318,325,334] |
| Hyperglycemic effect, prevents insulin resistance, antidiabetic effect | [166,167,169,174,184] |
| Decreases experimental colitis in rats | [61,62] |
| Antiallergic activity, decreases development of atopic dermatitis as skin lesions in rats | [334,340] |
| Usage | References |
|---|---|
| Pediatrics: | |
| Pre-term babies or inadequate food intake: improves overall condition, increase in weight, appetite, red blood cells, and hemoglobin | [6,112] |
| Geriatrics: | |
| Increase in overall well-being and recuperation from fatigue and menopausal problems | [90,121,202,211,223,224] |
| Against stenocardia and after heart attack; arteriosclerosis and atherosclerosis; hypertension | [187,188,190,195,197,202,203,204] |
| Against respiratory system diseases, asthma | [333,338,339] |
| Against diseases of the eye, e.g., blepharitis, conjunctivitis and retina burns, circulatory disorders in the eye | [97,126,161] |
| Bio-stimulating effect, increases physical endurance and work ability and increases resistance to hypoxia | [52,124,125,152] |
| Increase memory, neuro-vegetative activation | [137,138,141,145,147,154,157] |
| Anti diabetes 1 | [162,163,164,165,166,167,168,173,174] |
| Anticancer effects | [77,89] |
| Prevention of stomach and duodenum ulcer, stomach problems | [175,176,177] |
| Promote skin regeneration and skin lesion healing | [114,117,131,304,318,334] |
| Prevent degenerative processes and rheumatism | [85,88] |
| Prevent warts, acne, ulcers, seborea, neurodermatitis | [280,284,334] |
| Prevent kidney dysfunction | [264,265,269,288] |
| Biological Activity | Active Component | Molecular and Cellular Effect of RJ | References |
|---|---|---|---|
| Cellular senescence | |||
| Immune system | |||
| RJ and its components |
| [124,125] [98,132,133,134] [101,102] | |
| Nervous system | |||
| RJ and its components |
| [52,110] | |
| Cardiovascular system | |||
| RJ and its components |
| [98,132,133,134] | |
| Genes expresion and pathways involved in senescence | |||
| MRP1 |
| [98,102,110] [111] [115] [129,130,131,132] [43,44,105,106,107] [132,133,134] [109,110,117,118,119,120,121] | |
| Cognition and AD-related pathology | |||
| RJ and its lipids |
| [58,139,147] [58,135,136,137,139,140] [145,150] [58,145,146] [58] [146,155] [58,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,156] [147,154,156] [154,156] | |
| MRJPs |
| [139,159] | |
| Aging and longetivity | |||
| MRJPs |
| [11,111] [101,102,123] [110] | |
| Anti-diabetic | |||
| RJ |
| [170,171] [167,172] [28,127,172,173,174,203] [173] [167,168,169] [188] [183,189] [84,188,189] [181] [183] [184] [168] | |
| Obesity | |||
| HDAA HDEA |
| [182,183,184,185,186] [184] [189] [182] [186] [185] | |
| Hypotensive and hypolipidemic activity | |||
| MRJP1, MRJP2, and MRJP3 |
| [187,188,197] [197,198] [190,196,200] [199] | |
| Peptides (Ile-Tyr, Val-Tyr, and Ile-Val-Tyr) formed by hydrolysis of RJ by protease N |
| [190] | |
| Peptides derived from MRJP1 |
| [357] [28] [199,202,203,204] | |
| Anti-inflammatory | |||
| MRJP1 MRJP2, MRJP3, 10-HDA |
| [62,63,68,75] [85] [13,69] [85,88] | |
| Inflammatory bowel diseases | RJ |
| [72,73,74] [77,78,79,80,81,82,83,84,85,86,87,88,89] [77,78] |
| MRJP1, MRJP2, and MRJP3 |
| [76] | |
| Non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) | RJ and its 10-HDA, 10-HDAA, 2-DA, and SA |
| [80,81,82,83,84,85] [82] [35] |
| Rheumatoid arthritis (RA) | 10-HDA |
| [86] [89] |
| Multiple sclerosis | RJ and 10-HDA |
| [90,91] |
| Herpes stromal keratitis (HSK) | RJ and MRJP3 or its C-terminal tandem pentapeptide repeats (TPRs) sequence |
| [92,93,363] |
| Anti-cancer | |||
| MRJPs MRJP-1 10-HDA |
| [232,252] [242,243] [240] [232] [233] [234,235] [68] [275] [241] [252] [47,88] | |
| Anti-oxidative | |||
| MRJP-2 dipeptides Free amino acids |
| [62,63,64,65,66,67,68] [85] [67,88] [67] | |
| 4-hydroperoxy-2-decenoic acid ethyl ester (HPO-DAEE), and RJ fatty acid derivative |
| [67,68] [237,238] [98] [117,224] [233] | |
| Immunoregulatory activity | |||
| RJ and 10-HDA |
| [226] [36,226,227,228,229,230,246] [125] [246] [229,243,244,245] [230] [13] [76,238,243,246,250] [267,268] | |
| MRP3 |
| [37] | |
| MRJPs |
| [74,77,246] | |
| Anti-allergic effect | |||
| RJ |
| [37,333,334,335,336,337,338] [333] [339] | |
| Osteoporosis | |||
| 10-HDA |
| [206] [206,222] [206,220,221] [37,66,86,121,223,224,225] | |
| RJ and RJP |
| [220,221] | |
| Estrogen effect | |||
| RJ and 10-HDA |
| [121,211,212,213,214] [212,213] [208,209,210,211] | |
| Spematogenesis | |||
| RJ |
| [215,216] [217] [217,218,219] | |
| Growth-promoting and wound healing activities | |||
| RJ MRJP1 |
| [21,26,33,111,246,332] [36,313,314,315,316,317,318,319,320,321,322,323,324,325] [111] [313,314,315] [313,314,327,329,330] [326,327,328,329,330,331] | |
| SA, 10-HDA, and 10-HDAA |
| [53] | |
| Royalactin, royalisin, 10-HDA |
| [44,105,313,314,315,316,317,318,319,320,321,322,323,324,325] | |
| MRJP mixture (MRP1-MRP7) |
| [26,111,313,314,315,316,317,318,319,320,321,322,323,324,325] [29] [44] [26] [313,314,315] | |
| Antimicrobial activity | |||
| cysteine-rich AMPs and royalisin Jelleine-I-IV Chlorine-jelleine-I (Cl-J-I), bromine-jelleine-I (Br-J-I), and iodine-jelleine-I (I-J-I), MRJP1 |
| [48,63,68,292,293,294,295,296,297,298,299,300] [301,304,305] [307] | |
| glucose oxidase enzyme (GOx) |
| [292,298,299] | |
| Antiviral activity | - | ||
| 10-HDA |
| [48,63,68,292,293,294,295] [307] | |
| MRJPs MRJP2 and MRJP2 X1 MRJP1 |
| [305,306,307,308,309,310,311] [308] [307] | |
| RJ |
| [292,298,299,327,328,329,330,331] |
| Application | Dose/Period of Treatment | Conditions/Diseases | References |
|---|---|---|---|
| Infants: | 0.5 g/day for 2–12 months |
| [6] |
| For premature babies | 50 mg to 1 g per day |
| [6] |
| Children: Children aged 1–5 yrs old Children aged 5–12 yrs old | 0.5 g/day 0.5–1 g/day different period, condition-depedent |
| [6] |
| Children aged 1–5 yrs old Children aged 5–12 yrs old | 2.5 g/day, 1–3 days 5 g/day, 1–3 days |
| [6] |
| Children | 2 g, 3 months |
| [336] |
| Adults: | 1 to 2 g/day |
| [6,29,36] |
| 3–5 g/day |
| [6] | |
| 3000 mg, 6 months |
| [223] | |
| Up to 10 g/day for 1–3 days |
| [6] | |
| 10 g/day for 1–3 days 5–10 g/day up to 3–5 days |
| [6] | |
| 1000 mg, 8 weeks |
| [91,211,224,225] | |
| 5000, 8 weeks |
| [6,364] | |
| 1000 mg, 8 weeks |
| [365] | |
| 3600 mg, 24 months |
| [366] | |
| 500 mg, 3 weeks |
| [90] | |
| 10–15 g/day, long-term |
| [6,36] | |
| 800 mg, 12 weeks |
| [367] | |
| 3000 mg, 2 weeks |
| [368] | |
| 100 mg, 8 weeks |
| [369] | |
| 1000 mg, 8 weeks |
| [91,92] | |
| 900 mg, 13 weeks |
| [252] | |
| 3000 mg, 6–8 weeks |
| [252,264,281] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oršolić, N.; Jazvinšćak Jembrek, M. Royal Jelly: Biological Action and Health Benefits. Int. J. Mol. Sci. 2024, 25, 6023. https://doi.org/10.3390/ijms25116023
Oršolić N, Jazvinšćak Jembrek M. Royal Jelly: Biological Action and Health Benefits. International Journal of Molecular Sciences. 2024; 25(11):6023. https://doi.org/10.3390/ijms25116023
Chicago/Turabian StyleOršolić, Nada, and Maja Jazvinšćak Jembrek. 2024. "Royal Jelly: Biological Action and Health Benefits" International Journal of Molecular Sciences 25, no. 11: 6023. https://doi.org/10.3390/ijms25116023
APA StyleOršolić, N., & Jazvinšćak Jembrek, M. (2024). Royal Jelly: Biological Action and Health Benefits. International Journal of Molecular Sciences, 25(11), 6023. https://doi.org/10.3390/ijms25116023







