Gene Expression and Prognostic Value of NADPH Oxidase Enzymes in Breast Cancer
Abstract
1. Introduction
2. Results
2.1. Expression of NOX Family Genes in the Breast Tissue
2.2. Comparison of NOX mRNA Levels in Non-Tumoral and Breast Tumor Tissues
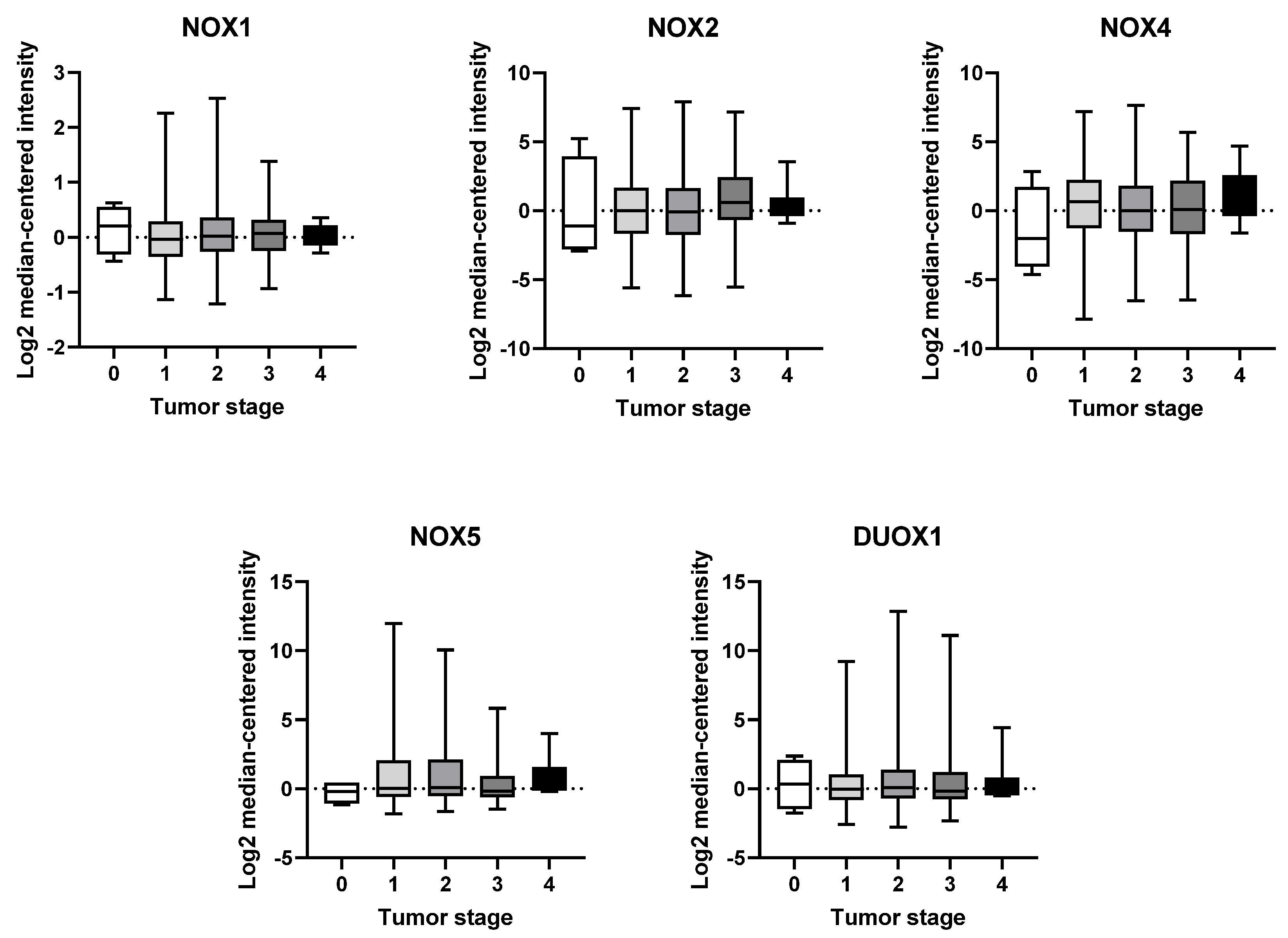
2.3. mRNA Expression of NOX Genes at Different Stages of Breast Tumor Development
2.4. Survival Analysis of NOX Members in BC Patients
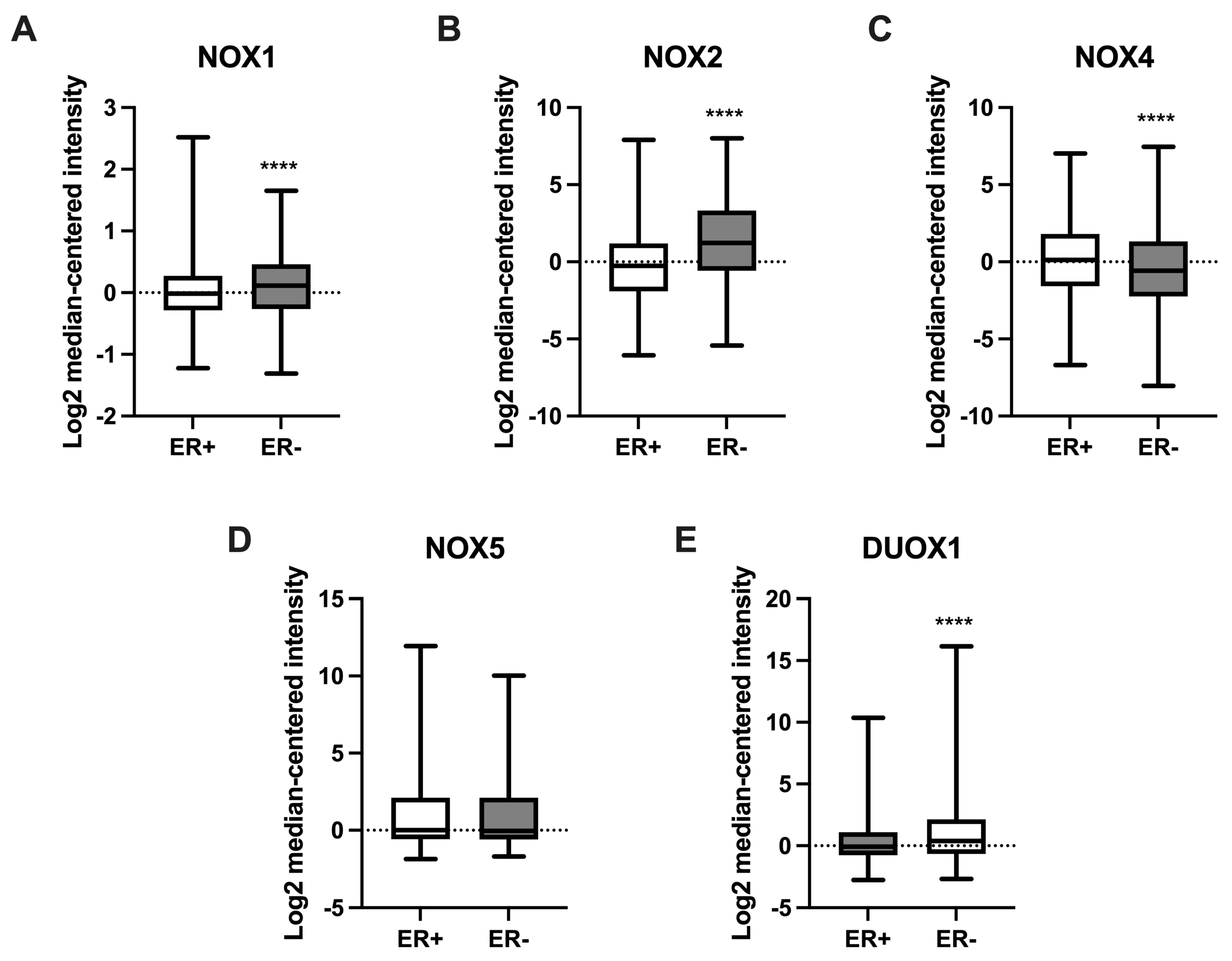
2.5. NOX Gene Expression in Relation to Estrogen Receptor Status in Breast Cancer Samples
3. Discussion
4. Materials and Methods
4.1. Gene Expression Profiles in Breast Tissue
4.2. Analysis of Gene Expression in Breast Tumor Tissue
4.3. Analysis of Patient Survival According to NOX Expression
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Turkoz, F.P.; Solak, M.; Petekkaya, I.; Keskin, O.; Kertmen, N.; Sarici, F.; Arik, Z.; Babacan, T.; Ozisik, Y.; Altundag, K. Association between common risk factors and molecular subtypes in breast cancer patients. Breast 2013, 22, 344–350. [Google Scholar] [CrossRef]
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA—J. Am. Med. Assoc. 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Blows, F.M.; Driver, K.E.; Schmidt, M.K.; Broeks, A.; van Leeuwen, F.E.; Wesseling, J.; Cheang, M.C.; Gelmon, K.; Nielsen, T.O.; Blomqvist, C.; et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: A collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010, 7, e1000279. [Google Scholar] [CrossRef]
- Cheang, M.C.U.; Martin, M.; Nielsen, T.O.; Prat, A.; Voduc, D.; Rodriguez-Lescure, A.; Ruiz, A.; Chia, S.; Shepherd, L.; Ruiz-Borrego, M.; et al. Defining Breast Cancer Intrinsic Subtypes by Quantitative Receptor Expression. Oncologist 2015, 20, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, G.K.; Zhao, X.; Band, H.; Band, V. Histological, molecular and functional subtypes of breast cancers. Cancer Biol. Ther. 2010, 10, 955–960. [Google Scholar] [CrossRef]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Parascandolo, A.; Laukkanen, M.O. Carcinogenesis and reactive oxygen species signaling: Interaction of the NADPH oxidase NOX1-5 and superoxide dismutase 1-3 signal transduction pathways. Antioxid. Redox Signal. 2019, 30, 443–486. [Google Scholar] [CrossRef] [PubMed]
- Block, K.; Gorin, Y. Aiding and abetting roles of NOX oxidases in cellular transformation. Nat. Rev. Cancer 2012, 12, 627–637. [Google Scholar] [CrossRef]
- Graham, K.A.; Kulawiec, M.; Owens, K.M.; Li, X.; Desouki, M.M.; Chandra, D.; Singh, K.K. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol. Ther. 2010, 10, 223–231. [Google Scholar] [CrossRef]
- Boudreau, H.E.; Casterline, B.W.; Rada, B.; Korzeniowska, A.; Leto, T.L. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic. Biol. Med. 2012, 53, 1489–1499. [Google Scholar] [CrossRef]
- Dho, S.H.; Kim, J.Y.; Lee, K.P.; Kwon, E.S.; Lim, J.C.; Kim, C.J.; Jeong, D.; Kwon, K.S. STAT5A-mediated NOX5-L expression promotes the proliferation and metastasis of breast cancer cells. Exp. Cell Res. 2017, 351, 51–58. [Google Scholar] [CrossRef]
- Giuliano, A.E.; Connolly, J.L.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Hortobagyi, G.N. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 290–303. [Google Scholar] [CrossRef]
- Schröder, K.; Wandzioch, K.; Helmcke, I.; Brandes, R.P. Nox4 acts as a switch between differentiation and proliferation in preadipocytes. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 239–245. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Ago, T.; Kuroda, J.; Wakisaka, Y.; Tachibana, M.; Komori, M.; Shibahara, T.; Nakashima, H.; Nakashima, K.; Kitazono, T. Nox4 Promotes Neural Stem/Precursor Cell Proliferation and Neurogenesis in the Hippocampus and Restores Memory Function Following Trimethyltin-Induced Injury. Neuroscience 2019, 398, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.R.; Marden, C.C.; Ward-Bailey, P.; Gagnon, L.H.; Bronson, R.T.; Donahue, L.R. Congenital hypothyroidism, dwarfism, and hearing impairment caused by a missense mutation in the mouse dual oxidase 2 gene, Duox2. Mol. Endocrinol. 2007, 21, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Grasberger, H. Defects of thyroidal hydrogen peroxide generation in congenital hypothyroidism. Mol. Cell. Endocrinol. 2010, 322, 99–106. [Google Scholar] [CrossRef]
- De Faria, C.C.; Fortunato, R.S. The role of dual oxidases in physiology and cancer. Genet. Mol. Biol. 2020, 43, e20190096. [Google Scholar] [CrossRef] [PubMed]
- Jagnandan, D.; Church, J.E.; Banfi, B.; Stuehr, D.J.; Marrero, M.B.; Fulton, D.J.R. Novel mechanism of activation of NADPH oxidase 5: Calcium sensitization via phosphorylation. J. Biol. Chem. 2007, 282, 6494–6507. [Google Scholar] [CrossRef]
- Schwerd, T.; Bryant, R.V.; Pandey, S.; Capitani, M.; Meran, L.; Cazier, J.B.; Jung, J.; Mondal, K.; Parkes, M.; Mathew, C.G.; et al. NOX1 loss-of-function genetic variants in patients with inflammatory bowel disease. Mucosal Immunol. 2018, 11, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Paffenholz, R.; Bergstrom, R.A.; Pasutto, F.; Wabnitz, P.; Munroe, R.J.; Jagla, W.; Heinzmann, U.; Marquardt, A.; Bareiss, A.; Laufs, J.; et al. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004, 18, 486–491. [Google Scholar] [CrossRef]
- Dumitrescu, R.G. Interplay between genetic and epigenetic changes in breast cancer subtypes. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2018; pp. 19–34. [Google Scholar] [CrossRef]
- Prat, A.; Pineda, E.; Adamo, B.; Galván, P.; Fernández, A.; Gaba, L.; Díez, M.; Viladot, M.; Arance, A.; Muñoz, M. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast 2015, 24, S26–S35. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, R.S.; Gomes, L.R.; Munford, V.; Pessoa, C.F.; Quinet, A.; Hecht, F.; Kajitani, G.S.; Milito, C.B.; Carvalho, D.P.; Menck, C.F.M. DUOX1 silencing in mammary cell alters the response to genotoxic stress. Oxidative Med. Cell. Longev. 2018, 2018, 3570526. [Google Scholar] [CrossRef]
- Desouki, M.M.; Kulawiec, M.; Bansal, S.; Das, G.; Singh, K.K. Cross talk between mitochondria and superoxide generating NADPH oxidase in breast and ovarian tumors. Cancer Biol. Ther. 2005, 4, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Pervin, S.; Tran, L.; Urman, R.; Braga, M.; Parveen, M.; Li, S.A.; Chaudhuri, G.; Singh, R. Oxidative stress specifically downregulates survivin to promote breast tumour formation. Br. J. Cancer 2013, 108, 848–858. [Google Scholar] [CrossRef]
- Choi, J.A.; Lee, J.W.; Kim, H.; Kim, E.Y.; Seo, J.M.; Ko, J.; Kim, J.H. Pro-survival of estrogen receptor-negative breast cancer cells is regulated by a BLT2-reactive oxygen species-linked signaling pathway. Carcinogenesis 2009, 31, 543–551. [Google Scholar] [CrossRef]
- Pluchino, L.A.; Wang, H.-C.R. Chronic Exposure to Combined Carcinogens Enhances Breast Cell Carcinogenesis with Mesenchymal and Stem-Like Cell Properties. PLoS ONE 2014, 9, e108698. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Nakamura, Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008, 266, 37–52. [Google Scholar] [CrossRef]
- Kusmartsev, S.; Nefedova, Y.; Yoder, D.; Gabrilovich, D.I. Antigen-Specific Inhibition of CD8 + T Cell Response by Immature Myeloid Cells in Cancer Is Mediated by Reactive Oxygen Species. J. Immunol. 2004, 172, 989–999. [Google Scholar] [CrossRef]
- Van Der Weyden, L.; Arends, M.J.; Campbell, A.D.; Bald, T.; Wardle-Jones, H.; Griggs, N.; Velasco-Herrera, M.D.C.; Tüting, T.; Sansom, O.J.; Karp, N.A.; et al. Genome-wide in vivo screen identifies novel host regulators of metastatic colonization. Nature 2017, 541, 233–236. [Google Scholar] [CrossRef]
- Maraldi, T.; Prata, C.; Sega, F.V.D.; Caliceti, C.; Zambonin, L.; Fiorentini, D.; Hakim, G. NAD(P)H oxidase isoform Nox2 plays a prosurvival role in human leukaemia cells. Free Radic. Res. 2009, 43, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Brar, S.S.; Kennedy, T.P.; Sturrock, A.B.; Huecksteadt, T.P.; Quinn, M.T.; Whorton, A.R.; Hoidal, J.R. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am. J. Physiol. Cell Physiol. 2002, 282, C1212–C1224. [Google Scholar] [CrossRef] [PubMed]
- Malla, R.R.; Raghu, H.; Rao, J.S. Regulation of NADPH oxidase (Nox2) by lipid rafts in breast carcinoma cells. Int. J. Oncol. 2010, 37, 1483–1493. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Z.; Hu, X. Inhibiting cancer metastasis via targeting NAPDH oxidase 4. Biochem. Pharmacol. 2013, 86, 253–266. [Google Scholar] [CrossRef]
- Juhasz, A.; Ge, Y.; Markel, S.; Chiu, A.; Matsumoto, L.; van Balgooy, J.; Roy, K.; Doroshow, J.H. Expression of NADPH oxidase homologues and accessory genes in human cancer cell lines, tumours and adjacent normal tissues. Free Radic. Res. 2009, 43, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Mahbouli, S.; Der Vartanian, A.; Ortega, S.; Rougé, S.; Vasson, M.P.; Rossary, A. Leptin induces ROS via NOX5 in healthy and neoplastic mammary epithelial cells. Oncol. Rep. 2017, 38, 3254–3264. [Google Scholar] [CrossRef] [PubMed]
- Fragomeni, S.M.; Sciallis, A.; Jeruss, J.S. Molecular Subtypes and Local-Regional Control of Breast Cancer. Surg. Oncol. Clin. N. Am. 2018, 27, 95–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pei, C.; Yan, S.; Liu, G.; Liu, G.; Chen, W.; Cui, Y.; Liu, Y. NADPH oxidase 1-dependent ROS is crucial for TLR4 signaling to promote tumor metastasis of non-small cell lung cancer. Tumor Biol. 2015, 36, 1493–1502. [Google Scholar] [CrossRef]
- You, X.; Ma, M.; Hou, G.; Hu, Y.; Shi, X. Gene expression and prognosis of NOX family members in gastric cancer. OncoTargets Ther. 2018, 11, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Eun, H.S.; Cho, S.Y.; Joo, J.S.; Kang, S.H.; Moon, H.S.; Lee, E.S.; Kim, S.H.; Lee, B.S. Gene expression of NOX family members and their clinical significance in hepatocellular carcinoma. Sci. Rep. 2017, 7, 11060. [Google Scholar] [CrossRef] [PubMed]
- Pulcrano, M.; Boukheris, H.; Talbot, M.; Caillou, B.; Dupuy, C.; Virion, A.; De Vathaire, F.; Schlumberger, M. Poorly differentiated follicular thyroid carcinoma: Prognostic factors and relevance of histological classification. Thyroid 2007, 17, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.; Chin, S.F.; Rueda, O.M.; Vollan, H.K.M.; Provenzano, E.; Bardwell, H.A.; Pugh, M.; Jones, L.; Russell, R.; Sammut, S.J.; et al. The somatic mutation profiles of 2433 breast cancers refines their genomic and transcriptomic landscapes. Nat. Commun. 2016, 7, 11479. [Google Scholar] [CrossRef]

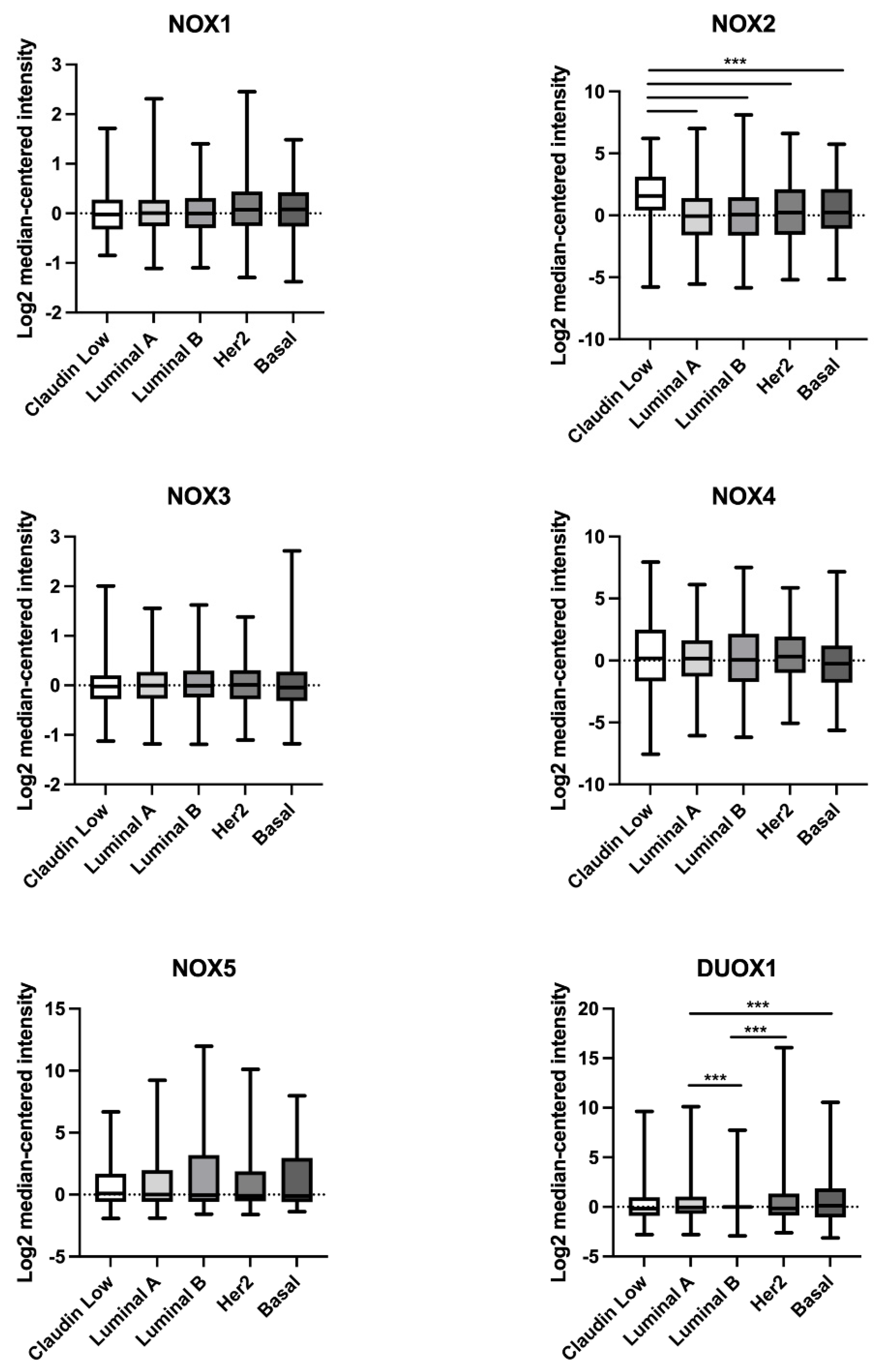

| Gene | BC Subtype | Non-Tumor | Tumor | p Value |
|---|---|---|---|---|
| NOX1 | Claudin-low | 0.03836 | −0.02103 | 0.1291 |
| Luminal A | −0.02086 | 0.00429 | 0.9626 | |
| Luminal B | 0.01854 | −0.0031 | 0.2762 | |
| HER2 | −0.09212 | 0.07419 | 0.0059 | |
| Basal | −0.09197 | 0.08255 | 0.011 | |
| NOX2 | Claudin-low | −2.2 | 1.576 | <0.0001 |
| Luminal A | 0.1081 | −0.06415 | 0.763 | |
| Luminal B | −0.1862 | 0.05626 | 0.0888 | |
| HER2 | −0.1628 | 0.2237 | 0.0483 | |
| Basal | −0.2893 | 0.24 | 0.001 | |
| NOX4 | Claudin-low | −0.3601 | 0.1767 | 0.036 |
| Luminal A | −1.125 | 0.1626 | <0.0001 | |
| Luminal B | −0.3539 | 0.0578 | 0.0564 | |
| HER2 | −0.9915 | 0.3326 | <0.0001 | |
| Basal | 0.2339 | −0.2542 | 0.0298 | |
| DUOX1 | Claudin-low | 0.2405 | −0.1769 | 0.0112 |
| Luminal A | 0.3179 | −0.04951 | 0.0154 | |
| Luminal B | 0.1411 | −0.00134 | 0.0104 | |
| HER2 | 0.3012 | −0.1476 | 0.0329 | |
| Basal | −0.2282 | 0.1196 | 0.0686 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Vasconcelos e Souza, A.; de Faria, C.C.; Pereira, L.M.; Ferreira, A.C.F.; Torres, P.H.M.; Fortunato, R.S. Gene Expression and Prognostic Value of NADPH Oxidase Enzymes in Breast Cancer. Int. J. Mol. Sci. 2024, 25, 3464. https://doi.org/10.3390/ijms25063464
de Vasconcelos e Souza A, de Faria CC, Pereira LM, Ferreira ACF, Torres PHM, Fortunato RS. Gene Expression and Prognostic Value of NADPH Oxidase Enzymes in Breast Cancer. International Journal of Molecular Sciences. 2024; 25(6):3464. https://doi.org/10.3390/ijms25063464
Chicago/Turabian Stylede Vasconcelos e Souza, Andressa, Caroline Coelho de Faria, Leonardo Matta Pereira, Andrea Claudia Freitas Ferreira, Pedro Henrique Monteiro Torres, and Rodrigo Soares Fortunato. 2024. "Gene Expression and Prognostic Value of NADPH Oxidase Enzymes in Breast Cancer" International Journal of Molecular Sciences 25, no. 6: 3464. https://doi.org/10.3390/ijms25063464
APA Stylede Vasconcelos e Souza, A., de Faria, C. C., Pereira, L. M., Ferreira, A. C. F., Torres, P. H. M., & Fortunato, R. S. (2024). Gene Expression and Prognostic Value of NADPH Oxidase Enzymes in Breast Cancer. International Journal of Molecular Sciences, 25(6), 3464. https://doi.org/10.3390/ijms25063464







