Polylevolysine and Fibronectin-Loaded Nano-Hydroxyapatite/PGLA/Dextran-Based Scaffolds for Improving Bone Regeneration: A Histomorphometric in Animal Study
Abstract
1. Introduction
2. Results
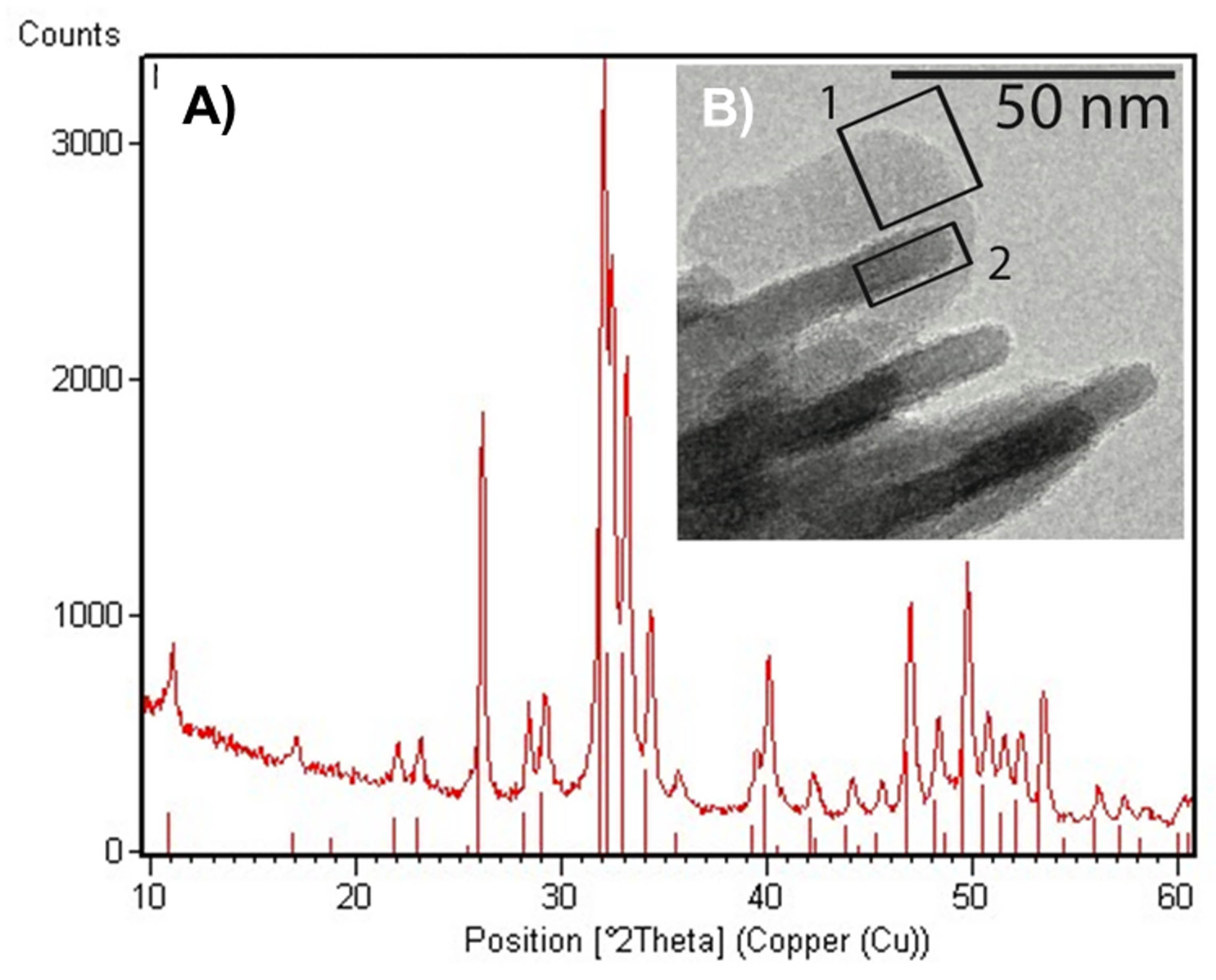
2.1. Scaffolds Physicochemical Characterization
2.1.1. Structural Characterization of HA
2.1.2. Morphological Characterization
2.2. Surgery
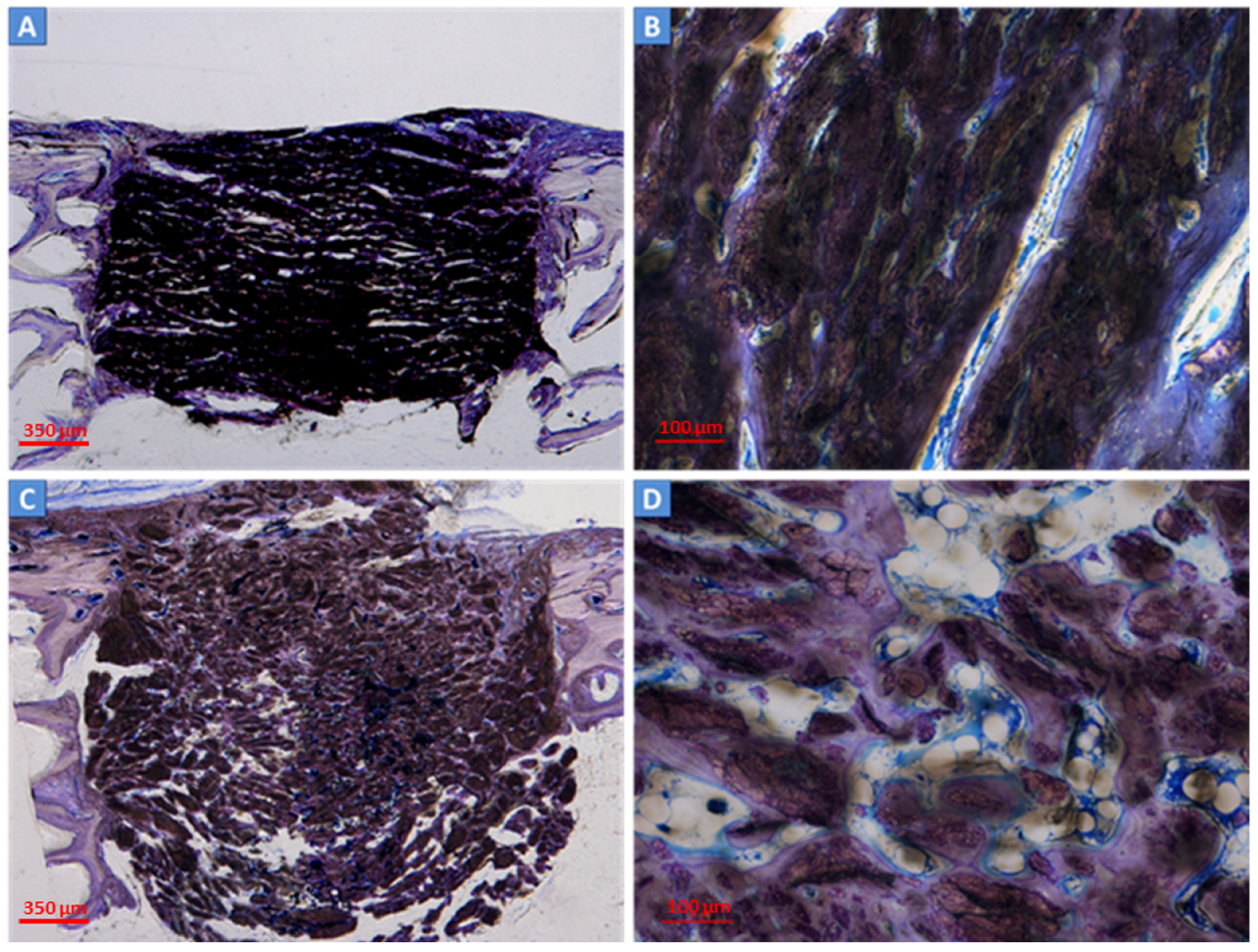
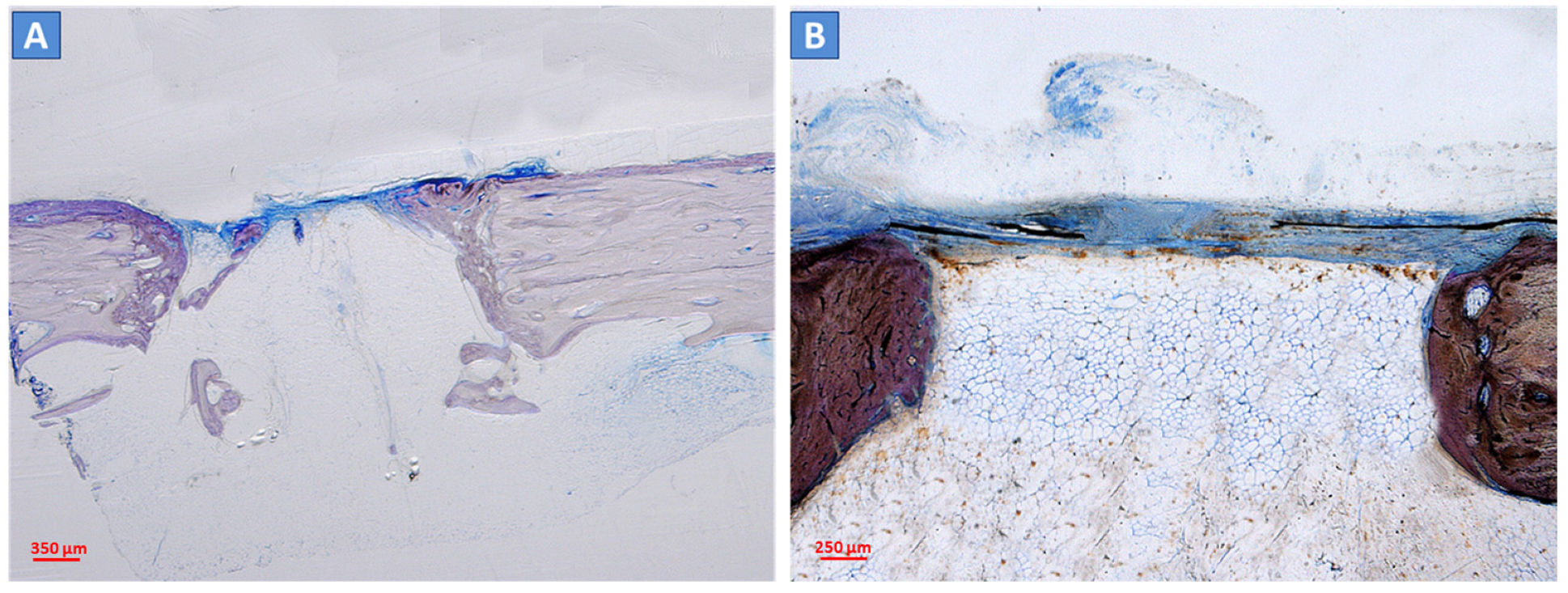
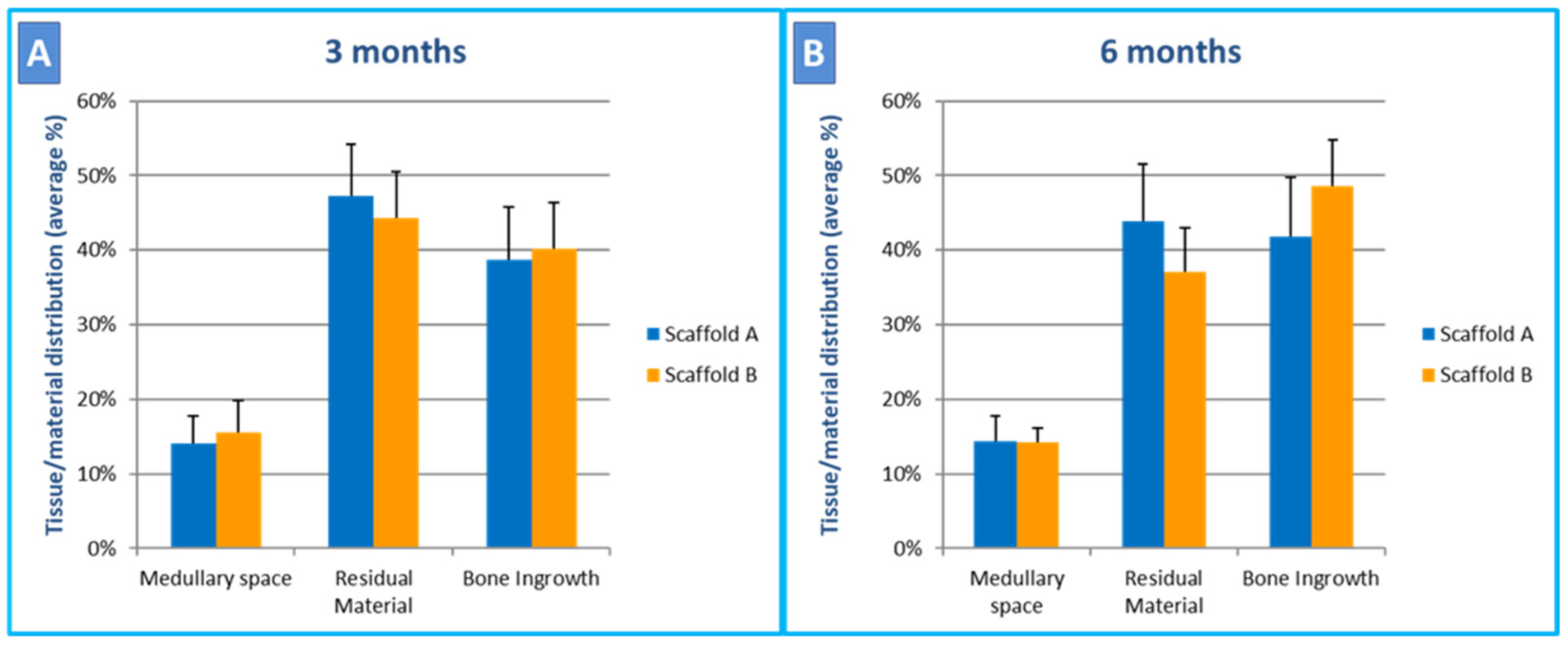
2.3. Histological and Histomorphometric Analyses
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Scaffold Fabrication
4.3. Scaffold Physicochemical Characterization
4.3.1. Structural Characterization of HA
4.3.2. Morphological Characterization
4.4. In Vivo Experiments
4.4.1. Animals
4.4.2. Surgery
4.4.3. Postoperative Histological Evaluation
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32 (Suppl. 1), S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Kengelbach-Weigand, A.; Thielen, C.; Bäuerle, T.; Götzl, R.; Gerber, T.; Körner, C.; Beier, J.P.; Horch, R.E.; Boos, A.M. Personalized Medicine for Reconstruction of Critical-Size Bone Defects—A Translational Approach with Customizable Vascularized Bone Tissue. Npj Regen. Med. 2021, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.A.; Narváez-Tovar, C.A.; Garzón-Alvarado, D.A. Design, Materials, and Mechanobiology of Biodegradable Scaffolds for Bone Tissue Engineering. BioMed Res. Int. 2015, 2015, e729076. [Google Scholar] [CrossRef] [PubMed]
- Dec, P.; Modrzejewski, A.; Pawlik, A. Existing and Novel Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2022, 24, 529. [Google Scholar] [CrossRef]
- Akilbekova, D.; Shaimerdenova, M.; Adilov, S.; Berillo, D. Biocompatible Scaffolds Based on Natural Polymers for Regenerative Medicine. Int. J. Biol. Macromol. 2018, 114, 324–333. [Google Scholar] [CrossRef]
- Balogová, A.F.; Trebuňová, M.; Bačenková, D.; Kohan, M.; Hudák, R.; Tóth, T.; Schnitzer, M.; Živčák, J. Impact of In Vitro Degradation on the Properties of Samples Produced by Additive Production from PLA/PHB-Based Material and Ceramics. Polymers 2022, 14, 5441. [Google Scholar] [CrossRef]
- Rumpel, E.; Wolf, E.; Kauschke, E.; Bienengräber, V.; Bayerlein, T.; Gedrange, T.; Proff, P. The Biodegradation of Hydroxyapatite Bone Graft Substitutes in Vivo. Folia Morphol. 2006, 65, 43–48. [Google Scholar]
- Akpan, E.S.; Dauda, M.; Kuburi, L.S.; Obada, D.O.; Dodoo-Arhin, D. A Comparative Study of the Mechanical Integrity of Natural Hydroxyapatite Scaffolds Prepared from Two Biogenic Sources Using a Low Compaction Pressure Method. Results Phys. 2020, 17, 103051. [Google Scholar] [CrossRef]
- Lyons, J.G.; Plantz, M.A.; Hsu, W.K.; Hsu, E.L.; Minardi, S. Nanostructured Biomaterials for Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 922. [Google Scholar] [CrossRef]
- Mo, X.; Zhang, D.; Liu, K.; Zhao, X.; Li, X.; Wang, W. Nano-Hydroxyapatite Composite Scaffolds Loaded with Bioactive Factors and Drugs for Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 1291. [Google Scholar] [CrossRef]
- Ignjatovic, N.L.; Ajdukovic, Z.R.; Savic, V.P.; Uskokovic, D.P. Size effect of calcium phosphate coated with poly-DL-lactide- co-glycolide on healing processes in bone reconstruction. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 108–117. [Google Scholar] [CrossRef]
- Rajula, M.P.B.; Narayanan, V.; Venkatasubbu, G.D.; Mani, R.C.; Sujana, A. Nano-Hydroxyapatite: A Driving Force for Bone Tissue Engineering. J. Pharm. Bioallied Sci. 2021, 13, S11–S14. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.-K. Nano-Hydroxyapatite Composite Biomaterials for Bone Tissue Engineering—A Review. J. Biomed. Nanotechnol. 2014, 10, 3124–3140. [Google Scholar] [CrossRef]
- Urbánek, T.; Jäger, E.; Jäger, A.; Hrubý, M. Selectively Biodegradable Polyesters: Nature-Inspired Construction Materials for Future Biomedical Applications. Polymers 2019, 11, 1061. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, T.; Li, J.; Cui, L.; Zhang, Z.; Zhuang, X.; Ding, J. Poly(Lactic-Co-Glycolic Acid)-Based Composite Bone-Substitute Materials. Bioact. Mater. 2021, 6, 346–360. [Google Scholar] [CrossRef]
- Pan, Z.; Ding, J. Poly(Lactide-Co-Glycolide) Porous Scaffolds for Tissue Engineering and Regenerative Medicine. Interface Focus 2012, 2, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Born, G.; Chaaban, M.; Scherberich, A. Natural Polymeric Scaffolds in Bone Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 474. [Google Scholar] [CrossRef]
- Sun, G.; Mao, J.J. Engineering Dextran-Based Scaffolds for Drug Delivery and Tissue Repair. Nanomedicine 2012, 7, 1771–1784. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Tsuji, N.; Shimomura, Y.; Hayashi, H.; Ohgushi, H. Osteogenesis Depending on Geometry of Porous Hydroxyapatite Scaffolds. Calcif. Tissue Int. 2008, 83, 139–145. [Google Scholar] [CrossRef]
- Schönmeyr, B.H.; Wong, A.K.; Li, S.; Gewalli, F.; Cordiero, P.G.; Mehrara, B.J. Treatment of Hydroxyapatite Scaffolds with Fibronectin and Fetal Calf Serum Increases Osteoblast Adhesion and Proliferation in Vitro. Plast. Reconstr. Surg. 2008, 121, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.; Canciani, E.; Palazzo, B.; Varasano, V.; Chevallier, P.; Petrizzi, L.; Dellavia, C.; Mantovani, D.; Rimondini, L. Effect of Poly-L-Lysine Coating on Titanium Osseointegration: From Characterization to in Vivo Studies. J. Oral Implantol. 2015, 41, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Roumani, S.; Jeanneau, C.; Giraud, T.; Cotten, A.; Laucournet, M.; Sohier, J.; Pithioux, M.; About, I. Osteogenic Potential of a Polyethylene Glycol Hydrogel Functionalized with Poly-Lysine Dendrigrafts (DGL) for Bone Regeneration. Mater. Basel Switz. 2023, 16, 862. [Google Scholar] [CrossRef]
- Woldetsadik, A.D.; Sharma, S.K.; Khapli, S.; Jagannathan, R.; Magzoub, M. Hierarchically Porous Calcium Carbonate Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2017, 3, 2457–2469. [Google Scholar] [CrossRef] [PubMed]
- Shirbhate, U.; Bajaj, P.; Pandher, J.; Durge, K. Fibronectin and Its Applications in Dentistry and Periodontics: A Cell Behaviour Conditioner. Cureus 2022, 14, e30702. [Google Scholar] [CrossRef]
- Palomino-Durand, C.; Pauthe, E.; Gand, A. Fibronectin-Enriched Biomaterials, Biofunctionalization, and Proactivity: A Review. Appl. Sci. 2021, 11, 12111. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Ghorbani, S.; Barani, M.; Singh Chauhan, N.P.; Khodadadi Yazdi, M.; Saeb, M.R.; Ramsey, J.D.; Hamblin, M.R.; Mozafari, M.; Mostafavi, E. Polylysine for Skin Regeneration: A Review of Recent Advances and Future Perspectives. Bioeng. Transl. Med. 2021, 7, e10261. [Google Scholar] [CrossRef]
- Abdala, P.M.F.; Iyomasa, M.M.; Sato, S.; Bentley, M.V.L.B.; Pitol, D.L.; Regalo, S.C.H.; Siéssere, S.; Issa, J.P.M. Osteoinductivity Potential of RhBMP-2 Associated with Two Carriers in Different Dosages. Anat. Sci. Int. 2010, 85, 181–188. [Google Scholar] [CrossRef]
- Petrizzi, L.; Mariscoli, M.; Valbonetti, L.; Varasano, V.; Langhoff, J.D.; Von Rechenberg, B. Preliminary Study on the Effect of Parenteral Naloxone, Alone and in Association with Calcium Gluconate, on Bone Healing in an Ovine “Drill Hole” Model System. BMC Musculoskelet. Disord. 2007, 8, 43. [Google Scholar] [CrossRef]
- Theiss, F.; Apelt, D.; Brand, B.; Kutter, A.; Zlinszky, K.; Bohner, M.; Matter, S.; Frei, C.; Auer, J.A.; von Rechenberg, B. Biocompatibility and Resorption of a Brushite Calcium Phosphate Cement. Biomaterials 2005, 26, 4383–4394. [Google Scholar] [CrossRef]
- Plunkett, N.A.; Partap, S.; O’Brien, F.J. Osteoblast Response to Rest Periods during Bioreactor Culture of Collagen-Glycosaminoglycan Scaffolds. Tissue Eng. Part A 2010, 16, 943–951. [Google Scholar] [CrossRef]
- Kavasi, R.-M.; Coelho, C.C.; Platania, V.; Quadros, P.A.; Chatzinikolaidou, M. In Vitro Biocompatibility Assessment of Nano-Hydroxyapatite. Nanomaterials 2021, 11, 1152. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, Biodegradation and Biomedical Applications of Poly(Lactic Acid)/Poly(Lactic-Co-Glycolic Acid) Micro and Nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Arredondo, R.; Poggioli, F.; Martínez-Díaz, S.; Piera-Trilla, M.; Torres-Claramunt, R.; Tío, L.; Monllau, J.C. Fibronectin-Coating Enhances Attachment and Proliferation of Mesenchymal Stem Cells on a Polyurethane Meniscal Scaffold. Regen. Ther. 2021, 18, 480–486. [Google Scholar] [CrossRef]
- Woodard, J.R.; Hilldore, A.J.; Lan, S.K.; Park, C.J.; Morgan, A.W.; Eurell, J.A.C.; Clark, S.G.; Wheeler, M.B.; Jamison, R.D.; Wagoner Johnson, A.J. The Mechanical Properties and Osteoconductivity of Hydroxyapatite Bone Scaffolds with Multi-Scale Porosity. Biomaterials 2007, 28, 45–54. [Google Scholar] [CrossRef]
- Lewandrowski, K.-U.; Bondre, S.P.; Wise, D.L.; Trantolo, D.J. Enhanced Bioactivity of a Poly(Propylene Fumarate) Bone Graft Substitute by Augmentation with Nano-Hydroxyapatite. Biomed. Mater. Eng. 2003, 13, 115–124. [Google Scholar] [PubMed]
- Li, M.; Liu, W.; Sun, J.; Xianyu, Y.; Wang, J.; Zhang, W.; Zheng, W.; Huang, D.; Di, S.; Long, Y.-Z.; et al. Culturing Primary Human Osteoblasts on Electrospun Poly(Lactic-Co-Glycolic Acid) and Poly(Lactic-Co-Glycolic Acid)/Nanohydroxyapatite Scaffolds for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2013, 5, 5921–5926. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Hu, Y.; Zeng, M.; Li, M.; Lin, S.; Zhou, Y.; Xie, J. Design and Evaluation of Nano-Hydroxyapatite/Poly(Vinyl Alcohol) Hydrogels Coated with Poly(Lactic-Co-Glycolic Acid)/Nano-Hydroxyapatite/Poly(Vinyl Alcohol) Scaffolds for Cartilage Repair. J. Orthop. Surg. 2019, 14, 446. [Google Scholar] [CrossRef]
- Mohamadyar-Toupkanlou, F.; Vasheghani-Farahani, E.; Hanaee-Ahvaz, H.; Soleimani, M.; Dodel, M.; Havasi, P.; Ardeshirylajimi, A.; Taherzadeh, E.S. Osteogenic Differentiation of MSCs on Fibronectin-Coated and NHA-Modified Scaffolds. ASAIO J. 2017, 63, 684–691. [Google Scholar] [CrossRef]
- Ai, C.; Liu, L.; Goh, J.C.-H. Pore Size Modulates in Vitro Osteogenesis of Bone Marrow Mesenchymal Stem Cells in Fibronectin/Gelatin Coated Silk Fibroin Scaffolds. Mater. Sci. Eng. C 2021, 124, 112088. [Google Scholar] [CrossRef]
- Tian, B.; Wang, N.; Jiang, Q.; Tian, L.; Hu, L.; Zhang, Z. The Immunogenic Reaction and Bone Defect Repair Function of ε-Poly-L-Lysine (EPL)-Coated Nanoscale PCL/HA Scaffold in Rabbit Calvarial Bone Defect. J. Mater. Sci. Mater. Med. 2021, 32, 63. [Google Scholar] [CrossRef]
- Zhu, J.; Qi, Z.; Zheng, C.; Xue, P.; Fu, C.; Pan, S.; Yang, X. Enhanced Cell Proliferation and Osteogenesis Differentiation through a Combined Treatment of Poly-L-Lysine-Coated PLGA/Graphene Oxide Hybrid Fiber Matrices and Electrical Stimulation. J. Nanomater. 2020, 2020, e5892506. [Google Scholar] [CrossRef]
- Lee, M.-J.; Sohn, S.-K.; Kim, K.-T.; Kim, C.-H.; Ahn, H.-B.; Rho, M.-S.; Jeong, M.-H.; Sun, S.-K. Effect of Hydroxyapatite on Bone Integration in a Rabbit Tibial Defect Model. Clin. Orthop. Surg. 2010, 2, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Canciani, E.; Ragone, V.; Biffi, C.A.; Valenza, F.; D’Ambrosi, R.; Olimpo, M.; Cristofalo, A.; Galliera, E.; Dellavia, C. Understanding the Role of Surface Modification of Randomized Trabecular Titanium Structures in Bone Tissue Regeneration: An Experimental Study. Medicina 2022, 58, 315. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canciani, E.; Straticò, P.; Varasano, V.; Dellavia, C.; Sciarrini, C.; Petrizzi, L.; Rimondini, L.; Varoni, E.M. Polylevolysine and Fibronectin-Loaded Nano-Hydroxyapatite/PGLA/Dextran-Based Scaffolds for Improving Bone Regeneration: A Histomorphometric in Animal Study. Int. J. Mol. Sci. 2023, 24, 8137. https://doi.org/10.3390/ijms24098137
Canciani E, Straticò P, Varasano V, Dellavia C, Sciarrini C, Petrizzi L, Rimondini L, Varoni EM. Polylevolysine and Fibronectin-Loaded Nano-Hydroxyapatite/PGLA/Dextran-Based Scaffolds for Improving Bone Regeneration: A Histomorphometric in Animal Study. International Journal of Molecular Sciences. 2023; 24(9):8137. https://doi.org/10.3390/ijms24098137
Chicago/Turabian StyleCanciani, Elena, Paola Straticò, Vincenzo Varasano, Claudia Dellavia, Chiara Sciarrini, Lucio Petrizzi, Lia Rimondini, and Elena M. Varoni. 2023. "Polylevolysine and Fibronectin-Loaded Nano-Hydroxyapatite/PGLA/Dextran-Based Scaffolds for Improving Bone Regeneration: A Histomorphometric in Animal Study" International Journal of Molecular Sciences 24, no. 9: 8137. https://doi.org/10.3390/ijms24098137
APA StyleCanciani, E., Straticò, P., Varasano, V., Dellavia, C., Sciarrini, C., Petrizzi, L., Rimondini, L., & Varoni, E. M. (2023). Polylevolysine and Fibronectin-Loaded Nano-Hydroxyapatite/PGLA/Dextran-Based Scaffolds for Improving Bone Regeneration: A Histomorphometric in Animal Study. International Journal of Molecular Sciences, 24(9), 8137. https://doi.org/10.3390/ijms24098137













