The Role of HIF-1α in Bone Regeneration: A New Direction and Challenge in Bone Tissue Engineering
Abstract
1. Introduction
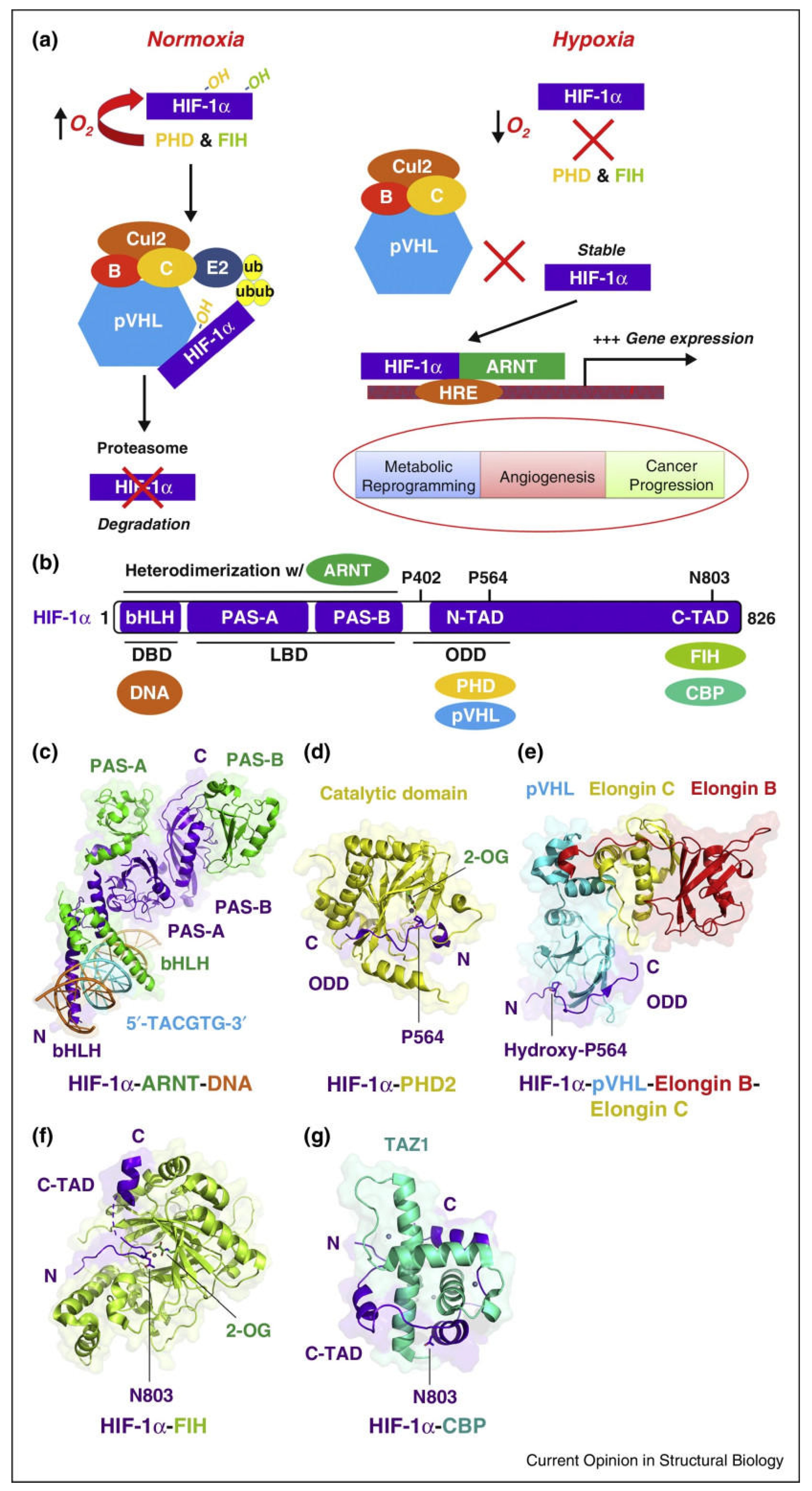
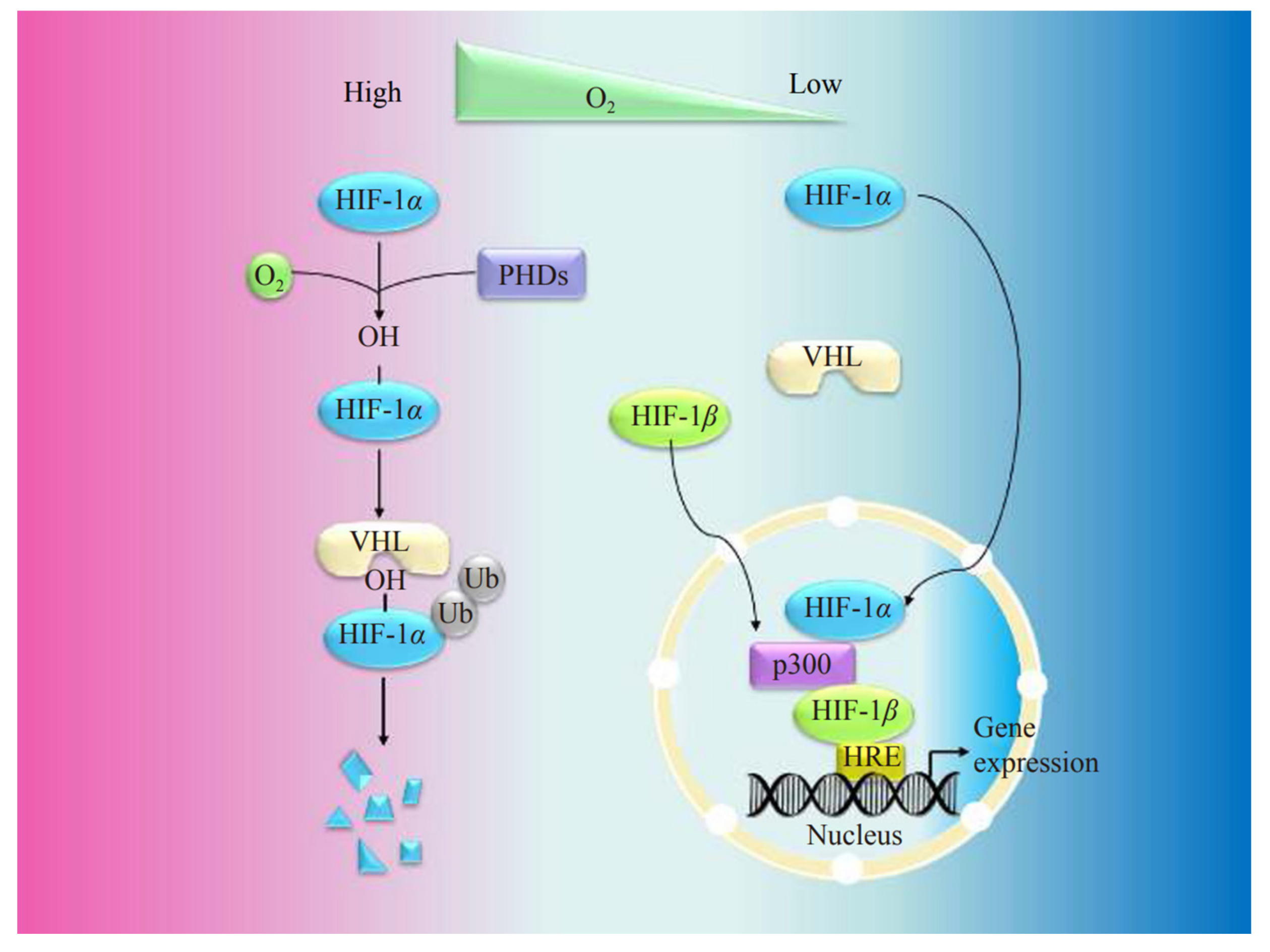
2. Basic Structure and Biological Properties of HIF
3. Bone Formation
3.1. Intramembranous Ossification
3.2. Endochondral Ossification
4. The Role of HIF-1α in Bone Formation
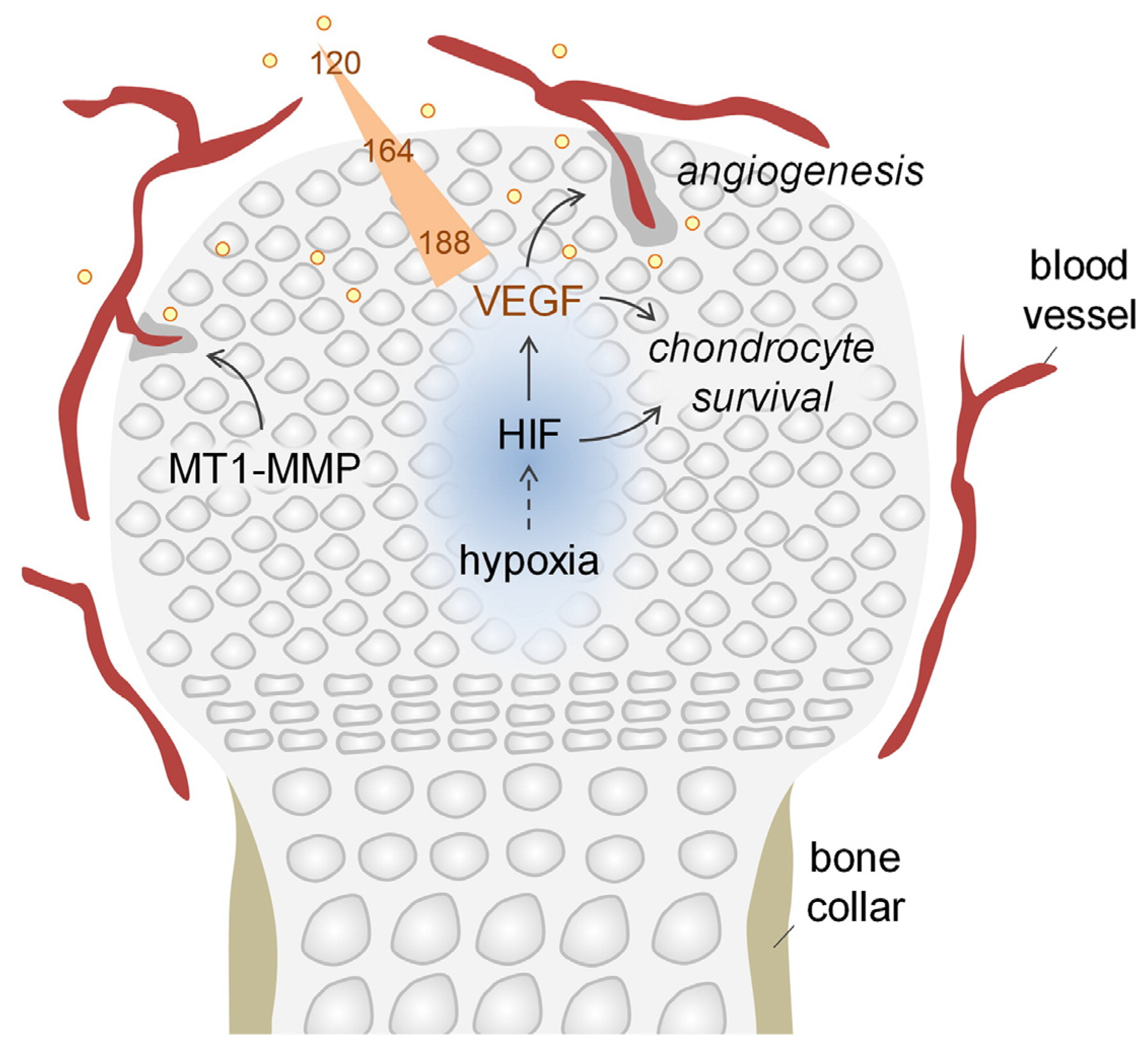
4.1. Promotion of Angiogenesis
4.2. Promotion of Osteogenesis
4.3. Change in Metabolism
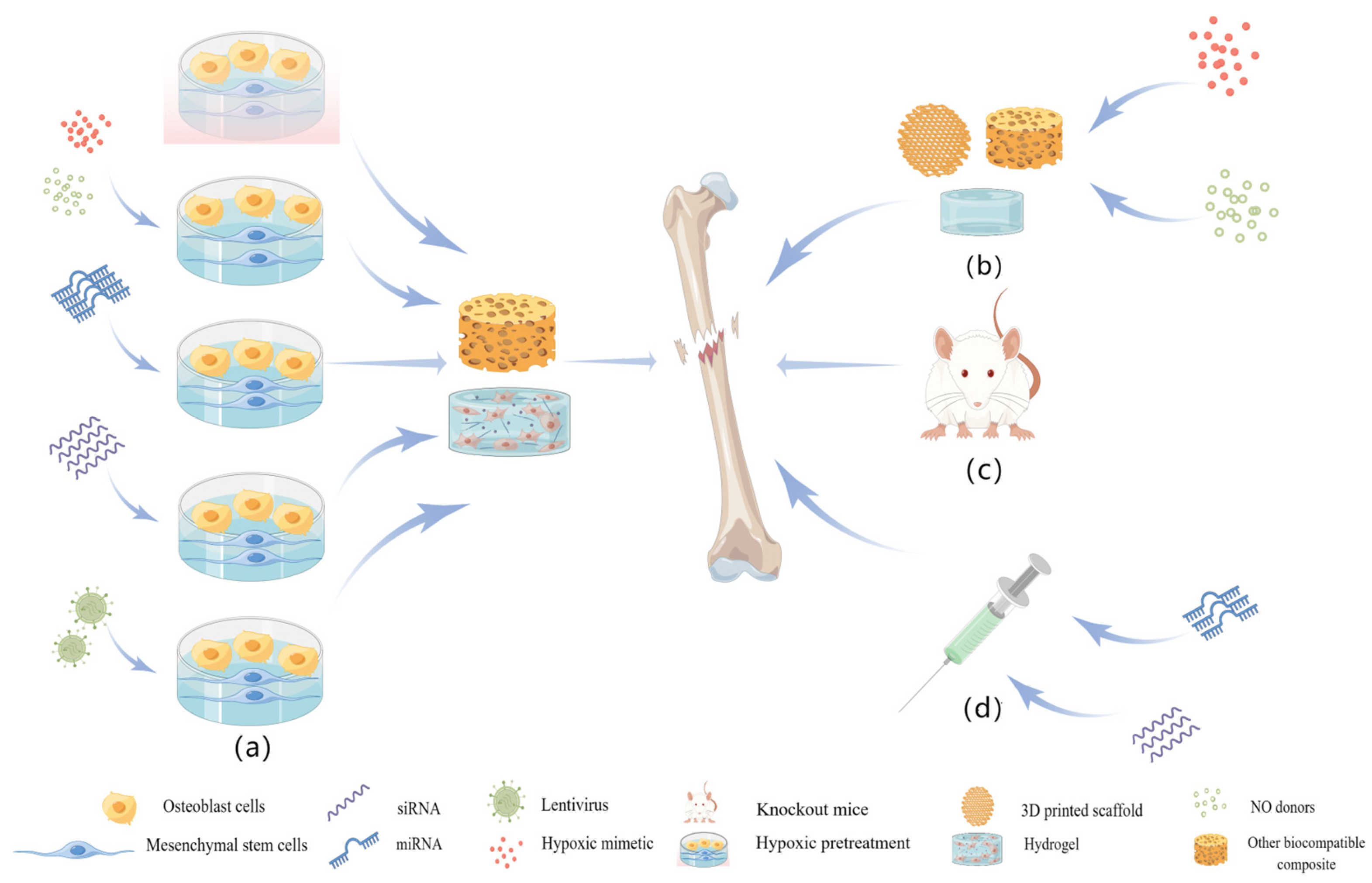
5. Method of Stabilizing HIF in Bone Tissue Engineering
5.1. Gene Therapy
5.2. Hypoxic Mimetics
5.2.1. Iron Chelator
5.2.2. 2-OG Analogs
5.3. Others
6. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hernlund, E.; Svedbom, A.; Ivergård, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jönsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef]
- Smith, C.A.; Richardson, S.M.; Eagle, M.J.; Rooney, P.; Board, T.; Hoyland, J.A. The use of a novel bone allograft wash process to generate a biocompatible, mechanically stable and osteoinductive biological scaffold for use in bone tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.M.; Skaer, N.J.V.; Gheysens, T.; Knight, D.; Bertram, C.; Roach, H.I.; Oreffo, R.O.C.; Von-Aulock, S.; Baris, T.; Skinner, J.; et al. Bone-like Resorbable Silk-based Scaffolds for Load-bearing Osteoregenerative Applications. Adv. Mater. 2009, 21, 75–78. [Google Scholar] [CrossRef]
- Basal, O.; Ozmen, O.; Deliormanlı, A.M. Bone Healing in Rat Segmental Femur Defects with Graphene-PCL-Coated Borate-Based Bioactive Glass Scaffolds. Polymers 2022, 14, 3898. [Google Scholar] [CrossRef]
- Nadine, S.; Correia, C.R.; Mano, J.F. Engineering immunomodulatory hydrogels and cell-laden systems towards bone regeneration. Biomater. Adv. 2022, 140, 213058. [Google Scholar] [CrossRef] [PubMed]
- Nadine, S.; Fernandes, I.J.; Correia, C.R.; Mano, J.F. Close-to-native bone repair via tissue-engineered endochondral ossification approaches. iScience 2022, 25, 105370. [Google Scholar] [CrossRef]
- Kronenberg, H.M. Developmental regulation of the growth plate. Nature 2003, 423, 332–336. [Google Scholar] [CrossRef]
- Duchamp de Lageneste, O.; Julien, A.; Abou-Khalil, R.; Frangi, G.; Carvalho, C.; Cagnard, N.; Cordier, C.; Conway, S.J.; Colnot, C. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat. Commun. 2018, 9, 773. [Google Scholar] [CrossRef]
- Grcevic, D.; Pejda, S.; Matthews, B.G.; Repic, D.; Wang, L.; Li, H.; Kronenberg, M.S.; Jiang, X.; Maye, P.; Adams, D.J.; et al. In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells 2012, 30, 187–196. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- O’Keefe, R.J. Fibrinolysis as a Target to Enhance Fracture Healing. N. Engl. J. Med. 2015, 373, 1776–1778. [Google Scholar] [CrossRef] [PubMed]
- de Jong, T.; Bakker, A.D.; Everts, V.; Smit, T.H. The intricate anatomy of the periodontal ligament and its development: Lessons for periodontal regeneration. J. Periodontal Res. 2017, 52, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.M.; Matsiko, A.; Farrell, E.; Kelly, D.J.; O’Brien, F.J. Recapitulating endochondral ossification: A promising route to in vivo bone regeneration. J. Tissue Eng. Regen. Med. 2015, 9, 889–902. [Google Scholar] [CrossRef]
- Visser, J.; Gawlitta, D.; Benders, K.E.; Toma, S.M.; Pouran, B.; van Weeren, P.R.; Dhert, W.J.; Malda, J. Endochondral bone formation in gelatin methacrylamide hydrogel with embedded cartilage-derived matrix particles. Biomaterials 2015, 37, 174–182. [Google Scholar] [CrossRef]
- Yu, B.; Wang, X.; Song, Y.; Xie, G.; Jiao, S.; Shi, L.; Cao, X.; Han, X.; Qu, A. The role of hypoxia-inducible factors in cardiovascular diseases. Pharmacol. Ther. 2022, 238, 108186. [Google Scholar] [CrossRef] [PubMed]
- Acker, T.; Plate, K.H. Hypoxia and hypoxia inducible factors (HIF) as important regulators of tumor physiology. Cancer Treat. Res. 2004, 117, 219–248. [Google Scholar] [CrossRef]
- You, L.; Wu, W.; Wang, X.; Fang, L.; Adam, V.; Nepovimova, E.; Wu, Q.; Kuca, K. The role of hypoxia-inducible factor 1 in tumor immune evasion. Med. Res. Rev. 2021, 41, 1622–1643. [Google Scholar] [CrossRef]
- Wu, D.; Rastinejad, F. Structural characterization of mammalian bHLH-PAS transcription factors. Curr. Opin. Struct. Biol. 2017, 43, 1–9. [Google Scholar] [CrossRef]
- Keith, B.; Johnson, R.S.; Simon, M.C. HIF1α and HIF2α: Sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 2011, 12, 9–22. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Y.; Zhao, Y.; Chen, X.; Xiao, Y.; Bao, C. HIF signaling: A new propellant in bone regeneration. Biomater. Adv. 2022, 138, 212874. [Google Scholar] [CrossRef]
- Yellowley, C.E.; Genetos, D.C. Hypoxia Signaling in the Skeleton: Implications for Bone Health. Curr. Osteoporos. Rep. 2019, 17, 26–35. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia-inducible factors in physiology and medicine. Cell 2012, 148, 399–408. [Google Scholar] [CrossRef]
- Schito, L.; Semenza, G.L. Hypoxia-Inducible Factors: Master Regulators of Cancer Progression. Trends Cancer 2016, 2, 758–770. [Google Scholar] [CrossRef]
- Yang, S.L.; Wu, C.; Xiong, Z.F.; Fang, X. Progress on hypoxia-inducible factor-3: Its structure, gene regulation and biological function (Review). Mol. Med. Rep. 2015, 12, 2411–2416. [Google Scholar] [CrossRef]
- Lin, Q.; Cong, X.; Yun, Z. Differential hypoxic regulation of hypoxia-inducible factors 1αlpha and 2αlpha. Mol. Cancer Res. MCR 2011, 9, 757–765. [Google Scholar] [CrossRef]
- Noguera, R.; Fredlund, E.; Piqueras, M.; Pietras, A.; Beckman, S.; Navarro, S.; Påhlman, S. HIF-1αlpha and HIF-2αlpha are differentially regulated in vivo in neuroblastoma: High HIF-1αlpha correlates negatively to advanced clinical stage and tumor vascularization. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 7130–7136. [Google Scholar] [CrossRef] [PubMed]
- Koh, M.Y.; Lemos, R., Jr.; Liu, X.; Powis, G. The hypoxia-associated factor switches cells from HIF-1α- to HIF-2α-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res. 2011, 71, 4015–4027. [Google Scholar] [CrossRef]
- Yang, C.; Zhong, Z.F.; Wang, S.P.; Vong, C.T.; Yu, B.; Wang, Y.T. HIF-1: Structure, biology and natural modulators. Chin. J. Nat. Med. 2021, 19, 521–527. [Google Scholar] [CrossRef]
- Epstein, A.C.; Gleadle, J.M.; McNeill, L.A.; Hewitson, K.S.; O’Rourke, J.; Mole, D.R.; Mukherji, M.; Metzen, E.; Wilson, M.I.; Dhanda, A.; et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001, 107, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Hon, W.C.; Wilson, M.I.; Harlos, K.; Claridge, T.D.; Schofield, C.J.; Pugh, C.W.; Maxwell, P.H.; Ratcliffe, P.J.; Stuart, D.I.; Jones, E.Y. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature 2002, 417, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Min, J.H.; Yang, H.; Ivan, M.; Gertler, F.; Kaelin, W.G., Jr.; Pavletich, N.P. Structure of an HIF-1αlpha -pVHL complex: Hydroxyproline recognition in signaling. Science 2002, 296, 1886–1889. [Google Scholar] [CrossRef]
- Lando, D.; Peet, D.J.; Gorman, J.J.; Whelan, D.A.; Whitelaw, M.L.; Bruick, R.K. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002, 16, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Freedman, S.J.; Sun, Z.Y.; Poy, F.; Kung, A.L.; Livingston, D.M.; Wagner, G.; Eck, M.J. Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proc. Natl. Acad. Sci. USA 2002, 99, 5367–5372. [Google Scholar] [CrossRef]
- Wu, D.; Su, X.; Lu, J.; Li, S.; Hood, B.L.; Vasile, S.; Potluri, N.; Diao, X.; Kim, Y.; Khorasanizadeh, S.; et al. Bidirectional modulation of HIF-2 activity through chemical ligands. Nat. Chem. Biol. 2019, 15, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Wenger, R.H.; Stiehl, D.P.; Camenisch, G. Integration of oxygen signaling at the consensus HRE. Sci. STKE Signal Transduct. Knowl. Environ. 2005, 2005, re12. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Miao, R.; Liu, G.; Qiu, X.; Yang, B.; Tan, X.; Liu, L.; Long, J.; Tang, W.; Jing, W. Spatiotemporal correlation between HIF-1α and bone regeneration. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22520. [Google Scholar] [CrossRef]
- Taylor, C.T.; Scholz, C.C. The effect of HIF on metabolism and immunity. Nat. Rev. Nephrol. 2022, 18, 573–587. [Google Scholar] [CrossRef]
- Kim, Y.H.; Oreffo, R.O.C.; Dawson, J.I. From hurdle to springboard: The macrophage as target in biomaterial-based bone regeneration strategies. Bone 2022, 159, 116389. [Google Scholar] [CrossRef]
- Bjørge, I.M.; Choi, I.S.; Correia, C.R.; Mano, J.F. Nanogrooved microdiscs for bottom-up modulation of osteogenic differentiation. Nanoscale 2019, 11, 16214–16221. [Google Scholar] [CrossRef]
- Piard, C.; Jeyaram, A.; Liu, Y.; Caccamese, J.; Jay, S.M.; Chen, Y.; Fisher, J. 3D printed HUVECs/MSCs cocultures impact cellular interactions and angiogenesis depending on cell-cell distance. Biomaterials 2019, 222, 119423. [Google Scholar] [CrossRef]
- Jaiswal, N.; Haynesworth, S.E.; Caplan, A.I.; Bruder, S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J. Cell Biochem. 1997, 64, 295–312. [Google Scholar] [CrossRef]
- Fan, Q.; Bai, J.; Shan, H.; Fei, Z.; Chen, H.; Xu, J.; Ma, Q.; Zhou, X.; Wang, C. Implantable blood clot loaded with BMP-2 for regulation of osteoimmunology and enhancement of bone repair. Bioact. Mater. 2021, 6, 4014–4026. [Google Scholar] [CrossRef]
- Nadine, S.; Patrício, S.G.; Correia, C.R.; Mano, J.F. Dynamic microfactories co-encapsulating osteoblastic and adipose-derived stromal cells for the biofabrication of bone units. Biofabrication 2019, 12, 015005. [Google Scholar] [CrossRef] [PubMed]
- Nadine, S.; Fernandes, I.; Patrício, S.G.; Correia, C.R.; Mano, J.F. Liquefied Microcapsules Compartmentalizing Macrophages and Umbilical Cord-Derived Cells for Bone Tissue Engineering. Adv. Healthc. Mater. 2022, 11, e2200651. [Google Scholar] [CrossRef] [PubMed]
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Parada, C.; Chai, Y. Mandible and Tongue Development. Curr. Top. Dev. Biol. 2015, 115, 31–58. [Google Scholar] [CrossRef]
- Veselá, B.; Švandová, E.; Bobek, J.; Lesot, H.; Matalová, E. Osteogenic and Angiogenic Profiles of Mandibular Bone-Forming Cells. Front. Physiol. 2019, 10, 124. [Google Scholar] [CrossRef]
- Lopes, D.; Martins-Cruz, C.; Oliveira, M.B.; Mano, J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials 2018, 185, 240–275. [Google Scholar] [CrossRef]
- Debnath, S.; Yallowitz, A.R.; McCormick, J.; Lalani, S.; Zhang, T.; Xu, R.; Li, N.; Liu, Y.; Yang, Y.S.; Eiseman, M.; et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 2018, 562, 133–139. [Google Scholar] [CrossRef]
- Fu, R.; Liu, C.; Yan, Y.; Li, Q.; Huang, R.-L. Bone defect reconstruction via endochondral ossification: A developmental engineering strategy. J. Tissue Eng. 2021, 12, 20417314211004211. [Google Scholar] [CrossRef]
- Berendsen, A.D.; Olsen, B.R. Bone development. Bone 2015, 80, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Carmeliet, G.; Schipani, E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat. Reviews. Rheumatol. 2012, 8, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Bahney, C.S.; Hu, D.P.; Miclau, T., 3rd; Marcucio, R.S. The multifaceted role of the vasculature in endochondral fracture repair. Front. Endocrinol. 2015, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Bai, Y.; Li, X.; Lim, L.W.; Li, W.; Ge, Z.; Zhang, X.; Deng, X. Electroactive Biomaterials for Facilitating Bone Defect Repair under Pathological Conditions. Adv. Sci. (Weinh. Baden-Wurtt. Ger.) 2022, 10, e2204502. [Google Scholar] [CrossRef] [PubMed]
- Scotti, C.; Piccinini, E.; Takizawa, H.; Todorov, A.; Bourgine, P.; Papadimitropoulos, A.; Barbero, A.; Manz, M.G.; Martin, I. Engineering of a functional bone organ through endochondral ossification. Proc. Natl. Acad. Sci. USA 2013, 110, 3997–4002. [Google Scholar] [CrossRef]
- Scotti, C.; Tonnarelli, B.; Papadimitropoulos, A.; Scherberich, A.; Schaeren, S.; Schauerte, A.; Lopez-Rios, J.; Zeller, R.; Barbero, A.; Martin, I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 7251–7256. [Google Scholar] [CrossRef]
- Dunwoodie, S.L. The role of hypoxia in development of the Mammalian embryo. Dev. Cell 2009, 17, 755–773. [Google Scholar] [CrossRef]
- Stegen, S.; Carmeliet, G. The skeletal vascular system—Breathing life into bone tissue. Bone 2018, 115, 50–58. [Google Scholar] [CrossRef]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-inducible factors and the response to hypoxic stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef]
- Semenza, G.L. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 1999, 15, 551–578. [Google Scholar] [CrossRef]
- Riddle, R.C.; Khatri, R.; Schipani, E.; Clemens, T.L. Role of hypoxia-inducible factor-1αlpha in angiogenic-osteogenic coupling. J. Mol. Med. (Berl. Ger.) 2009, 87, 583–590. [Google Scholar] [CrossRef]
- Schipani, E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2009, 24, 1347–1353. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef]
- Ramasamy, S.K.; Kusumbe, A.P.; Schiller, M.; Zeuschner, D.; Bixel, M.G.; Milia, C.; Gamrekelashvili, J.; Limbourg, A.; Medvinsky, A.; Santoro, M.M.; et al. Blood flow controls bone vascular function and osteogenesis. Nat. Commun. 2016, 7, 13601. [Google Scholar] [CrossRef] [PubMed]
- Wolff, L.I.; Hartmann, C. A Second Career for Chondrocytes-Transformation into Osteoblasts. Curr. Osteoporos. Rep. 2019, 17, 129–137. [Google Scholar] [CrossRef]
- Aghajanian, P.; Mohan, S. The art of building bone: Emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Res. 2018, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Sheehy, E.J.; Kelly, D.J.; O’Brien, F.J. Biomaterial-based endochondral bone regeneration: A shift from traditional tissue engineering paradigms to developmentally inspired strategies. Mater. Today Bio 2019, 3, 100009. [Google Scholar] [CrossRef] [PubMed]
- Rolian, C. Endochondral ossification and the evolution of limb proportions. Wiley Interdiscip. Rev. Dev. Biol. 2020, 9, e373. [Google Scholar] [CrossRef]
- De Spiegelaere, W.; Cornillie, P.; Casteleyn, C.; Burvenich, C.; Van den Broeck, W. Detection of hypoxia inducible factors and angiogenic growth factors during foetal endochondral and intramembranous ossification. Anat. Histol. Embryol. 2010, 39, 376–384. [Google Scholar] [CrossRef]
- Emans, P.J.; Spaapen, F.; Surtel, D.A.; Reilly, K.M.; Cremers, A.; van Rhijn, L.W.; Bulstra, S.K.; Voncken, J.W.; Kuijer, R. A novel in vivo model to study endochondral bone formation; HIF-1αlpha activation and BMP expression. Bone 2007, 40, 409–418. [Google Scholar] [CrossRef]
- Taheem, D.K.; Foyt, D.A.; Loaiza, S.; Ferreira, S.A.; Ilic, D.; Auner, H.W.; Grigoriadis, A.E.; Jell, G.; Gentleman, E. Differential Regulation of Human Bone Marrow Mesenchymal Stromal Cell Chondrogenesis by Hypoxia Inducible Factor-1α Hydroxylase Inhibitors. Stem Cells 2018, 36, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Robins, J.C.; Akeno, N.; Mukherjee, A.; Dalal, R.R.; Aronow, B.J.; Koopman, P.; Clemens, T.L. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone 2005, 37, 313–322. [Google Scholar] [CrossRef]
- Taheem, D.K.; Jell, G.; Gentleman, E. Hypoxia Inducible Factor-1α in Osteochondral Tissue Engineering. Tissue Eng. Part B Rev. 2020, 26, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Duval, E.; Baugé, C.; Andriamanalijaona, R.; Bénateau, H.; Leclercq, S.; Dutoit, S.; Poulain, L.; Galéra, P.; Boumédiene, K. Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering. Biomaterials 2012, 33, 6042–6051. [Google Scholar] [CrossRef] [PubMed]
- Merceron, C.; Ranganathan, K.; Wang, E.; Tata, Z.; Makkapati, S.; Khan, M.P.; Mangiavini, L.; Yao, A.Q.; Castellini, L.; Levi, B.; et al. Hypoxia-inducible factor 2α is a negative regulator of osteoblastogenesis and bone mass accrual. Bone Res. 2019, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Hu, N.; Liao, J.Y.; Lin, L.B.; Zhao, C.; Si, W.K.; Yang, Z.; Yi, S.X.; Fan, T.X.; Bao, W.; et al. HIF-1α as a Regulator of BMP2-Induced Chondrogenic Differentiation, Osteogenic Differentiation, and Endochondral Ossification in Stem Cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2015, 36, 44–60. [Google Scholar] [CrossRef]
- Tseng, W.P.; Yang, S.N.; Lai, C.H.; Tang, C.H. Hypoxia induces BMP-2 expression via ILK, Akt, mTOR, and HIF-1 pathways in osteoblasts. J. Cell. Physiol. 2010, 223, 810–818. [Google Scholar] [CrossRef]
- Bernhard, J.C.; Marolt Presen, D.; Li, M.; Monforte, X.; Ferguson, J.; Leinfellner, G.; Heimel, P.; Betti, S.L.; Shu, S.; Teuschl-Woller, A.H.; et al. Effects of Endochondral and Intramembranous Ossification Pathways on Bone Tissue Formation and Vascularization in Human Tissue-Engineered Grafts. Cells 2022, 11, 3070. [Google Scholar] [CrossRef]
- Lin, D.; Chai, Y.; Ma, Y.; Duan, B.; Yuan, Y.; Liu, C. Rapid initiation of guided bone regeneration driven by spatiotemporal delivery of IL-8 and BMP-2 from hierarchical MBG-based scaffold. Biomaterials 2019, 196, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Z.; Wang, L.; Sun, L.; Kim, B.Y.S.; Jiang, W.; Yuan, Y.; Liu, C. Spatiotemporal Immunomodulation Using Biomimetic Scaffold Promotes Endochondral Ossification-Mediated Bone Healing. Adv. Sci. 2021, 8, e2100143. [Google Scholar] [CrossRef]
- Lu, W.; Zhou, C.; Ma, Y.; Li, J.; Jiang, J.; Chen, Y.; Dong, L.; He, F. Improved osseointegration of strontium-modified titanium implants by regulating angiogenesis and macrophage polarization. Biomater. Sci. 2022, 10, 2198–2214. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Luo, K.; Tan, J.; Zhou, R.; Chen, Y.; Chen, C.; Rong, Z.; Deng, M.; Yu, X.; Zhang, C.; et al. Laminin alpha 4 promotes bone regeneration by facilitating cell adhesion and vascularization. Acta Biomater. 2021, 126, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.; Ma, X.; Li, S.; Li, T.; Li, Z.; Qian, Y.; Shafiq, M.; Wang, J.; Zhou, X.; He, C. Bone Microenvironment-Mimetic Scaffolds with Hierarchical Microstructure for Enhanced Vascularization and Bone Regeneration. Adv. Funct. Mater. 2022, 32, 2200011. [Google Scholar] [CrossRef]
- Han, X.; Sun, M.; Chen, B.; Saiding, Q.; Zhang, J.; Song, H.; Deng, L.; Wang, P.; Gong, W.; Cui, W. Lotus seedpod-inspired internal vascularized 3D printed scaffold for bone tissue repair. Bioact. Mater. 2021, 6, 1639–1652. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, Y.; Selvaratnam, B.; Koodali, R.T.; Sun, H. Mesoporous silicate nanoparticles/3D nanofibrous scaffold-mediated dual-drug delivery for bone tissue engineering. J. Control. Release Off. J. Control. Release Soc. 2018, 279, 69–78. [Google Scholar] [CrossRef]
- Harada, N.; Watanabe, Y.; Sato, K.; Abe, S.; Yamanaka, K.; Sakai, Y.; Kaneko, T.; Matsushita, T. Bone regeneration in a massive rat femur defect through endochondral ossification achieved with chondrogenically differentiated MSCs in a degradable scaffold. Biomaterials 2014, 35, 7800–7810. [Google Scholar] [CrossRef]
- Daly, A.C.; Pitacco, P.; Nulty, J.; Cunniffe, G.M.; Kelly, D.J. 3D printed microchannel networks to direct vascularisation during endochondral bone repair. Biomaterials 2018, 162, 34–46. [Google Scholar] [CrossRef]
- Sivaraj, K.K.; Adams, R.H. Blood vessel formation and function in bone. Development 2016, 143, 2706–2715. [Google Scholar] [CrossRef]
- Lyons, F.G.; Al-Munajjed, A.A.; Kieran, S.M.; Toner, M.E.; Murphy, C.M.; Duffy, G.P.; O’Brien, F.J. The healing of bony defects by cell-free collagen-based scaffolds compared to stem cell-seeded tissue engineered constructs. Biomaterials 2010, 31, 9232–9243. [Google Scholar] [CrossRef]
- Farrell, E.; Both, S.K.; Odörfer, K.I.; Koevoet, W.; Kops, N.; O’Brien, F.J.; Baatenburg de Jong, R.J.; Verhaar, J.A.; Cuijpers, V.; Jansen, J.; et al. In-vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet. Disord. 2011, 12, 31. [Google Scholar] [CrossRef]
- Li, J.; Xu, Q.; Teng, B.; Yu, C.; Li, J.; Song, L.; Lai, Y.X.; Zhang, J.; Zheng, W.; Ren, P.G. Investigation of angiogenesis in bioactive 3-dimensional poly(d,l-lactide-co-glycolide)/nano-hydroxyapatite scaffolds by in vivo multiphoton microscopy in murine calvarial critical bone defect. Acta Biomater. 2016, 42, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Roux, B.M.; Posukonis, M.; Bodamer, E.; Brey, E.M.; Fisher, J.P.; Dean, D. Effect of prevascularization on in vivo vascularization of poly(propylene fumarate)/fibrin scaffolds. Biomaterials 2016, 77, 255–266. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yuan, S.; Tang, H.; Wang, S.; Mu, Z.; Li, D.; Wang, S.; Jing, X.; Hu, S.; Ji, P.; et al. Safeguarding Osteointegration in Diabetic Patients: A Potent “Chain Armor” Coating for Scavenging ROS and Macrophage Reprogramming in a Microenvironment-Responsive Manner. Adv. Funct. Mater. 2021, 31, 2101611. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H.; Li, P.; Diao, H.; Zhu, S.; Dong, L.; Wang, R.; Guo, T.; Zhao, J.; Zhang, J. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials 2011, 32, 4793–4805. [Google Scholar] [CrossRef] [PubMed]
- Raftery, R.M.; Mencía Castaño, I.; Chen, G.; Cavanagh, B.; Quinn, B.; Curtin, C.M.; Cryan, S.A.; O’Brien, F.J. Translating the role of osteogenic-angiogenic coupling in bone formation: Highly efficient chitosan-pDNA activated scaffolds can accelerate bone regeneration in critical-sized bone defects. Biomaterials 2017, 149, 116–127. [Google Scholar] [CrossRef]
- Kuttappan, S.; Mathew, D.; Jo, J.I.; Tanaka, R.; Menon, D.; Ishimoto, T.; Nakano, T.; Nair, S.V.; Nair, M.B.; Tabata, Y. Dual release of growth factor from nanocomposite fibrous scaffold promotes vascularisation and bone regeneration in rat critical sized calvarial defect. Acta Biomater. 2018, 78, 36–47. [Google Scholar] [CrossRef]
- Li, H.; Liao, L.; Hu, Y.; Xu, Y.; Zhang, Y.; Huo, F.; Tian, W.; Guo, W. Identification of Type H Vessels in Mice Mandibular Condyle. J. Dent. Res. 2021, 100, 983–992. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, S.; Li, Y.; Crane, J.L. Type H blood vessels in bone modeling and remodeling. Theranostics 2020, 10, 426–436. [Google Scholar] [CrossRef]
- Watson, E.C.; Adams, R.H. Biology of Bone: The Vasculature of the Skeletal System. Cold Spring Harb. Perspect. Med. 2018, 8, a031559. [Google Scholar] [CrossRef]
- Ceradini, D.J.; Kulkarni, A.R.; Callaghan, M.J.; Tepper, O.M.; Bastidas, N.; Kleinman, M.E.; Capla, J.M.; Galiano, R.D.; Levine, J.P.; Gurtner, G.C. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat. Med. 2004, 10, 858–864. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Chen, H.; Kang, H.; Qi, J.; Zhao, P.; Jiang, M.; Guo, L.; Zhou, Q.; Qian, N.D.; Zhou, H.B.; et al. Deferoxamine released from poly(lactic-co-glycolic acid) promotes healing of osteoporotic bone defect via enhanced angiogenesis and osteogenesis. J. Biomed. Mater. Research. Part A 2016, 104, 2515–2527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jin, L.; Guo, J.; Bao, K.; Hu, J.; Zhang, Y.; Hou, Z.; Zhang, L. Chronic Intermittent Hypobaric Hypoxia Enhances Bone Fracture Healing. Front. Endocrinol. 2020, 11, 582670. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, H.; Zhang, X.; Li, G.; Chang, Q.; Zhao, J.; Qiao, Y.; Ding, X.; Yang, G.; Liu, X.; et al. A strontium-incorporated nanoporous titanium implant surface for rapid osseointegration. Nanoscale 2016, 8, 5291–5301. [Google Scholar] [CrossRef]
- Ying, C.; Wang, R.; Wang, Z.; Tao, J.; Yin, W.; Zhang, J.; Yi, C.; Qi, X.; Han, D. BMSC-Exosomes Carry Mutant HIF-1α for Improving Angiogenesis and Osteogenesis in Critical-Sized Calvarial Defects. Front. Bioeng. Biotechnol. 2020, 8, 565561. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ma, X.; Ma, J.; Sun, X.; Li, F.; Lv, J. Naringin enhances endothelial progenitor cell (EPC) proliferation and tube formation capacity through the CXCL12/CXCR4/PI3K/Akt signaling pathway. Chem.-Biol. Interact. 2018, 286, 45–51. [Google Scholar] [CrossRef]
- Ju, C.; Shen, Y.; Ma, G.; Liu, Y.; Cai, J.; Kim, I.M.; Weintraub, N.L.; Liu, N.; Tang, Y. Transplantation of Cardiac Mesenchymal Stem Cell-Derived Exosomes Promotes Repair in Ischemic Myocardium. J. Cardiovasc. Transl. Res. 2018, 11, 420–428. [Google Scholar] [CrossRef]
- Fu, H.; Deng, C.; Teng, L.; Cai, Z.; Chen, J.; Lu, G. Effect of heparan sulfate mimetics from Escherichia coli K5 polysaccharide on SDF-1/CXCL12-induced endothelial progenitor cells in vitro. Int. J. Biol. Macromol. 2018, 107, 2492–2500. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, Y.; Yang, Z.; Aimaijiang, M.; Ma, R.; Yang, Y.; Zhang, Y.; Zhou, Y. Hypoxia-Inducible Factors Signaling in Osteogenesis and Skeletal Repair. Int. J. Mol. Sci. 2022, 23, 11201. [Google Scholar] [CrossRef]
- Feng, Z.T.; Yang, T.; Hou, X.Q.; Wu, H.Y.; Feng, J.T.; Ou, B.J.; Cai, S.J.; Li, J.; Mei, Z.G. Sinomenine mitigates collagen-induced arthritis mice by inhibiting angiogenesis. Biomed. Pharmacother. 2019, 113, 108759. [Google Scholar] [CrossRef]
- Chai, M.; Gu, C.; Shen, Q.; Liu, J.; Zhou, Y.; Jin, Z.; Xiong, W.; Zhou, Y.; Tan, W. Hypoxia alleviates dexamethasone-induced inhibition of angiogenesis in cocultures of HUVECs and rBMSCs via HIF-1α. Stem Cell Res. Ther. 2020, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.N.; Howard, B.W.; Yang, H.T.; Eby-Wilkens, E.; Loos, P.; Varbanov, A.; Qu, A.; DeMuth, J.P.; Davis, M.G.; Proia, A.; et al. Efficacy of systemic administration of SDF-1 in a model of vascular insufficiency: Support for an endothelium-dependent mechanism. Cardiovasc. Res. 2006, 69, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Drager, J.; Harvey, E.J.; Barralet, J. Hypoxia signalling manipulation for bone regeneration. Expert Rev. Mol. Med. 2015, 17, e6. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ma, L.; Zhang, H.; Sun, W.; Zheng, L.; Liu, C.; Miao, L. EPO could be regulated by HIF-1 and promote osteogenesis and accelerate bone repair. Artif. Cells Nanomed. Biotechnol. 2020, 48, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Patterson, J.L.; Vines, J.B.; Javed, A.; Gilbert, S.R.; Jun, H.W. Biphasic peptide amphiphile nanomatrix embedded with hydroxyapatite nanoparticles for stimulated osteoinductive response. ACS Nano 2011, 5, 9463–9479. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Ruiz-Hernández, E. Bioceramics: From bone regeneration to cancer nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef]
- Kim, J.; Jung, Y.; Sun, H.; Joseph, J.; Mishra, A.; Shiozawa, Y.; Wang, J.; Krebsbach, P.H.; Taichman, R.S. Erythropoietin mediated bone formation is regulated by mTOR signaling. J. Cell Biochem. 2012, 113, 220–228. [Google Scholar] [CrossRef]
- Hannah, S.S.; McFadden, S.; McNeilly, A.; McClean, C. “Take My Bone Away?” Hypoxia and bone: A narrative review. J. Cell. Physiol. 2021, 236, 721–740. [Google Scholar] [CrossRef]
- Zheng, D.H.; Wang, X.X.; Ma, D.; Zhang, L.N.; Qiao, Q.F.; Zhang, J. Erythropoietin enhances osteogenic differentiation of human periodontal ligament stem cells via Wnt/β-catenin signaling pathway. Drug Des. Dev. Ther. 2019, 13, 2543–2552. [Google Scholar] [CrossRef]
- Tian, Y.; Shao, Q.; Tang, Y.; Li, X.; Qi, X.; Jiang, R.; Liang, Y.; Kang, F. HIF-1α regulates osteoclast activation and mediates osteogenesis during mandibular bone repair via CT-1. Oral Dis. 2022, 28, 428–441. [Google Scholar] [CrossRef]
- Shao, J.; Liu, S.; Zhang, M.; Chen, S.; Gan, S.; Chen, C.; Chen, W.; Li, L.; Zhu, Z. A dual role of HIF1α in regulating osteogenesis-angiogenesis coupling. Stem Cell Res. Ther. 2022, 13, 59. [Google Scholar] [CrossRef] [PubMed]
- Kuss, M.A.; Harms, R.; Wu, S.; Wang, Y.; Untrauer, J.B.; Carlson, M.A.; Duan, B. Short-term hypoxic preconditioning promotes prevascularization in 3D bioprinted bone constructs with stromal vascular fraction derived cells. RSC Adv. 2017, 7, 29312–29320. [Google Scholar] [CrossRef]
- Nicolaije, C.; van de Peppel, J.; van Leeuwen, J.P. Oxygen-induced transcriptional dynamics in human osteoblasts are most prominent at the onset of mineralization. J. Cell. Physiol. 2013, 228, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.Y.; Chun, S.Y.; Ha, Y.S.; Kim, D.H.; Kim, J.; Song, P.H.; Kim, H.T.; Yoo, E.S.; Kim, B.S.; Kwon, T.G. Hypoxia Enhances Cell Properties of Human Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2017, 14, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Yasui, Y.; Chijimatsu, R.; Hart, D.A.; Koizumi, K.; Sugita, N.; Shimomura, K.; Myoui, A.; Yoshikawa, H.; Nakamura, N. Preparation of Scaffold-Free Tissue-Engineered Constructs Derived from Human Synovial Mesenchymal Stem Cells Under Low Oxygen Tension Enhances Their Chondrogenic Differentiation Capacity. Tissue Eng. Part A 2016, 22, 490–500. [Google Scholar] [CrossRef]
- Antebi, B.; Rodriguez, L.A., 2nd; Walker, K.P., 3rd; Asher, A.M.; Kamucheka, R.M.; Alvarado, L.; Mohammadipoor, A.; Cancio, L.C. Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 265. [Google Scholar] [CrossRef]
- Yang, M.; Liu, H.; Wang, Y.; Wu, G.; Qiu, S.; Liu, C.; Tan, Z.; Guo, J.; Zhu, L. Hypoxia reduces the osteogenic differentiation of peripheral blood mesenchymal stem cells by upregulating Notch-1 expression. Connect. Tissue Res. 2019, 60, 583–596. [Google Scholar] [CrossRef]
- Jiang, C.; Sun, J.; Dai, Y.; Cao, P.; Zhang, L.; Peng, S.; Zhou, Y.; Li, G.; Tang, J.; Xiang, J. HIF-1α and C/EBPs transcriptionally regulate adipogenic differentiation of bone marrow-derived MSCs in hypoxia. Stem Cell Res. Ther. 2015, 6, 21. [Google Scholar] [CrossRef]
- Costa, V.; Raimondi, L.; Conigliaro, A.; Salamanna, F.; Carina, V.; De Luca, A.; Bellavia, D.; Alessandro, R.; Fini, M.; Giavaresi, G. Hypoxia-inducible factor 1A may regulate the commitment of mesenchymal stromal cells toward angio-osteogenesis by mirna-675-5P. Cytotherapy 2017, 19, 1412–1425. [Google Scholar] [CrossRef]
- Zhu, J.; Tang, Y.; Wu, Q.; Ji, Y.C.; Feng, Z.F.; Kang, F.W. HIF-1α facilitates osteocyte-mediated osteoclastogenesis by activating JAK2/STAT3 pathway in vitro. J. Cell. Physiol. 2019, 234, 21182–21192. [Google Scholar] [CrossRef]
- Fayed, H.A.; Barakat, B.M.; Elshaer, S.S.; Abdel-Naim, A.B.; Menze, E.T. Antiosteoporotic activities of isoquercitrin in ovariectomized rats: Role of inhibiting hypoxia inducible factor-1 alpha. Eur. J. Pharmacol. 2019, 865, 172785. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Cheng, M.; Li, M.; Cui, J.; Huang, J.; Zhang, C.; Si, J.; Lin, K.; Yu, H. Small extracellular vesicles derived from hypoxic mesenchymal stem cells promote vascularized bone regeneration through the miR-210-3p/EFNA3/PI3K pathway. Acta Biomater. 2022, 150, 413–426. [Google Scholar] [CrossRef] [PubMed]
- McCadden, L.; Leonard, C.G.; Primrose, W.J. Bisphosphonate-induced osteonecrosis of the ear canal: Our experience and a review of the literature. J. Laryngol. Otol. 2018, 132, 372–374. [Google Scholar] [CrossRef]
- Zhao, H.; Yeersheng, R.; Xia, Y.; Kang, P.; Wang, W. Hypoxia Enhanced Bone Regeneration Through the HIF-1α/β-Catenin Pathway in Femoral Head Osteonecrosis. Am. J. Med. Sci. 2021, 362, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Furusho, H.; Hirota, K.; Sasaki, H. Activation of hypoxia-inducible factor 1 attenuates periapical inflammation and bone loss. Int. J. Oral Sci. 2018, 10, 12. [Google Scholar] [CrossRef]
- Görlach, A.; Bonello, S. The cross-talk between NF-kappaB and HIF-1: Further evidence for a significant liaison. Biochem. J. 2008, 412, e17–e19. [Google Scholar] [CrossRef]
- Loeffler, J.; Duda, G.N.; Sass, F.A.; Dienelt, A. The Metabolic Microenvironment Steers Bone Tissue Regeneration. Trends Endocrinol. Metab. 2018, 29, 99–110. [Google Scholar] [CrossRef]
- Stegen, S.; van Gastel, N.; Eelen, G.; Ghesquière, B.; D’Anna, F.; Thienpont, B.; Goveia, J.; Torrekens, S.; Van Looveren, R.; Luyten, F.P.; et al. HIF-1α Promotes Glutamine-Mediated Redox Homeostasis and Glycogen-Dependent Bioenergetics to Support Postimplantation Bone Cell Survival. Cell Metab. 2016, 23, 265–279. [Google Scholar] [CrossRef]
- Choudhry, H.; Harris, A.L. Advances in Hypoxia-Inducible Factor Biology. Cell Metab. 2018, 27, 281–298. [Google Scholar] [CrossRef]
- Rolfe, D.F.; Brown, G.C. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol. Rev. 1997, 77, 731–758. [Google Scholar] [CrossRef]
- Cummins, E.P.; Keogh, C.E.; Crean, D.; Taylor, C.T. The role of HIF in immunity and inflammation. Mol. Asp. Med. 2016, 47–48, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Kong, N.; Ding, L.; Guo, Y.; Yang, W.; Yan, F. Ultrathin 2D Titanium Carbide MXene (Ti3C2Tx) Nanoflakes Activate WNT/HIF-1α-Mediated Metabolism Reprogramming for Periodontal Regeneration. Adv. Healthc. Mater. 2021, 10, e2101215. [Google Scholar] [CrossRef]
- Végran, F.; Boidot, R.; Michiels, C.; Sonveaux, P.; Feron, O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 2011, 71, 2550–2560. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D.Z.; Kwan, K.Y. Neurogenic-angiogenic synchrony via lactate. Nat. Neurosci. 2022, 25, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, M.; Feng, H.; Peng, Y.; Sun, J.; Qu, X.; Li, C. Lactate induces osteoblast differentiation by stabilization of HIF1α. Mol. Cell. Endocrinol. 2017, 452, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Yu, W.W.; Yan, X.Y.; Liang, X.Q.; Ma, X.F.; Long, J.P.; Du, X.Y.; Mao, H.Y.; Liu, H.B. Lactate-driven macrophage polarization in the inflammatory microenvironment alleviates intestinal inflammation. Front. Immunol. 2022, 13, 1013686. [Google Scholar] [CrossRef]
- Caslin, H.L.; Abebayehu, D.; Pinette, J.A.; Ryan, J.J. Lactate Is a Metabolic Mediator That Shapes Immune Cell Fate and Function. Front. Physiol. 2021, 12, 688485. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S. Lactic acid promotes macrophage polarization through MCT-HIF1α signaling in gastric cancer. Exp. Cell Res. 2020, 388, 111846. [Google Scholar] [CrossRef]
- Ke, W.; Ma, L.; Wang, B.; Song, Y.; Luo, R.; Li, G.; Liao, Z.; Shi, Y.; Wang, K.; Feng, X.; et al. N-cadherin mimetic hydrogel enhances MSC chondrogenesis through cell metabolism. Acta Biomater. 2022, 150, 83–95. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Sanz-Ros, J.; Román-Domínguez, A.; Gimeno-Mallench, L.; Inglés, M.; Viña, J.; Borrás, C. Extracellular Vesicles from Healthy Cells Improves Cell Function and Stemness in Premature Senescent Stem Cells by miR-302b and HIF-1α Activation. Biomolecules 2020, 10, 957. [Google Scholar] [CrossRef]
- Lanigan, T.M.; Kopera, H.C.; Saunders, T.L. Principles of Genetic Engineering. Genes 2020, 11, 291. [Google Scholar] [CrossRef]
- Damasceno, P.K.F.; de Santana, T.A.; Santos, G.C.; Orge, I.D.; Silva, D.N.; Albuquerque, J.F.; Golinelli, G.; Grisendi, G.; Pinelli, M.; Ribeiro Dos Santos, R.; et al. Genetic Engineering as a Strategy to Improve the Therapeutic Efficacy of Mesenchymal Stem/Stromal Cells in Regenerative Medicine. Front. Cell Dev. Biol. 2020, 8, 737. [Google Scholar] [CrossRef] [PubMed]
- Plenge, R.M. Biomedicine: Human genes lost and their functions found. Nature 2017, 544, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Rankin, E.B.; Castellini, L.; Alcudia, J.F.; LaGory, E.L.; Andersen, R.; Rhodes, S.D.; Wilson, T.L.; Mohammad, K.S.; Castillo, A.B.; et al. Oxygen-sensing PHDs regulate bone homeostasis through the modulation of osteoprotegerin. Genes Dev. 2015, 29, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Mayuranathan, T.; Huang, P.; Doerfler, P.A.; Li, Y.; Yao, Y.; Zhang, J.; Palmer, L.E.; Xu, P.; Mayberry, K.; et al. Regulation of Fetal Hemoglobin Expression By the VHL-HIF1α Oxygen Sensing System. Blood 2021, 138, 574. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, F.; Wu, Y.; Luo, J.; Mao, X.; Long, L.; Gou, M.; Yang, L.; Deng, D.Y.B. RGD-Modified Nanocarrier-Mediated Targeted Delivery of HIF-1α-AA Plasmid DNA to Cerebrovascular Endothelial Cells for Ischemic Stroke Treatment. ACS Biomater. Sci. Eng. 2019, 5, 6254–6264. [Google Scholar] [CrossRef] [PubMed]
- Heun, Y.; Pogoda, K.; Anton, M.; Pircher, J.; Pfeifer, A.; Woernle, M.; Ribeiro, A.; Kameritsch, P.; Mykhaylyk, O.; Plank, C.; et al. HIF-1α Dependent Wound Healing Angiogenesis In Vivo Can Be Controlled by Site-Specific Lentiviral Magnetic Targeting of SHP-2. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 1616–1627. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, I.; Wang, K. Recent Advances in RNA Therapy and Its Carriers to Treat the Single-Gene Neurological Disorders. Biomedicines 2022, 10, 158. [Google Scholar] [CrossRef]
- Smith, E.S.; Whitty, E.; Yoo, B.; Moore, A.; Sempere, L.F.; Medarova, Z. Clinical Applications of Short Non-Coding RNA-Based Therapies in the Era of Precision Medicine. Cancers 2022, 14, 1588. [Google Scholar] [CrossRef]
- Tam, C.; Wong, J.H.; Cheung, R.C.F.; Zuo, T.; Ng, T.B. Therapeutic potentials of short interfering RNAs. Appl. Microbiol. Biotechnol. 2017, 101, 7091–7111. [Google Scholar] [CrossRef]
- Poliseno, L.; Mercatanti, A.; Citti, L.; Rainaldi, G. RNA-based drugs: From RNA interference to short interfering RNAs. Curr. Pharm. Biotechnol. 2004, 5, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Ríos, C.N.; Skoracki, R.J.; Mathur, A.B. GNAS1 and PHD2 short-interfering RNA support bone regeneration in vitro and in an in vivo sheep model. Clin. Orthop. Relat. Res. 2012, 470, 2541–2553. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, M.A.; Ding, M.H.; Gutierrez, A.S.; Chew, S.A. The Application of microRNAs in Biomaterial Scaffold-Based Therapies for Bone Tissue Engineering. Biotechnol. J. 2019, 14, e1900084. [Google Scholar] [CrossRef] [PubMed]
- Bravo Vázquez, L.A.; Moreno Becerril, M.Y.; Mora Hernández, E.O.; León Carmona, G.G.; Aguirre Padilla, M.E.; Chakraborty, S.; Bandyopadhyay, A.; Paul, S. The Emerging Role of MicroRNAs in Bone Diseases and Their Therapeutic Potential. Molecules 2021, 27, 211. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef]
- Samad, A.F.A.; Kamaroddin, M.F. Innovative approaches in transforming microRNAs into therapeutic tools. Wiley Interdiscip. Rev. RNA 2022, 14, e1768. [Google Scholar] [CrossRef]
- Sun, G.; Zhou, Y.; Li, H.; Guo, Y.; Shan, J.; Xia, M.; Li, Y.; Li, S.; Long, D.; Feng, L. Over-expression of microRNA-494 up-regulates hypoxia-inducible factor-1 alpha expression via PI3K/Akt pathway and protects against hypoxia-induced apoptosis. J. Biomed. Sci. 2013, 20, 100. [Google Scholar] [CrossRef]
- Ren, S.; Liu, Y.; Zhu, Y.; Wang, Y.; Liu, M.; Zhou, Y. Application status of hypoxia mimetic agents in bone tissue engineering. Zhongguo Xiufu Chongjian Waike Zazhi Chin. J. Reparative Reconstr. Surg. 2020, 34, 1190–1194. [Google Scholar] [CrossRef]
- Li, R.L.; He, L.Y.; Zhang, Q.; Liu, J.; Lu, F.; Duan, H.X.; Fan, L.H.; Peng, W.; Huang, Y.L.; Wu, C.J. HIF-1α is a Potential Molecular Target for Herbal Medicine to Treat Diseases. Drug Des. Dev. Ther. 2020, 14, 4915–4949. [Google Scholar] [CrossRef]
- Zenk, S.F.; Hauck, S.; Mayer, D.; Grieshober, M.; Stenger, S. Stabilization of Hypoxia-Inducible Factor Promotes Antimicrobial Activity of Human Macrophages Against Mycobacterium tuberculosis. Front Immunol. 2021, 12, 678354. [Google Scholar] [CrossRef]
- Urso, E.; Maffia, M. Behind the Link between Copper and Angiogenesis: Established Mechanisms and an Overview on the Role of Vascular Copper Transport Systems. J. Vasc. Res. 2015, 52, 172–196. [Google Scholar] [CrossRef] [PubMed]
- Rigiracciolo, D.C.; Scarpelli, A.; Lappano, R.; Pisano, A.; Santolla, M.F.; De Marco, P.; Cirillo, F.; Cappello, A.R.; Dolce, V.; Belfiore, A.; et al. Copper activates HIF-1α/GPER/VEGF signalling in cancer cells. Oncotarget 2015, 6, 34158–34177. [Google Scholar] [CrossRef] [PubMed]
- Teti, G.; Focaroli, S.; Salvatore, V.; Mazzotti, E.; Ingra, L.; Mazzotti, A.; Falconi, M. The Hypoxia-Mimetic Agent Cobalt Chloride Differently Affects Human Mesenchymal Stem Cells in Their Chondrogenic Potential. Stem Cells Int. 2018, 2018, 3237253. [Google Scholar] [CrossRef] [PubMed]
- Nakuluri, K.; Mukhi, D.; Mungamuri, S.K.; Pasupulati, A.K. Stabilization of hypoxia-inducible factor 1α by cobalt chloride impairs podocyte morphology and slit-diaphragm function. J. Cell Biochem. 2018, 120, 7667–7678. [Google Scholar] [CrossRef] [PubMed]
- Otarola, G.A.; Hu, J.C.; Athanasiou, K.A. Ion modulatory treatments toward functional self-assembled neocartilage. Acta Biomater. 2022, 153, 85–96. [Google Scholar] [CrossRef]
- Liu, G.S.; Peshavariya, H.M.; Higuchi, M.; Chan, E.C.; Dusting, G.J.; Jiang, F. Pharmacological priming of adipose-derived stem cells for paracrine VEGF production with deferoxamine. J. Tissue Eng. Regen. Med. 2016, 10, E167–E176. [Google Scholar] [CrossRef]
- Yoo, H.I.; Moon, Y.H.; Kim, M.S. Effects of CoCl2 on multi-lineage differentiation of C3H/10T1/2 mesenchymal stem cells. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacal. 2016, 20, 53–62. [Google Scholar] [CrossRef]
- Zhang, W.; Chang, Q.; Xu, L.; Li, G.; Yang, G.; Ding, X.; Wang, X.; Cui, D.; Jiang, X. Graphene Oxide-Copper Nanocomposite-Coated Porous CaP Scaffold for Vascularized Bone Regeneration via Activation of Hif-1α. Adv. Healthc. Mater. 2019, 8, e1900067. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, X.; Wang, Y.; Liu, Y.; Pan, Y.; Li, Y.; Ji, M.; Zhao, X.; Huang, S.; Yao, Q. Hypoxia-mimicking 3D bioglass-nanoclay scaffolds promote endogenous bone regeneration. Bioact. Mater. 2021, 6, 3485–3495. [Google Scholar] [CrossRef]
- Geng, M.; Zhang, Q.; Gu, J.; Yang, J.; Du, H.; Jia, Y.; Zhou, X.; He, C. Construction of a nanofiber network within 3D printed scaffolds for vascularized bone regeneration. Biomater. Sci. 2021, 9, 2631–2646. [Google Scholar] [CrossRef]
- Peyvandi, A.A.; Abbaszadeh, H.A.; Roozbahany, N.A.; Pourbakht, A.; Khoshsirat, S.; Niri, H.H.; Peyvandi, H.; Niknazar, S. Deferoxamine promotes mesenchymal stem cell homing in noise-induced injured cochlea through PI3K/AKT pathway. Cell Prolif. 2018, 51, e12434. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K.; Fujisawa, K.; Takami, T.; Burganova, G.; Sasai, N.; Matsumoto, T.; Yamamoto, N.; Sakaida, I. NUPR1 acts as a pro-survival factor in human bone marrow-derived mesenchymal stem cells and is induced by the hypoxia mimetic reagent deferoxamine. J. Clin. Biochem. Nutr. 2019, 64, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.K.; Cavadas, M.A.; Scholz, C.C.; Fitzpatrick, S.F.; Bruning, U.; Cummins, E.P.; Tambuwala, M.M.; Manresa, M.C.; Kholodenko, B.N.; Taylor, C.T.; et al. A dynamic model of the hypoxia-inducible factor 1α (HIF-1α) network. J. Cell Sci. 2013, 126, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.B.; Wang, J.A.; Ogle, M.E.; Wei, L. Prolyl hydroxylase inhibitor dimethyloxalylglycine enhances mesenchymal stem cell survival. J. Cell Biochem. 2009, 106, 903–911. [Google Scholar] [CrossRef]
- Li, Y.; Han, W.; Wu, Y.; Zhou, K.; Zheng, Z.; Wang, H.; Xie, L.; Li, R.; Xu, K.; Liu, Y.; et al. Stabilization of Hypoxia Inducible Factor-1α by Dimethyloxalylglycine Promotes Recovery from Acute Spinal Cord Injury by Inhibiting Neural Apoptosis and Enhancing Axon Regeneration. J. Neurotrauma 2019, 36, 3394–3409. [Google Scholar] [CrossRef]
- Tian, Y.M.; Yeoh, K.K.; Lee, M.K.; Eriksson, T.; Kessler, B.M.; Kramer, H.B.; Edelmann, M.J.; Willam, C.; Pugh, C.W.; Schofield, C.J.; et al. Differential sensitivity of hypoxia inducible factor hydroxylation sites to hypoxia and hydroxylase inhibitors. J. Biol. Chem. 2011, 286, 13041–13051. [Google Scholar] [CrossRef]
- Zippusch, S.; Besecke, K.F.W.; Helms, F.; Klingenberg, M.; Lyons, A.; Behrens, P.; Haverich, A.; Wilhelmi, M.; Ehlert, N.; Böer, U. Chemically induced hypoxia by dimethyloxalylglycine (DMOG)-loaded nanoporous silica nanoparticles supports endothelial tube formation by sustained VEGF release from adipose tissue-derived stem cells. Regen. Biomater. 2021, 8, rbab039. [Google Scholar] [CrossRef]
- Rafique, M.; Wei, T.; Sun, Q.; Midgley, A.C.; Huang, Z.; Wang, T.; Shafiq, M.; Zhi, D.; Si, J.; Yan, H.; et al. The effect of hypoxia-mimicking responses on improving the regeneration of artificial vascular grafts. Biomaterials 2021, 271, 120746. [Google Scholar] [CrossRef]
- Costa, M.H.G.; Serra, J.; McDevitt, T.C.; Cabral, J.M.S.; da Silva, C.L.; Ferreira, F.C. Dimethyloxalylglycine, a small molecule, synergistically increases the homing and angiogenic properties of human mesenchymal stromal cells when cultured as 3D spheroids. Biotechnol. J. 2021, 16, e2000389. [Google Scholar] [CrossRef]
- Abu-Shahba, A.G.; Gebraad, A.; Kaur, S.; Paananen, R.O.; Peltoniemi, H.; Seppänen-Kaijansinkko, R.; Mannerström, B. Proangiogenic Hypoxia-Mimicking Agents Attenuate Osteogenic Potential of Adipose Stem/Stromal Cells. Tissue Eng. Regen. Med. 2020, 17, 477–493. [Google Scholar] [CrossRef]
- Yeh, T.L.; Leissing, T.M.; Abboud, M.I.; Thinnes, C.C.; Atasoylu, O.; Holt-Martyn, J.P.; Zhang, D.; Tumber, A.; Lippl, K.; Lohans, C.T.; et al. Molecular and cellular mechanisms of HIF prolyl hydroxylase inhibitors in clinical trials. Chem. Sci. 2017, 8, 7651–7668. [Google Scholar] [CrossRef]
- Petersen, A.; Princ, A.; Korus, G.; Ellinghaus, A.; Leemhuis, H.; Herrera, A.; Klaumünzer, A.; Schreivogel, S.; Woloszyk, A.; Schmidt-Bleek, K.; et al. A biomaterial with a channel-like pore architecture induces endochondral healing of bone defects. Nat. Commun. 2018, 9, 4430. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, F.; Xue, E.; Huang, L.; Yan, P.; Pan, X.; Zhou, Y. Hypoxia preconditioning promotes bone marrow mesenchymal stem cells survival by inducing HIF-1α in injured neuronal cells derived exosomes culture system. Cell Death Dis. 2019, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Feng, Z.; Wei, J.; Yu, Y.; Luo, J.; Zhou, J.; Li, Y.; Zheng, X.; Tang, W.; Liu, L.; et al. Repair of Critical-Sized Mandible Defects in Aged Rat Using Hypoxia Preconditioned BMSCs with Up-regulation of Hif-1α. Int. J. Biol. Sci. 2018, 14, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sonveaux, P.; Rabbani, Z.N.; Liu, S.; Yan, B.; Huang, Q.; Vujaskovic, Z.; Dewhirst Mark, W.; Li, C.-Y. Regulation of HIF-1α Stability through S-Nitrosylation. Mol. Cell 2007, 26, 63–74. [Google Scholar] [CrossRef]
- Park, Y.K.; Ahn, D.R.; Oh, M.; Lee, T.; Yang, E.G.; Son, M.; Park, H. Nitric oxide donor, (+/−)-S-nitroso-N-acetylpenicillamine, stabilizes transactive hypoxia-inducible factor-1αlpha by inhibiting von Hippel-Lindau recruitment and asparagine hydroxylation. Mol. Pharmacol. 2008, 74, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Flashman, E.; Mecinović, J.; Kramer, H.B.; Kessler, B.M.; Frapart, Y.M.; Boucher, J.L.; Clifton, I.J.; McDonough, M.A.; Schofield, C.J. Studies on the reaction of nitric oxide with the hypoxia-inducible factor prolyl hydroxylase domain 2 (EGLN1). J. Mol. Biol. 2011, 410, 268–279. [Google Scholar] [CrossRef]
- Metzen, E.; Zhou, J.; Jelkmann, W.; Fandrey, J.; Brüne, B. Nitric oxide impairs normoxic degradation of HIF-1αlpha by inhibition of prolyl hydroxylases. Mol. Biol. Cell 2003, 14, 3470–3481. [Google Scholar] [CrossRef]
- Sun, L.; Ma, Y.; Niu, H.; Liu, Y.; Yuan, Y.; Liu, C. Recapitulation of In Situ Endochondral Ossification Using an Injectable Hypoxia-Mimetic Hydrogel. Adv. Funct. Mater. 2021, 31, 2008515. [Google Scholar] [CrossRef]
- Bai, H.; Guo, X.; Tan, Y.; Wang, Y.; Feng, J.; Lei, K.; Liu, X.; Xiao, Y.; Bao, C. Hypoxia inducible factor-1 signaling pathway in macrophage involved angiogenesis in materials-instructed osteo-induction. J. Mater. Chem. B 2022, 10, 6483–6495. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, J.; Liu, M.; Li, M.; Zhai, S.; Quni, S.; Zhang, L.; Liu, X.; Jia, K.; Zhang, Y.; Zhou, Y. The Role of HIF-1α in Bone Regeneration: A New Direction and Challenge in Bone Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 8029. https://doi.org/10.3390/ijms24098029
You J, Liu M, Li M, Zhai S, Quni S, Zhang L, Liu X, Jia K, Zhang Y, Zhou Y. The Role of HIF-1α in Bone Regeneration: A New Direction and Challenge in Bone Tissue Engineering. International Journal of Molecular Sciences. 2023; 24(9):8029. https://doi.org/10.3390/ijms24098029
Chicago/Turabian StyleYou, Jiaqian, Manxuan Liu, Minghui Li, Shaobo Zhai, Sezhen Quni, Lu Zhang, Xiuyu Liu, Kewen Jia, Yidi Zhang, and Yanmin Zhou. 2023. "The Role of HIF-1α in Bone Regeneration: A New Direction and Challenge in Bone Tissue Engineering" International Journal of Molecular Sciences 24, no. 9: 8029. https://doi.org/10.3390/ijms24098029
APA StyleYou, J., Liu, M., Li, M., Zhai, S., Quni, S., Zhang, L., Liu, X., Jia, K., Zhang, Y., & Zhou, Y. (2023). The Role of HIF-1α in Bone Regeneration: A New Direction and Challenge in Bone Tissue Engineering. International Journal of Molecular Sciences, 24(9), 8029. https://doi.org/10.3390/ijms24098029





