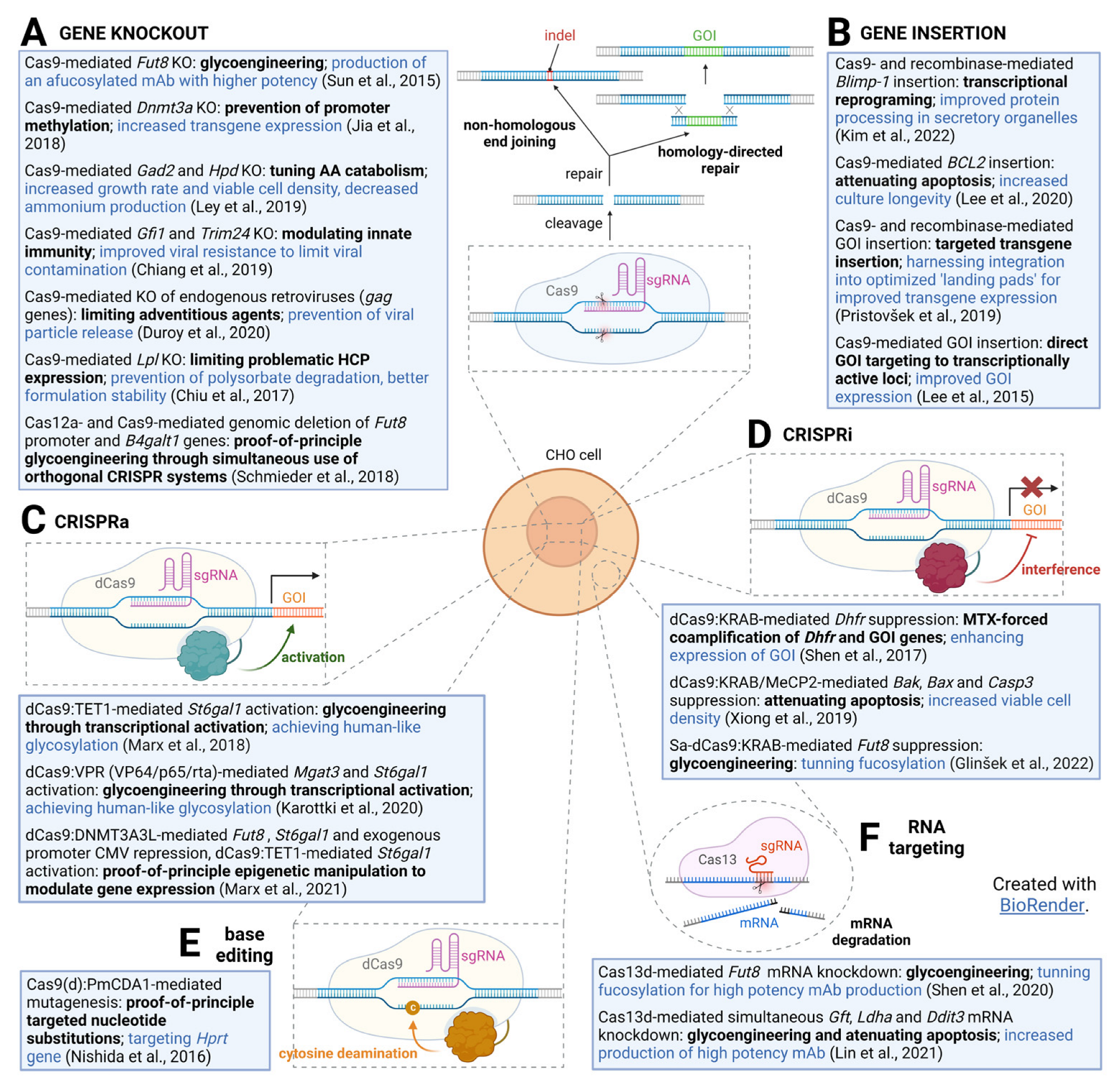
CRISPR Technologies in Chinese Hamster Ovary Cell Line Engineering
Abstract
1. Introduction
2. CRISPR-Cas9 System
3. CRISPR-Cas9-Mediated Gene Deletion in CHO Cells
3.1. Glycosylation
3.2. Enhancing Productivity and Cell Growth
3.3. Tackling Adventitious Agents by CRISPR-Cas9
3.4. CRISPR-Cas9-Mediated Elimination of Problematic Host Cell Proteins
| Application | Target Gene | Gene Editing Method | Outcome | Reference |
|---|---|---|---|---|
| Fucosylation and formation of elongated glycans in O-glycosylation | Fut8, Cosmc | CRISPR-Cas9-mediated KO | Indel frequency of up to 47.3% in Cosmc and 42.5% in Fut8. Applying lectin selection, the frequency was improved by up to 99.7% in Fut8. | [57] |
| Fucosylation | Fut8 | CRISPR-Cas9-mediated KO | Indel frequencies up to 25%, improved up to 52% with lectin selection. | [17] |
| Fucosylation | Slc35c1 | ZFNs, TALENs, and CRISPR-Cas9-mediated KO | Cas9-mediated indel frequency up 18.4%; production of EPO-Fc fusion protein and anti-Her2 antibody without core fucose. | [58] |
| α-2,6-sialylation | St3gal4, st3gal6, St6gal1 | CRISPR-Cas9-mediated KO and St6gal1 overexpression | Recombinant IgG with predominantly α-2,6 sialylation. | [64] |
| NGNA sialylation | Cmah | CRISPR-Cas9-mediated KO | Complete loss of the NGNA sialylation on the IgG4 antibody. | [65] |
| Glycoengineering of alpha-1-antitrypsin and plasma protease C1 inhibitor | KO: Mgat4A, Mgat4B, Mgat5, St3gal3, St3gal4, St3gal6, B3gnt2, Fut8, Sppl3, and Glul; OE: ST6GAL1 | CRISPR-Cas9-mediated KO and overexpression | Achieving glycosylation profile of recombinant proteins similar to the plasma-derived A1AT and C1INH. | [68] |
| Galactosylation | B4galt1, B4galt2, B4galt3, and B4galt4 | Combinatorial CRISPR-Cas9-mediated KO | Reducing the levels of galactosylated N-glycans to ~6% and ~3% on transiently expressed erythropoietin (EPO) and rituximab from triple KO clone. | [71] |
| Glycoengineering options for lysosomal replacement enzymes | 43 genes involved in N-glycosylation and mannose 6-phosphate processing | Individual and multiple CRISPR-Cas9-mediated KO | Improved circulation time and delivery to the heart of glycoengineered alpha-galactosidase in a Fabry disease mouse model. | [72] |
| Glycoengineering of therapeutic protein | 19 glycosyltransferase genes controlling N-glycosylation | ZFNs, TALENs, and CRISPR-Cas9-mediated KO | Target changes in the glycosylation profile of EPO. Identified key glycogenes controlling steps in N-glycosylation of proteins in CHO cells. | [49] |
| O-glycosylation | A number of genes involved in O-glycosylation | CRISPR-Cas9-mediated KO | O-glycoengineered CHO cell line platform for the production of engineered proteins with desired O-glycans. | [75] |
| Improving productivity | cgr-miR-744 | CRISPR-Cas9-mediated KO | Up to a 2-fold increase in antibody production. | [83] |
| Productivity | Casp-7 | Multiplex CRISPR homology-independent target integration (HITI) with KO | KO of Casp-7 lowered proliferation by up to 30% and reduced apoptosis resistance in KO clones. | [87] |
| Improving productivity and viability | Bax, Bak | CRISPR-Cas9-mediated double KO | Double KO clones with improved viability and up to 80% increase of productivity in intensified fed-batch. | [88] |
| Reducing apoptosis | Bak1, Bax, and Bok | Combinatorial CRISPR-Cas9-mediated KO | No detected impact on cell culture performance of Bok KO. Slower and delayed apoptosis in Bak1 and Bax KOs. | [89] |
| Improving secretory capacity | Blimp1 | CRISPR/Cas9-based recombinase-mediated KI | Up to 4-fold increased specific productivity of DTE recombinant human bone morphogenetic protein-4. | [24] |
| Reducing apoptosis | BCL2 | CRISPR-Cas9-mediated knock-in | Integration of human BCL2 into endogenous promoter locus reduced apoptosis. | [25] |
| Improving protein expression stability | Dnmt3a | CRISPR-Cas9-mediated KO | Enhanced long-term stability of transgene expression under CMV promoter. | [18] |
| Improving genome instability | CRISPR: Atm, Prkdc; OE: Xrcc6 and Lig4 | CRISPR-Cas9-mediated HDR-based gene correction and gene overexpression | DNA repair gene correction improved DNA repair and karyotypic instability. Overexpression of Xrcc6 and Lig4 led to improved stability of transgene copy number and productivity. | [95] |
| Reprogramming amino acid catabolism | Aass, Afmid, Ddc, Gad1, Gad2, Prodh, LOC100759874, Gapd2 and Hpd | CRISPR-Cas9-mediated KO | KOs of Gapd2 and Hpd increased growth rates by up to 19%, VCDs up to 50%, and up to 26% and 22% decrease in specific ammonium and lactate production, respectively. | [19] |
| Monitoring ER stress | BiP | CRISPR-Cas9-mediated KI | Generation of monitoring system for UPR activation detection upon ER stress. | [100] |
| Improving productivity | Cyp1a2, Atp5s, and Dgki | CRISPR-Cas9-mediated KO | Cyp1a2, Atp5s, or Dgki KOs led to more than 90% increased specific antibody productivity. | [101] |
| Improving resistance to adventitious agents | Slc35c1, Mgat1 and Cosmc | CRISPR-Cas9-mediated KO | Slc35a1 KO led to complete resistance to MVM infection, while Mgat1 and Cosmc KO led to significant inhibition of infection. | [76] |
| Improving resistance to adventitious agents | Gfi1 and Trim24 | CRISPR-Cas9-mediated KO | Increased antiviral resistance. | [20] |
| Eliminating viral particle contaminants | Gag | CRISPR-Cas9-mediated KO | Loss of function mutation in Gag gene led to reduced viral particle release. | [21] |
| Problematic HCP removal | Lpl | CRISPR-Cas9-mediated KO | Improved stability of PS20 (up to 57%) and PS80 (up to 47%) without significant impact on cell viability. | [22] |
| Problematic HCP removal | Ctsd, Anxa2, Calr | CRISPR-Cas9-mediated KO | Ctsd and Anxa2 KOs led to complete elimination of corresponding HCPs in cell lysates without affecting cell growth and viability. | [116] |
| Problematic HCP removal | Ctsd | shRNA interference and CRISPR-mediated KO | Ctsd KO led to almost complete elimination of the associated proteolytic degradation in purified mAbs. | [118] |
| Problematic HCP removal and glycoengineering of cell lines for HIV vaccine production | C1s, Mgat1 | CRISPR-Cas9-mediated KO | C1s/Mgat1 KO led to production of unclipped gp120 protein with high mannose glycans. | [119,120] |
| HCP removal for protein production enhancement | Timp1, Lgals3bp, Bgn, Nid1.1, Nid1.2, Ctsd, Tinagl1, Erp29, Aga, Lgmn, Gpr56, Yeats2, Sparc, Lpl | Multiple CRISPR-Cas9-mediated KOs | 6, 11 and 14 KOs led to 40–70% reduction of total HCP content and improved productivity and cell growth of selected clones. | [123] |
| Problematic HCP removal | CpD | CRISPR-Cas9-mediated KO | CpD KO led to complete abolishment of C-terminal lysine cleavage on IgG1. | [125] |
4. Toward Antibiotic-Free Recombinant Protein Production
5. From Random Integration toward CRISPR-Cas9-Mediated Site-Specific Knock-In
6. Applications of CRISPR-Mediated Gene Activation and Repression in CHO Cells
7. CRISPR for Studying Gene Function in CHO Cells
8. Major Challenges of CRISPR-Cas in CHO Cell Line Engineering and Potential Future Directions
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fischer, S.; Handrick, R.; Otte, K. The art of cho cell engineering: A comprehensive retrospect and future perspectives. Biotechnol. Adv. 2015, 33, 1878–1896. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kim, Y.G.; Lee, G.M. CHO cells in biotechnology for production of recombinant proteins: Current state and further potential. Appl. Microbiol. Biotechnol. 2012, 93, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Jayapal, K.P.; Wlaschin, K.F.; Hu, W.S.; Yap, M.G.S. Recombinant protein therapeutics from CHO cells—20 years and counting. Chem. Eng. Prog. 2007, 103, 40–47. [Google Scholar]
- Walsh, G. Biopharmaceutical benchmarks 2018. Nat. Biotechnol. 2018, 36, 1136–1145. [Google Scholar] [CrossRef]
- Datta, P.; Linhardt, R.J.; Sharfstein, S.T. An’omics approach towards CHO cell engineering. Biotechnol. Bioeng. 2013, 110, 1255–1271. [Google Scholar] [CrossRef]
- Walsh, G.; Walsh, E. Biopharmaceutical benchmarks 2022. Nat. Biotechnol. 2022, 40, 1722–1760. [Google Scholar] [CrossRef]
- Kwang Hong, J.; Lakshmanan, M.; Goudar, C.; Lee, D.Y.; Betenbaugh, M.; Titchener-Hooker, N. Towards next generation CHO cell line development and engineering by systems approaches. Curr. Opin. Chem. Eng. 2018, 22, 1–10. [Google Scholar] [CrossRef]
- Xu, X.; Nagarajan, H.; Lewis, N.E.; Pan, S.; Cai, Z.; Liu, X.; Chen, W.; Xie, M.; Wang, W.; Hammond, S.; et al. The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nat. Biotechnol. 2011, 29, 735–741. [Google Scholar] [CrossRef]
- Lonowski, L.A.; Narimatsu, Y.; Riaz, A.; Delay, C.E.; Yang, Z.; Niola, F.; Duda, K.; Ober, E.A.; Clausen, H.; Wandall, H.H.; et al. Genome editing using FACS enrichment of nuclease-expressing cells and indel detection by amplicon analysis. Nat. Protoc. 2017, 12, 581–603. [Google Scholar] [CrossRef]
- Carroll, D. Genome engineering with targetable nucleases. Annu. Rev. Biochem. 2014, 83, 409–439. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.S. A guide to genome engineering with programmable nucleases. Nat. Rev. Genet. 2014, 15, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, M.; McManus, M.T. Choosing the right tool for the job: RNAi, TALEN, or CRISPR. Mol. Cell. 2015, 58, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Sander, J.D.; Joung, J.K. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat. Biotechnol. 2014, 32, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Sanjana, N.E.; Cong, L.; Zhou, Y.; Cunniff, M.M.; Feng, G.; Zhang, F. A transcription activator-like effector toolbox for genome engineering. Nat. Protoc. 2012, 7, 171–192. [Google Scholar] [CrossRef] [PubMed]
- Urnov, F.D.; Rebar, E.J.; Holmes, M.C.; Zhang, H.S.; Gregory, P.D. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 2010, 9, 636–646. [Google Scholar] [CrossRef]
- Ferreira, P.; Choupina, A.B. CRISPR/Cas9 a simple, inexpensive and effective technique for gene editing. Mol. Biol. Rep. 2022, 49, 7079–7086. [Google Scholar] [CrossRef]
- Sun, T.; Li, C.; Han, L.; Jiang, H.; Xie, Y.; Zhang, B.; Qian, X.; Lu, H.; Zhu, J. Functional knockout of FUT8 in Chinese hamster ovary cells using CRISPR/Cas9 to produce a defucosylated antibody. Eng. Life Sci. 2015, 15, 660–666. [Google Scholar] [CrossRef]
- Jia, Y.; Guo, X.; Lu, J.; Wang, X.; Qiu, L.; Wang, T. CRISPR/Cas9-mediated gene knockout for DNA methyltransferase Dnmt3a in CHO cells displays enhanced transgenic expression and long-term stability. J. Cell. Mol. Med. 2018, 22, 4106–4116. [Google Scholar] [CrossRef]
- Ley, D.; Pereira, S.; Pedersen, L.E.; Arnsdorf, J.; Hefzi, H.; Davy, A.M.; Ha, T.K.; Wulff, T.; Kildegaard, H.F.; Andersen, M.R. Reprogramming AA catabolism in CHO cells with CRISPR/Cas9 genome editing improves cell growth and reduces byproduct secretion. Metab. Eng. 2019, 56, 120–129. [Google Scholar] [CrossRef]
- Chiang, A.W.T.; Li, S.; Kellman, B.P.; Chattopadhyay, G.; Zhang, Y.; Kuo, C.C.; Gutierrez, J.M.; Ghazi, F.; Schmeisser, H.; Ménard, P.; et al. Combating viral contaminants in CHO cells by engineering innate immunity. Sci. Rep. 2019, 9, 8827. [Google Scholar] [CrossRef]
- Duroy, P.; Bosshard, S.; Schmid-Siegert, E.; Neuenschwander, S.; Arib, G.; Lemercier, P.; Masterneak, J.; Roesch, L.; Buron, F.; Girod, P.; et al. Characterization and mutagenesis of Chinese hamster ovary cells endogenous retroviruses to inactivate viral particle release. Biotechnol. Bioeng. 2020, 117, 466–485. [Google Scholar] [CrossRef]
- Chiu, J.; Valente, K.N.; Levy, N.E.; Min, L.; Lenhoff, A.M.; Lee, K.H. Knockout of a difficult-to-remove CHO host cell protein, lipoprotein lipase, for improved polysorbate stability in monoclonal antibody formulations. Biotechnol. Bioeng. 2017, 114, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, V.; Bydlinski, N.; Strasser, R.; Baumann, M.; Kildegaard, H.F.; Jadhav, V.; Borth, N. Enhanced genome editing tools for multi-gene deletion knock-out approaches using paired CRISPR sgRNAs in CHO cells. Biotechnol. J. 2018, 13, 1700211. [Google Scholar] [CrossRef]
- Kim, S.H.; Baek, M.; Park, S.; Shin, S.; Lee, J.S.; Lee, G.M. Improving the secretory capacity of CHO producer cells: The effect of controlled blimp1 expression, a master transcription factor for plasma cells. Metab. Eng. 2022, 69, 73–86. [Google Scholar] [CrossRef]
- Lee, Y.; Kwak, J.M.; Lee, J.S. Endogenous p21-dependent transgene control for CHO cell engineering. ACS Synth. Biol. 2020, 9, 1572–1580. [Google Scholar] [CrossRef]
- Pristovšek, N.; Nallapareddy, S.; Grav, L.M.; Hefzi, H.; Lewis, N.E.; Rugbjerg, P.; Hansen, H.G.; Lee, G.M.; Andersen, M.R.; Faustrup Kildegaard, H. Systematic evaluation of site-specific recombinant gene expression for programmable mammalian cell engineering. ACS Synth. Biol. 2019, 8, 757–774. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kallehauge, T.B.; Pedersen, L.E.; Kildegaard, H.F. Site-specific integration in CHO cells mediated by CRISPR/Cas9 and homology-directed DNA repair pathway. Sci. Rep. 2015, 5, 8572. [Google Scholar] [CrossRef] [PubMed]
- Marx, N.; Grünwald-Gruber, C.; Bydlinski, N.; Dhiman, H.; Ngoc Nguyen, L.; Klanert, G.; Borth, N. CRISPR-based targeted epigenetic editing enables gene expression modulation of the silenced beta-galactoside alpha-2,6-sialyltransferase 1 in CHO cells. Biotechnol. J. 2018, 13, 1700217. [Google Scholar] [CrossRef]
- Marx, N.; Dhiman, H.; Schmieder, V.; Freire, C.M.; Nguyen, L.N.; Klanert, G.; Borth, N. Enhanced targeted DNA methylation of the CMV and endogenous promoters with dCas9-DNMT3A3L entails distinct subsequent histone modification changes in CHO cells. Metab. Eng. 2021, 66, 268–282. [Google Scholar] [CrossRef]
- Karottki, K.J.L.C.; Hefzi, H.; Xiong, K.; Shamie, I.; Hansen, A.H.; Li, S.; Pedersen, L.E.; Li, S.; Lee, J.S.; Lee, G.M.; et al. Awakening dormant glycosyltransferases in CHO cells with CRISPRa. Biotechnol. Bioeng. 2020, 117, 593–598. [Google Scholar] [CrossRef]
- Shen, C.C.; Sung, L.Y.; Lin, S.Y.; Lin, M.W.; Hu, Y.C. Enhancing protein production yield from Chinese hamster ovary cells by CRISPR interference. Cells 2017, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Marquart, K.F.; la Cour Karottki, K.J.; Li, S.; Shamie, I.; Lee, J.S.; Gerling, S.; Yeo, N.C.; Chavez, A.; Lee, G.M.; et al. Reduced apoptosis in Chinese hamster ovary cells via optimized CRISPR interference. Biotechnol. Bioeng. 2019, 116, 1813–1819. [Google Scholar] [CrossRef]
- Glinšek, K.; Kramer, L.; Krajnc, A.; Kranjc, E.; Pirher, N.; Marušič, J.; Hellmann, L.; Podobnik, B.; Štrukelj, B.; Ausländer, D.; et al. Coupling CRISPR interference with FACS enrichment: New approach in glycoengineering of CHO cell lines for therapeutic glycoprotein production. Biotechnol. J. 2022, 17, e2100499. [Google Scholar] [CrossRef]
- Nishida, K.; Arazoe, T.; Yachie, N.; Banno, S.; Kakimoto, M.; Tabata, M.; Mochizuki, M.; Miyabe, A.; Araki, M.; Hara, K.Y.; et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 2016, 353, aaf8729. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Lin, M.W.; Nguyen, B.K.T.; Chang, C.W.; Shih, J.R.; Nguyen, M.T.T.; Chang, Y.H.; Hu, Y.C. CRISPR-Cas13d for gene knockdown and engineering of CHO cells. ACS Synth. Biol. 2020, 9, 2808–2818. [Google Scholar] [CrossRef]
- Lin, M.W.; Shen, C.C.; Lin, Y.J.; Chou, M.Y.; Pham, N.N.; Chang, Y.H.; Chang, C.W.; Hwu, J.R.; Nguyen, M.T.T.; Hu, Y.C. Enhancing the yield and activity of defucosylated antibody produced by CHO-K1 cells using Cas13d-mediated multiplex gene targeting. J. Taiwan Inst. Chem. Eng. 2021, 121, 38–47. [Google Scholar] [CrossRef]
- Jinek, M.; East, A.; Cheng, A.; Lin, S.; Ma, E.; Doudna, J. RNA-programmed genome editing in human cells. eLife 2013, 2, e00471. [Google Scholar] [CrossRef] [PubMed]
- Cong, L.; Ran, F.A.; Cox, D.; Lin, S.; Barretto, R.; Habib, N.; Hsu, P.D.; Wu, X.; Jiang, W.; Marraffini, L.A.; et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [Google Scholar] [CrossRef]
- Mali, P.; Yang, L.; Esvelt, K.M.; Aach, J.; Guell, M.; DiCarlo, J.E.; Norville, J.E.; Church, G.M. RNA-guided human genome engineering via Cas9. Science 2013, 339, 823–826. [Google Scholar] [CrossRef]
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1–9. [Google Scholar] [CrossRef]
- Vora, S.; Tuttle, M.; Cheng, J.; Church, G. Next stop for the CRISPR revolution: RNA-guided epigenetic regulators. FEBS J. 2016, 283, 3181–3193. [Google Scholar] [CrossRef]
- Mali, P.; Aach, J.; Stranges, P.B.; Esvelt, K.M.; Moosburner, M.; Kosuri, S.; Yang, L.; Church, G.M. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 2013, 31, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G. Post-translational modifications of protein biopharmaceuticals. Drug Discov. Today 2010, 15, 773–780. [Google Scholar] [CrossRef]
- Walsh, G.; Jefferis, R. Post-translational modifications in the context of therapeutic proteins. Nat. Biotechnol. 2006, 24, 1241–1252. [Google Scholar] [CrossRef]
- Delobel, A. Glycosylation of therapeutic proteins: A critical quality attribute. Methods Mol. Biol. 2021, 2271, 1–21. [Google Scholar] [PubMed]
- Tejwani, V.; Andersen, M.R.; Nam, J.H.; Sharfstein, S.T. Glycoengineering in CHO cells: Advances in systems biology. Biotechnol. J. 2018, 13, 1700234. [Google Scholar] [CrossRef]
- Schulz, M.A.; Tian, W.; Mao, Y.; Van Coillie, J.; Sun, L.; Larsen, J.S.; Andersen, M.R.; Chin, P.T.K.; Andersen, M.R.; Kildegaard, H.F. Glycoengineering design options for IgG1 in CHO cells using precise gene editing. Glycobiology 2018, 28, 542–549. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, S.; Halim, A.; Schulz, M.A.; Frodin, M.; Rahman, S.H.; Vester-Christensen, M.B.; Behrens, C.; Kristensen, C.; Vakhrushev, S.Y.; et al. Engineered CHO cells for production of diverse, homogeneous glycoproteins. Nat. Biotechnol. 2015, 33, 842–844. [Google Scholar] [CrossRef]
- Pereira, N.A.; Chan, K.F.; Lin, P.C.; Song, Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. MAbs 2018, 10, 693–711. [Google Scholar] [CrossRef]
- Krapp, S.; Mimura, Y.; Jefferis, R.; Huber, R.; Sondermann, P. Structural analysis of human IgG-Fc glycoforms reveals a correlation between glycosylation and structural integrity. J. Mol. Biol. 2003, 325, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.L.; Lai, J.; Keck, R.; O’Connell, L.Y.; Hong, K.; Meng, Y.G.; Weikert, S.H.A.; Presta, L.G. Lack of fucose on human IgG1 N-linked oligosaccharide improves binding to human FcγRIII and antibody-dependent cellular toxicity. J. Biol. Chem. 2002, 277, 26733–26740. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, I.; Dhiman, H.; Klanert, G.; Jadhav, V.; Auer, N.; Hanscho, M.; Baumann, M.; Esteve-Codina, A.; Dabad, M.; Gómez, J.; et al. Epigenetic regulation of gene expression in Chinese Hamster Ovary cells in response to the changing environment of a batch culture. Biotechnol. Bioeng. 2019, 116, 677–692. [Google Scholar] [CrossRef]
- Yamane-Ohnuki, N.; Kinoshita, S.; Inoue-Urakubo, M.; Kusunoki, M.; Iida, S.; Nakano, R.; Wakitani, M.; Niwa, R.; Sakurada, M.; Uchida, K.; et al. Establishment of FUT8 knockout Chinese hamster ovary cells: An ideal host cell line for producing completely defucosylated antibodies with enhanced antibody-dependent cellular cytotoxicity. Biotechnol. Bioeng. 2004, 87, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Malphettes, L.; Freyvert, Y.; Chang, J.; Liu, P.Q.; Chan, E.; Miller, J.C.; Zhou, Z.; Nguyen, T.; Tsai, C.; Snowden, A.W.; et al. Highly efficient deletion of FUT8 in CHO cell lines using zinc-finger nucleases yields cells that produce completely nonfucosylated antibodies. Biotechnol. Bioeng. 2010, 106, 774–783. [Google Scholar] [CrossRef]
- Imai-Nishiya, H.; Mori, K.; Inoue, M.; Wakitani, M.; Iida, S.; Shitara, K.; Satoh, M. Double knockdown of alpha1,6-fucosyltransferase (FUT8) and GDP-mannose 4,6-dehydratase (GMD) in antibody-producing cells: A new strategy for generating fully non-fucosylated therapeutic antibodies with enhanced ADCC. BMC Biotechnol. 2007, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Ronda, C.; Pedersen, L.E.; Hansen, H.G.; Kallehauge, T.B.; Betenbaugh, M.J.; Nielsen, A.T.; Kildegard, H.F. Accelerating genome editing in CHO cells using CRISPR Cas9 and CRISPy, a web-based target finding tool. Biotechnol. Bioeng. 2014, 111, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.F.; Shahreel, W.; Wan, C.; Teo, G.; Hayati, N.; Tay, S.J.; Tong, W.H.; Yang, Y.; Rudd, P.M.; Zhang, P.; et al. Inactivation of GDP-fucose transporter gene (Slc35c1) in CHO cells by ZFNs, TALENs and CRISPR-Cas9 for production of fucose-free antibodies. Biotechnol. J. 2016, 11, 399–414. [Google Scholar] [CrossRef]
- Joubert, S.; Guimond, J.; Perret, S.; Malenfant, F.; Elahi, S.M.; Marcil, A.; Parat, M.; Gilbert, M.; Lenferink, A.E.G.; Baardsnes, J.; et al. Production of afucosylated antibodies in CHO cells by coexpression of an anti-FUT8 intrabody. Biotechnol. Bioeng. 2022, 119, 2206–2220. [Google Scholar] [CrossRef]
- Varki, A. Sialic acids in human health and disease. Trends Mol. Med. 2008, 14, 351–360. [Google Scholar] [CrossRef]
- Bork, K.; Horstkorte, R.; Weidemann, W. Increasing the sialylation of therapeutic glycoproteins: The potential of the sialic acid biosynthetic pathway. J. Pharm. Sci. 2009, 98, 3499–3508. [Google Scholar] [CrossRef]
- Lin, N.; Mascarenhas, J.; Sealover, N.R.; George, H.J.; Brooks, J.; Kayser, K.J.; Gau, B.; Yasa, I.; Azadi, P.; Archer-Hartmann, S. Chinese hamster ovary (CHO) host cell engineering to increase sialylation of recombinant therapeutic proteins by modulating sialyltransferase expression. Biotechnol. Prog. 2015, 31, 334–346. [Google Scholar] [CrossRef]
- Anthony, R.M.; Nimmerjahn, F.; Ashline, D.J.; Reinhold, V.N.; Paulson, J.C.; Ravetch, J.V. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 2008, 320, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.Y.; Wang, Q.; Yang, S.; Yin, B.; Zhang, H.; Betenbaugh, M. Integrated genome and protein editing swaps α-2,6 sialylation for α-2,3 sialic acid on recombinant antibodies from CHO. Biotechnol. J. 2017, 12, 1600502. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Mathias, S.; Stadermann, A.; Yang, S.; Schmieder, V.; Zeh, N.; Schmidt, N.; Richter, P.; Wright, S.; Zimmermann, E.; et al. Loss of a newly discovered microRNA in Chinese hamster ovary cells leads to upregulation of N-glycolylneuraminic acid sialylation on monoclonal antibodies. Biotechnol. Bioeng. 2022, 119, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Vijayasankaran, N.; Shen, A.; Kiss, R.; Amanullah, A. Cell culture processes for monoclonal antibody production. MAbs 2010, 2, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Glycan-based Interactions Involving Vertebrate Sialic-acid-Recognizing Proteins. Nature 2007, 446, 1023–1029. [Google Scholar] [CrossRef]
- Amann, T.; Hansen, A.H.; Kol, S.; Hansen, H.G.; Arnsdorf, J.; Nallapareddy, S.; Voldborg, B.; Lee, G.M.; Andersen, M.R.; Kildegaard, H.F. Glyco-engineered CHO cell lines producing alpha-1-antitrypsin and C1 esterase inhibitor with fully humanized N-glycosylation profiles. Metab. Eng. 2019, 52, 143–152. [Google Scholar] [CrossRef]
- Clerc, F.; Reiding, K.R.; Jansen, B.C.; Kammeijer, G.S.M.; Bondt, A.; Wuhrer, M. Human plasma protein N-glycosylation. Glycoconj. J. 2016, 33, 309–343. [Google Scholar] [CrossRef]
- Koyuturk, I.; Kedia, S.; Robotham, A.; Star, A.; Brochu, D.; Sauvageau, J.; Kelly, J.; Gilbert, M.; Durocher, Y. High-level production of wild-type and oxidation-resistant recombinant alpha-1-antitrypsin in glycoengineered CHO cells. Biotechnol. Bioeng. 2022, 119, 2331–2344. [Google Scholar] [CrossRef]
- Amann, T.; Hansen, A.H.; Kol, S.; Lee, G.M.; Andersen, M.R.; Kildegaard, H.F. CRISPR/Cas9-Multiplexed editing of Chinese hamster ovary B4Gal-T1, 2, 3, and 4 tailors N-glycan profiles of therapeutics and secreted host cell proteins. Biotechnol. J. 2018, 13, 1800111. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Ye, Z.; Wang, S.; Schulz, M.A.; van Coillie, J.; Sun, L.; Chen, Y.H.; Narimatsu, Y.; Hansen, L.; Kristensen, C.; et al. The glycosylation design space for recombinant lysosomal replacement enzymes produced in CHO cells. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Parenti, G.; Piganta, C.; Vajro, P.; Salerno, M. New strategies for the treatment of lysosomal storage diseases. Int. J. Mol. Med. 2013, 31, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Platt, F.M. Emptying the stores: Lysosomal diseases and therapeutic strategies. Nat. Rev. Drug Discov. 2018, 17, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Biel, T.G.; Faison, T.; Matthews, A.M.; Zou, G.; Ortega-Rodriguez, U.; Pegues, M.A.; Azer, N.; Gomez, F.; Johnson, S.; Rogstad, S.; et al. An etanercept O-glycovariant with enhanced potency. Mol. Ther. Methods Clin. Dev. 2022, 25, 124–135. [Google Scholar] [CrossRef]
- Mascarenhas, J.X.; Korokhov, N.; Burger, L.; Kassim, A.; Tuter, J.; Miller, D.; Borgschulte, T.; George, H.J.; Chang, A.; Pintel, D.J.; et al. Genetic engineering of CHO cells for viral resistance to minute virus of mice. Biotechnol. Bioeng. 2017, 114, 576–588. [Google Scholar] [CrossRef]
- Yang, Z.; Halim, A.; Narimatsu, Y.; Joshi, H.J.; Steentoft, C.; Schjoldager, K.T.B.G.; Schulz, M.A.; Sealover, N.R.; Kayser, K.J.; Bennett, E.P.; et al. The GalNAc-type O-glycoproteome of CHO cells characterized by the SimpleCell strategy. Mol. Cell. Proteomics 2014, 13, 3224–3235. [Google Scholar] [CrossRef]
- Bratkovič, T.; Glavan, G.; Štrukelj, B.; Živin, M.; Rogelj, B. Exploiting microRNAs for cell engineering and therapy. Biotechnol. Adv. 2012, 30, 753–765. [Google Scholar] [CrossRef]
- Fischer, S.; Marquart, K.F.; Pieper, L.A.; Fieder, J.; Gamer, M.; Gorr, I.; Schulz, P.; Bradl, H. miRNA engineering of CHO cells facilitates production of difficult-to-express proteins and increases success in cell line development. Biotechnol. Bioeng. 2017, 114, 1495–1510. [Google Scholar] [CrossRef]
- Fischer, S.; Buck, T.; Wagner, A.; Ehrhart, C.; Giancaterino, J.; Mang, S.; Schad, M.; Mathias, S.; Aschrafi, A.; Handrick, R.; et al. A functional high-content mirna screen identifies miR-30 family to boost recombinant protein production in CHO Cells. Biotechnol. J. 2014, 9, 1279–1292. [Google Scholar] [CrossRef]
- Druz, A.; Son, Y.J.; Betenbaugh, M.; Shiloach, J. Stable inhibition of mmu-miR-466h-5p improves apoptosis resistance and protein production in CHO cells. Metab. Eng. 2013, 16, 87–94. [Google Scholar] [CrossRef]
- Sanchez, N.; Kelly, P.; Gallagher, C.; Lao, N.T.; Clarke, C.; Clynes, M.; Barron, N. CHO Cell culture longevity and recombinant protein yield are enhanced by depletion of miR-7 activity via sponge decoy vectors. Biotechnol. J. 2014, 9, 396–404. [Google Scholar] [CrossRef]
- Raab, N.; Mathias, S.; Alt, K.; Handrick, R.; Fischer, S.; Schmieder, V.; Jadhav, V.; Borth, N.; Otte, K. CRISPR/Cas9-mediated knockout of microRNA-744 improves antibody titer of cho production cell lines. Biotechnol. J. 2019, 14, 1800477. [Google Scholar] [CrossRef] [PubMed]
- Grilo, A.L.; Mantalaris, A. Apoptosis: A mammalian cell bioprocessing perspective. Biotechnol. Adv. 2019, 37, 459–475. [Google Scholar] [CrossRef] [PubMed]
- Cade, C.E.; Clark, A.C. Caspases-Key Players in Apoptosis. In Proteases in Apoptosis: Pathways, Protocols and Translational Advances; Bose, K., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 31–51. [Google Scholar]
- Sung, Y.H.; Lee, J.S.; Park, S.H.; Koo, J.; Lee, G.M. Influence of co-down-regulation of caspase-3 and caspase-7 by siRNAs on sodium butyrate-induced apoptotic cell death of Chinese hamster Ovary cells producing thrombopoietin. Metab. Eng. 2007, 9, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Safari, F.; Farajnia, S.; Behbahani, A.B.; Zarredar, H.; Barekati-Mowahed, M.; Dehghani, H. Caspase-7 deficiency in Chinese hamster ovary cells reduces cell proliferation and viability. Biol. Res. 2020, 53, 52. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Lam, C.; Bauer, N.; Auslaender, S.; Snedecor, B.; Laird, M.W.; Misaghi, S. Bax and Bak Knockout apoptosis-resistant Chinese hamster ovary cell lines significantly improve culture viability and titer in intensified fed-batch culture process. Biotechnol. Prog. 2022, 38, e3228. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.A.; Barry, C.; Groves, T.; Martínez, V.S.; Gray, P.P.; Baker, K.; Shave, E.; Mahler, S.; Munro, T.; Marcellin, E.; et al. Modeling apoptosis resistance in CHO cells with CRISPR-mediated knockouts of Bak1, Bax, and Bok. Biotechnol. Bioeng. 2022, 119, 1380–1391. [Google Scholar] [CrossRef]
- Miao, Z.; Li, Q.; Zhao, J.; Wang, P.; Wang, L.; He, H.P.; Wang, N.; Zhou, H.; Zhang, T.; Lou, X. Stable expression of infliximab in CRISPR/Cas9-mediated BAK1-deficient CHO cells. Biotechnol. Lett. 2018, 40, 1209–1218. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Baumann, M.; Dhiman, H.; Marx, N.; Schmieder, V.; Hussein, M.; Eisenhut, P.; Hernandez, I.; Koehn, J.; Borth, N. Novel promoters derived from Chinese hamster ovary cells via in silico and in vitro analysis. Biotechnol. J. 2019, 14, 1900125. [Google Scholar] [CrossRef]
- Dahodwala, H.; Lee, K.H. The fickle CHO: A review of the causes, implications, and potential alleviation of the CHO cell line instability problem. Curr. Opin. Biotechnol. 2019, 60, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chusainow, J.; Yap, M.G. DNA methylation contributes to loss in productivity of monoclonal antibody-producing CHO cell lines. J. Biotechnol. 2010, 147, 180–185. [Google Scholar] [CrossRef]
- Moritz, B.; Becker, P.B.; Göpfert, U. CMV promoter mutants with a reduced propensity to productivity loss in CHO cells. Sci. Rep. 2015, 5, 16952. [Google Scholar] [CrossRef] [PubMed]
- Spahn, P.N.; Zhang, X.; Hu, Q.; Lu, H.; Hamaker, N.K.; Hefzi, H.; Li, S.; Kuo, C.; Huang, Y.; Lee, J.C.; et al. Restoration of DNA repair mitigates genome instability and increases productivity of Chinese hamster ovary cells. Biotechnol. Bioeng. 2022, 119, 963–982. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Kildegaard, H.F.; Andersen, M.R. Impact of CHO metabolism on cell growth and protein production: An overview of toxic and inhibiting metabolites and nutrients. Biotechnol. J. 2018, 13, 1700499. [Google Scholar] [CrossRef] [PubMed]
- Mulukutla, B.C.; Kale, J.; Kalomeris, T.; Jacobs, M.; Hiller, G.W. Identification and control of novel growth inhibitors in fed-batch cultures of Chinese hamster ovary cells. Biotechnol. Bioeng. 2017, 114, 1779–1790. [Google Scholar] [CrossRef]
- Young, J.D. Metabolic flux rewiring in mammalian cell cultures. Curr. Opin. Biotechnol. 2013, 24, 1108–1115. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Kyeong, M.; Lee, J.S. Endogenous BiP reporter system for simultaneous identification of ER stress and antibody production in Chinese hamster ovary cells. Metab. Eng. 2022, 72, 35–45. [Google Scholar] [CrossRef]
- Lin, P.; Liu, R.; Alvin, K.; Wahyu, S.; Murgolo, N.; Ye, J.; Du, Z.; Song, Z. Improving antibody production in stably transfected CHO cells by CRISPR-Cas9-mediated inactivation of genes identified in a large-scale screen with Chinese hamster-specific siRNAs. Biotechnol. J. 2021, 16, 2000267. [Google Scholar] [CrossRef]
- Klanert, G.; Fernandez, D.J.; Weinguny, M.; Eisenhut, P.; Bühler, E.; Melcher, M.; Titus, S.A.; Diendorfer, A.B.; Gludovacz, E.; Jadhav, V.; et al. A cross-species whole genome siRNA screen in suspension-cultured Chinese hamster ovary cells identifies novel engineering targets. Sci. Rep. 2019, 9, 8689. [Google Scholar] [CrossRef]
- Berting, A.; Farcet, M.R.; Kreil, T.R. Virus susceptibility of Chinese hamster ovary (CHO) cells and detection of viral contaminations by adventitious agent testing. Biotechnol. Bioeng. 2010, 106, 598–607. [Google Scholar] [CrossRef]
- Bethencourt, V. Virus stalls Genzyme plant. Nat. Biotechnol. 2009, 27, 681. [Google Scholar] [CrossRef]
- Nims, R.W. Detection of adventitious viruses in biologicals—A rare occurrence. Dev. Biol. 2006, 123, 153–164. [Google Scholar]
- Rabenau, H.; Ohlinger, V.; Anderson, J.; Selb, B.; Cinatl, J.; Wolf, W.; Frost, J.; Mellor, P.; Doerr, H.W. Contamination of genetically engineered CHO-cells by epizootic haemorrhagic disease virus (EHDV). Biologicals 1993, 21, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; Tattersall, P. Parvoviral host range and cell entry mechanisms. Adv. Virus Res. 2007, 70, 183–232. [Google Scholar]
- Merten, O.W. Virus contaminations of cell cultures—A biotechnological view. Cytotechnology 2002, 39, 91–116. [Google Scholar] [CrossRef] [PubMed]
- ICH Guideline Q11 on Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/Biological Entities). ICH/425213/2011. 2011. Available online: https://www.ema.europa.eu/en/ich-q11-development-manufacture-drug-substances-chemical-entities-biotechnological-biological (accessed on 26 April 2023).
- Singh, S.K. Impact of product-related factors on immunogenicity of biotherapeutics. J. Pharm. Sci. 2011, 100, 354–387. [Google Scholar] [CrossRef]
- Gao, S.X.; Zhang, Y.; Stansberry-Perkins, K.; Buko, A.; Bai, S.; Nguyen, V.; Brader, M.L. Fragmentation of a highly purified monoclonal antibody attributed to residual CHO cell protease activity. Biotechnol. Bioeng. 2011, 108, 977–982. [Google Scholar] [CrossRef]
- Robert, F.; Bierau, H.; Rossi, M.; Agugiaro, D.; Soranzo, T.; Broly, H.; Mitchell-Loean, C. Degradation of an Fc-fusion recombinant protein by host cell proteases: Identification of a CHO cathepsin D protease. Biotechnol. Bioeng. 2009, 104, 1132–1141. [Google Scholar] [CrossRef]
- Jones, M.; Palackal, N.; Wang, F.; Gaza-Bulseco, G.; Hurkmans, K.; Zhao, Y.; Chitikila, C.; Clavier, S.; Liu, S.; Menesale, E.; et al. “High-risk” host cell proteins (HCPs): A multi-company collaborative view. Biotechnol. Bioeng. 2021, 118, 2870–2885. [Google Scholar] [CrossRef] [PubMed]
- Levy, N.E.; Valente, K.N.; Lee, K.H.; Lenhoff, A.M. Host cell protein impurities in chromatographic polishing steps for monoclonal antibody purification. Biotechnol. Bioeng. 2016, 113, 1260–1272. [Google Scholar] [CrossRef] [PubMed]
- Valente, K.N.; Lenhoff, A.M.; Lee, K.H. Expression of difficult-to-remove host cell protein impurities during extended Chinese hamster ovary cell culture and their impact on continuous bioprocessing. Biotechnol. Bioeng. 2015, 112, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Senga, Y.; Honda, S. Anxa2 and Ctsd-knockout CHO cell lines to diminish the risk of contamination with host cell proteins. Biotechnol. Prog. 2019, 35, e2820. [Google Scholar] [CrossRef] [PubMed]
- Bailey-Kellogg, C.; Gutiérrez, A.H.; Moise, L.; Terry, F.; Martin, W.D.; de Groot, A.S. CHOPPI: A web tool for the analysis of immunogenicity risk from host cell proteins in CHO-based protein production. Biotechnol. Bioeng. 2014, 111, 2170–2182. [Google Scholar] [CrossRef]
- Dovgan, T.; Golghalyani, V.; Zurlo, F.; Hatton, D.; Lindo, V.; Turner, R.; Harris, C.; Cui, T. Targeted CHO cell engineering approaches can reduce HCP-related enzymatic degradation and improve mAb product quality. Biotechnol. Bioeng. 2021, 118, 3821–3831. [Google Scholar] [CrossRef]
- Li, S.W.; Yu, B.; Byrne, G.; Wright, M.; O’Rourke, S.; Mesa, K.; Berman, P.W. Identification and CRISPR/Cas9 inactivation of the C1s protease responsible for proteolysis of recombinant proteins produced in CHO cells. Biotechnol. Bioeng. 2019, 116, 2130–2145. [Google Scholar] [CrossRef]
- Li, S.W.; Wright, M.; Healey, J.F.; Hutchinson, J.M.; O’Rourke, S.; Mesa, K.A.; Lollar, P.; Berman, P.W. Gene editing in CHO cells to prevent proteolysis and enhance glycosylation: Production of HIV envelope proteins as vaccine immunogens. PLoS ONE 2020, 15, e0233866. [Google Scholar] [CrossRef]
- Pugach, P.; Ozorowski, G.; Cupo, A.; Ringe, R.; Yasmeen, A.; de Val, N.; Derking, R.; Kim, H.J.; Korzun, J.; Golabek, M.; et al. A native-like SOSIP.664 trimer based on an HIV-1 subtype B env gene. J. Virol. 2015, 89, 3380–3395. [Google Scholar] [CrossRef]
- Byrne, G.; O’Rourke, S.M.; Alexander, D.L.; Yu, B.; Doran, R.C.; Wright, M.; Chen, Q.; Azadi, P.; Berman, P.W. CRISPR/Cas9 gene editing for the creation of an MGAT1-deficient CHO cell line to control HIV-1 vaccine glycosylation. PLoS Biol. 2018, 16, e2005817. [Google Scholar] [CrossRef]
- Kol, S.; Ley, D.; Wulff, T.; Decker, M.; Arnsdorf, J.; Schoffelen, S.; Hansen, A.H.; Jensen, T.L.; Gutierrez, J.M.; Chiang, A.W.T.; et al. Multiplex secretome engineering enhances recombinant protein production and purity. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dick, L.W.; Qiu, D.; Mahon, D.; Adamo, M.; Cheng, K.C. C-terminal lysine variants in fully human monoclonal antibodies: Investigation of test methods and possible causes. Biotechnol. Bioeng. 2008, 100, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, H.; Haley, B.; Macchi, F.; Yang, F.; Misaghi, S.; Elich, J.; Yang, R.; Tang, Y.; Joly, J.C.; et al. Carboxypeptidase D is the only enzyme responsible for antibody C-terminal lysine cleavage in Chinese hamster ovary (CHO) cells. Biotechnol. Bioeng. 2016, 113, 2100–2106. [Google Scholar] [CrossRef]
- Teixeira, A.P.; Stücheli, P.; Ausländer, S.; Ausländer, D.; Schönenberger, P.; Hürlemann, S.; Fussenegger, M. CelloSelect—A synthetic cellobiose metabolic pathway for selection of stable transgenic CHO cell lines. Metab. Eng. 2022, 70, 23–30. [Google Scholar] [CrossRef]
- Lai, T.; Yang, Y.; Ng, S. Advances in mammalian cell cine development technologies for recombinant protein production. Pharmaceuticals 2013, 6, 579–603. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jiang, B.; Nelson, L.; Huhn, S.; Du, Z.; Chasin, L.A. A multiauxotrophic CHO cell line for the rapid isolation of producers of diverse or high levels of recombinant proteins. Biotechnol. Prog. 2022, 38, e3281. [Google Scholar] [CrossRef]
- Hamaker, N.K.; Lee, K.H. Site-specific integration ushers in a new era of precise CHO cell line engineering. Curr. Opin. Chem. Eng. 2018, 22, 152–160. [Google Scholar] [CrossRef]
- Hilliard, W.; Lee, K.H. Systematic identification of safe harbor regions in the CHO genome through a comprehensive epigenome analysis. Biotechnol. Bioeng. 2021, 118, 659–675. [Google Scholar] [CrossRef]
- Gaidukov, L.; Wroblewska, L.; Teague, B.; Nelson, T.; Zhang, X.; Liu, Y.; Jagtap, K.; Mamo, S.; Tseng, W.A.; Lowe, A.; et al. A multi-landing pad DNA integration platform for mammalian cell engineering. Nucleic Acids Res. 2018, 46, 4072–4086. [Google Scholar] [CrossRef]
- Crawford, Y.; Zhou, M.; Hu, Z.; Joly, J.; Snedecor, B.; Shen, A.; Gao, A. Fast identification of reliable hosts for targeted cell line development from a limited-genome screening using combined φC31 integrase and cre-lox technologies. Biotechnol. Prog. 2013, 29, 1307–1315. [Google Scholar] [CrossRef]
- Sengupta, S.; George, R.E. Super-enhancer-driven transcriptional dependencies in cancer. Trends Cancer 2017, 3, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.; Raabe, M.; Hu, W. Epigenomic features revealed by ATAC-seq impact transgene expression in CHO cells. Biotechnol. Bioeng. 2021, 118, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Inniss, M.C.; Bandara, K.; Jusiak, B.; Lu, T.K.; Weiss, R.; Wroblewska, L.; Zhang, L. A novel Bxb1 integrase RMCE system for high fidelity site-specific integration of mAb expression cassette in CHO cells. Biotechnol. Bioeng. 2017, 114, 1837–1846. [Google Scholar] [CrossRef]
- Chi, X.; Zheng, Q.; Jiang, R.; Chen-Tsai, R.Y.; Kong, L.J. A system for site-specific integration of transgenes in mammalian cells. PLoS ONE 2019, 14, e0219842. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, D.; Lee, G.M.; Nielsen, L.K.; Grav, L.M. Multicopy targeted integration for accelerated development of high-producing Chinese Hamster Ovary cells. ACS Synth. Biol. 2020, 9, 2546–2561. [Google Scholar] [CrossRef]
- Lee, J.S.; Grav, L.M.; Pedersen, L.E.; Lee, G.M.; Kildegaard, H.F. Accelerated homology-directed targeted integration of transgenes in Chinese Hamster Ovary cells via CRISPR/Cas9 and fluorescent enrichment. Biotechnol. Bioeng. 2016, 113, 2518–2523. [Google Scholar] [CrossRef]
- Chavez, A.; Scheiman, J.; Vora, S.; Pruitt, B.W.; Tuttle, M.; Iyer, E.P.R.; Lin, S.; Kiani, S.; Guzman, C.D.; Wiegand, D.J.; et al. Highly efficient Cas9-mediated transcriptional programming. Nat. Methods 2015, 12, 326–328. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A.; et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 2013, 154, 442–451. [Google Scholar] [CrossRef]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L.; Bassik, M.C.; et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 2014, 159, 647–661. [Google Scholar] [CrossRef]
- Dominguez, A.A.; Lim, W.A.; Qi, L.S. Beyond Editing: Repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat. Rev. Mol. Cell Biol. 2016, 17, 5–15. [Google Scholar] [CrossRef]
- Yeo, N.C.; Chavez, A.; Lance-Byrne, A.; Chan, Y.; Menn, D.; Milanova, D.; Kuo, C.; Guo, X.; Sharma, S.; Tung, A.; et al. An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat. Methods 2018, 15, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Pickar-Oliver, A.; Gersbach, C.A. The next generation of CRISPR–Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 2019, 20, 490–507. [Google Scholar] [CrossRef] [PubMed]
- Ran, F.A.; Cong, L.; Yan, W.X.; Scott, D.A.; Gootenberg, J.S.; Kriz, A.J.; Zetsche, B.; Shalem, O.; Wu, X.; Makarova, K.S.; et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 2015, 520, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, L.; Zou, X.; Duan, S.; Li, Z.; Deng, Z.; Luo, J.; Lee, S.Y.; Chen, S. Advances in CRISPR-Cas systems for RNA targeting, tracking and editing. Biotechnol. Adv. 2019, 37, 708–729. [Google Scholar] [CrossRef]
- Konermann, S.; Lotfy, P.; Brideau, N.J.; Oki, J.; Shokhirev, M.N.; Hsu, P.D. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell 2018, 173, 665–676. [Google Scholar] [CrossRef]
- Liu, L.; Pei, D.S. Insights gained from RNA editing targeted by the CRISPR-Cas13 family. Int. J. Mol. Sci. 2022, 23, 11400. [Google Scholar] [CrossRef]
- Chen, J.S.; Doudna, J.A. The chemistry of Cas9 and its CRISPR colleagues. Nat. Rev. Chem. 2017, 1, 0078. [Google Scholar] [CrossRef]
- Chavez, A.; Tuttle, M.; Pruitt, B.W.; Ewen-Campen, B.; Chari, R.; Ter-Ovanesyan, D.; Haque, S.J.; Cecchi, R.J.; Kowal, E.J.K.; Buchthal, J.; et al. Comparison of Cas9 activators in multiple species. Nat. Methods 2016, 13, 563–567. [Google Scholar] [CrossRef]
- Shamie, I.; Duttke, S.H.; Karottki, K.J.L.C.; Han, C.Z.; Hansen, A.H.; Hefzi, H.; Xiong, K.; Li, S.; Roth, S.J.; Tao, J.; et al. A Chinese hamster transcription start site atlas that enables targeted editing of CHO cells. NAR Genom. Bioinform. 2021, 3, lqab061. [Google Scholar] [CrossRef]
- Karottki, K.J.L.C.; Hefzi, H.; Li, S.; Pedersen, L.E.; Spahn, P.N.; Joshi, C.; Duckerbauer, D.; Bort, J.A.H.; Thomas, A.; Lee, J.S.; et al. A metabolic CRISPR-Cas9 screen in chinese hamster ovary cells identifies glutamine-sensitive genes. Metab. Eng. 2021, 66, 114–122. [Google Scholar] [CrossRef]
- Doench, J.G. Am I Ready for CRISPR? A user’s guide to genetic screens. Nat. Rev. Genet. 2017, 19, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, V.; Novak, N.; Dhiman, H.; Nguyen, L.N.; Serafimova, E.; Klanert, G.; Baumann, M.; Kildegaard, H.F.; Borth, N. A pooled CRISPR/AsCpf1 screen using paired grnas to induce genomic deletions in Chinese hamster ovary cells. Biotechnol. Rep. 2021, 31, e00649. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; la Cour Karottki, K.J.; Hefzi, H.; Li, S.; Grav, L.M.; Li, S.; Spahn, P.; Lee, J.S.; Ventina, I.; Lee, G.M.; et al. An optimized genome-wide, virus-free CRISPR screen for mammalian cells. Cell Rep. Methods 2021, 1, 100062. [Google Scholar] [CrossRef] [PubMed]
- Canver, M.C.; Bauer, D.E.; Dass, A.; Yien, Y.Y.; Chung, J.; Masuda, T.; Maeda, T.; Paw, B.H.; Orkin, S.H. Characterization of genomic deletion efficiency mediated by clustered regularly interspaced palindromic repeats (CRISPR)/Cas9 nuclease system in mammalian cells. J. Biol. Chem. 2014, 289, 21312–21324. [Google Scholar] [CrossRef]
- Koonin, E.V.; Makarova, K.S.; Zhang, F. Diversity, classification and evolution of CRISPR-Cas systems. Curr. Opin. Microbiol. 2017, 37, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Hille, F.; Charpentier, E. CRISPR-Cas: Biology, mechanisms and relevance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150496. [Google Scholar] [CrossRef]
- Zetsche, B.; Gootenberg, J.S.; Abudayyeh, O.O.; Slaymaker, I.M.; Makarova, K.S.; Essletzbichler, P.; Volz, S.E.; Joung, J.; van der Oost, J.; Regev, A.; et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 2015, 163, 759–771. [Google Scholar] [CrossRef]
- Schweickert, P.G.; Wang, N.; Sandefur, S.L.; Lloyd, M.E.; Konieczny, S.F.; Frye, C.C.; Cheng, Z. CRISPR/Cas12a-mediated CHO genome engineering can be effectively integrated at multiple stages of the cell line generation process for bioproduction. Biotechnol. J. 2021, 16, 2000308. [Google Scholar] [CrossRef]
- Bydlinski, N.; Coats, M.T.; Maresch, D.; Strasser, R.; Borth, N. Transfection of glycoprotein encoding mRNA for swift evaluation of N-glycan engineering strategies. Biotechnol. Prog. 2020, 36, e2990. [Google Scholar] [CrossRef]
- Zhang, X.H.; Tee, L.Y.; Wang, X.G.; Huang, Q.S.; Yang, S.H. Off-target effects in CRISPR/Cas9-mediated genome engineering. Mol. Ther. Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef]
- Lee, N.; Shin, J.; Park, J.H.; Lee, G.M.; Cho, S.; Cho, B.K. Targeted gene deletion using DNA-free RNA-guided Cas9 nuclease accelerates adaptation of CHO cells to suspension culture. ACS Synth. Biol. 2016, 5, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, S.; Dad, A.B.K.; Beloor, J.; Gopalappa, R.; Lee, S.K.; Kim, H. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 2014, 24, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Rees, H.A.; Liu, D.R. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018, 19, 770–788. [Google Scholar] [CrossRef] [PubMed]
- Rojek, J.B.; Basavaraju, Y.; Nallapareddy, S.; Dubhe, D.; Ocaña, B.B.; Baumgartner, R.; Grabenhorst, E.; Tharmalingam, T.; Münch, R.; Rhiel, L.; et al. Expanding the CRISPR toolbox for Chinese hamster ovary cells with comprehensive tools for Mad7 genome editing. Biotechnol. Bioeng. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zetsche, B.; Heidenreich, M.; Mohanraju, P.; Fedorova, I.; Kneppers, J.; DeGennaro, E.M.; Winblad, N.; Choudhury, S.R.; Abudayyeh, O.O.; Gootenberg, J.S.; et al. Multiplex gene editing by CRISPR–Cpf1 using a single crRNA array. Nat. Biotechnol. 2017, 35, 31–34. [Google Scholar] [CrossRef]
- Gao, Y.; Xiong, X.; Wong, S.; Charles, E.J.; Lim, W.A.; Qi, L.S. Complex transcriptional modulation with orthogonal and inducible dCas9 regulators. Nat. Methods. 2016, 13, 1043–1049. [Google Scholar] [CrossRef]
- Rupp, O.; MacDonald, M.L.; Li, S.; Dhiman, H.; Polson, S.; Griep, S.; Heffner, K.; Hernandez, I.; Brinkrolf, K.; Jadhav, V.; et al. A reference genome of the Chinese hamster based on a hybrid assembly strategy. Biotechnol. Bioeng. 2018, 115, 2087–2100. [Google Scholar] [CrossRef]
- Hilliard, W.; MacDonald, M.L.; Lee, K.H. Chromosome-scale scaffolds for the Chinese hamster reference genome assembly to facilitate the study of the CHO epigenome. Biotechnol. Bioeng. 2020, 117, 2331–2339. [Google Scholar] [CrossRef]
- Kleinstiver, B.P.; Pattanayak, V.; Prew, M.S.; Tsai, S.Q.; Nguyen, N.T.; Zheng, Z.; Joung, J.K. High-fidelity CRISPR–Cas9 nucleases with no detectable genome-wide off-target effects. Nature 2016, 529, 490–495. [Google Scholar] [CrossRef]
- Nishimasu, H.; Shi, X.; Ishiguro, S.; Gao, L.; Hirano, S.; Okazaki, S.; Noda, T.; Abdudayyeh, O.O.; Gootenberg, J.S.; Mori, H.; et al. Engineered CRISPR-Cas9 nuclease with expanded targeting space. Science 2018, 361, 1259–1262. [Google Scholar] [CrossRef]
- Hu, J.H.; Miller, S.M.; Geurts, M.H.; Tang, W.; Chen, L.; Sun, N.; Zeina, C.M.; Gao, X.; Rees, H.A.; Lin, Z.; et al. Evolved Cas9 variants with broad PAM compatibility and high DNA specificity. Nature 2018, 556, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.W.; Gaidukov, L.; Lai, Y.; Wu, M.R.; Cao, J.; Gutbrod, M.J.; Choi, G.C.G.; Utomo, R.P.; Chen, Y.C.; Wroblewska, L.; et al. A synthetic transcription platform for programmable gene expression in mammalian cells. Nat. Commun. 2022, 13, 6167. [Google Scholar] [CrossRef] [PubMed]
- Carver, J.; Kern, M.; Ko, P.; Greenwood-Goodwin, M.; Yu, X.C.; Duan, D.; Tang, D.; Misaghi, S.; Auslaender, S.; Haley, B.; et al. A ribonucleoprotein-based decaplex CRISPR/Cas9 knockout strategy for CHO host engineering. Biotechnol. Bioeng. 2021, 38, e3212. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glinšek, K.; Bozovičar, K.; Bratkovič, T. CRISPR Technologies in Chinese Hamster Ovary Cell Line Engineering. Int. J. Mol. Sci. 2023, 24, 8144. https://doi.org/10.3390/ijms24098144
Glinšek K, Bozovičar K, Bratkovič T. CRISPR Technologies in Chinese Hamster Ovary Cell Line Engineering. International Journal of Molecular Sciences. 2023; 24(9):8144. https://doi.org/10.3390/ijms24098144
Chicago/Turabian StyleGlinšek, Katja, Krištof Bozovičar, and Tomaž Bratkovič. 2023. "CRISPR Technologies in Chinese Hamster Ovary Cell Line Engineering" International Journal of Molecular Sciences 24, no. 9: 8144. https://doi.org/10.3390/ijms24098144
APA StyleGlinšek, K., Bozovičar, K., & Bratkovič, T. (2023). CRISPR Technologies in Chinese Hamster Ovary Cell Line Engineering. International Journal of Molecular Sciences, 24(9), 8144. https://doi.org/10.3390/ijms24098144