Novel Double Hybrid-Type Bone Cements Based on Calcium Phosphates, Chitosan and Citrus Pectin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Setting Times
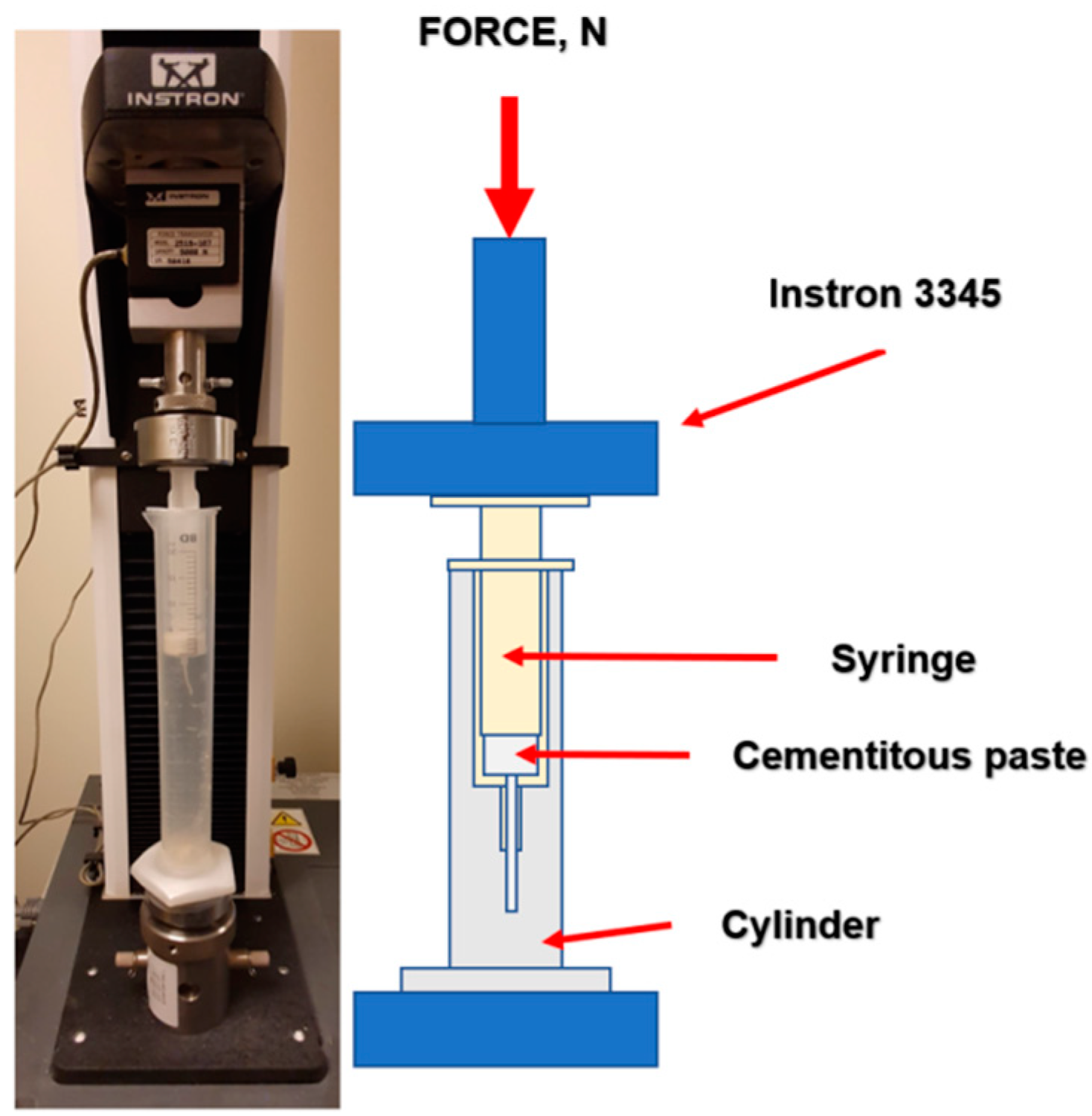
2.2. Injectability
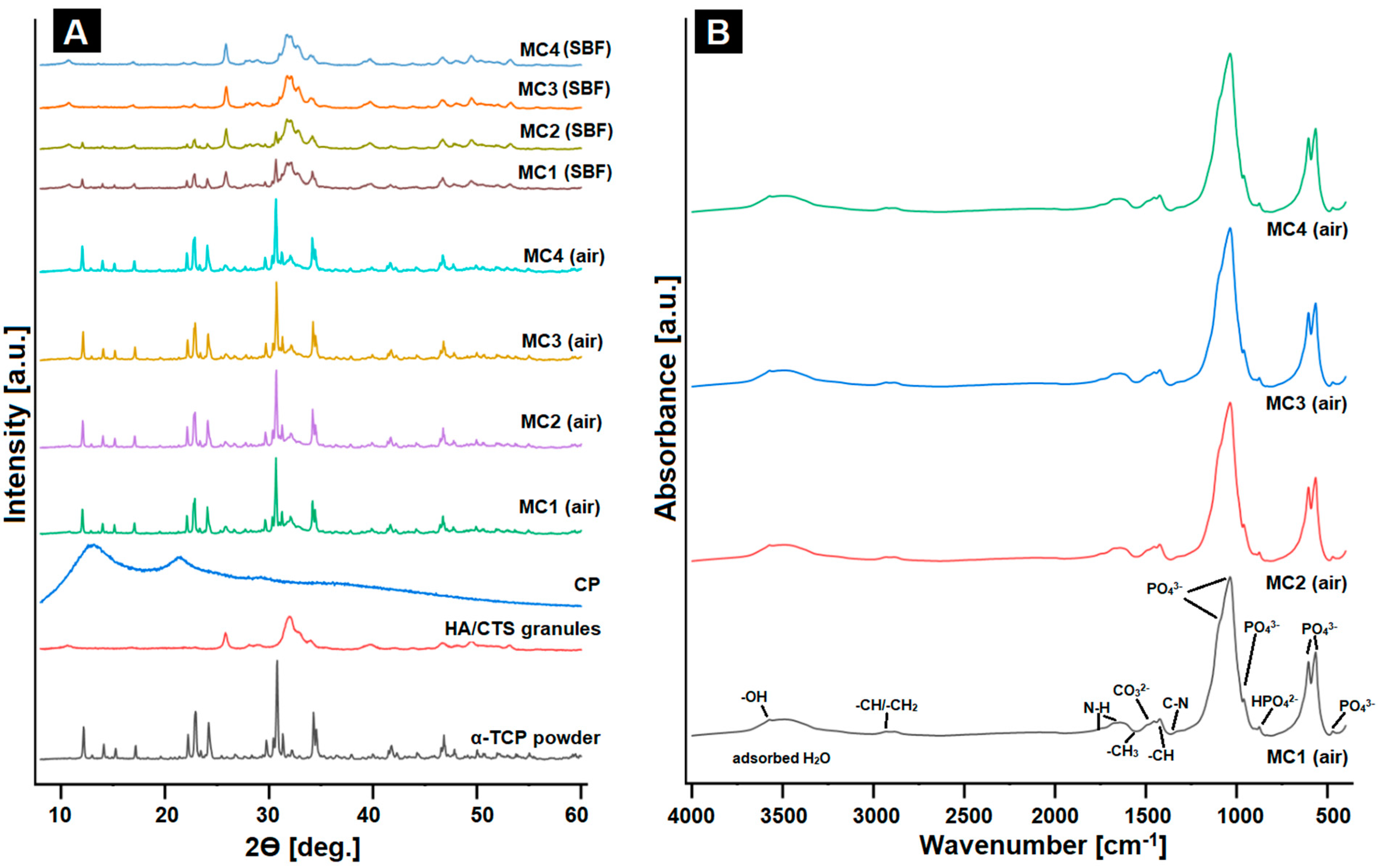
2.3. Phase Composition and Structural Studies
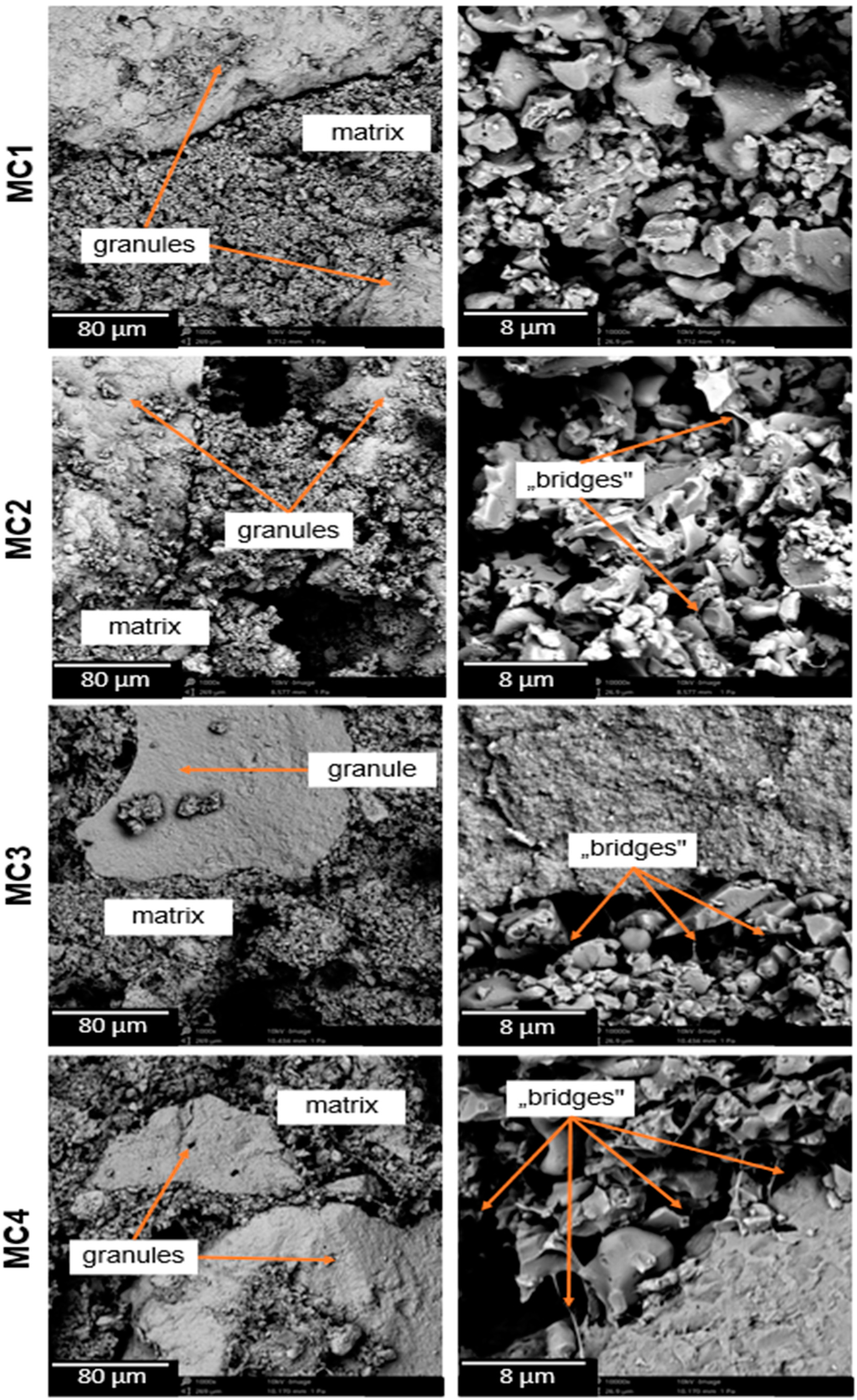
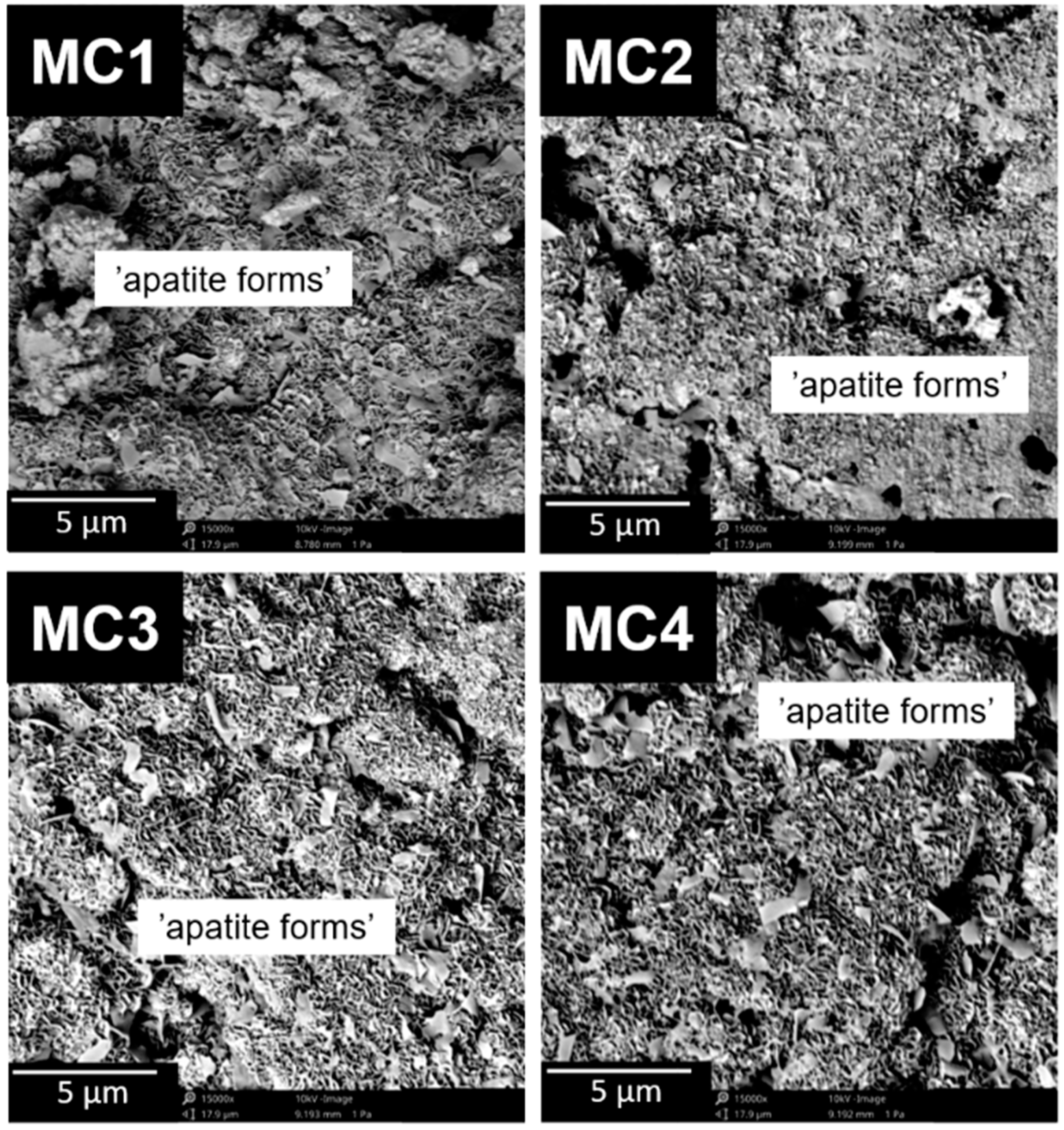
2.4. Microstructure
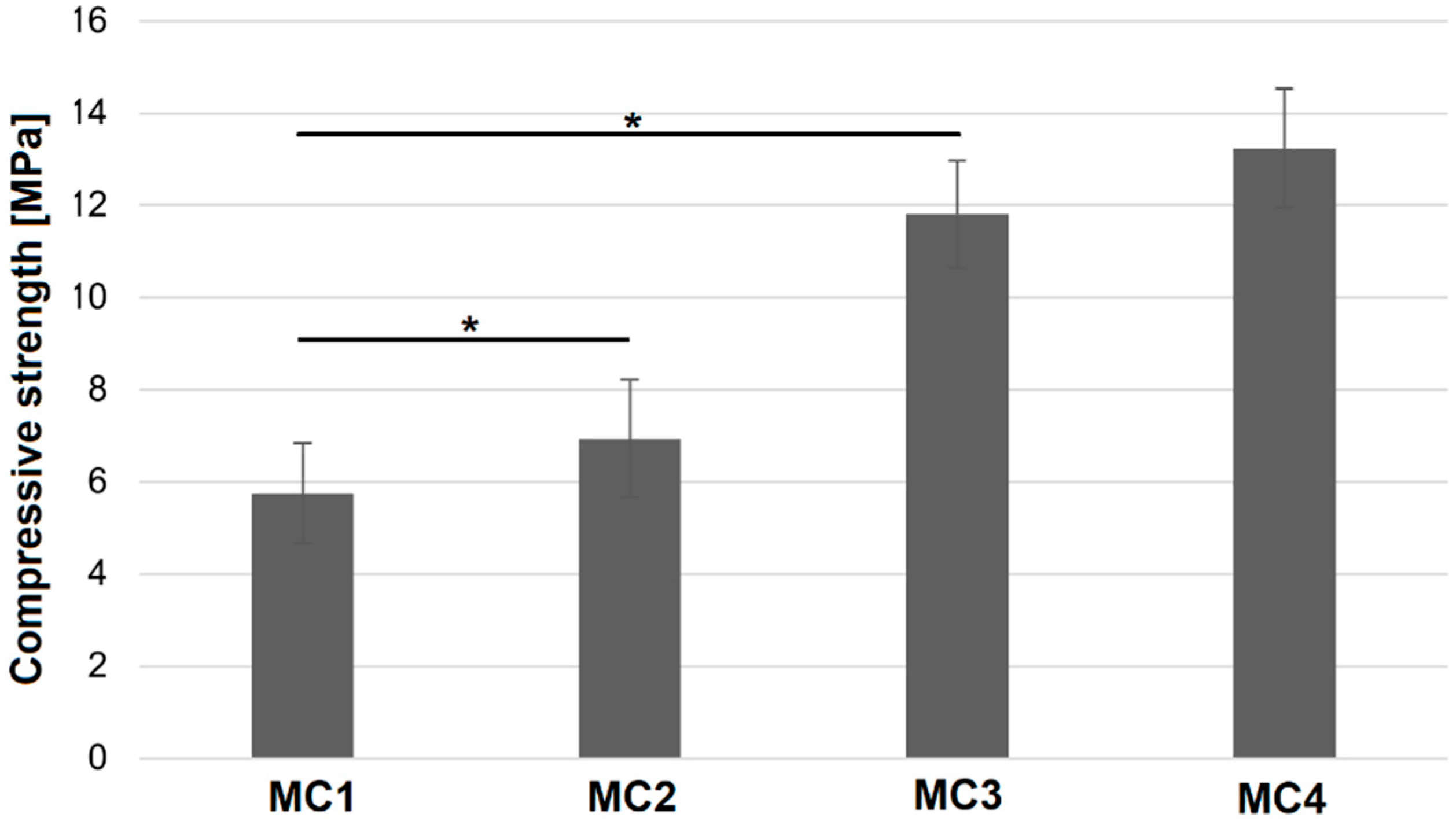
2.5. Compressive Strength
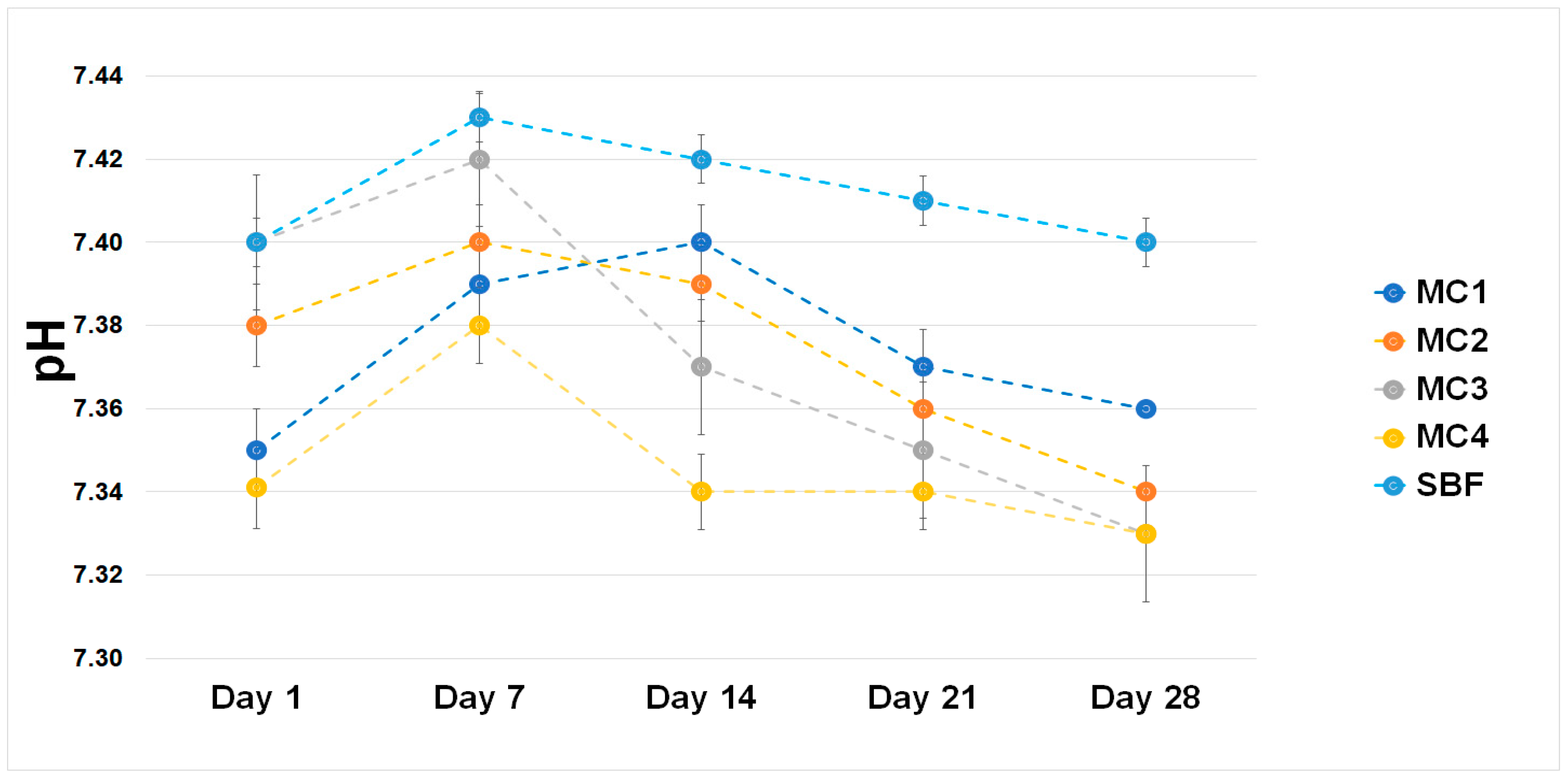
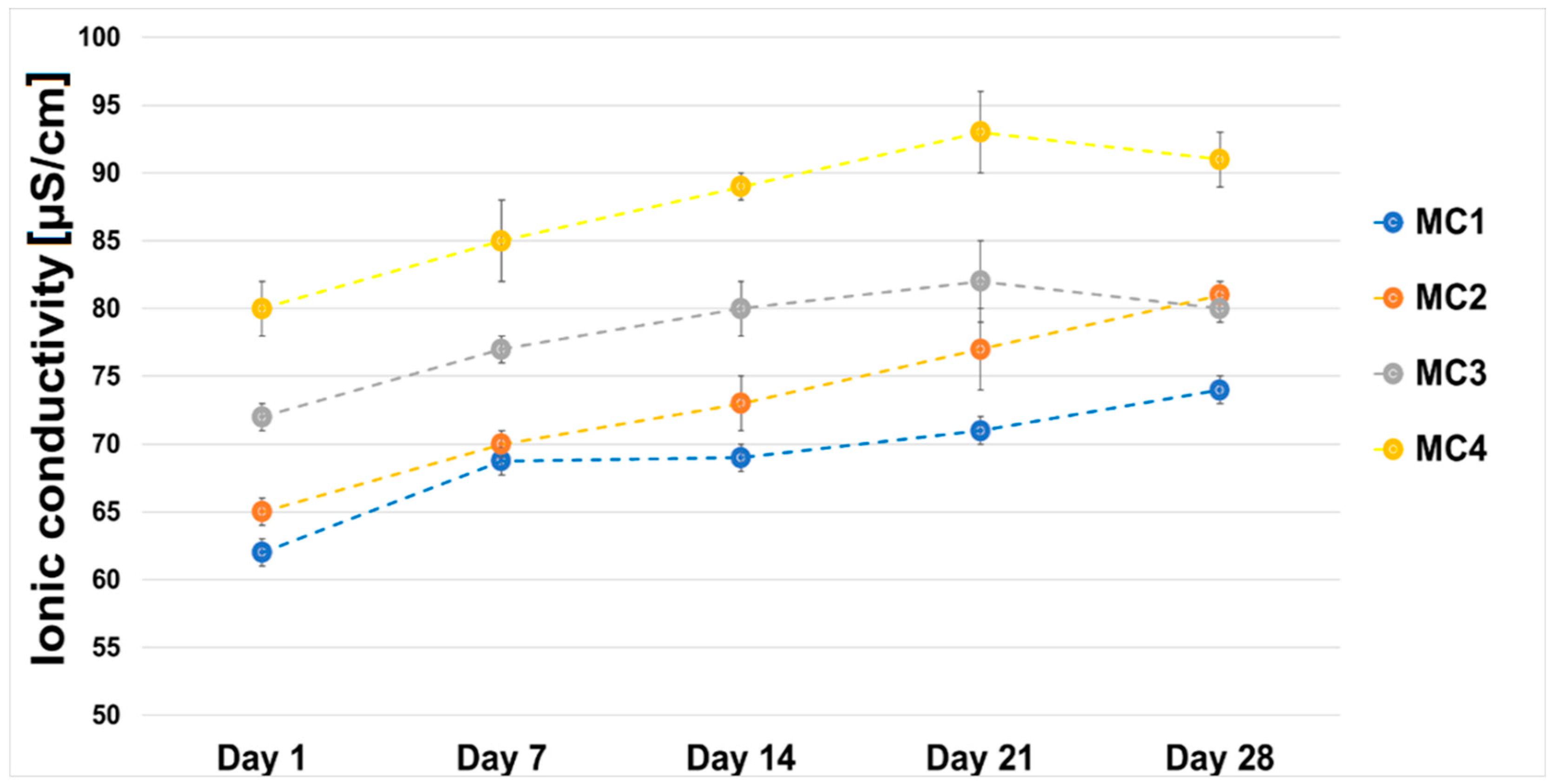
2.6. Chemical Stability and Bioactivity In Vitro
3. Materials and Methods
3.1. Materials
3.2. Preparation of Biomicroconcretes
3.3. Methods
3.3.1. Setting Times
3.3.2. Injectability
3.3.3. Phase Composition
3.3.4. Structural Analysis
3.3.5. Compressive Strength
3.3.6. Microstructure
3.3.7. Chemical Stability and Bioactivity In Vitro
3.3.8. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kashimbetova, A.; Slámečka, K.; Casas-Luna, M.; Oliver-Urrutia, C.; Ravaszová, S.; Dvořák, K.; Čelko, L.; Montufar, E.B. Implications of unconventional setting conditions on the mechanical strength of synthetic bone grafts produced with self-hardening calcium phosphate pastes. Ceram. Int. 2022, 48, 6225–6235. [Google Scholar] [CrossRef]
- Ambard, A.J.; Mueninghoff, L. Calcium Phosphate Cement: Review of Mechanical and Biological Properties. J. Prosthodont. 2006, 15, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Tronco, M.C.; Cassel, J.B.; Santos, L.A.D. α-TCP-based calcium phosphate cements: A critical review. Acta Biomater. 2022, 151, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Vitiello, L.; Sbardella, F.; Santos, J.I.; Tirillò, J.; Bracciale, M.P.; Rivilla, I.; Sarasini, F. Effect of Carbon Nanostructures and Fatty Acid Treatment on the Mechanical and Thermal Performances of Flax/Polypropylene Composites. Polymers 2020, 12, 438. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef]
- Zima, A.; Czechowska, J.; Szponder, T.; Ślósarczyk, A. In vivo behavior of biomicroconcretes based on α-tricalcium phosphate and hybrid hydroxyapatite/chitosan granules and sodium alginate. J. Biomed. Mater. Res. 2020, 108, 1243–1255. [Google Scholar] [CrossRef]
- Czechowska, J.; Cichoń, E.; Belcarz, A.; Ślósarczyk, A.; Zima, A. Effect of Gold Nanoparticles and Silicon on the Bioactivity and Antibacterial Properties of Hydroxyapatite/Chitosan/Tricalcium Phosphate-Based Biomicroconcretes. Materials 2021, 14, 3854. [Google Scholar] [CrossRef]
- De Santis, R.; Russo, T.; Rau, J.V.; Papallo, I.; Martorelli, M.; Gloria, A. Design of 3D Additively Manufactured Hybrid Structures for Cranioplasty. Materials 2021, 14, 181. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Gauthier, O.; Sourice, S.; Pilet, P.; Rethore, G.; Khairoun, K.; Bouler, J.-M.; Tancret, F.; Weiss, P. A simple and effective approach to prepare injectable macroporous calcium phosphate cement for bone repair: Syringe-foaming using a viscous hydrophilic polymeric solution. Acta Biomater. 2016, 31, 326–338. [Google Scholar] [CrossRef]
- Jang, J.-H.; Shin, S.; Kim, H.-J.; Jeong, J.; Jin, H.-E.; Desai, M.S.; Lee, S.-W.; Kim, S.-Y. Improvement of physical properties of calcium phosphate cement by elastin-like polypeptide supplementation. Sci. Rep. 2018, 8, 5216. [Google Scholar] [CrossRef]
- Saveleva, M.S.; Eftekhari, K.; Abalymov, A.; Douglas, T.E.L.; Volodkin, D.; Parakhonskiy, B.V.; Skirtach, A.G. Hierarchy of Hybrid Materials—The Place of Inorganics-in-Organics in it, Their Composition and Applications. Front. Chem. 2019, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Hashim, A.F.; Youssef, K.; Roberto, S.R.; Abd-Elsalam, K.A. Hybrid inorganic-polymer nanocomposites: Synthesis, characterization, and plant-protection applications. In Multifunctional Hybrid Nanomaterials for Sustainable Agri-Food and Ecosystems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 33–49. [Google Scholar] [CrossRef]
- An, J.; Wolke, J.G.C.; Jansen, J.A.; Leeuwenburgh, S.C.G. Influence of polymeric additives on the cohesion and mechanical properties of calcium phosphate cements. J. Mater. Sci. Mater. Med. 2016, 27, 58. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Dziadek, K.; Salagierski, S.; Drozdowska, M.; Serafim, A.; Stancu, I.-C.; Szatkowski, P.; Kopec, A.; Rajzer, I.; Douglas, T.E.; et al. Newly crosslinked chitosan- and chitosan-pectin-based hydrogels with high antioxidant and potential anticancer activity. Carbohydr. Polym. 2022, 290, 119486. [Google Scholar] [CrossRef]
- Pellis, A.; Guebitz, G.M.; Nyanhongo, G.S. Chitosan: Sources, Processing and Modification Techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; El-Zairy, E.M.R. Chitosan as a Biomaterial—Structure, Properties, and Electrospun Nanofibers. In Concepts, Compounds and the Alternatives of Antibacterials; Bobbarala, V., Ed.; InTech: London, UK, 2015. [Google Scholar] [CrossRef]
- Singhal, S.; Hulle, N.R.S. Citrus pectins: Structural properties, extraction methods, modifications and applications in food systems—A review. Appl. Food Res. 2022, 2, 100215. [Google Scholar] [CrossRef]
- Minzanova, S.; Mironov, V.F.; Arkhipova, D.M.; Khabibullina, A.V.; Mironova, L.G.; Zakirova, Y.M.; Milyukov, V.A. Biological Activity and Pharmacological Application of Pectic Polysaccharides: A Review. Polymers 2018, 10, 1407. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Marudova, M.; MacDougall, A.J.; Ring, S.G. Pectin–chitosan interactions and gel formation. Carbohydr. Res. 2004, 339, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Czechowska, J.; Zima, A.; Paszkiewicz, Z.; Lis, J.; Ślósarczyk, A. Physicochemical properties and biomimetic behaviour of α-TCP-chitosan based materials. Ceram. Int. 2014, 40, 5523–5532. [Google Scholar] [CrossRef]
- de Almeida, D.A.; Sabino, R.M.; Souza, P.R.; Bonafé, E.G.; Venter, S.A.; Popat, K.C.; Martins, A.F.; Monteiro, J.P. Pectin-capped gold nanoparticles synthesis in-situ for producing durable, cytocompatible, and superabsorbent hydrogel composites with chitosan. Int. J. Biol. Macromol. 2020, 147, 138–149. [Google Scholar] [CrossRef]
- Martins, J.G.; Camargo, S.E.A.; Bishop, T.T.; Popat, K.C.; Kipper, M.J.; Martins, A.F. Pectin-chitosan membrane scaffold imparts controlled stem cell adhesion and proliferation. Carbohydr. Polym. 2018, 197, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Wong, Y.H.; Chin, K.-Y.; Ima-Nirwana, S. A Review on the Enhancement of Calcium Phosphate Cement with Biological Materials in Bone Defect Healing. Polymers 2021, 13, 3075. [Google Scholar] [CrossRef]
- Nicolle, L.; Journot, C.M.A.; Gerber-Lemaire, S. Chitosan Functionalization: Covalent and Non-Covalent Interactions and Their Characterization. Polymers 2021, 13, 4118. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, W.; Liu, C.-M.; Li, T.; Liang, R.-H.; Luo, S.-J. Pectin Modifications: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1684–1698. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Annapure, U.S. Trends in “green” and novel methods of pectin modification—A review. Carbohydr. Polym. 2022, 278, 118967. [Google Scholar] [CrossRef] [PubMed]
- Geffers, M.; Barralet, J.E.; Groll, J.; Gbureck, U. Dual-setting brushite–silica gel cements. Acta Biomater. 2015, 11, 467–476. [Google Scholar] [CrossRef]
- Christel, T.; Kuhlmann, M.; Vorndran, E.; Groll, J.; Gbureck, U. Dual setting α-tricalcium phosphate cements. J. Mater. Sci. Mater. Med. 2013, 24, 573–581. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Fernández, E.; Boltong, M.G.; Bermúdez, O.; Planell, J.A.; Driessens, F.C.M. Compliance of an apatitic calcium phosphate cement with the short-term clinical requirements in bone surgery, orthopaedics and dentistry. Clin. Mater. 1994, 17, 99–104. [Google Scholar] [CrossRef]
- Sugawara, A.; Asaoka, K.; Ding, S.-J. Calcium phosphate-based cements: Clinical needs and recent progress. J. Mater. Chem. B 2013, 1, 1081–1089. [Google Scholar] [CrossRef]
- Smith, B.T.; Lu, A.; Watson, E.; Santoro, M.; Melchiorri, A.J.; Grosfeld, E.C.; Beucken, J.J.v.D.; Jansen, J.A.; Scott, D.W.; Fisher, J.P.; et al. Incorporation of fast dissolving glucose porogens and poly(lactic-co-glycolic acid) microparticles within calcium phosphate cements for bone tissue regeneration. Acta Biomater. 2018, 78, 341–350. [Google Scholar] [CrossRef]
- Şahin, E. Calcium Phosphate Bone Cements. In Cement Based Materials; Saleh, H.E.-D.M., Rahman, R.O.A., Eds.; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- He, Z.; Zhai, Q.; Hu, M.; Cao, C.; Wang, J.; Yang, H.; Li, B. Bone cements for percutaneous vertebroplasty and balloon kyphoplasty: Current status and future developments. J. Orthop. Transl. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Durucan, C.; Brown, P.W. Reactivity of α-tricalcium phosphate. J. Mater. Sci. 2002, 37, 963–969. [Google Scholar] [CrossRef]
- Rabiee, S.M.; Baseri, H. Prediction of the Setting Properties of Calcium Phosphate Bone Cement. Comput. Intell. Neurosci. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Zima, A.; Cichoń, E.; Czechowska, J.; Ślósarczyk, A. Biomicroconcretes based on the hybrid HAp/CTS granules, α-TCP and pectins as a novel injectable bone substitutes. Mater. Lett. 2020, 265, 127457. [Google Scholar] [CrossRef]
- Larsson, S.; Hannink, G. Injectable bone-graft substitutes: Current products, their characteristics and indications, and new developments. Injury 2011, 42, S30–S34. [Google Scholar] [CrossRef]
- Ginebra, M.-P.; Montufar, E.B. Cements as bone repair materials. In Bone Repair Biomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 233–271. [Google Scholar] [CrossRef]
- O’Neill, R.; McCarthy, H.; Montufar, E.; Ginebra, M.-P.; Wilson, D.; Lennon, A.; Dunne, N. Critical review: Injectability of calcium phosphate pastes and cements. Acta Biomater. 2017, 50, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Arkin, V.H.; Narendrakumar, U.; Madhyastha, H.; Manjubala, I. Characterization and In Vitro Evaluations of Injectable Calcium Phosphate Cement Doped with Magnesium and Strontium. ACS Omega 2021, 6, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Yao, B.; Gao, M.; Sun, X.; Gou, D.; Hu, J.; Zhou, Y.; Liu, Y. Effects of pectin structure and crosslinking method on the properties of crosslinked pectin nanofibers. Carbohydr. Polym. 2017, 157, 766–774. [Google Scholar] [CrossRef]
- da Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Alginate and pectin composite films crosslinked with Ca2+ ions: Effect of the plasticizer concentration. Carbohydr. Polym. 2009, 77, 736–742. [Google Scholar] [CrossRef]
- Zima, A.; Czechowska, J.; Siek, D.; Ślósarczyk, A. Influence of magnesium and silver ions on rheological properties of hydroxyapatite/chitosan/calcium sulphate based bone cements. Ceram. Int. 2017, 43, 16196–16203. [Google Scholar] [CrossRef]
- Stephen, A.M.; Cummings, J.H. Water-holding by dietary fibre in vitro and its relationship to faecal output in man. Gut 1979, 20, 722–729. [Google Scholar] [CrossRef]
- Boulos, N.N.; Greenfield, H.; Wills, R.B.H. Water holding capacity of selected soluble and insoluble dietary fibre. Int. J. Food Prop. 2000, 3, 217–231. [Google Scholar] [CrossRef]
- Gao, J.; Qiao, L.; Li, L.; Wang, Y. Hemolysis effect and calcium-phosphate precipitation of heat-organic-film treated magnesium. Trans. Nonferrous Met. Soc. China 2006, 16, 539–544. [Google Scholar] [CrossRef]
- Cichoń, E.; Ślósarczyk, A.; Zima, A. Influence of Selected Surfactants on Physicochemical Properties of Calcium Phosphate Bone Cements. Langmuir 2019, 35, 13656–13662. [Google Scholar] [CrossRef]
- Rashidova, S.S.; Milusheva, R.Y.; Semenova, L.N.; Mukhamedjanova, M.Y.; Voropaeva, N.L.; Vasilyeva, S.; Faizieva, R.; Ruban, I.N. Characteristics of Interactions in the Pectin?Chitosan System. Chromatographia 2004, 59, 11–12. [Google Scholar] [CrossRef]
- Vaishya, R.; Chauhan, M.; Vaish, A. Bone cement. J. Clin. Orthop. Trauma 2013, 4, 157–163. [Google Scholar] [CrossRef]
- Gerhardt, L.-C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef]
- Hopkins, E.; Sanvictores, T.; Sharma, S. Physiology, Acid Base Balance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: http://www.ncbi.nlm.nih.gov/books/NBK507807/ (accessed on 25 November 2022).
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Li, X.; Bi, J.; Jin, X.; Li, X.; Zhao, Y.; Song, Y. Effect of pectin osmosis or degradation on the water migration and texture properties of apple cube dried by instant controlled pressure drop drying (DIC). LWT 2020, 125, 109202. [Google Scholar] [CrossRef]
- Khattab, A.M. The Microbial Degradation for Pectin. In Pectins—The New-Old Polysaccharides; Masuelli, M.A., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Klinchongkon, K.; Khuwijitjaru, P.; Adachi, S. Degradation kinetics of passion fruit pectin in subcritical water. Biosci. Biotechnol. Biochem. 2017, 81, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Kolmas, J.; Kaflak, A.; Zima, A.; Ślósarczyk, A. Alpha-tricalcium phosphate synthesized by two different routes: Structural and spectroscopic characterization. Ceram. Int. 2015, 41, 5727–5733. [Google Scholar] [CrossRef]
- Zima, A. Hydroxyapatite-chitosan based bioactive hybrid biomaterials with improved mechanical strength. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2018, 193, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Komath, M.; Varma, H.K.; Sivakumar, R. On the development of an apatitic calcium phosphate bone cement. Bull. Mater. Sci. 2000, 23, 135–140. [Google Scholar] [CrossRef]
- C01 Committee. Test Method for Time of Setting of Hydraulic-Cement Paste by Gillmore Needles; ASTM International: Singapore, 2020. [Google Scholar] [CrossRef]

| Material | Initial Setting Time (ti) [min] | Final Setting Time (tf) [min] |
|---|---|---|
| MC1 | 4.5 ± 1.0 | 7.5 ± 0.5 |
| MC2 | 9.0 ± 0.5 | 16.5 ± 1.0 |
| MC3 | 11.5 ± 0.5 | 21.0 ± 1.0 |
| MC4 | 17.5 ± 1.0 | 32.0 ± 1.5 |
| MC5 | 30.5 ± 0.5 | 55.5 ± 1.0 |
| Material | 7 Days in Air | 7 Days in SBF | ||
|---|---|---|---|---|
| α-TCP, wt% | Hydroxyapatite, wt% | α-TCP, wt% | Hydroxyapatite, wt% | |
| MC1 | 54 ± 1 | 46 ± 1 | 2 ± 1 | 98 ± 1 |
| MC2 | 56 ± 1 | 44 ± 1 | 2 ± 1 | 98 ± 1 |
| MC3 | 59 ± 1 | 41 ± 1 | 3 ± 1 | 97 ± 1 |
| MC4 | 61 ± 1 | 39 ± 1 | 3 ± 1 | 97 ± 1 |
| Material | Solid Phase (P) | Liquid Phase (L) |
|---|---|---|
| MC1 (Control) | α-TCP: HA/CTS granules 3:2 | 2.0 wt% Na2HPO4 solution |
| MC2 | 1.5 wt% Na2HPO4 solution in 1.25 wt% citrus pectin gel | |
| MC3 | 1.0 wt% Na2HPO4 solution in 2.5 wt% citrus pectin gel | |
| MC4 | 0.5 wt% Na2HPO4 solution in 3.75 wt% citrus pectin gel | |
| MC5 | 5.0 wt% citrus pectin gel |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pańtak, P.; Czechowska, J.P.; Cichoń, E.; Zima, A. Novel Double Hybrid-Type Bone Cements Based on Calcium Phosphates, Chitosan and Citrus Pectin. Int. J. Mol. Sci. 2023, 24, 13455. https://doi.org/10.3390/ijms241713455
Pańtak P, Czechowska JP, Cichoń E, Zima A. Novel Double Hybrid-Type Bone Cements Based on Calcium Phosphates, Chitosan and Citrus Pectin. International Journal of Molecular Sciences. 2023; 24(17):13455. https://doi.org/10.3390/ijms241713455
Chicago/Turabian StylePańtak, Piotr, Joanna P. Czechowska, Ewelina Cichoń, and Aneta Zima. 2023. "Novel Double Hybrid-Type Bone Cements Based on Calcium Phosphates, Chitosan and Citrus Pectin" International Journal of Molecular Sciences 24, no. 17: 13455. https://doi.org/10.3390/ijms241713455
APA StylePańtak, P., Czechowska, J. P., Cichoń, E., & Zima, A. (2023). Novel Double Hybrid-Type Bone Cements Based on Calcium Phosphates, Chitosan and Citrus Pectin. International Journal of Molecular Sciences, 24(17), 13455. https://doi.org/10.3390/ijms241713455











